Optimizing Metal Sites in Hierarchical USY for Selective Hydrocracking of Naphthalene to BTX
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Catalysts
2.1.1. Structure Properties and Pore Structure
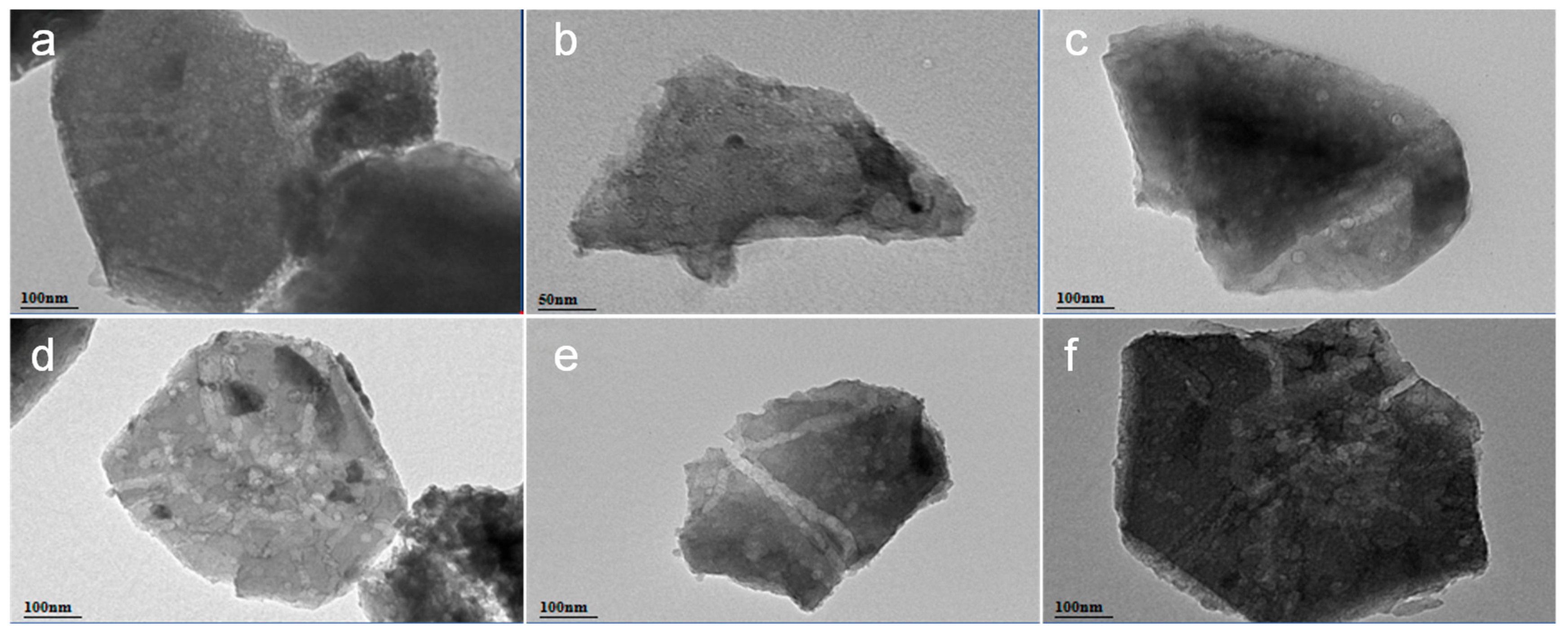
2.1.2. Microstructure and Morphology
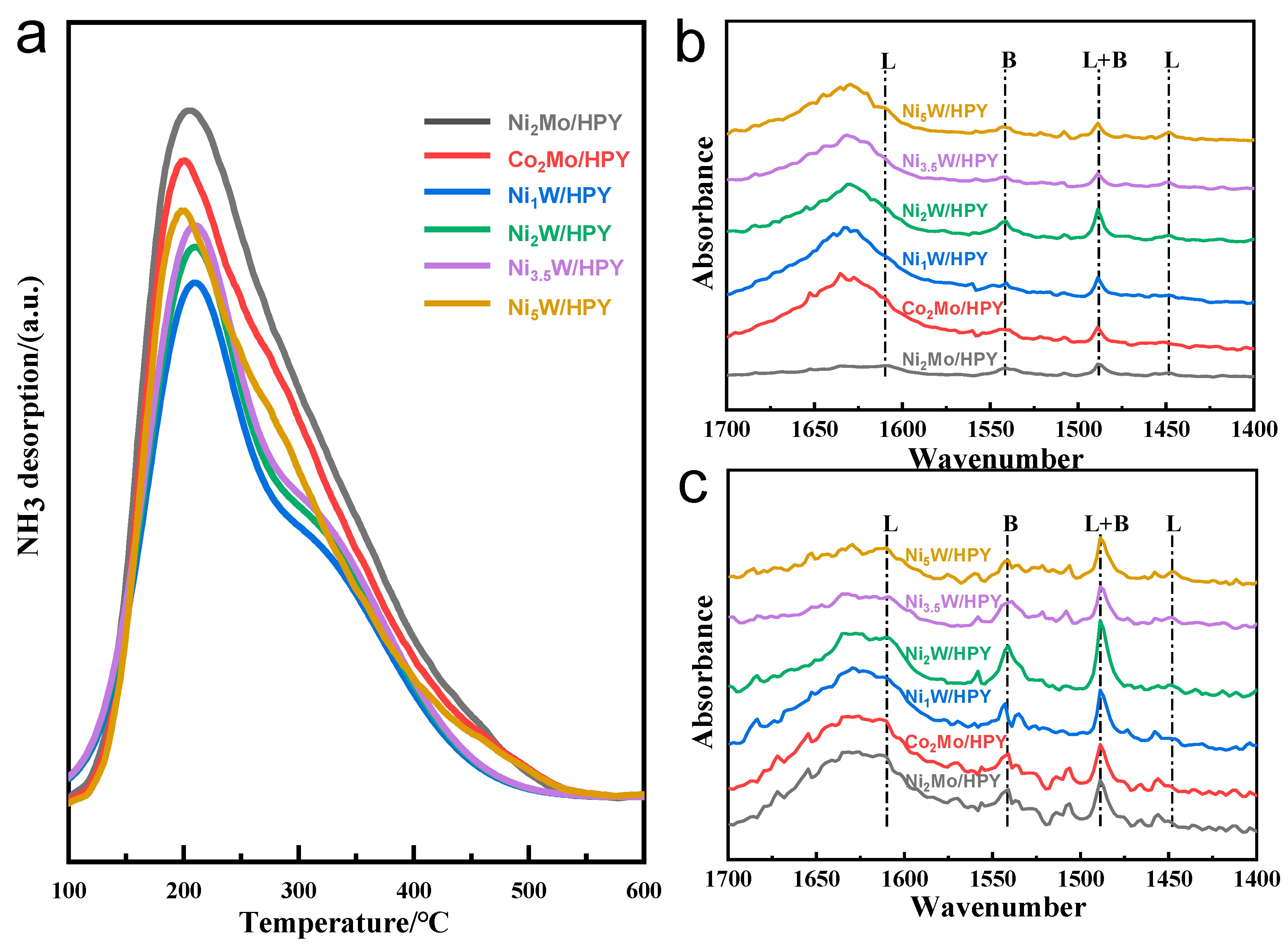
2.1.3. Surface Acidity Properties of AxB/HPY Catalysts
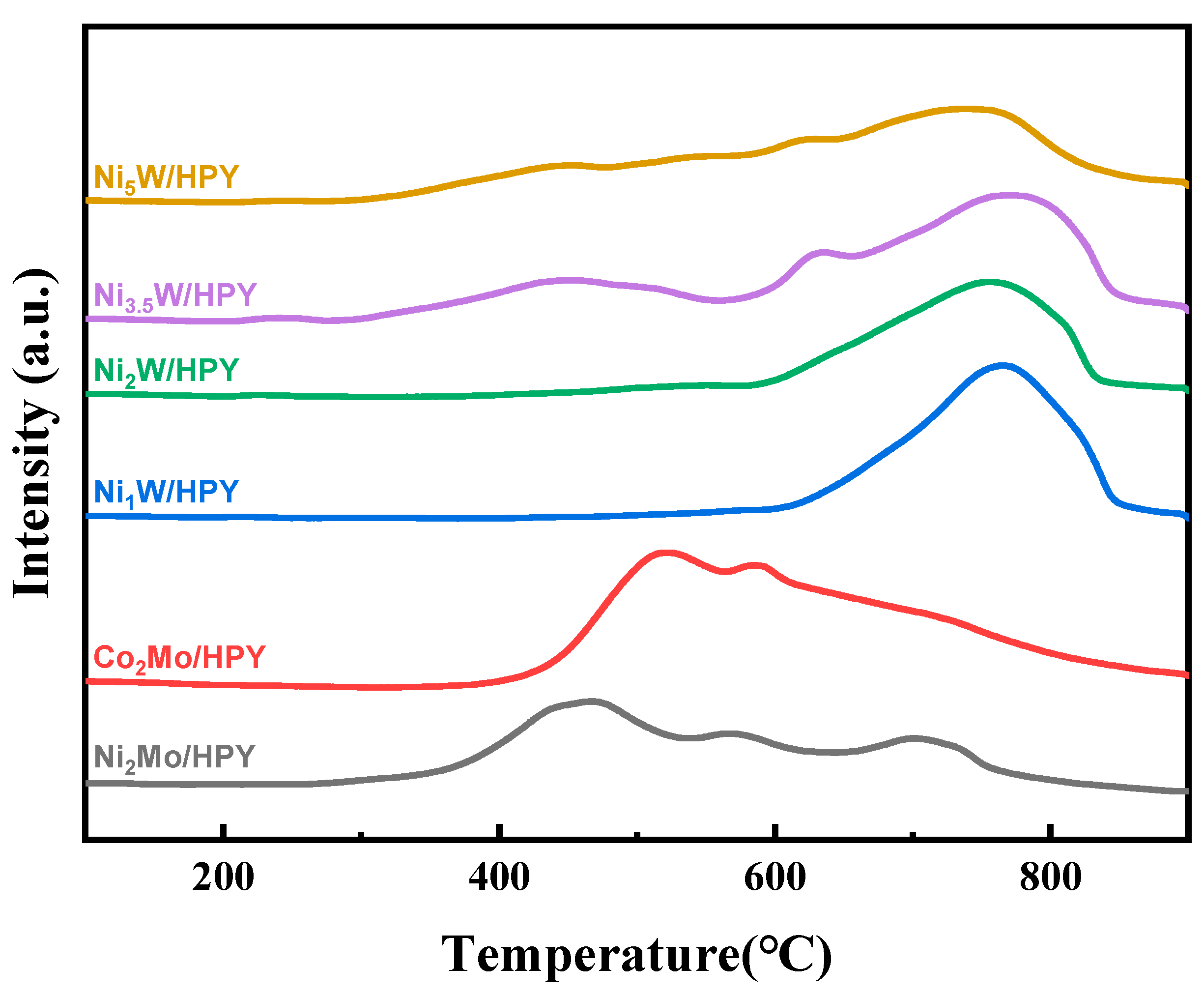
2.1.4. Metal–Support Interaction of AxB/HPY Catalysts
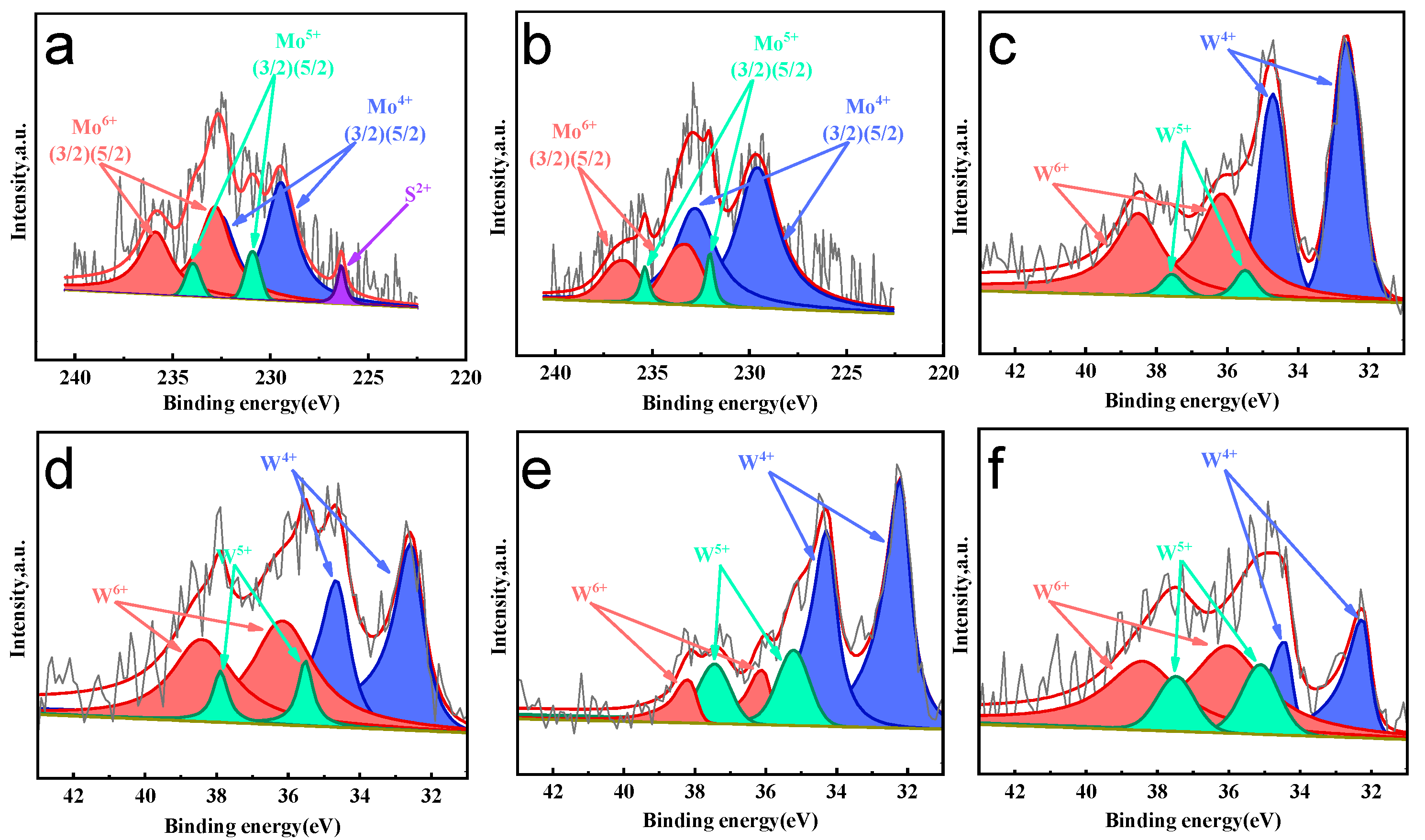
2.1.5. Chemical State Analysis of Active Phase
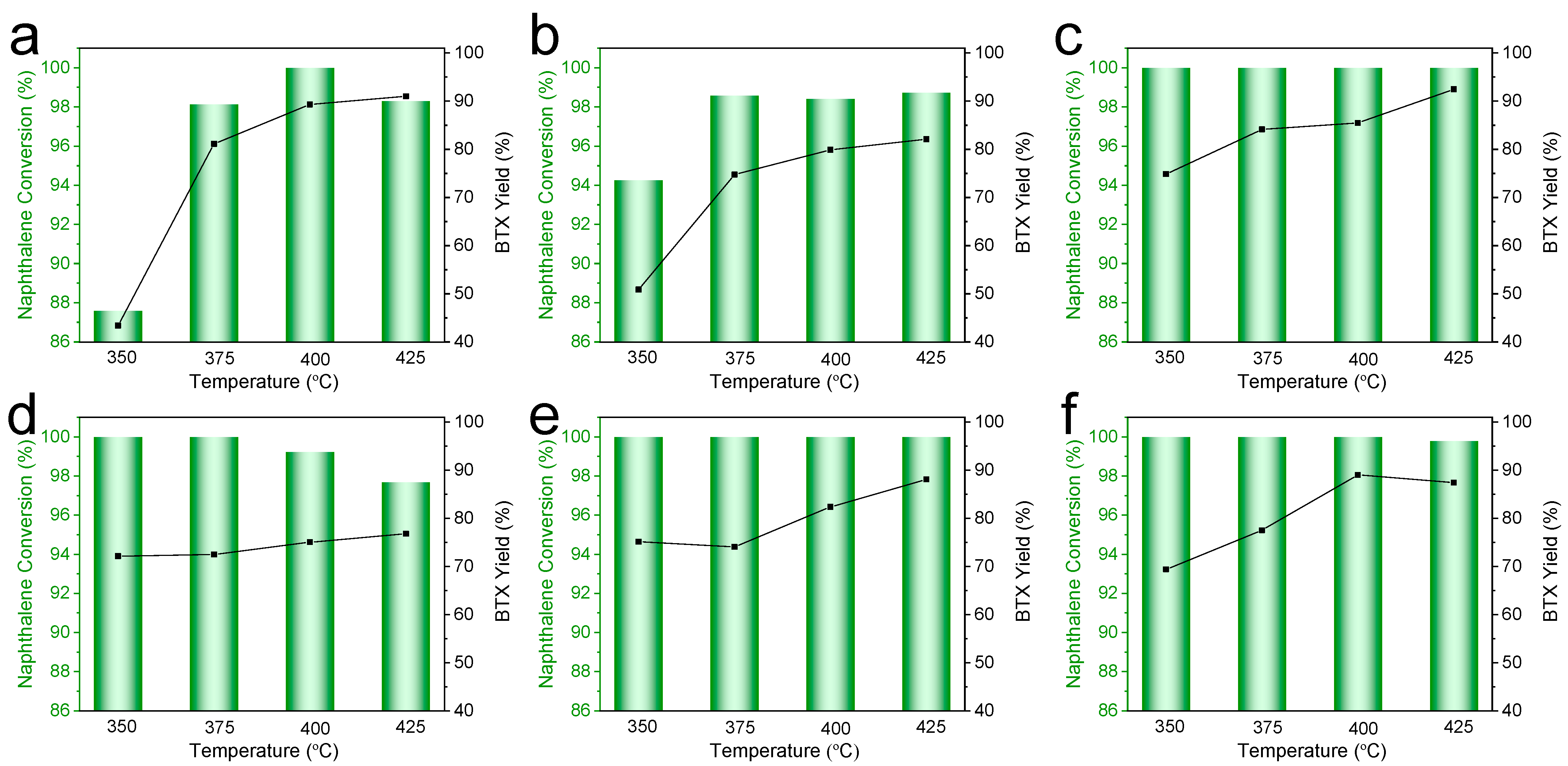
2.2. Catalytic Performance in Naphthalene Hydrocracking
3. Experimental Section
3.1. Materials
3.2. HPY Support Synthesis
3.3. Catalyst Synthesis (Sequential Incipient Wetness Impregnation)
3.4. Catalyst Characterization
3.5. Catalytic Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peng, C.; Liu, B.; Feng, X.; Du, Y.; Fang, X. Engineering dual bed hydrocracking catalyst towards enhanced high-octane gasoline generation from light cycle oil. Chem. Eng. J. 2020, 389, 123461. [Google Scholar] [CrossRef]
- Zhang, M.; Song, Q.; He, Z.; Wang, Q.; Wang, L.; Zhang, X.; Li, G. Tuning the mesopore-acid-metal balance in Pd/HY for efficient deep hydrogenation saturation of naphthalene. Int. J. Hydrogen Energy 2022, 47, 20881–20893. [Google Scholar] [CrossRef]
- Gordeeva, N.A.; Shesterkina, A.A.; Vikanova, K.V.; Kustov, A.L. Naphthalene and its derivatives hydrogenation for hydrogen storage: Comparative analysis of the role of noble and non-noble metal catalysts—A review. Int. J. Hydrogen Energy 2024, 69, 113–121. [Google Scholar] [CrossRef]
- Gutiérrez, A.; Arandes, J.M.; Castaño, P.; Olazar, M.; Bilbao, J. Preliminary studies on fuel production through LCO hydrocracking on noble-metal supported catalysts. Fuel 2012, 94, 504–515. [Google Scholar] [CrossRef]
- Chen, F.; Huo, J.; Zhao, L.; Gao, J.; Xu, C. Optimizing support acidity of NiMo catalysts for increasing the yield of benzene, toluene and xylene in tetralin hydrocracking. Front. Chem. Sci. Eng. 2024, 18, 7. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, C.; Geng, R.; Zhou, H.; Dong, Q.; Liu, S.; Fan, B.; Li, R. Hydrocracking of naphthalene over Beta zeolite coupled with NiMo/γ-Al2O3: Investigation of metal and acid balance based on the composition of industrial hydrocracking catalyst. Fuel 2023, 344, 128049. [Google Scholar] [CrossRef]
- Lee, J.; Choi, Y.; Shin, J.; Lee, J.K. Selective hydrocracking of tetralin for light aromatic hydrocarbons. Catal. Today 2016, 265, 144–153. [Google Scholar] [CrossRef]
- Upare, D.P.; Park, S.; Kim, M.; Jeon, Y.-P.; Kim, J.; Lee, D.; Lee, J.; Chang, H.; Choi, S.; Choi, W. Selective hydrocracking of pyrolysis fuel oil into benzene, toluene and xylene over CoMo/beta zeolite catalyst. J. Ind. Eng. Chem. 2017, 46, 356–363. [Google Scholar] [CrossRef]
- Zhu, L.; Shan, S.; Petkov, V.; Hu, W.; Kroner, A.; Zheng, J.; Yu, C.; Zhang, N.; Li, Y.; Luque, R. Ruthenium–nickel–nickel hydroxide nanoparticles for room temperature catalytic hydrogenation. J. Mater. Chem. A 2017, 5, 7869–7875. [Google Scholar] [CrossRef]
- Shin, J.; Oh, Y.; Choi, Y.; Lee, J.; Lee, J.K. Design of selective hydrocracking catalysts for BTX production from diesel-boiling-range polycyclic aromatic hydrocarbons. Appl. Catal. A Gen. 2017, 547, 12–21. [Google Scholar] [CrossRef]
- Anilkumar, M.; Loke, N.; Patil, V.; Panday, R. Hydrocracking of hydrotreated light cycle oil to mono aromatics over non-noble bi-functional (ni-w supported) zeolite catalysts. Catal. Today 2020, 358, 221–227. [Google Scholar] [CrossRef]
- Oh, Y.; Noh, H.; Park, H.; Han, H.; Nguyen, T.-B.; Lee, J.K. Molecular-size selective hydroconversion of FCC light cycle oil into petrochemical light aromatic hydrocarbons. Catal. Today 2020, 352, 329–336. [Google Scholar] [CrossRef]
- Jia, H.; Yan, W.; Dong, Q.; Qing, B.; Hao, W.; Du, Y.; Zhang, X.; Ma, J.; Li, R. Selective ring-opening in 9, 10-dihydrophenanthrene hydrocracking over a practical NiMo/Al2O3-USY catalyst under mild conditions. J. Energy Inst. 2024, 116, 101716. [Google Scholar] [CrossRef]
- Kuchinskaya, T.; Kniazeva, M.; Samoilov, V.; Maximov, A. In situ generated nanosized sulfide Ni-W catalysts based on zeolite for the hydrocracking of the pyrolysis fuel oil into the BTX fraction. Catalysts 2020, 10, 1152. [Google Scholar] [CrossRef]
- Golubev, I.; Dik, P.; Kazakov, M.; Pereyma, V.Y.; Klimov, O.; Smirnova, M.Y.; Prosvirin, I.; Gerasimov, E.Y.; Kondrashev, D.; Golovachev, V. The effect of Si/Al ratio of zeolite Y in NiW catalyst for second stage hydrocracking. Catal. Today 2021, 378, 65–74. [Google Scholar] [CrossRef]
- Cao, Z.; Zhang, X.; Xu, C.; Huang, X.; Wu, Z.; Peng, C.; Duan, A. Selective hydrocracking of light cycle oil into high-octane gasoline over bi-functional catalysts. J. Energy Chem. 2021, 52, 41–50. [Google Scholar] [CrossRef]
- Laredo, G.C.; Águeda-Rangel, R.; García-López, A.; García-Gutiérrez, J.L.; Olmos-Cerda, E.H. Effect of the chemical composition of six hydrotreated light cycle oils for benzene, toluene, ethylbenzene, and xylene production by a hydrocracking process. Appl. Petrochem. Res. 2021, 11, 249–263. [Google Scholar] [CrossRef]
- Shanshan, D.; Yongkang, L.; Xun, W.; Ruipeng, R. Effects of W loading amounts on catalytic performances of Ni-W bimetallic catalysts for CH 4-CO 2 reforming reaction. Low-Carbon Chem. Chem. Eng. 2024, 49, 12–18. [Google Scholar]
- Kong, X.; Li, Y.; Liu, Z.; Zou, Y.; Li, D.; Liu, J.; Wang, W.; Wang, C.; Xu, C.; Wang, X. Pore-Structure Engineering of Hierarchical Ni/Y Catalysts for the Enhanced Selective Hydrocracking of Naphthalene to BTX. ACS Sustain. Chem. Eng. 2025, 13, 23. [Google Scholar] [CrossRef]
- Hu, Z.; Hu, P.; Wang, X.; Wu, T.; Ge, S.; Xie, H.; Liu, B.; Chen, S.; Wu, Z. Selective hydrocracking of 1-methylnaphthalene to benzene/toluene/xylenes (BTX) over NiW/Beta bifunctional catalyst: Effects of metal–acid balance. Fuel 2024, 363, 130947. [Google Scholar] [CrossRef]
- Jurković, D.L.; Kostyniuk, A.; Likozar, B. Mechanisms, reaction micro-kinetics and modelling of hydrocracking of aromatic biomass tar model compounds into benzene, toluene and xylenes (BTX) over H-ZSM-5 catalyst. Chem. Eng. J. 2022, 445, 136898. [Google Scholar] [CrossRef]
- Kong, X.; Mei, J.; Liu, Z.; Zou, Y.; Wang, E.; Wang, W.; Wang, C.; Xu, C.; Wang, X. Regulating metal–acid active sites in hierarchical porous Ni/Y for selective hydrocracking of naphthalene. Carbon Resour. Convers. 2025, 8, 100326. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, G.; Weng, X.; Zhang, Y.; Zhao, L.; Cao, L.; Gao, J.; Xu, C.; Liu, X.; Gao, X. High value utilization of inferior diesel for BTX production: Mechanisms, catalysts, conditions and challenges. Appl. Catal. A Gen. 2021, 616, 118095. [Google Scholar] [CrossRef]
- Lee, S.-U.; Lee, Y.-J.; Kim, J.-R.; Jeong, S.-Y. Rational synthesis of silylated Beta zeolites and selective ring opening of 1-methylnaphthalene over the NiW-supported catalysts. Appl. Catal. B Environ. 2017, 219, 1–9. [Google Scholar] [CrossRef]
- Wu, T.; Chen, S.-L.; Yuan, G.-m.; Pan, X.; Du, J.; Zhang, Y.; Zhang, N. High metal–acid balance and selective hydrogenation activity catalysts for hydrocracking of 1-methylnaphthalene to benzene, toluene, and xylene. Ind. Eng. Chem. Res. 2020, 59, 5546–5556. [Google Scholar] [CrossRef]
- Zhang, Y.-T.; Zhang, N.-N.; Chen, S.-L.; Dang, H.; Wu, T. Surface dealuminated Beta zeolites supported WO3 catalyst and its catalytic performance in tetralin hydrocracking. Pet. Sci. 2022, 19, 3116–3123. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.; Huang, L.; Zhou, S.; Sheng, X.; Wang, Q.; Zhang, C. Structure and catalytic properties of the Zn-modified ZSM-5 supported platinum catalyst for propane dehydrogenation. Chem. Eng. J. 2015, 270, 352–361. [Google Scholar] [CrossRef]
- Jin, F.; Zhang, P.; Wu, G. Fundamental kinetics model of acidity-activity relation for ethylene oligomerization and aromatization over ZSM-5 zeolites. Chem. Eng. Sci. 2021, 229, 116144. [Google Scholar] [CrossRef]
- Peng, C.; Zhou, Z.; Cheng, Z.; Fang, X. Upgrading of light cycle oil to high-octane gasoline through selective hydrocracking over non-noble metal bifunctional catalysts. Energy Fuels 2019, 33, 1090–1097. [Google Scholar] [CrossRef]
- Klimova, T.; Reyes, J.; Gutiérrez, O.; Lizama, L. Novel bifunctional NiMo/Al-SBA-15 catalysts for deep hydrodesulfurization: Effect of support Si/Al ratio. Appl. Catal. A Gen. 2008, 335, 159–171. [Google Scholar] [CrossRef]
- Ding, L.; Zheng, Y.; Zhang, Z.; Ring, Z.; Chen, J. Hydrotreating of light cycled oil using WNi/Al2O3 catalysts containing zeolite beta and/or chemically treated zeolite Y. J. Catal. 2006, 241, 435–445. [Google Scholar] [CrossRef]
- Arribas, M.a.A.; Concepción, P.; Martínez, A.N. The role of metal sites during the coupled hydrogenation and ring opening of tetralin on bifunctional Pt (Ir)/USY catalysts. Appl. Catal. A Gen. 2004, 267, 111–119. [Google Scholar] [CrossRef]
- Kubička, D.; Kumar, N.; Venäläinen, T.; Karhu, H.; Kubičková, I.; Österholm, H.; Murzin, D.Y. Metal− support interactions in zeolite-supported noble metals: Influence of metal crystallites on the support acidity. J. Phys. Chem. B 2006, 110, 4937–4946. [Google Scholar] [CrossRef]
- Lee, S.-U.; Lee, Y.-J.; Kim, J.-R.; Kim, E.-S.; Kim, T.-W.; Kim, H.J.; Kim, C.-U.; Jeong, S.-Y. Selective ring opening of phenanthrene over NiW-supported mesoporous HY zeolite catalyst depending on their mesoporosity. Mater. Res. Bull. 2017, 96, 149–154. [Google Scholar] [CrossRef]
- Zhengkai, C.; Xia, Z.; Chunming, X.; Aijun, D.; Rong, G.; Zhen, Z.; Ziming, W.; Chong, P.; Jianmei, L.; Xilong, W. The Synthesis of Al-SBA-16 Materials with a Novel Method and Their Catalytic Application on Hydrogenation for FCC Diesel. Energy Fuels 2017, 31, 805–814. [Google Scholar]
- Pedroarena, I.; Grande, L.; Torrez-Herera, J.J.; Korili, S.A.; Gil, A. Analysis by temperature-programmed reduction of the catalytic system Ni-Mo-Pd/Al2O3. Fuel 2023, 334, 126789. [Google Scholar] [CrossRef]
- Zheng, L.; Li, Z.; Fu, P.; Sun, F.; Liu, M.; Guo, T.; Fan, Q. Development of Mo-modified pseudoboehmite supported Ni catalysts for efficient hydrogen production from formic acid. ACS Omega 2022, 7, 27172–27184. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Pham, G.H.; Ran, R.; Vagnoni, R.; Pareek, V.; Liu, S. Dry reforming of methane over Co–Mo/Al 2 O 3 catalyst under low microwave power irradiation. Catal. Sci. Technol. 2018, 8, 5315–5324. [Google Scholar] [CrossRef]
- Soghrati, E.; Kok Poh, C.; Du, Y.; Gao, F.; Kawi, S.; Borgna, A. C− O Hydrogenolysis of Tetrahydrofurfuryl Alcohol to 1, 5-Pentanediol Over Bi-functional Nickel-Tungsten Catalysts. ChemCatChem 2018, 10, 4652–4664. [Google Scholar] [CrossRef]
- Fang, M.; Ma, S.; Wang, T.; Xia, Z.; Tang, W.; Xia, L.; Luo, Z. Hydrotreatment of model compounds with catalysts of NiW/Al2O3 and NiWP/Al2O3 to simulate low temperature coal tar oil. RSC Adv. 2017, 7, 54512–54521. [Google Scholar] [CrossRef]
- De León, J.D.; Picquart, M.; Massin, L.; Vrinat, M.; De los Reyes, J. Hydrodesulfurization of sulfur refractory compounds: Effect of gallium as an additive in NiWS/γ-Al2O3 catalysts. J. Mol. Catal. A Chem. 2012, 363, 311–321. [Google Scholar] [CrossRef]
- Tayeb, K.B.; Lamonier, C.; Lancelot, C.; Fournier, M.; Bonduelle-Skrzypczak, A.; Bertoncini, F. Active phase genesis of NiW hydrocracking catalysts based on nickel salt heteropolytungstate: Comparison with reference catalyst. Appl. Catal. B Environ. 2012, 126, 55–63. [Google Scholar] [CrossRef]
- Wang, Y.; Lancelot, C.; Lamonier, C.; Yang, M.; Sun, Y.; Morin, J.-C.; Rives, A. Restraining deactivation of hierarchical zeolite supported NiW catalysts in the HDS of thiophene. RSC Adv. 2015, 5, 74150–74158. [Google Scholar] [CrossRef]
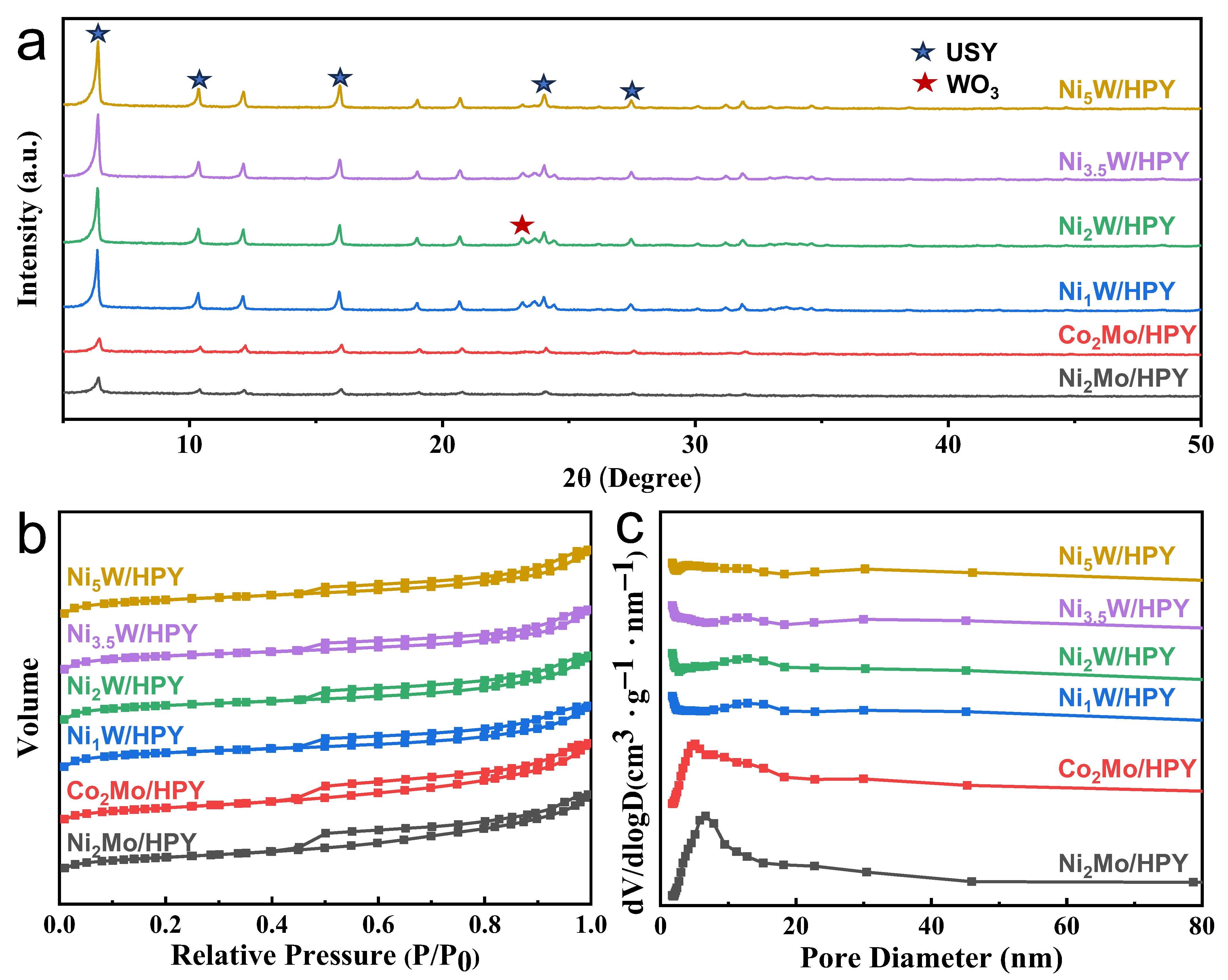
| Samples /HPY | Pore Size (nm) | Vtotal (cm3/g) | Vmic (cm3/g) | Vmes (cm3/g) | SBET (m2/g) | Smic (m2/g) | Sext (m2/g) |
|---|---|---|---|---|---|---|---|
| HPY | 7.5 | 0.45 | 0.26 | 0.19 | 709 | 554 | 155 |
| Ni2Mo | 5.8 | 0.27 | 0.07 | 0.20 | 258 | 170 | 88 |
| Co2Mo | 6.5 | 0.25 | 0.08 | 0.17 | 277 | 194 | 83 |
| Ni1W | 7.0 | 0.32 | 0.20 | 0.12 | 536 | 427 | 109 |
| Ni2W | 7.2 | 0.33 | 0.21 | 0.12 | 548 | 437 | 111 |
| Ni3W | 7.3 | 0.31 | 0.20 | 0.11 | 540 | 434 | 106 |
| Ni5W | 7.4 | 0.32 | 0.20 | 0.12 | 539 | 433 | 106 |
| Samples /HPY | Total Acid μmol/g | Weak Acid, μmol/g | Strong Acid, μmol/g | ||||
|---|---|---|---|---|---|---|---|
| L | B | L + B | L | B | L + B | ||
| Ni2Mo | 619.2 | 72.0 | 158.1 | 230.1 | 165.7 | 223.4 | 389.1 |
| Co2Mo | 557.2 | 113.9 | 93.2 | 207.1 | 60.5 | 289.6 | 350.1 |
| Ni1W | 431.0 | 59.0 | 111.3 | 170.3 | 64.2 | 196.5 | 260.7 |
| Ni2W | 461.9 | 61.5 | 120.3 | 181.8 | 38.0 | 242.2 | 280.2 |
| Ni3.5W | 480.6 | 125.2 | 63.5 | 188.7 | 56.1 | 235.8 | 291.9 |
| Ni5W | 495.3 | 114.9 | 80.7 | 195.6 | 65.9 | 233.8 | 299.7 |
| Samples /HPY | Mo(W)4+% | Mo(W)5+% | Mo(W)6+% |
|---|---|---|---|
| Ni2Mo | 48.8 | 7.1 | 44.1 |
| Co2Mo | 72.4 | 5.4 | 22.2 |
| Ni1W | 70.3 | 9.7 | 20.1 |
| Ni2W | 50.2 | 8.5 | 41.3 |
| Ni3.5W | 52.1 | 5.4 | 42.5 |
| Ni5W | 59.5 | 26.7 | 13.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, K.; Liu, M.; Li, H.; Chen, X.; Wang, X. Optimizing Metal Sites in Hierarchical USY for Selective Hydrocracking of Naphthalene to BTX. Molecules 2025, 30, 4023. https://doi.org/10.3390/molecules30194023
Zheng K, Liu M, Li H, Chen X, Wang X. Optimizing Metal Sites in Hierarchical USY for Selective Hydrocracking of Naphthalene to BTX. Molecules. 2025; 30(19):4023. https://doi.org/10.3390/molecules30194023
Chicago/Turabian StyleZheng, Kunyi, Mingjia Liu, Haidong Li, Xiu Chen, and Xilong Wang. 2025. "Optimizing Metal Sites in Hierarchical USY for Selective Hydrocracking of Naphthalene to BTX" Molecules 30, no. 19: 4023. https://doi.org/10.3390/molecules30194023
APA StyleZheng, K., Liu, M., Li, H., Chen, X., & Wang, X. (2025). Optimizing Metal Sites in Hierarchical USY for Selective Hydrocracking of Naphthalene to BTX. Molecules, 30(19), 4023. https://doi.org/10.3390/molecules30194023





