Characterization of Essential Oils and Ethanolic Extracts from Nine Pepper Species: Antioxidant and Antimicrobial Activity and Spectroscopic Analysis
Abstract
1. Introduction
2. Results and Discussion
2.1. Essential Oil and Ethanolic Extract Yields
| Pepper Species | Essential Oil Yield, % | Refs. | Ethanolic Extract Yield, % | Ref. | ||
|---|---|---|---|---|---|---|
| Exp | Lit 1 | Exp | Lit 2 | |||
| Black pepper | 2.18 | 0.91–3.68 | [22,23] | 12.5 | 9.80 | [5] |
| Green pepper | 1.86 | 0.47–3.76 | [24,25] | 12.6 | - | - |
| White pepper | 2.44 | 0.44–4.12 | [22,23] | 6.4 | - | - |
| Melegueta pepper | 0.27 | 0.21–0.30 | [26,27,28] | 4.8 | - | - |
| Voatsiperifery pepper | 6.09 | 3.04–11.30 | [29,30] | 18.0 | - | - |
| Javanese pepper | 9.73 | 0.20–11.80 | [21,31] | 17.4 | - | - |
| Pink pepper | 6.63 | 0.16–6.54 | [32,33] | 41.0 | - | - |
| Bengali pepper | 0.86 | 0.10–0.80 | [34,35] | 27.71 | - | - |
| Sichuan pepper | 3.93 | - | - | 26.6 | - | - |
| Pepper Species | Extraction Method | Yield, % | Major Compounds | Ref. |
|---|---|---|---|---|
| Black pepper | Commercial oil | - | (E)-caryophyllene (27.404%); limonene (14.74%); β-phellandren (10.69%); pinene (7.73%) | [37] |
| HD—6 h | 1.69–3.68 | β-caryophyllene, α-pinene, β-pinene, sabinene, 3-carene, and limonene | [22] | |
| HD—4 h | 0.905 | α-pinene (6.61%), β-pinene (15.87%), 3-carene (17.57%), limonene (35.6%), β-caryophyllene (9.48%) | [23] | |
| Commercial oil | - | β-caryophyllene (30.33%), limonene (12.12%), sabinene (7.52%), β-pinene (7.42%) | [38] | |
| UMAHD | 4.00 | α-pinene (8.6%), β-pinene (14.0%), 3-δ-carene (33.2%), limonene (19.2%), caryophyllene (13.0%) | [36] | |
| HD—5 h | 0.359–2.079 | 3-carene (6.2–26.84%), limonene (4.39–25.83%), caryophyllene (25.58–62.23%), (1R)-2,6,6-trimethylbicyclo[3.1.1]hept-2-ene (0–40.85%) | [39] | |
| HD | 1.98–3.57 | β-pinene (5.4–7.2%), α-phellandrene (11.4–18.2%), limonene (15.9–20.0%), β-caryophyllene (9.5–15.9%) | [25] | |
| HD—7 h | 1.11 | β-caryophyllene (51.12%), β-thujene (20.58%) | [40] | |
| Green pepper | Commercial oil | - | β-pinene (24.42%), δ3-carene (19.72%), limonene (18.73%), α-pinene (10.39%) | [38] |
| HD | 2.76–3.76 | β-pinene (6.2–7.4%), α-phellandrene (11.8–14.7%), limonene (16.4–19.1%), β-caryophyllene (10.0–16.3%) | [25] | |
| HD—3 h | 0.75 | 3-carene (35.21%), D-limonene (21.54%), β-caryophyllene (10.05%), β-pinene (9.17%), sabinene (7.37%) | [41] | |
| White pepper | HD—6 h | 1.68–4.12 | β-caryophyllene, α-pinene, β-pinene, sabinene, 3-carene, and limonene | [22] |
| HD—4 h | 0.44 | α-pinene (7.31%), β-pinene (16.18%), 3-carene (18.02%), limonene (26.03%), β-caryophyllene (14.42%) | [23] | |
| UAHD, MAHD, UMAHD | 3.4–4.1 | β-pinene (6.9–9.3%), 3-δ-carene (23.1–25.1%), limonene (15.9–23.2%), caryophyllene (25.1–33.4%) | [36] | |
| HD—5 h | 0.538–2.25 | 3-carene (0–25.09%), limonene (8.77–20.84%), caryophyllene (43.96–58.24%), (1R)-2,6,6-trimethylbicyclo[3.1.1]hept-2-ene (0–17.39%) | [39] | |
| HD | 2.25–2.92 | β-pinene (6.4–7.0%), α-phellandrene (10.3–11.5%), limonene (17.0–18.9%), β-caryophyllene (16.2–17.3%) | [25] | |
| HD—6 h | - | sabinene (12.6%), β-pinene (7.3%), limonene (11.9%), β-bisabolene (7.4%), torreyol (9.3%) | [42] | |
| SD—6 h | - | α-pinene (5.20–10.65%), sabinene (0.14–21.58%), β-pinene (8.18–14.82%), Δ-3-carene (21.37–27.83%), DL-limonene (15.41–21.68%), caryophyllene (6.99–30.90%) | [43] | |
| Melegueta pepper | HD | 0.30 | α-caryophyllene (48.78%), β-caryophyllene (32.50%), linalool (5.40%), E-nerolidol (4.33%) | [26] |
| HD—3 h | 0.21 | α-humulene (60.9%), β-caryophyllene (21.7%), humulene oxide II (5.5%) | [27] | |
| HD—4 h | 0.30 | humulene (16.30%), gingerol (15.40%), gingerone (24.27%), gingerdione (22.46%) | [28] | |
| Voatsiperifery pepper | HD | 3.04 | limonene (27.31%), α-phellandrene (14.47%), asaricin (13.47%), β-pinene (6.81%), α-pinene (6.78%) | [29] |
| HD | 11.6 | limonene + eucalyptol (29.54%), α-phellandrene (14.38%), asaricin (13.94%), β-pinene (6.46%), α-pinene (6.00%) | [30] | |
| Javanese pepper | HD—4 h | 1.01 | β-myrcene (21.19%), 1,8-cineole (6.41%), eugenol (10.66%) | [23] |
| HD—4 h | 11.8 | sabinene (9.1%), β-elemene (9.4%), β-caryophyllene (3.1%), epi-cubebol (4.3%), cubebol (5.6%) | [31] | |
| HD—3 h | 9.6 | sabinene (46.3%), 4-terpineol (17.0%), γ-terpinene (4.2%) | [44] | |
| HD—3 h | 2.4 | β-cubebene (18.94%), cubebol (13.32%), sabinene (9.60%), α-copaene (7.41%), β-caryophyllene (5.28%) | [45] | |
| HD—7 h | 1.23 | terpinen-4-ol (42.41%), α-copaene (20.04%), γ-elemene (17.68%) | [40] | |
| HD—4 h | 2.3 | methyleugenol (41.31%), eugenol (33.95%), (E)-caryophyllene (5.65%) | [46] | |
| HD | 2.3 | sabinene (19.4%), β-cubebene (18.3%), α-copaene (8.8%), β-phellandrene (5.9%) | [47] | |
| Pink pepper | HD—2 h | - | β-myrcene (41%), 218 β-cubebene (12%), limonene (9%), α-pinene (8%) | [48] |
| HD—3 h | 2.93 | α-pinene (14.22%), sabinene (31.39%), β-myrcene (7.83%), α-phellandrene (11.27%), β-phellandrene (7.57%), germacrene D (8.62%) | [49] | |
| HD | 1.77–4.77 | α-pinene (20.7–57%), δ-3-carene (11.07–17%), cis-ocimene (3.3–27.9%), p-cymene (2.6–7.1%), limonene (8–11%) | [50] | |
| HD—6 h | 6.54 ± 1.06 | α-phelandrene (35.84%), limonene (17.31%), α-pinene (1.98%), monoterpenes and β-phelandrene (13.04%) | [33] | |
| HD | 0.16 | limonene (16.99%), germacrene D (10.85%), δ-cadinene (9.21%), myrcene (20.43%) | [32] | |
| Bengali pepper | HD—4 h | 0.285 | β-caryophyllene (11.85%), α-humulene (6.25%), 1-heptadecene (11.03%), n-heptadecane (11.93%) | [23] |
| HD—4 h | 0.49 | (Z)-β-farnesene (25.08%), β-caryophyllene (13.57%), α-humulene (13.37%), 8-heptadecene (9.28%), heptadecane (7.07%) | [51] | |
| HD—6 h | 0.10 | α-pinene (15.3%), β-pinene (43.1%), limonene (9.6%), nerolidol (8.8%) | [34] | |
| SD | 1.01 | caryophyllene (10.78%), 3-heptadecene (9.95%), zingiberene (9.54%), germacrene D (8.96%), pentadecane (8.76%), heptadecane (8.73%) | [52] | |
| SD | - | (n)-trans-nerolidol (12.7%), β-linalool (8,4%) | [53] | |
| HD | 0.15–0.80 | β-caryophyllene (15–25%), hexadecen-1-ol (3.75%), α-caryophyllene (9.58%), β-humulene (6.17%), pentadecane (6.48%) | [35] |
| Pepper Species | Extraction Method | Yield (EE), % | Yield (Pip), % | Ref. |
|---|---|---|---|---|
| Black pepper | RE with 96% ethanol | 9.8 | - | [8] |
| MAC, RE, UAE, SE; results are given for UAE with 70% ethanol | 9.8 | - | [5] | |
| Mixing, ethanol purity not specified | 10–12.48 | 21–37.50 | [58] | |
| MAE, UAE, UMAE, SE with >95% ethanol—results are given for UAE | - | 3.70 | [54] | |
| Shaking with 80% ethanol | - | 3.29–7.39 | [59] | |
| SE with 96% ethanol | 7.12 | [60] | ||
| MAE with 96% ethanol | 16.28 | 5.41 | [61] | |
| Green pepper | Shaking with 80% ethanol | - | 5.09–8.61 | [59] |
| MAC, UAE, MAE, UMAE with anhydrous ethanol—results are given for UAE | Up to 11.4 | up to 19.25 | [6] | |
| White pepper | MAC in 96% ethanol | - | 0.92 | [62] |
| PER in (60–90%) ethanol—results are given for 70% ethanol | - | 2.7 | [57] | |
| SE with >99.8% ethanol | 5.7 | 43.5 | [42] | |
| Melegueta pepper | Mixing with 95% ethanol | 2.0 | [63] | |
| MAC with absolute ethanol | 6.16 | [64] | ||
| SE with 95% ethanol | 12.3 | [65] | ||
| Javanese pepper | RE with 96% ethanol | 12.0 | [8] | |
| SE with 96% ethanol | 8.70 | [60] | ||
| MAC with 70 and 96% ethanol—results are given for 70% ethanol | 14.89 | [21] | ||
| SE with 99.5% ethanol | 1.00 | [45] | ||
| MAE with 96% ethanol | 13.94 | 0.03 | [61] | |
| Pink pepper | UAE and SE with >95% ethanol—results are given for UAE | 28.6 | [55] | |
| PLE and SE with 99.9% ethanol—results are given for SE | 36.00 | [9] | ||
| SE, UAE, SEE with 44% ethanol—results are given for UAE | 21.00 | [19] | ||
| Bengali pepper | SE with 90% ethanol | 8.6 | [66] | |
| UAE, SE, mixing with ethanol | 0.58 | [56] | ||
| MAC with 30% ethanol | 4.7 | [67] |
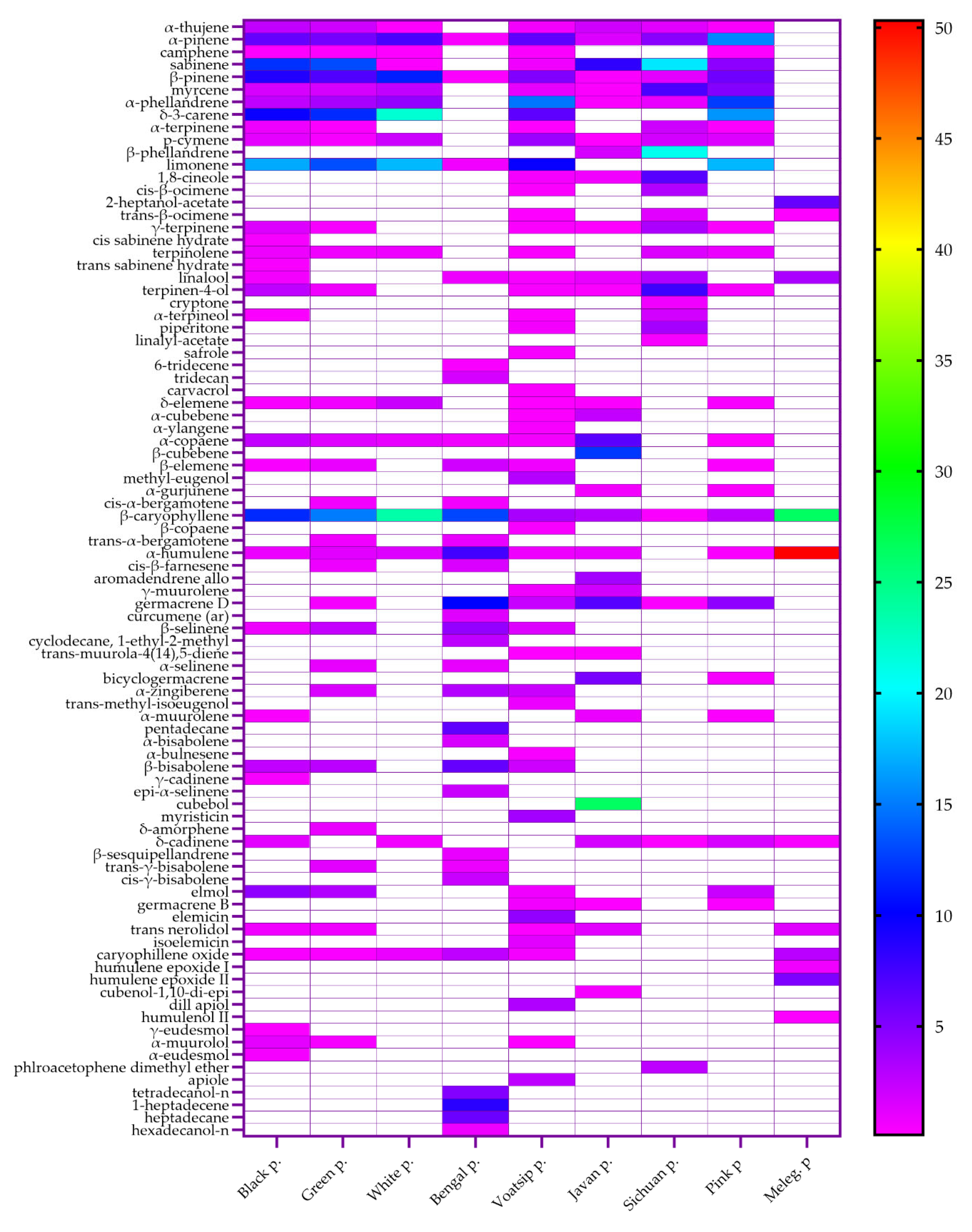
2.2. Chemical Composition of Essential Oils
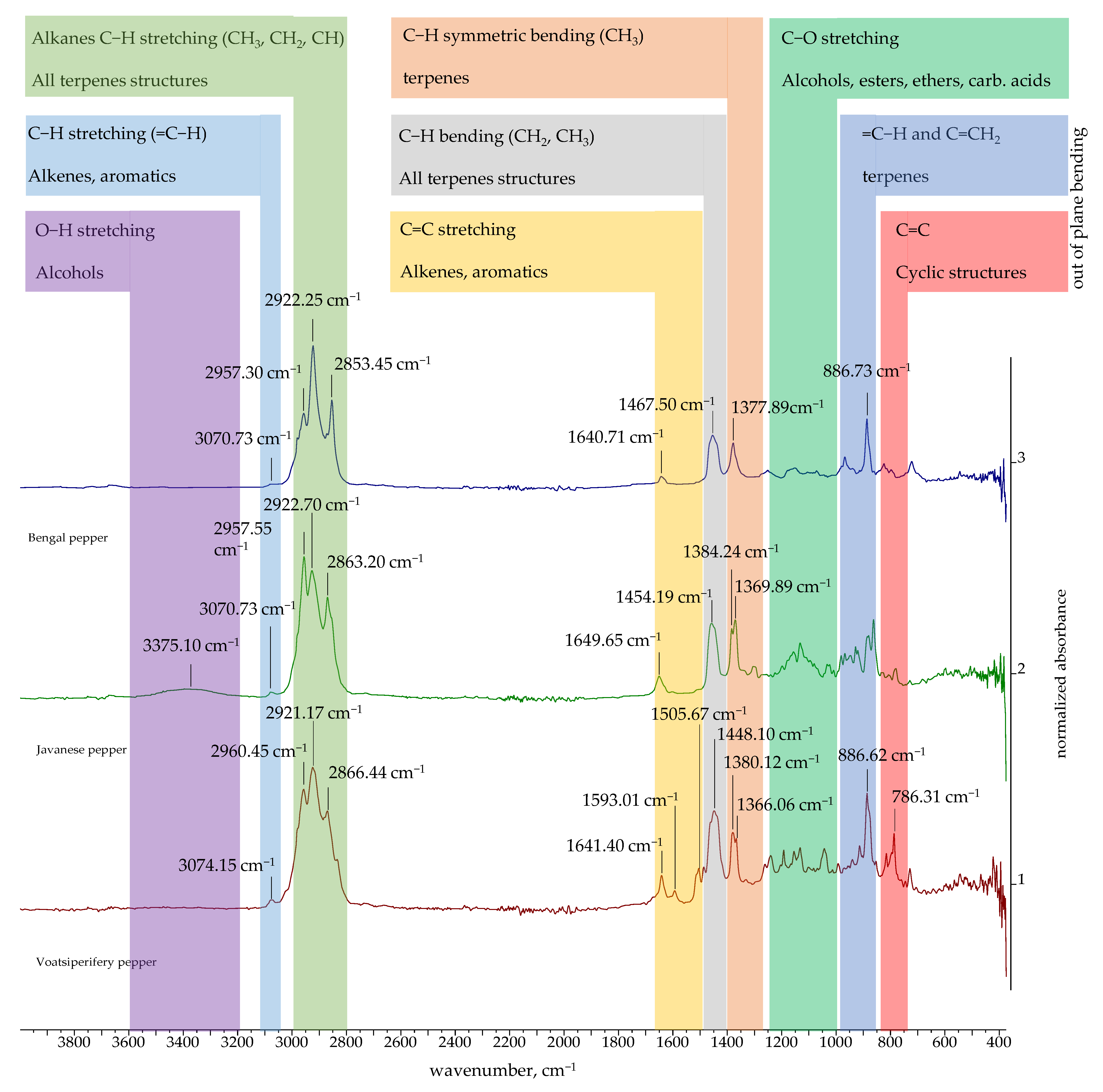
2.3. FTIR and 1H NMR Spectra of Essential Oils
2.4. Piperine Content in Ethanolic Extracts
2.4.1. Determination of Piperine Content by HPLC
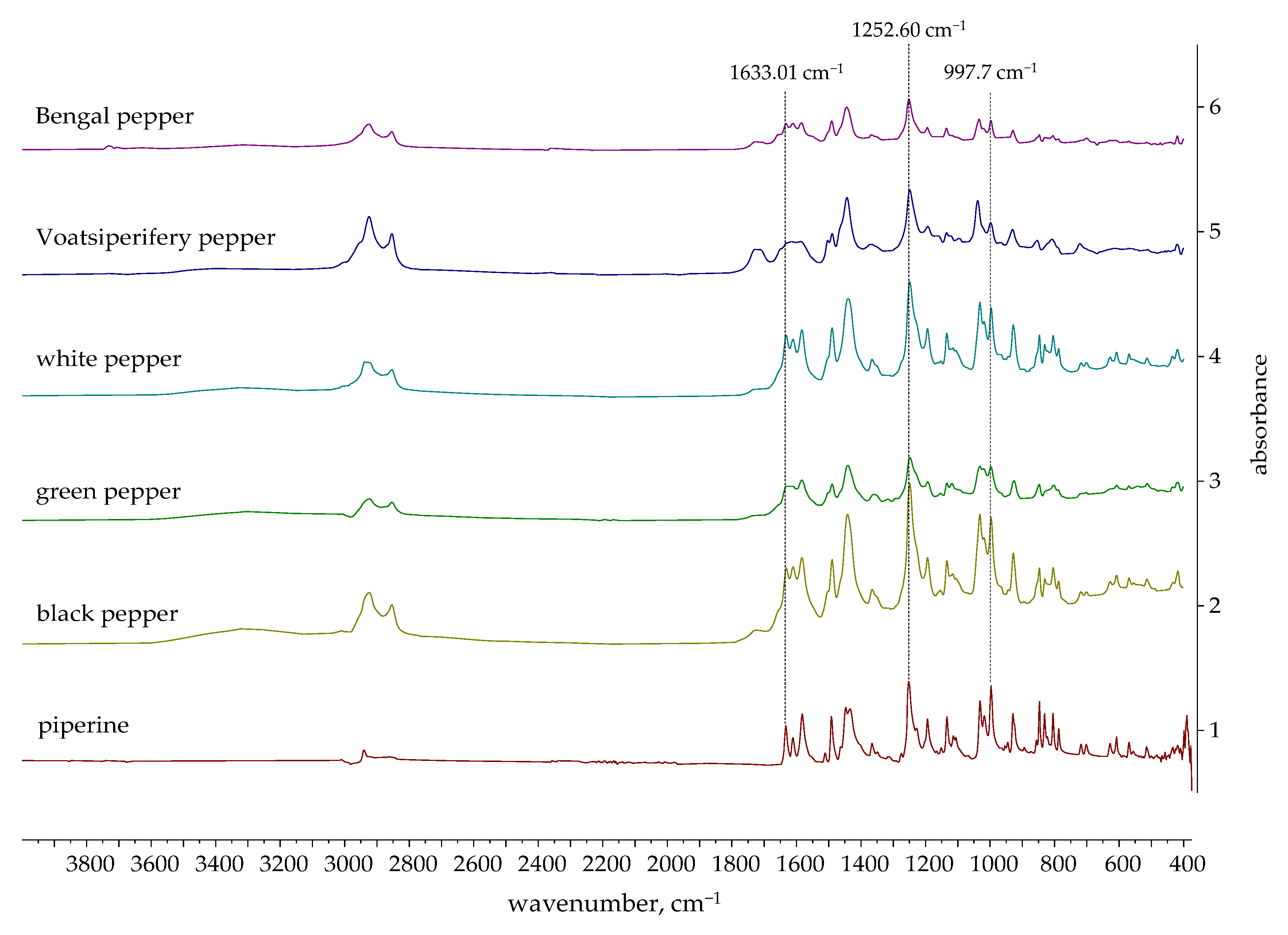
2.4.2. Verification of Piperine Presence in Ethanolic Extracts by FTIR
- 1633.01 cm−1: A very strong band that is highly characteristic of conjugated C=O stretching (amide I band) from the amide functional group in piperine;
- 1252.60 cm−1: A strong absorption band characteristic of the asymmetric stretching of the C-O-C bonds within the methylenedioxy group, a unique structural feature of piperine;
- 997.07 cm−1: A strong and sharp peak characteristic of the out-of-plane bending of the trans C=C double bond in piperine’s alkene side chain.
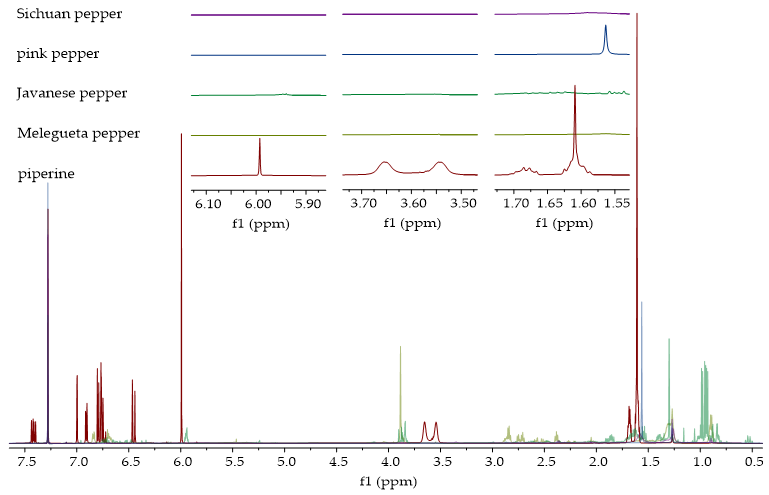
2.4.3. Verification of Piperine Presence in Ethanolic Extracts by 1H NMR
2.5. Antioxidant Activity of EOs and EEs
2.5.1. Total Phenolic and Flavonoid Content in EOs and EEs
- Essential oils: TPC for Javanese, pink, Bengal, and Sichuan pepper;
- Ethanolic extracts: TPC and TFC for white, Voatsiperifery, and Sichuan pepper.
2.5.2. Radical Scavenging Activity of EOs and EEs
2.6. Antimicrobial Activity of Essential Oils and Ethanolic Extracts
2.7. Trace Element Content
3. Materials and Methods
3.1. Chemicals
3.2. Essential Oil Extraction
3.3. Solvent Extraction
3.4. Characterization of Essential Oils and Ethanolic Extracts
3.4.1. Chemical Composition
3.4.2. FTIR Spectrophotometry
3.4.3. NMR Spectrophotometry
3.4.4. Determination of Piperine by High-Performance Liquid Chromatography
3.5. Antioxidant Activity
3.6. Antimicrobial Activity
3.7. Trace Element Content
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Díaz-Guerrero, P.; Panzani, S.; Sanmartin, C.; Muntoni, C.; Taglieri, I.; Venturi, F. “Pepper”: Different Spices, One Name—Analysis of Sensory and Biological Aspects. Molecules 2025, 30, 1891. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Zakaria, Z.A.; Gyawali, R.; Ibrahim, S.A.; Rajkovic, J.; Shinwari, Z.K.; Khan, T.; Sharifi-Rad, J.; Ozleyen, A.; Turkdonmez, E.; et al. Piper Species: A Comprehensive Review on Their Phytochemistry, Biological Activities and Applications. Molecules 2019, 24, 1364. [Google Scholar] [CrossRef] [PubMed]
- Stojanović-Radić, Z.; Pejčić, M.; Dimitrijević, M.; Aleksić, A.V.; Anil Kumar, N.; Salehi, B.C.; Cho, W.; Sharifi-Rad, J. Piperine-A Major Principle of Black Pepper: A Review of Its Bioactivity and Studies. Appl. Sci. 2019, 9, 4270. [Google Scholar] [CrossRef]
- Lee, J.-E.; Jayakody, J.; Kim, J.-I.; Jeong, J.-W.; Choi, K.-M.; Kim, T.-S.; Seo, C.; Azimi, I.; Hyun, J.; Ryu, B. The Influence of Solvent Choice on the Extraction of Bioactive Compounds from Asteraceae: A Comparative Review. Foods 2024, 13, 3151. [Google Scholar] [CrossRef] [PubMed]
- Milenković, A.; Aleksovski, S.; Miteva, K.; Milenković, L.; Stanojević, J.; Nikolić, G.; Ilić, Z.S.; Stanojević, L. The Effect of Extraction Technique on the Yield, Extraction Kinetics and Antioxidant Activity of Black Pepper (Piper nigrum L.) Ethanolic Extracts. Horticulturae 2025, 11, 125. [Google Scholar] [CrossRef]
- Zhang, C.; Gu, F.; Hu, W.; Wu, G.; Chen, W.; Dong, C.; Niu, Z. Effect of Extraction Technique on Chemical Compositions and Antioxidant Activities of Freeze-Dried Green Pepper. Front. Nutr. 2022, 9, 998840. [Google Scholar] [CrossRef]
- Adigun, N.S.; Oladiji, A.T.; Ajiboye, T.O. Antioxidant and Anti-Hyperlipidemic Activity of Hydroethanolic Seed Extract of Aframomum melegueta K. Schum in Triton X-100 Induced Hyperlipidemic Rats. S. Afr. J. Bot. 2016, 105, 324–332. [Google Scholar] [CrossRef]
- Stanojević, L.; Milenković, A.; Pejčić, M.; Stojanović-Radić, Z.; Petrović, S.; Nikolić, V.; Troter, D. Ethanolic Extracts from Black (Piper nigrum L.) and Cubeb Pepper (Piper cubeba L.) Fruits Available in Serbia: Comparative Study Regarding Phytochemical Properties, Antimicrobial and Antioxidant Activities. Adv. Techol. 2024, 13, 42–49. [Google Scholar] [CrossRef]
- Dias, S.L.G.; Stevanato, N.; Barão, C.E.; Hoscheid, J.; Raspe, D.T.; Cardozo-Filho, L.; Silva, C.D. Application of Ethanol under Pressurized Conditions for the Extraction of Phytochemical Compounds from Pink Pepper Fruit. Acta Sci. Technol. 2024, 47, e70241. [Google Scholar] [CrossRef]
- Magdalena, H.; Fajrina, A.; Eriadi, A.; Asra, R. Potential of Piperaceae Plants as Antibacterial against Staphylococcus aureus and Escherichia coli: A Review. Asian J. Pharm. Res. Dev. 2021, 9, 83–100. [Google Scholar] [CrossRef]
- Rodrigues De Oliveira, M.; Anjos Da Silva, L.; Santos Da Silva, R.; Branco De Queiroz, C.C.; Takeara, R. Chemical Composition and Biological Activities of Essential Oils of Piper Species from the Amazon. J. Essent. Oil Res. 2021, 33, 536–548. [Google Scholar] [CrossRef]
- Ramsey, J.T.; Shropshire, B.C.; Nagy, T.R.; Chambers, K.D.; Li, Y.; Korach, K.S. Essential Oils and Health. Yale J. Biol. Med. 2020, 93, 291–305. [Google Scholar] [PubMed]
- Bunse, M.; Daniels, R.; Gründemann, C.; Heilmann, J.; Kammerer, D.R.; Keusgen, M.; Lindequist, U.; Melzig, M.F.; Morlock, G.E.; Schulz, H.; et al. Essential Oils as Multicomponent Mixtures and Their Potential for Human Health and Well-Being. Front. Pharmacol. 2022, 13, 956541. [Google Scholar] [CrossRef] [PubMed]
- Bechis, G.; Minteguiaga, M.A.; Sgorbini, B.; Marengo, A.; Rubiolo, P.; Cagliero, C. Make the Quality Control of Essential Oils Greener: Fast Enantioselective GC-MS Analysis of Sweet and Bitter Orange as a Case Study. Molecules 2023, 28, 6231. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.K.; Ghuge, A.; Parab, M.; Al-Refaei, Y.; Khandare, A.; Dand, N.; Waghmare, N. A Comparative Review on High-Performance Liquid Chromatography (HPLC), Ultra Performance Liquid Chromatography (UPLC) & High-Performance Thin Layer Chromatography (HPTLC) with Current Updates. Curr. Issues Pharm. Med. Sci. 2022, 35, 224–228. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Ristivojevic, P.; Gegechkori, V.; Litvinova, T.M.; Morton, D.W. Essential Oil Quality and Purity Evaluation via FT-IR Spectroscopy and Pattern Recognition Techniques. Appl. Sci. 2020, 10, 7294. [Google Scholar] [CrossRef]
- Colson, K.L.; Yuk, J.; Markus, M.A.; Le, P.M. Identification and Quantification of Plant Extracts Using Automated Nuclear Magnetic Resonance; Bruker Biospin: Billerica, MA, USA, 2015. [Google Scholar] [CrossRef]
- Valentino, G.; Graziani, V.; D’Abrosca, B.; Pacifico, S.; Fiorentino, A.; Scognamiglio, M. NMR-Based Plant Metabolomics in Nutraceutical Research: An Overview. Molecules 2020, 25, 1444. [Google Scholar] [CrossRef]
- Andrade, K.S.; Poncelet, D.; Ferreira, S.R.S. Sustainable Extraction and Encapsulation of Pink Pepper Oil. J. Food Eng. 2017, 204, 38–45. [Google Scholar] [CrossRef]
- Jaafaru, M.S. Flavonoids-Rich Extract of Aframomum melegueta (Black Pepper) Improves Antioxidant Status and Modulates Aging Process in Lead-Induced Neurotoxic Drosophila Melanogaster. Eurasian J. Sci. Eng. 2024, 10, 34–45. [Google Scholar] [CrossRef]
- Dwita, L.P. Brain Antioxidant Properties of Piper cubeba L. Extracts and Essential Oil. Farmacia 2023, 71, 296–302. [Google Scholar] [CrossRef]
- Chen, S.-X.; Yang, K.; Xiang, J.-Y.; Raymond Kwaku, O.; Han, J.-X.; Zhu, X.-A.; Huang, Y.-T.; Liu, L.-J.; Shen, S.-B.; Li, H.-Z.; et al. Comparison of Chemical Compositions of the Pepper EOs From Different Cultivars and Their AChE Inhibitory Activity. Nat. Prod. Commun. 2020, 15, 1–6. [Google Scholar] [CrossRef]
- Al-Sayed, E.; Gad, H.A.; El-Kersh, D.M. Characterization of Four Piper Essential Oils (GC/MS and ATR-IR) Coupled to Chemometrics and Their Anti-Helicobacter pylori Activity. ACS Omega 2021, 6, 25652–25663. [Google Scholar] [CrossRef] [PubMed]
- Ademović, Z.; Horozić, E. Antioxidant and Antimicrobial Potential of Essential Oils of Different Types of Pepper (Piper sp.). RŠF 2023, 53, 41–45. [Google Scholar] [CrossRef]
- Buckle, K.A.; Rathnawathie, M.; Brophy, J.J. Compositional Differences of Black, Green and White Pepper (Piper nigrum L.) Oil from Three Cultivars. Int. J. Food Sci. Technol. 1985, 20, 599–613. [Google Scholar] [CrossRef]
- Owokotomo, I.A.; Ekundayo, O.; Oguntuase, B.J. Chemical Constituents of the Leaf, Stem, Root and Seed Essential Oils of Aframomum melegueta (K. Schum) from South West Nigeria. Int. Res. J. Pure Appl. Chem. 2014, 4, 395–401. [Google Scholar] [CrossRef]
- Ajaiyeoba, E.O.; Ekundayo, O. Essential Oil Constituents of Aframomum melegueta (Roscoe) K. Schum. Seeds (Alligator Pepper) from Nigeria. Flavour Fragr. J. 1999, 14, 109–111. [Google Scholar] [CrossRef]
- Banahene, J.C.M.; Ofosu, I.W.; Lutterodt, H.E.; Ellis, W.O. Exploring Essential Oils from Piper guineense and Aframomum melegueta as Natural Biocontrol Agents against Cocoa Fungal Isolates towards the Safety of Cocoa Beans. Food Humanit. 2025, 4, 100601. [Google Scholar] [CrossRef]
- Weil, M.; Boulanger, R.; Servent, A.; Shum Cheong Sing, A.; Bohuon, P. Quality, Typicity and Potential Valorization of Piper Borbonense, a Poorly Known Wild Pepper from Reunion Island. Fruits 2020, 75, 95–103. [Google Scholar] [CrossRef]
- Weil, M.; Shum Cheong Sing, A.; Méot, J.M.; Boulanger, R.; Bohuon, P. Impact of Blanching, Sweating and Drying Operations on Pungency, Aroma and Color of Piper Borbonense. Food Chem. 2017, 219, 274–281. [Google Scholar] [CrossRef]
- Bos, R.; Woerdenbag, H.J.; Kayser, O.; Quax, W.J.; Ruslan, K. Elfami Essential Oil Constituents of Piper cubeba L. Fils. from Indonesia. J. Essent. Oil Res. 2007, 19, 14–17. [Google Scholar] [CrossRef]
- Santos, A.C.A.D.; Rossato, M.; Agostini, F.; Serafini, L.A.; Santos, P.L.D.; Molon, R.; Dellacassa, E.; Moyna, P. Chemical Composition of the Essential Oils from Leaves and Fruits of Schinus molle L. and Schinus terebinthifolius Raddi from Southern Brazil. J. Essent. Oil Bear. Plants 2009, 12, 16–25. [Google Scholar] [CrossRef]
- Cole, E.R.; Santos, R.B.D.; Lacerda Júnior, V.; Martins, J.D.L.; Greco, S.J.; Cunha Neto, A. Chemical Composition of Essential Oil from Ripe Fruit of Schinus Terebinthifolius Raddi and Evaluation of Its Activity against Wild Strains of Hospital Origin. Braz. J. Microbiol. 2014, 45, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Varughese, T.; Unnikrishnan, P.K.; Deepak, M.; Balachandran, I.; Rema Shree, A.B. Chemical Composition of the Essential Oils from Stem, Root, Fruit and Leaf of Piper longum Linn. J. Essent. Oil Bear. Plants 2016, 19, 52–58. [Google Scholar] [CrossRef]
- Tran, T.H.; Khang, V.C.; Ngo, H.D.; Le, X.T. Extraction Process and Analysis of Components in Essential Oils of Piper longum Linn. Harvested in Dak Lak Province, Vietnam. Asian J. Chem. 2020, 32, 2639–2646. [Google Scholar] [CrossRef]
- Wang, Y.; Li, R.; Jiang, Z.-T.; Tan, J.; Tang, S.-H.; Li, T.-T.; Liang, L.-L.; He, H.-J.; Liu, Y.-M.; Li, J.-T.; et al. Green and Solvent-Free Simultaneous Ultrasonic-Microwave Assisted Extraction of Essential Oil from White and Black Peppers. Ind. Crops Prod. 2018, 114, 164–172. [Google Scholar] [CrossRef]
- Bhatia, S.; Shah, Y.A.; Al-Harrasi, A.; Jawad, M.; Koca, E.; Aydemir, L.Y. Novel Applications of Black Pepper Essential Oil as an Antioxidant Agent in Sodium Caseinate and Chitosan Based Active Edible Films. Int. J. Biol. Macromol. 2024, 254, 128045. [Google Scholar] [CrossRef]
- Nikolić, M.; Stojković, D.; Glamočlija, J.; Ćirić, A.; Marković, T.; Smiljković, M.; Soković, M. Could Essential Oils of Green and Black Pepper Be Used as Food Preservatives? J. Food Sci. Technol. 2015, 52, 6565–6573. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, C.; Pan, S.; Chen, L.; Liu, M.; Yang, K.; Zeng, X.; Tian, J. Analysis of Chemical Components and Biological Activities of Essential Oils from Black and White Pepper (Piper nigrum L.) in Five Provinces of Southern China. Lwt 2020, 117, 108644. [Google Scholar] [CrossRef]
- Andriana, Y.; Xuan, T.D.; Quy, T.N.; Tran, H.-D.; Le, Q.-T. Biological Activities and Chemical Constituents of Essential Oils from Piper Cubeba Bojer and Piper nigrum L. Molecules 2019, 24, 1876. [Google Scholar] [CrossRef] [PubMed]
- Dao, T.P.; Tran, T.H.; Nhan, N.P.T.; Quyen, N.T.C.; Tien, L.X.; Anh, T.T.; Quan, P.M.; Nguyen, N.H.; Anh, L.L.T.; Linh, H.T.K. Optimization of Essential Oil Yield from Vietnamese Green Pepper (Piper nigrum) Using Hydro-Distillation Method. IOP Conf. Ser. Mater. Sci. Eng. 2020, 736, 022039. [Google Scholar] [CrossRef]
- Singh, S.; Kapoor, I.P.S.; Singh, G.; Schuff, C.; De Lampasona, M.P.; Catalan, C.A.N. Chemistry, Antioxidant and Antimicrobial Potentials of White Pepper (Piper nigrum L.) Essential Oil and Oleoresins. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2013, 83, 357–366. [Google Scholar] [CrossRef]
- Permana, G.E.; Pamudjo, I. Analysis of White Pepper Essential Oil Components Using Gas Chromatography-Mass Spectroscopy. Pharm. Educ. 2021, 21, 230–234. [Google Scholar] [CrossRef]
- Mothana, A.R.; Al-Said, M.S.; Khaled, J.M.; Alharbi, N.S.; Alatar, A.; Ahmad, A.; Alsohaibani, M.; Al-Yahya, M.; Rafatullah, S. Chemical Composition, Anti-Inflammatory and Antioxidant Activities of the Essential Oil of Piper cubeba L. Rom. Biotechnol. Lett. 2017, 22, 12366–12376. [Google Scholar]
- Singh, G.; Kiran, S.; Marimuthu, P.; de Lampasona, M.P.; de Heluani, C.S.; Catalán, C.A.N. Chemistry, Biocidal and Antioxidant Activities of Essential Oil and Oleoresins from Piper cubeba (Seed). Int. J. Essent. Oil Ther. 2008, 2, 50–59. [Google Scholar]
- Alminderej, F.; Bakari, S.; Almundarij, T.I.; Snoussi, M.; Aouadi, K.; Kadri, A. Antioxidant Activities of a New Chemotype of Piper cubeba L. Fruit Essential Oil (Methyleugenol/Eugenol): In Silico Molecular Docking and ADMET Studies. Plants 2020, 9, 1534. [Google Scholar] [CrossRef]
- Singh, G.; Marimuthu, P.; Schuff, C.; Catalan, C.A.N. Chemical Constituents, Antioxidative and Antimicrobial Activities of Essential Oil and Oleoresin of Tailed Pepper (Piper cubeba L.). Int. J. Food Eng. 2007, 3, 1–22. [Google Scholar] [CrossRef]
- Dannenberg, G.D.S.; Funck, G.D.; Silva, W.P.D.; Fiorentini, Â.M. Essential Oil from Pink Pepper (Schinus terebinthifolius Raddi): Chemical Composition, Antibacterial Activity and Mechanism of Action. Food Control 2019, 95, 115–120. [Google Scholar] [CrossRef]
- Carneiro, M.J.; Pinheiro, G.P.; Baseggio, A.M.; Maróstica-Júnior, M.R.; Sawaya, A.C.H.F. Chemical Composition and Antioxidant Activity of Essential Oil from Male and Female Schinus Terebinthifolius. Pharmacogn. Res. 2023, 15, 484–491. [Google Scholar] [CrossRef]
- Borges De Oliveira, K.; Carocho, M.; Finimundy, T.; Resende, O.; Célia, J.A.; Gomes, F.P.; Quequeto, W.D.; José De Campos Bastos, F.; Barros, L.; Ferreira Junior, W.N. Analysis of Volatiles of Rose Pepper Fruits by GC/MS: Drying Kinetics, Essential Oil Yield, and External Color Analysis. J. Food Qual. 2022, 2022, 1–10. [Google Scholar] [CrossRef]
- Kumar, V.; Mondal, P.C.; Kumar, R.; Kaushik, P.; Shakil, N.A.; Gowda, A.P.A.; Rana, V.S. Composition and Nematicidal Activity of the Essential Oil from Piper longum against Root Knot Nematode. Indian J. Agri. Sci. 2023, 93, 774–779. [Google Scholar] [CrossRef]
- Do Thi, T.-V.; Nguyen Thi, T.-H.; Nguyen, A.-H.; Tran Thi, N.-B. Chemical Compositions and Anti-Acetylcholinesterase, Nitric Oxide Suppressing Activities of Piper longum Fruits Oil. HPU2 J. Sci. Nat. Sci. Technol. 2023, 1, 38–45. [Google Scholar] [CrossRef]
- Jayarathna, N.; Athapattu, R.; Senanayake, P.; Paranagama, P. Volatile Chemical Constituents and Bioactivity of Selected Piper Species in Sri Lanka. Int. J. Mod. Bot. 2021, 1, 1–8. [Google Scholar]
- Gorgani, L.; Mohammadi, M.; Najafpour, G.D.; Nikzad, M. Sequential Microwave-Ultrasound-Assisted Extraction for Isolation of Piperine from Black Pepper (Piper nigrum L.). Food Bioprocess Technol. 2017, 10, 2199–2207. [Google Scholar] [CrossRef]
- De Mello, A.F.A.; Hoscheid, J.; Raspe, D.T.; Stevanato, N.; Da Silva, C. Green Extraction of Oleoresin from Pink Pepper Fruits: Effect of Experimental Conditions and Characterization. AppliedChem 2024, 4, 56–69. [Google Scholar] [CrossRef]
- Rathod, S.S.; Rathod, V.K. Extraction of Piperine from Piper longum Using Ultrasound. Ind. Crops Prod. 2014, 58, 259–264. [Google Scholar] [CrossRef]
- Le, X.-T.; Nguyen, N.-T.; Nguyen-Thi, B.-T.; Dang, M.-K.; Ngo-Thi, T.-N.; Vu, D.-P.; Duong-Nguyen, H.-N.; Pham-Vu, M.-C. Optimization of Piperine Extraction Process from Vietnamese White Pepper. IOP Conf. Ser. Earth Environ. Sci. 2024, 1340, 012020. [Google Scholar] [CrossRef]
- Gafar, P.A. Extraction of Black Pepper Non-Volatile Components as an Industrial Material. IOP Conf. Ser. Earth Environ. Sci. 2022, 950, 012035. [Google Scholar] [CrossRef]
- Kim, D.W.; Kim, M.J.; Shin, Y.; Jung, S.K.; Kim, Y.-J. Green Pepper (Piper nigrum L.) Extract Suppresses Oxidative Stress and LPS-Induced Inflammation via Regulation of JNK Signaling Pathways. Appl. Sci. 2020, 10, 2519. [Google Scholar] [CrossRef]
- Nahak, G.; Sahu, R.K. Phytochemical Evaluation and Antioxidant Activity of Piper cubeba and Piper longum. J. Appl. Pharm. Sci. 2011, 1, 153–157. [Google Scholar]
- Kusumawati, I.; Mahatmaputra, S.; Hadi, R.; Rohmania, R.; Rullyansyah, S.; Yusuf, H.; Rahman, A. Aphrodisiac Activity of Ethanolic Extracts from the Fruits of Three Pepper Plants from Piperaceae Family. J. Farm. Dan Ilmu Kefarmasian Indones. 2021, 8, 194. [Google Scholar] [CrossRef]
- Arsana, I.N.; Juliasih, N.; Ayu Sauca Sunia Widyantari, A.; Suriani, N.; Manto, A. GC-MS Analysis of the Active Compound in Ethanol Extracts of White Pepper (Piper nigrum L.) and Pharmacological Effects. Cell. Mol. Biomed. Rep. 2022, 2, 151–161. [Google Scholar] [CrossRef]
- Ilic, N.M.; Dey, M.; Poulev, A.A.; Logendra, S.; Kuhn, P.E.; Raskin, I. Anti-Inflammatory Activity of Grains of Paradise (Aframomum melegueta Schum) Extract. J. Agric. Food Chem. 2014, 62, 10452–10457. [Google Scholar] [CrossRef] [PubMed]
- Asiat, N.; Yusuf Oloruntoyin, A.; Damilola Ibukun, K.; Sobiat, S.; Bukola, B.; Mutiu Adewunmi, A.; Abdul-Azeez, B.; Azeemat Titilola, A.; Mohd Nizam, M. Phytochemical Screening and in Silico Pharmacological Profiling of Ethanolic Extract of Aframomum melegueta for Prostate Carcinoma. J. Appl. Pharm. Sci. 2020, 11, 132–145. [Google Scholar] [CrossRef]
- Adegoke, G.O.; Makinde, O.; Falade, K.O.; Uzo-Peters, P.I. Extraction and Characterization of Antioxidants from Aframomum melegueta and Xylopia aethiopica. Eur. Food Res. Technol. 2003, 216, 526–528. [Google Scholar] [CrossRef]
- Ramesh, V.; Hari, R.; Pandian, S.; Arumugam, G. Antioxidant Activity of Combined Ethanolic Extract of Eclipta alba and Piper longum Linn. J. Complement. Integr. Med. 2011, 8, 1. [Google Scholar] [CrossRef]
- Yadav, M.K.; Choi, J.; Song, J.-J. Protective Effect of Hexane and Ethanol Extract of Piper longum L. on Gentamicin-Induced Hair Cell Loss in Neonatal Cultures. Clin. Exp. Otorhinolaryngol. 2014, 7, 13. [Google Scholar] [CrossRef]
- Thanh-Tam Huynh, T.; Mai, T.-C.; Dang, C.-H.; Thuy-Trang Vo, T.; Nguyen, D.-T.; Dang, V.-S.; Duy Vu Nguyen, K.; Tran, V.-T.; Nguyen, T.-D. Influence of Extractions on Physicochemical Characterization and Bioactivity of Piper nigrum Oils: Study on the Non-Isothermal Decomposition Kinetic. Arab. J. Chem. 2020, 13, 7289–7301. [Google Scholar] [CrossRef]
- Angaye, S.S.; Inengite, A.K. Spectraland Antimicrobial Studies of Alligator Pepper Oil. IOSR J. Appl. Chem. 2017, 10, 61–65. [Google Scholar] [CrossRef]
- Cerceau, C.I.; Barbosa, L.C.A.; Filomeno, C.A.; Alvarenga, E.S.; Demuner, A.J.; Fidencio, P.H. An Optimized and Validated 1H NMR Method for the Quantification of α-Pinene in Essentials Oils. Talanta 2016, 150, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Baky, M.H.; Kamal, I.M.; Wessjohann, L.A.; Farag, M.A. Assessment of Metabolome Diversity in Black and White Pepper in Response to Autoclaving Using MS- and NMR-Based Metabolomics and in Relation to Its Remote and Direct Antimicrobial Effects against Food-Borne Pathogens. RSC Adv. 2024, 14, 10799–10813. [Google Scholar] [CrossRef]
- Rivera-Pérez, A.; Romero-González, R.; Garrido Frenich, A. A Metabolomics Approach Based on 1H NMR Fingerprinting and Chemometrics for Quality Control and Geographical Discrimination of Black Pepper. J. Food Compos. Anal. 2022, 105, 104235. [Google Scholar] [CrossRef]
- Bi, Y.; Wang, Y.; Zhou, G.; Pan, D.; Liu, J.; Zhang, Y.; Cao, J. The Effect of Coating Incorporated with Black Pepper Essential Oil on the Taste Quality of Jinhua Ham After Storage for Four Months. J. Food Sci. 2019, 84, 3109–3116. [Google Scholar] [CrossRef] [PubMed]
- Shingate, P.N.; Dongre, P.P.; Kannur, D.M. New Method Development for Extraction and Isolation of Piperine From Black Pepper. Int. J. Pharm. Sci. Res. 2014, 4, 3165–3170. [Google Scholar]
- Rajopadhye, A.; Upadhye, A.; Mujumdar, A. HPTLC Method for Analysis of Piperine in Fruits of Piper Species. J. Planar Chromatogr. Mod. TLC 2011, 24, 57–59. [Google Scholar] [CrossRef]
- Aziz, D.M.; Hama, J.R.; Alam, S.M. Synthesising a Novel Derivatives of Piperine from Black Pepper (Piper nigrum L.). Food Meas. 2015, 9, 324–331. [Google Scholar] [CrossRef]
- Bahri, S.; Ambarwati, Y.; Iqbal, M.; Baihaqy, A.A. Synthesis 4-Piperoilmorpholine from Piperine. J. Phys. Conf. Ser. 2019, 1338, 012010. [Google Scholar] [CrossRef]
- Wdowiak, K.; Pietrzak, R.; Tykarska, E.; Cielecka-Piontek, J. Hot-Melt Extrusion as an Effective Technique for Obtaining an Amorphous System of Curcumin and Piperine with Improved Properties Essential for Their Better Biological Activities. Molecules 2023, 28, 3848. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.; Dominguez-López, I.; Lamuela-Raventós, R.M. The Chemistry Behind the Folin–Ciocalteu Method for the Estimation of (Poly)Phenol Content in Food: Total Phenolic Intake in a Mediterranean Dietary Pattern. J. Agric. Food Chem. 2023, 71, 17543–17553. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhao, J.; Famous, E.; Pan, S.; Peng, X.; Tian, J. Antioxidant, Hepatoprotective and Antifungal Activities of Black Pepper (Piper nigrum L.) Essential Oil. Food Chem. 2021, 346, 128845. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kim, D.W.; Kim, J.G.; Shin, Y.; Jung, S.K.; Kim, Y.-J. Analysis of the Chemical, Antioxidant, and Anti-Inflammatory Properties of Pink Pepper (Schinus molle L.). Antioxidants 2021, 10, 1062. [Google Scholar] [CrossRef]
- Adefegha, S.A.; Olasehinde, T.A.; Oboh, G. Essential Oil Composition, Antioxidant, Antidiabetic and Antihypertensive Properties of Two Afromomum Species. J. Oleo Sci. 2017, 66, 51–63. [Google Scholar] [CrossRef]
- Ajayi, T.O.; Moody, J.; Abiose, I.M. Comparative Total Phenolic Content, Anti-Lipase and Antioxidant Activities of Two Nigerian Aframomum Species. Niger. J. Nat. Prod. Med. 2016, 20, 1–8. [Google Scholar]
- Salsabila, A.K.M.; Anwar, K. Kiki Damayanti Antioxidant Activity of Cubeb Fruit Extract (Piper cubeba L.) Using the ABTS Method and Determining Total Phenolic and Flavonoid Levels. Open Access Indones. J. Med. Rev. 2024, 4, 627–633. [Google Scholar] [CrossRef]
- Mittal, A. Differential Effects of Solvents on Extraction, Pharmacognostic Evaluation and Antioxidant Activity of Long Pepper Piper longum Fruit Extract. Biosc. Biotech. Res. Comm. 2022, 15, 171–176. [Google Scholar] [CrossRef]
- Gutiérrez-del-Río, I.; López-Ibáñez, S.; Magadán-Corpas, P.; Fernández-Calleja, L.; Pérez-Valero, Á.; Tuñón-Granda, M.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Terpenoids and Polyphenols as Natural Antioxidant Agents in Food Preservation. Antioxidants 2021, 10, 1264. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Tan, J.; Li, R.; Jiang, Z.-T.; Tang, S.-H. Comparative Analysis of Intracellular and in Vitro Antioxidant Activities of Essential Oil from White and Black Pepper (Piper nigrum L.). Front. Pharmacol. 2021, 12, 680754. [Google Scholar] [CrossRef]
- Deen, J.I.; Zawad, A.N.M.S.; Uddin, M.; Chowdhury, M.A.H.; Al Araby, S.Q.; Rahman, M.A. Terpinen-4-Ol, A Volatile Terpene Molecule, Extensively Electrifies the Biological Systems against the Oxidative Stress-Linked Pathogenesis. Adv. Redox Res. 2023, 9, 100082. [Google Scholar] [CrossRef]
- Chadha, J.; Khullar, L.; Mudgil, U.; Harjai, K. A Comprehensive Review on the Pharmacological Prospects of Terpinen-4-Ol: From Nature to Medicine and Beyond. Fitoterapia 2024, 176, 106051. [Google Scholar] [CrossRef] [PubMed]
- Del Prado-Audelo, M.L.; Cortés, H.; Caballero-Florán, I.H.; González-Torres, M.; Escutia-Guadarrama, L.; Bernal-Chávez, S.A.; Giraldo-Gomez, D.M.; Magaña, J.J.; Leyva-Gómez, G. Therapeutic Applications of Terpenes on Inflammatory Diseases. Front. Pharmacol. 2021, 12, 704197. [Google Scholar] [CrossRef] [PubMed]
- Abd-ElGawad, A.M.; Elshamy, A.I.; El-Amier, Y.A.; El Gendy, A.E.-N.G.; Al-Barati, S.A.; Dar, B.A.; Al-Rowaily, S.L.; Assaeed, A.M. Chemical Composition Variations, Allelopathic, and Antioxidant Activities of Symphyotrichum squamatum (Spreng.) Nesom Essential Oils Growing in Heterogeneous Habitats. Arab. J. Chem. 2020, 13, 4237–4245. [Google Scholar] [CrossRef]
- De Oliveira, M.S.; Kumar, R.; Mali, S.; De Aguiar Andrade, E.H. Methyl Eugenol: Potential to Inhibit Oxidative Stress, Address Related Diseases, and Its Toxicological Effects. Future Integr. Med. 2024, 3, 274–280. [Google Scholar] [CrossRef]
- Port-Lougarre, Y.; Gourlaouen, C.; Vileno, B.; Giménez-Arnau, E. Antioxidant Activity and Skin Sensitization of Eugenol and Isoeugenol: Two Sides of the Same Coin? Chem. Res. Toxicol. 2023, 36, 1804–1813. [Google Scholar] [CrossRef]
- Al-Qahtani, W.H.; Dinakarkumar, Y.; Arokiyaraj, S.; Saravanakumar, V.; Rajabathar, J.R.; Arjun, K.; Gayathri, P.K.; Nelson Appaturi, J. Phyto-Chemical and Biological Activity of Myristica Fragrans, an Ayurvedic Medicinal Plant in Southern India and Its Ingredient Analysis. Saudi J. Biol. Sci. 2022, 29, 3815–3821. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-K.; Yang, X.-N.; Zhu, X.; Xiao, X.-R.; Yang, X.-W.; Qin, H.-B.; Gonzalez, F.J.; Li, F. Role of Metabolic Activation in Elemicin-Induced Cellular Toxicity. J. Agric. Food Chem. 2019, 67, 8243–8252. [Google Scholar] [CrossRef] [PubMed]
- Parise-Filho, R.; Pastrello, M.; Pereira Camerlingo, C.E.; Silva, G.J.; Agostinho, L.A.; De Souza, T.; Motter Magri, F.M.; Ribeiro, R.R.; Brandt, C.A.; Polli, M.C. The Anti-Inflammatory Activity of Dillapiole and Some Semisynthetic Analogues. Pharm. Biol. 2011, 49, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Khairan, K.; Ginting, B.; Sufriadi, E.; Amalia, A.; Sofyan, H.; Muhammad, S.; Diah, M.; Ernawati, E. Studies on the Antioxidant Activity of Safrole, Myristicin and Terpeniol from Myristica fragrans Houtt: A Review. IOP Conf. Ser. Earth Environ. Sci. 2023, 1183, 012062. [Google Scholar] [CrossRef]
- Ibikunle, G.J.; Oladoye, S.O.; Akintola, A.O.; Olajide, P.O.; Aako, D.P. Antioxidant, Chelating and Phytotoxic Potentials of Ethanolic Seeds Extract of Aframomum melegueta. Int. J. Adv. Eng. Manag. 2021, 3, 1112–1115. [Google Scholar]
- Tamang, L.D.; Boro, S.; Bharadwaj, B.; Sharma, S.K. GC-MS Profiling and Assessment of Antioxidant and Antibacterial Properties of Oil Extracts of Sichuan Pepper. Int. J. Bot. Stud. 2021, 6, 1292–1297. [Google Scholar]
- Boubker, A.; El Ouardi, A.; El Kamli, T.; Kaicer, M.; Kichou, F.; Errafii, K.; El Hamidi, A.; Ben Aakame, R.; Sifou, A. Integrated Phytochemical Profiling, UPLC-HRMS Characterization, and Bioactivity Evaluation of Zingiber officinale and Piper nigrum. Int. J. Mol. Sci. 2025, 26, 7782. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Upadhya, V.; Pai, S.R.; Naik, P.M.; Al-Mssallem, M.Q.; Alessa, F.M. Comparative Quantification of the Phenolic Compounds, Piperine Content, and Total Polyphenols along with the Antioxidant Activities in the Piper trichostachyon and P. nigrum. Molecules 2022, 27, 5965. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Wu, G.; Ding, Y.; Niu, Z.; Feng, J.; Guo, R.; He, S.; Wang, X.; Zhu, H.; Dong, W.; et al. Phenolic Constituents in Pepper (Piper nigrum L.) Berries: UPLC-MS/MS Analysis, Antioxidant Properties, Antibacterial Activity against Pseudomonas Fragi and Association Analyzed by WCGNA. Food Chem. X 2025, 29, 102810. [Google Scholar] [CrossRef] [PubMed]
- Phan, U.T.T.; Nguyen, H.D.; Nguyen, T.K.O.; Tran, T.H.; Le, T.H.; Tran, T.T.P. Anti-Inflammatory Effect of Piper longum L. Fruit Methanolic Extract on Lipopolysaccharide-Treated RAW 264.7 Murine Macrophages. Heliyon 2024, 10, e26174. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Naim, A.; Alghamdi, A.; Algandaby, M.; Al-Abbasi, F.; Al-Abd, A.; Abdallah, H.; El-Halawany, A.; Hattori, M. Phenolics Isolated from Aframomum meleguta Enhance Proliferation and Ossification Markers in Bone Cells. Molecules 2017, 22, 1467. [Google Scholar] [CrossRef]
- Nurcholis, W.; Alfadzrin, R.; Izzati, N.; Arianti, R.; Vinnai, B.Á.; Sabri, F.; Kristóf, E.; Artika, I.M. Effects of Methods and Durations of Extraction on Total Flavonoid and Phenolic Contents and Antioxidant Activity of Java Cardamom (Amomum compactum Soland Ex Maton) Fruit. Plants 2022, 11, 2221. [Google Scholar] [CrossRef]
- Ri, H.; Kim, C.; Pak, U.; Kang, M.; Kim, T. Effect of Different Polarity Solvents on Total Phenols and Flavonoids Content, and In-Vitro Antioxidant Properties of Flowers Extract from Aurea Helianthus. arXiv 2019, arXiv:1906.12006. [Google Scholar] [CrossRef]
- Butt, M.S.; Pasha, I.; Sultan, M.T.; Randhawa, M.A.; Saeed, F.; Ahmed, W. Black Pepper and Health Claims: A Comprehensive Treatise. Crit. Rev. Food Sci. Nutr. 2013, 53, 875–886. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Sharma, A.; Baek, K.-H. Antibacterial Mode of Action of Cudrania Tricuspidata Fruit Essential Oil, Affecting Membrane Permeability and Surface Characteristics of Food-Borne Pathogens. Food Control 2013, 32, 582–590. [Google Scholar] [CrossRef]
- Zhang, J.; Ye, K.-P.; Zhang, X.; Pan, D.-D.; Sun, Y.-Y.; Cao, J.-X. Antibacterial Activity and Mechanism of Action of Black Pepper Essential Oil on Meat-Borne Escherichia coli. Front. Microbiol. 2017, 7, 2094. [Google Scholar] [CrossRef]
- Sultanbawa, F.; Sultanbawa, Y. Mineral Nutrient-Rich Plants—Do They Occur? Appl. Food Res. 2023, 3, 100347. [Google Scholar] [CrossRef]
- Napiórkowska-Baran, K.; Treichel, P.; Dardzińska, A.; Majcherczak, A.; Pilichowicz, A.; Szota, M.; Szymczak, B.; Alska, E.; Przybyszewska, J.; Bartuzi, Z. Immunomodulatory Effects of Selected Non-Nutritive Bioactive Compounds and Their Role in Optimal Nutrition. Curr. Issues Mol. Biol. 2025, 47, 89. [Google Scholar] [CrossRef]
- Dresen, E.; Pimiento, J.M.; Patel, J.J.; Heyland, D.K.; Rice, T.W.; Stoppe, C. Overview of Oxidative Stress and the Role of Micronutrients in Critical Illness. J. Parenter. Enter. Nutr. 2023, 47, S38–S49. [Google Scholar] [CrossRef]
- Parveen, R.; Abbasi, A.M.; Shaheen, N.; Shah, M.H. Accumulation of Selected Metals in the Fruits of Medicinal Plants Grown in Urban Environment of Islamabad, Pakistan. Arab. J. Chem. 2020, 13, 308–317. [Google Scholar] [CrossRef]
- European Pharmacopoeia Online. Available online: https://pheur.edqm.eu/home (accessed on 6 August 2025).
- Agazzi, A.; Pirola, C. Fundamentals, Methods and Future Trends of Environmental Microwave Sample Preparation. Microchem. J. 2000, 67, 337–341. [Google Scholar] [CrossRef]







| Plant Material | Piperine Content, % | Refs. | |
|---|---|---|---|
| Exp | Lit | ||
| Black pepper | 3.10 ± 0.01 | 3.2–37.5 | [58,74] |
| Green pepper | 3.48 ± 0.03 | 5.09–28.32 | [6,59] |
| White pepper | 3.05 ± 0.02 | 0.92–51.92 | [57,62] |
| Voatsiperifery pepper | 0.86 ± 0.01 | - | |
| Javanese pepper | - | 0.03–1.12 | [61,75] |
| Bengali pepper | 3.38 ± 0.02 | 0.10–3.71 | [56,75] |
| Pepper Species | TPC | TFC | |
|---|---|---|---|
| EOs, mg GAE/mL 1 | EEs, mg GAE/g 2 | EEs, mg RE/g 2 | |
| Black pepper | 1.15 ± 0.08 | 93.87 ± 4.21 | 75.41 ± 7.31 |
| Green pepper | 0.96 ± 0.06 | 143.55 ± 13.53 | 264.98 ± 16.37 |
| White pepper | 0.86 ± 0.07 | 45.55 ± 2.05 | 221.53 ± 14.67 |
| Melegueta pepper | 0.29 ± 0.03 | 213.00 ± 14.51 | 346.78 ± 16.27 |
| Voatsiperifery pepper | 1.27 ± 0.05 | 36.16 ± 2.67 | 34.08 ± 5.24 |
| Javanese pepper | 1.59 ± 0.16 | 51.46 ± 3.99 | 60.28 ± 4.18 |
| Pink pepper | 1.29 ± 0.09 | 50.03 ± 3.89 | 19.81 ± 1.94 |
| Bengali pepper | 1.32 ± 0.10 | 31.46 ± 2.83 | 9.37 ± 0.21 |
| Sichuan pepper | 1.83 ± 0.03 | 162.63 ± 16.86 | 204.92 ± 18.76 |
| Pepper Species | EO/EE | Extraction Method | Results (TPC, TFC, EC50, or AA) | Ref. |
|---|---|---|---|---|
| Black pepper | EO | HD | TPC = 237.556 mg GAE/g | [80] |
| HD | TPC = 1.21 mg GAE/g | [24] | ||
| EE | REX with 96% ethanol | TPC = 89.8 mg GAE/g TFC = 17.2 mg RE/g | [8] | |
| MAC, REX, UAE, SE with ethanol of various purities—results are given for 70% ethanol and UAE | TPC = 85.64 mg GAE/g TFC = 73.15 mg RE/g | [5] | ||
| Shaking with 80% ethanol | TPC = 7.94 mg GAE/g TFC = 3.44 mg CE/g | [81] | ||
| Shaking with 80% ethanol | TPC = 5.89–9.86 mg GAE/g TFC = 2.75–5.77 mg CE/g | [59] | ||
| SE with ethanol | TPC = 62.3 mg CE/g | [60] | ||
| Green pepper | EO | HD | TPC = 0.4 mg GAE/g | [24] |
| EE | Shaking with 80% ethanol | TPC = 14.15 mg GAE/g TFC = 9.23–10.83 mg CE/g | [59] | |
| MAC, UAE, MAE, UMAE with anhydrous ethanol—results are given for UAE | TPC = 3.75 mg GAE/g | [6] | ||
| White pepper | EO | HD | TPC = 0.94 mg GAE/g | [24] |
| Melegueta pepper | EO | HD | TPC = 5.45 mg GAE/L | [82] |
| EE | MAC in ethanol (not specified) | TPC = 60.375 mg GAE/g | [83] | |
| MAC with ethanol of different purities; results are given for 70% ethanol | TPC = 90 mg GAE/g TFC = 65 mg GAE/g | [20] | ||
| Voatsiperifery pepper | EO | HD | TPC = 1.56 mg GAE/100 g | [29] |
| Javanese pepper | EE | REX with 96% ethanol | TPC = 75.6 mg GAE/g TFC = 4.5 mg RE/g | [8] |
| MAC with 96% ethanol | TPC = 183.039 mg GAE/g TFC = 3.53 mg QE/g | [84] | ||
| SE with ethanol | TPC = 123.1 mg CE/g | [60] | ||
| MAC with 70 and 96% ethanol; results are given for 70% ethanol | TPC = 206.99 mg GAE/g TFC = 146.96 mg QE/g | [21] | ||
| Pink pepper | EE | SE and UAE with hexane, ethanol (>99.5%), and ethyl acetate, SFE; results are given for ethanol | TPC = 14.2 mg GAE/g | [19] |
| SE, PLE with 99.9% ethanol | TPC = 23.26–54.85 mg GAE/g | [9] | ||
| Shaking with 80% ethanol | TPC = 12.50–16.08 mg GAE/g TFC = 2.30–2.67 mg CE/g | [81] | ||
| UAE with >95% ethanol | TPC = 22.29 mg GAE/g TFC = 3.93 mg QE/g | [55] | ||
| Bengal pepper | EE | MAC with 99.97% ethanol | TPC = 74.8 mg GAE/g TFC = 55.4 mg CE/g | [66] |
| MAC with ethanol | TPC = 153.8 mg GAE/g | [85] |
| Pepper Species | EC50, mg/mL | EC50, μg/mL |
|---|---|---|
| EOs | EEs | |
| Black pepper | 68.75 ± 0.33 | 886.24 ± 22.85 |
| Green pepper | 47.29 ± 0.28 | 197.43 ± 11.48 |
| White pepper | 84.28 ± 5.65 | 1049.43 ± 64.78 |
| Melegueta pepper | 66.08 ± 6.62 | 96.33 ± 5.08 |
| Voatsiperifery pepper | 9.11 ± 2.86 | 1278.55 ± 139.53 |
| Javanese pepper | 25.57 ± 2.65 | 1127.85 ± 12.45 |
| Pink pepper | 8.45 ± 0.20 | 561.95 ± 82.83 |
| Bengali pepper | 8.78 ± 0.49 | >12,300 |
| Sichuan pepper | 7.31 ± 0.08 | 99.37 ± 0.48 |
| Pepper Species | EO/EE | Extraction Method | Results (TPC, TFC, EC50, or AA) | Ref. |
|---|---|---|---|---|
| Black pepper | EO | Commercial oils | EC50 = 36.84 mg/mL | [38] |
| HD | EC50 = 7.67 mg/mL | [24] | ||
| UMAHD | EC50 = 6.348 mg/mL | [87] | ||
| HD | EC50 = 1.15 mg/mL | [40] | ||
| SD | EC50 = 4.18 mg/mL | [53] | ||
| EE | REX with 96% ethanol | EC50 = 0.104 mg/mL | [8] | |
| MAC, REX, UAE, SE with ethanol of various purities—results are given for 70% ethanol and UAE | EC50 = 0.142 mg/mL | [5] | ||
| SE with ethanol | EC50 = 14.15 μg/mL | [60] | ||
| Green pepper | EO | Commercial oils | EC50 = 38.77 mg/mL | [38] |
| HD | EC50 = 40.86 mg/mL | [24] | ||
| EE | MAC, UAE, MAE, UMAE with anhydrous ethanol—results are given for UAE | AA = 7.11.mg Trolox/mL | [6] | |
| White pepper | EO | HD | EC50 = 45.02 mg/mL | [24] |
| UMAHD | EC50 = 7.332 mg/mL | [87] | ||
| Melegueta pepper | EE | MAC in absolute ethanol | EC50 = 20.4 mg/mL | [98] |
| 40% ethanol; method not specified | EC50 = 150 μg/mL | [7] | ||
| MAC in ethanol (not specified) | EC50 = 24.44 μg/mL | [83] | ||
| Javanese pepper | EO | HD | EC50 = 78.9 μg/mL | [44] |
| HD | EC50 = 0.82 mg/mL | [40] | ||
| HD | EC50 = 110 μg/mL | [46] | ||
| HD | EC50 = 7.95 μL/mL | [47] | ||
| EE | REX with 96% ethanol | EC50 = 0.378 mg/mL | [8] | |
| MAC with 96% ethanol | EC50 = 82.10 μg/mL | [84] | ||
| SE with ethanol | EC50 = 10.54 μg/mL | [60] | ||
| Pink pepper | EO | HD | EC50 = 44.1 μg/mL | [49] |
| EE | SE and UAE with hexane, ethanol (>99.5%), and ethyl acetate, SFE; results are given for ethanol | EC50 = 339 g/mL | [19] | |
| SE, PLE with 99.9% ethanol | AA = 108.33–317.14 μmol Trolox/g | [9] | ||
| UAE with >95% ethanol | EC50 = 103.43 μmol Trolox/mL | [55] | ||
| Bengal pepper | EO | SD | EC50 = 0.6 mg/mL | [53] |
| Sichuan pepper | EO | MAHD | EC50 = 5 μL/mL | [99] |
| Pepper Species | MIC, μg/mL | ||||
|---|---|---|---|---|---|
| Escherichia coli | Enterococcus faecalis | Klebsiella pneumoniae | Pseudomonas aeruginosa | Staphylococcus aureus | |
| Black pepper | 128 | 256 | 128 | 128 | 64 |
| Green pepper | 128 | >128 | >64 | 128 | >32 |
| White pepper | 128 | >128 | >64 | 64 | >64 |
| Melegueta pepper | 128 | >128 | >64 | >64 | >64 |
| Voatsiperifery pepper | 128 | >128 | >64 | >32 | >64 |
| Javanese pepper | 128 | >128 | >64 | >64 | >64 |
| Pink pepper | 128 | >128 | >128 | >64 | >128 |
| Bengali pepper | 128 | 256 | 128 | >64 | >64 |
| Sichuan pepper | 128 | 256 | 128 | >128 | >128 |
| CAZ | 0.5 | 256 | 256 | 4 | 64 |
| CIP | <0.125 | 0.5 | 256 | <125 | 0.5 |
| Pepper Species | MIC, μg/mL | ||||
|---|---|---|---|---|---|
| Escherichia coli | Enterococcus faecalis | Klebsiella pneumoniae | Pseudomonas aeruginosa | Staphylococcus aureus | |
| Black pepper | 128 | 256 | 128 | 64 | 128 |
| Green pepper | 128 | 256 | 128 | 128 | 128 |
| White pepper | >64 | 256 | 128 | 128 | >64 |
| Melegueta pepper | >64 | >128 | 256 | >64 | >64 |
| Voatsiperifery pepper | >64 | 256 | 128 | >64 | >64 |
| Javanese pepper | >8 | >128 | >32 | >16 | >8 |
| Pink pepper | >16 | >128 | >64 | >16 | >32 |
| Bengali pepper | >8 | >128 | >64 | >64 | >64 |
| Sichuan pepper | >8 | 256 | >32 | >8 | >32 |
| Concentration, mg/kg | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Element | White p. | Voats. p. | Javan. p. | Pink p. | Green p. | Black p. | Sichu. p. | Meleg. p. | Bengali p. |
| Cr | 1.89 | 0.53 | / | / | / | 0.56 | 0.79 | / | / |
| Mn | 77.86 | 133.79 | 68.69 | 14.62 | 82.06 | 38.19 | 36.21 | 136.61 | 24.22 |
| Fe | 61.05 | 142.86 | 127.32 | 93.00 | 54.58 | 55.38 | 154.65 | 58.94 | 71.97 |
| Ni | 1.58 | 3.05 | 2.19 | 1.02 | 2.59 | 1.06 | 1.90 | 1.33 | 0.66 |
| Cu | 9.30 | 13.68 | 21.07 | 8.50 | 18.04 | 11.00 | 4.22 | 7.68 | 11.15 |
| Zn | 48.84 | 22.51 | 99.83 | 15.78 | 21.15 | 12.98 | 11.28 | 26.96 | 20.79 |
| Br | 2.97 | 4.81 | 5.79 | 37.54 | 6.12 | 28.65 | 4.06 | 4.62 | 12.80 |
| Rb | 2.40 | 65.00 | 72.13 | 29,13 | 31.17 | 21.45 | 4.37 | 16.46 | 133.81 |
| Sr | 14.68 | 31.94 | 95.41 | 37.54 | 52.08 | 24.19 | 27.62 | 9.90 | 56.25 |
| Ba | 4.40 | 4.81 | 33.61 | / | 55.71 | / | / | / | 27.84 |
| Chemical | Manufacturer | CAS Number |
|---|---|---|
| DPPH 1, >97% | Fluka (Buchs, Switzerland) | 1898-66-4 |
| Gallic acid, 98% | Thermo Scientific (Waltham, MA, USA) | 149-91-7 |
| Folin–Ciocalteu reagent, 98% | VWR Chemicals (Radnor, PA, USA) | 12111-13-6 |
| BHT 2, 99% | Thermo Scientific (Waltham, MA, USA) | 128-37-0 |
| Aluminum chloride hexahydrate, >97% | BIOCHEM Chemopharma (Cosne-Cours-sur-Loire, France) | 7784-13-6 |
| Sodium carbonate, ≥99% | Lach-ner (Boston, MA, USA) | 497-19-8 |
| Sodium hydroxide, >98% | T.T.T. (London, UK) | 1310-73-2 |
| Sodium nitrite, >98% | Alkaloid Skopje (Skopje, North Macedonia) | 7632-00-0 |
| Sodium sulfate, 99.9% | Kemika d.d. (Zagreb, Croatia) | 7757-82-6 |
| Methanol, HPLC grade, 99.8% | J. T. Baker (Phillipsburg, NJ, USA) | 67-56-1 |
| n-Hexane, GC grade, ≥95% | Fisher Scientific (Hampton, NH, USA) | 110-54-3 |
| Rutin hydrate, >94% | TCI (Tokyo, Japan) | 207671-50-9 |
| Hydrogen peroxide, 30%, TraceSELECT | Sigma-Aldrich (St. Louis, MO, USA) | 7722-84-1 |
| Nitric acid, 69%, HIPERPUR | Panreac (Barcelona, Spain) | 7697-37-2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sander, A.; Bival Štefan, M.; Sander, T.; Kučić Grgić, D.; Parlov Vuković, J.; Blažević, I.; Jablan, J. Characterization of Essential Oils and Ethanolic Extracts from Nine Pepper Species: Antioxidant and Antimicrobial Activity and Spectroscopic Analysis. Molecules 2025, 30, 4140. https://doi.org/10.3390/molecules30204140
Sander A, Bival Štefan M, Sander T, Kučić Grgić D, Parlov Vuković J, Blažević I, Jablan J. Characterization of Essential Oils and Ethanolic Extracts from Nine Pepper Species: Antioxidant and Antimicrobial Activity and Spectroscopic Analysis. Molecules. 2025; 30(20):4140. https://doi.org/10.3390/molecules30204140
Chicago/Turabian StyleSander, Aleksandra, Maja Bival Štefan, Tea Sander, Dajana Kučić Grgić, Jelena Parlov Vuković, Iva Blažević, and Jasna Jablan. 2025. "Characterization of Essential Oils and Ethanolic Extracts from Nine Pepper Species: Antioxidant and Antimicrobial Activity and Spectroscopic Analysis" Molecules 30, no. 20: 4140. https://doi.org/10.3390/molecules30204140
APA StyleSander, A., Bival Štefan, M., Sander, T., Kučić Grgić, D., Parlov Vuković, J., Blažević, I., & Jablan, J. (2025). Characterization of Essential Oils and Ethanolic Extracts from Nine Pepper Species: Antioxidant and Antimicrobial Activity and Spectroscopic Analysis. Molecules, 30(20), 4140. https://doi.org/10.3390/molecules30204140







