Application of HPSEC Technique and In Silico Analysis in the Evaluation of Bioactive Peptides and Polysaccharide Profile in Wort Supplemented with Malted and Unmalted Hemp Seeds
Abstract
1. Introduction
2. Results and Discussion
2.1. Bioactive Peptides in Wort Depending on Grist Composition
2.1.1. HPSEC Analysis
2.1.2. In Silico Analyses
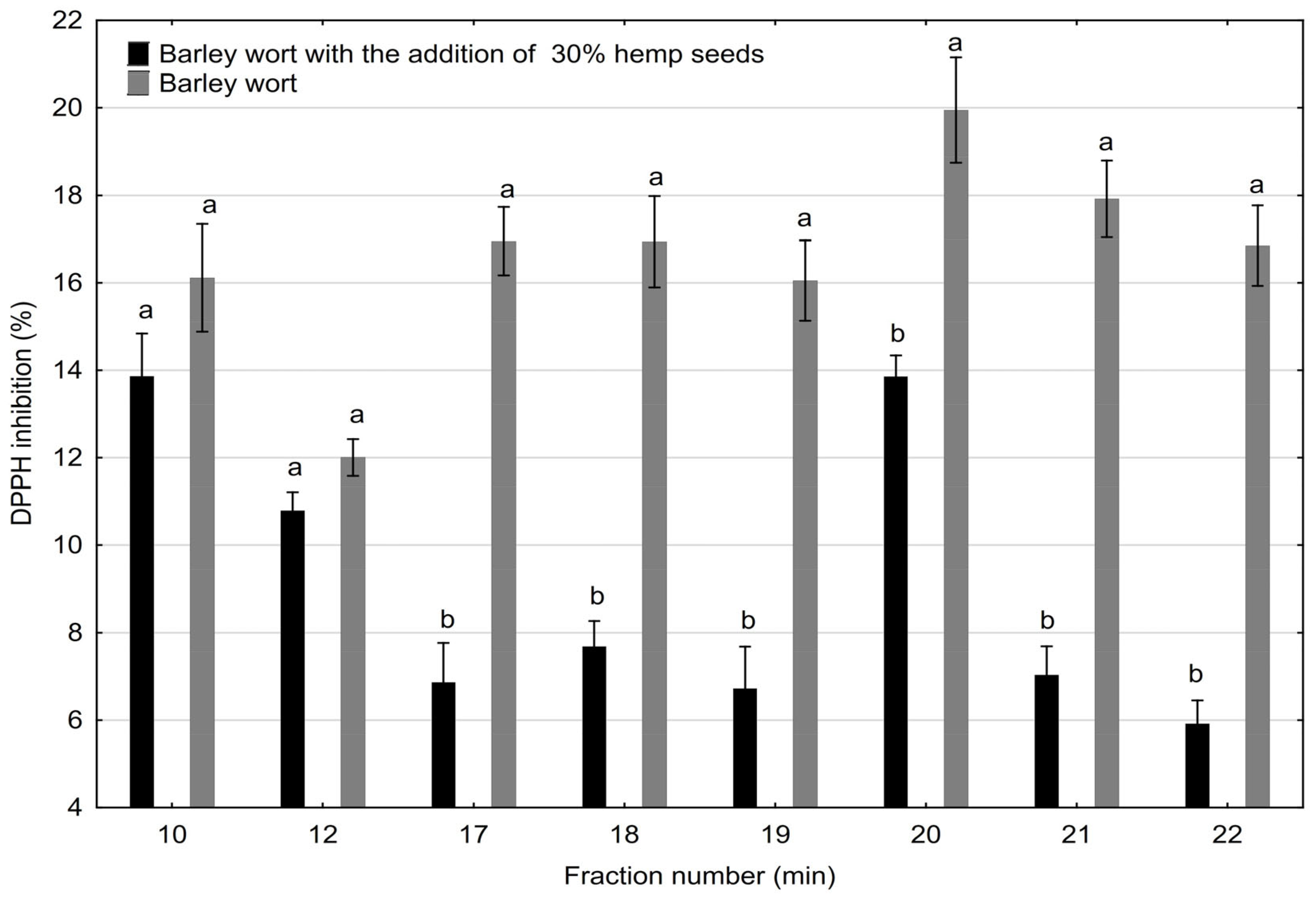
2.2. Antioxidant Activity
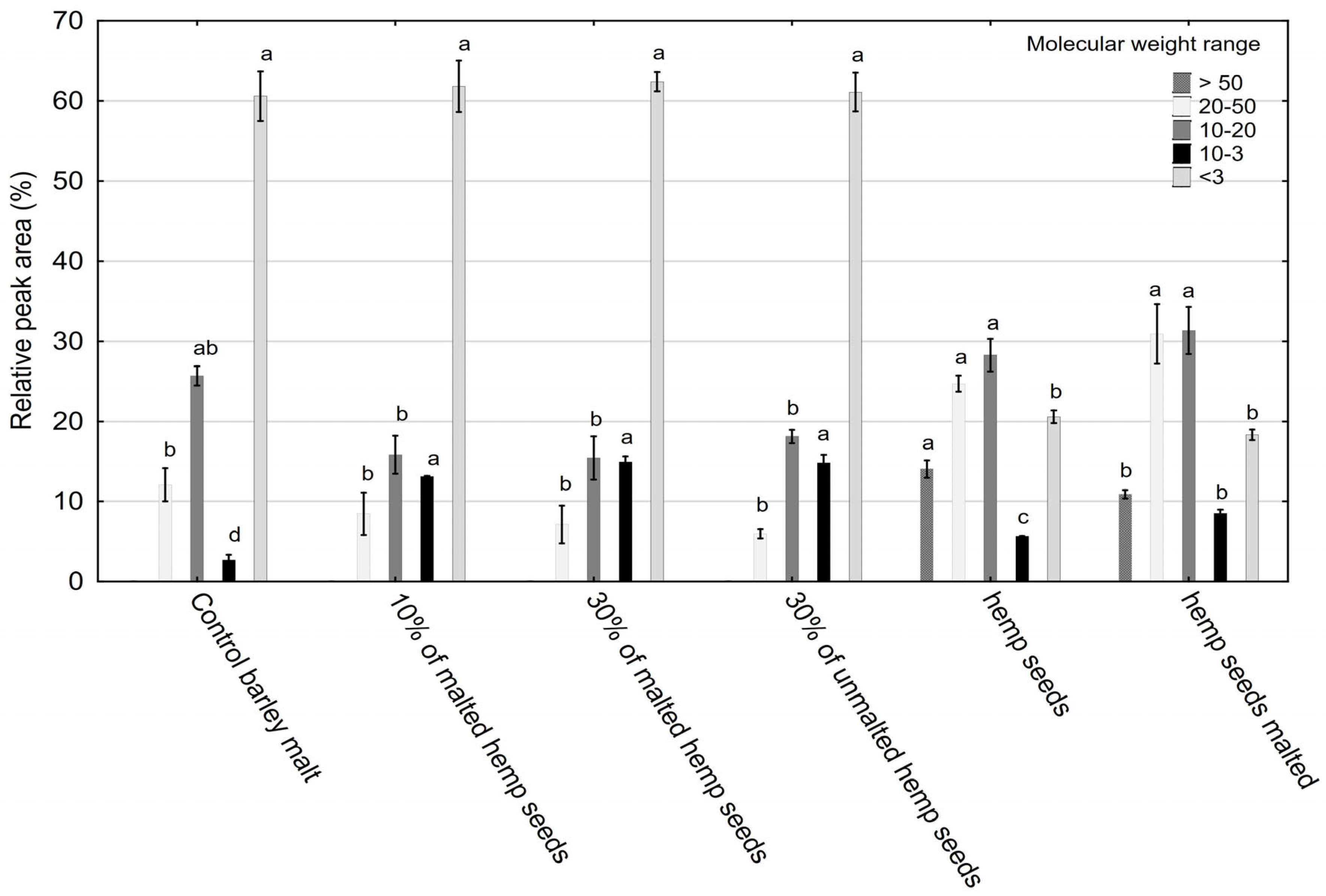
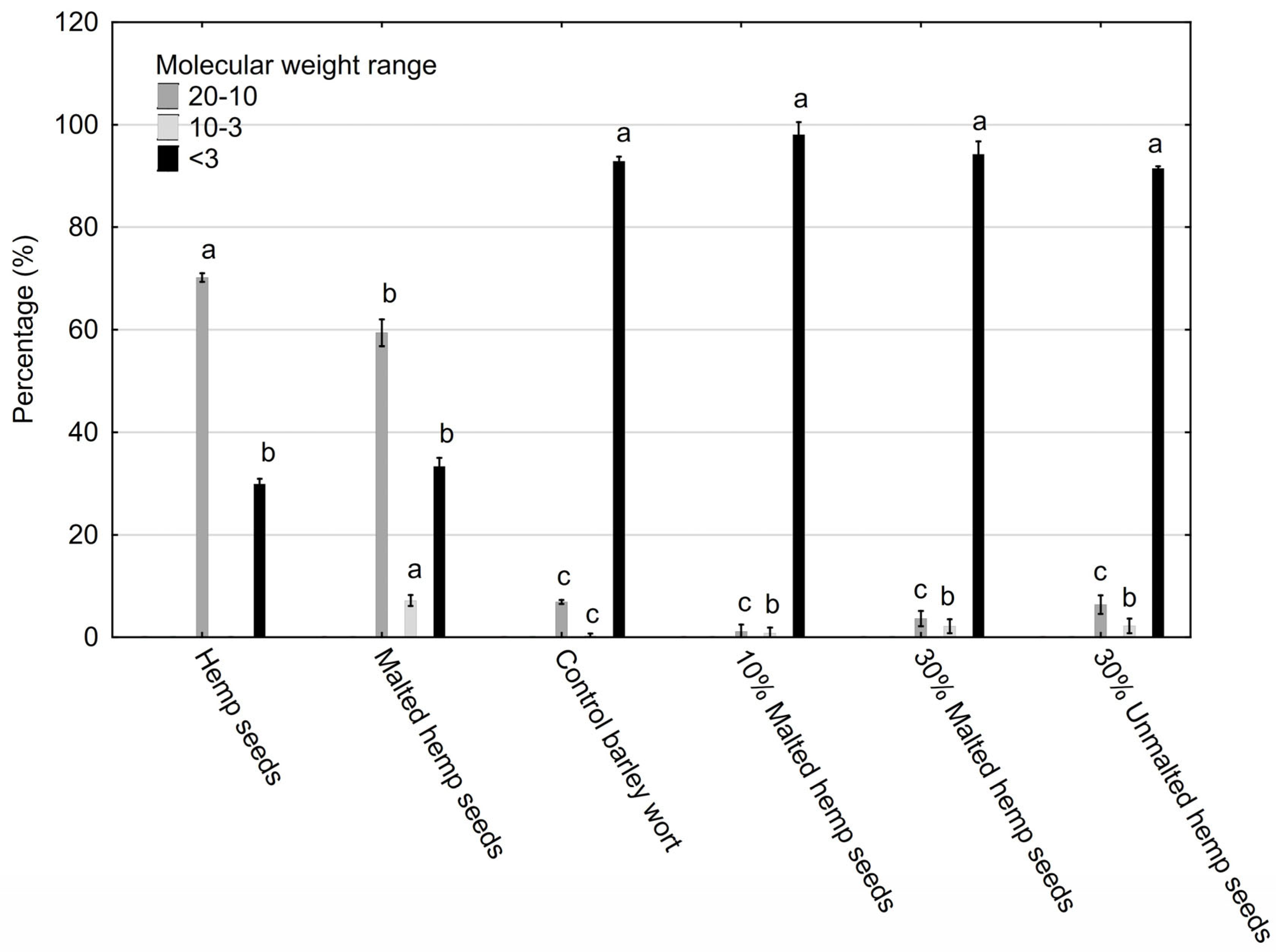
2.3. HPSEC Results—Polysaccharide Fractions
- -
- High-MW fractions (10–50 kDa): These enhance foam retention, colloidal stability, and mouthfeel and may bind bioactive compounds [54].
- -
- Low-MW oligosaccharides (<3 kDa): These improve fermentability and alcohol yield and contribute mild sweetness and clean taste.
3. Materials and Methods
3.1. Materials
3.2. Malting Procedure
3.3. Preparation of Laboratory Worts
Mashing
3.4. In Silico Analyses
3.5. SEC-HPLC Separation
Preparation of Extracts for Antioxidant Activity Determination
3.6. Statistical Analysis
4. Summary and Conclusions
- The addition of hemp—particularly at a 10% inclusion rate of malted seeds—may enrich wort with bioactive peptides and functional polysaccharides, potentially enhancing sensory attributes and health-related properties without compromising fermentation performance or clarity. While these results are promising, they must be validated through controlled fermentation trials and sensory evaluations before hemp can be credibly proposed as a novel raw material for craft and functional beer production.
- Increasing the hemp content may alter the molecular structure of saccharides, which could in turn influence fermentability and sensory attributes.
- The use of unmalted hemp requires enzymatic support to recover fermentable sugars.
- HPSEC is a valuable tool for tailoring wort functionality in hemp-based beer development.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACE | Angiotensin-converting enzyme |
| ANOVA | Analysis of variance |
| BIOPEP-UWM | Bioactive peptide database of University of Warmia and Mazury in Olsztyn (UWM) |
| DHt | Theoretical degree of hydrolysis of protein |
| DPP-IV | Dipeptidyl peptidase-IV, an enzyme that plays a key role in glucose metabolism and is an important target in the treatment of type 2 diabetes |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl, a stable radical used in biochemistry to study the antioxidant properties of various substances |
| EBC | European Brewery Convention, a scale used to determine the color of malt and beer |
| FAN | Free amino nitrogen |
| Hep3B | The name of a human hepatocellular carcinoma (HCC) cell line that is extensively used in biomedical research |
| HMG-CoA reductase | Reductase of 3-hydroxy-3-methylglutaryl-coenzyme A, an enzyme that converts HMG-CoA to mevalonate, which is the controlling (limiting) step in cholesterol synthesis |
| HPSEC | High-performance size-exclusion chromatography |
| HSD | Honest significant difference |
| HSPs | Hemp seed polysaccharides |
| IL- | IL-10 (interleukin 10), IL-4 (interleukin 4), etc., which are cytokines or signaling proteins that play a key role in regulating the immune system |
| LC-MS/MS | Liquid chromatography–tandem mass spectrometry |
| MW | Molecular weight |
| PVPP | Polyvinylpolypyrrolidone |
| SEC-HPLC | Size-exclusion chromatography–high-performance liquid chromatography |
| TNF-α | Tumor necrosis factor alpha, is a key pro-inflammatory cytokine, which is a signaling protein produced primarily by immune system cells such as macrophages |
| UniProt | Universal Protein Resource, a universal protein database |
References
- Galasso, I.; Russo, R.; Mapelli, S.; Ponzoni, E.; Brambilla, I.M.; Battelli, G.; Reggiani, R. Variability in Seed Traits in a Collection of Cannabis sativa L. Genotypes. Front. Plant Sci. 2016, 7, 688. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, M.J.; Kim, C.Y. The Development of New Functional Foods and Ingredients. Foods 2024, 13, 3038. [Google Scholar] [CrossRef]
- Mahou, Y.; Marrakchi Ben Jaafar, D.; Al Moudani, N.; Jeddi, M.; Chda, A.; Fettoukh, N.; Fikri Benbrahim, K.; Aarab, L.; El Bouri, A.; Stambouli, H.; et al. Chemical Profile and Bioactive Properties of Cannabis sativa Threshing Residue: Vasorelaxant, Antioxidant, Immunomodulatory, and Antibacterial Activities. J. Herbmed Pharmacol. 2025, 14, 29–42. [Google Scholar] [CrossRef]
- Morimoto, S.; Tanaka, Y.; Sasaki, K.; Tanaka, H.; Fukamizu, T.; Shoyama, Y.; Shoyama, Y.; Taura, F. Identification and Characterization of Cannabinoids that Induce Cell Death through Mitochondrial Permeability Transition in Cannabis Leaf Cells*. J. Biol. Chem. 2007, 282, 20739–20751. [Google Scholar] [CrossRef]
- Mygdalia, A.; Panoras, I.; Vazanelli, E.; Tsaliki, E. Nutritional and Industrial Insights into Hemp Seed Oil: A Value-Added Product of Cannabis sativa L. Seeds 2025, 4, 5. [Google Scholar] [CrossRef]
- Burton, R.A.; Andres, M.; Cole, M.; Cowley, J.M.; Augustin, M.A. Industrial Hemp Seed: From the Field to Value-Added Food Ingredients. J. Cannabis Res. 2022, 4, 45. [Google Scholar] [CrossRef]
- Górski, K.; Kowalczyk, T.; Gładys, A.; Glica, M.; Muskała, M.; Picot, L.; Mori, M.; Hatziantoniou, S.; Sitarek, P. Industrial Applications of Cannabis sativa (L.): Exploring Its Biological and Nanotechnological Potential. Ind. Crops Prod. 2025, 225, 120566. [Google Scholar] [CrossRef]
- Irakli, M.; Tsaliki, E.; Kalivas, A.; Kleisiaris, F.; Sarrou, E.; Cook, C.M. Effect οf Genotype and Growing Year on the Nutritional, Phytochemical, and Antioxidant Properties of Industrial Hemp (Cannabis sativa L.) Seeds. Antioxidants 2019, 8, 491. [Google Scholar] [CrossRef]
- Aladić, K.; Jarni, K.; Barbir, T.; Vidović, S.; Vladić, J.; Bilić, M.; Jokić, S. Supercritical CO2 Extraction of Hemp (Cannabis sativa L.) Seed Oil. Ind. Crops Prod. 2015, 76, 472–478. [Google Scholar] [CrossRef]
- Orhan, I.; Sener, B. Fatty Acid Content of Selected Seed Oils. J. Herb. Pharmacother. 2002, 2, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Asadi, S.; Moghadam, H.; Naghdi Badi, H.; Naghavi, M.; Salami, S. A Review on Agronomic, Phytochemical and Pharmacological Aspects of Cannabis (Cannabis sativa L.). J. Med. Plants 2019, 18, 1–20. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.V.; Davis, A.; Kumar, S.K.; Murray, B.; Zheljazkov, V.D. Industrial Hemp (Cannabis sativa Subsp. Sativa) as an Emerging Source for Value-Added Functional Food Ingredients and Nutraceuticals. Molecules 2020, 25, 4078. [Google Scholar] [CrossRef]
- Ertaş, N.; Aslan, M. Antioxidant and Physicochemical Properties of Cookies Containing Raw and Roasted Hemp Flour. Acta Sci. Pol. Technol. Aliment. 2020, 19, 177–184. [Google Scholar] [CrossRef]
- Korus, J.; Witczak, M.; Ziobro, R.; Juszczak, L. Hemp (Cannabis sativa Subsp. Sativa) Flour and Protein Preparation as Natural Nutrients and Structure Forming Agents in Starch Based Gluten-Free Bread. LWT 2017, 84, 143–150. [Google Scholar] [CrossRef]
- Nissen, L.; di Carlo, E.; Gianotti, A. Prebiotic Potential of Hemp Blended Drinks Fermented by Probiotics. Food Res. Int. 2020, 131, 109029. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Jiang, J.; Xiong, Y.L. High Pressure Homogenization Combined with pH Shift Treatment: A Process to Produce Physically and Oxidatively Stable Hemp Milk. Food Res. Int. 2018, 106, 487–494. [Google Scholar] [CrossRef]
- Zahari, I.; Ferawati, F.; Helstad, A.; Ahlström, C.; Östbring, K.; Rayner, M.; Purhagen, J.K. Development of High-Moisture Meat Analogues with Hemp and Soy Protein Using Extrusion Cooking. Foods 2020, 9, 772. [Google Scholar] [CrossRef]
- Norajit, K.; Gu, B.-J.; Ryu, G.-H. Effects of the Addition of Hemp Powder on the Physicochemical Properties and Energy Bar Qualities of Extruded Rice. Food Chem. 2011, 129, 1919–1925. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, J.; Hu, R.; Wang, W.; Griffin, J.; Li, Y.; Sun, X.S.; Wang, D. Effect of Genotype on the Physicochemical, Nutritional, and Antioxidant Properties of Hempseed. J. Agric. Food Res. 2021, 3, 100119. [Google Scholar] [CrossRef]
- Mamone, G.; Picariello, G.; Ramondo, A.; Nicolai, M.A.; Ferranti, P. Production, Digestibility and Allergenicity of Hemp (Cannabis sativa L.) Protein Isolates. Food Res. Int. 2019, 115, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Girgih, A.T.; Udenigwe, C.C.; Li, H.; Adebiyi, A.P.; Aluko, R.E. Kinetics of Enzyme Inhibition and Antihypertensive Effects of Hemp Seed (Cannabis sativa L.) Protein Hydrolysates. J. Am. Oil Chem. Soc. 2011, 88, 1767–1774. [Google Scholar] [CrossRef]
- Jurgoński, A.; Opyd, P.M.; Fotschki, B. Effects of Native or Partially Defatted Hemp Seeds on Hindgut Function, Antioxidant Status and Lipid Metabolism in Diet-Induced Obese Rats. J. Funct. Foods 2020, 72, 104071. [Google Scholar] [CrossRef]
- Ren, Y.; Liang, K.; Jin, Y.; Zhang, M.; Chen, Y.; Wu, H.; Lai, F. Identification and Characterization of Two Novel α-Glucosidase Inhibitory Oligopeptides from Hemp (Cannabis sativa L.) Seed Protein. J. Funct. Foods 2016, 26, 439–450. [Google Scholar] [CrossRef]
- Wei, L.-H.; Dong, Y.; Sun, Y.-F.; Mei, X.-S.; Ma, X.-S.; Shi, J.; Yang, Q.; Ji, Y.-R.; Zhang, Z.-H.; Sun, H.-N.; et al. Anticancer Property of Hemp Bioactive Peptides in Hep3B Liver Cancer Cells through Akt/GSK3β/β-Catenin Signaling Pathway. Food Sci. Nutr. 2021, 9, 1833–1841. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Martin, N.M.; Montserrat-de la Paz, S.; Toscano, R.; Grao-Cruces, E.; Villanueva, A.; Pedroche, J.; Millan, F.; Millan-Linares, M.C. Hemp (Cannabis sativa L.) Protein Hydrolysates Promote Anti-Inflammatory Response in Primary Human Monocytes. Biomolecules 2020, 10, 803. [Google Scholar] [CrossRef]
- Iwaniak, A.; Minkiewicz, P.; Darewicz, M. BIOPEP-UWM Database—Present and Future. Curr. Opin. Food Sci. 2024, 55, 101108. [Google Scholar] [CrossRef]
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM Database of Bioactive Peptides: Current Opportunities. Int. J. Mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef]
- Julakanti, S.; Charles, A.P.R.; Syed, R.; Bullock, F.; Wu, Y. Hempseed Polysaccharide (Cannabis sativa L.): Physicochemical Characterization and Comparison with Flaxseed Polysaccharide. Food Hydrocoll. 2023, 143, 108900. [Google Scholar] [CrossRef]
- Wishart, D.S.; Hiebert-Giesbrecht, M.; Inchehborouni, G.; Cao, X.; Guo, A.C.; LeVatte, M.A.; Torres-Calzada, C.; Gautam, V.; Johnson, M.; Liigand, J.; et al. Chemical Composition of Commercial Cannabis. J. Agric. Food Chem. 2024, 72, 14099–14113. [Google Scholar] [CrossRef]
- Habschied, K.; Jokić, S.; Aladić, K.; Šplajt, I.; Krstanović, V.; Mastanjević, K. Addition of Industrial Hemp (Cannabis sativa L.) Dry Inflorescence in Beer Production. Appl. Sci. 2025, 15, 624. [Google Scholar] [CrossRef]
- Staples, A.J. Beer Drinker Perceptions of Cannabis-Infused Beverages. Br. Food J. 2024, 127, 451–475. [Google Scholar] [CrossRef]
- Merenkova, S.; Fatkullin, R.; Kalinina, I. Effect of Fermentation on the Biochemical Parameters Antioxidant Capacity and Dispersed Composition of Plant Beverages Based on Barley and Hemp Seeds. Fermentation 2022, 8, 384. [Google Scholar] [CrossRef]
- Pontonio, E.; Verni, M.; Dingeo, C.; Diaz-de-Cerio, E.; Pinto, D.; Rizzello, C.G. Impact of Enzymatic and Microbial Bioprocessing on Antioxidant Properties of Hemp (Cannabis sativa L.). Antioxidants 2020, 9, 1258. [Google Scholar] [CrossRef]
- Zdaniewicz, M.; Duliński, R.; Lakatošová, J.; Gołaszewski, J.; Żuk-Gołaszewska, K. Evaluation of the Profile of Selected Bioactive Compounds and the Potential of Barley Wort Enriched with Malted and Unmalted Hemp Seeds for Brewing Applications. Molecules 2025, 30, 3261. [Google Scholar] [CrossRef]
- Ferreira, I.M.P.L.V.O.; Jorge, K.; Nogueira, L.C.; Silva, F.; Trugo, L.C. Effects of the Combination of Hydrophobic Polypeptides, Iso-α Acids, and Malto-Oligosaccharides on Beer Foam Stability. J. Agric. Food Chem. 2005, 53, 4976–4981. [Google Scholar] [CrossRef]
- Wei, P.; Tang, Y.; Zhou, K.; Wei, Z.; Liu, G. Characteristics of Polysaccharides from Industrial Hemp (Cannabis sativa L.) Kernels. Foods 2024, 13, 3429. [Google Scholar] [CrossRef]
- McMurrough, I.; Madigan, D.; Kelly, R.J. Evaluation of Rapid Colloidal Stabilization with Polyvinylpolypyrrolidone (PVPP). J. Am. Soc. Brew. Chem. 1997, 55, 38–43. [Google Scholar] [CrossRef]
- Tanase Apetroaei, V.; Istrati, D.I.; Vizireanu, C. Plant-Derived Compounds in Hemp Seeds (Cannabis sativa L.): Extraction, Identification and Bioactivity—A Review. Molecules 2025, 30, 124. [Google Scholar] [CrossRef] [PubMed]
- Malomo, S.A.; Aluko, R.E. A Comparative Study of the Structural and Functional Properties of Isolated Hemp Seed (Cannabis sativa L.) Albumin and Globulin Fractions. Food Hydrocoll. 2015, 43, 743–752. [Google Scholar] [CrossRef]
- Guerdrum, L.J.; Bamforth, C.W. Prolamin Levels Through Brewing and the Impact of Prolyl Endoproteinase. Cerevisia 2013, 38, 52. [Google Scholar] [CrossRef]
- Stewart, G.G.; Hill, A.; Lekkas, C. Wort FAN—Its Characteristics and Importance during Fermentation. J. Am. Soc. Brew. Chem. 2013, 71, 179–185. [Google Scholar] [CrossRef]
- Hellwig, M.; Witte, S.; Henle, T. Free and Protein-Bound Maillard Reaction Products in Beer: Method Development and a Survey of Different Beer Types. J. Agric. Food Chem. 2016, 64, 7234–7243. [Google Scholar] [CrossRef] [PubMed]
- Koller, H.; Perkins, L.B. Brewing and the Chemical Composition of Amine-Containing Compounds in Beer: A Review. Foods 2022, 11, 257. [Google Scholar] [CrossRef] [PubMed]
- Daliri, E.B.; Oh, D.H.; Lee, B.H. Bioactive Peptides. Foods 2017, 6, 32. [Google Scholar] [CrossRef]
- Liu, S.; Sun, H.; Ma, G.; Zhang, T.; Wang, L.; Pei, H.; Li, X.; Gao, L. Insights into Flavor and Key Influencing Factors of Maillard Reaction Products: A Recent Update. Front. Nutr. 2022, 9, 973677. [Google Scholar] [CrossRef]
- Sheehan, M.C.; Skerritt, J.H. Identification and Characterisation of Beer Polypeptides Derived from Barley Hordeins. J. Inst. Brew. 1997, 103, 297–306. [Google Scholar] [CrossRef]
- Agbana, M.R.; Angeletti, B.S.; Buecker, H.C.; Tseng, Y.-C.; Davis, B.E.; Schendel, R.R. Characterizing the Non-Starch Polysaccharides of Hempseed Cell Walls. Eur. Food Res. Technol. 2024, 250, 2405–2419. [Google Scholar] [CrossRef]
- Kosiv, R. Comparison of the Hydrocolloids Application Efficiency for Stabilizing the Foam of Beer. SR 2021, 6, 25–30. [Google Scholar] [CrossRef]
- Blšáková, L.; Gregor, T.; Mešťánek, M.; Hřivna, L.; Kumbár, V. The Use of Unconventional Malts in Beer Production and Their Effect on the Wort Viscosity. Foods 2022, 11, 31. [Google Scholar] [CrossRef]
- Langenaeken, N.A.; De Schepper, C.F.; De Schutter, D.P.; Courtin, C.M. Carbohydrate Content and Structure during Malting and Brewing: A Mass Balance Study. J. Inst. Brew. 2020, 126, 253–262. [Google Scholar] [CrossRef]
- Hejazi, S.N.; Orsat, V. Malting Process Optimization for Protein Digestibility Enhancement in Finger Millet Grain. J. Food Sci. Technol. 2016, 53, 1929–1938. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xu, B.; Wang, Y.; Li, W.; He, D.; Zhang, Y.; Zhang, X.; Xing, X. Emerging Natural Hemp Seed Proteins and Their Functions for Nutraceutical Applications. FSHW 2023, 12, 929–941. [Google Scholar] [CrossRef]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Langstaff, S.A.; Guinard, J.-X.; Lewis, M.J. Sensory Evaluation of the Mouthfeel of Beer. J. Am. Soc. Brew. Chem. 1991, 49, 54–59. [Google Scholar] [CrossRef]
- Zdaniewicz, M.; Duliński, R.; Żuk-Gołaszewska, K.; Tarko, T. Characteristics of Selected Bioactive Compounds and Malting Parameters of Hemp (Cannabis sativa L.) Seeds and Malt. Molecules 2024, 29, 4345. [Google Scholar] [CrossRef]
- Minkiewicz, P.; Darewicz, M.; Iwaniak, A. In Silico Analysis of Individual Fractions of Bovine Casein as Precursors of Bioactive Peptides—Influence of Post-Translational Modifications. Appl. Sci. 2023, 13, 8091. [Google Scholar] [CrossRef]
- Minkiewicz, P.; Dziuba, J.; Michalska, J. Bovine Meat Proteins as Potential Precursors of Biologically Active Peptides—A Computational Study Based on the BIOPEP Database. Food Sci. Technol. Int. 2011, 17, 39–45. [Google Scholar] [CrossRef]
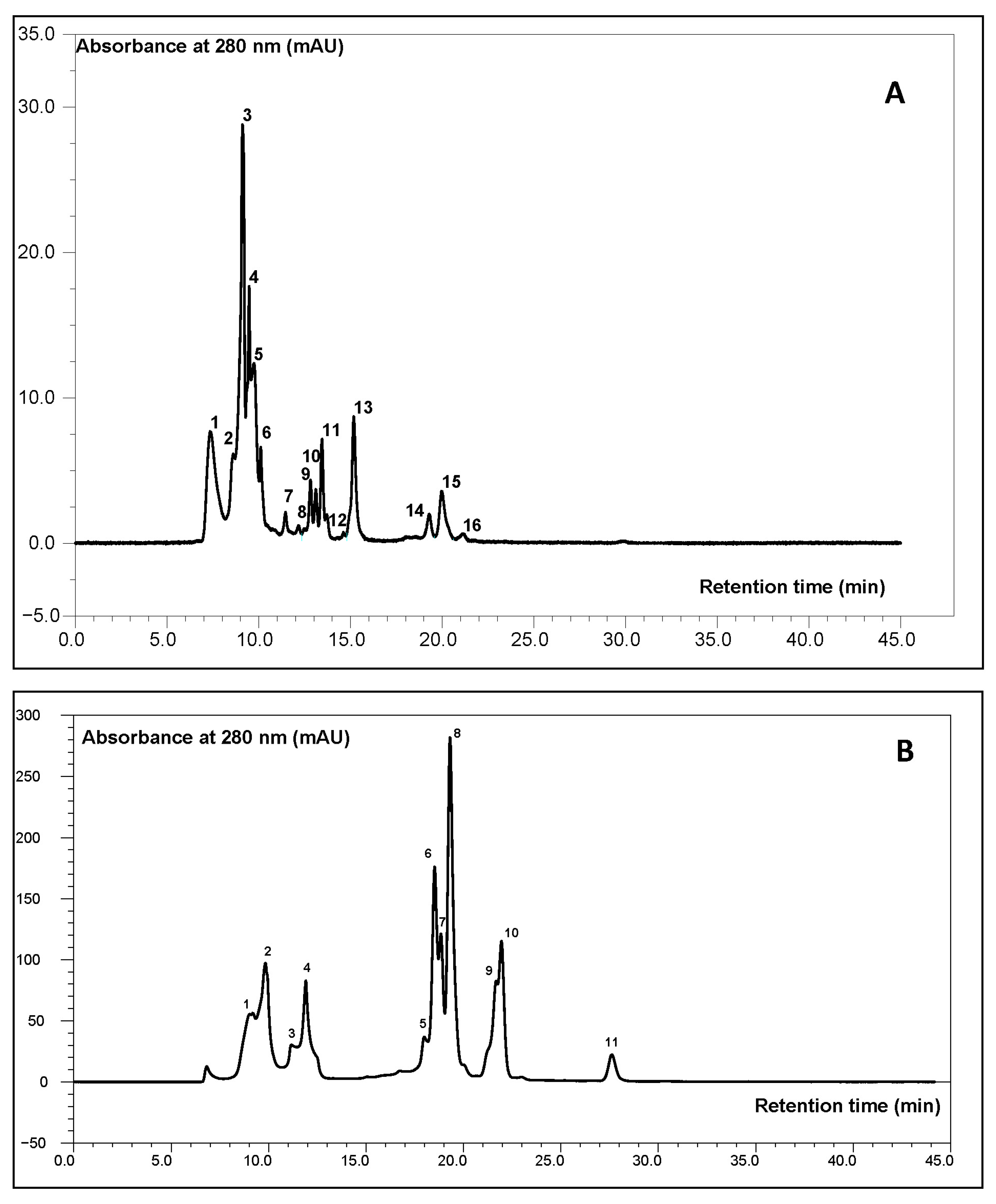
| Protein Code | DHt [%] | Type of Peptide Bioactivity | Number of Peptides | Ae | W |
|---|---|---|---|---|---|
| P06470 | 26.03 | antiamnestic | 1 | 0.0034 | 0.5000 |
| dipeptidyl peptidase IV inhibitor | 14 | 0.0478 | 0.0593 | ||
| calpain 1 inhibitor | 1 | 0.0034 | 1.0000 | ||
| Stimulating | 3 | 0.0102 | 0.2991 | ||
| ACE inhibitor | 10 | 0.0341 | 0.0568 | ||
| antioxidative | 2 | 0.0068 | 0.0664 | ||
| dipeptidyl peptidase III inhibitor | 1 | 0.0034 | 0.0433 | ||
| inhibitor of tripeptidyl peptidase II | 3 | 0.0102 | 0.5965 | ||
| neprilysin 2 inhibitor | 1 | 0.0034 | 1.0000 | ||
| xaa-pro inhibitor | 1 | 0.0034 | 0.3333 | ||
| neuropeptide | 1 | 0.0034 | 0.0907 | ||
| leucyltransferase inhibitor | 1 | 0.0034 | 1.0000 | ||
| neprilysin inhibitor | 1 | 0.0034 | 0.1245 | ||
| P06472 | 20.19 | ACE inhibitor | 3 | 0.0286 | 0.0423 |
| dipeptidyl peptidase IV inhibitor | 3 | 0.0286 | 0.0306 | ||
| xaa-pro inhibitor | 4 | 0.0286 | 0.6008 | ||
| lactocepin inhibitor | 3 | 0.0286 | 0.4288 | ||
| I6SW23 | 24.40 | dipeptidyl peptidase IV inhibitor | 36 | 0.0482 | 0.0663 |
| inhibitor of tripeptidyl peptidase II | 1 | 0.0013 | 0.0422 | ||
| ACE inhibitor | 4 | 0.0054 | 0.0099 | ||
| antioxidative | 2 | 0.0027 | 0.0255 | ||
| stimulating | 1 | 0.0013 | 0.1215 | ||
| phospholipase A2 inhibitor | 1 | 0.0013 | 0.4815 | ||
| renin inhibitor | 1 | 0.0013 | 0.1083 | ||
| dipeptidyl peptidase III inhibitor | 1 | 0.0013 | 0.0347 | ||
| neuropeptide | 1 | 0.0013 | 0.0147 | ||
| anti-inflammatory | 1 | 0.0013 | 0.1940 | ||
| xaa-pro inhibitor | 1 | 0.0013 | 0.4815 | ||
| lactocepin inhibitor | 1 | 0.0013 | 0.1625 | ||
| pancreatic lipase inhibitor | 2 | 0.0027 | 0.3375 | ||
| hypotensive | 1 | 0.0013 | 0.1215 |
| Protein Code | DHt [%] | Type of Peptide Bioactivity | Number of Peptides | Ae | W |
|---|---|---|---|---|---|
| A0A090CXP9 | 27.14 | calpain 1 inhibitor | 2 | 0.0041 | 1.0000 |
| ACE inhibitor | 16 | 0.0326 | 0.0711 | ||
| antioxidative | 2 | 0.0041 | 0.0530 | ||
| stimulating | 3 | 0.0061 | 0.1362 | ||
| dipeptidyl peptidase IV inhibitor | 30 | 0.0611 | 0.0980 | ||
| dipeptidyl peptidase III inhibitor | 4 | 0.0081 | 0.0864 | ||
| inhibitor of tripeptidyl peptidase II | 4 | 0.0081 | 0.2207 | ||
| neprilysin 2 inhibitor | 2 | 0.0041 | 1.0000 | ||
| HMG-CoA reductase inhibitor | 1 | 0.002 | 0.4878 | ||
| leucyltransferase inhibitor | 1 | 0.002 | 0.0578 | ||
| xaa-pro inhibitor | 1 | 0.002 | 0.4878 | ||
| lactocepin inhibitor | 1 | 0.002 | 0.1093 | ||
| acylaminoacyl peptidase inhibitor | 1 | 0.002 | 0.4878 | ||
| tubulin-tyrosine ligase inhibitor | 1 | 0.002 | 0.3279 | ||
| hypouricemic | 1 | 0.002 | 0.0893 | ||
| pancreatic lipase inhibitor | 1 | 0.002 | 0.2469 | ||
| antiviral | 1 | 0.002 | 0.1399 | ||
| A0A803Q1B3 | 34.58 | neuropeptide | 1 | 0.0047 | 1.0000 |
| dipeptidyl peptidase IV inhibitor | 9 | 0.0419 | 0.0634 | ||
| calpain 1 inhibitor | 1 | 0.0047 | 1.0000 | ||
| ACE inhibitor | 4 | 0.0186 | 0.0400 | ||
| stimulating | 2 | 0.0093 | 0.1332 | ||
| inhibitor of tripeptidyl peptidase II | 1 | 0.0047 | 0.0842 | ||
| neprilysin 2 inhibitor | 1 | 0.0047 | 1.0000 | ||
| antidiabetic | 1 | 0.0047 | 0.5054 | ||
| A0A7J6FEU0 | 31.16 | calpain 1 inhibitor | 1 | 0.0025 | 1.0000 |
| ACE inhibitor | 15 | 0.0376 | 0.0725 | ||
| Antioxidative | 3 | 0.0075 | 0.0623 | ||
| dipeptidyl peptidase IV inhibitor | 24 | 0.0602 | 0.0961 | ||
| alpha-glucosidase inhibitor | 1 | 0.0025 | 0.0767 | ||
| dipeptidyl peptidase III inhibitor | 1 | 0.0025 | 0.0285 | ||
| anti inflammatory | 1 | 0.0025 | 0.5000 | ||
| inhibitor of tripeptidyl peptidase II | 2 | 0.005 | 0.1247 | ||
| neprilysin 2 inhibitor | 1 | 0.0025 | 1.0000 | ||
| acylaminoacyl peptidase inhibitor | 1 | 0.0025 | 1.0000 | ||
| tubulin-tyrosine ligase inhibitor | 1 | 0.0025 | 0.1429 | ||
| glutamate carboxypeptidase II inhibitor | 2 | 0.005 | 0.1247 | ||
| hypouricemic | 1 | 0.0025 | 0.1244 | ||
| binding | 1 | 0.0025 | 1.0000 | ||
| neuropeptide | 1 | 0.0025 | 0.1244 | ||
| pancreatic lipase inhibitor | 1 | 0.0025 | 0.2500 |
| Protein Name or Function | Access. No in UniProt | Length (aa) | Mass (kDa) |
|---|---|---|---|
| B1-hordein | P06470 · HOR1_HORVU | 293 | 33.422 |
| Sequence: MKTFLIFALLAIAATSTIAQQQPFPQQPIPQQPQPYPQQPQPYPQQPFPPQQPFPQQPVPQQPQPYPQQPFPPQQPFPQQPPFWQQKPFPQQPPFGLQQPILSQQQPCTPQQTPLPQGQLYQTLLQLQIQYVHPSILQQLNPCKVFLQQQCSPVPVPQRIARSQMLQQSSCHVLQQQCCQQLPQIPEQFRHEAIRAIVYSIFLQEQPQQLVEGVSQPQQQLWPQQVGQCSFQQPQPQQVGQQQQVPQSAFLQPHQIAQLEATTSIALRTLPMMCSVNVPLYRILRGVGPSVGV | |||
| C-hordein | P06472 · HOR7_HORVU | 105 | 12.180 |
| Sequence: QPQQSYPVQPQQPFPQPQPVPQQRPQQASPLQPQQPFPQGSEQIIPQQPFPLQPQPFPQQPQQPLPQPQQPFRQQAELIIPQQPQQPLPLQPHQPYTQQTIWSMV | |||
| D-hordein | I6SW23 · I6SW23_HORVU | 747 | 79.350 |
| Sequence: MAKRLVLFVAVIVALVALTTAEREINGNNIFLDSRSRQLQCERELQESSLEACRRVVDQQLVGQLPWSTGLQMQCCQQLRDVSPECRPVALSQVVRQYEQQTEVPSKGGSFYPGGTAPPLQQGGWWGTSVKWYYPDQTSSQQSWQGQQGYHQSVTSSQQPGQGQQGSYPGSTFPQQPGQGQQPGQRQPWSYPSATFPQQPGQGQGQQGYYPGATSLLQPGQGQQGPYQSATSPQQPGQGQGQQETYPIATSPHQPGQWQQPGQGQQGYYPSVTSPQQSGQGQQGYPSTTSPQQSGQGQQLGQGQQPGQGQQGYPSATFPQQPGQWQQGSYPSTTSPQQSGQGQQGYNPSGTSTQQPGQVQQLGQGQQGYYPIATSPQQPGQGQQLGQGQQPGHGQQLVQGQQQGQGQQGHYPSMTSPHQTGQGQKGYYPSAISPQQSGQGQQGYQPSGASSQGSVQGACQHSTSSPQQQAQGCQASSPKQGLGSLYYPSGAYTQQKPGQGYNPGGTSPLHQQGGGFGGGLTTEQPQGGKQPFHCQQTTVSPHQGQQTTVSPHQGQQTTVSPHQGQQTTVSPHQGQQTTVSPHQGQQTTVSPHQGQQTTVSPHPGQQTTVSPHQGQQTTVSPHPGQQTTVSPHQGQQTTVSPHQGQQTTVSPHQGQQTTVSPHQGQQTTVSPHQGQQPGEQPCGFPGQQTTVSLHHGQQSNELYYGSPYHVSVEQPSASLKVAKAQQLAAQLPAMCRLEGGGGLLASQ | |||
| Protein Name or Function | Access. No in UniProt | Length (aa) | Mass (kDa) |
|---|---|---|---|
| 11S seed storage protein | A0A090CXP9 · CANSA | 491 | 55.974 |
| Sequence: MARSSTSLLCFTLFSLLLSHACFAQIEQMPQRSQRGGQQRQQHRWQSQCQFQRLNARQPNRRVECEAGVSEYWDIQNTEDDELHCAGVETARHTIQRRGLLLPSFLNAPMMFYVIQGRGIHGAVIPGCPETFERGTSSPSSRGYRSEGASSDEQHQKVREIKEGDMVAMPAGVADWVYNNGDSPLVLIAFVDVGNQANQLDQFSRRFHLAGNPHREQKTQQQVRARSQSRSQLRRESGEQTPNGNIFSGFDTRILAESFNVDTELAHKLQNRDDMRERIVRVRGEDLQIIAPSRIQEEERRHYSRDNGLEETFCTLRLRQNIDRPSQADIFNPRGGRLNTLNNYNLPILRFLQLTAERGVLYKNGMMAPHFNLDSHSVIYVTRGSARLQVVDDNGRNVFDGELREGQIFVVPQNFAVVKKASAQGFEWIAVKTNDNAMRNPLAGKVSAMRAMPDDVLANAFQTSREQARRLKYGRDEISVFSPSSQQTRYE | |||
| Bifunctional protein | A0A803Q1B3 · CANSA | 215 | 21.640 |
| Sequence: MESLVHLPRLLVAALAIFAVLITPVFGQVSTPCNASMISSFTPCMNFVTNSSSAGTSPTSDCCNALKTLTSSGMDCLCLIVTGSVPFQVPINRSLAISLPRACNMAGVPVQCKATAAPIPAPAPASFGPALSPGDSPSSGLSPTGSSIPQPVSPALSPESDTTPLLTPPTTTGGSEAPTATTGSRSVLPPSAATTLYSSSSFLLFAMGCLVMELY | |||
| Storage protein | A0A7J6FEU0 · CANSA | 399 | 45.680 |
| Sequence: MANSHRSGLIKRSSDGARVVIGTIMGVIFGFFIGMSFPSVSLNKINLPSSLISSLDVAITDIHGSSISRSFEDNGPSNVPRIYVPTNPRGAELLPPGIIVSESDFYLRRLWGEPSEDLKKKPKYLMTFTVGLDQKNNIDAAAKKLSEDFQIMLFHYDDRVTEWDEFEWSKDAIHVSVRKQTKWWYAKRFLHPDIVAAYEYIFIWDEDLGVENFNGDKYIELVKKHGLEISQPGLEPNNGLTWEMTKRRGEQEVHKDAVERPGWCDNPRQPPCAAFVEIMAPVFSRKAWRCVWHMIQNDLVHGWGLDFALRRCVEPAYEKIGVVDSQWIVHQTIPSLGNQASHHQYLLLFKQKISHSTGNSEDGKAPWEGVRARCRNEWTEFQSRLNKADEEYFAHVGKG | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duliński, R.; Zdaniewicz, M.; Byczyński, Ł.; Żuk-Gołaszewska, K.; Bukowska, B. Application of HPSEC Technique and In Silico Analysis in the Evaluation of Bioactive Peptides and Polysaccharide Profile in Wort Supplemented with Malted and Unmalted Hemp Seeds. Molecules 2025, 30, 3676. https://doi.org/10.3390/molecules30183676
Duliński R, Zdaniewicz M, Byczyński Ł, Żuk-Gołaszewska K, Bukowska B. Application of HPSEC Technique and In Silico Analysis in the Evaluation of Bioactive Peptides and Polysaccharide Profile in Wort Supplemented with Malted and Unmalted Hemp Seeds. Molecules. 2025; 30(18):3676. https://doi.org/10.3390/molecules30183676
Chicago/Turabian StyleDuliński, Robert, Marek Zdaniewicz, Łukasz Byczyński, Krystyna Żuk-Gołaszewska, and Bożena Bukowska. 2025. "Application of HPSEC Technique and In Silico Analysis in the Evaluation of Bioactive Peptides and Polysaccharide Profile in Wort Supplemented with Malted and Unmalted Hemp Seeds" Molecules 30, no. 18: 3676. https://doi.org/10.3390/molecules30183676
APA StyleDuliński, R., Zdaniewicz, M., Byczyński, Ł., Żuk-Gołaszewska, K., & Bukowska, B. (2025). Application of HPSEC Technique and In Silico Analysis in the Evaluation of Bioactive Peptides and Polysaccharide Profile in Wort Supplemented with Malted and Unmalted Hemp Seeds. Molecules, 30(18), 3676. https://doi.org/10.3390/molecules30183676






