Recent Development on the Synthesis Strategies and Mechanisms of Co3O4-Based Electrocatalysts for Oxygen Evolution Reaction: A Review
Abstract
1. Introduction
2. External Catalytic Performance
2.1. Active Surface Areas and Active Sites Enhancements
2.1.1. Morphology Engineering
2.1.2. Catalyst Anchoring
2.2. Material Transport Channels
2.2.1. Catalyst Structure for Material Transfer Control
2.2.2. Combination with Conductive Materials to Reduce Resistance
3. Intrinsic Activity Enhancement
3.1. Defect Effect
3.2. Synergistic Effect
4. Conclusions and Outlook
- (1)
- Establish a set of standard catalytic evaluation systems.
- (2)
- Integrating favorable factors.
- (3)
- Industrialization synthesis of the catalyst.
- (4)
- Better understanding of the OER mechanism of Co3O4-based catalyst.
- (5)
- Challenges and opportunities for practical applications of Co3O4-based catalysts.
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, C.; Liu, J.; Li, B.; Ren, D.; Chen, X.; Yu, J.; Zhang, Q. Multiscale Construction of Bifunctional Electrocatalysts for Long-Lifespan Rechargeable Zinc–Air Batteries. Adv. Funct. Mater. 2020, 30, 2003619. [Google Scholar] [CrossRef]
- Zhao, C.X.; Liu, J.N.; Wang, J.; Ren, D.; Yu, J.; Chen, X.; Li, B.Q.; Zhang, Q. A ΔE= 0.63 V Bifunctional Oxygen Electrocatalyst Enables High-Rate and Long-Cycling Zinc–Air Batteries. Adv. Mater. 2021, 33, 2008606. [Google Scholar] [CrossRef]
- Ni, Z.; Liang, H.; Yi, Z.; Guo, R.; Liu, C.; Liu, Y.; Sun, H.; Liu, X. Research progress of electrochemical CO2 reduction for copper-based catalysts to multicarbon products. Coord. Chem. Rev. 2021, 441, 213983. [Google Scholar] [CrossRef]
- Chi, J.; Yu, H. Water electrolysis based on renewable energy for hydrogen production. Chin. J. Catal. 2018, 39, 390–394. [Google Scholar] [CrossRef]
- Grigoriev, S.; Fateev, V.; Bessarabov, D.; Millet, P. Current status, research trends, and challenges in water electrolysis science and technology. Int. J. Hydrogen Energy 2020, 45, 26036–26058. [Google Scholar] [CrossRef]
- Ursua, A.; Gandia, L.M.; Sanchis, P. Hydrogen Production from Water Electrolysis: Current Status and Future Trends. Proc. IEEE 2012, 100, 410–426. [Google Scholar] [CrossRef]
- Wei, Y.; Zheng, M.; Zhu, W.; Zhang, Y.; Hu, W.; Pang, H. Preparation of hierarchical hollow CoFe Prussian blue analogues and its heat-treatment derivatives for the electrocatalyst of oxygen evolution reaction. J. Colloid Interface Sci. 2022, 631, 8–16. [Google Scholar] [CrossRef]
- Xu, Q.-Z.; Su, Y.-Z.; Wu, H.; Cheng, H.; Guo, Y.-P.; Li, N.; Liu, Z.-Q. Effect of Morphology of Co3O4 for Oxygen Evolution Reaction in Alkaline Water Electrolysis. Curr. Nanosci. 2014, 11, 107–112. [Google Scholar] [CrossRef]
- Chen, D.; Chen, C.; Baiyee, Z.M.; Shao, Z.; Ciucci, F. Nonstoichiometric Oxides as Low-Cost and Highly-Efficient Oxygen Reduction/Evolution Catalysts for Low-Temperature Electrochemical Devices. Chem. Rev. 2015, 115, 9869–9921. [Google Scholar] [CrossRef]
- Tong, W.; Forster, M.; Dionigi, F.; Dresp, S.; Erami, R.S.; Strasser, P.; Cowan, A.J.; Farràs, P. Electrolysis of low-grade and saline surface water. Nat. Energy 2020, 5, 367–377. [Google Scholar] [CrossRef]
- Sun, H.; Yan, Z.; Liu, F.; Xu, W.; Cheng, F.; Chen, J. Self-Supported Transition-Metal-Based Electrocatalysts for Hydrogen and Oxygen Evolution. Adv. Mater. 2019, 32, e1806326. [Google Scholar] [CrossRef]
- Yu, Z.; Duan, Y.; Feng, X.; Yu, X.; Gao, M.; Yu, S. Clean and Affordable Hydrogen Fuel from Alkaline Water Splitting: Past, Recent Progress, and Future Prospects. Adv. Mater. 2021, 33, 2007100. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, P.; Wang, Z.; Gao, L. Shape–Preserved CoFeNi–MOF/NF Exhibiting Superior Performance for Overall Water Splitting across Alkaline and Neutral Conditions. Materials 2024, 17, 2195. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Dong, Y.; Wang, X.; Chen, L. Electrocatalysts for the Oxygen Evolution Reaction in Acidic Media. Adv. Mater. 2023, 35, e2210565. [Google Scholar] [CrossRef]
- Brauns, J.; Turek, T. Alkaline Water Electrolysis Powered by Renewable Energy: A Review. Processes 2020, 8, 248. [Google Scholar] [CrossRef]
- Jamesh, M.-I.; Harb, M. Tuning the electronic structure of the earth-abundant electrocatalysts for oxygen evolution reaction (OER) to achieve efficient alkaline water splitting—A review. J. Energy Chem. 2021, 56, 299–342. [Google Scholar] [CrossRef]
- Wei, J.; Zhou, M.; Long, A.; Xue, Y.; Liao, H.; Wei, C.; Xu, Z.J. Heterostructured Electrocatalysts for Hydrogen Evolution Reaction Under Alkaline Conditions. Nano-Micro Lett. 2018, 10, 1–15. [Google Scholar] [CrossRef]
- Li, X.-R.; Zhai, X.-J.; Liu, H.-Z.; Wen, X.; Qi, Y.; Zhang, C.; Li, G.; Chai, Y.-M.; Dong, B. The effect of ligand regulation on mechanism of alkaline oxygen evolution reaction under industrial scale. Chem. Eng. J. 2025, 519, 165624. [Google Scholar] [CrossRef]
- Li, Z.; Wei, P.; Wang, G. Recent Advances on Perovskite Electrocatalysts for Water Oxidation in Alkaline Medium. Energy Fuels 2022, 36, 11724–11744. [Google Scholar] [CrossRef]
- Linke, J.; Rohrbach, T.; Ranocchiari, M.; Schmidt, T.J.; Fabbri, E. Enlightening the journey of metal-organic framework (derived) catalysts during the oxygen evolution reaction in alkaline media via operando X-ray absorption spectroscopy. Curr. Opin. Electrochem. 2021, 30, 100845. [Google Scholar] [CrossRef]
- Chang, C.-J.; Chu, Y.-C.; Yan, H.-Y.; Liao, Y.-F.; Chen, H.M. Revealing the structural transformation of rutile RuO2 via in situ X-ray absorption spectroscopy during the oxygen evolution reaction. Dalton Trans. 2019, 48, 7122–7129. [Google Scholar] [CrossRef]
- Lee, Y.; Suntivich, J.; May, K.J.; Perry, E.E.; Shao-Horn, Y. Synthesis and Activities of Rutile IrO2 and RuO2 Nanoparticles for Oxygen Evolution in Acid and Alkaline Solutions. J. Phys. Chem. Lett. 2012, 3, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Stoerzinger, K.A.; Qiao, L.; Biegalski, M.D.; Shao-Horn, Y. Orientation-Dependent Oxygen Evolution Activities of Rutile IrO2 and RuO2. J. Phys. Chem. Lett. 2014, 5, 1636–1641. [Google Scholar] [CrossRef] [PubMed]
- Alex, C.; Sarma, S.C.; Peter, S.C.; John, N.S. Competing Effect of Co3+ Reducibility and Oxygen-Deficient Defects Toward High Oxygen Evolution Activity in Co3O4Systems in Alkaline Medium. ACS Appl. Energy Mater. 2020, 3, 5439–5447. [Google Scholar] [CrossRef]
- Liu, S.; Li, L.; Patterson, N.A.; Manthiram, A. Morphological Transformations during In Situ Electrochemical Generation of 2-Dimensional Co3O4Hexagonal Nanoplates. J. Electrochem. Soc. 2015, 163, A150–A155. [Google Scholar] [CrossRef]
- Peña, N.O.; Ihiawakrim, D.; Han, M.; Lassalle-Kaiser, B.; Carenco, S.; Sanchez, C.; Laberty-Robert, C.; Portehault, D.; Ersen, O. Morphological and Structural Evolution of Co3O4 Nanoparticles Revealed by in situ Electrochemical Transmission Electron Microscopy during Electrocatalytic Water Oxidation. ACS Nano 2019, 13, 11372–11381. [Google Scholar] [CrossRef]
- Sagar, P.; Shreenivasa, L.; Syed, A.; Marraiki, N.; Ashoka, S. Validation of enhanced OER performance of the amorphous Al2O3-added Co3O4/NiO two-dimensional ternary nanocomposite. Chem. Pap. 2021, 76, 1433–1441. [Google Scholar] [CrossRef]
- Srinivasa, N.; Shreenivasa, L.; Ashoka, S.; Yogesh, K. Studies on Co3O4–NiO nanocomposites for potential electrocatalyst for alkaline water electrolysis. Appl. Phys. A 2022, 128, 1–9. [Google Scholar] [CrossRef]
- Rathla, K.S.G.; Jagadisha, A.S.; Nagaraja, E.; Kumar, B.N.P.; Prasanna, D.G.; Umesha, S.D. Studies on oxygen evolution reaction performance of porous Co3O4–NiO–B2O3 composites. Chem. Pap. 2022, 77, 867–875. [Google Scholar] [CrossRef]
- Liu, N.; Guan, J. Core–shell Co3O4@FeOx catalysts for efficient oxygen evolution reaction. Mater. Today Energy 2021, 21, 100715. [Google Scholar] [CrossRef]
- Niyitanga, T.; Kim, H. FeO-Co3O4 Hybrid Composite on Oxidized Graphite: Single-Stage Hydrothermal Synthesis and Application as an Electrocatalyst for Oxygen Evolution Reaction. J. Electrochem. Soc. 2022, 169, 106510. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Chen, W.; Xiao, Z.; Hu, Z.; Lu, Y.-R.; Chen, J.-L.; Chen, C.-L.; Lin, H.-J.; Chen, C.-T.; Arul, K.T.; et al. In Situ/Operando Soft X-ray Spectroscopic Identification of a Co4+ Intermediate in the Oxygen Evolution Reaction of Defective Co3O4 Nanosheets. J. Phys. Chem. Lett. 2022, 13, 8386–8396. [Google Scholar] [CrossRef]
- Du, H.; Pu, W.; Yang, C. Morphology control of Co3O4 with nickel incorporation for highly efficient oxygen evolution reaction. Appl. Surf. Sci. 2021, 541, 148221. [Google Scholar] [CrossRef]
- Du, J.; Li, C.; Tang, Q. Oxygen vacancies enriched Co3O4 nanoflowers with single layer porous structures for water splitting. Electrochim. Acta 2020, 331, 135456. [Google Scholar] [CrossRef]
- Feng, Y.; Li, Z.; Cheng, C.-Q.; Kang, W.-J.; Mao, J.; Shen, G.-R.; Yang, J.; Dong, C.-K.; Liu, H.; Du, X.-W. Strawberry-like Co3O4-Ag bifunctional catalyst for overall water splitting. Appl. Catal. B Environ. 2021, 299, 120658. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, X.; Bao, A.; Dong, L.; Zhang, X.; Pan, H.; Cui, W.; Qi, X. Enhancing electrical conductivity of single-atom doped Co3O4 nanosheet arrays at grain boundary by phosphor doping strategy for efficient water splitting. Nano Res. 2022, 15, 9511–9519. [Google Scholar] [CrossRef]
- Guo, B.; Ma, R.; Li, Z.; Luo, J.; Yang, M.; Wang, J. Dual-doping of ruthenium and nickel into Co3O4 for improving the oxygen evolution activity. Mater. Chem. Front. 2020, 4, 1390–1396. [Google Scholar] [CrossRef]
- Hu, Z.; Hao, L.; Quan, F.; Guo, R. Recent developments of Co3O4-based materials as catalysts for the oxygen evolution reaction. Catal. Sci. Technol. 2021, 12, 436–461. [Google Scholar] [CrossRef]
- Kéranguéven, G.; Filimonenkov, I.S.; Savinova, E.R. Investigation of the stability of the boron-doped diamond support for Co3O4-based oxygen evolution reaction catalysts synthesized through in situ autocombustion method. J. Electroanal. Chem. 2022, 916, 116367. [Google Scholar] [CrossRef]
- Gong, Y.; Xu, Z.; Pan, H. Facile Synthesis and Characterization of MOF-Derived Porous Co3O4 Composite for Oxygen Evolution Reaction. Chem. Select 2019, 4, 1131–1137. [Google Scholar] [CrossRef]
- Yan, X.; Zhuang, L.; Zhu, Z.; Yao, X. Defect engineering and characterization of active sites for efficient electrocatalysis. Nanoscale 2021, 13, 3327–3345. [Google Scholar] [CrossRef]
- Qiao, C.; Hao, Y.; Cao, C.; Zhang, J. Transformation mechanism of high-valence metal sites for the optimization of Co- and Ni-based OER catalysts in an alkaline environment: Recent progress and perspectives. Nanoscale 2022, 15, 450–460. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, Y.; Li, X.; Cao, H.; Guo, Y.; Zhang, C. Mn-incorporated Co3O4 bifunctional electrocatalysts for zinc-air battery application: An experimental and DFT study. Appl. Catal. B Environ. 2022, 319, 121909. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, L.; Wang, Z.; Bu, D.; Zhan, K.; Yan, Y.; Yang, J.; Zhao, B. N and Mn dual-doped cactus-like cobalt oxide nanoarchitecture derived from cobalt carbonate hydroxide as efficient electrocatalysts for oxygen evolution reactions. J. Colloid Interface Sci. 2021, 597, 361–369. [Google Scholar] [CrossRef]
- Yu, Z.; Lin, Y.-H.; Gao, X.-T.; Guo, B.; Ma, J.-L.; Zhang, Y.; Bai, F.-Y.; Dong, Y.-W.; Zhao, Z. Prussian-blue-analog derived hollow Co3O4/NiO decorated CeO2 nanoparticles for boosting oxygen evolution reaction. J. Alloys Compd. 2022, 914, 165344. [Google Scholar] [CrossRef]
- Sun, X.; Xu, T.; Sun, W.; Bai, J.; Li, C. Ce-doped ZIF-67 derived Co3O4 nanoparticles supported by carbon nanofibers: A synergistic strategy towards bifunctional oxygen electrocatalysis and Zn-Air batteries. J. Alloys Compd. 2022, 898, 162778. [Google Scholar] [CrossRef]
- Zhang, Y.; Liao, W.; Zhang, G. A general strategy for constructing transition metal Oxide/CeO2 heterostructure with oxygen vacancies toward hydrogen evolution reaction and oxygen evolution reaction. J. Power Sources 2021, 512, 230514. [Google Scholar] [CrossRef]
- Zhang, B.; Lu, T.; Ren, Y.; Huang, L.; Pang, H.; Zhao, Q.; Tian, S.; Yang, J.; Xu, L.; Tang, Y.; et al. Encapsulation of Co/Co3O4 hetero-nanoparticles within the inner tips of N-doped carbon nanotubes: Engineering Mott-Schottky nanoreactors for efficient bifunctional oxygen electrocalysis toward flexible zinc-air batteries. Chem. Eng. J. 2022, 448, 137709. [Google Scholar] [CrossRef]
- Qi, L.; Wang, M.; Li, X. Graphene-induced growth of Co3O4 nanoplates with modulable oxygen vacancies for improved OER properties. CrystEngComm 2021, 23, 7928–7931. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, J.; Peng, X.; Liu, Y.; Cen, T.; Ye, Z.; Yuan, D. Co3O4–NiCo2O4 Hybrid Nanoparticles Anchored on N-Doped Reduced Graphene Oxide Nanosheets as an Efficient Catalyst for Zn–Air Batteries. Energy Fuels 2021, 35, 4550–4558. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, W.; Song, Y.; Yin, F.; Zhang, C.; Zhang, L. In situ construction of Co/Co3O4 with N-doped porous carbon as a bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions. Catal. Today 2020, 355, 286–294. [Google Scholar] [CrossRef]
- Peng, X.; Jin, X.; Gao, B.; Liu, Z.; Chu, P.K. Strategies to improve cobalt-based electrocatalysts for electrochemical water splitting. J. Catal. 2021, 398, 54–66. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, Q.; Li, K.; Zhang, S.; Ma, X. First-row transition metal oxide oxygen evolution electrocatalysts: Regulation strategies and mechanistic understandings. Sustain. Energy Fuels 2020, 4, 5417–5432. [Google Scholar] [CrossRef]
- Kong, Q.; Feng, W.; Xie, X.; Zhang, S.; Yuan, X.; Sun, C. Morphology-Controlled Synthesis of Co3O4 Materials and its Electrochemical Catalytic Properties Towards Oxygen Evolution Reaction. Catal. Lett. 2018, 148, 3771–3778. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Zhao, T.-J.; Wang, H.-H.; Lin, Y.-X.; Zhai, G.-Y.; Jiang, Z.-D.; Hirano, S.-I.; Li, X.-H.; Chen, J.-S. Oriented arrays of Co3O4 nanoneedles for highly efficient electrocatalytic water oxidation. Chem. Commun. 2019, 55, 3971–3974. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Jiang, L.; Jia, X.; Xiao, H.; Liang, Y.; Yang, B.; Guo, P.; Zhang, L.; Yang, H. In situ growth of Co3O4 nanoneedles on titanium mesh for electrocatalytic oxygen evolution. J. Mater. Sci. Mater. Electron. 2021, 32, 23275–23284. [Google Scholar] [CrossRef]
- Mu, Y.; Pei, X.; Zhao, Y.; Dong, X.; Kou, Z.; Cui, M.; Meng, C.; Zhang, Y. In situ confined vertical growth of Co2.5Ni0.5Si2O5(OH)4 nanoarrays on rGO for an efficient oxygen evolution reaction. Nano Mater. Sci. 2023, 5, 351–360. [Google Scholar] [CrossRef]
- You, N.; Cao, S.; Huang, M.; Fan, X.; Shi, K.; Huang, H.; Chen, Z.; Yang, Z.; Zhang, W. Constructing P-CoMoO4@NiCoP heterostructure nanoarrays on Ni foam as efficient bifunctional electrocatalysts for overall water splitting. Nano Mater. Sci. 2023, 5, 278–286. [Google Scholar] [CrossRef]
- He, Y.; Zhang, J.; He, G.; Han, X.; Zheng, X.; Zhong, C.; Hu, W.; Deng, Y. Ultrathin Co3O4 nanofilm as an efficient bifunctional catalyst for oxygen evolution and reduction reaction in rechargeable zinc–air batteries. Nanoscale 2017, 9, 8623–8630. [Google Scholar] [CrossRef]
- Xu, L.; Jiang, Q.; Xiao, Z.; Li, X.; Huo, J.; Wang, S.; Dai, L. Plasma-Engraved Co3O4 Nanosheets with Oxygen Vacancies and High Surface Area for the Oxygen Evolution Reaction. Angew. Chem. Int. Ed. Engl. 2016, 55, 5277–5281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Li, J.-H. Tailoring oxygen vacancy on Co3O4 nanosheets with high surface area for oxygen evolution reaction. Chin. J. Chem. Phys. 2018, 31, 517–522. [Google Scholar] [CrossRef]
- Zheng, Y.; Gao, R.; Zheng, L.; Sun, L.; Hu, Z.; Liu, X. Ultrathin Co3O4 Nanosheets with Edge-Enriched {111} Planes as Efficient Catalysts for Lithium–Oxygen Batteries. ACS Catal. 2019, 9, 3773–3782. [Google Scholar] [CrossRef]
- Luo, J.; Wang, X.; Gu, Y.; Wang, D.; Wang, S.; Li, W.; Zhou, Y.; Zhang, J. Constructing hollow nanocages of Co3O4-CoMoO4 heterostructure for efficient electrocatalytic oxygen evolution reaction. Appl. Surf. Sci. 2022, 606, 154562. [Google Scholar] [CrossRef]
- Wu, Z.; Feng, L.; Lu, Z.; Yu, X.; Zhao, Y.; Luo, J.; Wang, S.; Tian, X.; Chen, Q. Covalent organic frameworks/carbon nanotubes composite with cobalt (II) pyrimidine sites for bifunctional oxygen electrocatalysis. Nano Mater. Sci. 2024, 6, 419–427. [Google Scholar] [CrossRef]
- Malik, B.; Sadhanala, H.K.; Sun, R.; Deepak, F.L.; Gedanken, A.; Nessim, G.D. Co3O4|CoP Core–Shell Nanoparticles with Enhanced Electrocatalytic Water Oxidation Performance. ACS Appl. Nano Mater. 2022, 5, 9150–9158. [Google Scholar] [CrossRef]
- Dhawale, D.S.; Bodhankar, P.; Sonawane, N.; Sarawade, P.B. Fast microwave-induced synthesis of solid cobalt hydroxide nanorods and their thermal conversion into porous cobalt oxide nanorods for efficient oxygen evolution reaction. Sustain. Energy Fuels 2019, 3, 1713–1719. [Google Scholar] [CrossRef]
- Feng, L.-L.; Yu, G.; Wu, Y.; Li, G.-D.; Li, H.; Sun, Y.; Asefa, T.; Chen, W.; Zou, X. High-Index Faceted Ni3S2 Nanosheet Arrays as Highly Active and Ultrastable Electrocatalysts for Water Splitting. J. Am. Chem. Soc. 2015, 137, 14023–14026. [Google Scholar] [CrossRef]
- Guo, C.; Zheng, Y.; Ran, J.; Xie, F.; Jaroniec, M.; Qiao, S. Engineering High-Energy Interfacial Structures for High-Performance Oxygen-Involving Electrocatalysis. Angew. Chem. Int. Ed. Engl. 2017, 56, 8539–8543. [Google Scholar] [CrossRef]
- Wei, R.; Fang, M.; Dong, G.; Lan, C.; Shu, L.; Zhang, H.; Bu, X.; Ho, J.C. High-Index Faceted Porous Co3O4 Nanosheets with Oxygen Vacancies for Highly Efficient Water Oxidation. ACS Appl. Mater. Interfaces 2018, 10, 7079–7086. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; He, W.; Yang, Y.; Zhang, H.; Liu, D.; Yu, W.; Ma, Q.; Yin, D.; Dong, X. Flower-shaped and sea urchin-shaped structures coexisting in cobalt-based phosphate and oxides achieve efficient electrochemical overall water electrolysis. Int. J. Hydrogen Energy 2022, 47, 34856–34865. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Q.; Sun, F.; Qi, J.; Zhang, D.; Sun, H.; Li, Z.; Wang, Q.; Wang, B. Construction of Co3O4/CeO2 heterostructure nanoflowers facilitates deployment of oxygen defects to enhance the oxygen evolution kinetics. J. Alloys Compd. 2023, 933, 167700. [Google Scholar] [CrossRef]
- Que, R.; Liu, S.; Yang, Y.; Pan, Y. Core-shell structure Co3O4@NiCo LDH was used as a high efficiency catalyst for overall water splitting. Mater. Lett. 2021, 288, 129364. [Google Scholar] [CrossRef]
- Feng, C.; Yang, J.; Xiao, C.; Xin, B.; Zhang, S.; Wang, L.; Geng, B. Glycerate-derived Co3O4 nano-microspheres as efficient catalysts for oxygen evolution reaction. Appl. Surf. Sci. 2022, 598, 153795. [Google Scholar] [CrossRef]
- Audichon, T.; Napporn, T.W.; Canaff, C.; Morais, C.; Comminges, C.; Kokoh, K.B. IrO2 Coated on RuO2 as Efficient and Stable Electroactive Nanocatalysts for Electrochemical Water Splitting. J. Phys. Chem. C 2016, 120, 2562–2573. [Google Scholar] [CrossRef]
- Bai, J.; Zhou, W.; Xu, J.; Zhou, P.; Deng, Y.; Xiang, M.; Xiang, D.; Su, Y. RuO2 catalysts for electrocatalytic oxygen evolution in acidic media: Mechanism, activity promotion strategy and research progress. Molecules 2024, 29, 537. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, L.; Jin, Q.; Yun, J.; Liang, X. MoS2–Co3S4 hollow polyhedrons derived from ZIF-67 towards hydrogen evolution reaction and hydrodesulfurization. Int. J. Hydrogen Energy 2019, 44, 24246–24255. [Google Scholar] [CrossRef]
- Zhang, K.; Ma, W.; Tan, G.; Cheng, Z.; Ma, Y.; Li, W.; Feng, X.; Li, Z. Interfacial engineering of Ru-doped Co3O4/CoP nanowires heterostructure as efficient bifunctional electrocatalysts for alkaline water splitting. J. Mol. Catal. 2022, 530, 112640. [Google Scholar] [CrossRef]
- Cho, S.-H.; Jeon, H.-J.; Son, Y.; Lee, S.-E.; Kim, T.-O. Water splitting over an ultrasonically synthesized NiFe/MoO3@CFP electrocatalyst. Int. J. Hydrogen Energy 2023, 48, 26032–26045. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; He, G.; Zhu, Y.; Xiao, J.; Han, L. Borate anion dopant inducing oxygen vacancies over Co3O4 nanocages for enhanced oxygen evolution. Int. J. Hydrogen Energy 2021, 11, 659. [Google Scholar] [CrossRef]
- Peng, J.; Yuan, Y.; Yuan, W.; Peng, K. Vacancies and phosphorus atoms assembled in amorphous urchin-like Co3O4 for highly efficient overall water splitting. Int. J. Hydrogen Energy 2021, 46, 24117–24127. [Google Scholar] [CrossRef]
- Wu, H.; Sun, W.; Shen, J.; Rooney, D.W.; Wang, Z.; Sun, K. Role of flower-like ultrathin Co3O4 nanosheets in water splitting and non-aqueous Li–O2 batteries. Nanoscale 2018, 10, 10221–10231. [Google Scholar] [CrossRef]
- Liang, J.; Liu, Y.; Wang, Z.; Jia, Y.; Ding, Z.; Gao, L. Ni3S2@MoO3@Co3O4@AMO/NF core–shell heterostructure for high performance alkaline overall water splitting. Discov. Nano 2025, 20, 112. [Google Scholar] [CrossRef]
- Rittiruam, M.; Saelee, T.; Márquez, V.; Shaikh, N.; Santos, J.; Kanjanaboos, P.; Nazeeruddin, M.; Praserthdam, S.; Praserthdam, P. Ru tailored hydrous cobalt phosphate as a rational approach for high-performance alkaline oxygen evolution reaction. Mater. Today Chem. 2022, 26, 101267. [Google Scholar] [CrossRef]
- de Freitas, I.C.; Parreira, L.S.; Barbosa, E.C.M.; Novaes, B.A.; Mou, T.; Alves, T.V.; Quiroz, J.; Wang, Y.-C.; Slater, T.J.; Thomas, A.; et al. Design-controlled synthesis of IrO2 sub-monolayers on Au nanoflowers: Marrying plasmonic and electrocatalytic properties. Nanoscale 2020, 12, 12281–12291. [Google Scholar] [CrossRef]
- Chen, F.-Y.; Wu, Z.-Y.; Adler, Z.; Wang, H. Stability challenges of electrocatalytic oxygen evolution reaction: From mechanistic understanding to reactor design. Joule 2021, 5, 1704–1731. [Google Scholar] [CrossRef]
- Liu, S.; Li, L.; Ahn, H.S.; Manthiram, A. Delineating the roles of Co3O4and N-doped carbon nanoweb (CNW) in bifunctional Co3O4/CNW catalysts for oxygen reduction and oxygen evolution reactions. J. Mater. Chem. A 2015, 3, 11615–11623. [Google Scholar] [CrossRef]
- Chen, H.; Chen, S.; Zhang, Z.; Sheng, L.; Zhao, J.; Fu, W.; Xi, S.; Si, R.; Wang, L.; Fan, M.; et al. Single-Atom-Induced Adsorption Optimization of Adjacent Sites Boosted Oxygen Evolution Reaction. ACS Catal. 2022, 12, 13482–13491. [Google Scholar] [CrossRef]
- Dai, Y.; Yu, J.; Wang, J.; Shao, Z.; Guan, D.; Huang, Y.; Ni, M. Bridging the Charge Accumulation and High Reaction Order for High-Rate Oxygen Evolution and Long Stable Zn-Air Batteries. Adv. Funct. Mater. 2022, 32, 2111989. [Google Scholar] [CrossRef]
- Jiang, W.; Lehnert, W.; Shviro, M. The Influence of Loadings and Substrates on the Performance of Nickel-Based Catalysts for the Oxygen Evolution Reaction. ChemElectroChem 2023, 10, e202200991. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, J.; Zhong, M.; Guo, S. Co3O4 Nanosheet Arrays on Ni Foam as Electrocatalyst for Oxygen Evolution Reaction. J. Electrocatal. 2018, 9, 653–661. [Google Scholar] [CrossRef]
- Ali, B.A.; Ahmed, N.; Allam, N.K. Deciphering the hype effect of Ni-foam substrate in electrochemical supercapacitors: Is there a way out? Mater. Today Commun. 2022, 32, 103972. [Google Scholar] [CrossRef]
- Chutia, B.; Patowary, S.; Misra, A.; Rao, K.N.; Bharali, P. Morphology Effect of Co3O4 Nanooctahedron in Boosting Oxygen Reduction and Oxygen Evolution Reactions. Energy Fuels 2022, 36, 13863–13872. [Google Scholar] [CrossRef]
- Liu, S.; Pan, X. Self-assembling Co3O4 Nanorods Electrode with High-Performance Anode for Lithium-Ion Batteries and Efficient Electrocatalysts Toward Oxygen Evolution Reaction. J. Electron. Mater. 2019, 48, 7404–7410. [Google Scholar] [CrossRef]
- Zhang, Q.; Jing, B.; Qiu, S.; Cui, C.; Zhu, Y.; Deng, F. A mechanism in boosting H2 generation: Nanotip-enhanced local temperature and electric field with the boundary layer. J. Colloid Interface Sci. 2022, 629, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Koumoto, K.; Yanagida, H. Electrical Conduction in Pure and Li-Substituted Co3O4. J. Am. Ceram. Soc. 1981, 64, C-156–C-157. [Google Scholar] [CrossRef]
- Yang, G.; Zhu, B.; Fu, Y.; Zhao, J.; Lin, Y.; Gao, D.; Li, J. High-valent Zirconium-doping modified Co3O4 weave-like nanoarray boosts oxygen evolution reaction. J. Alloys Compd. 2021, 886, 161172. [Google Scholar] [CrossRef]
- Yang, H.; Ding, Z.; Li, Y.-T.; Li, S.-Y.; Wu, P.-K.; Hou, Q.-H.; Zheng, Y.; Gao, B.; Huo, K.-F.; Du, W.-J.; et al. Recent advances in kinetic and thermodynamic regulation of magnesium hydride for hydrogen storage. Rare Met. 2023, 42, 2906–2927. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Choi, B.; Kim, Y.-B. Development of Highly Active Bifunctional Electrocatalyst Using Co3O4 on Carbon Nanotubes for Oxygen Reduction and Oxygen Evolution. Sci. Rep. 2018, 8, 2543. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, Z.; Wu, J.-M.; Jiang, R.; Yang, P.; Gu, Y.; Liu, W.; Wu, J.; Wen, W. Cobalt/nickel oxide nanosheet arrays for electrocatalytic water oxidation: Size modulation, composition/phase control, and surface decoration. Chem. Phys. Lett. 2020, 754, 137734. [Google Scholar] [CrossRef]
- Hu, Q.; Liu, Y.; Ma, L.; Zhang, X.; Huang, H. PPy enhanced Fe, W Co-doped Co3O4 free-standing electrode for highly-efficient oxygen evolution reaction. J. Appl. Electrochem. 2018, 48, 1189–1195. [Google Scholar] [CrossRef]
- Li, X.; Yang, B.; Wu, Y.; Lin, S.; Zhang, L. Homogeneous Co3O4 film electrode with enhanced oxygen evolution electrocatalysis via surface reduction. Chin. J. Chem. Eng. 2021, 29, 221–227. [Google Scholar] [CrossRef]
- Yang, X.; Chen, J.; Chen, Y.; Feng, P.; Lai, H.; Li, J.; Luo, X. Novel Co3O4 Nanoparticles/Nitrogen-Doped Carbon Composites with Extraordinary Catalytic Activity for Oxygen Evolution Reaction (OER). Nano-Micro Lett. 2017, 10, 15. [Google Scholar] [CrossRef]
- Filimonenkov, I.S.; Bouillet, C.; Kéranguéven, G.; Simonov, P.A.; Tsirlina, G.A.; Savinova, E.R. Carbon materials as additives to the OER catalysts: RRDE study of carbon corrosion at high anodic potentials. Electrochim. Acta 2019, 321, 134657. [Google Scholar] [CrossRef]
- Min, M.; You, E.; Lee, S.W.; Pak, C. Cathode durability enhancement under start-up/shut-down process using IrRuOx/C catalyst in polymer electrolyte membrane fuel cell. J. Power Sources 2021, 488, 229423. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, Y.; Yuan, J.; Shen, J.; Wang, A.-J.; Niu, L.; Huang, S. Uniform Deposition of Co3O4 Nanosheets on Exfoliated MoS2 Nanosheets as Advanced Catalysts for Water Splitting. Electrochim. Acta 2016, 212, 890–897. [Google Scholar] [CrossRef]
- Chen, Y.; Yao, H.; Kong, F.; Tian, H.; Meng, G.; Wang, S.; Mao, X.; Cui, X.; Hou, X.; Shi, J. V2C MXene synergistically coupling FeNi LDH nanosheets for boosting oxygen evolution reaction. Appl. Catal. B Environ. 2021, 297, 120474. [Google Scholar] [CrossRef]
- Du, C.; Song, Q.; Liang, Q.; Zhao, X.; Wang, J.; Zhi, R.; Wang, Y.; Yu, H. The Passive Effect of MXene on Electrocatalysis: A Case of Ti3C2Tx/CoNi−MOF nanosheets for Oxygen Evolution Reaction. Chemnanomat 2021, 7, 539–544. [Google Scholar] [CrossRef]
- Wei, B.; Fu, Z.; Legut, D.; Germann, T.C.; Du, S.; Zhang, H.; Francisco, J.S.; Zhang, R. Rational Design of Highly Stable and Active MXene-Based Bifunctional ORR/OER Double-Atom Catalysts. Adv. Mater. 2021, 33, 2102595. [Google Scholar] [CrossRef]
- Lu, Y.; Fan, D.; Chen, Z.; Xiao, W.; Cao, C.; Yang, X. Anchoring Co3O4 nanoparticles on MXene for efficient electrocatalytic oxygen evolution. Sci. Bull. 2020, 65, 460–466. [Google Scholar] [CrossRef]
- Saad, A.; Liu, D.; Wu, Y.; Song, Z.; Li, Y.; Najam, T.; Zong, K.; Tsiakaras, P.; Cai, X. Ag nanoparticles modified crumpled borophene supported Co3O4 catalyst showing superior oxygen evolution reaction (OER) performance. Appl. Catal. B Environ. 2021, 298, 120529. [Google Scholar] [CrossRef]
- Aman, S.; Gouadria, S.; Manzoor, S.; Abdullah, M.; Khosa, R.Y.; Ashiq, M.N.; Ansari, M.Z.; Alfantazi, A. Facile synthesis of CuZrO3@PPY nanohybrid balls embedded 3-dimensional network with synergistic effect for efficient oxygen evolution reaction. Surfaces Interfaces 2022, 36, 102607. [Google Scholar] [CrossRef]
- Antar, A.; Naimi, Y.; Takky, D. Development of nickel-cobalt bimetallic/conducting polymer composite used as a catalyst in the oxygen evolution reaction (OER). IOP Conf. Ser. Earth Environ. Sci. 2018, 161, 012027. [Google Scholar] [CrossRef]
- Tong, Y.; Liu, H.; Dai, M.; Xiao, L.; Wu, X. Metal-organic framework derived Co3O4/PPy bifunctional electrocatalysts for efficient overall water splitting. Chin. Chem. Lett. 2020, 31, 2295–2299. [Google Scholar] [CrossRef]
- Tomboc, G.M.; Venkateshalu, S.; Ngo, Q.-T.; Choi, S.; Pollet, B.G.; Lee, H.; Lee, K. Defect-induced electronic modification and surface reconstruction of catalysts during water oxidation process. Chem. Eng. J. 2022, 454, 140254. [Google Scholar] [CrossRef]
- Yan, D.; Li, Y.; Huo, J.; Chen, R.; Dai, L.; Wang, S. Defect Chemistry of Nonprecious-Metal Electrocatalysts for Oxygen Reactions. Adv. Mater. 2017, 29, 1606459. [Google Scholar] [CrossRef]
- Li, Z.; Xu, X.; Lu, X.; He, C.; Huang, J.; Sun, W.; Tian, L. Synergistic coupling of FeNi3 alloy with graphene carbon dots for advanced oxygen evolution reaction electrocatalysis. J. Colloid Interface Sci. 2022, 615, 273–281. [Google Scholar] [CrossRef]
- Wang, J.; Li, K.; Zhong, H.; Xu, D.; Wang, Z.; Jiang, Z.; Wu, Z.; Zhang, X. Synergistic Effect between Metal–Nitrogen–Carbon Sheets and NiO Nanoparticles for Enhanced Electrochemical Water-Oxidation Performance. Angew. Chem. Int. Ed. Engl. 2015, 54, 10530–10534. [Google Scholar] [CrossRef]
- Zhang, S.; Wei, N.; Yao, Z.; Zhao, X.; Du, M.; Zhou, Q. Oxygen vacancy-based ultrathin Co3O4 nanosheets as a high-efficiency electrocatalyst for oxygen evolution reaction. Int. J. Hydrogen Energy 2021, 46, 5286–5295. [Google Scholar] [CrossRef]
- Zhu, K.; Shi, F.; Zhu, X.; Yang, W. The roles of oxygen vacancies in electrocatalytic oxygen evolution reaction. Nano Energy 2020, 73, 104761. [Google Scholar] [CrossRef]
- He, Q.; Han, L.; Tao, K. Oxygen vacancy modulated Fe-doped Co3O4 hollow nanosheet arrays for efficient oxygen evolution reaction. Chem. Commun. 2023, 60, 1116–1119. [Google Scholar] [CrossRef]
- Cai, Z.; Bi, Y.; Hu, E.; Liu, W.; Dwarica, N.; Tian, Y.; Li, X.; Kuang, Y.; Li, Y.; Yang, X.; et al. Single-Crystalline Ultrathin Co3O4 Nanosheets with Massive Vacancy Defects for Enhanced Electrocatalysis. Adv. Energy Mater. 2017, 8, 1701694. [Google Scholar] [CrossRef]
- Ding, Z.; Li, Y.; Yang, H.; Lu, Y.; Tan, J.; Li, J.; Li, Q.; Chen, Y.; Shaw, L.L.; Pan, F. Tailoring MgH2 for hydrogen storage through nanoengineering and catalysis. J. Magnes. Alloys 2022, 10, 2946–2967. [Google Scholar] [CrossRef]
- Chen, L.; Ren, W.; Xu, C.; Hu, W.; Hu, L.; Wang, W.; Huang, J.; Hou, Z. A simple room–temperature acid capture strategy to controllably tune oxygen vacancy in Co3O4 for oxygen evolution reaction. J. Alloys Compd. 2022, 932, 167657. [Google Scholar] [CrossRef]
- Zeng, H.; Oubla, M.; Zhong, X.; Alonso-Vante, N.; Du, F.; Xie, Y.; Huang, Y.; Ma, J. Rational defect and anion chemistries in Co3O4 for enhanced oxygen evolution reaction. Appl. Catal. B Environ. 2021, 281, 119535. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Q.; Wang, J.; Li, Y.; Jiang, H.; Li, C. Dual-defective Co3O4 nanoarrays enrich target intermediates and promise high-efficient overall water splitting. Chem. Eng. J. 2021, 424, 130328. [Google Scholar] [CrossRef]
- Xie, C.; Yan, D.; Li, H.; Du, S.; Chen, W.; Wang, Y.; Zou, Y.; Chen, R.; Wang, S. Defect Chemistry in Heterogeneous Catalysis: Recognition, Understanding, and Utilization. ACS Catal. 2020, 10, 11082–11098. [Google Scholar] [CrossRef]
- Li, X.; Su, X.; Pei, Y.; Liu, J.; Zheng, X.; Tang, K.; Guan, G.; Hao, X. Generation of edge dislocation defects in Co3O4 catalysts: An efficient tactic to improve catalytic activity for oxygen evolution. J. Mater. Chem. A 2019, 7, 10745–10750. [Google Scholar] [CrossRef]
- Guo, J.; Wang, G.; Cui, S.; Xia, B.; Liu, Z.; Zang, S.-Q. Vacancy and strain engineering of Co3O4 for efficient water oxidation. J. Colloid Interface Sci. 2022, 629, 346–354. [Google Scholar] [CrossRef]
- Jiang, H.; Ding, Z.; Li, Y.; Lin, G.; Li, S.; Du, W.; Chen, Y.; Shaw, L.L.; Pan, F. Hierarchical interface engineering for advanced magnesium-based hydrogen storage: Synergistic effects of structural design and compositional modification. Chem. Sci. 2025, 16, 7610–7636. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, Y.; Xu, H.; Guan, D.; Hu, Z.; Jing, C.; Sha, Y.; Gu, Y.; Huang, Y.; Chang, Y.; et al. Combined Corner-Sharing and Edge-Sharing Networks in Hybrid Nanocomposite with Unusual Lattice-Oxygen Activation for Efficient Water Oxidation. Adv. Funct. Mater. 2022, 32, 2207618. [Google Scholar] [CrossRef]
- Ahmed, I.; Biswas, R.; Patil, R.A.; Singh, H.; Banerjee, B.; Kumar, B.; Ma, Y.-R.; Haldar, K.K. Graphitic Carbon Nitride Composites with MoO3-Decorated Co3O4 Nanorods as Catalysts for Oxygen and Hydrogen Evolution. ACS Appl. Nano Mater. 2021, 4, 12672–12681. [Google Scholar] [CrossRef]
- Dai, F.F.; Xue, Y.X.; Gao, D.L.; Liu, Y.X.; Chen, J.H.; Lin, Q.J.; Lin, W.W.; Yang, Q. Facile fabrication of self-supporting porous CuMoO4@Co3O4 nanosheets as a bifunctional electrocatalyst for efficient overall water splitting. Dalton Trans. 2022, 51, 12736–12745. [Google Scholar] [CrossRef]
- Gao, W.-K.; Lin, J.-H.; Wang, K.; Liu, Z.-Z.; Qin, J.-F.; Xie, J.-Y.; Zhang, X.-Y.; Chai, Y.-M.; Dong, B. Controllable phosphorsulfurization of uniform binary Ni-Fe nanocubes for enhanced water oxidation. Mater. Lett. 2018, 229, 248–251. [Google Scholar] [CrossRef]
- Wang, P.; He, H.; Pu, Z.; Chen, L.; Zhang, C.; Wang, Z.; Mu, S. Phosphorization engineering ameliorated the electrocatalytic activity for overall water splitting on Ni3S2 nanosheets. Dalton Trans. 2019, 48, 13466–13471. [Google Scholar] [CrossRef]
- Alhakemy, A.Z.; Senthilkumar, N.; Ding, Y.; Li, J.; Wen, Z. Co3O4–C@FeMoP on nickel foam as bifunctional electrocatalytic electrode for high-performance alkaline water splitting. Int. J. Hydrogen Energy 2021, 46, 32846–32857. [Google Scholar] [CrossRef]
- Muthurasu, A.; Maruthapandian, V.; Kim, H.Y. Metal-organic framework derived Co3O4/MoS2 heterostructure for efficient bifunctional electrocatalysts for oxygen evolution reaction and hydrogen evolution reaction. Appl. Catal. B Environ. 2019, 248, 202–210. [Google Scholar] [CrossRef]
- Xia, Z.; Deng, B.; Wang, Y.; Jiang, Z.; Jiang, Z.-J. Synergistic co-doping induced high catalytic activities of La/Fe doped Co3O4 towards oxygen reduction/evolution reactions for Zn–air batteries. J. Mater. Chem. A 2022, 10, 23483–23493. [Google Scholar] [CrossRef]
- Li, G.; Yin, F.; Lei, Z.; Zhao, X.; He, X.; Li, Z.; Yu, X. Se-doped cobalt oxide nanoparticle as highly-efficient electrocatalyst for oxygen evolution reaction. Int. J. Hydrogen Energy 2022, 47, 216–227. [Google Scholar] [CrossRef]
- Shao, Z.; Gao, X.; Zhu, Q.; Zhao, W.; Wu, X.; Huang, K.; Feng, S. Synergistic regulation of coordination sites of Co3O4 by selective doping for efficient water oxidation. Chem. Commun. 2022, 58, 10408–10411. [Google Scholar] [CrossRef]
- Silva, A.L.; Esteves, L.M.; Silva, L.P.C.; Ramos, V.S.; Passos, F.B.; Carvalho, N.M.F. Mn-doped Co3O4 for acid, neutral and alkaline electrocatalytic oxygen evolution reaction. RSC Adv. 2022, 12, 26846–26858. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, R.A.; Raja, K.C.N.; Devi, N.; Lin, H.-T.; Huang, C.-C.; Jiang, X.-Y.; Li, Y.-Y.; Arthanareeswaran, G.; Ponvijayakanthan, L.; Jaiswal, N.K.; et al. Development of Ni-doped Co3O4 oxygen evolution catalysts for anion exchange membrane water electrolysis. Int. J. Hydrogen Energy 2024, 72, 677–686. [Google Scholar] [CrossRef]
- Qi, J.; Wang, H.; Lin, J.; Li, C.; Si, X.; Cao, J.; Zhong, Z.; Feng, J. Mn and S dual-doping of MOF-derived Co3O4 electrode array increases the efficiency of electrocatalytic generation of oxygen. J. Colloid Interface Sci. 2019, 557, 28–33. [Google Scholar] [CrossRef]
- Guan, D.; Xu, H.; Huang, Y.; Jing, C.; Tsujimoto, Y.; Xu, X.; Lin, Z.; Tang, J.; Wang, Z.; Sun, X.; et al. Operando Studies Redirect Spatiotemporal Restructuration of Model Coordinated Oxides in Electrochemical Oxidation. Adv. Mater. 2024, 37, e2413073. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Hu, L.; Zhu, X.; Shang, C.; Li, J.; Wang, E. Rapid synthesis of Co3O4 nanosheet arrays on Ni foam by in situ electrochemical oxidization of air-plasma engraved Co(OH)2 for efficient oxygen evolution. Chem. Commun. 2018, 54, 12698–12701. [Google Scholar] [CrossRef] [PubMed]
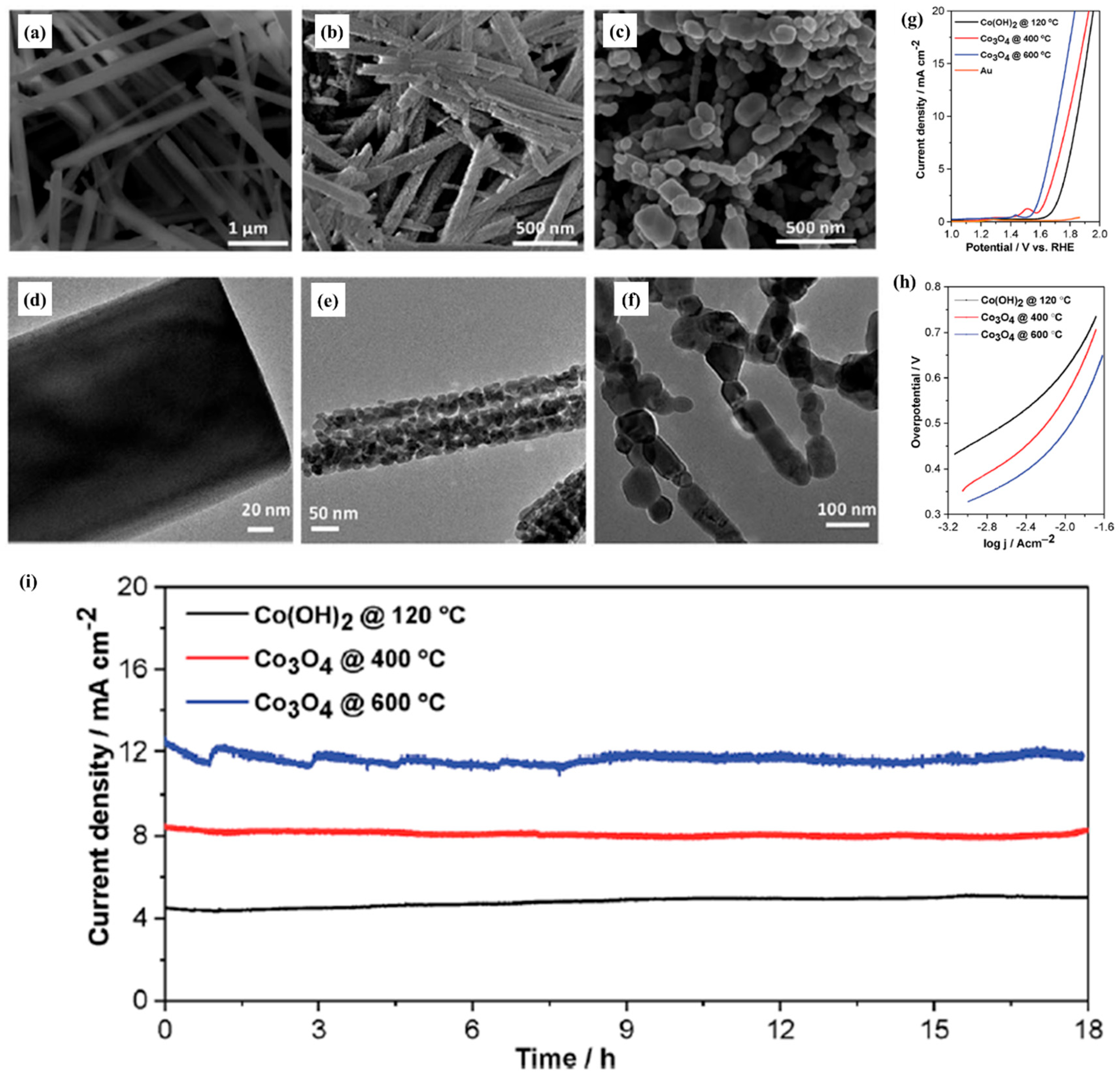
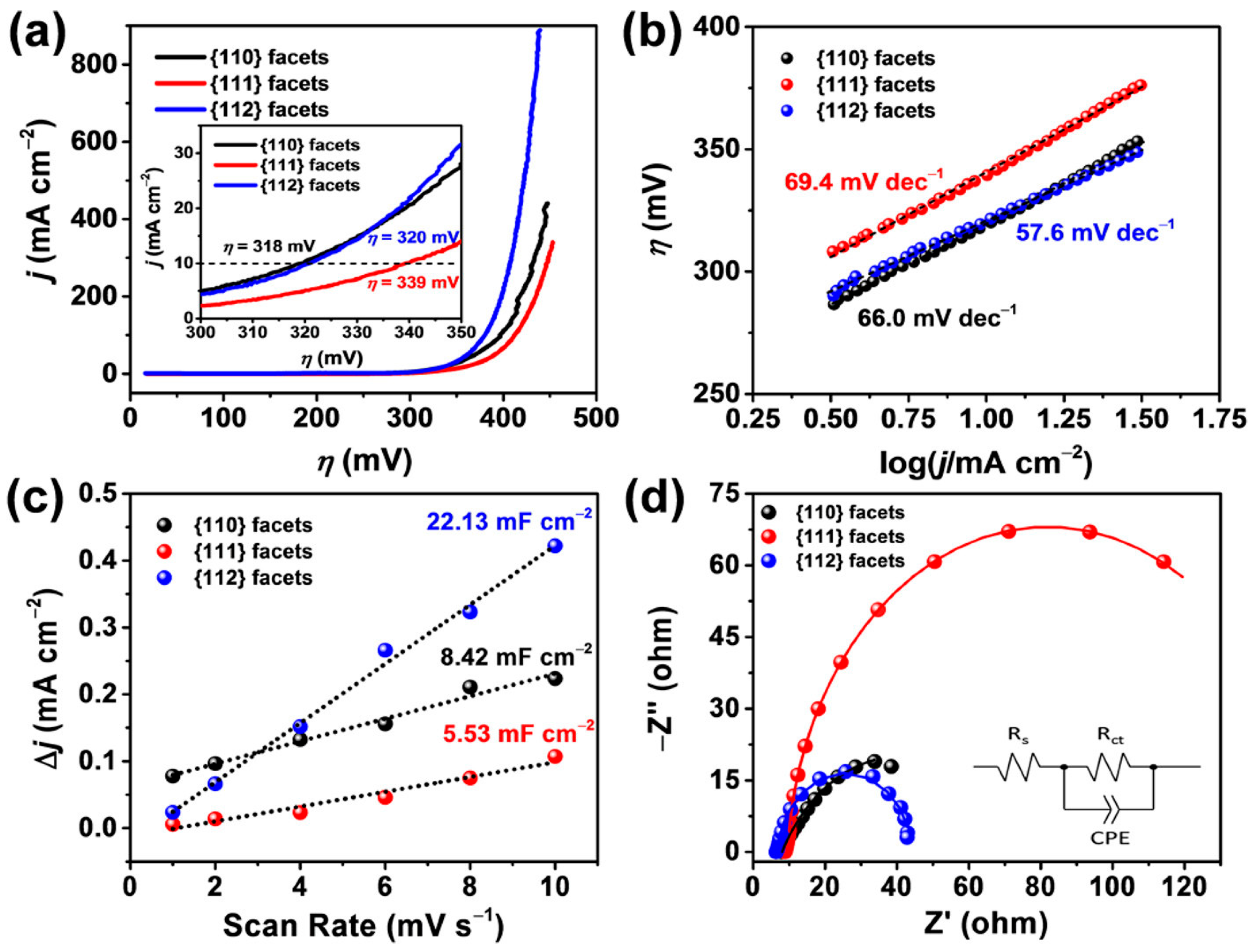
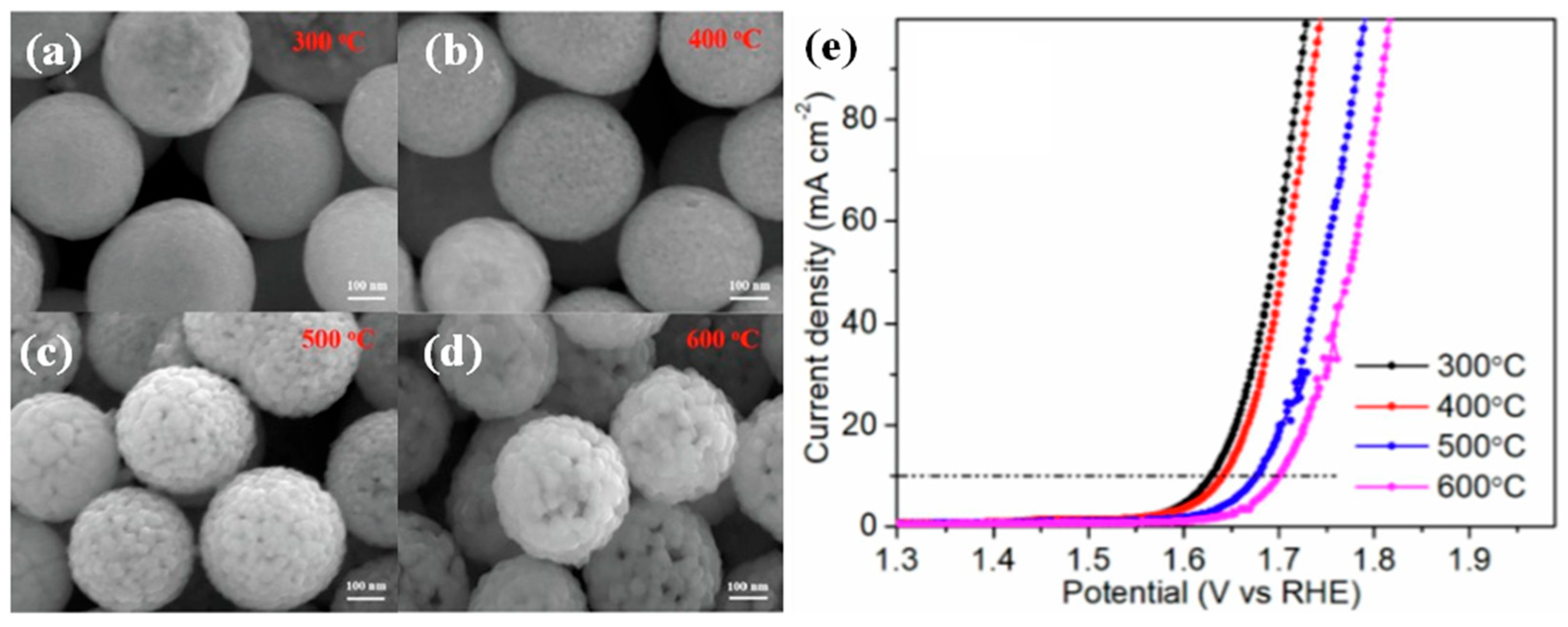
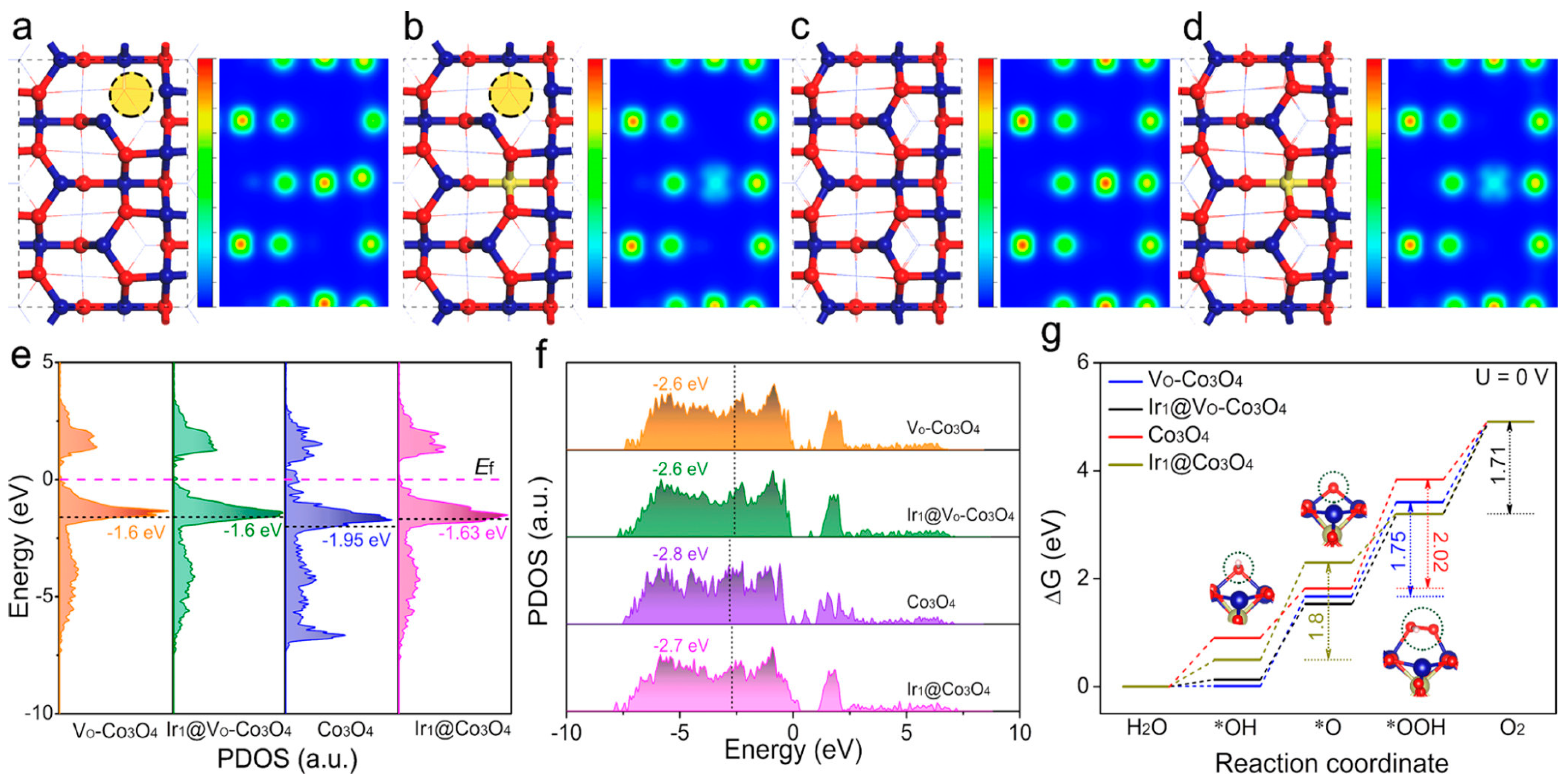
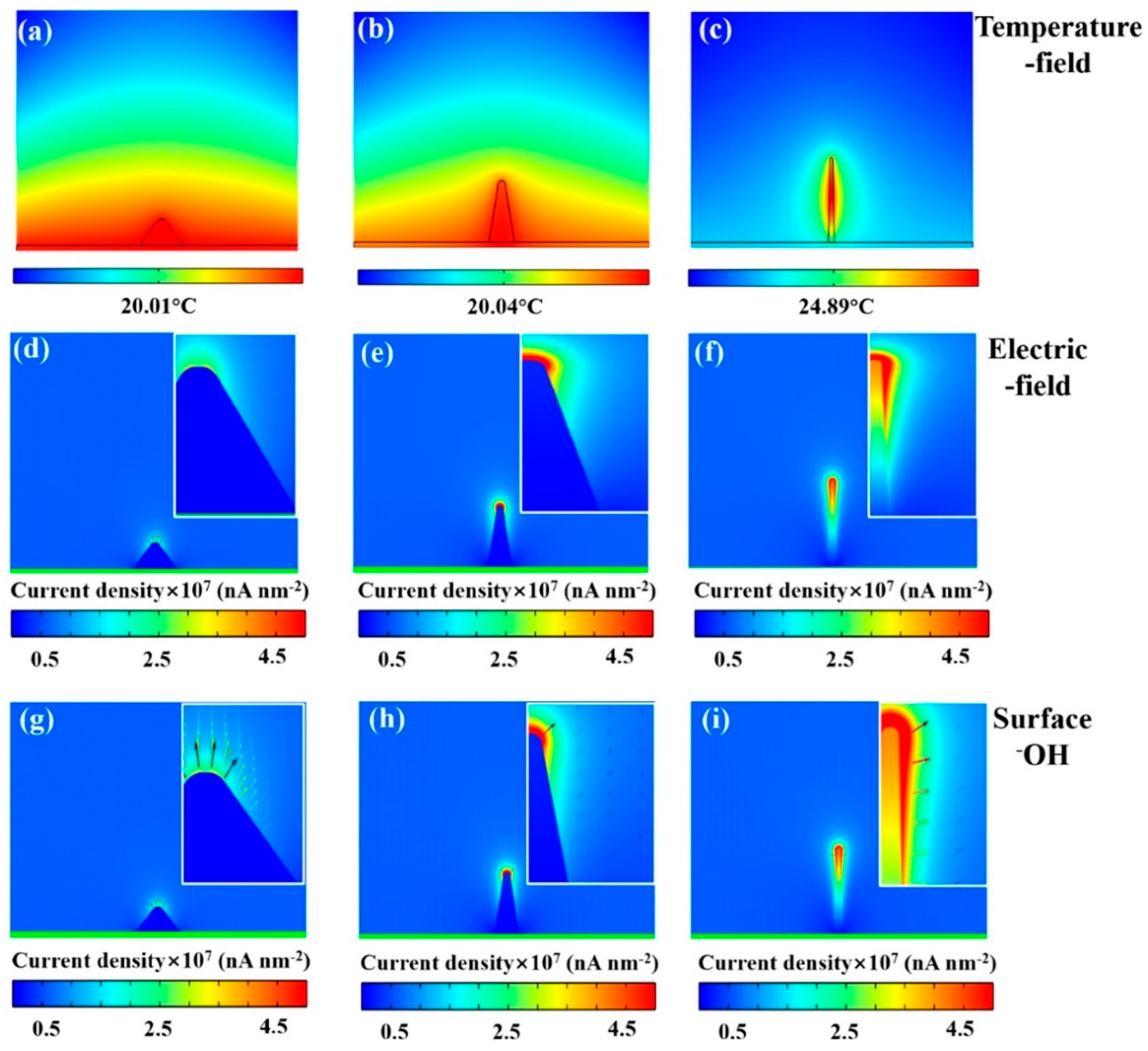
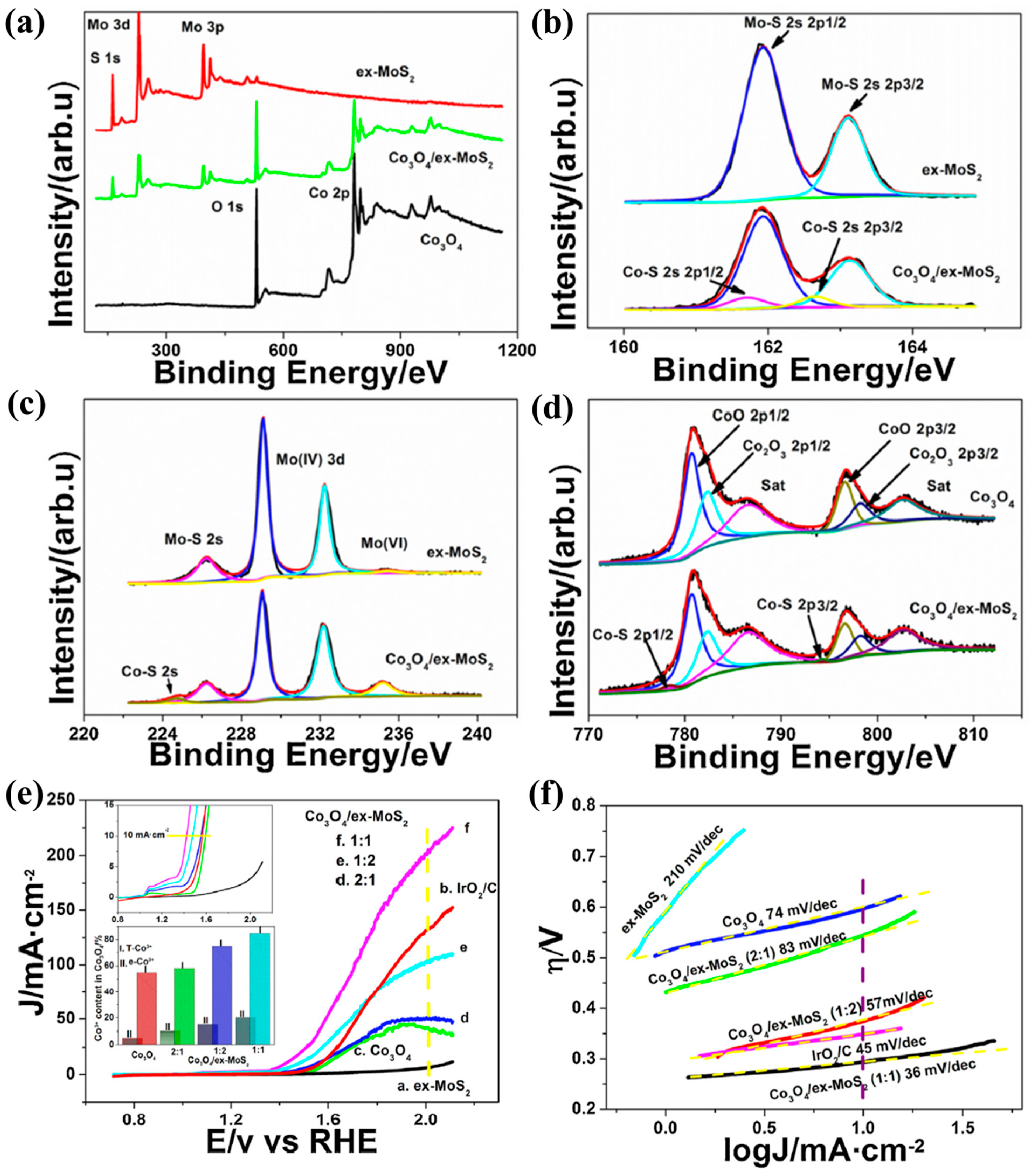
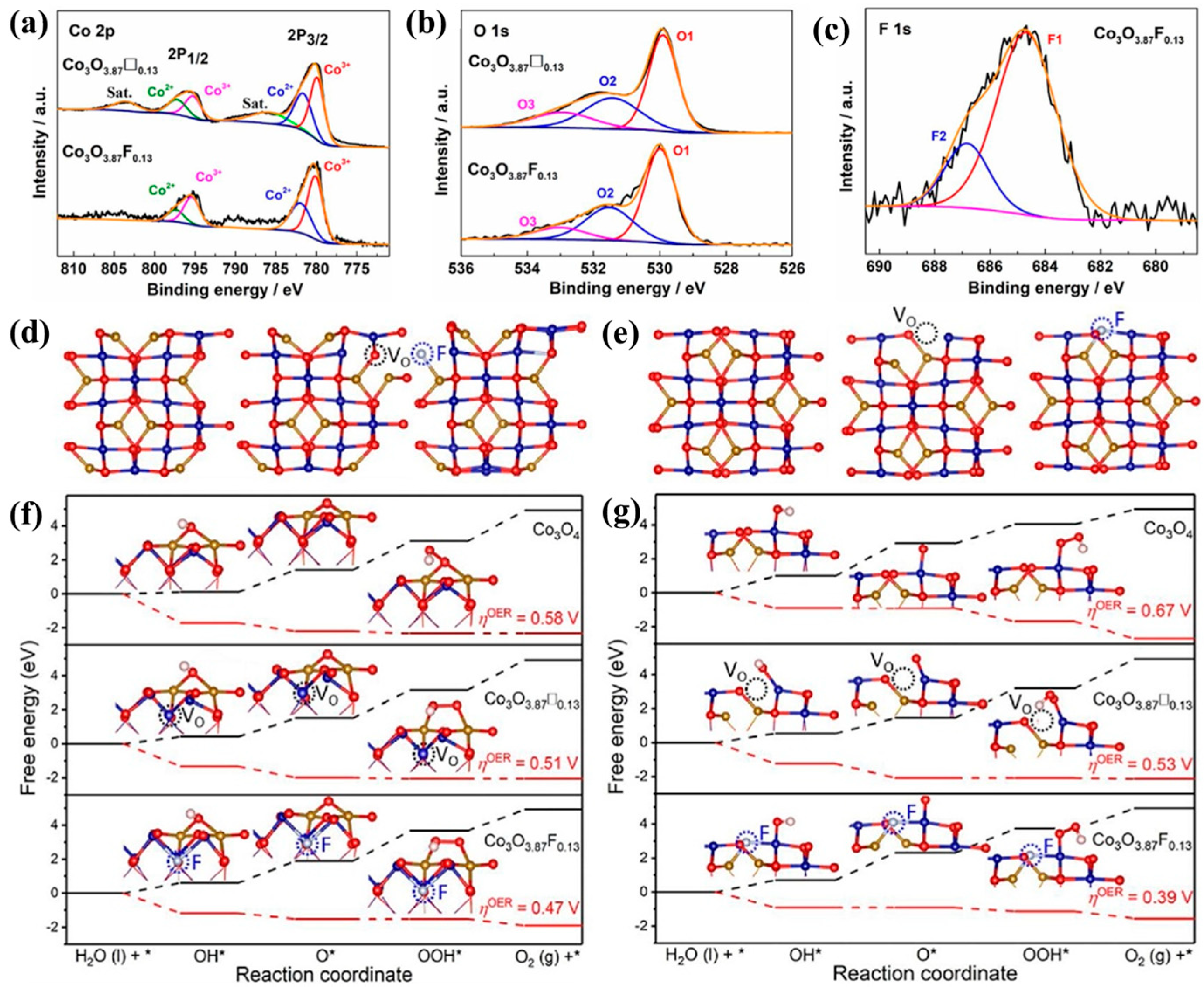
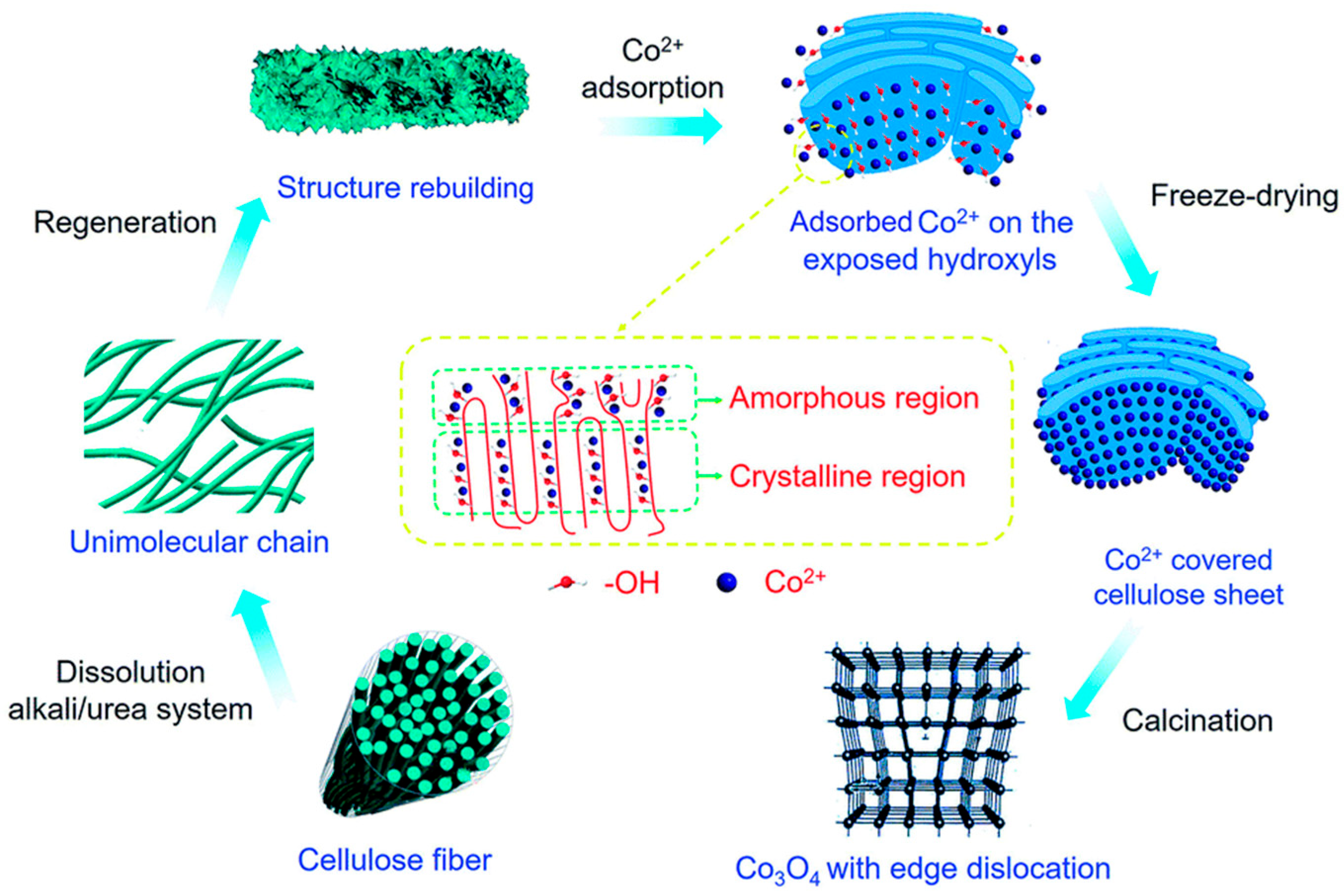
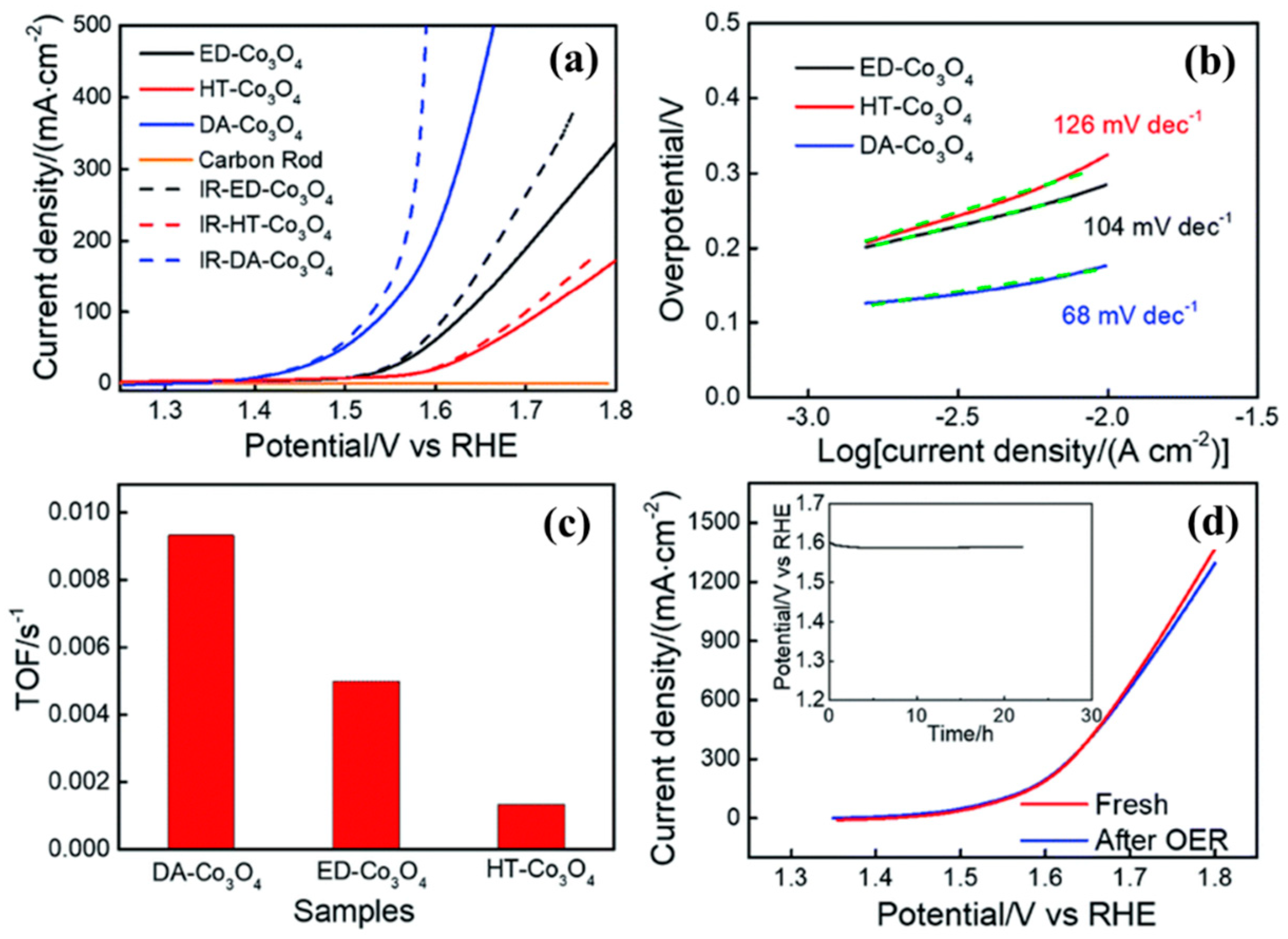
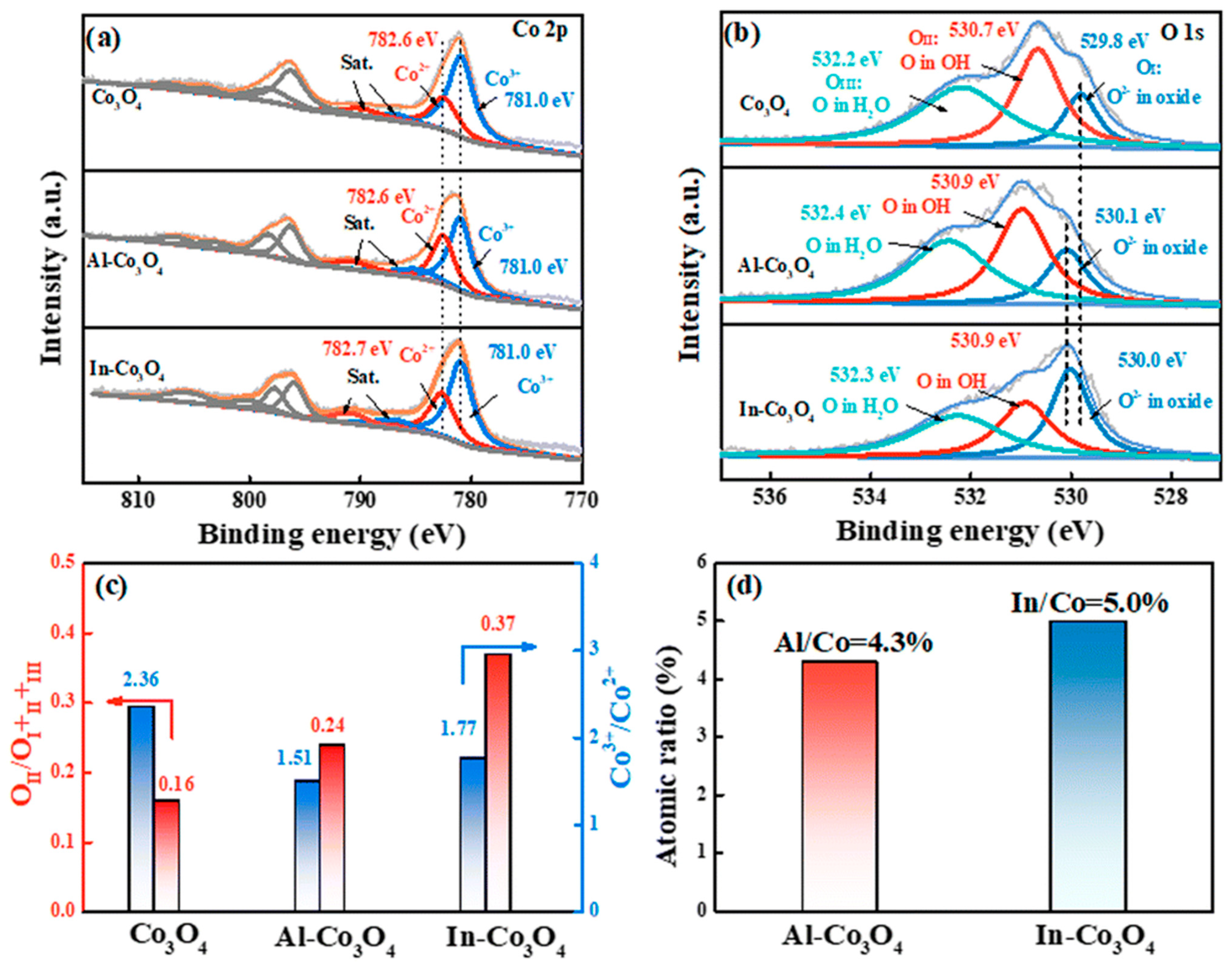
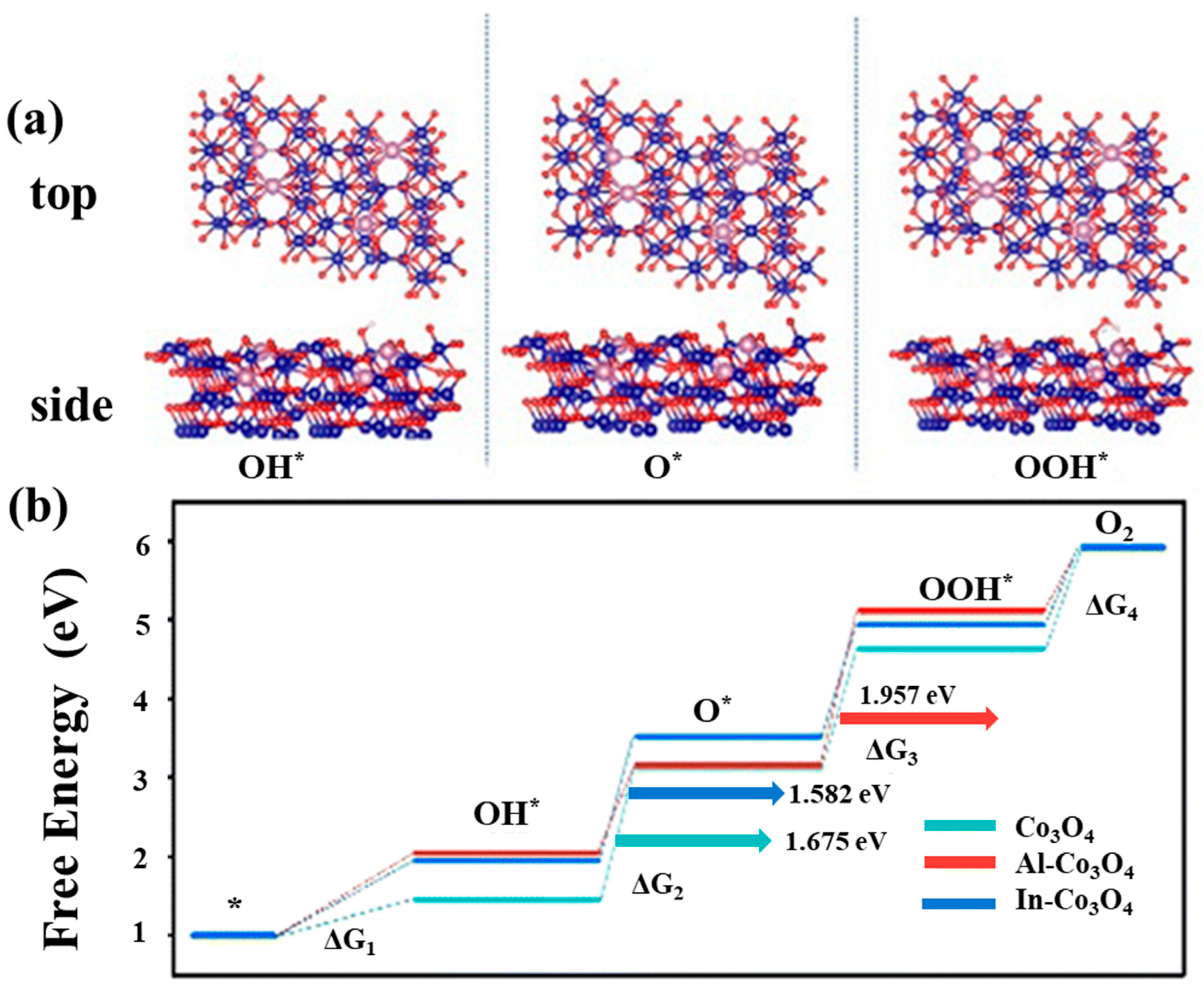
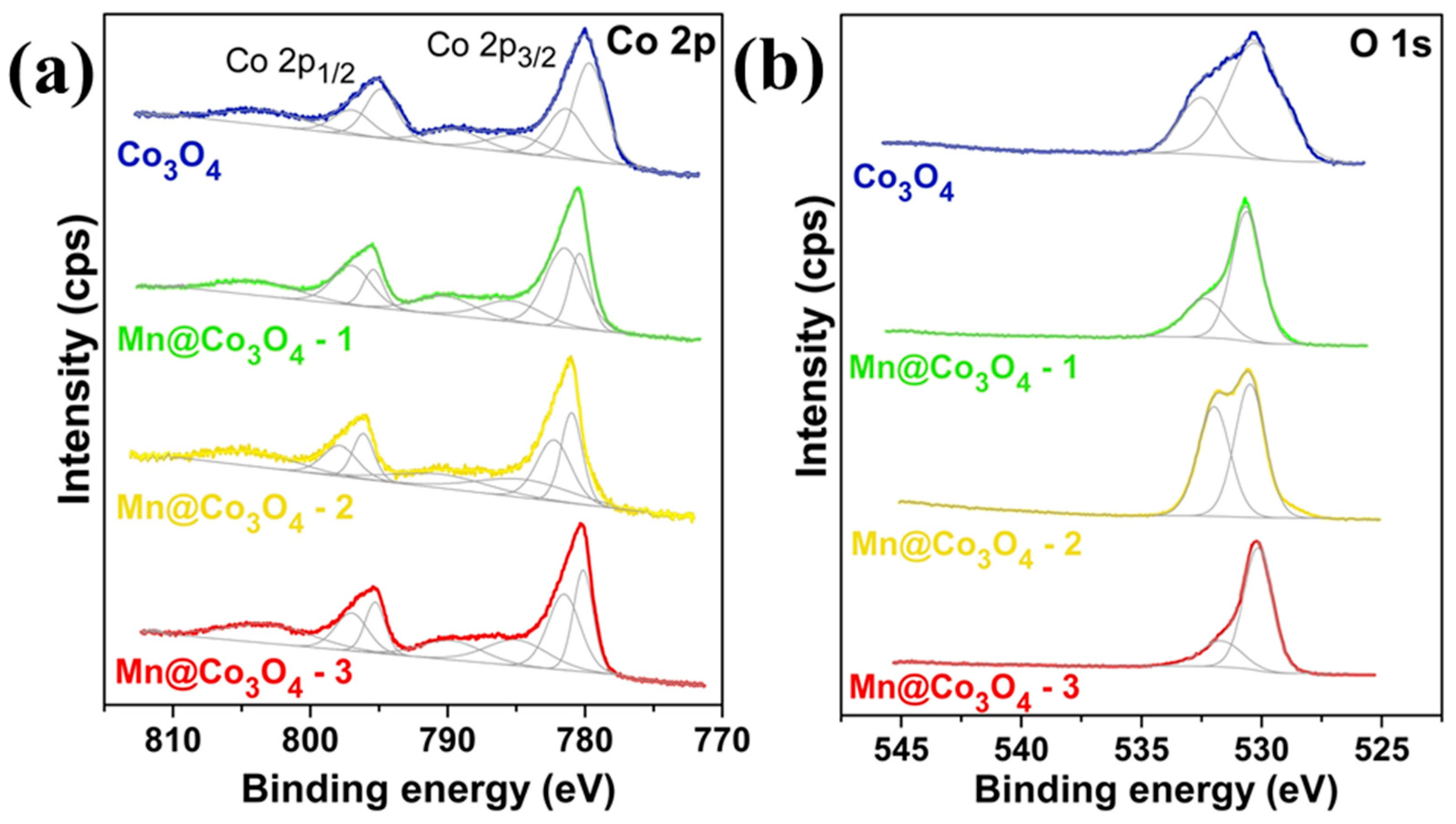
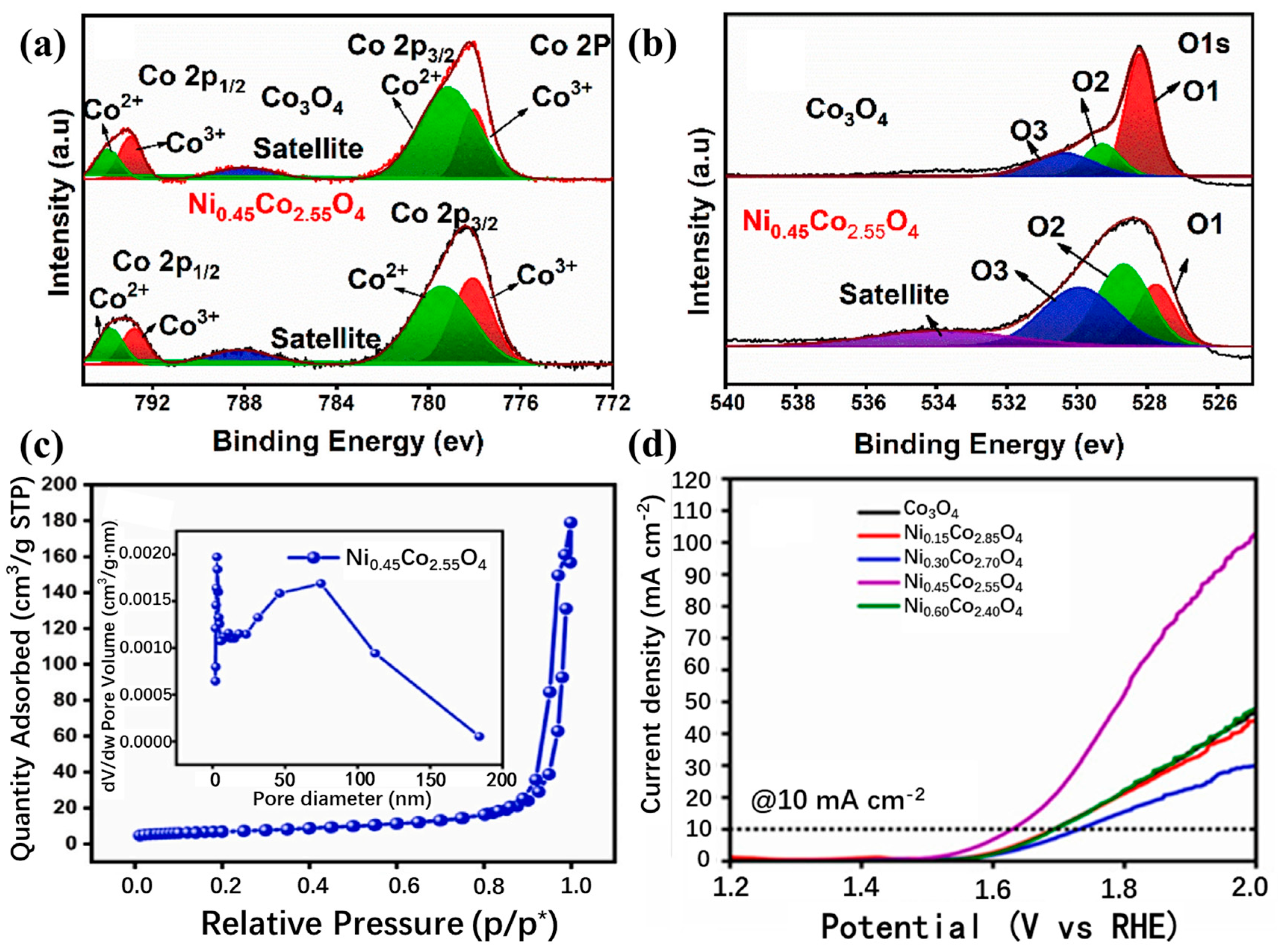
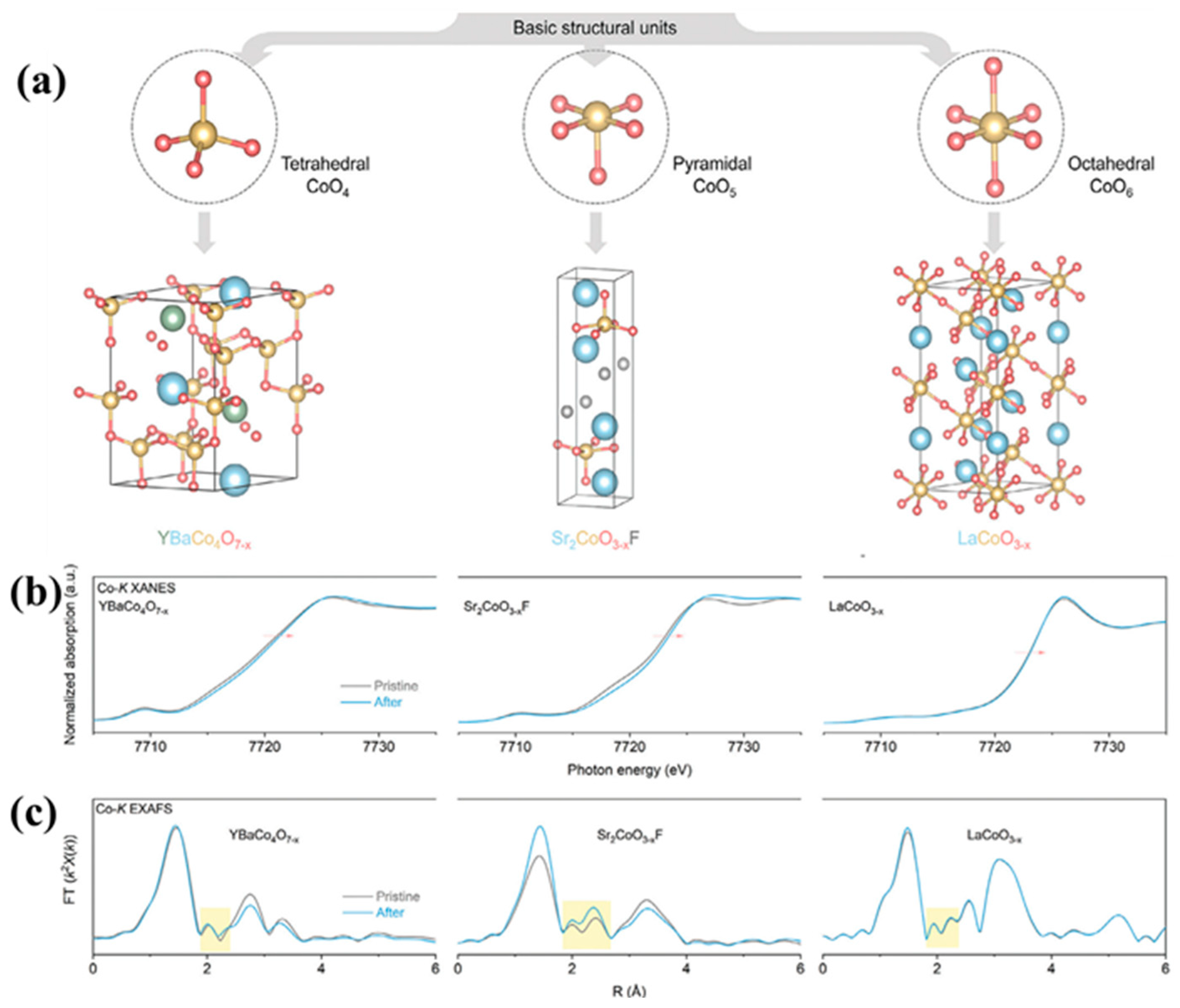
| Catalyst | Morphology | j (mA cm−2) | η (mV) | Electrolyte | Ref. |
|---|---|---|---|---|---|
| Co3O4@Ti | Nanoneedles | 20 | 416 | 1 M KOH | [57] |
| Ti@M-Co3O4 | Nanoneedles | 10 | 450 | 0.1 M KOH | [77] |
| NiOx@Co3O4/CC | Nanowire | 10 | 360 | 0.1 M KOH | [78] |
| Ru-Co3O4/CoP/TM | Nanowire | 10 | 293 | 1 M KOH | [78] |
| Ultrathin Co3O4 nanofilm | Nanofilms | 40 | 461 | 1 M KOH | [79] |
| {112} high-index faceted porous Co3O4 | Nanofilms | 10 | 318 | 1 M KOH | [70] |
| B-Co3O4@ZIF-67 | Nanocages | 10 | 334 | 1 M KOH | [80] |
| Co-CNT@COF-Pyr | Nanocages | 10 | 438 | 1 M KOH | [65] |
| Co3O4-Ov | Sea urchin-shaped structures | 20 | 280 | 1 M KOH | [81] |
| Co3O4/NF | Sea urchin-shaped structures | 20 | 327 | 1 M KOH | [71] |
| Co3O4 nanosheets | Flower-shaped structures | 10 | 380 | 0.1 M KOH | [82] |
| CoCe HNF | Flower-shaped structures | 10 | 315 | 1 M KOH | [72] |
| Co3O4|CoP | Core-shell structure | 10 | 320 | 1 M KOH | [66] |
| Co3O4@NiCo LDH | Core-shell structure | 15 | 279 | 1 M KOH | [73] |
| Ni3S2@MoO3@Co3O4@AMO/NF | Core-shell structure | 10 | 248 | 1 M KOH | [83] |
| Ru–CoPO | Nanowire | 10 | 310 | 1 M KOH | [84] |
| Au–IrO2 | Flower-shaped structures | 10 | 286 | 0.1 M KOH | [85] |
| Catalyst | Electrode Type (Work, Counter, and Reference Electrodes) | j (mA cm−2) | η (mV) | Electrolyte | Ref. |
|---|---|---|---|---|---|
| Mn@Co3O4 | Mn@Co3O4, platinum bar, and Ag|AgCl electrode (3 mol L−1 KCl) | 10 | 320 | 1 M KOH | [145] |
| Hybrid-phase SrCo0.55Fe0.5O3−δ | Hybrid-phase SrCo0.55Fe0.5O3−δ, graphite rod, and Ag/AgCl | 10 | 290 | 1 M KOH | [131] |
| Vo-Fe–Co3O4 | Vo-Fe–Co3O4, Pt wire, and Hg/HgO | 10 | 231 | 1 M KOH | [121] |
| Co3O4/PPy-120 | Co3O4/PPy-120, graphite rod, and Ag/AgCl | 10 | 140 | 1 M KOH | [114] |
| M-Co3O4/NPC | Co3O4/NPC, platinum foil, and reversible hydrogen electrode (RHE) | 10 | 302 | 1 M KOH | [103] |
| Ir0.33@Co3O4 | Glassy carbon, Pt mesh, and Ag/AgCl | 10 | 296 | 1 M KOH | [88] |
| In-Co3O4 | In-Co3O4, platinum slice, and Hg/HgO | 10 | 340 | 1 M KOH | [140] |
| Co3O3.87◻0.13 | Co3O3.87◻0.13, Pt wire equipped with isolation tube, and Hg/HgO | 10 | 440 | 0.1 M KOH | [125] |
| Co3O4 NS/NF | Co3O4 NS/NF, Pt foil, and saturated Ag/AgCl (3 M KCl) electrode | 10 | 190 | 0.1 M KOH | [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Jia, Y.; Jia, H.; Gao, L. Recent Development on the Synthesis Strategies and Mechanisms of Co3O4-Based Electrocatalysts for Oxygen Evolution Reaction: A Review. Molecules 2025, 30, 3238. https://doi.org/10.3390/molecules30153238
Liu Y, Jia Y, Jia H, Gao L. Recent Development on the Synthesis Strategies and Mechanisms of Co3O4-Based Electrocatalysts for Oxygen Evolution Reaction: A Review. Molecules. 2025; 30(15):3238. https://doi.org/10.3390/molecules30153238
Chicago/Turabian StyleLiu, Yu, Yifan Jia, Hongxing Jia, and Liangjuan Gao. 2025. "Recent Development on the Synthesis Strategies and Mechanisms of Co3O4-Based Electrocatalysts for Oxygen Evolution Reaction: A Review" Molecules 30, no. 15: 3238. https://doi.org/10.3390/molecules30153238
APA StyleLiu, Y., Jia, Y., Jia, H., & Gao, L. (2025). Recent Development on the Synthesis Strategies and Mechanisms of Co3O4-Based Electrocatalysts for Oxygen Evolution Reaction: A Review. Molecules, 30(15), 3238. https://doi.org/10.3390/molecules30153238







