Understanding the Paradigm of Molecular-Network Conformations in Nanostructured Se-Rich Arsenoselenides AsxSe100−x (x < 10)
Abstract
1. Introduction
2. Results and Discussion
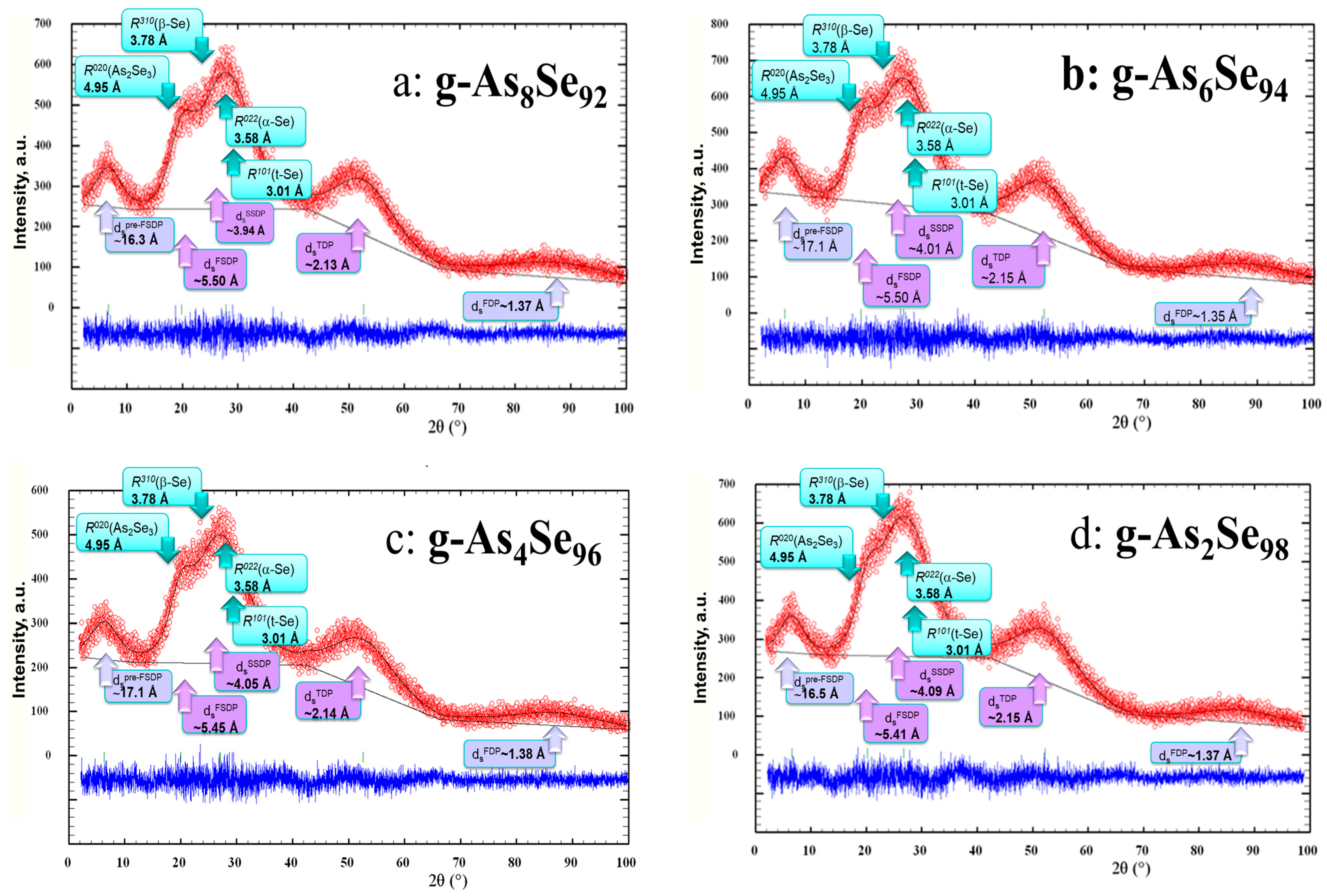
2.1. Compositional Changes in the XRD Patterning in Se-Rich AsxSe100−x Alloys (x < 10)
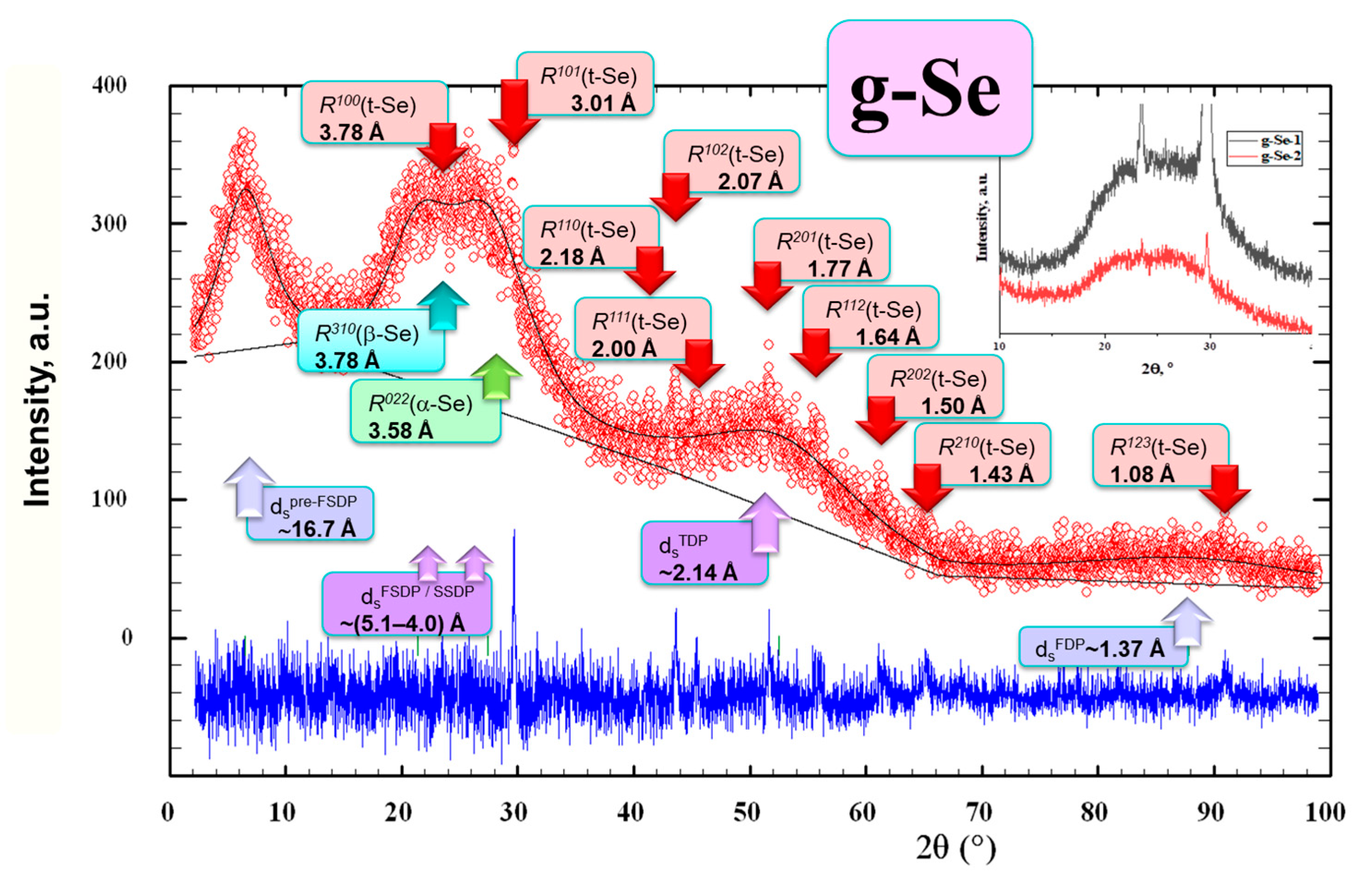
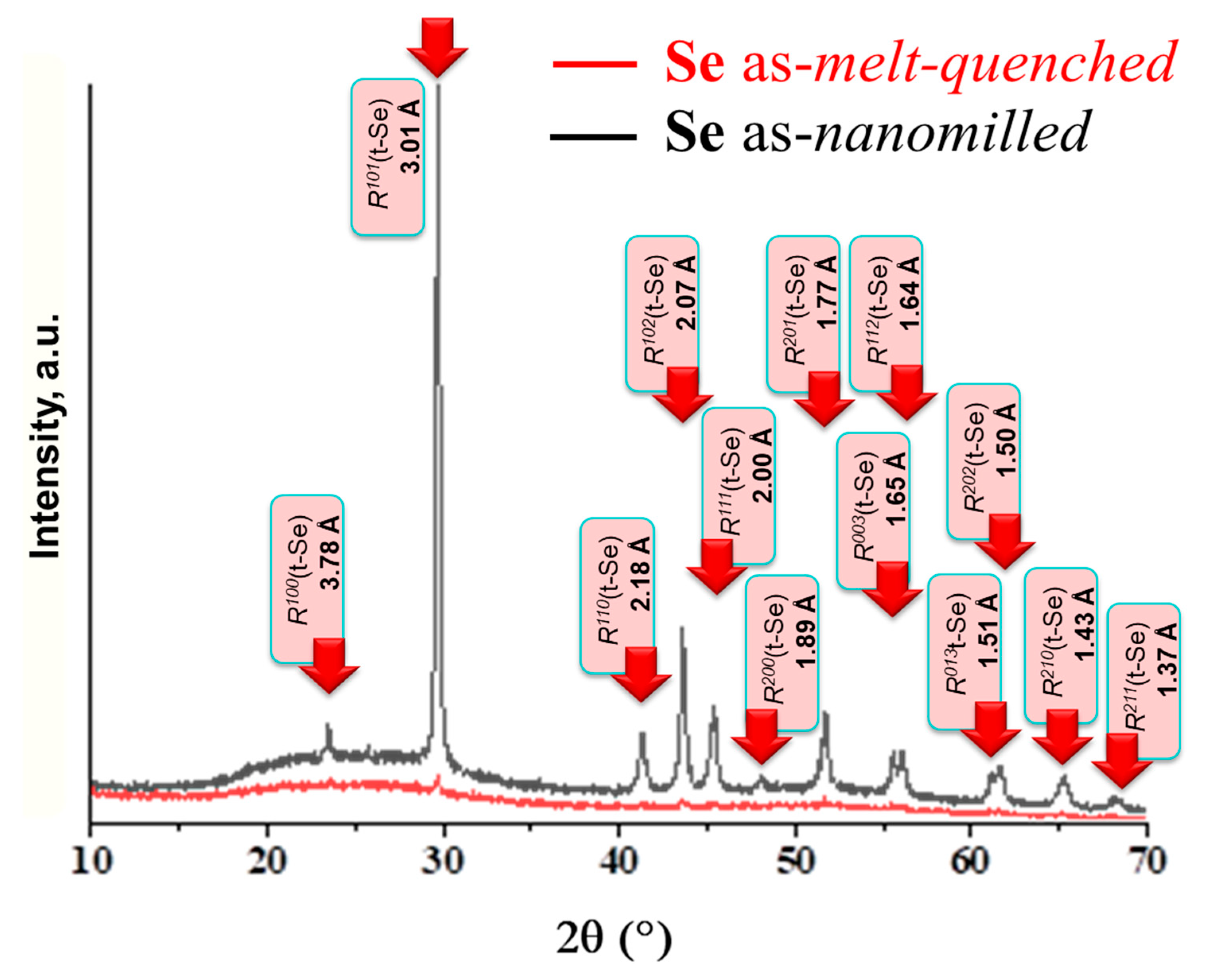
2.2. XRD Patterning of Nanostructurization Response in Se-Rich AsxSe100−x Alloys (x < 10)
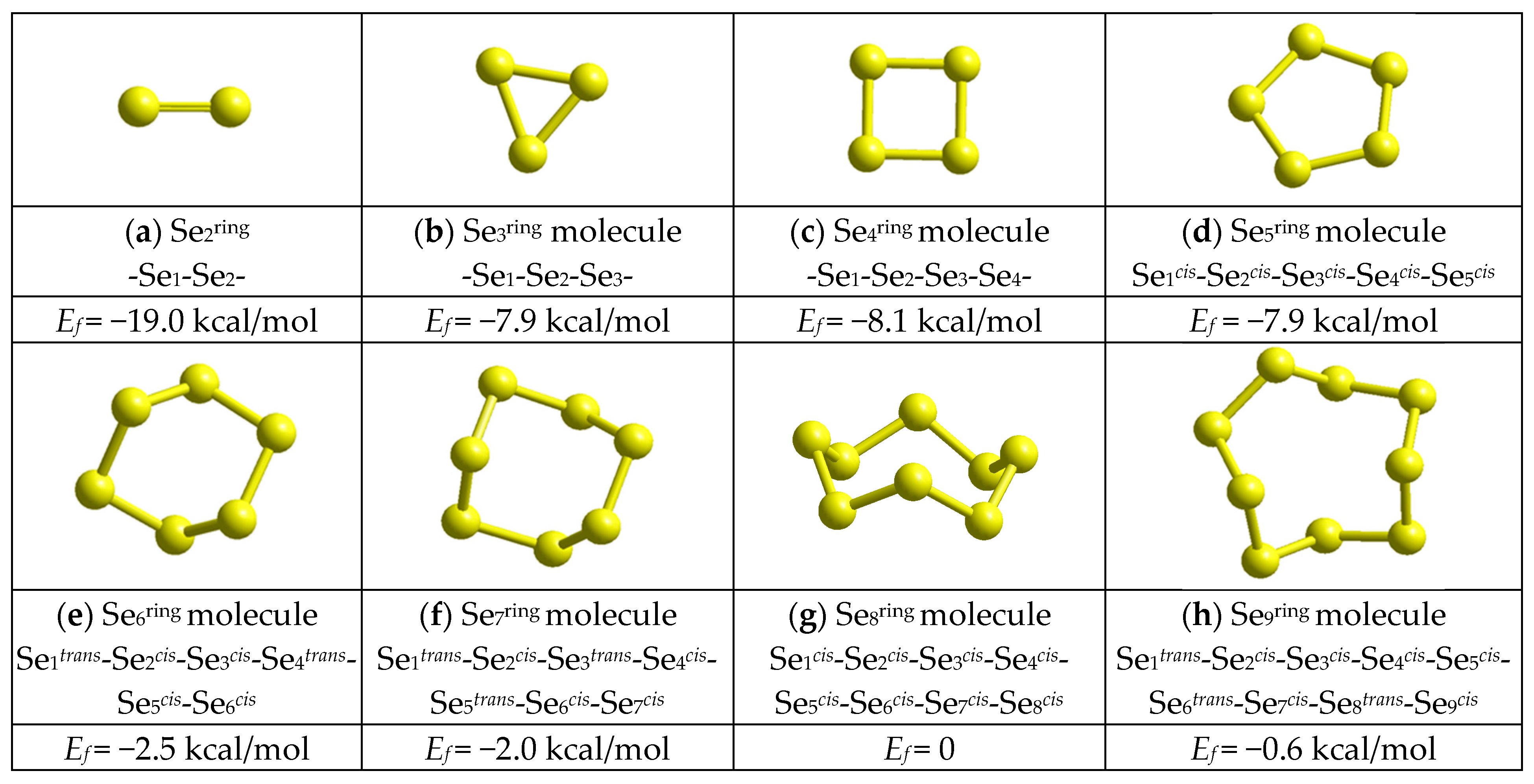
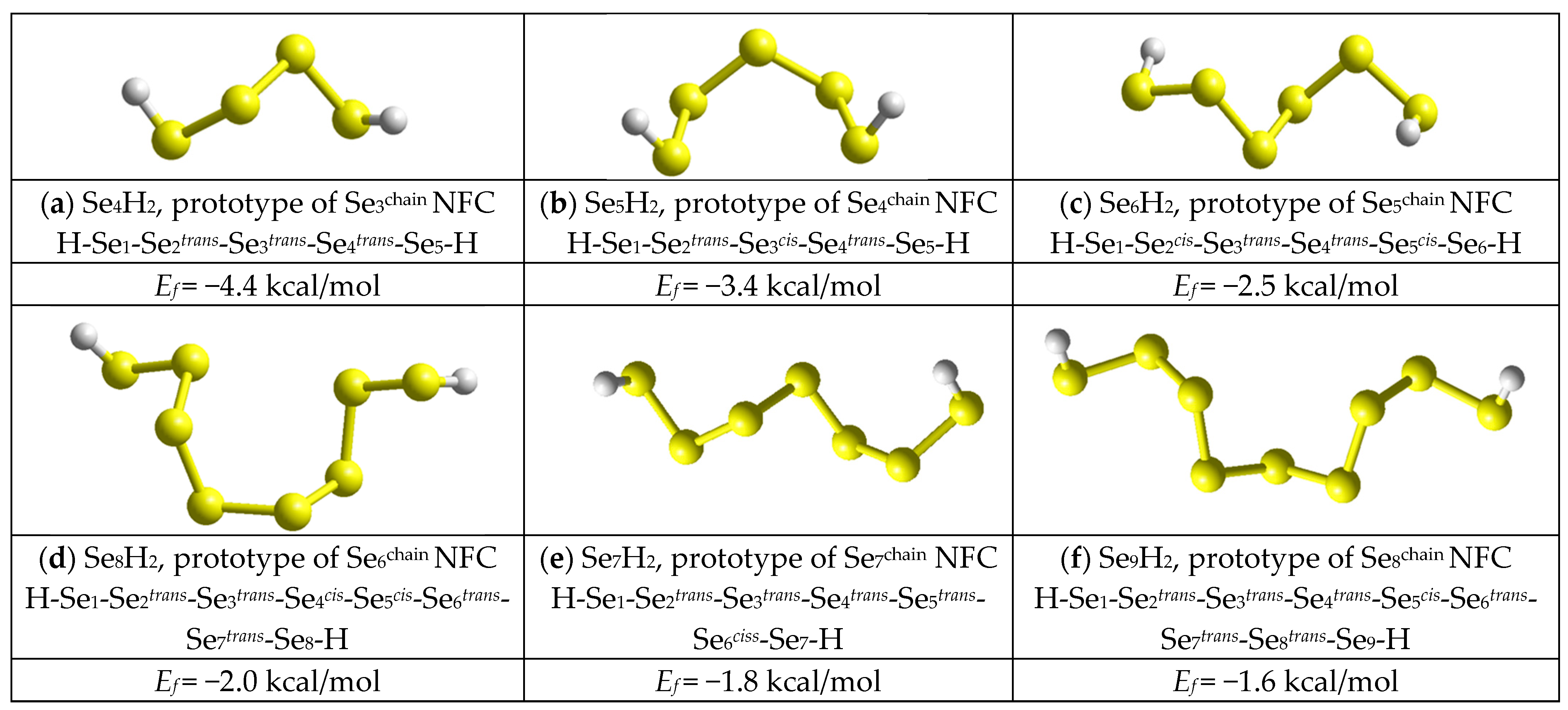
2.3. Computational Insight on Nanostructurization in Se-Rich AsxSe100−x Alloys (x < 10)
3. Methods
3.1. Preparation and Characterization of Se-Rich Glassy Arsenoselenides AsxSe100−x (x < 10)
3.2. Medium-Range Structure of Nanostructured Amorphous Alloys by the XRD Analysis
3.3. Cluster Modeling of Molecular-Network Conformations in Glassy Arsenoselenides
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feltz, A. Amorphous Inorganic Materials and Glasses; VCH: Weinheim, Germany, 1993; pp. 1–446. [Google Scholar]
- Adam, J.-L.; Zhang, X. Chalcogenide Glasses: Preparation, Properties and Application; Woodhead Publishing Series in Electronics and Optical Materials; Woodhead Publishing: Philadelphia, PA, USA; New Delhi, India, 2013; pp. 1–717. [Google Scholar]
- Baláž, P.; Baláž, M.; Achimovičová, M.; Bujňáková, Z.; Dutková, E. Chalcogenide mechanochemistry in materials science: Insight into synthesis and applications (a review). J. Mater. Sci. 2017, 52, 11851–11890. [Google Scholar] [CrossRef]
- Baláž, P.; Achimovicova, M.; Baláž, M.; Billik, P.; Cherkezova-Zheleva, Z.; Manuel Criado, J.; Delogu, F.; Dutkova, E.; Gaffet, E.; Gotor, F.J.; et al. Hallmarks of mechanochemistry: From nanoparticles to technology. Chem. Soc. Rev. 2023, 42, 7571–7637. [Google Scholar] [CrossRef]
- Seddon, A.B.; Farries, M.C.; Nunes, J.J.; Xiao, B.; Furniss, D.; Barney, E.; Phang, S.; Chahal, S.; Kalfagiannis, N.; Sojka, L.; et al. Short review and prospective: Chalcogenide glass mid-infrared fibre lasers. Eur. Phys. J. Plus 2024, 139, 142. [Google Scholar] [CrossRef]
- Gholipour, B.; Elliott, S.R.; Müller, M.J.; Wuttig, M.; Hewak, D.W.; Hayden, B.E.; Li, Y.; Jo, S.S.; Jaramillo, R.; Simpson, R.E.; et al. Roadmap on chalcogenide photonics. J. Phys. Photonics 2023, 5, 012501. [Google Scholar] [CrossRef]
- Mishra, A.; Frechero, M.A.; Caron, A.; Singh, P.K.; Tiwari, A. Recent progress and future directions in nanoglass materials: A deep insight into synthesis, characterization, and application. Nanotechnol. Precis. Eng. 2025, 8, 015002. [Google Scholar] [CrossRef]
- Liu, G.; Yurong Song, Y.; Li, C.; Liu, R.; Chen, Y.; Yu, L.; Huang, Q.; Zhu, D.; Lu, C.; Yu, X.; et al. Arsenic compounds: The wide application and mechanisms applied in acute promyelocytic leukemia and carcinogenic toxicology. Eur. J. Med. Chem. 2021, 221, 113519. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, Y.; Li, R.; Wang, Z. Research progress on arsenic, arsenic-containing medicinal materials, and arsenic-containing preparations: Clinical application, pharmacological effects, and toxicity. Front. Pharmacol. 2024, 15, 1338725. [Google Scholar] [CrossRef]
- Zhang, J.; Qin, Y.; Chen, Y.; Zhao, X.; Wang, J.; Wang, Z.; Li, J.; Zhao, J.; Liu, S.; Guo, Z.; et al. Ultrathin 2D As2Se3 Nanosheets for Photothermal-Triggered Cancer Immunotherapy. ACS Nano 2024, 18, 4398–4413. [Google Scholar] [CrossRef]
- Shpotyuk, Y.; Demchenko, P.; Bujňáková, Z.; Baláž, P.; Boussard-Pledel, C.; Bureau, B.; Shpotyuk, O. Effect of high-energy mechanical milling on the medium-range ordering in glassy As-Se. J. Am. Ceram. Soc. 2020, 103, 1631–1646. [Google Scholar] [CrossRef]
- Shpotyuk, Y.; Demchenko, P.; Shpotyuk, O.; Balitska, V.; Boussard-Pledel, C.; Bureau, B.; Lukáčová Bujňáková, Z.; Baláž, P. High-energy mechanical milling-driven reamorphization in glassy arsenic monoselenide g-AsSe: On the path tailoring special molecular-network glasses. Materials 2021, 14, 4478. [Google Scholar] [CrossRef]
- Shpotyuk, O.; Hyla, M.; Lukáčová Bujňáková, Z.; Shpotyuk, Y.; Boyko, V. Diversity of Molecular-Network Conformations in the Over-Stoichiometric Arsenoselenides covering a full Thioarsenides Row As4Sen (0 ≤ n ≤ 6). Molecules 2025, 30, 1963. [Google Scholar] [CrossRef] [PubMed]
- Shpotyuk, Y.; Shpotyuk, O.; Lukáčová Bujňáková, Z.; Baláž, P.; Hyla, M.; Boussard-Pledel, C.; Bureau, B. Tailoring Se-rich glassy arsenoselenides employing the nanomilling platform. Mater. Sci. Eng. B 2024, 300, 117069. [Google Scholar] [CrossRef]
- Minaev, V.S.; Timoshenkov, P.; Kalugin, V. Structural and phase transformations in condensed selenium. J. Optoelectron. Adv. Mater. 2005, 7, 1717–1741. [Google Scholar]
- Burbank, R.D. The crystal structure of α-monoclinic selenium. Acta Cryst. 1951, 4, 140–148. [Google Scholar] [CrossRef]
- Cherin, P.; Phyllis, U. Refinement of the crystal structure of α-monoclinie Se. Acta Cryst. B 1972, 28, 313–317. [Google Scholar] [CrossRef]
- Burbank, R.D. The crystal structure of β-monoclinic selenium. Acta Cryst. 1952, 5, 236–246. [Google Scholar] [CrossRef]
- Marsh, R.E.; Paulling, L. The crystal structure of β selenium. Acta Cryst. 1953, 6, 71–75. [Google Scholar] [CrossRef]
- Cherin, P.; Phyllis, U. The Crystal Structure of Trigonal Selenium. Inorg. Chem. 1967, 6, 1589–1591. [Google Scholar] [CrossRef]
- Misawa, M.; Suzuki, K. Ring-chain transition in liquid selenium by a disordered chain model. J. Phys. Soc. Jpn. 1978, 44, 1612–1618. [Google Scholar] [CrossRef]
- Misawa, M.; Suzuki, K. Structure of Chain Molecule in Liquid Selenium by Time-of-Flight Pulsed Neutron Diffraction. Trans. Jpn. Inst. Met. 1977, 18, 427–434. [Google Scholar] [CrossRef]
- Semlyen, J.A. Rotational isomeric state models of sulphur and selenium chains. Part 2. Calculation of entropy changes in formation of cyclooctasulphur and cyclooctaselenium. Trans. Faraday Soc. 1967, 63, 2342–2345. [Google Scholar] [CrossRef]
- Lucovsky, G.; Mooradian, A.; Taylor, W.; Wright, G.B.; Keezer, R.C. Identification of the fundamental vibrational modes of trigonal, α-monoclinic and amorphous selenium. Solid State Commun. 1967, 5, 113–117. [Google Scholar] [CrossRef]
- Tanaka, K. Structural studies of amorphous Se under pressure. Phys. Rev. B 1990, 42, 11245–11251. [Google Scholar] [CrossRef]
- Fukunaga, T.; Utsumi, M.; Akatsuka, H.; Misawa, M.; Mizutani, U. Structure of amorphous Se prepared by milling. J. Non-Cryst. Solids 1996, 205–207, 531–535. [Google Scholar] [CrossRef]
- Guo, F.Q.; Lu, K. Microstructural evolution in melt-quenched amorphous Se during mechanical attrition. Phys. Rev. B 1998, 57, 10414–10420. [Google Scholar] [CrossRef]
- de Lima, J.C.; Grandi, T.A.; de Biasi, R.S. Influence of aging on the thermal and structural properties of amorphous selenium prepared by ball milling. J. Non-Cryst. Solids 2001, 286, 93–98. [Google Scholar] [CrossRef]
- Chen, P.; Boolchand, P.; Georgiev, D.G. Long term aging of selenide glasses: Evidence of sub-Tg endotherms and pre-Tg exotherms. J. Phys. Condens. Matter 2010, 22, 065104. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dash, S.; Chen, P.; Boolchand, P. Molecular origin of aging of pure Se glass: Growth of inter-chain structural correlations, network compaction, and partial ordering. J. Chem. Phys. 2017, 146, 224506. [Google Scholar] [CrossRef]
- Elliott, S.R. Extended-range order, interstitial voids and the first sharp diffraction peak of network glasses. J. Non-Cryst. Solids 1995, 182, 40–48. [Google Scholar] [CrossRef]
- Elliott, S.R. Second sharp diffraction peak in the structure factor of binary covalent network glasses. Phys. Rev. B 1995, 51, 8599–8601. [Google Scholar] [CrossRef]
- Zeidler, A.; Salmon, P.S. Pressure-driven transformation of the ordering in amorphous network-forming materials. Phys. Rev. B 2016, 93, 214204. [Google Scholar] [CrossRef]
- Salmon, P.S. Real space manifestation of the first sharp diffraction peak in the structure factor of liquid and glassy materials. Proc. R. Soc. Lond. A 1994, 445, 351–365. [Google Scholar] [CrossRef]
- Renninger, A.L.; Averbach, B.L. Crystalline structures of As2Se3 and As4Se4. Acta Cryst. B 1973, 29, 1583–1589. [Google Scholar] [CrossRef]
- Stergiou, A.C.; Rentzeperis, P.J. The crystal structure of arsenic selenide, As2Se3. Zeitsch. Krist. 1985, 173, 185–191. [Google Scholar] [CrossRef]
- Bychkov, E.; Benmore, C.J.; Price, D.L. Compositional changes in the first sharp diffraction peak in binary selenide glasses. Phys. Rev. B 2005, 72, 172107. [Google Scholar] [CrossRef]
- Golovchak, R.; Lucas, P.; Oelgoetz, J.; Kovalskiy, A.; York-Winegar, J.; Saiyasombat, C.; Shpotyuk, O.; Feygenson, M.; Neuefeind, J.; Jain, H. Medium range order and structural relaxation in As–Se network glasses through FSDP analysis. Mater. Chem. Phys. 2015, 153, 432–442. [Google Scholar] [CrossRef]
- Popescu, M. Medium range order in chalcogenide glasses. In Physics and Applications of Non-Crystalline Semiconductors in Optoelectronics; Andriesh, A., Bertolotti, M., Eds.; Springer: Dordrecht, The Netherlands, 1997; pp. 215–232. [Google Scholar] [CrossRef]
- Lukyanov, A.; Lubchenko, V. Amorphous chalcogenides as random octahedrally bonded solids: I. Implications for the first sharp diffraction peak, photodarkening, and Boson peak. J. Chem. Phys. 2017, 147, 114505. [Google Scholar] [CrossRef]
- Corb, B.W.; Wei, W.D.; Averbach, B.L. Atomic models of amorphous selenium. J. Non-Cryst. Solids 1982, 53, 29–43. [Google Scholar] [CrossRef]
- Andonov, P. Studies of non-crystalline forms of selenium. J. Non-Cryst. Solids 1982, 47, 297–339. [Google Scholar] [CrossRef]
- Yannopoulos, S.N. Structure and photo-induced effects in elemental chalcogens: A review on Raman scattering. J. Mater. Sci. Mater. Electron. 2020, 31, 7565–7595. [Google Scholar] [CrossRef]
- Shpotyuk, O.; Hyla, M.; Boyko, V. Structural-topological genesis of network-forming nano-clusters in chalcogenide semiconductor glasses. J. Optoelectron. Adv. Mater. 2013, 15, 1429–1437. [Google Scholar]
- Shpotyuk, O.; Hyla, M.; Boyko, V. Compositionally-dependent structural variations in glassy chalcogenides: The case of binary As-Se system. Comput. Mater. Sci. 2015, 110, 144–151. [Google Scholar] [CrossRef]
- Kozdras, A.; Shpotyuk, O.; Mahlovanyi, B.; Shpotyuk, Y.; Kovalskiy, A. Thermodynamic heat-transfer phenomena in nanostructured glassy substances: A comparative study on g-As5Se95 and g-As55Se45. J. Therm. Anal. Calorim. 2023, 148, 2265–2271. [Google Scholar] [CrossRef]
- Downs, R.T.; Hall-Wallace, M. The American mineralogist crystal structure database. Am. Mineral. 2003, 88, 247–250. [Google Scholar]
- Villars, P.; Cenzual, K. (Eds.) Pearson’s Crystal Data: Crystal Structure Database for Inorganic Compounds; Release 2014/15; ASM Intern.: Materials Park, OH, USA, 2014. [Google Scholar]
- Roisnel, T.; Rodriguez-Carvajal, J. WinPLOTR: A Windows tool for powder diffraction patterns analysis. Mater. Sci. Forum 2001, 118, 378–381. [Google Scholar]
- Kraus, W.; Nolze, G. Powder cell—A program for the representation and manipulation of rystal structures and calculation of the resulting X-ray powder patterns. J. Appl. Cryst. 1996, 29, 301–303. [Google Scholar] [CrossRef]
- Bletry, J. Sphere and distance models for binary disordered systems. Phil. Mag. B 1990, 62, 469–508. [Google Scholar] [CrossRef]
- Rachek, O.P. X-ray diffraction study of amorphous alloys Al-Ni-Ce-Sc with using Ehrenfest’s formula. J. Non-Cryst. Solids 2006, 352, 3781–3786. [Google Scholar] [CrossRef]
- Feng, R.; Stachurski, Z.H.; Rodrigues, M.D.; Kluth, P.; Araujo, L.L.; Bulla, D.; Ridway, M.C. X-ray scattering from amorphous solids. J. Non-Cryst. Solids 2013, 383, 21–27. [Google Scholar] [CrossRef]
- Ehrenfest, P. On interference phenomena to be expected when Roentgen rays pass through a diatomic gas. KNAW Proc. 1915, 17, 1184–1190. [Google Scholar]
- Hehre, W.J.; Stewart, R.F.; Pople, J.A. Self-consistent molecular-orbital methods. I. Use of Gaussian expansions of slater-type atomic orbitals. J. Chem. Phys. 1969, 51, 2657–2665. [Google Scholar] [CrossRef]
- McLean, A.D.; Chandler, G.S. Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z=11-18. J. Chem. Phys. 1980, 72, 5639–5648. [Google Scholar] [CrossRef]
- Jackson, K. Electric fields in electronic structure calculations: Electric polarizabilities and IR and Raman spectra from first principles. Phys. Stat. Solidi B 2000, 217, 293–310. [Google Scholar] [CrossRef]
- Holomb, R.; Veres, M.; Mitsa, V. Ring-, branchy-, and cage-like AsnSm nanoclusters in the structure of amorphous semiconductors: Ab initio and Raman study. J. Optoelectron. Adv. Mater. 2009, 11, 917–923. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shpotyuk, O.; Lukáčová Bujňáková, Z.; Shpotyuk, Y.; Kovalskiy, A. Understanding the Paradigm of Molecular-Network Conformations in Nanostructured Se-Rich Arsenoselenides AsxSe100−x (x < 10). Molecules 2025, 30, 3380. https://doi.org/10.3390/molecules30163380
Shpotyuk O, Lukáčová Bujňáková Z, Shpotyuk Y, Kovalskiy A. Understanding the Paradigm of Molecular-Network Conformations in Nanostructured Se-Rich Arsenoselenides AsxSe100−x (x < 10). Molecules. 2025; 30(16):3380. https://doi.org/10.3390/molecules30163380
Chicago/Turabian StyleShpotyuk, Oleh, Zdenka Lukáčová Bujňáková, Yaroslav Shpotyuk, and Andriy Kovalskiy. 2025. "Understanding the Paradigm of Molecular-Network Conformations in Nanostructured Se-Rich Arsenoselenides AsxSe100−x (x < 10)" Molecules 30, no. 16: 3380. https://doi.org/10.3390/molecules30163380
APA StyleShpotyuk, O., Lukáčová Bujňáková, Z., Shpotyuk, Y., & Kovalskiy, A. (2025). Understanding the Paradigm of Molecular-Network Conformations in Nanostructured Se-Rich Arsenoselenides AsxSe100−x (x < 10). Molecules, 30(16), 3380. https://doi.org/10.3390/molecules30163380






