Chemical Constituents and Pharmacological Effects of Camellia oleifera Fruits: A Review
Abstract
1. Introduction
2. Phytochemical Composition
2.1. Triterpenoids and Triterpenoid Saponins
2.2. Flavonoids and Their Glycosides
2.3. Polysaccharides
2.4. Lignans
2.5. Phytosterols
2.6. Others
3. Pharmacological Activities
3.1. Anti-Tumor Activity
3.2. Antioxidant Activity
3.3. Hypolipidemic Activity
3.4. Hypoglycemic Activity
3.5. Anti-Inflammatory Activity
3.6. Neuroprotective Activity
3.7. Antimicrobial Activity
3.8. Other Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kong, Q.; Chen, T.; Wang, H.; Zheng, S.; Wang, H.; Liang, H.; Zhou, L.; Yang, H.; Jiang, X.; Ding, C.; et al. Variation of Camellia oleifera fruit traits and nutritional constituents in seed oil during development and post-harvest. Sci. Hortic. 2025, 339, 113903. [Google Scholar] [CrossRef]
- Luan, F.; Zeng, J.S.; Yang, Y.; He, X.R.; Wang, B.J.; Gao, Y.B.; Nan, Z. Recent advances in Camellia oleifera Abel a review of nutritional constituents, biofunctional properties and potential industrial applications. J. Funct. Foods 2020, 75, 104242. [Google Scholar] [CrossRef]
- Jiang, N.; Xu, Z.; Lu, S.; Xia, X.; Li, M.; Miao, Y.; Xiang, X. Advances in valorization of Camellia oleifera Abel. Seed cake: A review on the bioactive components, health benefits, extraction methods, and potential food applications. Food Res. Int. 2025, 208, 116134. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tan, Y.; Ma, G.; Bai, X.; Cao, J. Advances in research and application of Camellia oil. J. Chin. Cereals Oils Assoc. 2017, 32, 191–196. [Google Scholar]
- Wang, L.Q.; Xu, Q.L.; Dong, L.M.; Zhang, Q.; Tan, J.W. Chemical constituents from the fruit shell of Camellia oleifera. J. Trop. Subtrop. Bot. 2017, 25, 81–86. [Google Scholar]
- Kuo, P.C.; Lin, T.C.; Yang, C.W.; Lin, C.L.; Chen, G.F.; Huang, J.W. Bioactive saponin from tea seed pomace with inhibitory effects against Rhizoctonia solani. J. Agric. Food Chem. 2010, 58, 8618–8622. [Google Scholar] [CrossRef]
- Hu, J.L.; Nie, S.P.; Huang, D.F.; Li, C.; Xie, M.Y. Extraction of saponin from Camellia oleifera cake and evaluation of its antioxidant activity. Int. J. Food Sci. Tech. 2012, 47, 1676–1687. [Google Scholar] [CrossRef]
- Zhang, X.F.; Han, Y.Y.; Di, T.M.; Gao, L.P.; Xia, T. Triterpene saponins from tea seed pomace (Camellia oleifera Abel) and their cytotoxic activity on MCF-7 cells in vitro. Nat. Prod. Res. 2019, 35, 2730–2733. [Google Scholar] [CrossRef]
- Jian, H.L.; Liao, X.X.; Zhu, L.W.; Zhang, W.M.; Jiang, J.X. Synergism and foaming properties in binary mixtures of a biosurfactant derived from Camellia oleifera Abel and synthetic surfactants. J. Colloid. Interf. Sci. 2011, 359, 487–492. [Google Scholar] [CrossRef]
- Chen, Y.F.; Yang, C.H.; Chang, M.S.; Ciou, Y.P.; Huang, Y.C. Foam properties and detergent abilities of the saponins from Camellia oleifera. Int. J. Mol. Sci. 2010, 11, 4417–4425. [Google Scholar] [CrossRef]
- Zhang, L.X.; He, Y.F.; Zhu, Y.J.; Liu, Y.T.; Wang, X.C. Camellia oleifera shell as an alternative feedstock for furfural production using a high surface acidity solid acid catalyst. Bioresour. Technol. 2018, 249, 536–541. [Google Scholar] [CrossRef]
- Jung, K.; Yeh, W.J.; Huang, W.C.; Yang, H.Y. Camellia oleifera seed extract mildly ameliorates carbon tetrachloride-induced hepatotoxicity in rats by suppressing inflammation. J. Food Sci. 2019, 84, 1586–1591. [Google Scholar]
- Yang, Y.; Cui, J.; Zhang, J.; Jiang, J.; Chen, X.; Shan, K. Advancements in extraction and sustainable applications of Camellia oleifera: A comprehensive review. Food Chem. 2025, 488, 144940. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Deng, S.; Fan, B.; Xu, D.; Du, G.; Li, X. Agricultural waste of Camellia oleifera fruit shell extract with potassium iodide as a novel synergistic composite inhibitor for the corrosion of steel in methylsulfonic acid solution. Ind. Crop. Prod. 2024, 222, 119834. [Google Scholar] [CrossRef]
- State Pharmacopoeia Commission of PR China. Pharmacopoeia of the People’s Republic of China; Chemical Industry Press: Beijing, China, 2020; Volume 1, p. 429. [Google Scholar]
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef]
- Zuo, J.; Zhu, Y.; Yang, N.; Xu, D.; Zhao, J. Pharmacological mechanisms and pharmacokinetic analysis of anti-tumor components in Chinese herbal medicine. Chinese J. Anal. Chem. 2025, 53, 100590. [Google Scholar]
- Chen, J. Essential role of medicine and food homology in health and wellness. Chin. Herb. Med. 2023, 15, 347–348. [Google Scholar] [CrossRef]
- Aoyama, S. Camellia Saponin. Yakugaku Zasshi. 1930, 50, 378–379. [Google Scholar] [CrossRef]
- Chen, J.; Wu, H.; Liau, B.; Chang, C.J.; Jong, T.; Wu, L. Identification and evaluation of antioxidants defatted Camellia oleifera seeds by isopropanol salting-out pretreatment. Food Chem. 2010, 121, 1246–1254. [Google Scholar] [CrossRef]
- Di, T.M.; Yang, S.L.; Du, F.Y.; Zhao, L.; Xia, T.; Zhang, X.F. Cytotoxic and hypoglycemic activity of triterpenoid saponins from Camellia oleifera Abel. seed pomace. Molecules 2017, 22, 1562. [Google Scholar] [CrossRef]
- Zhang, X.F.; Han, Y.Y.; Bao, G.H.; Ling, T.J.; Zhang, L.; Gao, L.P.; Xia, T. A new saponin from tea seed pomace (Camellia oleifera Abel) and its protective effect on PC12 cells. Molecules 2012, 17, 11721–11728. [Google Scholar] [CrossRef] [PubMed]
- Di, T.M.; Yang, S.L.; Du, F.Y.; Zhao, L.; Li, X.H.; Xia, T.; Zhang, X.F. Oleiferasaponin A2, a novel saponin from Camellia oleifera Abel. seeds, inhibits lipid accumulation of HepG2 cells through regulating fatty acid metabolism. Molecules 2018, 23, 3296. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, C.Z.; Ye, J.Z.; Chen, H.X. New triterpene saponins from the seed cake of Camellia Oleifera and their cytotoxic activity. Phytochem. Lett. 2014, 8, 46–51. [Google Scholar] [CrossRef]
- Zong, J.F.; Wang, R.; Bao, G.; Ling, T.; Zhang, L.; Zhang, X.; Hou, R. Novel triterpenoid saponins from residual seed cake of Camellia oleifera Abel. show anti-proliferative activity against tumor cells. Fitoterapia 2015, 104, 7–13. [Google Scholar] [CrossRef]
- Zong, J.F.; Peng, Y.; Bao, G.; Hou, R.; Wan, X. Two new oleanane-type saponins with anti-proliferative activity from Camellia oleifera Abel. seed cake. Molecules 2016, 21, 188. [Google Scholar] [CrossRef]
- Zong, J.F.; Wang, D.; Jiao, W.; Zhang, L.; Bao, G.; Ho, C.; Hou, R.; Wan, X. Oleiferasaponin C6 from the seeds of Camellia oleifera Abel.: A novel compound inhibits proliferation through inducing cell-cycle arrest and apoptosis on human cancer cell lines in vitro. Rsc. Advances 2016, 6, 91386–91393. [Google Scholar] [CrossRef]
- Fu, H.Z.; Wan, K.H.; Yan, Q.W.; Zhou, G.P.; Feng, T.T.; Dai, M.; Zhong, R.J. Cytotoxic triterpenoid saponins from the defatted seeds of Camellia oleifera Abel. J. Asian Nat. Prod. Res. 2018, 20, 412–422. [Google Scholar] [CrossRef]
- Ye, Y.; Fang, F.; Li, Y. Isolation of the sapogenin from defatted seeds of Camellia oleifera and its neuroprotective effects on dopaminergic neurons. J. Agric. Food. Chem. 2014, 62, 6175–6182. [Google Scholar] [CrossRef]
- Xiong, L.; Fu, H.Z.; Yan, Q.W. A new triterpene saponin from defatted seeds of Camellia oleifera. Chin. Tradit. Herb. Drugs 2017, 48, 4375–4380. [Google Scholar]
- Wu, Z.; Tan, X.; Zhou, J.; Yuan, J.; Yang, G.; Li, Z.; Long, H.; Yi, Y.; Lv, C.; Zeng, C.; et al. Discovery of new triterpenoids extracted from Camellia oleifera seed cake and the molecular mechanism underlying their antitumor activity. Antioxidants 2023, 12, 7. [Google Scholar] [CrossRef]
- Zong, J.F.; Guo, X.X.; Zou, K.K.; Cui, C.J.; Hu, Z.H.; Hou, R.Y. Isolation and identification of triterpenoid saponins with antiproliferative and hemolytic activities from Camellia oleifera Abel seeds. Phytochemistry 2025, 235, 114476. [Google Scholar] [CrossRef]
- Chen, S.P.; Huang, Y.; Wu, L.; Wu, J. Chemical constituents from fruit shells of Camellia oleifera. Biol. Chem. Eng. 2017, 3, 21–23. [Google Scholar]
- Fang, Y.D.; Liu, L.Q.; Li, H. Study on pentacyclic triterpene alcohol and tetracyclic triterpene alcohol in the unsaponifiable of Camellia seed oil. J. Chin. Cereal Oil Ass. 1999, 14, 18–22, 26. [Google Scholar]
- Ai, J.X.; Zheng, D.J.; Wu, X. Progress in germplasm resources, chemical composition and pharmacological activities of Camellia oleifera. Food Drug 2025, 27, 17–26. [Google Scholar]
- Luo, Y.M.; Li, B.; Xie, Y.H. Research on the chemical components of Camellia oleifera. Chin. Traditi. Herbal. Drugs 2003, 34, 117–118. [Google Scholar]
- Wang, Y.; Fei, X.; Lu, K.; Yao, X.; Guo, S.; Wang, K. Inhibitory effect of Aspergillus flavus and component analysis of methanol extraction from camellia seed cake. Trans. Chin. Soc. Agric. Eng. 2019, 35, 322–329. [Google Scholar]
- Cheng, H.Y. Study on the Chemical Constituents of Camellia oleifera Abel. Master’s Thesis, Jinan University, Guangzhou, China, 2022. [Google Scholar]
- Ye, Y.; Guo, Y.; Luo, Y.T.; Wang, Y.F. Isolation and free radical scavenging activities of a novel biflavonoid from the shells of Camellia oleifera Abel. Fitoterapia 2012, 83, 1585–1589. [Google Scholar] [CrossRef]
- Ye, Y.; Guo, Y.; Luo, Y.T. Anti-inflammatory and analgesic activities of a novel biflavonoid from shells of Camellia oleifera. Int. J. Mol. Sci. 2012, 13, 12401–12411. [Google Scholar] [CrossRef]
- Shu, Q.; Wu, Y.Y.; Tang, Y.J.; Yuan, Y.F.; Wang, M.; Zhou, G.X. Chemical constituents from hull of Camellia oleifera Able. Nat. Prod. Res. Dev. 2025, 37, 1243–1251. [Google Scholar]
- Jiao, B.; Xu, C.; Li, Q.; Qin, J.; Luo, Y.; Yang, W. Chemical constituents of the flavonoids from Camellia oleifera and their anti-inflammatory activities in vitro. Chin. Tradit. Pat. Med. 2019, 41, 327–333. [Google Scholar]
- Luo, Y.; Li, B. Studies on chemical constituents of Camellia oleifera Abel. Chem. J. Int. 2003, 5, 20–26. [Google Scholar]
- Zhu, W.F.; Wang, C.L.; Ye, F.; Sun, H.P.; Ma, C.Y.; Liu, W.Y.; Feng, F.; Abe, M.; Akihisa, T.; Zhang, J. Chemical constituents of the seed cake of Camellia oleifera and their antioxidant and antimelanogenic activities. Chem. Biodivers. 2018, 15, e1800137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.D.; Nie, S.P.; Xie, M.Y.; Hu, J.L. Antioxidant and antibacterial capabilities of phenolic compounds and organic acids from Camellia oleifera cake. Food Sci. Biotechnol. 2020, 29, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.F.; Chen, L.; Li, J.; Liang, L.; Fan, Y.; Qiu, L.; Deng, Z. Two kaempferol glycosides separated from Camellia oleifera meal by high-speed countercurrent chromatography and their possible application for antioxidant. J. Food Sci. 2019, 84, 2805–2811. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.J. Structure and growth of plant cell walls. Nat. Rev. Mol. Cell Biol. 2024, 25, 340–358. [Google Scholar] [CrossRef]
- Sha, M.; Li, X.; Liu, Y.; Tian, H.; Liang, X.; Li, X.; Gao, W. Comparative chemical characters of Pseudostellaria heterophylla from geographical origins of China. Chin. Herb. Med. 2023, 15, 439–446. [Google Scholar] [CrossRef]
- Zhang, S.; Li, X. Hypoglycemic activity in vitro of polysaccharides from Camellia oleifera Abel. seed cake. Int. J. Biol. Macromol. 2018, 115, 811–819. [Google Scholar] [CrossRef]
- Feng, S.; Zhang, J.; Luo, X.; Xu, Z.; Liu, K.; Chen, T.; Zhou, L.; Ding, C. Green extraction of polysaccharides from Camellia oleifera fruit shell using tailor-made deep eutectic solvents. Int. J. Biol. Macromol. 2023, 253, 127286. [Google Scholar] [CrossRef]
- Zhang, L.M.; Yang, T.T.; Hu, J.N.; Liu, Z.G.; Deng, Z.Y. Ultrasonic-assisted extraction and antioxidant activity of polysaccharides from seed cake of Camellia oleifera Abel. Food Sci. 2013, 34, 36–40. [Google Scholar]
- Shen, S.; Cheng, H.; Li, X.; Li, T.; Yuan, M.; Zhou, Y.; Ding, C. Effects of extraction methods on antioxidant activities of polysaccharides from camellia seed cake. Eur. Food Res. Technol. 2014, 238, 1015–1021. [Google Scholar] [CrossRef]
- Xu, B.Y. Extraction, Separation, Structure Analysis and Hypoglycemic Activity of Polysaccharide from Camellia Seed Meal. Master’s Thesis, Hefei University of Technology, Hefei, China, 2016. [Google Scholar]
- Xu, Z.; Li, X.; Feng, S.; Liu, J.; Zhou, L.; Yuan, M.; Ding, C. Characteristics and bioactivities of different molecular weight polysaccharides from camellia seed cake. Int. J. Biol. Macromol. 2016, 91, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.S.; Guo, Y.H.; Xu, B.Y.; Wang, H.X.; Yuan, C.X. Physicochemical properties of polysaccharides separated from Camellia oleifera Abel seed cake and its hypoglycemic activity on streptozotocin-induced diabetic mice. Int. J. Biol. Macromol. 2019, 125, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.H. Separation, Purification, Physicochemical Properties and Antioxidant Activity of Polysaccharides Separated from Camellia oleifera Abel Seed Cake. Master’s Thesis, Hefei University of Technology, Hefei, China, 2019. [Google Scholar]
- Jin, R.S.; Shan, Q.; Yuan, C.X. Structural characterization of camellia seed meal polysaccharide and its effect on intestinal microflora. Farm. Prod. Process. 2022, 543, 7–13. [Google Scholar]
- Guo, X.; Zhao, J.Y.; Zhao, B.; Zhang, G.C. Optimization of ultrasonic-assisted extraction process and antioxidant activity of polysaccharides from oil-tea camellia seed hull. China Oils Fats 2024, 49, 104–109, 117. [Google Scholar]
- Cheng, H.Y.; Xu, T.Q.; Hu, Y.L.; Shu, Q.; Xu, W.; Fan, C.L.; Zhou, G.X. Two new aryltetralin-type lignans from Camellia oleifera husk. Nat. Prod. Res. 2024, 38, 2264–2271. [Google Scholar] [CrossRef]
- Lee, C.P.; Yen, G.C. Antioxidant activity and bioactive compounds of tea seed (Camellia oleifera Abel.) oil. J. Agr. Food Chem. 2006, 54, 779–784. [Google Scholar] [CrossRef]
- Xiao, Y.P.; Zhang, B.; Deng, D.W.; Luo, J.X.; Bai, X. Separation and analysis of unsaponifiable matters in camellia seed oil. J. Nanchang Univ. Eng. Technol. 2015, 37, 16–19. [Google Scholar]
- Luo, Y.; Wei, Z.; Liu, J.; Yang, X.; Du, C.; Liu, Y.; Xue, Y.; Wang, X.; Zheng, Z.; Duan, Z. Chemometric assessment of quality biomarkers in Camellia oleifera seed oils in the leading production regions of China. J. Food Compos. Anal. 2025, 146, 107949. [Google Scholar] [CrossRef]
- Gao, S.; Wang, B.F.; Liu, F.D.; Zhao, J.R.; Yuan, J.; Xiao, S.X.; Masabni, J.; Zou, F.; Yuan, D.Y. Variation in fruit morphology and seed oil fatty acid composition of Camellia oleifera collected from diverse regions in southern China. Horticulturae 2022, 8, 818. [Google Scholar] [CrossRef]
- Cao, M.; Tang, Y.; Luo, Y.; Gu, F.; Zhu, Y.; Liu, X.; Yan, C.; Hu, W.; Wang, S.; Chao, X.; et al. Natural compounds modulating mitophagy: Implications for cancer therapy. Cancer. Lett. 2024, 582, 216500. [Google Scholar] [CrossRef]
- Wang, D.X.; Huo, R.W.; Cui, C.J.; Gao, Q.; Zong, J.F.; Wang, Y.J.; Sun, Y.; Hou, R.Y. Anticancer activity and mechanism of total saponins from the residual seed cake of Camellia oleifera Abel. in hepatoma-22 tumor-bearing mice. Food Funct. 2019, 10, 2480–2490. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, S.; Chen, S.; Zuo, Y.; Mu, X.; Zhou, H.; Yao, Y.; Peng, X.; Li, W. UPLC-Q-TOF/MS-based serum metabolomics revealed anti-tumor mechanism of ethanol-soluble acidic components from Camellia oleifera cake and synergy with PD-1 inhibitor. Food Biosci. 2025, 65, 106005. [Google Scholar] [CrossRef]
- Li, T.T.; Zhang, H.; Wu, C. Screening of antioxidant and antitumor activities of major ingredients from defatted Camellia oleifera seeds. Food Sci. Biotechnol. 2014, 23, 873–880. [Google Scholar] [CrossRef]
- Jin, X.; Ning, Y. Antioxidant and antitumor activities of the polysaccharide from seed cake of Camellia oleifera Abel. Int. J. Biol. Macromol. 2012, 51, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Kavyani, B.; Ahmadi, S.; Nabizadeh, E.; Abdi, M. Anti-oxidative activity of probiotics; focused on cardiovascular disease, cancer, aging, and obesity. Microb. Pathog. 2024, 196, 107001. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Kang, J.; Guo, W.; Wang, F.; Guo, M.; Feng, S.; Zhou, W.; Li, J.; Tahir, A.T.; Wang, S.; et al. An optimal medicinal and edible Chinese herbal formula attenuates particulate matter-induced lung injury through its anti-oxidative, anti-inflammatory and anti-apoptosis activities. Chin. Herb. Med. 2023, 15, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Tu, P.S.; Tung, Y.T.; Lee, W.T.; Yen, G.C. Protective effect of camellia oil (Camellia oleifera Abel.) against ethanol-induced acute oxidative injury of the gastric mucosa in mice. J. Agric. Food Chem. 2017, 65, 4932–4941. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, Y.; Xie, J.; Yu, Q.; Lu, H.; Zhong, J.; Chen, Y. Camellia oleifera seeds cake: Polyphenol profile and in vitro antioxidant activities as determined by different harvest periods. Food Biosci. 2023, 55, 103081. [Google Scholar] [CrossRef]
- Wei, M.; Hu, Y.; Zou, W.; Li, Y.; Cao, Y.; Li, S.; Huang, J.; Xing, L.; Huang, B.; Wang, X. Physicochemical property and antioxidant activity of polysaccharide from the seed cakes of Camellia oleifera Abel. Food Sci. Nutr. 2022, 10, 1667–1682. [Google Scholar] [CrossRef]
- Yao, L.; Huang, Q.; Wang, H.; Feng, T.; Yu, C.; Xie, K.; Liu, H.; Song, S.; Shao, L.; Sun, M. Microbial hydrolysis of Camellia seed cake with Bacillus subtilis: Fermentation process optimization and bioactivity assessment of the hydrolysates. Biocatal. Agr. Biotech. 2025, 66, 103579. [Google Scholar] [CrossRef]
- Nechchadi, H.; Boulbaroud, S.; Nadir, Y.; Benhssaine, K.; Berrougui, H.; Alem, C.; Ramchoun, M. Hypolipidemic activity of phytochemical combinations: A mechanistic review of preclinical and clinical studies. Food Chem. 2024, 459, 140264. [Google Scholar] [CrossRef]
- Yang, H.Y.; Yeh, W.J.; Ko, J.; Chen, J.R. Camellia oleifera seed extract attenuated abdominal and hepatic fat accumulation in rats fed a high-fat diet. Appl. Physiol. Nutr. Metab. 2019, 44, 320–325. [Google Scholar] [CrossRef]
- Shi, H.; He, X.E.; Ding, R.H.; Wang, W.L.; Huang, Q.; You, J. Effect of Camellia polyphenols on blood lipid reduction and antioxidation in SD rats. Food Res. Dev. 2021, 42, 28–34. [Google Scholar]
- Wang, J.; Zhang, Y.H.; Liu, Y.H.; Zhang, Y.; Xu, Q.; Zhang, X.S.; Yu, X.M.; Zhang, R.X.; Xue, C.Y. Effects of teaseed oil on triglyceride and weight in hypertriglyceridemic subjects. J. Hyg. Res. 2014, 43, 92–95. [Google Scholar]
- Bumrungpert, A.; Pavadhgul, P.; Kalpravidh, R.W. Camellia oil-enriched diet attenuates oxidative stress and inflammatory markers in hypercholesterolemic subjects. J. Med. Food 2016, 19, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Tan, D.H.; Li, B.; Wang, Y.Q.; Shi, L. Gypenoside ameliorates insulin resistance and hyperglycemia via the AMPK-mediated signaling pathways in the liver of type 2 diabetes mellitus mice. Food Sci. Hum. Well. 2022, 11, 1347–1354. [Google Scholar] [CrossRef]
- Zhang, S.; Li, X.Z. Inhibition of α-glucosidase by polysaccharides from the fruit hull of Camellia oleifera Abel. Carbohy. Polym. 2015, 115, 38–43. [Google Scholar] [CrossRef]
- Chandimali, N.; Bak, S.G.; Park, E.H.; Lim, H.J.; Won, Y.S.; Kim, E.K.; Park, S.I.; Lee, S.J. Free radicals and their impact on health and antioxidant defenses: A review. Cell Death Discov. 2025, 24, 19. [Google Scholar] [CrossRef]
- Duan, Y.; Zhou, Y.H.; Li, S.X.; Li, J. Advances in phytochemical constituents and antibacterial and anti-inflammatory activity of Camellia oleifera Abel. Nat. Prod. Res. Dev. 2021, 33, 1603–1615. [Google Scholar]
- Lin, X.; Li, Y.; Zhan, M.; Fu, X.J.; Zhong, H.Y.; Yao, W.; Liu, C. Metabolite composition and anti-inflammatory activity of ethanol extract of Camellia oleifera seed. Food Sci. 2023, 44, 304–311. [Google Scholar]
- Ye, Y.; Xing, H.T.; Chen, X.L. Anti-inflammatory and analgesic activities of the hydrolyzed sasanquasaponins from the defatted seeds of Camellia oleifera. Arch. Pharm. Res. 2013, 36, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Dharmalingam, K. Herbal remedies for Alzheimer’s disease: Neuroprotective mechanisms and cognitive enhancement potential. Digital Chin. Med. 2025, 8, 183. [Google Scholar]
- Amtul, Z.; Westaway, D.; Cechetto, D.F.; Rozamhel, R.F. Oleic acid ameliorates amyloidosis in cellular and mouse models of Alzheimer’s disease. Brain Pathol. 2011, 21, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhou, N.; Chen, L.; Liu, T.; Guo, P.; Zhang, B.; Zhang, Z.; Zeng, M.; Xiong, W.; Zheng, X.; et al. Urine metabolomics analysis of the intervention effect of camellia oil in mice with Alzheimer’s disease. Food Sci. 2024, 45, 114–121. [Google Scholar]
- Weng, M.H.; Chen, S.Y.; Li, Z.Y.; Yen, G.C. Camellia oil alleviates the progression of Alzheimer’s disease in aluminum chloride-treated rats. Free Radic. Biol. Med. 2020, 152, 411–421. [Google Scholar] [CrossRef]
- Guo, P.; Zeng, M.; Cao, B.; Liu, M.; Zhang, Y.; Jia, J.; Zhang, Q.; Zhang, B.; Wang, R.; Xiong, W.; et al. Camellia oil improves Aβ25–35-induced memory impairment by regulating the composition of the gut microbiota and lipid metabolism in mice. J. Funct. Foods 2022, 96, 105214. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, C.; Zhao, J.; Ye, Y. Synthesis and neuroprotective effects of the complex nanoparticles of iron and sapogenin isolated from the defatted seeds of Camellia oleifera. Pharm. Biol. 2017, 55, 428–434. [Google Scholar] [CrossRef]
- Yang, Q.; Fang, F.; Li, Y.; Ye, Y. Neuroprotective effects of the nanoparticles of Zinc sapogenin from seeds of Camellia oleifera. J. Nanosci. Nanotechnol. 2017, 17, 2394–2400. [Google Scholar] [CrossRef]
- James, R.; Hardefeldt, L.Y.; Ierano, C.; Charani, E.; Dowson, L.; Elkins, S.; Thursky, K. Antimicrobial stewardship from a One Health perspective. Nat. Rev. Microbiol. 2025, 9, 10. [Google Scholar] [CrossRef]
- Akihisa, T.; Tokuda, H.; Ukiya, M.; Suzuki, T.; Enjo, F.; Koike, K.; Nikaido, T.; Nishino, H. 3-Epicabraleahydroxylactone and other triterpenoids from camellia oil and their inhibitory effects on Epstein–Barr virus activation. Chem. Pharm. Bull. 2004, 52, 153–156. [Google Scholar] [CrossRef]
- Yang, C.; Wang, W.J.; Tang, W.L.; Chen, H. Comparison of antibacterial activity of tea oil extracted by two different methods. J. Gannan Norm. Univ. 2021, 3, 121–124. [Google Scholar]
- Duan, Y.; Huang, D.; Zhou, Y.H.; Yang, F.M.; Zhou, T.T.; Li, S.X.; Li, J. Effect of refining process on chemical components and antibacterial activity of solvent extracted oil-tea camellia seed oil. China Oils Fats 2022, 47, 45–51. [Google Scholar]
- Cheng, Y.T.; Wu, S.L.; Ho, C.Y.; Huang, S.M.; Cheng, C.L.; Yen, G.C. Beneficial effects of camellia oil (Camellia oleifera Abel.) on ketoprofen-induced gastrointestinal mucosal damage through upregulation of HO-1 and VEGF. J. Agric. Food Chem. 2014, 62, 642–650. [Google Scholar] [CrossRef]
- Lin, C.Y.; Chen, S.Y.; Lee, W.T.; Yen, G.C. Immunomodulatory effect of camellia oil (Camellia oleifera Abel.) on CD19+ B cells enrichment and IL-10 production in BALB/c mice. J. Funct. Foods 2022, 88, 104863. [Google Scholar] [CrossRef]

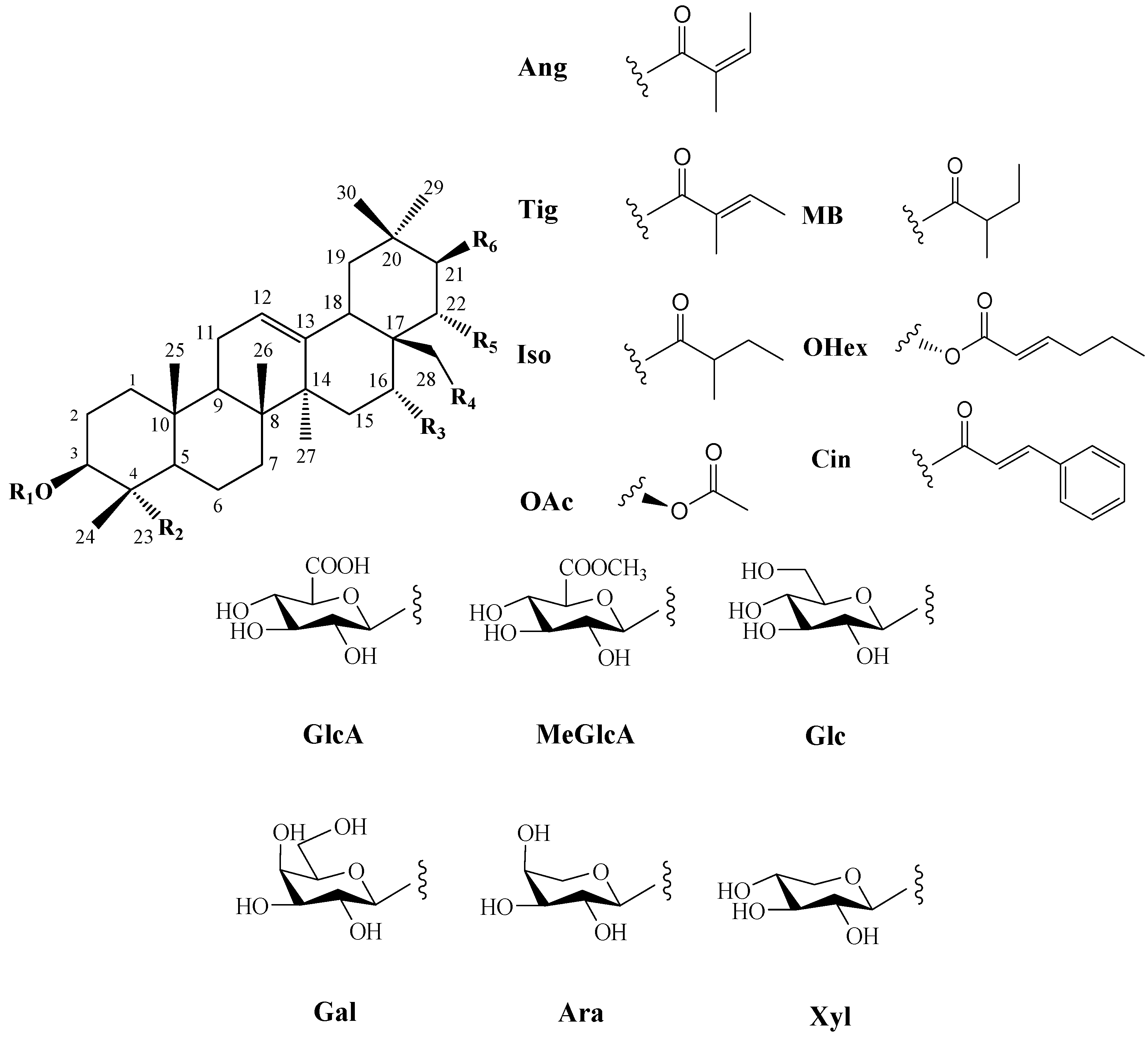
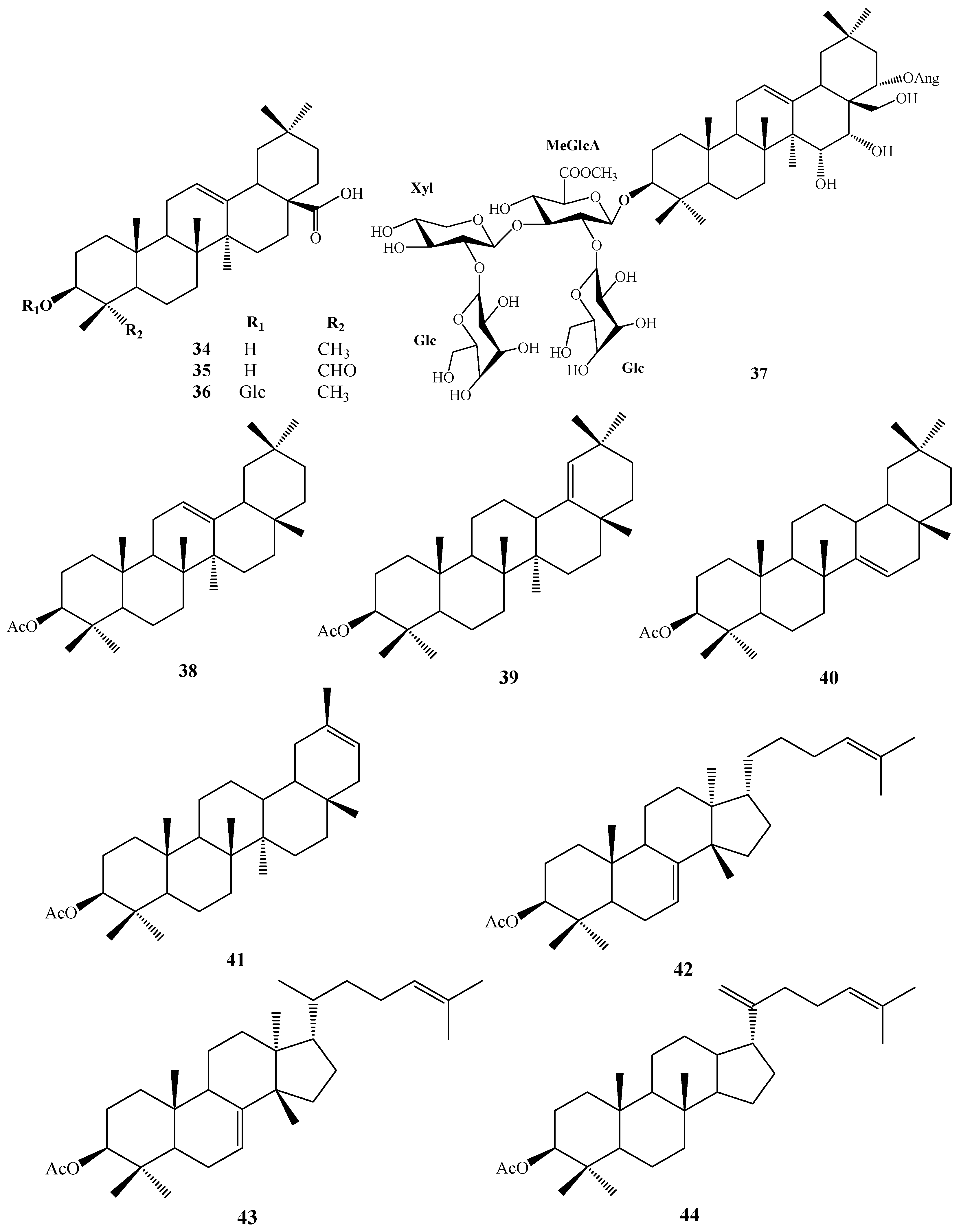
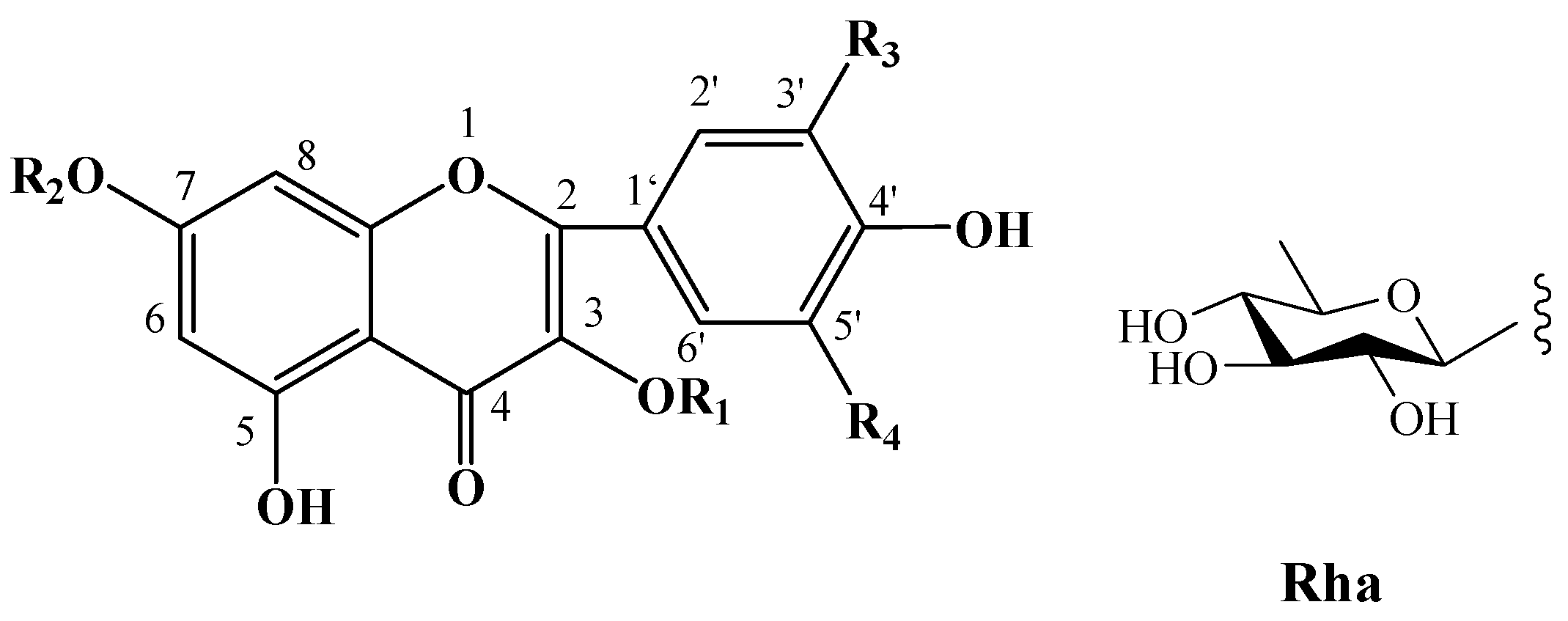
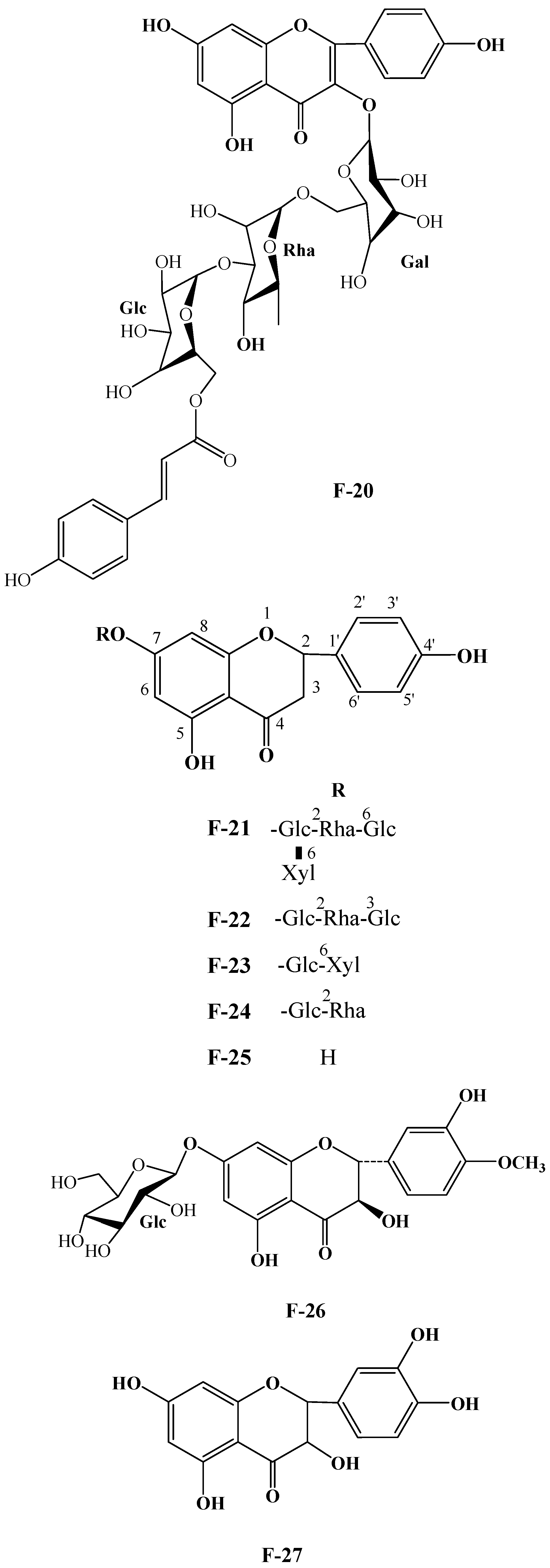
| No. | Compound Name | Chemical Formula | R1 | R2 | R3 | R4 | R5 | R6 |
|---|---|---|---|---|---|---|---|---|
| 1 [20] | 21-O-angeloyl-28-O-acetyltheasapogenol E 3-O-[β-D-galactopyranosyl(1 → 2)] [β-D-xylopyranosyl (1 → 2)-α-L-arabinopyranosyl (1 → 3)]-methyl β-D-glucopyranosiduronate | C60H92O27 | 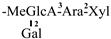 | CHO | OH | OAc | OH | OAng |
| 2 [6] | camelliasaponin B1 | C58H89O26 | 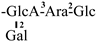 | CHO | OH | OH | OH | OAng |
| 3 [21] | camelliasaponin B2 | C58H90O26 | 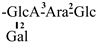 | CHO | OH | OH | OH | OTig |
| 4 [22] | oleiferasaponin A1 | C59H92O26 | 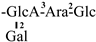 | CHO | OH | OH | OHex | H |
| 5 [23] | oleiferasaponin A2 | C63H99O28 | 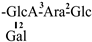 | CHO | OH | OH | OIso | OAng |
| 6 [21] | oleiferasaponin A3 | C67H96O28 | 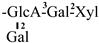 | CHO | OH | OH | OCin | OAng |
| 7 [24] | oleiferasaponin B1 | C58H90O26 | 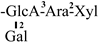 | CHO | OH | OH | OTig | CH2OH |
| 8 [24] | oleiferasaponin B2 | C61H90O24 | 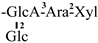 | CHO | OH | OH | OCin | H |
| 9 [25] | oleiferasaponin C1 | C59H91O26 | 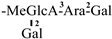 | CHO | OH | H | OAng | H |
| 10 [25] | oleiferasaponin C2 | C60H95O26 | 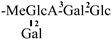 | CH3 | OH | H | OAng | H |
| 11 [25] | oleiferasaponin C3 | C63H91O26 | 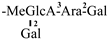 | CHO | OH | OCin | OH | H |
| 12 [26] | oleiferasaponin C4 | C60H94O27 | 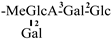 | CHO | OH | OH | OAng | H |
| 13 [26] | oleiferasaponin C5 | C54H84O22 | 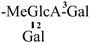 | CHO | OH | OH | OAng | H |
| 14 [27] | oleiferasaponin C6 | C65H94O28 | 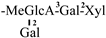 | CHO | OH | OH | OCin | OAc |
| 15 [28] | oleiferasaponin D1 | C58H91O25 | 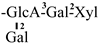 | CH3 | OH | OH | OAng | H |
| 16 [28] | oleiferasaponin D2 | C58H89O26 | 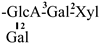 | CHO | OH | OH | OAng | H |
| 17 [28] | oleiferasaponin D3 | C58H89O26 | 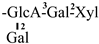 | CHO | OH | OH | OTig | H |
| 18 [28] | oleiferasaponin D4 | C58H91O26 | 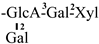 | CHO | OH | OH | OMB | H |
| 19 [28] | oleiferasaponin D5 | C58H91O27 | 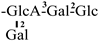 | CHO | OH | OH | OTig | H |
| 20 [29] | sasanqua saponin | C30H48O5 | H | CHO | OH | OH | OH | H |
| 21 [30] | gordonoside R | C62H96O26 | 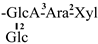 | CH3 | OH | OH | OAng | OAng |
| 22 [30] | gordonoside Q | C62H96O26 | 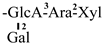 | CH3 | OH | OH | OAng | OAng |
| 23 [31] | C1 | C35H54O6 | H | CHO | OTig | OH | OH | H |
| 24 [31] | C2 (camelliagenin A 16-tiglate) | C35H56O5 | H | CH3 | OTig | OH | OH | H |
| 25 [31] | C3 (camelliagenin A 22-tiglate) | C35H56O5 | H | CH3 | OH | OH | OTig | H |
| 26 [31] | C4 | C47H70O14 | MeGlcA | CH3 | OH | OH | OTig | OTig |
| 27 [32] | oleiferasaponin G1 | C58H90O26 |  | CHO | OTig | OH | OH | H |
| 28 [32] | oleiferasaponin G2 | C59H92O27 | 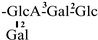 | CHO | OTig | OH | OH | H |
| 29 [32] | oleiferasaponin G3 | C59H92O27 | 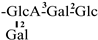 | CHO | OAng | OH | OH | H |
| 30 [32] | oleiferasaponin G4 | C59H92O27 | 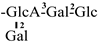 | CHO | OMB | OH | OH | H |
| 31 [32] | oleiferasaponin G5 | C58H90O26 | 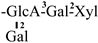 | CHO | OAng | OH | OH | H |
| 32 [32] | oleiferasaponin G6 | C60H92O28 | 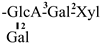 | CHO | OH | OH | OAng | OAc |
| 33 [32] | oleiferasaponin G7 | C59H92O27 | 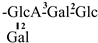 | CHO | OH | OH | OTig | H |
| No. | Compound Name | Chemical Formula | R1 | R2 | R3 | R4 |
|---|---|---|---|---|---|---|
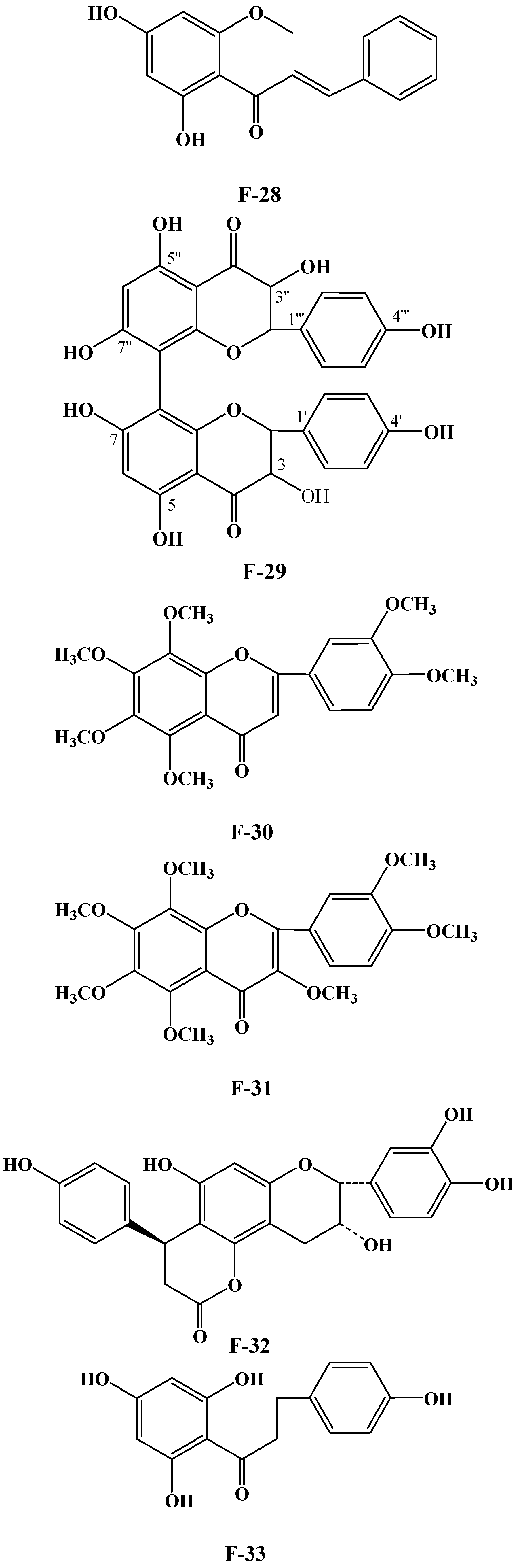
| F-1 [35] | kaempferol | C15H10O6 | H | H | H | H |
| F-2 [36] | quercetin | C15H10O7 | H | H | OH | H |
| F-3 [36] | rutin | C27H30O16 | 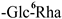 | H | OH | H |
| F-4 [36] | kaempferol-3-O-β-D-glucopyranosyl-(1 → 2)-α-L-arabinopyranoside | C26H30O16 | 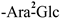 | H | H | H |
| F-5 [35] | kaempferol-3-O-α-L-rhamnopyranosyl-(1 → 6)-β-D-glucopyranoside | C27H30O15 | 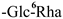 | H | H | H |
| F-6 [35] | kaempferol-3-O-[2-O-α-L-rhamnopyranosyl-6-O-β-D-glucopyranosyl]-β-D-glucopyranoside | C33H40O20 | 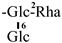 | H | H | H |
| F-7 [35] | kaempferol-3-O-[2-O-β-D-xylopyranosyl-6-O-α-L-rhamnopyranosyl]-β-D-glucopyranoside | C32H38O19 | 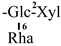 | H | H | H |
| F-8 [35] | kaempferol-3-O-[2-O-α-L-rhamnopyranosyl-6-O-β-D-glucopyranosyl]-β-D-glucopyranoside | C32H38O19 | 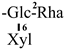 | H | H | H |
| F-9 [36] | kaempferol-3-O-[2-O-β-D-galacotopyranosyl-6-O-α-L-rhamnopyranosyl]-β-D-glucopyranoside | C33H40O20 | 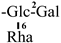 | H | H | H |
| F-10 [42] | kaempferol-3-O-[2-O-β-D-glucopyranosyl-6-O-α-L-rhamnopyranosyl]-β-D-glucopyranoside | C33H40O20 | 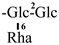 | H | H | H |
| F-11 [20,43] | kaempferol 3,7-O-bis-α-L-rhamnopyranoside (kaempferitrin) | C27H30O14 | Rha | Rha | H | H |
| F-12 [43] | kaempferol-3-O-β-D-glucopyranoside | C21H20O11 | Glc | H | H | H |
| F-13 [43] | isorhamnetin | C16H12O7 | H | H | H | OCH3 |
| F-14 [44] | quercetin-3-O-β-D-glucopyranoside | C21H20O12 | Glc | H | OH | H |
| F-15 [44] | quercetin-3-O-β-D-glucuronide | C21H18O13 | GlcA | H | OH | H |
| F-16 [44] | kaempferol-3-O-β-D-glucuronide | C21H18O12 | GlcA | H | H | H |
| F-17 [45] | kaempferol 3-O-[α-L-rhamnopyranosyl-(1 → 6)-β-D-glucopyranosyl]-7-O-β-D-glucopyranoside | C33H40O20 | 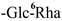 | Glc | H | H |
| F-18 [45] | kaempferol 3-O-[β-D-glucopyranosyl-(1 → 4)-α-L-rhamnopyranosyl]-7-O-α-L-rhamnopyranoside | C33H40O19 | 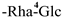 | Rha | H | H |
| F-19 [46] | kaempferol-3-O-[β-D-glucopyranosyl-(1 → 3)-α-L-rhamnopyranosyl-(1 → 6)-O-β-D-galactopyranoside | C33H40O20 | 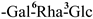 | H | H | H |
| No. | Polysaccharides | The Types and Proportions of Monosaccharides | M.W. (Da) | Total Sugar Content |
|---|---|---|---|---|
| 1 [49] | CCP | Xyl:GlcA:Gal:Man =10.9:4.4:2.6:1.8 | 4736 | – |
| 2 [51] | – | Glc:Rha:Man:GalA:Gal:Xyl:Ara = 1.0:0.40:0.28:0.09:0.17:0.10:0.07 | – | 81.20% |
| 3 [50] | CSP-1 | Rha:Ara:Gal:Glc:Xyl:Man:GalA:GlcA = 0.04:0.16:0.10:0.34:0.02:0.02:0.27:0.02 | 8.90 × 104, 13.51 × 104 | 75.93% |
| 4 [50] | CSP-2 | Rha:Ara:Gal:Glc:Xyl:Man:GalA:GlcA = 0.04:0.23:0.18:0.20:0.03:0.01:0.03:0.04 | 9.92 × 104 | 69.22% |
| 5 [50] | CSP-3 | Rha:Ara:Gal:Glc:Xyl:Man:GalA:GlcA = 0.03:0.12:0.07:0.20:0.01:0.01:0.02:0.01 | 1.83 × 105 | 80.61% |
| 6 [50] | CSP-4 | Rha:Ara:Gal:Glc:Xyl:Man:GalA:GlcA = 0.05:0.18:0.08:0.15:0.01:0.02:0.03:0.01 | 3.61 × 105, 7.426 × 105 | 75.93% |
| 7 [52] | COP-H | Glc:Gal:Man:Rha:Ara:Fuc = 46.55:18.89: 15.33:8.37:1.11:9.75 | 3.94 × 105 | – |
| 8 [52] | COP-U | Glc:Gal:Man:Rha:Ara:Fuc = 63.06:8.28:9.58:9.04:3.95:6.09 | 2.97 × 105 | – |
| 9 [52] | COP-E | Glc:Gal:Man:Rha:Ara:Fuc = 43.32:11.77: 14.45:5.97:5.11:19.37 | 4.62 × 105 | – |
| 10 [52] | COP-A | Glc:Gal:Man:Rha:Ara:Fuc = 38.31:14.41: 19.00:12.96:3.29:12.04 | 4.29 × 105 | – |
| 11 [53] | SCFP | Rha:Xyl:Glc = 11.19:17.06:5.81 | 2.0121 × 107 | – |
| 12 [54] | COP-1 | Man:Rha:GalA:Glc:Gal:Xyl:Ara = 2.16:1.67:2.34:78.55:8.97:1.39:4.91 | 7.90 × 103 | – |
| 13 [54] | COP-2 | Man:Rha:GalA:Glc:Gal:Xyl:Ara = 2.73:2.38:2.84:72.65:10.82:1.85:6.75 | 3.60 × 104 | – |
| 14 [54] | COP-3 | Man:Rha:GlcA:GalA:Glc:Gal:Xyl:Ara = 2.23:2.45:5.79:5.04:60.03:11.98:1.67:10.81 | 8.30 × 104 | – |
| 15 [54] | COP-4 | Man:Rha:GlcA:GalA:Glc:Gal:Xyl:Ara = 1.58:2.15:10.29:2.96:56.80:11.20:1.14:13.89 | 2.25 × 105 | – |
| 16 [55] | SCP-1 | Man:Glc:Xyl = 1.77:0.93:1.00 | 7.16 × 106 | – |
| 17 [55] | SCP-2 | Man:Rha:Glc:Xyl = 5.27:1.21:0.16:1.00 | 2.00 × 104 | – |
| 18 [56] | SCP-a | Man:Rha:Glc:Gal:Xyl = 1.00:16.82:19.80:103.46:41.96 | 8.26 × 104 | 80.12% |
| 19 [56] | SCP-b | Man:Rha:Glc:Gal:Xyl:Ara = 9.08:1.00:1.00:1.35:0:1.51 | 2.05 × 107 | 2.30% |
| 20 [56] | SCP-c | Man:Rha:Glc =35.74:1.00:5.26 | 1.44 × 107 | 76.12% |
| 21 [57] | CCPA | GalA:Glc:Rha:Gal:Man:Ara = 16.27:16.89:16.02:17.32:14.28:19.22 | 7.02 × 104 | 84.36% |
| 22 [57] | CCPB | GalA:Glc:Rha:Gal:Man:Ara = 6.02:18.88:14.38:20.49:17.79:22.44 | 2.46 × 106 | 80.30% |
| 23 [58] | CFP-U | Man:Rha:GlcA:GalA:Glc:Gal:Xyl:Fuc = 0.05:0.14:005:1.00:0.05:0.30:0.35:0.41 | 9.16 × 104 | – |
| No. | Chemical Constituents | Pharmacological Activities | Models | Mechanisms/Effects |
|---|---|---|---|---|
| 1 [24] | oleiferasaponin B1 | anti-tumor activity | human tumor cell lines (A549, SK-OV-3, SK-MEL-2, and HCT15), in vitro | IC50 values of 18.5 μM (A549), 11.3 μM (SK-OV-3), 13.9 μM (SK-MEL-2), and 1.6 μM (HCT15) |
| 2 [24] | oleiferasaponin B2 | IC50 values of 8.4 μM (A549), 6.3 μM (SK-OV-3), 9.2 μM (SK-MEL-2), and 0.8 μM (HCT15) | ||
| 3 [25] | oleiferasaponin C1 | anti-tumor activity | human cancer cell lines (BEL-7402, BGC-823, MCF-7, HL-60, and KB), in vitro | IC50 values of 6.641 μM (BEL-7402), 12.766 μM (BGC-823), 5.567 μM (MCF-7), 7.930 μM (HL-60), 12.215 μM (KB) |
| 4 [25] | oleiferasaponin C2 | IC50 values of 9.601 μM (BEL-7402), 13.558 μM (BGC-823), 11.223 μM (MCF-7), 4.590 μM (HL-60), 12.995 μM (KB) | ||
| 5 [25] | camelliasaponin B1 | IC50 values of 17.649 μM (BEL-7402), 25.788 μM (BGC-823), 12.995 μM (MCF-7), 18.071 μM (HL-60), 16.544 μM (KB) | ||
| 6 [26] | oleiferasaponin C4 | anti-tumor activity | IC50 values of 10.385 μM (BEL-7402), 11.242 μM (BGC-823), 15.094 μM (MCF-7), 6.489 μM (HL-60), 12.302 μM (KB) | |
| 7 [26] | oleiferasaponin C5 | IC50 values of 17.649 μM (BEL-7402), 25.788 μM (BGC-823), 12.995 μM (MCF-7), 18.071 μM (HL-60), 16.544 μM (KB) | ||
| 8 [27] | oleiferasaponin C6 | IC50 values of 4.023 μM (BEL-7402), 6.001 μM (BGC-823), 9.016 μM (MCF-7), 1.876 μM (HL-60), 6.119 μM (KB) | ||
| 9 [28] | oleiferasaponins D1 | anti-tumor activity | human tumor cell lines (HCT-116, HepG2, BGC-823, NCI-H1650, and A2780), in vitro | IC50 values of 8.33 μM (HCT-116), 6.11 μM (HepG2), 9.53 μM (BGC-823), 7.85 μM (NCI-H1650), 6.48 μM (A2780) |
| 10 [28] | oleiferasaponins D2 | IC50 values of 10.23 μM (HCT-116), 8.52 μM (HepG2), 4.86 μM (BGC-823), 3.31 μM (NCI-H1650), 8.49 μM (A2780) | ||
| 11 [22] | oleiferasaponin A1 | neuroprotective activity | PC12 cells injured by H2O2 at 5, 25, and 125 μM, in vitro | potentially prevent |
| [21] | ||||
| hypolipidemic activity | RIN-m5f was injured by high glucose, in vitro | protecting pancreatic-cell lines from high-glucose damage, insulin levels of RIN-m5f cells ↑ | ||
| 12 [23] | oleiferasaponin A2 | hypolipidemic activity | on HepG2 cell lines, in vitro | SREBP-1c, FAS ↓, CPT-1, and ACOX-1 ↑ |
| 13 [29] | Susanqua saponin | neuroprotective activity | injected with 20 mg/kg MPTP once every 2 h for three consecutive times, Kunming mice, in vivo | increase dopamine content in the striatum and tyrosine hydroxylase positive cells in the substantia nigra, and relieve inflammation and behavioral disorder |
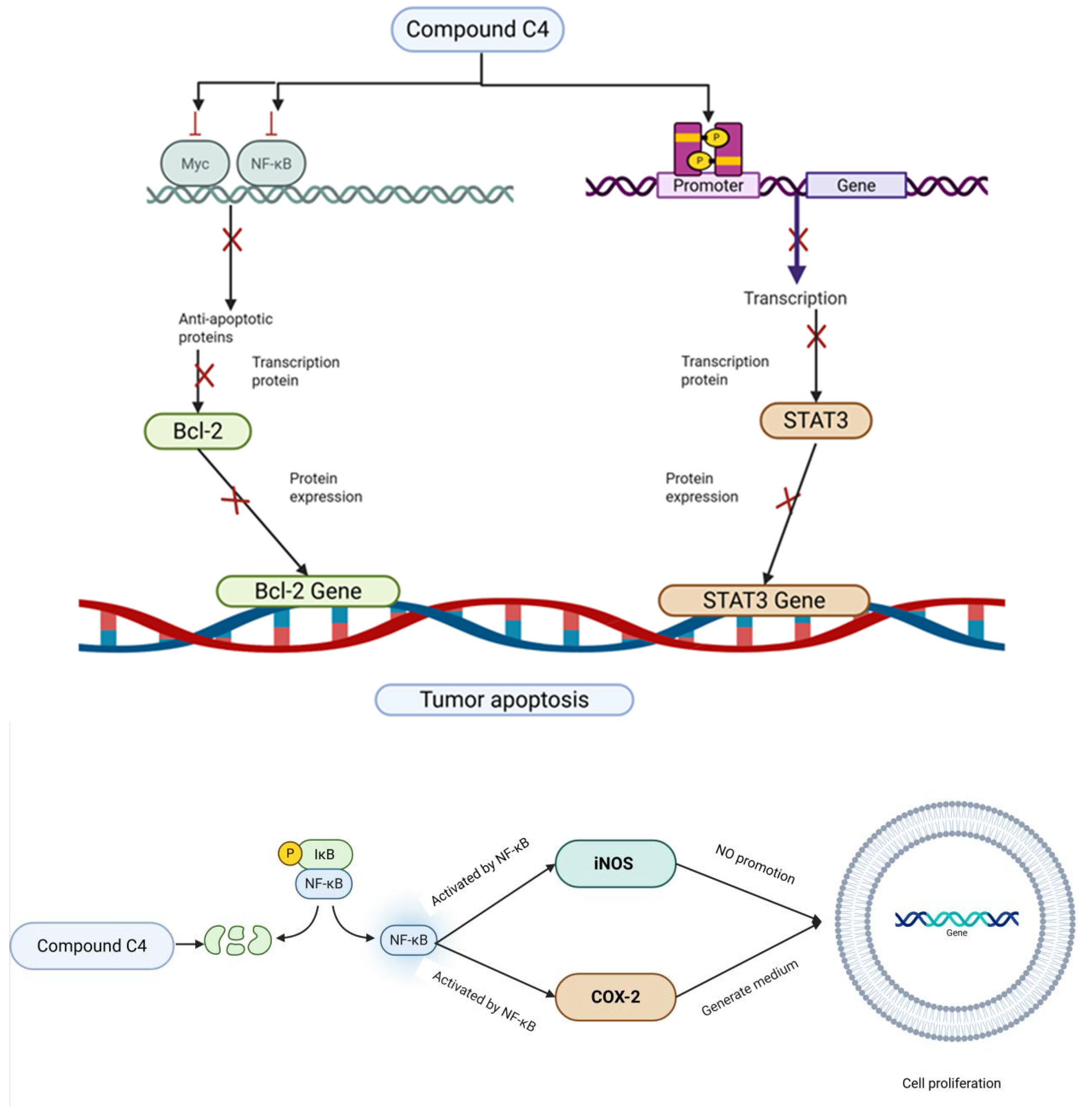
| 14 [31] | C4 | anti-tumor activity | human tumor cell lines (Huh-7, HepG2, Hela, A549, SGC7901, 239T, U251 and PAN02), in vivo | the ED50 values ranged from 1.5 to 11.3 μM p-mTOR, p-STAT3, and Bcl-2 ↓ |
| LPS-induced HepG2 cells, in vivo | β-catenin, Nrf2, HO-1 ↓ Caspase-3, TNF-α, NF-kβ, iNOS, and COX-2 ↑ | |||
| 15 [32] | oleiferasaponin G6 | anti-tumor activity | human tumor cell lines (HCT-116, HL-60, HepG2), in vitro | IC50 values of 1.200 μM (HCT-116), 3.515 μM (HL-60), and 2.518 μM (HepG2) |
| 16 [32] | oleiferasaponin G7 | IC50 values of 1.208 μM (HCT-116), 2.938 μM (HL-60), and 2.711 μM (HepG2) | ||
| 17 [32] | oleiferasaponins D2 | IC50 values of 1.264 μM (HCT-116), 2.775 μM (HL-60), and 1.204 μM (HepG2) | ||
| 18 [32] | oleiferasaponins D3 | IC50 values of 1.181 μM (HCT-116), 5.596 μM (HL-60), and 2.704 μM (HepG2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, B.; Du, A.-N.; Liu, T.-Z.; Wei, P.-H.; Zhu, B.-R.; Chen, K.; Shi, L. Chemical Constituents and Pharmacological Effects of Camellia oleifera Fruits: A Review. Molecules 2025, 30, 3965. https://doi.org/10.3390/molecules30193965
Xu B, Du A-N, Liu T-Z, Wei P-H, Zhu B-R, Chen K, Shi L. Chemical Constituents and Pharmacological Effects of Camellia oleifera Fruits: A Review. Molecules. 2025; 30(19):3965. https://doi.org/10.3390/molecules30193965
Chicago/Turabian StyleXu, Bing, A-Nan Du, Tian-Zhi Liu, Ping-Hui Wei, Bo-Rong Zhu, Kai Chen, and Lin Shi. 2025. "Chemical Constituents and Pharmacological Effects of Camellia oleifera Fruits: A Review" Molecules 30, no. 19: 3965. https://doi.org/10.3390/molecules30193965
APA StyleXu, B., Du, A.-N., Liu, T.-Z., Wei, P.-H., Zhu, B.-R., Chen, K., & Shi, L. (2025). Chemical Constituents and Pharmacological Effects of Camellia oleifera Fruits: A Review. Molecules, 30(19), 3965. https://doi.org/10.3390/molecules30193965







