Coupling Rare-Earth Complexes with Carbon Dots via Surface Imprinting: A New Strategy for Spectroscopic Cu2+ Sensors
Abstract
1. Introduction
2. Results and Discussion
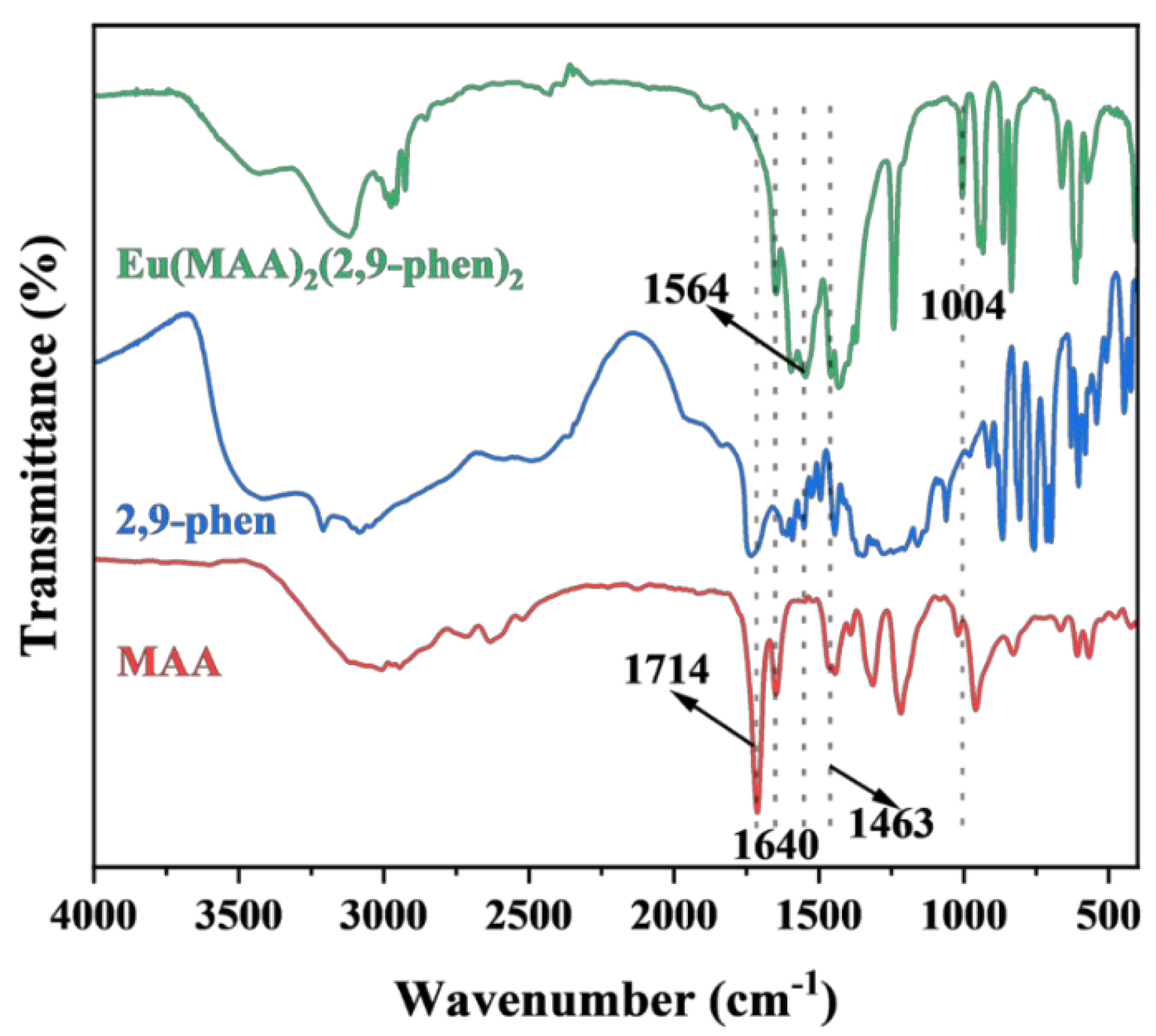
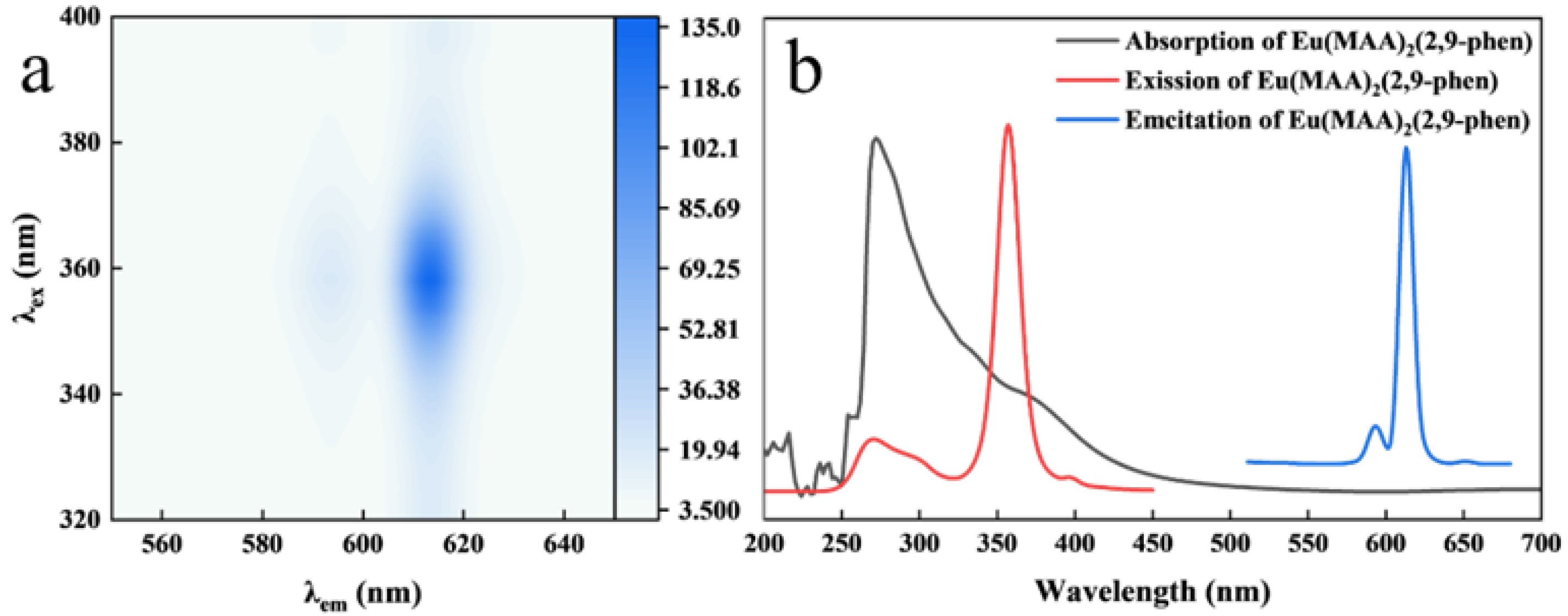
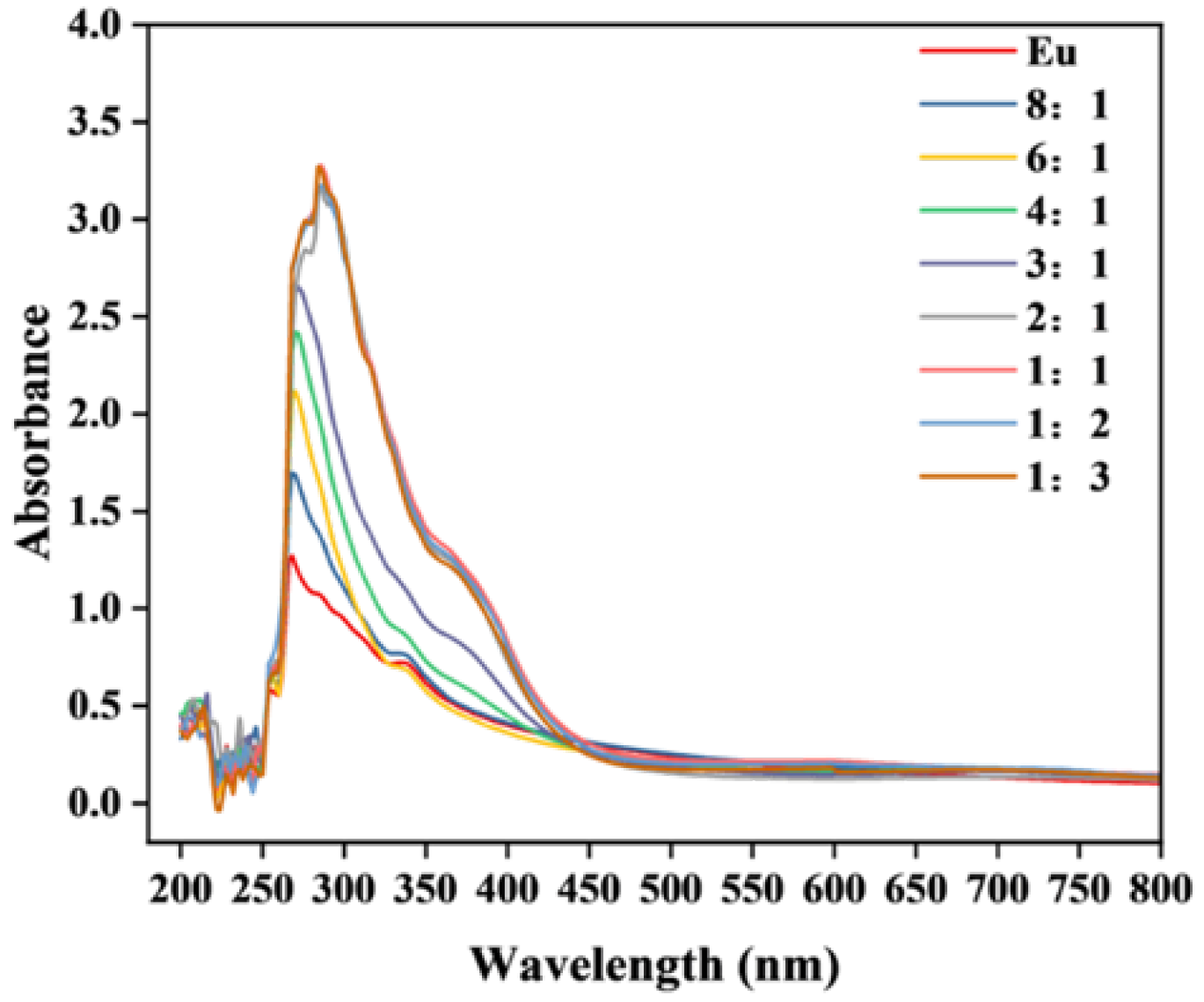
2.1. Characterization of Eu(MAA)2(2,9-phen)
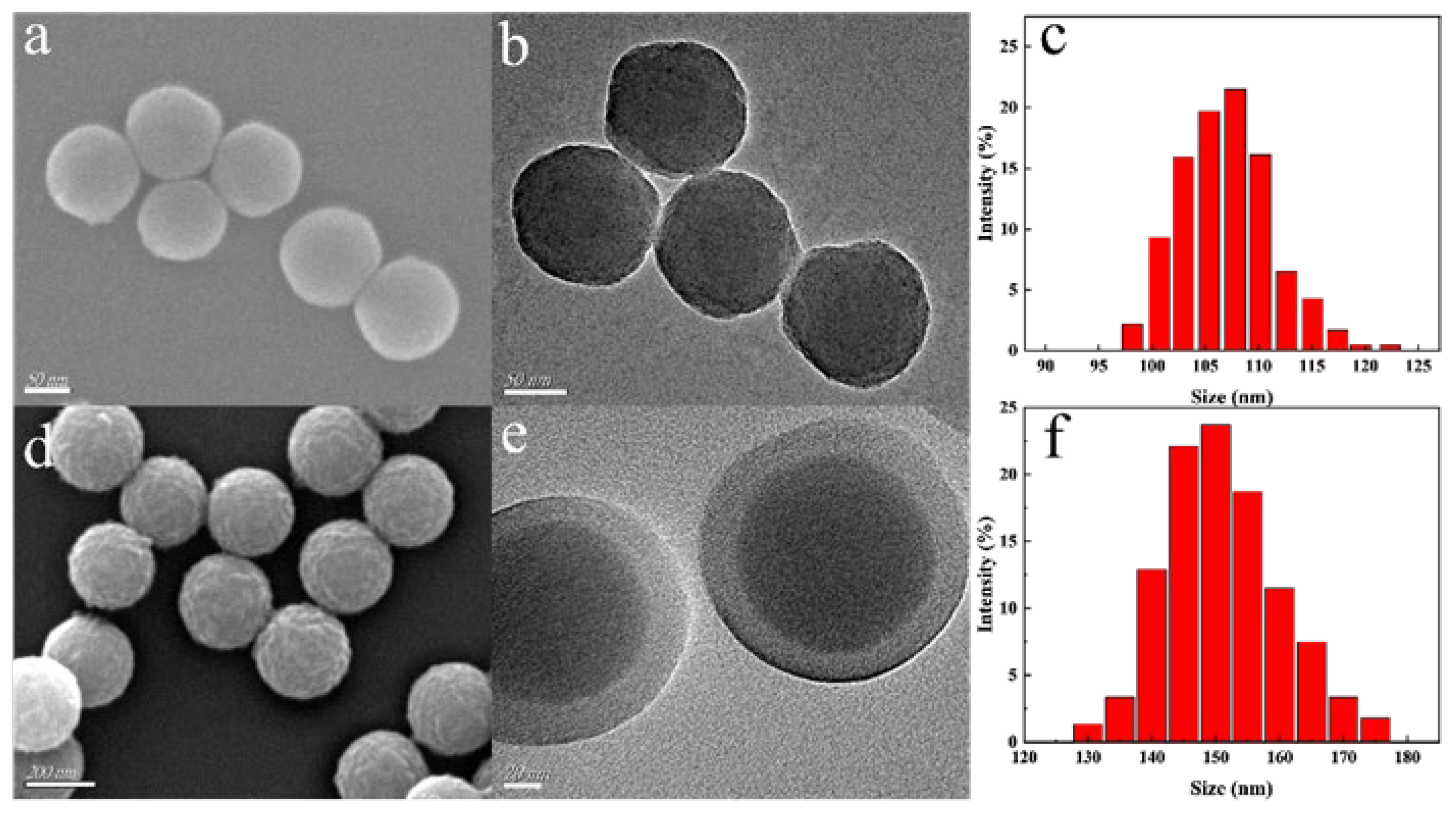
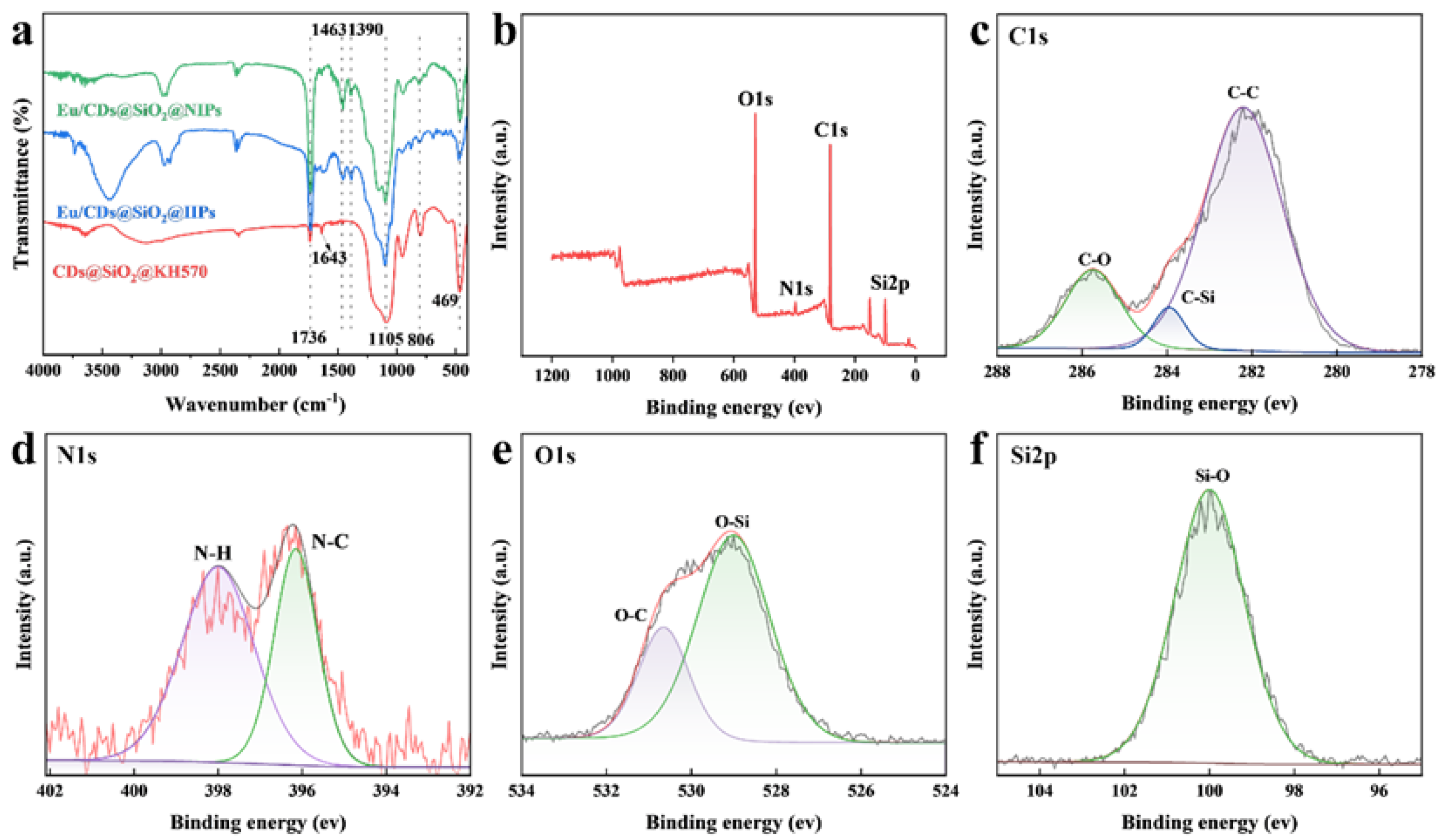
2.2. Structural and Physicochemical Characterization of Eu/CDs@SiO2@IIPs
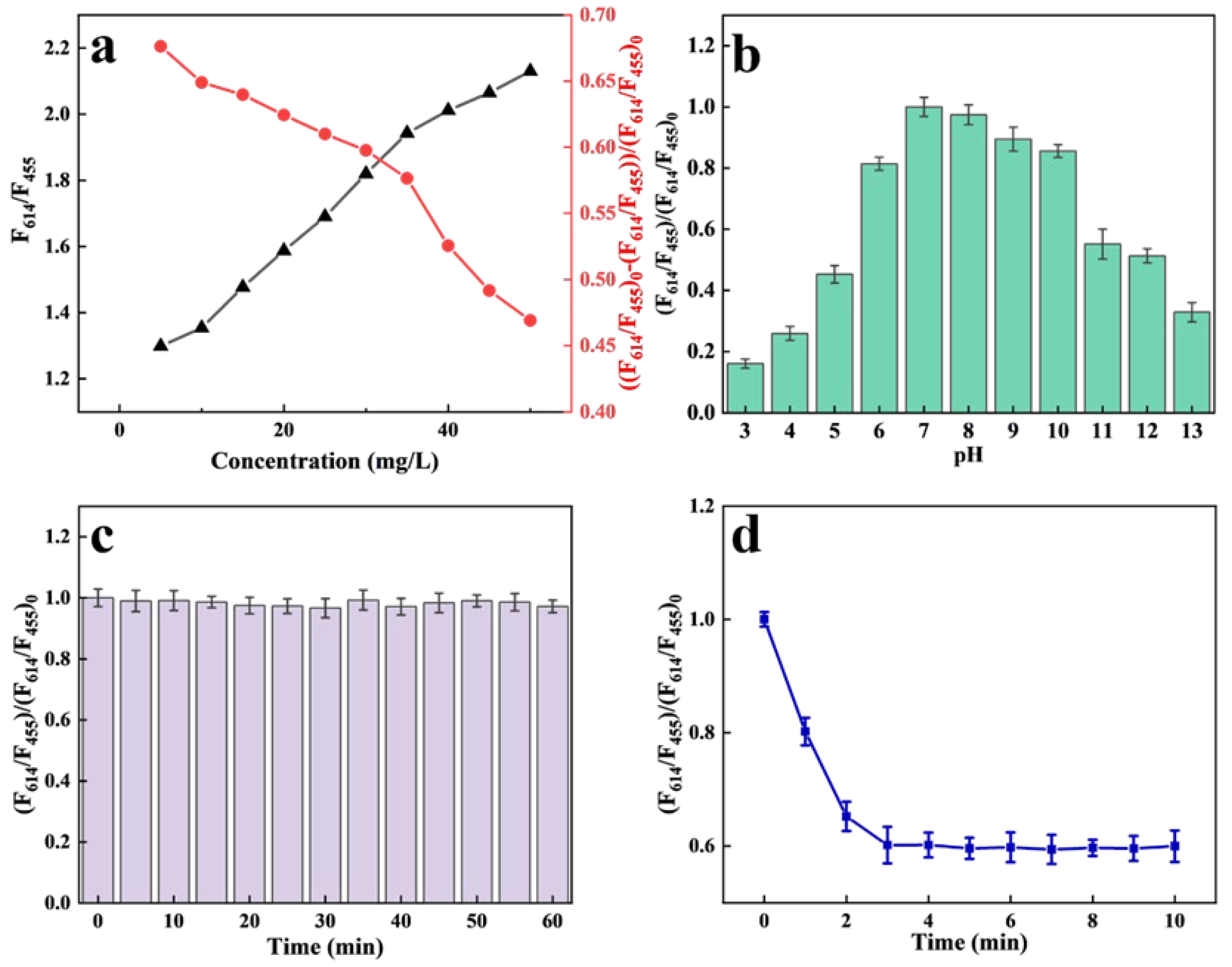
2.3. Optimisation of Fluorescence Detection Conditions
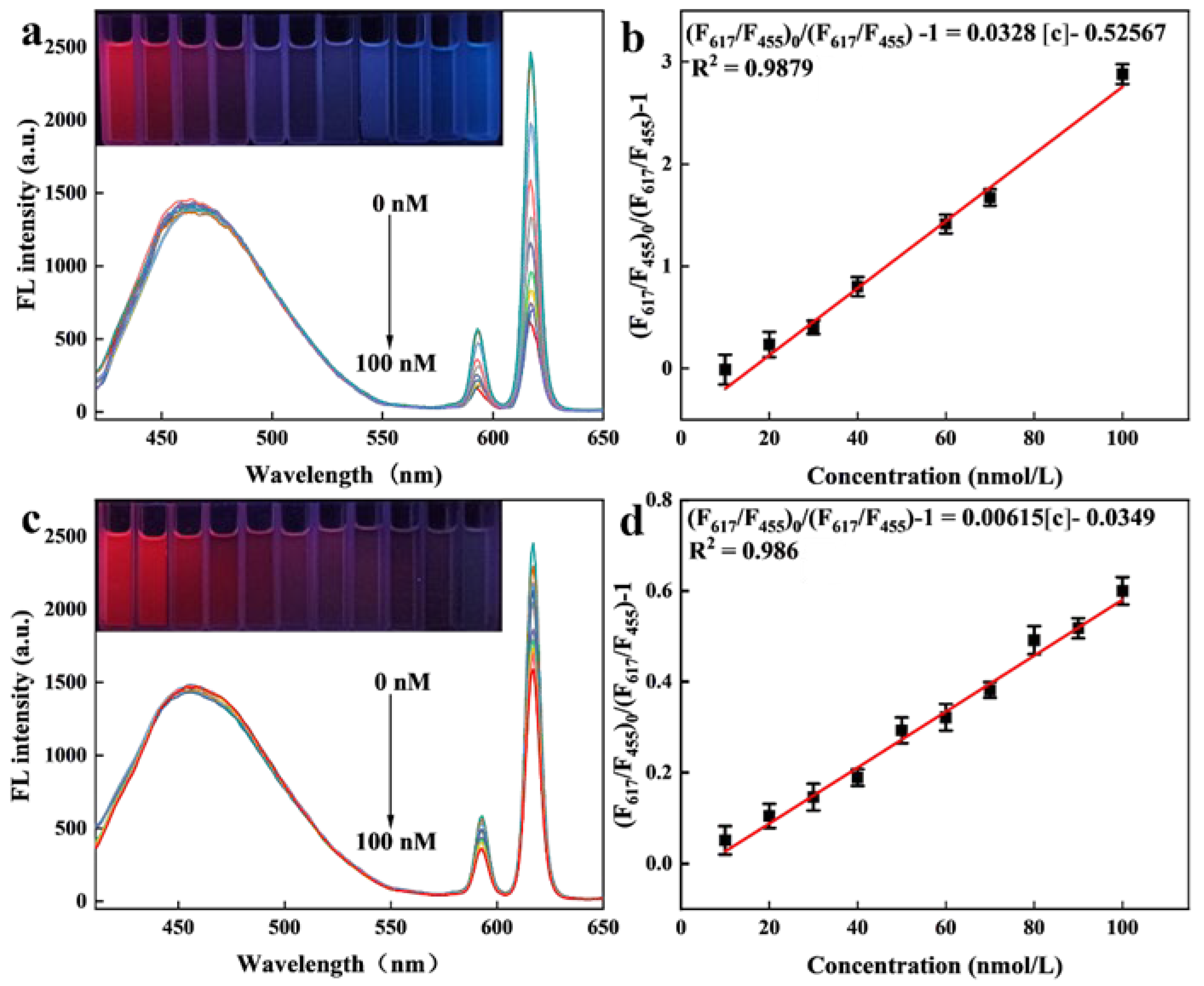
2.4. Fluorescence Detection Performance of Eu/CDs@SiO2@IIPs for Cu2+
2.5. Selectivity Assessment of Eu/CDs@SiO2@IIPs
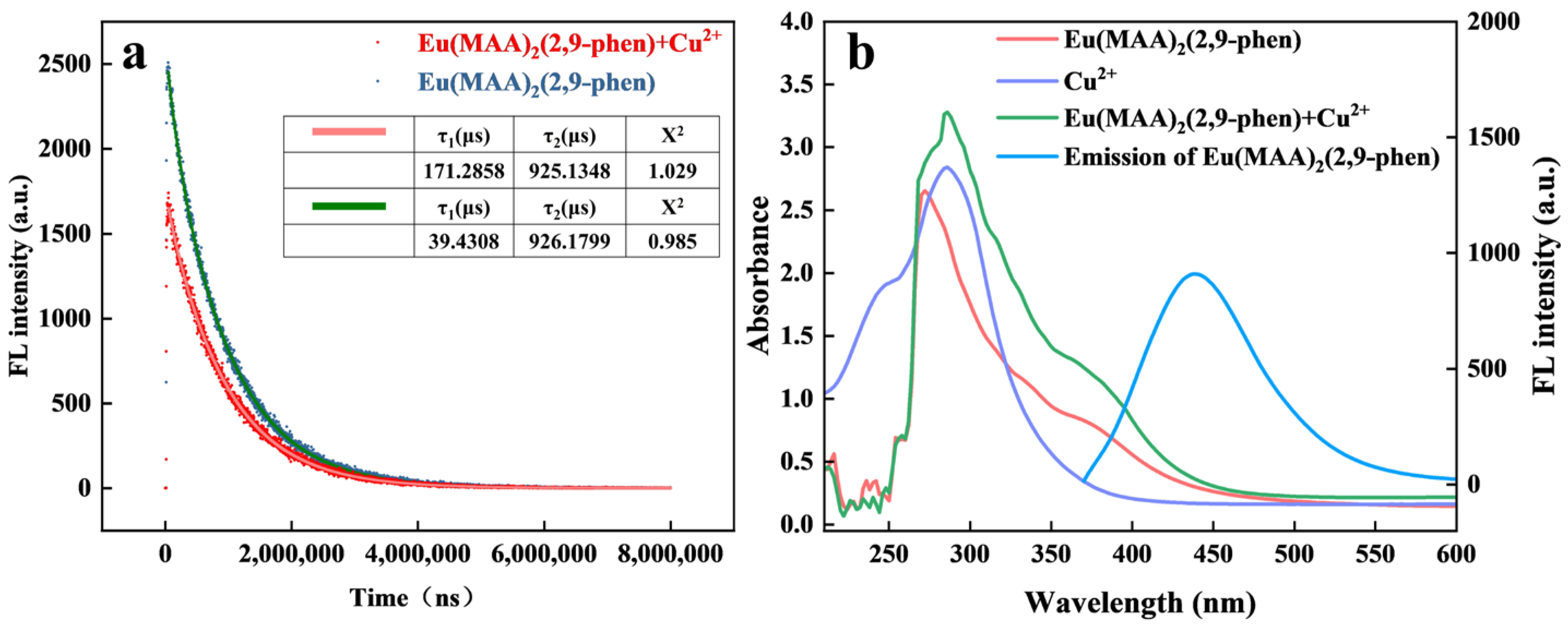
2.6. Mechanistic Study of Fluorescence Quenching
2.7. Evaluation of Response Efficiency and Sensitivity Threshold
2.8. Real Sample Validation
3. Materials and Methods
3.1. Materials
3.2. Apparatus
3.3. Preparation of the Lanthanide Complex Eu(MAA)2(2,9-phen)
3.4. Preparation of CDs
3.5. Preparation and Surface Functionalization of CDs@SiO2 Nanoparticles
3.6. Preparation of Eu/CDs@SiO2@IIPs
3.7. Fluorescent Detection of Cu2+
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rehman, M.; Liu, L.; Wang, Q.; Saleem, M.H.; Bashir, S.; Ullah, S.; Peng, D. Copper environmental toxicology, recent advances, and future outlook: A review. Environ. Sci. Pollut. Res. 2019, 26, 18003–18016. [Google Scholar] [CrossRef]
- Zeb, M.; Khan, K.; Younas, M.; Farooqi, A.; Cao, X.; Kavil, Y.N.; Alelyani, S.S.; Alkasbi, M.M.; Al-Sehemi, A.G. A review of heavy metals pollution in riverine sediment from various Asian and European countries: Distribution, sources, and environmental risk. Mar. Pollut. Bull. 2024, 206, 116775. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, D.; Ren, F.; Huang, L. Spatiotemporal variation of soil heavy metals in China: The pollution status and risk assessment. Sci. Total. Environ. 2023, 871, 161768. [Google Scholar] [CrossRef] [PubMed]
- Bandara, G.C.; Heist, C.A.; Remcho, V.T. Chromatographic separation and visual detection on wicking microfluidic devices: Quantitation of Cu2+ in surface, ground, and drinking water. Anal. Chem. 2018, 90, 2594–2600. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wei, X.; Chen, M.; Wang, J. Non-ceruloplasmin-bound copper and copper speciation in serum with extraction using functionalized dendritic silica spheres followed by ICP-MS detection. Anal. Chim. Acta 2023, 1251, 340993. [Google Scholar] [CrossRef]
- Bahçıvan, A.; Şaylan, M.; Sagdic, O.; Bakırdere, S. CoSn(OH)6 nanocubes as a solid sorbent for the effective preconcentration of copper ions in cinnamon (Cinnamomum zeylanicum) extract. Food Chem. 2024, 447, 139037. [Google Scholar] [CrossRef]
- Zhou, F.; Wu, B.; Zhou, J. Novel Spectrophotometric Method for Robust Detection of Trace Copper and Cobalt in High-Concentration Zinc Solution. Molecules 2024, 29, 5765. [Google Scholar] [CrossRef]
- Bettini, S.; Pagano, R.; Valli, L.; Giancane, G. Spectroscopic investigation of the selective interaction of mercuric and cupric ions with a porphyrin active layer. J. Phys. Chem. C 2014, 118, 12384–12390. [Google Scholar] [CrossRef]
- Wei, J.; Li, J.; Jiang, Z.; Tang, Z.; Guo, P.; Zhao, Y.-L.; Ke, P.; Wang, A. Improved electrochemical detection of copper ions in nitrogen doped ta-C films for marine corrosive monitoring. Electrochim. Acta 2023, 469, 143250. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.-M.; Han, J. Fluorescent chemosensors for copper(II) ion: Structure, mechanism and application. J. Photochem. Photobiol. C Photochem. Rev. 2017, 32, 78–103. [Google Scholar] [CrossRef]
- Chen, J.; Li, Z.; Li, X.; Du, Y. A reverse-signal response fluorescence sensor based on NH2-MIL-101(Fe) and silicon-doped carbon quantum dots for selective determination of copper ions. J. Photochem. Photobiol. A Chem. 2024, 452, 115579. [Google Scholar] [CrossRef]
- Li, J.; Zhao, L.; Zhang, T.; Yang, Z.; Liu, X.; Hu, B.; Zhao, L.; Che, G. Fabrication of Mesoporous ion-Imprinted Fluorescent Sensors for Rapid and Reliable Cu2+ Detection in Aqueous Environments. Results Surf. Interfaces 2025, 19, 100533. [Google Scholar] [CrossRef]
- Hassan, M.; Zareef, M.; Xu, Y.; Li, H.; Chen, Q. SERS based sensor for mycotoxins detection: Challenges and improvements. Food Chem. 2021, 344, 128652. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, C.; Guo, W.; Guo, Y.; Zou, X.; Sun, Z. Recyclable magnetic HNTs@MIPs-Based SERS sensors for selective, sensitive, and reliable detection of capsaicin for gutter oil discrimination. Food Biosci. 2025, 66, 106179. [Google Scholar] [CrossRef]
- Feng, N.; Dong, C.; Shuang, S.; Song, S. Fluorescence and colorimetric dual-mode sensing of copper ions and fingerprint visualization by benzimidazole derivatives. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 326, 125292. [Google Scholar] [CrossRef]
- Hu, B.; Zhao, W.; Chen, L.; Liu, Y.; Ma, Z.; Yan, Y.; Meng, M. Enhanced molecularly imprinted fluorescent test strip for rapid and visual detection of norfloxacin via a smartphone. Molecules 2024, 29, 661. [Google Scholar] [CrossRef]
- Lin, K.-T.; Nian, X.; Li, K.; Han, J.; Zheng, N.; Lu, X.; Guo, C.; Lin, H.; Jia, B. Highly efficient flexible structured metasurface by roll-to-roll printing for diurnal radiative cooling. eLight 2023, 3, 22. [Google Scholar] [CrossRef]
- Minopoli, A.; Wagner, S.; Erben, E.; Liao, W.; Stoev, I.D.; Lauga, E.; Kreysing, M. ISO-FLUCS: Symmetrization of optofluidic manipulations in quasi-isothermal micro-environments. eLight 2023, 3, 16. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, X.; Yu, F.; Quan, Y. Preparation of dummy molecularly imprinted polymers based on dextran-modified magnetic nanoparticles Fe3O4 for the selective detection of acrylamide in potato chips. Food Chem. 2020, 317, 126431. [Google Scholar] [CrossRef]
- Bai, Q.; Huang, C.; Ma, S.; Gong, B.; Ou, J. Rapid adsorption and detection of copper ions in water by dual-functional ion-imprinted polymers doping with carbon dots. Sep. Purif. Technol. 2023, 315, 123666. [Google Scholar] [CrossRef]
- Dong, H.; Ran, C.; Gao, W.; Li, M.; Xia, Y.; Huang, W. Metal Halide Perovskite for next-generation optoelectronics: Progresses and prospects. eLight 2023, 3, 3. [Google Scholar] [CrossRef]
- Bai, Y.; Lu, H.; Lei, M.; Qiu, J.; Lin, J. An ultrastable luminescent covalent organic polymer for selective Pd2+ detection in strong acid. EcoEnergy 2025, 3, 170–179. [Google Scholar] [CrossRef]
- Ahmad, W.; Aljuhani, E.; Alwael, H.; Assirey, E.; Nassef, H.; El-Shahawi, M. Redox impulse, computational calculation of molecular energy potentials and ultra-trace determination of the food colorant erythrosine b in fruit jams, soft drinks and water. J. Food Compos. Anal. 2023, 117, 105110. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, T.; Wang, S.; Yan, Y. Mesoporous silica-based molecularly imprinted fluorescence sensor for the ultrafast and sensitive recognition of oxytetracycline. J. Food Compos. Anal. 2022, 108, 104427. [Google Scholar] [CrossRef]
- Hu, B.; Chen, L.; Yu, Z.; Xu, Y.; Dai, J.; Yan, Y.; Ma, Z. Hollow molecularly imprinted fluorescent sensor using europium complex as functional monomer for the detection of trace 2,4,6-trichlorophenol in real water samples. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 246, 119051. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, W.; Liu, H.; Jing, Y.; Zhou, D.; Ji, Y.; Widengren, J.; Bai, X.; Song, H. A multiband NIR upconversion core-shell design for enhanced light harvesting of silicon solar cells. Light. Sci. Appl. 2024, 13, 312. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.-C.; Nguyen, H.-Q.; Kang, H.; Goddati, M.; Lee, S.-Y.; Yee, K.-J.; Lee, J. Metal plasmon-enhanced lanthanide fluorescent nanoparticles for monitoring aqueous copper ions. Mater. Today Nano 2023, 24, 100380. [Google Scholar] [CrossRef]
- Yang, L.; Hu, W.; Pei, F.; Liu, Z.; Wang, J.; Tong, Z.; Mu, X.; Du, B.; Xia, M.; Wang, F.; et al. A ratiometric fluorescence imprinted sensor based on N-CDs and metal–organic frameworks for visual smart detection of malathion. Food Chem. 2024, 438, 138068. [Google Scholar] [CrossRef]
- Fan, Y.; Huang, W.; Zhu, F.; Liu, X.; Jin, C.; Guo, C.; An, Y.; Kivshar, Y.; Qiu, C.-W.; Li, W. Dispersion-assisted high-dimensional photodetector. Nature 2024, 630, 77–83. [Google Scholar] [CrossRef]
- Chen, X.; Shu, X.; Zhou, J.; Wan, L.; Xiao, P.; Fu, Y.; Ye, J.; Huang, Y.-T.; Yan, B.; Xue, D.; et al. Additive engineering for Sb2S3 indoor photovoltaics with efficiency exceeding 17%. Light. Sci. Appl. 2024, 13, 281. [Google Scholar] [CrossRef]
- Bettini, S.; Syrgiannis, Z.; Pagano, R.; D̵oRd̵eVić, L.; Salvatore, L.; Prato, M.; Giancane, G.; Valli, L. Perylene bisimide aggregates as probes for subnanomolar discrimination of aromatic biogenic amines. ACS Appl. Mater. Interfaces 2019, 11, 17079–17089. [Google Scholar] [CrossRef]
- Han, E.; Pan, Y.; Li, L.; Cai, J. Bisphenol A detection based on nano gold-doped molecular imprinting electrochemical sensor with enhanced sensitivity. Food Chem. 2023, 426, 136608. [Google Scholar] [CrossRef]
- Dong, Z.; Lu, J.; Wu, Y.; Meng, M.; Yu, C.; Sun, C.; Chen, M.; Da, Z.; Yan, Y. Antifouling molecularly imprinted membranes for pretreatment of milk samples: Selective separation and detection of lincomycin. Food Chem. 2020, 333, 127477. [Google Scholar] [CrossRef]
- Su, X.; Zheng, K.; Tian, X.; Zhou, X.; Zou, X.; Xu, X.; Sun, Z.; Zhang, W. An advanced ratiometric molecularly imprinted sensor based on metal ion reoxidation for indirect and ultrasensitive glyphosate detection in fruit. Food Chem. 2023, 429, 136927. [Google Scholar] [CrossRef]
- Zhou, T.; Ding, L.; Che, G.; Jiang, W.; Sang, L. Recent advances and trends of molecularly imprinted polymers for specific recognition in aqueous matrix: Preparation and application in sample pretreatment. TrAC Trends Anal. Chem. 2019, 114, 11–28. [Google Scholar] [CrossRef]
- Yang, C.; Hu, W.; Liu, J.; Han, C.; Gao, Q.; Mei, A.; Zhou, Y.; Guo, F.; Han, H. Achievements, challenges, and future prospects for industrialization of perovskite solar cells. Light. Sci. Appl. 2024, 13, 227. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Jin, W.; Nolen, J.R.; Pan, H.; Yi, N.; An, Y.; Zhang, Z.; Kong, X.; Zhu, F.; Jiang, K.; et al. Subambient daytime radiative cooling of vertical surfaces. Science 2024, 386, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Cao, L.; Cai, H.; Yang, W.; Lu, H.; Adila, A.; Zhang, B.; Cao, Y.; Huang, W.; Xu, W.; et al. A rapid microfluidic paper-based chip sensor using ratiometric fluorescence and molecularly imprinted polymers for visual detection of sulfadiazine in actual samples. J. Food Compos. Anal. 2025, 139, 107108. [Google Scholar] [CrossRef]
- Qiu, H.; Gao, L.; Wang, J.; Pan, J.; Yan, Y.; Zhang, X. A precise and efficient detection of Beta-Cyfluthrin via fluorescent molecularly imprinted polymers with ally fluorescein as functional monomer in agricultural products. Food Chem. 2017, 217, 620–627. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, Y.; Li, T.; Li, H.; Yang, C.; Sun, D.; Wang, D.; Liu, C.; Che, G. Fabricating magnetic hydrophilic molecularly imprinted resin with enhanced adsorption and recognition performance for targeted detecting chlorophenols in environmental water. Chem. Eng. J. 2021, 420, 129904. [Google Scholar] [CrossRef]
- Xu, Q.; Li, Z.; Li, H. Water-Soluble Luminescent Hybrid Composites Consisting of Oligosilsesquioxanes and Lanthanide Complexes and their Sensing Ability for Cu2+. Chem.—A Eur. J. 2016, 22, 3037–3043. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Liu, W.; Zhang, H.; Yang, Z.; Wang, B.; Chen, F.; Chen, H. Triple-emitting dumbbell fluorescent nanoprobe for multicolor detection and imaging applications. Inorg. Chem. 2015, 54, 7725–7734. [Google Scholar] [CrossRef]
- Ren, H.; Wang, X.; Gong, R.; Li, M.; Zhu, H.; Zhang, J.; Duan, E. Atomically dispersed Eu(III) sites in natural deep eutectic solvents based fluorescent probe efficient identification of Fe3+ and Cu2+ in wastewater. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 229, 117874. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, W.; Wen, X. A dual-emission ratiometric luminescence probe based on Zr-MOFs for the efficient detection of Cu2+ in aqueous solutions. Funct. Mater. Lett. 2024, 17, 2450005. [Google Scholar] [CrossRef]
- Ye, Z.; Li, L.; Zhao, F.; Yang, Q.; Wang, Y.; Bohinc, K.; Guo, X. Dual-emission fluorescent probe templated by spherical polyelectrolyte brush for ratiometric detection of copper ions. J. Mater. Sci. 2020, 55, 10168–10184. [Google Scholar] [CrossRef]
- Wang, J.; Pei, J.; Li, G. Lanthanide ternary complex as a fluorescent probe for highly sensitive and selective detection of copper ions based on selective recognition and photoinduced electron transfer. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 290, 122287. [Google Scholar] [CrossRef]


| Sample | C (%) | H (%) | N (%) | O (%) | |
|---|---|---|---|---|---|
| Eu(MAA)2(2,9-phen) | found calc. | 44.20 44.92 | 3.65 2.74 | 5.61 4.76 | 21.10 21.74 |
| Sensor Name | Linear Range (nM) | Detection Limit (nM) | Response Time (min) | Bibliography | |
|---|---|---|---|---|---|
| 1 | Eu/CDs@SiO2@IIPs | 10–100 | 2.79 | 3.0 | this work |
| 2 | Ln(dpa)3@POSS-NH2 | 1 × 104–1 × 107 | not given | 30 | [41] |
| 3 | Eu:AMC(Tb:cs124)-DTPA-PEG-Fe3O4-Au | 30–100 | 30 | 10 | [42] |
| 4 | Glu/Gly/EuCl3·6H2O | 0–50,000 | not given | 20 | [43] |
| 5 | Eu(DPA)3@UIO-66 | 0–25,000 | 890 | 30 | [44] |
| 6 | Eu-doped PS@PSS@GSH-CdTe | 0–1000 | 1.45 | 10 | [45] |
| 7 | DPA-Eu3+-PEI probe | 20–10,000 | 8.0 | 1.0 | [46] |
| Eu/CDs@SiO2@IIPs | ICP-MS | |||||||
|---|---|---|---|---|---|---|---|---|
| Sample Number | Add Concentration (nM) | Detection Limit (nM) | Recovery Rate (%) | RSD (n = 3) | Detection Limit (μM) | Recovery Rate (%) | RSD (n = 3) | |
| Deionized water | 1 | 103.7 | 3.2 | 20.2 | 101 | 1.3 | ||
| 2 | 20 | 20.74 | 96.9 | 4.4 | 51 | 102 | 2.1 | |
| 3 | 50 | 48.48 | 102.1 | 3.2 | 102 | 102 | 1.1 | |
| Creek | 1 | 100 | 102.1 | 104 | 1.2 | 20.4 | 102 | 1.6 |
| 2 | 20 | 20.8 | 107 | 4.5 | 50.5 | 102 | 1.2 | |
| 3 | 50 | 53.5 | 99.3 | 2.4 | 103 | 101 | 2.5 | |
| Electroplating wastewater | 1 | 100 | 115.2 | - | 2.8 | 112.5 | 103 | 2.2 |
| 2 | 0 | 127.6 | - | 4.3 | 117.4 | - | 1.7 | |
| 3 | 0 | 109.3 | - | 3.7 | 113.9 | - | 1.3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Hu, B.; Meng, M. Coupling Rare-Earth Complexes with Carbon Dots via Surface Imprinting: A New Strategy for Spectroscopic Cu2+ Sensors. Molecules 2025, 30, 3967. https://doi.org/10.3390/molecules30193967
Liu Z, Hu B, Meng M. Coupling Rare-Earth Complexes with Carbon Dots via Surface Imprinting: A New Strategy for Spectroscopic Cu2+ Sensors. Molecules. 2025; 30(19):3967. https://doi.org/10.3390/molecules30193967
Chicago/Turabian StyleLiu, Zuoyi, Bo Hu, and Minjia Meng. 2025. "Coupling Rare-Earth Complexes with Carbon Dots via Surface Imprinting: A New Strategy for Spectroscopic Cu2+ Sensors" Molecules 30, no. 19: 3967. https://doi.org/10.3390/molecules30193967
APA StyleLiu, Z., Hu, B., & Meng, M. (2025). Coupling Rare-Earth Complexes with Carbon Dots via Surface Imprinting: A New Strategy for Spectroscopic Cu2+ Sensors. Molecules, 30(19), 3967. https://doi.org/10.3390/molecules30193967








