Mechanochemical Approach to a Monocationic Asymmetric Monomethine Cyanine Dye for Nucleic Acid Analysis and Visualization
Abstract
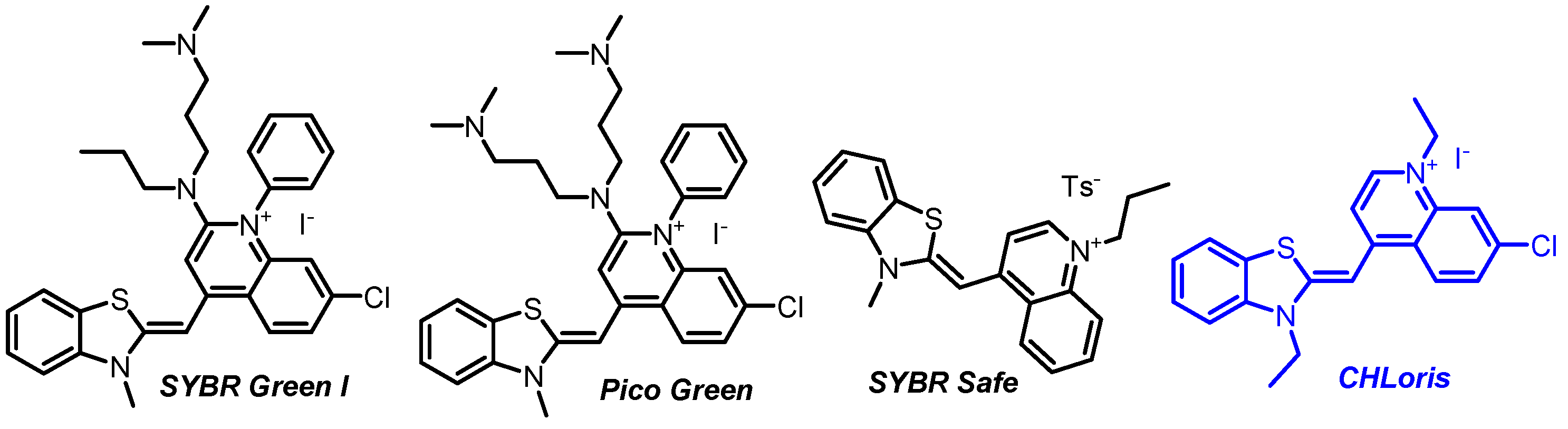
1. Introduction
2. Results and Discussion
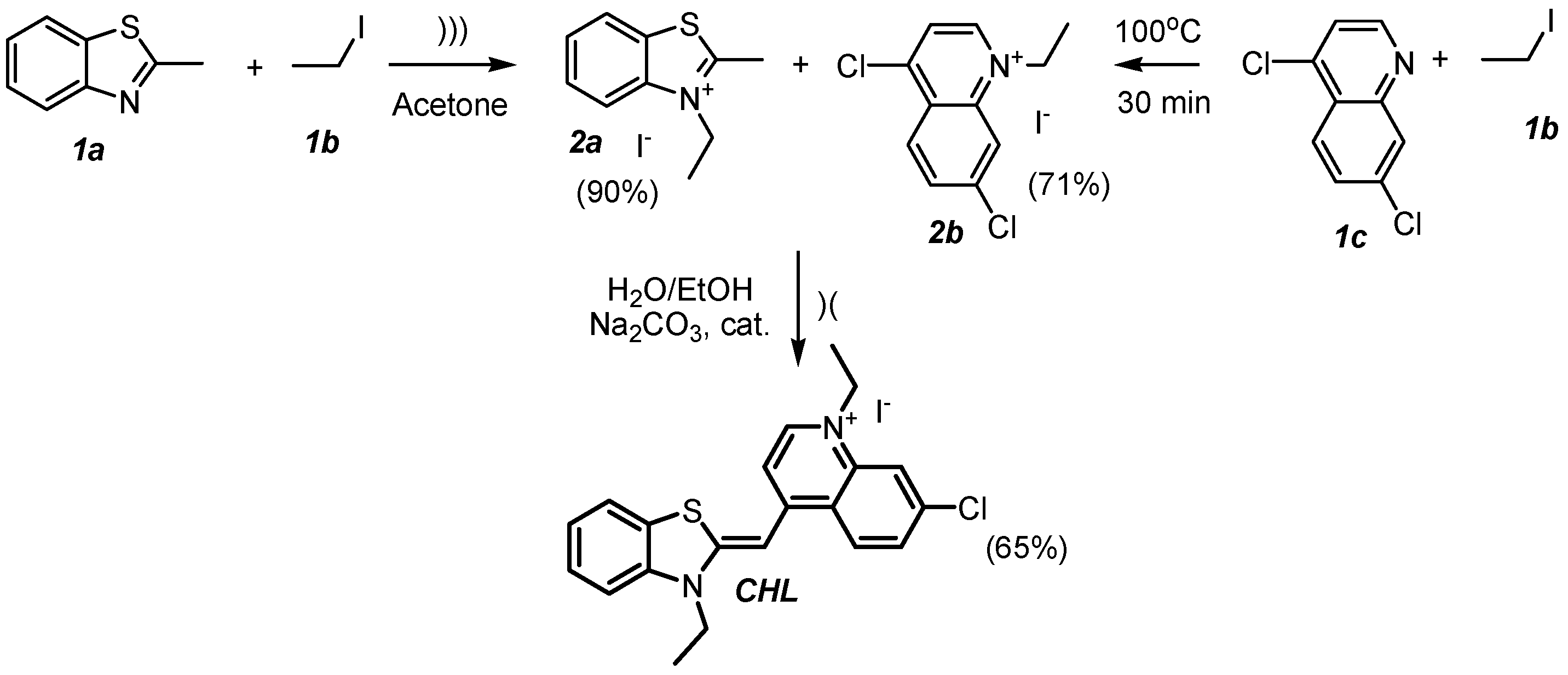
2.1. Synthesis
2.2. Photophysical Properties
2.2.1. Photophysical Properties of the Neat Dye CHL
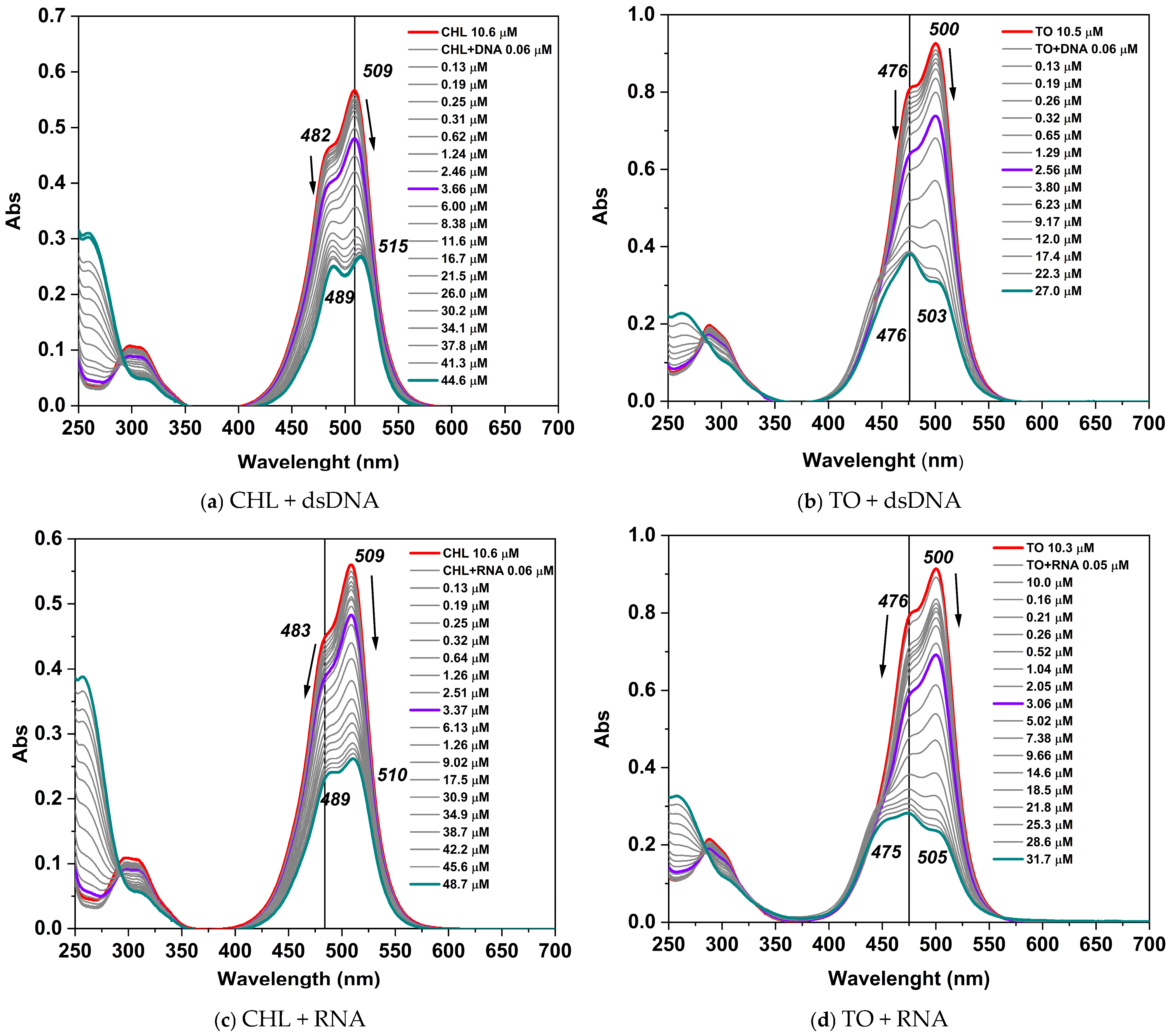
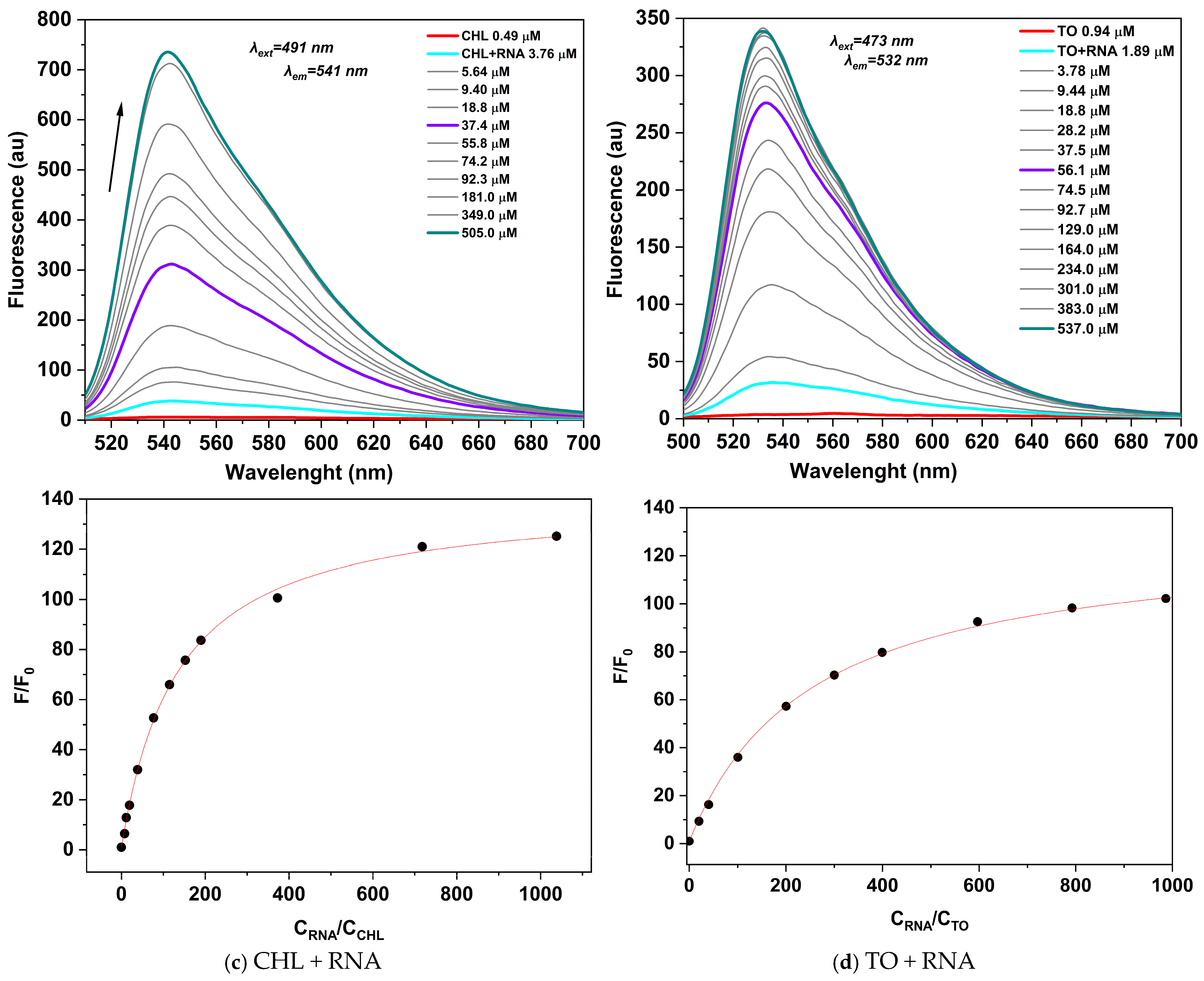
2.2.2. Photophysical Properties of CHL Dye Complexes with dsDNA and RNA
3. Materials and Methods
3.1. General
3.2. Synthesis of 3-Ethyl-2-methylbenzo[d]thiazol-3-ium Iodide (2a)
3.3. Synthesis of 4,7-Dichloro-1-ethylquinolin-1-ium Iodide (2b)
3.4. Synthesis of 7-Chloro-1-ethyl-4-((3-ethylbenzo[d]thiazol-2(3H)-ylidene)methyl)quinolin-1-ium Iodide (CHL)
3.4.1. Procedure A
3.4.2. Procedure B
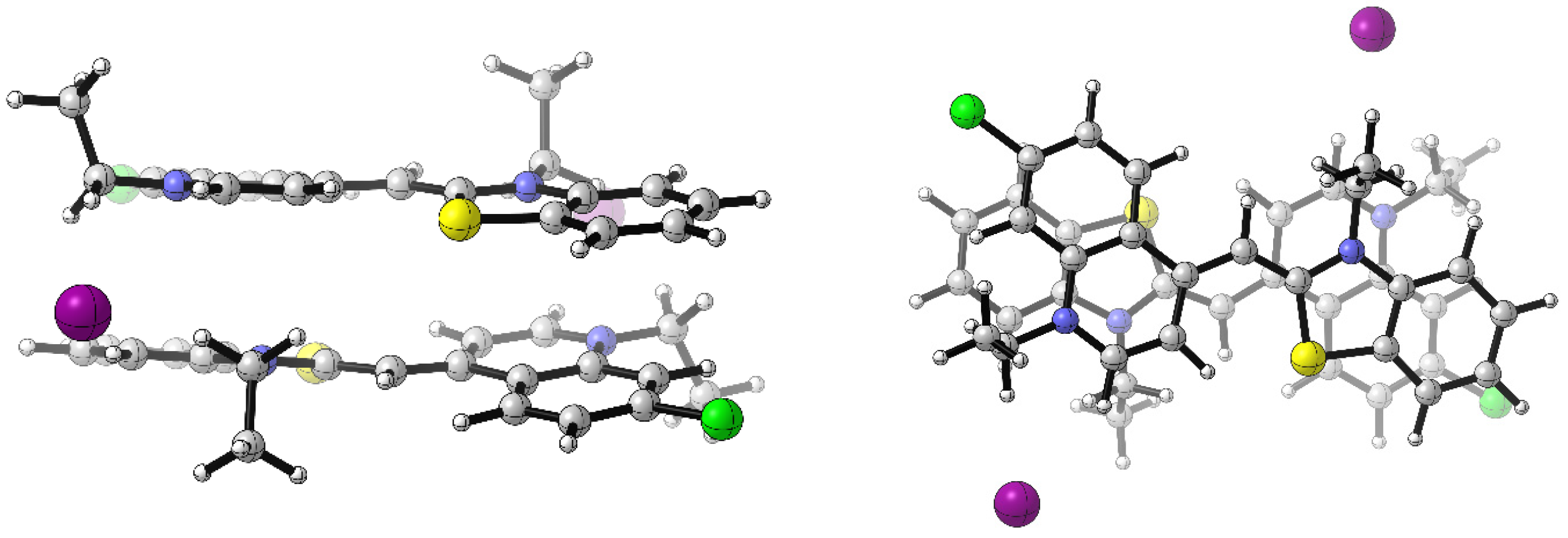
3.5. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, I.; Spence, M. (Eds.) A guide to fluorescent probes and labeling technologies. In Molecular Probes, 11th ed.; Thermo-Fisher: Eugene, OR, USA, 2010. [Google Scholar]
- Deligeorgiev, T.; Vasilev, A. Functional Dyes; Kim, S.-H., Ed.; Elsevier: Amsterdam, The Netherlands, 2006; Chapter 4; p. 137. [Google Scholar]
- Kurutos, A.; Nikodinovic-Runic, J.; Veselinovic, A.; Veselinović, J.B.; Kamounah, F.S.; Tatjana, I.-T. RNA-Targeting Low-Molecular-Weight Fluorophores for Nucleoli Staining: Synthesis, In Silico Modelling and Cellular Imaging. New J. Chem. 2021, 45, 12818–12829. [Google Scholar] [CrossRef]
- Yan, X.; Chen, X.; Shan, Z.; Bi, L. Innovative Cyanine-Based Fluorescent Dye for Targeted Mitochondrial Imaging and Its Utility in Whole-Brain Visualization. ACS Omega 2024, 9, 2585–2596. [Google Scholar] [CrossRef]
- Minoshima, M.; Reja, S.I.; Hashimoto, R.; Iijima, K.; Kikuchi, K. Hybrid Small-Molecule/Protein Fluorescent Probes. Chem. Rev. 2024, 124, 6198–6270. [Google Scholar] [CrossRef]
- Chang, C.J. Introduction: Fluorescent Probes in Biology. Chem. Rev. 2024, 124, 11639–11640. [Google Scholar] [CrossRef]
- Sun, R.; Yang, D.; Zhang, X.; Liu, J.; Liu, M.; Wang, L.; Yao, L.; Tang, Y.; Sun, H. A Fluorescent Probe for Rapid Staining and Real-Time Detection of Autophagy in Single Cells by Lighting-Up G-Quadruplexes. Sens. Actuators B Chem. 2025, 423, 136768. [Google Scholar] [CrossRef]
- Sharma, A.; Verwilst, P.; Li, M.; Ma, D.; Singh, N.; Yoo, J.; Kim, Y.; Yang, Y.; Zhu, J.H.; Huang, H.; et al. Theranostic Fluorescent Probes. Chem. Rev. 2024, 124, 2699–2804. [Google Scholar] [CrossRef]
- Tumir, L.; Crnolatac, I.; Deligeorgiev, T.; Vasilev, A.; Kaloyanova, S.; Grabar, M.; Tomić, S.; Mišković, K.; Glavaš-Obrovac, L.; Piantanida, I. Kinetic Differentiation between Homo- and Alternating AT DNA by Sterically Restricted Phosphonium Dyes. Chem. Eur. J. 2012, 18, 3859–3864. [Google Scholar] [CrossRef]
- Sonmezoglu, A.Ö.; Özkay, K. A new organic dye-based staining for the detection of plant DNA in agarose gels. Nucleosides Nucleotides Nucleic Acids 2015, 34, 515–522. [Google Scholar] [CrossRef]
- Tatikolov, A.S. Polymethine Dyes as Spectral-Fluorescent Probes for Biomacromolecules. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 55–90. [Google Scholar] [CrossRef]
- Pronkin, P.G.; Tatikolov, A.S. Fluorescent Probes for Biomacromolecules Based on Monomethine Cyanine Dyes. Chemosensors 2023, 11, 280. [Google Scholar] [CrossRef]
- Wang, G.W.; Wang, N.; Pan, H.; Shao, G.; Chen, J.-S. Chapter 3—Mechanochemistry in organic synthesis. In Introduction to Condensed Matter Chemistry; Xu, R., Yu, J., Yan, W., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 73–103. [Google Scholar]
- West, W.; Pearce, S. The Dimeric State of Cyanine Dyes. J. Phys. Chem. 1965, 69, 1894–1903. [Google Scholar] [CrossRef]
- Biver, T.; Boggioni, A.; Secco, F.; Turriani, E.; Venturini, M.; Yarmoluk, S. Influence of cyanine dye structure on self-aggregation and interaction with nucleic acids: A kinetic approach to TO and BO binding. Arch. Biochem. Biophys. 2007, 465, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Ilieva, S.; Kandinska, M.; Vasilev, A.; Cheshmedzhieva, D. Theoretical Modeling of Absorption and Fluorescent Characteristics of Cyanine Dyes. Photochem 2022, 2, 202–216. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Petersson, G.A.; Bennett, A.; Tensfeldt, T.G.; Al-Laham, M.A.; Shirley, W.A.; Mantzaris, J. A complete basis set model chemistry. I. The total energies of closed-shell atoms and hydrides of the first-row elements. J. Chem. Phys. 1988, 89, 2193–2218. [Google Scholar] [CrossRef]
- Asaduzzaman, A.M.; Schreckenbach, G. Computational study of the ground state properties of iodine and polyiodide ions. Theor. Chem. Acta 2009, 122, 119–125. [Google Scholar] [CrossRef]
- Legault, C.Y. CYLview, Version 1.0 b; Université de Sherbrooke: Sherbrooke, QC, Canada, 2009. Available online: http://www.cylview.org (accessed on 18 July 2025).
- Ilieva, S.; Bozova, N.; Rangelov, M.; Todorova, N.; Vasilev, A.; Cheshmedzhieva, D. Asymmetric Monomethine Cyanine Dyes with Hydrophobic Functionalities for Fluorescent Intercalator Displacement Assay. Molecules 2024, 29, 114. [Google Scholar] [CrossRef]
- Long, W.; Lu, Y.-J.; Zhang, K.; Huang, X.-H.; Hou, J.-Q.; Cai, S.-Y.; Li, Y.; Du, X.; Luyt, L.G.; Wong, W.-L.; et al. Boosting the turn-on fluorescent signaling ability of thiazole orange dyes: The effectiveness of structural modification site and its unusual interaction behavior with nucleic acids. Dye. Pigment. 2018, 159, 449–456. [Google Scholar] [CrossRef]
- Zhao, Z.; Cao, S.; Li, H.; Li, D.; He, Y.; Wang, X.; Chen, J.; Zhang, S.; Xu, J.; Knutson, J.R. Ultrafast excited-state dynamics of thiazole orange. Chem. Phys. 2022, 553, 111392. [Google Scholar] [CrossRef]
- OriginLab. OriginPro, Version 2023b; OriginLab Corporation: Northampton, MA, USA, 2023.
- Nygren, J.; Svanvik, N.; Kubista, M. The interactions between the fluorescent dye thiazole orange and DNA. Biopolymers 1998, 46, 39–51. [Google Scholar] [CrossRef]
- Kandinska, M.I.; Cheshmedzhieva, D.V.; Kostadinov, A.; Rusinov, K.; Rangelov, M.; Todorova, N.; Ilieva, S.; Ivanov, D.P.; Videva, V.; Lozanov, V.S.; et al. Tricationic asymmetric monomeric monomethine cyanine dyes with chlorine and trifluoromethyl functionality—Fluorogenic nucleic acids probes. J. Mol. Liq. 2021, 342, 117501–117516. [Google Scholar] [CrossRef]
- Hajian, R.; Huat, T.G. Electrochemical Study on the Interaction of Irinotecan with Calf Thymus Double Stranded DNA. Chin. J. Chem. 2012, 30, 738–742. [Google Scholar] [CrossRef]
- Zhao, N.A.; Wang, M.; Niu, X.; Sun, W.; Jiao, K. Spectrophotometric studies on the interaction of yeast RNA with crystal violet and its analytical application. J. Chil. Chem. Soc. 2008, 53, 1594–1598. [Google Scholar] [CrossRef][Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar][Green Version]
- Cossi, M.; Barone, V.; Cammi, R.; Tomasi, J. Ab initio study of solvated molecules: A new implementation of the polarizable continuum model. Chem. Phys. Lett. 1996, 255, 327–335. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-consistent equation ns including exchange and correlation effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
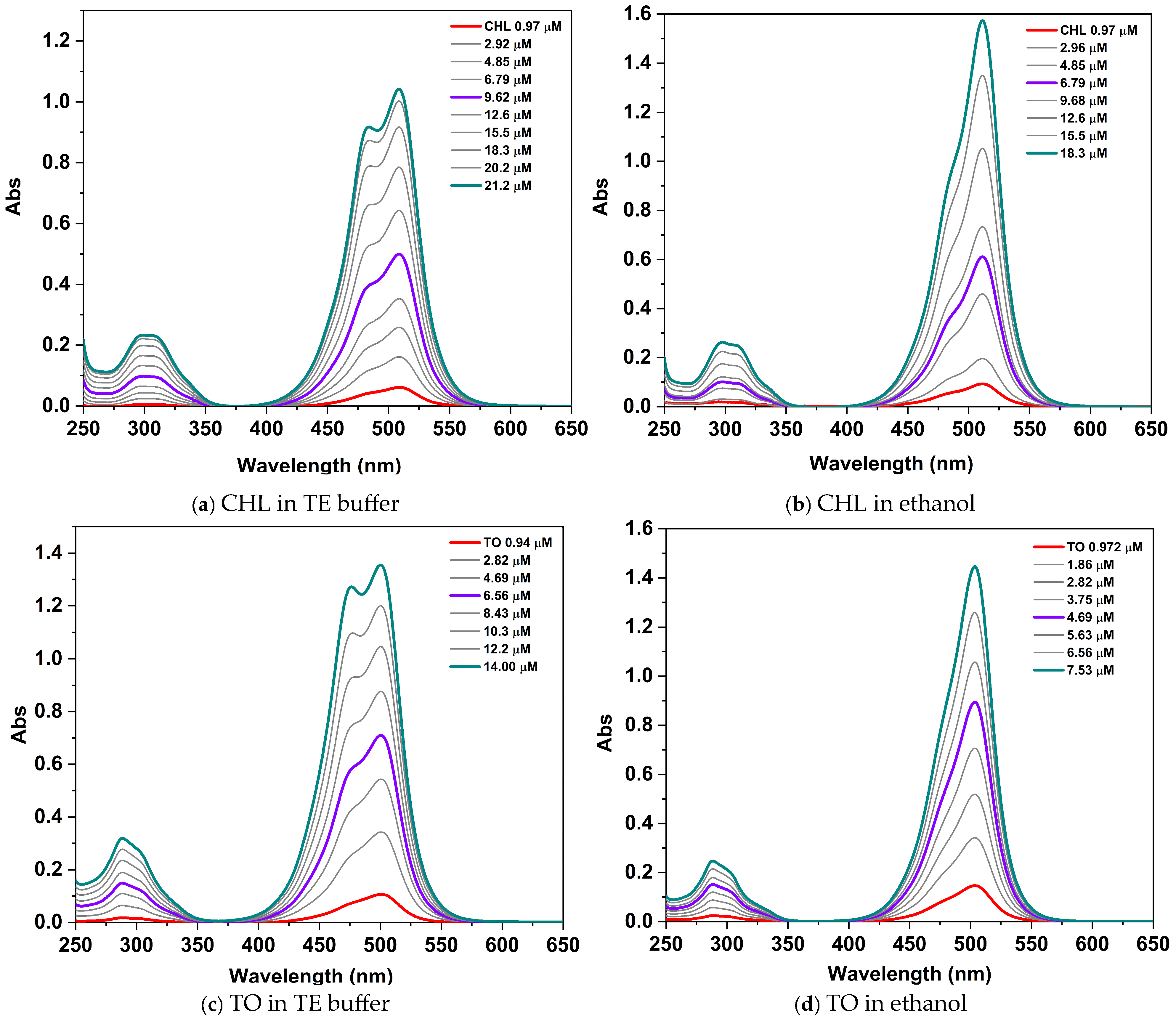


| Dye | Monomer TE Buffer | Monomer Ethanol | Dimer TE Buffer | ||
|---|---|---|---|---|---|
| [nm] | [nm] | [nm] | [nm] | [nm] | |
| CHL | 509 (48,694) | 505 | 511 (85,834) | 482 | 474 |
| TO | 500 (93,710) | 496 | 503 (196,691) | 476 | 471 |
| Dye | NA | (nm) | (nm) | (nm) | Imax/I0 | Kb × 105 | Φf |
|---|---|---|---|---|---|---|---|
| CHL a | DNA | 489 | 547 | 538 | 76 | 3.74 | 0.33 |
| RNA | 491 | 545 | 541 | 125 | 1.65 | 0.14 | |
| TO b | DNA | 476 | 537 | 533 | 216 | 3.13 | 0.25 |
| RNA | 476 | 534 | 532 | 93 | 4.25 | 0.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheshmedzhieva, D.; Bozova, N.; Ilieva, S.; Novakov, C.; Vasilev, A. Mechanochemical Approach to a Monocationic Asymmetric Monomethine Cyanine Dye for Nucleic Acid Analysis and Visualization. Molecules 2025, 30, 3966. https://doi.org/10.3390/molecules30193966
Cheshmedzhieva D, Bozova N, Ilieva S, Novakov C, Vasilev A. Mechanochemical Approach to a Monocationic Asymmetric Monomethine Cyanine Dye for Nucleic Acid Analysis and Visualization. Molecules. 2025; 30(19):3966. https://doi.org/10.3390/molecules30193966
Chicago/Turabian StyleCheshmedzhieva, Diana, Nadezhda Bozova, Sonia Ilieva, Christo Novakov, and Aleksey Vasilev. 2025. "Mechanochemical Approach to a Monocationic Asymmetric Monomethine Cyanine Dye for Nucleic Acid Analysis and Visualization" Molecules 30, no. 19: 3966. https://doi.org/10.3390/molecules30193966
APA StyleCheshmedzhieva, D., Bozova, N., Ilieva, S., Novakov, C., & Vasilev, A. (2025). Mechanochemical Approach to a Monocationic Asymmetric Monomethine Cyanine Dye for Nucleic Acid Analysis and Visualization. Molecules, 30(19), 3966. https://doi.org/10.3390/molecules30193966





