Chemical Composition and Biological Activities of the Essential Oils from Different Parts of Rosa bracteata J.C.Wendl
Abstract
1. Introduction
2. Results and Discussion
2.1. Analysis of Chemical Composition
2.2. Evaluation of Antibacterial Activity
2.3. Evaluation of Synergistic Interactions
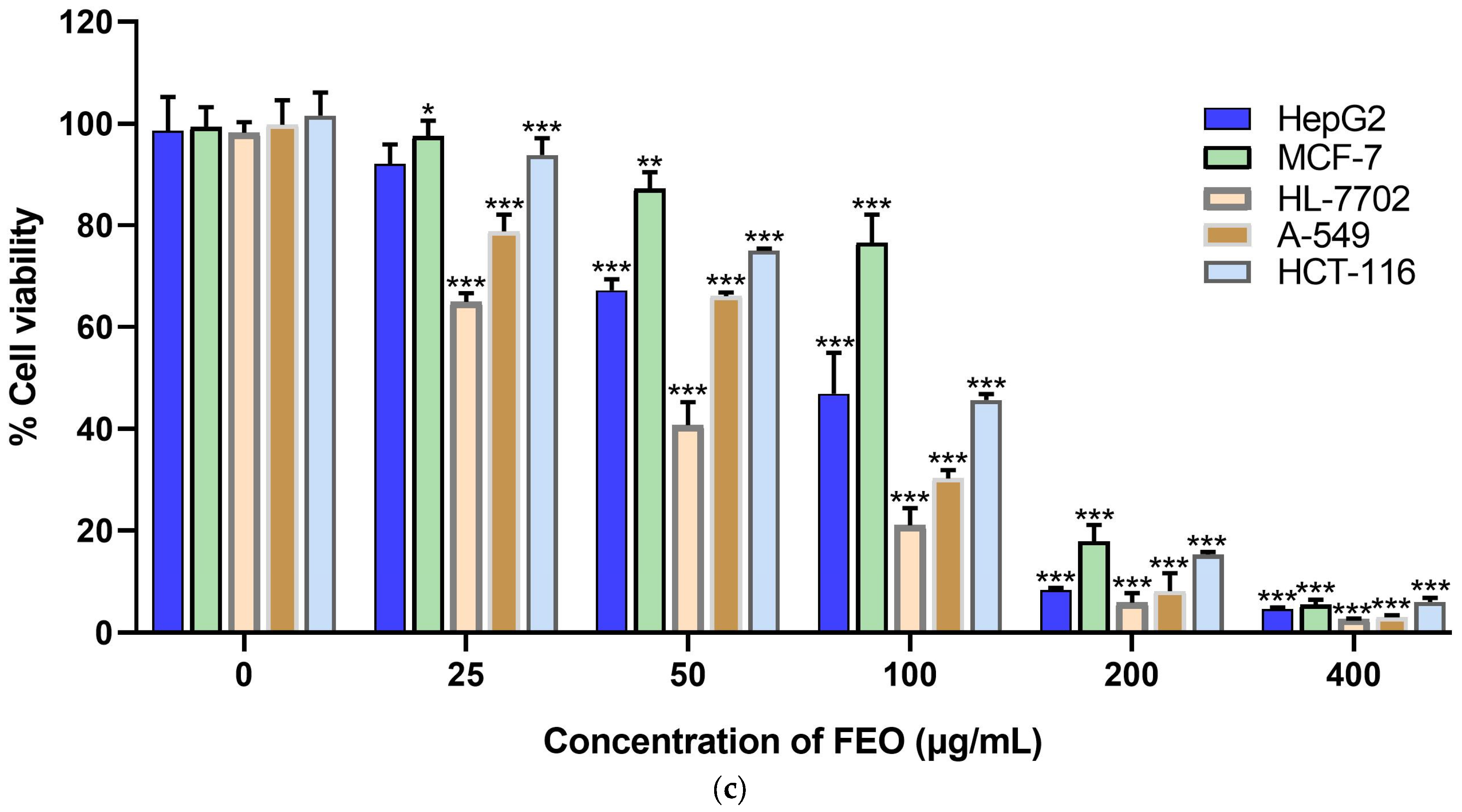
2.4. Evaluation of Cytotoxic Activity
2.5. Evaluation of Antioxidant Activity
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material
3.3. Essential Oil Extraction
3.4. Identification of EO Components
3.5. Antibacterial Activity Assays
3.6. Synergistic Effect Evaluation
3.7. Cytotoxic Activity Evaluation
3.8. Antioxidant Activity Evaluation
3.8.1. DPPH Radical Scavenging
3.8.2. ABTS Radical Cation Scavenging
3.8.3. Ferric Reducing Power
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef]
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019, 2, 49–55. [Google Scholar] [CrossRef]
- Tisserand, R.; Young, R. Essential Oil Profiles. Essential Oil Safety: A Guide for Health Care Professionals, 2nd ed.; Elsevier: London, UK, 2014; pp. 187–482. [Google Scholar]
- Sánchez-González, L.; Vargas, M.; González-Martínez, C.; Chiralt, A.; Chafer, M. Use of essential oils in bioactive edible coatings: A review. Food Eng. Rev. 2011, 3, 1–16. [Google Scholar] [CrossRef]
- Pichersky, E.; Gershenzon, J. The formation and function of plant volatiles: Perfumes for pollinator attraction and defense. Curr. Opin. Plant Biol. 2002, 5, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Bașer, K.H.C.; Buchbauer, G. Handbook of Essential Oils: Science, Technology, and Applications, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 1–1000. [Google Scholar]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Aziz, Z.A.; Ahmad, A.; Setapar, S.H.M.; Karakucuk, A.; Azim, M.M.; Lokhat, D.; Rafatullah, M.; Ganash, M.; Kamal, M.A.; Ashraf, G.M. Essential oils: Extraction techniques, pharmaceutical and therapeutic potential-a review. Curr. Drug Metab. 2018, 19, 1100–1110. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Gudin, S. Rose: Genetics and breeding. Plant Breed Rev. 2010, 17, 159–189. [Google Scholar]
- Cheng, B.C.Y.; Fu, X.Q.; Guo, H.; Li, T.; Wu, Z.Z.; Chan, K.; Yu, Z.L. The genus Rosa and arthritis: Overview on pharmacological perspectives. Pharmacol. Res. 2016, 114, 219–234. [Google Scholar] [CrossRef]
- Ayati, Z.; Amiri, M.S.; Ramezani, M.; Delshad, E.; Sahebkar, A.; Emami, S.A. Phytochemistry, traditional uses and pharmacological profile of rose hip: A review. Curr. Pharm. Des. 2018, 24, 4101–4124. [Google Scholar] [CrossRef]
- Mármol, I.; Sánchez-de-Diego, C.; Jiménez-Moreno, N.; Ancín-Azpilicueta, C.; Rodríguez-Yoldi, M.J. Therapeutic applications of rose hips from different Rosa species. Int. J. Mol. Sci. 2017, 18, 1137. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Liu, X.; Li, J.; Zhang, J.; Liu, D. Chemical constituents and pharmacological activities of medicinal plants from Rosa genus. Chin. Herb. Med. 2022, 14, 187–209. [Google Scholar] [CrossRef]
- Latifi, G.; Ghannadi, A.; Minaiyan, M. Anti-inflammatory effect of volatile oil and hydroalcoholic extract of Rosa damascena Mill. on acetic acid-induced colitis in rats. Res. Pharm. Sci. 2015, 10, 514–522. [Google Scholar]
- Dobreva, A.; Nedeva, D.; Mileva, M. Comparative study of the yield and chemical profile of rose oils and hydrosols obtained by industrial plantations of oil-bearing roses in Bulgaria. Resources 2023, 12, 83. [Google Scholar] [CrossRef]
- Xiao, Z.; Luo, J.; Niu, Y.; Wu, M. Characterization of key aroma compounds from different rose essential oils using gas chromatography-mass spectrometry, gas chromatography–olfactometry and partial least squares regression. Nat. Prod. Res. 2018, 32, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Öz, M.; Deniz, I.; Okan, O.T.; Baltaci, C.; Karatas, S.M. Determination of the chemical composition, antioxidant and antimicrobial activities of different parts of Rosa canina L. and Rosa pimpinellifolia L. essential oils. J. Essent. Oil Bear. Plants 2021, 24, 519–537. [Google Scholar] [CrossRef]
- Haghparasti, A.; Sichani, M.M.; Tavakoli, M. Chemical composition and antibacterial activity of wild Rose (Rosa canina L.) gall extracts against gram-negative pathogenic bacteria. J. Adv. Biomed. Sci. 2023, 13, 13–22. [Google Scholar] [CrossRef]
- Elhawary, E.A.; Mostafa, N.M.; Labib, R.M.; Singab, A.N. Metabolomic profiles of essential oils from selected rosa varieties and their antimicrobial activities. Plants 2021, 10, 1721. [Google Scholar] [CrossRef]
- Li, C.; Luo, Y.; Zhang, W.; Cai, Q.; Wu, X.; Tan, Z.; Chen, R.; Chen, Z.; Wang, S.; Zhang, L. A comparative study on chemical compositions and biological activities of four essential oils: Cymbopogon citratus (DC.) Stapf, Cinnamomum cassia (L.) Presl, Salvia japonica Thunb. and Rosa rugosa Thunb. J. Ethnopharmacol. 2021, 280, 114472. [Google Scholar] [CrossRef]
- Kumar, A.; Gautam, R.D.; Singh, S.; Chauhan, R.; Kumar, M.; Kumar, D.; Kumar, A.; Singh, S. Phenotyping floral traits and essential oil profiling revealed considerable variations in clonal selections of damask rose (Rosa damascena Mill.). Sci. Rep. 2023, 13, 8101. [Google Scholar] [CrossRef]
- Andoğan, B.C.; Baydar, H.; Kaya, S.; Demirci, M.; Özbaşar, D.; Mumcu, E. Antimicrobial activity and chemical composition of some essential oils. Arch. Pharm. Res. 2002, 25, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Boskabady, M.H.; Shafei, M.N.; Saberi, Z.; Amini, S. Pharmacological effects of Rosa damascena. Iran. J. Basic Med. Sci. 2011, 14, 295–307. [Google Scholar] [PubMed]
- Mohebitabar, S.; Shirazi, M.; Bioos, S.; Rahimi, R.; Malekshahi, F.; Nejatbakhsh, F. Therapeutic efficacy of rose oil: A comprehensive review of clinical evidence. Avicenna J. Phytomed. 2017, 7, 206–213. [Google Scholar] [PubMed]
- Enloe, S.F.; Kline, W.N.; Aulakh, J.S.; Bethke, R.K.; Gladney, J.B.; Lauer, D.K. Macartney rose (Rosa bracteata) response to herbicide and mowing treatments. Invasive Plant Sci. Manag. 2013, 6, 260–267. [Google Scholar] [CrossRef]
- Yuan, G.; Du, F. Studies on chemical constituents of the fruits of Rosa bracteata var. bracteata. Zhongyaocai 2000, 23, 454–456. [Google Scholar]
- Ulusoy, S.; Boşgelmez-Tınaz, G.; Seçilmiş-Canbay, H. Tocopherol, carotene, phenolic contents and antibacterial properties of rose essential oil, hydrosol and absolute. Curr. Microbiol. 2009, 59, 554–558. [Google Scholar] [CrossRef]
- Katekar, V.P.; Rao, A.B.; Sardeshpande, V.R. Review of the rose essential oil extraction by hydrodistillation: An investigation for the optimum operating condition for maximum yield. Sustain. Chem. Pharm. 2022, 29, 100783. [Google Scholar] [CrossRef]
- Xiao, Z.; Luo, J.; Niu, Y.; Wang, P.; Wang, R.; Sun, X. Olfactory impact of esters on rose essential oil floral alcohol aroma expression in model solution. Food Res. Int. 2019, 116, 211–222. [Google Scholar] [CrossRef]
- Basim, E.; Basim, H. Antibacterial activity of Rosa damascena essential oil. Fitoterapia 2003, 74, 394–396. [Google Scholar] [CrossRef]
- Baydar, N.G.; Baydar, H. Phenolic compounds, antiradical activity and antioxidant capacity of oil-bearing rose (Rosa damascena Mill.) extracts. Ind. Crops Prod. 2013, 41, 375–380. [Google Scholar] [CrossRef]
- Gochev, V.; Wlcek, K.; Buchbauer, G.; Stoyanova, A.; Dobreva, A.; Schmidt, E.; Jirovetz, L. Comparative evaluation of antimicrobial activity and composition of rose oils from various geographic origins, in particular Bulgarian rose oil. Nat. Prod. Commun. 2008, 3, 1063–1068. [Google Scholar] [CrossRef]
- Valcheva, V.; Mileva, M.; Dogonadze, M.; Dobreva, A.; Mokrousov, I. Antimycobacterial Activity of Essential Oils from Bulgarian Rosa Species Against Phylogenomically Different Mycobacterium tuberculosis Strains. Pharmaceutics 2024, 16, 1393. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 5th ed.; Texensis Publishing: Gruver, TX, USA, 2017. [Google Scholar]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention indices for frequently reported compounds of plant essential oils. J. Phys. Chem. Ref. Data 2011, 40, 43101. [Google Scholar] [CrossRef]
- Linstrom, P.J.; Mallard, W.G. NIST Chemistry WebBook, NIST Standard Reference Database Number 69; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2023. Available online: http://webbook.nist.gov/chemistry (accessed on 1 January 2023).
- Krist, S.; Banovac, D.; Tabanca, N.; Wedge, D.E.; Gochev, V.K.; Wanner, J.; Schmidt, E.; Jirovetz, L. Antimicrobial activity of nerolidol and its derivatives against airborne microbes and further biological activities. Nat. Prod. Commun. 2015, 10, 143–148. [Google Scholar] [CrossRef]
- de Moura, D.F.; Rocha, T.A.; de Melo Barros, D.; da Silva, M.M.; dos Santos Santana, M.; Neta, B.M.; Cavalcanti, I.M.F.; Martins, R.D.; da Silva, M.V. Evaluation of the antioxidant, antibacterial, and antibiofilm activity of the sesquiterpene nerolidol. Arch. Microbiol. 2021, 203, 4303–4311. [Google Scholar] [CrossRef]
- Almeida-Bezerra, J.W.; Menezes, S.A.; Silva, J.T.d.C.; de Sousa, S.G.; Alves, D.S.; Alencar, G.G.; Araújo, I.M.; Rodrigues, E.Y.d.S.; Oliveira-Tintino, C.D.d.M.; da Cruz, R.P. Analysis of the antibiotic-potentiating activity, absorption, distribution, metabolism, and excretion (ADME) and the molecular docking properties of phytol against multi-drug-resistant (MDR) strains. Antibiotics 2024, 13, 1171. [Google Scholar] [CrossRef]
- Lee, W.; Woo, E.R.; Lee, D.G. Phytol has antibacterial property by inducing oxidative stress response in Pseudomonas aeruginosa. Free Radic. Res. 2016, 50, 1309–1318. [Google Scholar] [CrossRef]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef]
- Saha, M.; Bandyopadhyay, P. In vivo and in vitro antimicrobial activity of phytol, a diterpene molecule, isolated and characterized from Adhatoda vasica Nees.(Acanthaceae), to control severe bacterial disease of ornamental fish, Carassius auratus, caused by Bacillus licheniformis PKBMS16. Microb. Pathog. 2020, 141, 103977. [Google Scholar]
- Su, Y.C.; Ho, C.L. Composition of the leaf essential oil of Phoebe formosana from Taiwan and its in vitro cytotoxic, antibacterial, and antifungal activities. Nat. Prod. Commun. 2016, 11, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Diastuti, H.; Chasani, M.; Suwandri, S. Antibacterial activity of benzyl benzoate and crotepoxide from Kaempferia rotunda L. Rhizome. Indones. J. Chem. 2020, 20, 9–15. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Parsons, J.B.; Yao, J.; Frank, M.W.; Jackson, P.; Rock, C.O. Membrane disruption by antimicrobial fatty acids releases low-molecular-weight proteins from Staphylococcus aureus. J. Bacteriol. 2012, 194, 5294–5304. [Google Scholar] [CrossRef]
- Kabara, J.J.; Swieczkowski, D.M.; Conley, A.J.; Truant, J.P. Fatty acids and derivatives as antimicrobial agents. Antimicrob. Agents Chemother. 1972, 2, 23–28. [Google Scholar] [CrossRef]
- Douglas, E.J.; Palk, N.; Rudolph, E.R.; Laabei, M. Anti-staphylococcal fatty acids: Mode of action, bacterial resistance and implications for therapeutic application. Microbiology 2025, 171, 001563. [Google Scholar] [CrossRef]
- Lamba, A.; Kopel, J.; Westenberg, D.; Kapila, S. Fatty acids, esters, and biogenic oil disinfectants: Novel agents against bacteria. Bayl. Univ. Med. Cent. Proc. 2023, 36, 375–379. [Google Scholar] [CrossRef]
- Bonapace, C.R.; Bosso, J.A.; Friedrich, L.V.; White, R.L. Comparison of methods of interpretation of checkerboard synergy testing. Diagn. Microbiol. Infect. Dis. 2002, 44, 363–366. [Google Scholar] [CrossRef]
- Giovagnorio, F.; De Vito, A.; Madeddu, G.; Parisi, S.G.; Geremia, N. Resistance in Pseudomonas aeruginosa: A narrative review of antibiogram interpretation and emerging treatments. Antibiotics 2023, 12, 1621. [Google Scholar] [CrossRef]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef]
- Qin, S.; Xiao, W.; Zhou, C.; Pu, Q.; Deng, X.; Lan, L.; Liang, H.; Song, X.; Wu, M. Pseudomonas aeruginosa: Pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct. Target. Ther. 2022, 7, 199. [Google Scholar] [CrossRef]
- Langeveld, W.T.; Veldhuizen, E.J.; Burt, S.A. Synergy between essential oil components and antibiotics: A review. Crit. Rev. Microbiol. 2014, 40, 76–94. [Google Scholar] [CrossRef]
- Alonso, L.; Fernandes, K.S.; Mendanha, S.A.; Gonçalves, P.J.; Gomes, R.S.; Dorta, M.L.; Alonso, A. In vitro antileishmanial and cytotoxic activities of nerolidol are associated with changes in plasma membrane dynamics. Biochim. Biophys. Acta Biomembr. 2019, 1861, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Sperotto, A.; Moura, D.; Péres, V.; Damasceno, F.; Caramão, E.; Henriques, J.; Saffi, J. Cytotoxic mechanism of Piper gaudichaudianum Kunth essential oil and its major compound nerolidol. Food Chem. Toxicol. 2013, 57, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chinnathambi, A.; Alharbi, S.A.; Natarajan, N.; Raman, M. Nerolidol, bioactive compound suppress growth of HCT-116 colorectal cancer cells through cell cycle arrest and induction of apoptosis. Appl. Biochem. Biotechnol. 2024, 196, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Alencar, M.V.; Islam, M.T.; Ali, E.S.; Santos, J.V.; Paz, M.F.; Sousa, J.M.; Dantas, S.M.; Mishra, S.K.; Cavalcante, A.A. Association of phytol with toxic and cytotoxic activities in an antitumoral perspective: A meta-analysis and systemic review. Anticancer Agents Med. Chem. 2018, 18, 1828–1837. [Google Scholar] [CrossRef]
- Islam, M.T.; Streck, L.; de Alencar, M.V.O.B.; Silva, S.W.C.; da Conceição Machado, K.; Júnior, A.L.G.; Paz, M.F.C.J.; da Mata, A.M.O.F.; e Sousa, J.M.d.C. Evaluation of toxic, cytotoxic and genotoxic effects of phytol and its nanoemulsion. Chemosphere 2017, 177, 93–101. [Google Scholar] [CrossRef]
- Pejin, B.; Kojic, V.; Bogdanovic, G. An insight into the cytotoxic activity of phytol at in vitro conditions. Nat. Prod. Res. 2014, 28, 2053–2056. [Google Scholar] [CrossRef]
- Yap, H.Y.Y.; Muria-Gonzalez, M.J.; Kong, B.H.; Stubbs, K.A.; Tan, C.S.; Ng, S.T.; Tan, N.H.; Solomon, P.S.; Fung, S.Y.; Chooi, Y.H. Heterologous expression of cytotoxic sesquiterpenoids from the medicinal mushroom Lignosus rhinocerotis in yeast. Microb. Cell Fact. 2017, 16, 103. [Google Scholar] [CrossRef]
- Acar, A.; Turkmen, Z.; Cavusoglu, K.; Yalcin, E. Investigation of benzyl benzoate toxicity with anatomical, physiological, cytogenetic and biochemical parameters in in vivo. Caryologia 2020, 73, 21–32. [Google Scholar]
- Balakrishnan, V.; Ganapathy, S.; Veerasamy, V.; Duraisamy, R.; Sathiavakoo, V.A.; Krishnamoorthy, V.; Lakshmanan, V. Anticancer and antioxidant profiling effects of nerolidol against DMBA induced oral experimental carcinogenesis. J. Biochem. Mol. Toxicol. 2022, 36, e23029. [Google Scholar] [CrossRef]
- Chan, W.K.; Tan, L.T.; Chan, K.G.; Lee, L.H.; Goh, B.H. Nerolidol: A sesquiterpene alcohol with multi-faceted pharmacological and biological activities. Molecules 2016, 21, 529. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.C.M.P.; Salvadori, M.S.; Mota, V.G.; Costa, L.M.; Almeida, A.A.C.; de Oliveira, G.A.L.; Costa, J.P.; de Sousa, D.P.; de Freitas, R.M.; de Almeida, R.N. Antinociceptive and antioxidant activities of phytol in vivo and in vitro models. Neurosci. J. 2013, 2013, 949452. [Google Scholar] [CrossRef] [PubMed]
- Elzaawely, A.A.; Xuan, T.D.; Koyama, H.; Tawata, S. Antioxidant activity and contents of essential oil and phenolic compounds in flowers and seeds of Alpinia zerumbet (Pers.) BL Burtt. & RM Sm. Food Chem. 2007, 104, 1648–1653. [Google Scholar]
- Hidajati, N.; Tukiran, T.; Setiabudi, D.A.; Wardana, A.P. Antioxidant activity of palmitic acid and pinostrobin from methanol extract of Syzygium litoralle (Myrtaceae). In Proceedings of the International Conference on Science and Technology (ICST 2018), Yogyakarta, Indonesia, 7–8 August 2018; pp. 183–187. [Google Scholar]
- Miguel, M.G. Antioxidant and anti-inflammatory activities of essential oils: A short review. Molecules 2010, 15, 9252–9287. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Zengin, H.; Baysal, A.H. Antibacterial and antioxidant activity of essential oil terpenes against pathogenic and spoilage-forming bacteria and cell structure-activity relationships evaluated by SEM microscopy. Molecules 2014, 19, 17773–17798. [Google Scholar] [CrossRef]
- Feng, L.; Xu, F.; Qiu, S.; Sun, C.; Lai, P. Chemical Composition and Antibacterial, Antioxidant, and Cytotoxic Activities of Essential Oils from Leaves and Stems of Aeschynomene indica L. Molecules 2024, 29, 3552. Molecules 2024, 29, 3552. [Google Scholar] [CrossRef]
- Humphries, R.M.; Ambler, J.; Mitchell, S.L.; Castanheira, M.; Dingle, T.; Hindler, J.A.; Koeth, L.; Sei, K. CLSI methods development and standardization working group best practices for evaluation of antimicrobial susceptibility tests. J. Clin. Microbio.l 2018, 56, e01934-17. [Google Scholar] [CrossRef]
- Hsieh, M.H.; Chen, M.Y.; Victor, L.Y.; Chow, J.W. Synergy assessed by checkerboard a critical analysis. Diagn. Microbiol. Infect. Dis. 1993, 16, 343–349. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of cell viability by the MTT assay. Cold Spring Harb. Protoc. 2018, 2018, prot095505. [Google Scholar] [CrossRef]
- Samarth, R.M.; Panwar, M.; Kumar, M.; Soni, A.; Kumar, M.; Kumar, A. Evaluation of antioxidant and radical-scavenging activities of certain radioprotective plant extracts. Food Chem. 2008, 106, 868–873. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
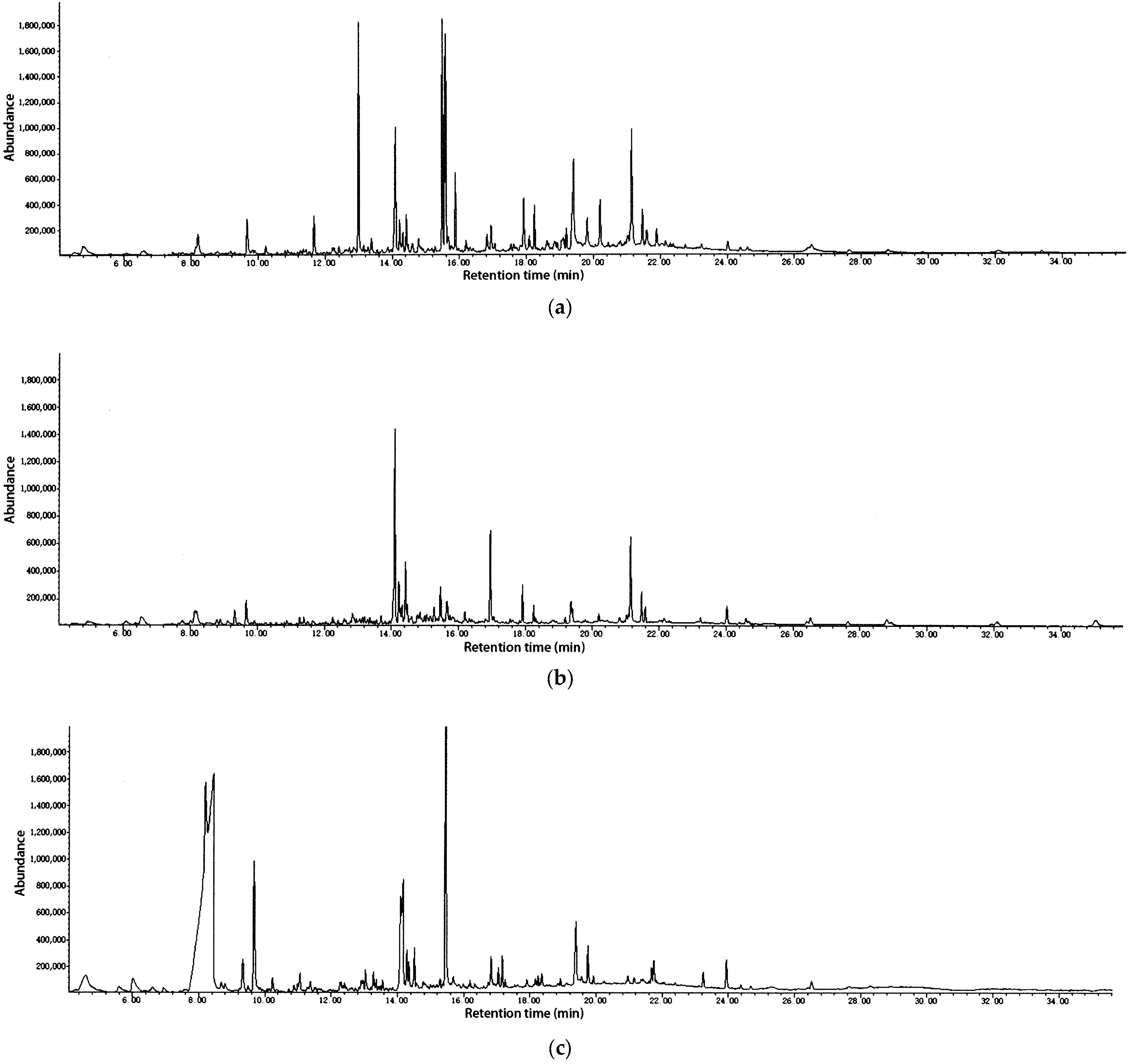

| No. | R.T. | Compound | RI a | RI b | % Leaves | % Stems | % Flowers |
|---|---|---|---|---|---|---|---|
| 1 | 4.60 | Hexanoic acid | 980 | 967 | 3.7 | ||
| 2 | 5.61 | Phenyl acetaldehyde | 1042 | 1046 | 0.6 | ||
| 3 | 4.80 | (E)-2-Octenal | 1053 | 1049 | 1.9 | ||
| 4 | 6.02 | 1-Octanol | 1067 | 1063 | 1.4 | ||
| 5 | 6.59 | Linalool | 1101 | 1095 | 1.2 | 2.6 | |
| 6 | 8.14 | α-Terpineol | 1192 | 1188 | 2.2 | ||
| 7 | 8.20 | Methyl salicylate | 1195 | 1191 | 2.2 | 2.6 | |
| 8 | 8.20 | Ethyl octanoate | 1195 | 1196 | 31.4 | ||
| 9 | 8.43 | Octanoic acid | 1209 | 1202 | 25.8 | ||
| 10 | 8.69 | 8,9-Dehydrothymol | 1224 | 1221 | 0.6 | ||
| 11 | 8.81 | Isobornyl formate | 1231 | 1235 | 0.5 | ||
| 12 | 8.90 | (Z)-3-Hexenyl isovalerate | 1236 | 1237 | 0.9 | ||
| 13 | 9.33 | (Z)-Chrysanthenyl acetate | 1261 | 1264 | 1.7 | 1.5 | |
| 14 | 9.50 | Nonanoic acid | 1271 | 1267 | 0.3 | ||
| 15 | 10.23 | (E,E)-2,4-Decadienal | 1315 | 1315 | 0.6 | 0.4 | |
| 16 | 10.89 | Dehydro-ar-ionene | 1355 | 1355 | 0.3 | ||
| 17 | 11.06 | Decanoic acid | 1366 | 1364 | 0.6 | ||
| 18 | 11.28 | 8-Hydroxylinalool | 1379 | 1367 | 0.8 | ||
| 19 | 11.38 | Butyl caprylate | 1385 | 1393 | 0.4 | ||
| 20 | 11.40 | (E)-2-Hexenyl hexanoate | 1386 | 1391 | 1.0 | ||
| 21 | 11.68 | α-Gurjunene | 1404 | 1409 | 2.0 | ||
| 22 | 12.26 | Thujopsene | 1441 | 1441 | 0.3 | ||
| 23 | 12.42 | Geranyl acetone | 1451 | 1453 | 0.4 | 0.1 | |
| 24 | 12.60 | Cabreuva oxide B | 1463 | 1464 | 0.8 | ||
| 25 | 12.85 | α-Amorphene | 1479 | 1483 | 0.8 | 2.0 | 0.4 |
| 26 | 13.00 | 1-Pentadecene | 1489 | 1489 | 10.6 | 0.6 | |
| 27 | 13.15 | Benzyl tiglate | 1499 | 1497 | 0.5 | 0.7 | |
| 28 | 13.21 | β-Guaiene | 1502 | 1502 | 0.7 | ||
| 29 | 13.29 | Tridecanal | 1508 | 1509 | 0.4 | 0.6 | |
| 30 | 13.36 | 1,1,4,5,6-Pentamethyl-Indan | 1513 | 1523 | 0.3 | ||
| 31 | 13.55 | δ-Cadinene | 1526 | 1522 | 0.3 | ||
| 32 | 13.70 | α-Copaen-11-ol | 1535 | 1539 | 0.9 | ||
| 33 | 14.10 | (E)-Nerolidol | 1562 | 1561 | 7.1 | 18.5 | |
| 34 | 14.17 | Dodecanoic acid | 1567 | 1565 | 4.4 | ||
| 35 | 14.23 | (Z)-3-Hexen-1-ol benzoate | 1571 | 1573 | 1.5 | 3.7 | |
| 36 | 14.27 | Caryophyllene oxide | 1574 | 1582 | 1.2 | ||
| 37 | 14.32 | Hexyl benzoate | 1577 | 1579 | 0.9 | 1.7 | 1.0 |
| 38 | 14.43 | (2E)-2-Hexenyl benzoate | 1585 | 1587 | 1.8 | 5.5 | |
| 39 | 14.47 | Isoaromadendrene epoxide | 1588 | 1594 | 1.8 | ||
| 40 | 14.52 | Ethyl dodecanoate | 1591 | 1594 | 1.2 | ||
| 41 | 14.61 | Viridiflorol | 1597 | 1592 | 0.8 | 1.5 | |
| 42 | 14.79 | Cedrol | 1609 | 1600 | 1.0 | 1.1 | 0.3 |
| 43 | 14.86 | (E)-Longipinocarveol | 1615 | 1618 | 1.9 | ||
| 44 | 15.01 | Junenol | 1625 | 1619 | 0.8 | ||
| 45 | 15.07 | Selin-6-en-4α-ol | 1630 | 1636 | 1.3 | ||
| 46 | 15.29 | epi-α-Cadinol | 1645 | 1640 | 0.3 | 2.0 | 0.4 |
| 47 | 15.50 | α-Cadinol | 1660 | 1665 | 10.4 | 4.9 | 10.4 |
| 48 | 15.59 | (Z)-7-Tetradecen-1-ol | 1666 | 1660 | 13.9 | ||
| 49 | 15.67 | α-Bisabolol | 1672 | 1673 | 1.2 | 3.6 | 0.8 |
| 50 | 15.76 | Cadalene | 1679 | 1676 | 0.9 | ||
| 51 | 15.89 | 1-Heptadecene | 1687 | 1687 | 3.4 | ||
| 52 | 16.20 | 1-Pentadecanal | 1710 | 1715 | 0.6 | 1.6 | 0.3 |
| 53 | 16.74 | α-Bisabolol oxide A | 1751 | 1748 | 0.2 | ||
| 54 | 16.83 | Tetradecanoic acid | 1758 | 1758 | 1.2 | 1.3 | |
| 55 | 16.95 | Benzyl Benzoate | 1767 | 1760 | 1.5 | 9.0 | |
| 56 | 17.05 | Octyl octanoate | 1774 | 1779 | 0.6 | ||
| 57 | 17.06 | (Z)-9-Hexadecenal | 1775 | 1759 | 0.4 | 0.7 | |
| 58 | 17.25 | Ethyl tetradecanoate | 1789 | 1795 | 0.2 | ||
| 59 | 17.92 | Hexahydrofarnesyl acetone | 1841 | 1845 | 3.1 | 3.0 | 0.3 |
| 60 | 18.09 | Z-9-Hexadecen-1-ol | 1854 | 1863 | 1.2 | 0.3 | |
| 61 | 18.36 | 1-Hexadecanol | 1876 | 1874 | 0.5 | ||
| 62 | 18.92 | Methyl hexadecanoate | 1920 | 1921 | 0.2 | ||
| 63 | 19.20 | Isophytol | 1943 | 1946 | 1.1 | 0.5 | |
| 64 | 19.41 | Hexadecanoic acid | 1961 | 1959 | 8.0 | 2.1 | 2.5 |
| 65 | 19.75 | Ethyl hexadecanoate | 1989 | 1992 | 1.0 | ||
| 66 | 19.82 | Panaxjapyne A | 1994 | 1994 | 2.7 | ||
| 67 | 19.92 | Hexadecyl acetate | 2003 | 2003 | 0.2 | ||
| 68 | 20.20 | Geranyl linallol | 2027 | 2020 | 2.6 | 0.6 | |
| 69 | 20.81 | Methyl linoleate | 2079 | 2085 | 0.6 | 0.7 | |
| 70 | 21.03 | γ-Palmitolactone | 2098 | 2104 | 0.8 | 0.9 | 0.3 |
| 71 | 21.15 | Phytol | 2109 | 2114 | 6.9 | 9.1 | 0.2 |
| 72 | 21.43 | Linoleic acid | 2134 | 2132 | 0.3 | ||
| 73 | 21.47 | Oleic Acid | 2138 | 2141 | 1.5 | 2.0 | |
| Total identified | 96.3 | 95.9 | 97.4 | ||||
| Microorganism | MIC (μg/mL) | MBC (μg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|
| LEO | SEO | FEO | Chl | LEO | SEO | FEO | Chl | |
| Gram positive | ||||||||
| B. subtilis ATCC 6633 | 80.00 | 40.00 | 80.00 | 2.00 | 80.00 | 80.00 | 80.00 | 2.00 |
| S. aureus ATCC 6538 | 160.00 | 80.00 | 160.00 | 2.00 | 160.00 | 160.00 | 160.00 | 16.00 |
| Gram negative | ||||||||
| E. coli ATCC 25922 | 320.00 | 160.00 | 640.00 | 8.00 | 640.00 | 320.00 | 640.00 | 32.00 |
| P. aeruginosa ATCC 27853 | 320.00 | 320.00 | 640.00 | 128.00 | 640.00 | 1280.00 | 1280.00 | 256.00 |
| Strains | Sample | MICa (μg/mL) | MICc (μg/mL) | FICI |
|---|---|---|---|---|
| Bacillus subtilis | LEO | 80.00 | 20.00 | 0.50 (S) |
| Chl | 2.00 | 0.50 | ||
| SEO | 40.00 | 10 | 0.38 (S) | |
| Chl | 2.00 | 0.25 | ||
| FEO | 80.00 | 20.00 | 0.38 (S) | |
| Chl | 2.00 | 0.25 | ||
| Staphylococcus aureus | LEO | 160.00 | 40.00 | 0.38 (S) |
| Chl | 2.00 | 0.25 | ||
| SEO | 80.00 | 20 | 0.38 (S) | |
| Chl | 2.00 | 0.25 | ||
| FEO | 160.00 | 40.00 | 0.50 (S) | |
| Chl | 2.00 | 0.50 | ||
| Escherichia coli | LEO | 320.00 | 20.00 | 0.08 (S) |
| Chl | 8.00 | 0.13 | ||
| SEO | 160.00 | 5.00 | 0.06 (S) | |
| Chl | 8.00 | 0.25 | ||
| FEO | 640.00 | 160.00 | 0.38 (S) | |
| Chl | 8.00 | 1.00 | ||
| Pseudomonas aeruginosa | LEO | 320.00 | 20.00 | 0.07 (S) |
| Chl | 128.00 | 0.50 | ||
| SEO | 320.00 | 20.00 | 0.06 (S) | |
| Chl | 128.00 | 0.25 | ||
| FEO | 640.00 | 40.00 | 0.13 (S) | |
| Chl | 128.00 | 8.00 |
| Strains | Sample | MICa (μg/mL) | MICc (μg/mL) | FICI |
|---|---|---|---|---|
| Bacillus subtilis | LEO | 80.00 | 10.00 | 0.38 (S) |
| SM | 1.00 | 0.25 | ||
| SEO | 40.00 | 5.00 | 0.26 (S) | |
| SM | 1.00 | 0.13 | ||
| FEO | 80.00 | 10.00 | 0.38 (S) | |
| SM | 1.00 | 0.25 | ||
| Staphylococcus aureus | LEO | 160.00 | 20.00 | 0.38 (S) |
| SM | 1.00 | 0.25 | ||
| SEO | 80.00 | 10.00 | 0.26 (S) | |
| SM | 1.00 | 0.13 | ||
| FEO | 160.00 | 40.00 | 0.50 (S) | |
| SM | 1.00 | 0.25 | ||
| Escherichia coli | LEO | 320.00 | 10.00 | 0.10 (S) |
| SM | 2.00 | 0.13 | ||
| SEO | 160.00 | 2.50 | 0.08 (S) | |
| SM | 2.00 | 0.13 | ||
| FEO | 640.00 | 40.00 | 0.31 (S) | |
| SM | 2.00 | 0.50 | ||
| Pseudomonas aeruginosa | LEO | 320.00 | 20.00 | 0.13 (S) |
| SM | 4.00 | 0.25 | ||
| SEO | 320.00 | 20.00 | 0.10 (S) | |
| SM | 4.00 | 0.13 | ||
| FEO | 640.00 | 20.00 | 0.09 (S) | |
| SM | 4.00 | 0.25 |
| A549 | MCF-7 | HepG2 | HCT-116 | HL-7702 | |
|---|---|---|---|---|---|
| LEO | 49.51 ± 2.25 | 44.25 ± 5.74 | 66.55 ± 0.32 | 59.60 ± 1.50 | 49.15 ± 2.97 |
| LEO-SI | 0.99 | 1.11 | 0.74 | 0.82 | |
| SEO | 43.91 ± 1.36 | 37.91 ± 2.10 | 51.15 ± 6.42 | 44.76 ± 4.49 | 49.01 ± 1.46 |
| SEO-SI | 1.12 | 1.30 | 0.96 | 1.10 | |
| FEO | 61.57 ± 2.91 | 127.93 ± 9.66 | 79.80 ± 3.01 | 90.10 ± 1.22 | 38.53 ± 0.55 |
| FEO-SI | 0.63 | 0.30 | 0.48 | 0.43 | |
| Doxorubicin | 1.47 ± 0.09 | 1.36 ± 0.03 | 1.87 ± 0.13 | 0.99 ± 0.04 | 0.80 ± 0.04 |
| Test Sample | DPPH IC50 (μg/mL) | ABTS IC50 (μg/mL) | FRAP (μmol Trolox·g−1) |
|---|---|---|---|
| LEO | (1.13 ± 0.12) × 103 | (0.29 ± 0.03) × 103 | 97.66 ± 11.27 |
| SEO | (1.38 ± 0.16) × 103 | (0.38 ± 0.05) × 103 | 79.52 ± 8.32 |
| FEO | (1.43 ± 0.37) × 103 | (0.69 ± 0.06) × 103 | 55.36 ± 6.38 |
| BHT * | 6.18 ± 0.39 | 4.16 ± 0.25 | |
| Trolox * | 5.19 ± 0.56 | 1.68 ± 0.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, S.; Chen, Y.; Chen, H.; Han, Q.; Lai, P. Chemical Composition and Biological Activities of the Essential Oils from Different Parts of Rosa bracteata J.C.Wendl. Molecules 2025, 30, 4021. https://doi.org/10.3390/molecules30194021
Song S, Chen Y, Chen H, Han Q, Lai P. Chemical Composition and Biological Activities of the Essential Oils from Different Parts of Rosa bracteata J.C.Wendl. Molecules. 2025; 30(19):4021. https://doi.org/10.3390/molecules30194021
Chicago/Turabian StyleSong, Shiyu, Yifang Chen, Hongrui Chen, Qinglei Han, and Pengxiang Lai. 2025. "Chemical Composition and Biological Activities of the Essential Oils from Different Parts of Rosa bracteata J.C.Wendl" Molecules 30, no. 19: 4021. https://doi.org/10.3390/molecules30194021
APA StyleSong, S., Chen, Y., Chen, H., Han, Q., & Lai, P. (2025). Chemical Composition and Biological Activities of the Essential Oils from Different Parts of Rosa bracteata J.C.Wendl. Molecules, 30(19), 4021. https://doi.org/10.3390/molecules30194021







