Spray-Dried Phenolic Compounds from Olive Mill Waste Water as Animal Feed Supplement: Impact on the Aromatic Profile of “Caciotta Cheese”
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Composition of Cheese
2.2. Spray-Dried Olive Mill Wastewater Phenols Composition
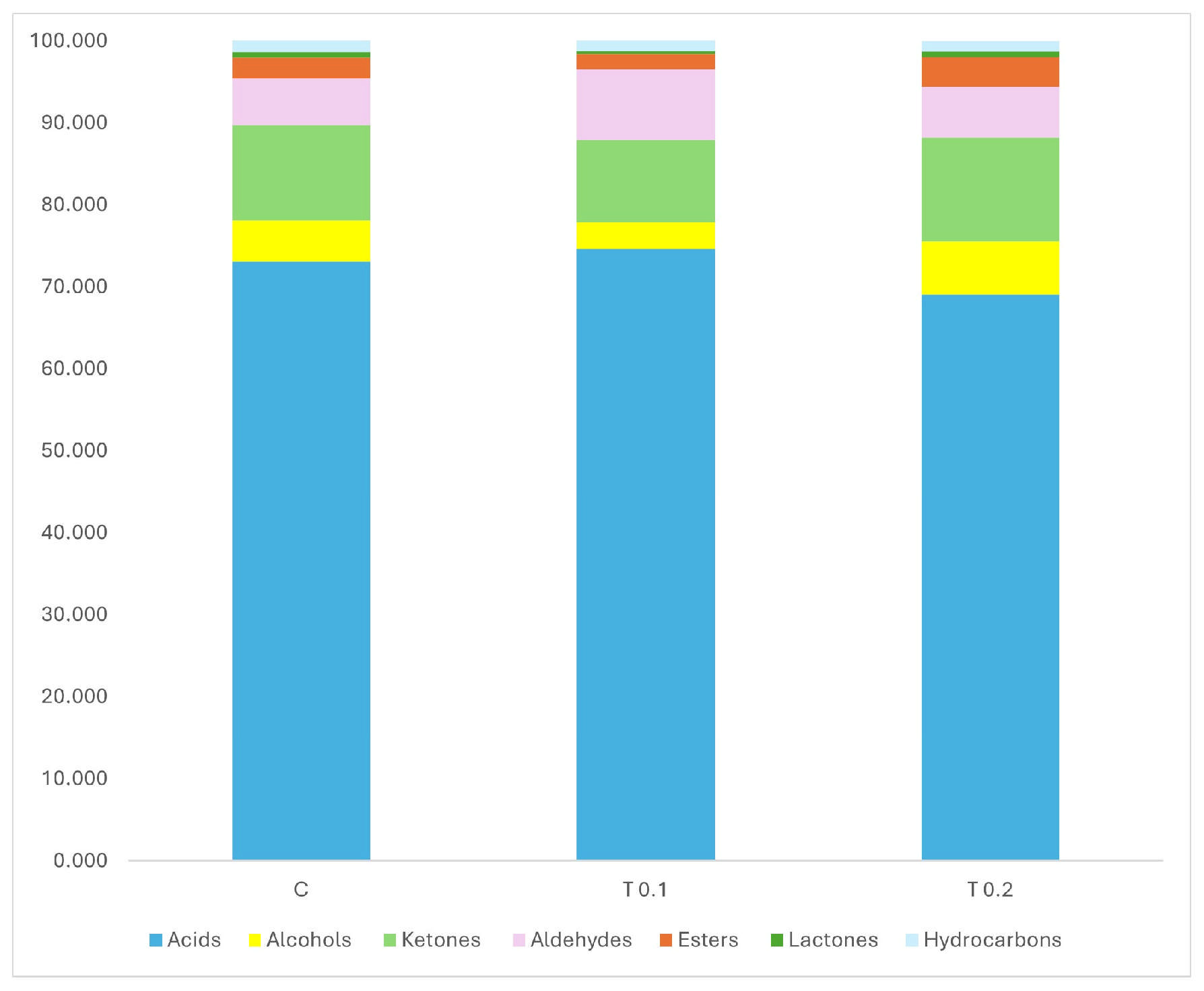
2.3. Volatile Profiles of Caciotta Cheese
2.4. Influence of Dietary Polyphenols on Odour Impact Ratio Values (OIRs) and Identification of Key Volatile Compounds in Caciotta Cheese
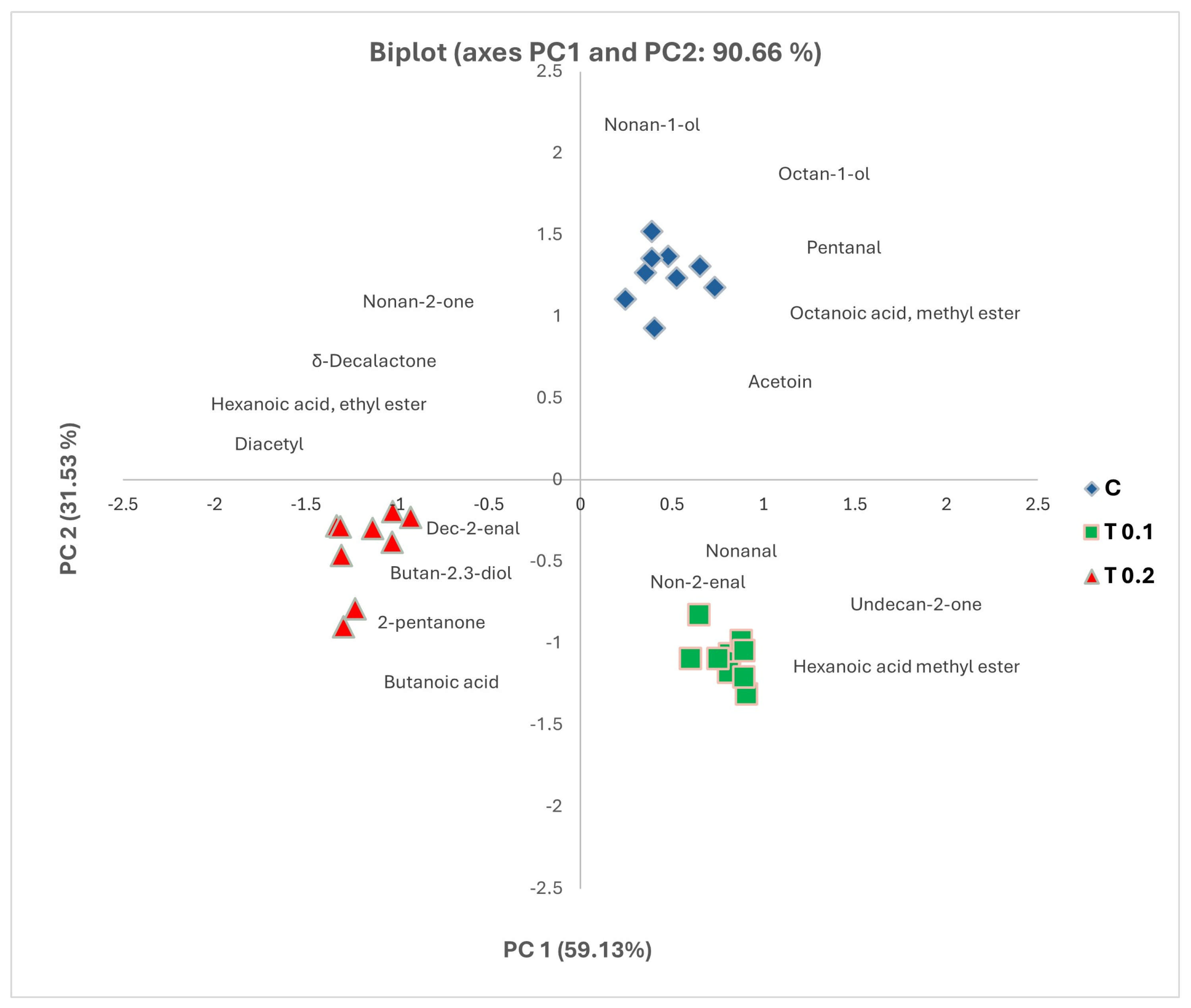
2.5. Principal Component Analysis
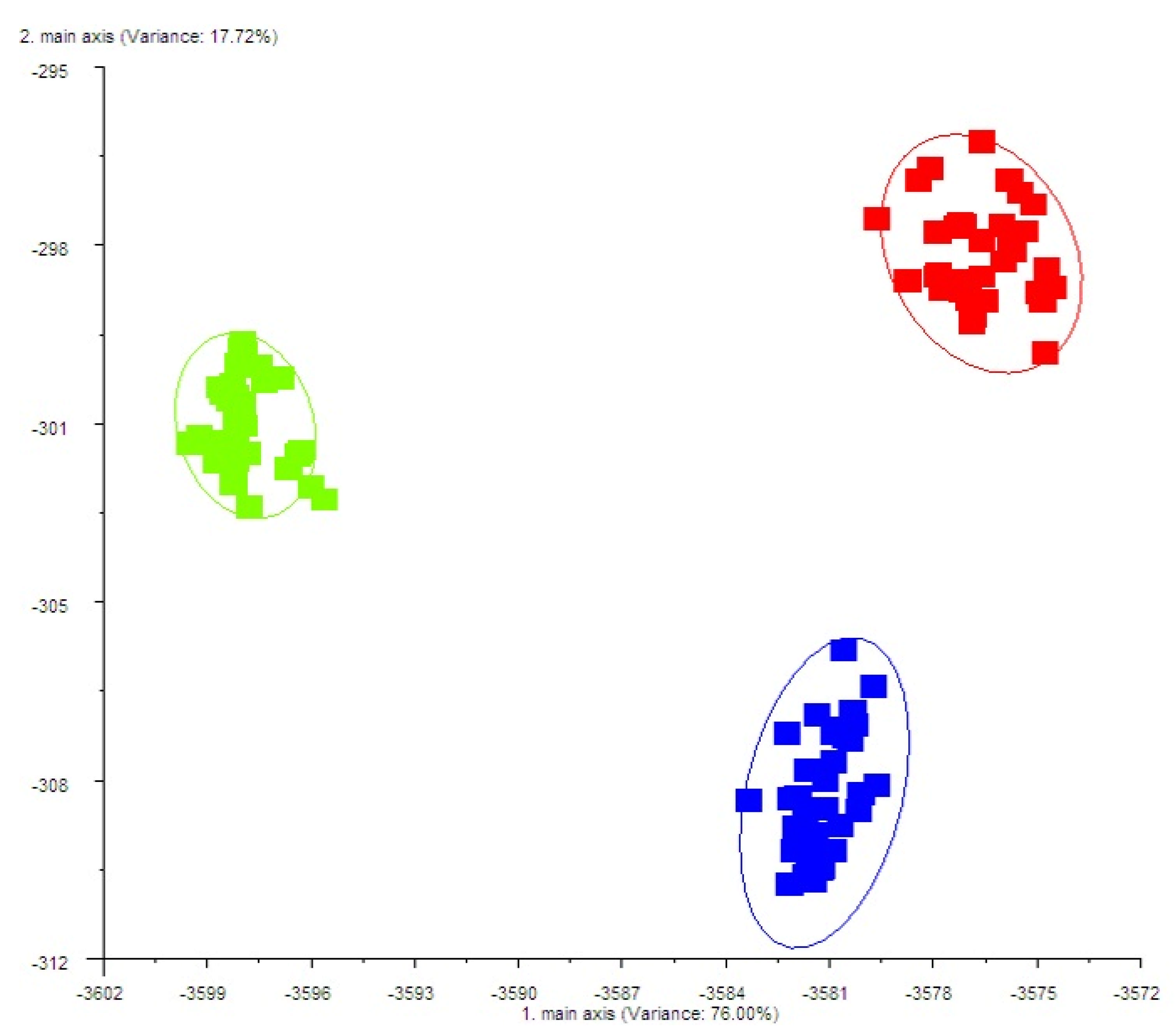
2.6. E-Nose Analysis
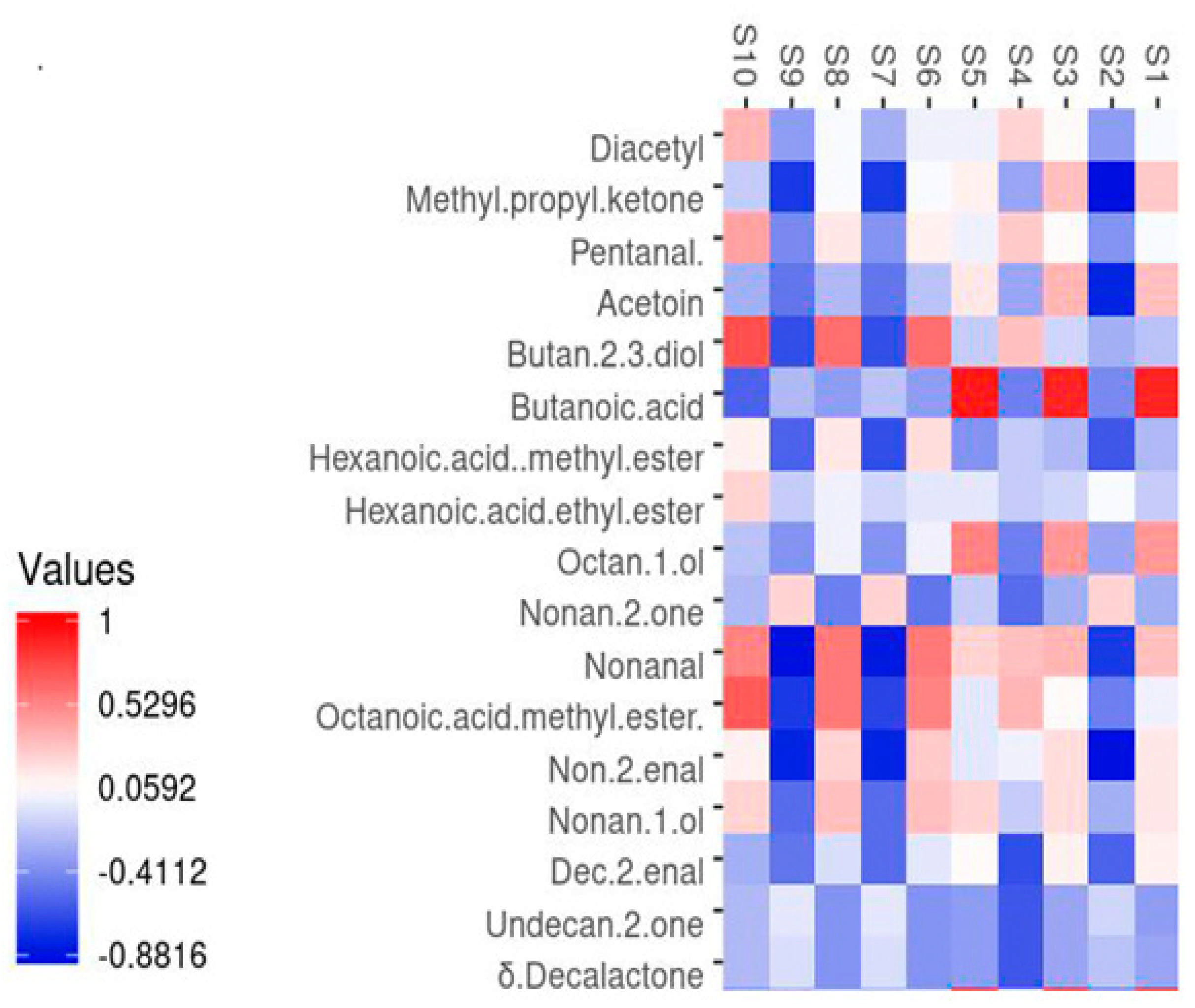
2.7. Correlation Between Key Volatile Compounds and Intelligent Sensory Signals
3. Materials and Methods
3.1. Experimental Design
3.2. Chemical Analysis of Feed and Cheese
3.3. Spray-Dried Olive Mill Wastewater Phenols Analysis
3.4. Cheese Making
3.5. Headspace-Solid Phase Microextraction-Gas Chromatography–Mass Spectrometry
3.6. E-Nose Analysis
3.7. Calculation of Odour Impact Ratio Values (OIRs)
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Saini, R.K.; Khan, M.I.; Kumar, V.; Shang, X.; Lee, J.-H.; Ko, E.-Y. Bioactive Compounds of Agro-Industrial By-Products: Current Trends, Recovery, and Possible Utilization. Antioxidants 2025, 14, 650. [Google Scholar] [CrossRef]
- Cuffaro, D.; Bertolini, A.; Bertini, S.; Ricci, C.; Cascone, M.G.; Danti, S.; Saba, A.; Macchia, M.; Digiacomo, M. Olive Mill Wastewater as Source of Polyphenols with Nutraceutical Properties. Nutrients 2023, 15, 3746. [Google Scholar] [CrossRef]
- Gerasopoulos, K.; Stagos, D.; Kokkas, S.; Petrotos, K.; Kantas, D.; Goulas, P.; Kouretas, D. Feed Supplemented with Byproducts from Olive Oil Mill Wastewater Processing Increases Antioxidant Capacity in Broiler Chickens. Food Chem. Toxicol. 2015, 82, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Albini, A.; Corradino, P.; Morelli, D.; Albini, F.; Noonan, D. Cosmeceutical and Dermatological Potential of Olive Mill Wastewater: A Sustainable and Eco-Friendly Source of Natural Ingredients. Cosmetics 2025, 12, 142. [Google Scholar] [CrossRef]
- Benincasa, C.; Pellegrino, M.; Romano, E.; Claps, S.; Fallara, C.; Perri, E. Qualitative and Quantitative Analysis of Phenolic Compounds in Spray-Dried Olive Mill Wastewater. Front. Nutr. 2022, 8, 782693. [Google Scholar] [CrossRef] [PubMed]
- Cifuni, G.F.; Claps, S.; Morone, G.; Sepe, L.; Caparra, P.; Benincasa, C.; Pellegrino, M.; Perri, E. Valorization of Olive Mill Byproducts: Recovery of Biophenol Compounds and Application in Animal Feed. Plants 2023, 12, 3062. [Google Scholar] [CrossRef]
- Tutino, V.; Caruso, M.G.; Messa, C.; Perri, E.; Notarnicola, M. Antiproliferative, antioxidant and anti-inflammatory effects of hydroxytyrosol on human hepatoma HepG2 and Hep3B cell lines. Anticancer Res. 2012, 32, 5371–5377. [Google Scholar]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.M. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr. Cancer Drug Targets 2008, 8, 634–646. [Google Scholar] [CrossRef]
- Fuccelli, R.; Fabiani, R.; Rosignoli, P. Hydroxytyrosol exerts anti-inflammatory and anti-oxidant activities in a mouse model of systemic inflammation. Molecules 2018, 23, 3212. [Google Scholar] [CrossRef]
- Batarfi, W.A.; Yunus, M.H.M.; Hamid, A.A.; Lee, Y.T.; Maarof, M. Hydroxytyrosol: A Promising Therapeutic Agent for Mitigating Inflammation and Apoptosis. Pharmaceutics 2024, 16, 1504. [Google Scholar] [CrossRef]
- Correddu, F.; Lunesu, M.F.; Buffa, G.; Atzori, A.S.; Nudda, A.; Battacone, G.; Pulina, G. Can Agro-Industrial By-Products Rich in Polyphenols be Advantageously Used in the Feeding and Nutrition of Dairy Small Ruminants? Animals 2020, 10, 131. [Google Scholar] [CrossRef]
- Scerra, M.; Foti, F.; Caparra, P.; Cilione, C.; Bognanno, M.; Paolo, F.; Paolo, D.C.; Natalello, A.; Musati, M.; Chies, L. Effects of Feeding Bergamot Pulp and Olive Leaves on Performance and Meat Quality in Apulo-Calabrese Pigs. Vet. Anim. Sci. 2024, 23, 100336. [Google Scholar] [CrossRef] [PubMed]
- Scerra, M.; Bognanno, M.; Foti, F.; Caparra, P.; Cilione, C.; De Caria, P.; Fortugno, P.; Luciano, G.; Natalello, A.; Chies, L. Effect of High Levels of Almond Hulls Supplementation on Performance and Meat Oxidative Stability in Lambs. Meat Sci. 2023, 205, 109295. [Google Scholar] [CrossRef] [PubMed]
- Tufarelli, V.; Laudadio, V.; Casalino, E. An extra-virgin olive oil rich in polyphenolic compounds has antioxidant effects in meat-type broiler chickens. Environ. Sci. Pollut. Res. 2016, 23, 6197–6204. [Google Scholar] [CrossRef] [PubMed]
- Dal Bosco, A.; Mourvaki, E.; Cardinali, R.; Servili, M.; Sebastiani, B.; Ruggeri, S.; Mattioli, S.; Taticchi, A.; Esposto, S.; Castellini, C. Effect of dietary supplementation with olive pomaces on the performance and meat quality of growing rabbits. Meat Sci. 2012, 92, 783–788. [Google Scholar] [CrossRef]
- De Caria, P.; Chies, L.; Cifuni, G.F.; Scerra, M.; Foti, F.; Cilione, C.; Fortugno, P.; Boninsegna, M.A.; Giacondino, C.; Claps, S.; et al. The Effects of Olive Cake and Linseed Dietary Supplementation on the Performance, Carcass Traits, and Oxidative Stability of Beef from Young Podolian Bulls. Animals 2025, 15, 2188. [Google Scholar] [CrossRef]
- Caparra, P.; Chies, L.; Scerra, M.; Foti, F.; Bognanno, M.; Cilione, C.; De Caria, P.; Claps, S.; Cifuni, G.F. Effect of Dietary Ensiled Olive Cake Supplementation on Performance and Meat Quality of Apulo-Calabrese Pigs. Animals 2023, 13, 2022. [Google Scholar] [CrossRef]
- Scerra, M.; Rao, R.; Foti, F.; Caparra, P.; Cilione, C.; Natalello, A.; Biondi, L.; Bella, M.S.; Chies, L. Influence of Dietary Inclusion of Exhausted Bergamot By-Product in Pigs on Animal Performance, Fatty Acid Profile and Oxidative Stability of Meat and Meat Products. Animals 2022, 12, 757. [Google Scholar] [CrossRef]
- Castellani, F.; Vitali, A.; Bernardi, N.; Marone, E.; Palazzo, F.; Grotta, L.; Martino, G. Dietary Supplementation with Dried Olive Pomace in Dairy Cows Modifies the Composition of Fatty Acids and the Aromatic Profile in Milk and Related Cheese. J. Dairy Sci. 2017, 100, 8658–8669. [Google Scholar] [CrossRef]
- Kazemi, M. Recycling Agricultural Waste: Sustainable Solutions for Enhancing Livestock Nutrition. Vet. Med. Sci. 2025, 11, e70321. [Google Scholar] [CrossRef]
- Ianni, A.; Bennato, F.; Martino, C.; Grotta, L.; Martino, G. Volatile Flavor Compounds in Cheese as Affected by Ruminant Diet. Molecules 2020, 25, 461. [Google Scholar] [CrossRef]
- Branciari, R.; Galarini, R.; Miraglia, D.; Ranucci, D.; Valiani, A.; Giusepponi, D.; Servili, M.; Acuti, G.; Pauselli, M.; Trabalza-Marinucci, M. Dietary Supplementation with Olive Mill Wastewater in Dairy Sheep: Evaluation of Cheese Characteristics and Presence of Bioactive Molecules. Animals 2020, 10, 1941. [Google Scholar] [CrossRef]
- Ianni, A.; Innosa, D.; Martino, C.; Bennato, F.; Martino, G. Short Communication: Compositional Characteristics and Aromatic Profile of Caciotta Cheese Obtained from Friesian Cows Fed with a Dietary Supplementation of Dried Grape Pomace. J. Dairy Sci. 2019, 102, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Ianni, A.; Martino, G. Dietary Grape Pomace Supplementation in Dairy Cows: Effect on Nutritional Quality of Milk and Its Derived Dairy Products. Foods 2020, 9, 168. [Google Scholar] [CrossRef] [PubMed]
- Bertuzzi, A.S.; McSweeney, P.L.; Rea, M.C.; Kilcawley, K.N. Detection of volatile compounds of cheese and their contribution to the flavor profile of surface-ripened cheese. Compr. Rev. Food Sci. Food Saf. 2018, 17, 371–390. [Google Scholar] [CrossRef] [PubMed]
- Curioni, P.M.G.; Bosset, J.O. Key Odorants in Various Cheese Types as Determined by Gas Chromatography-Olfactometry. Int. Dairy J. 2002, 12, 959–984. [Google Scholar] [CrossRef]
- Silva, L.F.; Sunakozawa, T.N.; Monteiro, D.A.; Casella, T.; Conti, A.C.; Todorov, S.D.; Barretto Penna, A.L. Potential of Cheese-Associated Lactic Acid Bacteria to Metabolize Citrate and Produce Organic Acids and Acetoin. Metabolites 2023, 13, 1134. [Google Scholar] [CrossRef]
- McSweeney, P.L.H.; Sousa, M.J. Biochemical Pathways for the Production of Flavour Compounds in Cheeses during Ripening: A Review. Lait 2000, 80, 293–324. [Google Scholar] [CrossRef]
- Barbaccia, P.; Francesca, N.; Gerlando, R.D.; Busetta, G.; Moschetti, G.; Gaglio, R.; Settanni, L. Biodiversity and dairy traits of indigenous milk lactic acid bacteria grown in presence of the main grape polyphenols. FEMS Microbiol. Lett. 2020, 367, fnaa066. [Google Scholar] [CrossRef]
- Guillén, M.D.; Ibargoitia, M.L.; Sopelana, P.; Palencia, G.; Fresno, M. Components Detected by Means of Solid-Phase Microextraction and Gas Chromatography/Mass Spectrometry in the Headspace of Artisan Fresh Goat Cheese Smoked by Traditional Methods. J. Dairy Sci. 2004, 87, 284–299. [Google Scholar] [CrossRef]
- Innosa, D.; Ianni, A.; Faccia, M.; Martino, C.; Grotta, L.; Saletti, M.A.; Pomilio, F.; Martino, G. Physical, Nutritional, and Sensory Properties of Cheese Obtained from Goats Fed a Dietary Supplementation with Olive Leaves. Animals 2020, 10, 2238. [Google Scholar] [CrossRef]
- Castillo, I.; Calvo, M.V.; Alonso, L.; Juárez, M.; Fontecha, J. Changes in Lipolysis and Volatile Fraction of a Goat Cheese Manufactured Employing a Hygienized Rennet Paste and a Defined Strain Starter. Food Chem. 2007, 100, 590–598. [Google Scholar] [CrossRef]
- Bennato, F.; Ianni, A.; Innosa, D.; Martino, C.; Grotta, L.; Pomilio, F.; Verna, M.; Martino, G. Influence of Licorice Root Feeding on Chemical-Nutritional Quality of Cow Milk and Stracciata Cheese, an Italian Traditional Fresh Dairy Product. Animals 2019, 9, 1153. [Google Scholar] [CrossRef] [PubMed]
- Holland, R.; Liu, S.-Q.; Crow, V.L.; Delabre, M.-L.; Lubbers, M.; Bennett, M.; Norris, G. Esterases of Lactic Acid Bacteria and Cheese Flavour: Milk Fat Hydrolysis, Alcoholysis and Esterification. Int. Dairy J. 2005, 15, 711–718. [Google Scholar] [CrossRef]
- Qian, M.; Reineccius, G. Identification of Aroma Compounds in Parmigiano-Reggiano Cheese by Gas Chromatography/Olfactometry. J. Dairy Sci. 2002, 85, 1362–1369. [Google Scholar] [CrossRef]
- McSweeney, P.L.H. Biochemistry of Cheese Ripening. Int. J. Dairy Technol. 2004, 57, 127–144. [Google Scholar] [CrossRef]
- Fox, P.F.; Guinee, T.P.; Cogan, T.M.; McSweeney, P.L.H. Fundamentals of Cheese Science; Springer: Boston, MA, USA, 2017. [Google Scholar] [CrossRef]
- Starowicz, M. Analysis of Volatiles in Food Products. Separations 2021, 8, 157. [Google Scholar] [CrossRef]
- Buzek, J.; Chastel, O. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes Text with EEA Relevance. Off. J. Eur. Union 2010, 276, 33–79. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32010L0063&from=IT (accessed on 25 July 2025).
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis, 20th ed.; AOAC International: Gaithersburg, MD, USA, 2016. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Gray, I.K.; Rumsby, M.G.; Hawke, J.C. The variations in linolenic acid and galactolipid levels in Graminaceae species with age of tissue and light environment. Phytochemistry 1967, 6, 107–113. [Google Scholar] [CrossRef]
- Santamarina-García, G.; Amores, G.; Hernández, I.; Morán, L.; Barrón, L.J.R.; Virto, M. Relationship between the Dynamics of Volatile Aroma Compounds and Microbial Succession during the Ripening of Raw Ewe Milk-Derived Idiazabal Cheese. Curr. Res. Food Sci. 2023, 6, 100425. [Google Scholar] [CrossRef]
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients, 4th ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
| Diets 1 | |||||
|---|---|---|---|---|---|
| C | T0.1 | T0.2 | SEM 2 | p | |
| Dry matter (%) | 47.465 | 47.506 | 47.540 | 0.044 | 0.498 |
| Protein (%) | 20.527 | 20.861 | 20.887 | 0.038 | 0.839 |
| Fat (%) | 21.955 | 21.853 | 21.962 | 0.042 | 0.207 |
| Ash (%) | 2.460 | 2.442 | 2.526 | 0.089 | 0.788 |
| Diets 1 | ||||||
|---|---|---|---|---|---|---|
| C | T0.1 | T0.2 | SEM 2 | p | ||
| R.T. 3 | ||||||
| 1.467 | Ethanol | 0.807 b | 1.019 ab | 1.372 a | 0.098 | 0.018 |
| 1.547 | Acetone | 0.436 B | 1.094 A | 1.130 A | 0.038 | 0.0001 |
| 2.107 | 2,3-Butanedione | 2.366 B | 1.641 B | 4.062 A | 0.192 | 0.0003 |
| 2.429 | Acetic acid | 13.508 | 9.689 | 12.175 | 1.093 | ns |
| 3.067 | 2-Pentanone | 0.688 b | 1.110 ab | 1.570 a | 0.161 | 0.024 |
| 3.123 | Pentanal | 1.771 A | 1.332 B | 1.015 B | 0.081 | 0.0018 |
| 3.418 | 2-Butanone, 3-hydroxy | 5.933 A | 4.359 B | 3.119 C | 0.277 | 0.0011 |
| 5.47 | Cyclobutanol | 0.597 A | 0.097 B | 0.082 B | 0.025 | 0.0001 |
| 5.827 | Octane | 0.729 | 0.828 | 0.612 | 0.066 | ns |
| 5.949 | Hexanal | 0.759 | 0.583 | 0.762 | 0.075 | ns |
| 6.698 | 2,3-Butanediol | 0.786 B | 1.004 B | 1.825 A | 0.134 | 0.0036 |
| 8.567 | Butanoic acid | 13.460 B | 19.339 A | 19.120 A | 0.708 | 0.0017 |
| 10.041 | 2-Heptanone | 0.813 | 0.997 | 0.800 | 0.099 | ns |
| 10.381 | Heptanal | 0.743 | 0.704 | 0.579 | 0.054 | ns |
| 11.451 | Hexanoic acid, methyl ester | 0.090 B | 0.422 A | 0.087 B | 0.039 | 0.0013 |
| 14.237 | Hexanoic acid, ethyl ester | 1.535 B | 0.716 C | 2.939 A | 0.079 | 0.0001 |
| 14.445 | Octanal | 0.785 | 0.911 | 1.235 | 0.195 | ns |
| 15.627 | Hexanoic acid | 19.633 | 20.184 | 19.658 | 0.791 | ns |
| 16.919 | 1-Octanol | 0.783 A | 0.482 B | 0.311 B | 0.052 | 0.0020 |
| 17.585 | 2-Nonanone | 1.151 A | 0.685 B | 1.289 A | 0.068 | 0.0019 |
| 17.956 | Nonanal | 0.533 B | 3.814 A | 0.488 B | 0.126 | 0.0001 |
| 18.522 | Octanoic acid, methyl ester | 0.776 A | 0.680 A | 0.060 B | 0.049 | 0.0001 |
| 19.777 | 2-Nonenal | 0.107 B | 0.727 A | 0.127 B | 0.094 | 0.0054 |
| 20.279 | 1-Nonanol | 0.290 a | 0.058 b | 0.131 ab | 0.038 | 0.0130 |
| 20.851 | Octanoic acid, ethyl ester | 0.174 B | 0.064 B | 0.550 A | 0.039 | 0.0001 |
| 21.234 | Octanoic Acid | 6.162 | 5.180 | 6.858 | 0.478 | ns |
| 22.797 | 2-Decenal | 0.359 B | 0.207 B | 1.568 A | 0.053 | 0.0001 |
| 23.485 | Nonanoic acid | 0.296 B | 0.146 C | 0.647 A | 0.024 | 0.0001 |
| 23.672 | 2-Undecanone | 0.575 B | 0.986 A | 0.139 C | 0.012 | <0.0001 |
| 26.605 | Decanoic acid | 2.267 B | 2.014 B | 2.874 A | 0.062 | 0.0002 |
| 26.75 | Dodecanal | 0.448 A | 0.214 B | 0.248 B | 0.020 | 0.0004 |
| 28.462 | 1-Dodecanol | 0.347 ab | 0.223 b | 0.424 a | 0.043 | 0.046 |
| 28.943 | Hexadecane | 0.266 ab | 0.145 b | 0.315 a | 0.032 | 0.026 |
| 29.155 | δ-Decalactone | 0.304 B | 0.207 C | 0.442 A | 0.012 | 0.0001 |
| 29.739 | Nonadecane | 0.067 B | 0.040 C | 0.214 A | 0.006 | <0.0001 |
| 31.063 | Dodecanoic acid | 1.134 A | 0.517 B | 0.948 A | 0.069 | 0.002 |
| 31.315 | Tridecane | 0.184 A | 0.085 B | 0.079 B | 0.006 | 0.0001 |
| 31.449 | Diethyl phthalate | 0.132 ab | 0.156 a | 0.077 b | 0.014 | 0.0208 |
| 31.703 | Pentadecanal | 0.144 b | 0.178 ab | 0.214 a | 0.014 | 0.0385 |
| 34.053 | δ-Nonalactone | 0.352 a | 0.088 b | 0.217 ab | 0.038 | 0.0078 |
| 35.398 | Tetradecanoic acid | 2.654 A | 1.485 B | 2.256 A | 0.095 | 0.0004 |
| 39.408 | Hexadecanoic acid | 12.488 b | 14.244 ab | 15.558 a | 0.520 | 0.016 |
| 41.272 | 1-Octadecanol | 1.373 B | 0.353 C | 2.335 A | 0.049 | 0.0001 |
| 42.689 | Oleic Acid | 0.513 | 0.477 | 0.516 | 0.029 | ns |
| 43.144 | Octadecanoic acid | 0.682 | 0.516 | 0.518 | 0.117 | ns |
| Odour Threshold | OIR | Odour Descriptors | |||
|---|---|---|---|---|---|
| (μg/kg) | C 1 | T0.1 | T0.2 | ||
| 2-Pentanone | 0.5 | 1.376 | 2.220 | 3.140 | Sweety, fruity |
| Pentanal | 0.13 | 13.623 | 10.246 | 7.808 | Chemical. pungent |
| 2-Butanone 3-hydroxy | 0.14 | 42.379 | 31.136 | 22.279 | Butter like. Sour milk |
| 2,3-Butanediol | 1 | 0.786 | 1.004 | 1.825 | Buttery, creamy |
| Butanoic acid | 18 | 0.748 | 1.074 | 1.062 | Strong cheese unpleasant |
| Hexanoic acid. methyl ester | 0.075 | 1.200 | 5.627 | 1.160 | Fruity, cheese |
| 1-Octanol | 0.5 | 1.566 | 0.964 | 0.622 | Fat, metal |
| 2-Nonanone | 0.5 | 2.302 | 1.370 | 2.578 | Fruity pleasant |
| Nonanal | 0.22 | 2.423 | 17.336 | 2.218 | Tallow, animal |
| Octanoic acid. methyl ester | 0.5 | 1.552 | 1.360 | 0.120 | Wax sweet |
| 2-Nonenal | 0.045 | 2.378 | 16.156 | 2.822 | Green |
| 1-Nonanol | 0.08 | 3.625 | 0.725 | 1.638 | citrus |
| 2-Decenal. (E)- | 0.092 | 3.902 | 2.250 | 17.043 | Pungent |
| 2-Undecanone | 0.5 | 1.150 | 1.972 | 0.278 | Floreal |
| δ-Decalactone | 0.064 | 4.750 | 3.234 | 6.906 | Peachy, coconut |
| Diets 1 | |||
|---|---|---|---|
| C | T0.1 | T0.2 | |
| Polyphite Hay | 60 | 60 | 60 |
| Maize grain | 27 | 27 | 27 |
| Wheat bran | 16.2 | 16.1 | 16 |
| Hulled soybean flour | 14 | 14 | 14 |
| Maize dried distillers’ grain | 14 | 14 | 14 |
| Distiller’s Dried Grains with Soluble | 14 | 14 | 14 |
| Hulled sunflower seed flour | 13.6 | 13.6 | 13.6 |
| Barley grain | 0.2 | 0.2 | 0.2 |
| Molasses | 0.2 | 0.2 | 0.2 |
| Dried beet pulp | 0.2 | 0.2 | 0.2 |
| Spray-dried olive mill wastewater phenolics | - | 0.1 | 0.2 |
| Vitamin-mineral supplement | 0.6 | 0.6 | 0.6 |
| Chemical Composition | |||
| Dry matter | 88.827 | 88.829 | 88.831 |
| Crude protein | 15.71 | 15.70 | 15.70 |
| Ether extract | 3.00 | 3.00 | 3.00 |
| Fibre | 27.01 | 27.00 | 27.00 |
| Ash | 7.293 | 7.295 | 7.297 |
| NDF | 51.20 | 51.19 | 51.17 |
| ADF | 6.03 | 6.02 | 6.02 |
| ADL | 4.44 | 4.43 | 4.43 |
| UFL (Kg/S.S.) | 0.84 | 0.84 | 0.84 |
| Fatty acids (g/100 g of fatty acids) | |||
| C16:0 | 11.64 | 11.64 | 11.64 |
| C18:0 | 21.69 | 21.69 | 21.69 |
| C18:1 n-9 | 10.53 | 10.55 | 10.56 |
| C18:2 n-6 | 28.15 | 28.13 | 28.12 |
| C18:3 n-3 | 7.00 | 7.00 | 7.00 |
| Others | 20.98 | 20.98 | 20.98 |
| Sensor Number | Sensor Name 1 | Sensor Sensitives | Detection Limits |
|---|---|---|---|
| 1 | W1C | Aromatic organic compounds | Toluene, 10 mg kg−1 |
| 2 | W5S | Very sensitive, broad range sensitivity, reacts to nitrogen oxides, very sensitive with negative signal | NO2, 1 mg kg−1 |
| 3 | W3C | Ammonia, also used as sensor for aromatic compounds | Benzene, 10 mg kg−1 |
| 4 | W6S | Detects mainly hydrogen gas | H2, 0.1 mg kg−1 |
| 5 | W5C | Alkanes, aromatic compounds, and nonpolar organic compounds | Propane, 1 mg kg−1 |
| 6 | W1S | Sensitive to methane, broad range of organic compounds detected | CH3, 100 mg kg−1 |
| 7 | W1W | Detects inorganic sulphur compounds, e.g., H2S. Also sensitive to many terpenes and sulfur-containing organic compounds | H2S, 1 mg kg−1 |
| 8 | W2S | Detects alcohol, partially sensitive to aromatic compounds, broad range | CO, 100 mg kg−1 |
| 9 | W2W | Aromatic compounds, inorganic sulphur and organic compounds | H2S, 1 mg kg−1 |
| 10 | W3S | Reacts to high concentrations (>100 mg kg−1) of methane and aliphatic organic compounds | Not determined |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cifuni, G.F.; Caparra, P.; Perri, E.; Benincasa, C.; Morone, G.; Claps, S. Spray-Dried Phenolic Compounds from Olive Mill Waste Water as Animal Feed Supplement: Impact on the Aromatic Profile of “Caciotta Cheese”. Molecules 2025, 30, 3991. https://doi.org/10.3390/molecules30193991
Cifuni GF, Caparra P, Perri E, Benincasa C, Morone G, Claps S. Spray-Dried Phenolic Compounds from Olive Mill Waste Water as Animal Feed Supplement: Impact on the Aromatic Profile of “Caciotta Cheese”. Molecules. 2025; 30(19):3991. https://doi.org/10.3390/molecules30193991
Chicago/Turabian StyleCifuni, Giulia Francesca, Pasquale Caparra, Enzo Perri, Cinzia Benincasa, Giuseppe Morone, and Salvatore Claps. 2025. "Spray-Dried Phenolic Compounds from Olive Mill Waste Water as Animal Feed Supplement: Impact on the Aromatic Profile of “Caciotta Cheese”" Molecules 30, no. 19: 3991. https://doi.org/10.3390/molecules30193991
APA StyleCifuni, G. F., Caparra, P., Perri, E., Benincasa, C., Morone, G., & Claps, S. (2025). Spray-Dried Phenolic Compounds from Olive Mill Waste Water as Animal Feed Supplement: Impact on the Aromatic Profile of “Caciotta Cheese”. Molecules, 30(19), 3991. https://doi.org/10.3390/molecules30193991








