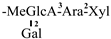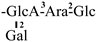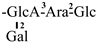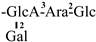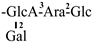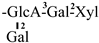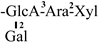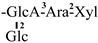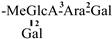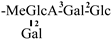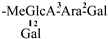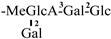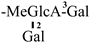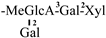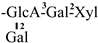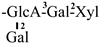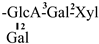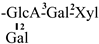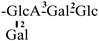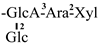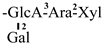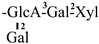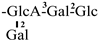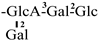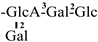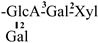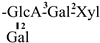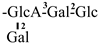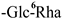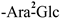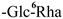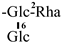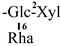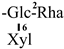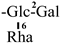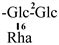Abstract
Camellia oleifera, a member of the Theaceae family and belonging to the Camellia Linn species, is a plant utilized for edible oil production and medicinal value. Its fruit is abundant in various bioactive compounds, including triterpene saponins, flavonoids, lignans, fatty acids, sterols, polysaccharides, and numerous other chemical constituents. Among these, triterpene saponins and flavonoids serve as the primary active ingredients. The pharmacological effects of C. oleifera fruits are diverse, encompassing anti-tumor properties, cardiovascular and cerebrovascular protection, anti-inflammatory, antioxidant activity, lipid-lowering capability, anti-fungal property, and neuroprotective function. In recent years, this area has garnered significant attention from scholars both domestically and internationally. This article reviews the chemical constituents and pharmacological effects of C. oleifera fruits, aiming to provide a comprehensive reference for further research and development. Additionally, it offers a scientific foundation and innovative insights for clinical applications and the identification of relevant bioactive components.
1. Introduction
Camellia oleifera Abel. (Theaceae family), a high-quality edible oil plant endemic to China, is recognized as one of the four major woody oil plants globally, alongside olive, oil palm, and coconut [1]. The seeds of C. oleifera are distinguished by their high oil content, with a significant proportion of unsaturated fatty acids, predominantly oleic acid (about 80%) [2].
The fruit shells, seeds, and tea seed cakes (defatted seeds) of C. oleifera fruits contain various components, including triterpene saponins, flavonoids, lignans, tannins, fatty acids, and polysaccharides [3,4,5]. The main active constituents are triterpene saponins and flavonoids. Tea saponin, a pentacyclic triterpene saponin and glycoside molecule, exhibits excellent natural surfactant properties and antibacterial, antitumor, anti-inflammatory, antioxidant, and other properties [6,7,8,9,10]. Therefore, it is widely used in the pharmaceutical, cosmetic, functional food, and pesticide industries, among other sectors [11,12,13,14].
The oil derived from the mature seeds of C. oleifera is known as “Eastern olive oil” and is rich in nutrients, clear in color, and aromatic in flavor. The 2020 edition of the Chinese Pharmacopoeia lists this premium edible vegetable oil as a foundation for ointments and a raw ingredient for tea oil for injection [15]. Tea seed cake also has therapeutic benefits. It can remove heat, encourage blood flow, eliminate blood stasis, and relieve pain. China is the original home and distribution center of C. oleifera, with a long history of cultivation and abundant genetic resources. However, the majority of processed goods made from it are restricted to primary goods like tea seed oil. The underdeveloped production and processing of by-products, such as tea seed cakes and fruit shells, limit the possibility for sustainable industry expansion and lead to wasteful resource management.
Human health and quality of life are still seriously threatened by cancer, hyperlipidemia, and inflammatory chronic diseases. Research on new drugs is now focused on finding natural compounds that contain multi-target synergistic inhibitors that are safer, more effective, and less expensive [16,17]. Nowadays, dual-use products for food and medicine play a significant role in the prevention and management of chronic diseases and age-related conditions [18]. In this paper, we reviewed the primary chemical constituents and pharmacological effects of C. oleifera fruits (Figure 1), thereby providing a scientific foundation and strategic insights for the further development and utilization of this plant, particularly in functional foods and pharmaceutical applications.

Figure 1.
The fruits of C. oleifera.
2. Phytochemical Composition
2.1. Triterpenoids and Triterpenoid Saponins
In 1930, Aoyama first isolated Camellia saponin from tea tree seeds and named it “Theasaponin”, which belongs to the triterpenoid saponins [19]. C. oleifera fruit also has many saponin components, primarily oleanane-type pentacyclic triterpene saponins. Figure 2 depicts the structural mother nucleus of the saponin compounds. This paper summarizes 44 triterpenoids and triterpenoid saponins that have been isolated and reported [20,21,22,23,24,25,26,27,28,29,30,31,32,33]. The following are 33 of them listed in Table 1. The chemical structures of oleanolic acid (34), quillaic acid (35), oleanolic acid 3-O-β-D-glucoside (36) [33], camelliasaponin Ab (37) [30], β-amyrin acetate (38), germanol acetate (39), taraxerol acetate (40), Ψ-taraxasterol acetate (41), butyrospermol acetate (42), kansonidiol acetate (43), and damadienol acetate (44) [34] are presented in Figure 3.
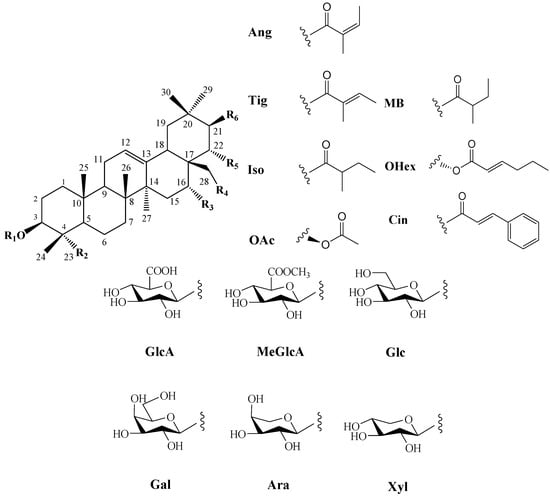
Figure 2.
Structures of triterpenoids in C. oleifera fruit. R1–R6 are substituent groups.

Table 1.
Triterpenoids and triterpenoid saponins from C. oleifera fruit.
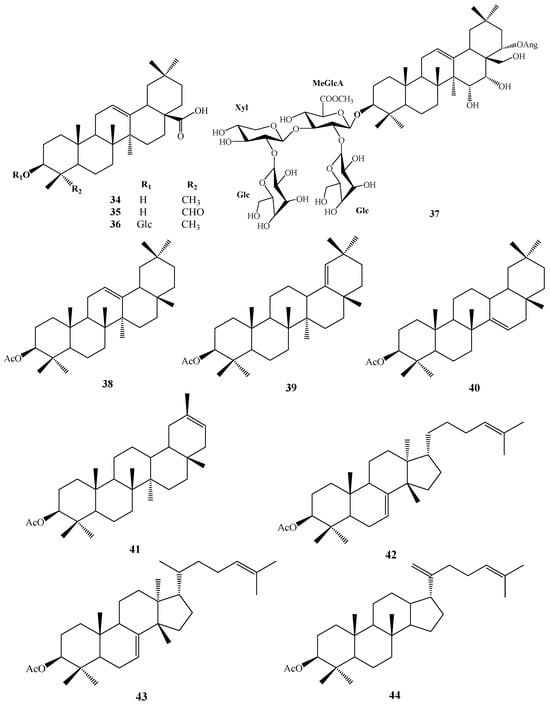
Figure 3.
Structures of 34–44.
The mother nuclei of these triterpenoids are predominantly pentacyclic triterpenoids of the oleanane type, which contain multiple active substitution sites primarily located at carbon positions C-3, C-16, C-21, C-22, C-23, and C-28. The molecular structure of most saponins consists of three distinct components: aglycones, sugar moieties, and organic acids [35]. Sugar substituents are mainly attached at the C-3 position, with the sugar chain composed predominantly of pentoses such as arabinose (Ara) and xylose (Xyl), as well as hexoses including glucose (Glc) and galactose (Gal). In addition, glucuronic acid (GlcA) and methylglucuronic acid (MeGlcA) are also commonly observed. These sugar units typically form oligosaccharide chains that are covalently linked to the aglycone, thereby generating triterpenoid saponins. The associated organic acids—such as acetic acid, angelic acid, isovaleric acid, hexenoic acid, 2-methylbutyric acid, tiglic acid, and cinnamic acid—can undergo esterification with specific hydroxyl groups on the saponin molecules, yielding ester derivatives.
2.2. Flavonoids and Their Glycosides
Numerous plants contain flavonoids, which are significant secondary metabolites. According to research, C. oleifera fruits have a comparatively large and diverse concentration of these components, primarily flavonols and their glycosides. Its structural mother nucleus is mainly composed of flavonols such as quercetin and kaunferol [36]. Arabinose (Ara), xylose (Xyl), glucose (Glc), rhamnose (Rha), and galactose (Gal) make up the majority of the sugar chain. The structural mother nucleus of the flavonoids found in C. oleifera fruit is displayed in Figure 4. The following are 19 of them listed in Table 2. The chemical structures of kaempferol-3-O-[6-trans-p-coumarol]-β-D-glucopyranosyl-(1 → 3)-α-L-rhamnopyranosyl-(1 → 6)-O-β-D-galactopyranoside (F-20) [37], naringenin-7-O-[β-D-xylopyranosyl-(1 → 6)][β-D-glucopyranosyl(1 → 3)-α-L-rhamnopyranosyl(1 → 2)-O-β-D-glucopyranoside (F-21), naringenin-7-O-[β-D-glucopyranosyl(1 → 3)-α-L-rhamnopyranosyl(1 → 2)-O-β-D-glucopyranoside (F-22), naringenin-7-O-β-D-xylopyranosyl-(1 → 6)-β-D-glucopyranoside (F-23) [20], naringoside (F-24) [33], naringenin (F-25) [5], (+)-4′-methylcatechin-7-O-β-D-glucopyranoside (F-26) [20], taxifolin (F-27) [38], cardamonin (F-28) [39], 4′,4′″,5,5″,7,7″-hexahydroxy-8,8″-biflavanonol (F-29) [39,40], 5,6,7,8,3′,4′-hexamethoxyflavone (F-30), 3,5,6,7,8,3′,4′-hexamethoxyflavone (F-31) [38], epicatechin-(5,6-bc)-4β-(p-hydroxyphenyl)-dihydro-2(3H)-pyranone (F-32), phloretin and (F-33) [41] are presented in Figure 5.
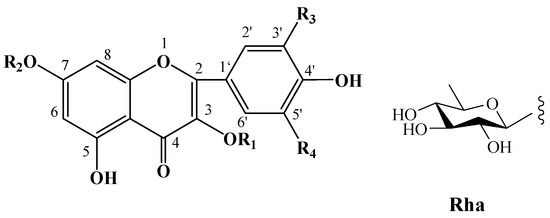
Figure 4.
Structures of flavonoids in C. oleifera fruits. R1–R4 are substituent groups.

Table 2.
Flavonoids and their glycosides from C. oleifera fruit.
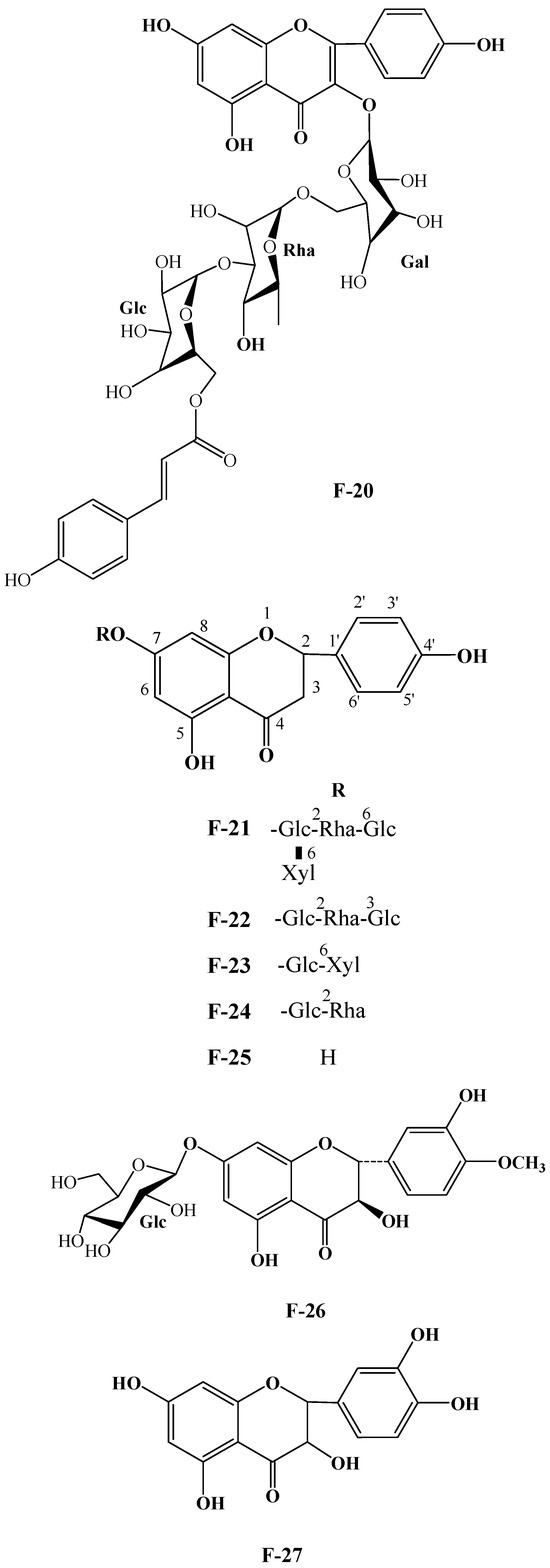
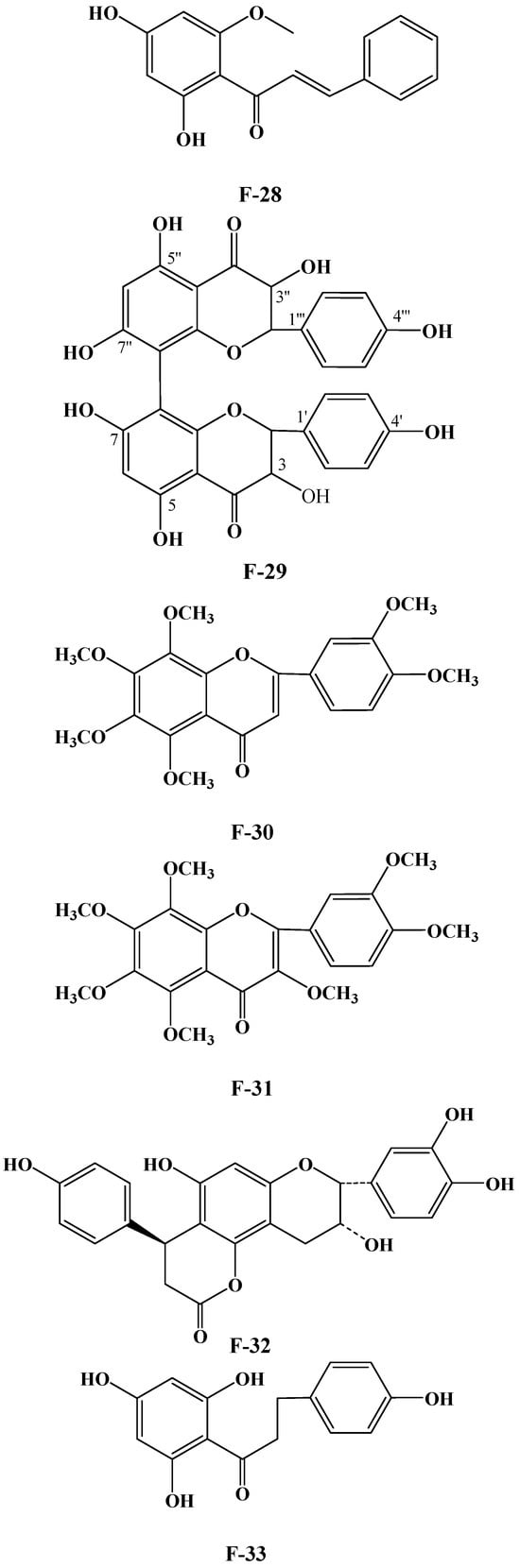
Figure 5.
Structures of F-20, F-21~25, F-26, F-27, F-28, F-29, F-30, F-31, F-32, and F-33.
2.3. Polysaccharides
Natural carbohydrates called polysaccharides are present in many plants and can be used as a criterion to assess the quality of herbs that contain them [47,48]. Specifically, polysaccharides from C. oleifera fruits are important components found in the cake meal and the fruit shells.
These days, the extraction, separation, and purification of C. oleifera polysaccharides are the primary areas of chemical research. As studies into the fundamental structure of these polysaccharides have progressed, some insight into their glycosyl composition has emerged. The main monosaccharides that have been identified are rhamnose, glucose, glucuronic acid, galacturonic acid, arabinose, xylose, mannose, galactose, and fucose. The typical relative molecular mass of the polysaccharides ranges from 103 to 107 Da [49,50]. The chemical structural characteristics of those that have been found thus far are shown in Table 3.

Table 3.
Polysaccharides from C. oleifera fruit.
2.4. Lignans
Fewer lignan compounds were isolated from the fruits of C. oleifera. Four lignans—(-)-pinoresinol-4-O-β-D-glucopyranoside, methylpinoresinol, (-)-pinoresinol, and (-)-pinoresinol diglucoside—were isolated from C. oleifera seed cake by Zhu et al. [44]. Cheng [39] isolated some lignans from the shells of C. oleifera fruits, including (+)-epipinoresinol, (1S,2R,5S,6R)-2-(4-hydroxyphenyl)-6-(3-methoxy-4-hydroxyphenyl)-3,7-dioxabicyclo [0,3] octane, syringaresinol, salicifoliol, (-)-secoisolariciresinol, arctigenin, (+)-isolariciresinol, pinnatifidanin B VI, pinnatifidanin B V, dihydrodehydrodiconiferl alcohol. (-)-isolariciresinol-9′-methyl succinate and (-)-isolariciresinol-9-methyl succinate, two new aryltetralin-type lignans were isolated from C. oleifera fruit husk [59]. (7S,8S)-3-methoxy-3′,7-epoxy-8,4′-oxyneoligna-4,9,9′-triol, massonianoside E, isolariciresinol-9-O-xyloside, nudiposide, 5′-methoxy-isolarchitin-9′-O-β-D-xyloside, aviculin, davidioside A, (7R,8R)-4-O-(glycer-2-yl)-7,9,9′-trihydroxy-3,3′-dimethoxy-8-O-4′-neolignan from the fruit hull of C. oleifera [48]. Sesamin and 2,5-bis-benzo[1,3]dioxol-5-yl-tetrahydro-furo [3,4-d][1,3]-dioxine were isolated from the methanol extract of tea seed oil [60].
2.5. Phytosterols
Phytosterols are naturally found in various parts of plants, including roots, stems, leaves, fruits, and seeds, and can be classified into three major categories: 4-methylsterols, 4,4′-dimethylsterols, and sterols without methyl substitutions. C. oleifera fruit contains geranyl linalool, ergosterol, β-amyrin, lanosterol [61], 3α-spinasterol, ergosta-4,6,8(14),22-tetraen-3-one [37], β-sitosterol, stigmast-7-en-3-ol [62].
2.6. Others
Fatty acids, phenylpropyl compounds, anthraquinones, volatile oils, alkaloids, and other bioactive substances have also been isolated from C. oleifera fruits. The main fatty acid constituents in its seeds are stearic acid, palmitic acid, oleic acid, and linoleic acid. Other components include palmitic acid, arachidic acid, α-linolenic acid, and others. Unsaturated fatty acids can make up more than 90% of the total. Oleic acid (C18:1), which ranged from 70.21% to 85.23%, palmitic acid (C16:0), which ranged from 6.93% to 13.89%, and linoleic acid (C18:2), which ranged from 5.02% to 14.26%, were the most prevalent fatty acids (FA) [63]. The fruit shells of C. oleifera were used to separate quinone components, including emodin, 6-ethyl-5-hydroxy-2, 7-dimethoxy-1, 4-naphthoquinone, and ω-hydroxyemodin [38]. Additionally, phenolic substances such as gallotannin, ellagitannin, and catechin were identified using HPLC–ESI–MS technology; oxalic acid, citric acid, acetic acid, malic acid, and succinic acid were found to be the major organic acids present [45].
3. Pharmacological Activities
Modern pharmacological studies have demonstrated that the extract of C. oleifera fruit has multiple pharmacological effects, including anti-tumor, anti-allergy, hypoglycemic, lipid-lowering, anti-inflammatory, antioxidant, and protective effects on the gastric mucosa.
3.1. Anti-Tumor Activity
A tumor is a mass formed by abnormal proliferation of cells within the body. This abnormal hyperplasia may be benign or malignant cancer. Cancer is recognized as the second leading cause of death globally, and the incidence of cancer continues to rise rapidly, imposing an escalating burden on global public health. Although chemotherapy remains the most commonly employed treatment for cancer, its efficacy is often constrained by the development of drug resistance and significant adverse effects [64]. Numerous natural compounds have been identified to influence cancer cell apoptosis and exhibit anticancer properties through multi-target synergistic mechanisms.
Previous studies have shown C. oleifera saponins exhibit significant anti-tumor effects both in vivo and in vitro, exerting direct cytotoxic actions and inhibiting the proliferation of tumor cells (Table 4). The cytotoxicity of oleiferasaponin B1 and B2 was evaluated in four human carcinoma cell lines: A549, SK-OV-3, SK-MEL-2, and HCT15. Both of them exhibited significant cytotoxic activity with IC50 values of 18.5 μM, 11.3 μM, 13.9 μM, and 1.6 μM; and IC50 values of 8.4 μM, 6.3 μM, 9.2 μM, and 0.8 μM [24]. Oleiferasaponin C1, C2, and camelliasaponin B1 showed significant cytotoxic activities against BEL-7402, BGC-823, MCF-7, HL-60, and KB, five human tumor cell lines [25]. Oleiferasaponin C4~C6 from the seeds of C. oleifera can inhibit proliferation through inducing cell-cycle arrest and apoptosis in human cancer cell lines (BEL-7402, BGC-823, MCF-7, HL-60, and KB) in vitro [26,27]. Oleiferasaponins D1 and D2 exhibited cytotoxic activity against five human cancer cell lines (HCT-116, HepG2, BGC-823, NCI-H1650, and A2780), with IC50 values ranging from 3.31 to 10.23 μM. Oleiferasaponins D3~D5 showed moderate cytotoxic activities toward the tested cell lines [28]. C4 exhibits the strongest anticancer activity among the five cell lines, as evidenced by its ED50 value, which varies from 1.5 to 11.3 μM against Huh-7, HepG2, HeLa, A549, and SGC7901. This value is comparable to that of cisplatinum (CDDP) in these cell lines. Compound C4 may increase cell proliferation via the NF-B/iNOS/COX-2 pathway and induce apoptosis via the Bcl-2/Caspase-3 and JAK2/STAT3 pathways [31] (Figure 6). Bioactivity evaluations demonstrated that oleiferasaponins G6~G9, as well as the total saponin, exhibited significant proliferative inhibitory activity against HCT-116, HL-60, and HepG2 cell lines [32].

Table 4.
Pharmacological activities of main saponin compounds from C. oleifera fruits.
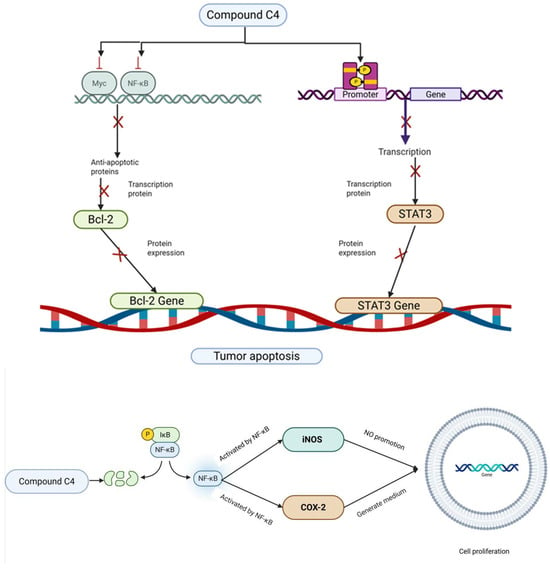
Figure 6.
The mechanisms of action of compound C4 against cancer.
Total saponins of C. oleifera (TSSC) have an inhibitory effect on the growth of solid tumors of H22 liver cancer in mice. TSSC can promote the apoptosis of H22 by up-regulating the expression of Bax protein and down-regulating the expression of Bcl-2 protein simultaneously [65]. In a recent study, it was demonstrated that the ethanol-soluble acidic components of C. oleifera cake exhibited anti-tumor activities both in vitro and in vivo through regulated cell death, which involved mechanisms of apoptosis, pyroptosis, and ferroptosis. Moreover, the combination of ESAC and PD-1 inhibitors showed greater efficacy than either treatment alone, promoting pyroptosis through the activation of the NLRP3/Caspase-1/GSDMD signaling pathway [66].
With an IC50 value of 5.826 μg/mL, the crude polysaccharides had substantial anti-proliferative activity against HepG2 cells [67]. On normal IAR20 cells, however, the inhibitory rate was 0.61%, suggesting low toxicity. The anticancer potential is further supported by in vivo research; in BALB/C mice, SCP1 demonstrated an 85.6% inhibition rate against Sarcoma 180 solid tumors at a dose of 40 mg/kg [68].
3.2. Antioxidant Activity
An imbalance between the body’s oxidation and antioxidation processes is known as oxidative stress, and it is thought to be a major contributing cause to aging and disease. It can cause neutrophils to infiltrate and induce inflammation, increase protease secretion, and produce a large number of oxidation intermediate products, which can lead to an excess of reactive oxygen species and other substances, and harm cells and tissues [69,70]. Polyphenolic chemicals and unsaturated fatty acids are abundant in C. oleifera oil. Tea polyphenols and oleic acid are strong natural antioxidants that have been shown to be highly effective in scavenging free radicals in the body [41,71].
Studies have shown that polyphenols extracted from seed cake can alleviate H2O2-induced oxidative stress in cells by modulating SOD activity, reducing the levels of MDA and ROS, suppressing apoptosis, and inhibiting the activation of the NF-κB signaling pathway [72].
Out of the five solvent extracts, the methanol extract of tea seed oil exhibited the highest yield and the strongest antioxidant activity based on DPPH scavenging activity and Trolox equivalent antioxidant capacity [60]. ABTS, DPPH, and OH radicals were all effectively scavenged by the polysaccharide derived from C. oleifera seed cakes, with IC50 values of 2.94, 2.24, and 5.09 mg/mL, respectively [73]. Because of its greater ability to donate hydrogen, COP-E showed the most positive antioxidant impact among the studied polysaccharides COP-H, COP-U, COP-E, and COP-A [52]. SCP-a demonstrated more ABTS scavenging activity than SCP-b and SCP-c at the same dose. Its compact, dense, and helical surface shape, along with the higher galactose content in its monosaccharide composition, may be responsible for this increased activity. In contrast, SCP-c demonstrated superior metal chelating ability, which could be attributed to its relatively higher uronic acid and sulfate content [56].
Bacillus subtilis was utilized for the submerged fermentation of camellia seed cake. The antioxidant capacity of the resulting product (CSCH) was evaluated using DPPH, ABTS, and hydroxyl radical scavenging assays. The results indicated that CSCH has the potential to be developed as a novel, fully natural antioxidant and anti-tyrosinase agent [74].
3.3. Hypolipidemic Activity
Elevated blood lipid levels are a defining feature of hyperlipidemia, a condition that significantly increases the risk of a number of illnesses, most notably heart disease and stroke. Promising substitutes for traditional lipid-lowering drugs, which have a long list of side effects, are phytochemical substances [75].
On HepG2 cell lines, oleiferasaponin A2 demonstrated anti-hyperlipidemic activity by dramatically increasing the expression of ACOX-1, CPT-1, and ACOX-1 protein, while significantly downregulating the expression of SREBP-1c, FAS, and FAS protein, which inhibited fatty acid synthesis [23]. For eight weeks, the rats were given a basal diet, a high-fat diet, and a high-fat diet combined with a hot water extract of tea seed cake (CSE). The findings demonstrated that rats administered CSE had reduced levels of circulating leptin, lower levels of malondialdehyde and hydroxyproline in the liver, and decreased levels of epididymal and retroperitoneal fat compared to the high-fat diet group [76].
A high-fat diet, a high-fat diet with atorvastatin, a high-fat diet supplemented with C. oleifera polyphenols (2.5, 7.5, and 15 mL/kg), or a basal diet were given to the rats. The findings showed that the high-fat diet combined with polyphenol or atorvastatin treatment decreased body weight and the liver-to-body weight ratio. The activities of alanine aminotransferase and aspartate aminotransferase were also reduced, as were the levels of total cholesterol, triglycerides, and low-density lipoprotein cholesterol. High-density lipoprotein cholesterol, on the other hand, was elevated. Genes linked to hepatic lipid metabolism, such as ACAT1, DGAT2, FAS, and SREBP, showed markedly decreased relative expression levels [77].
Camellia oil may elevate serum oleic acid levels and reduce body weight and BMI in individuals with hypertriglyceridemia who maintain stable dietary intake and physical activity [78]. Consuming camellia oil has been demonstrated to influence inflammatory indicators and oxidative stress in women with hypercholesterolemia. A diet high in tea oil has been shown to lower blood levels of malondialdehyde. Low-density lipoprotein cholesterol (LDL-C), malondialdehyde (MDA), and C-reactive protein (CRP) are examples of inflammatory indicators that can lower the risk of cardiovascular diseases [79].
3.4. Hypoglycemic Activity
Hyperglycemia, hyperlipidemia, and hepatic steatosis are among the signs of type 2 diabetic mellitus (T2DM), a metabolic disorder. Statistics show that 463 million people worldwide have diabetes, with type 2 diabetes accounting for the great majority of cases. Some complications are brought on by this illness, which not only affects blood sugar levels but also poses a major threat to life [80].
Oleiferasaponin A1, which may have hypoglycemic properties, protected pancreatic cell lines against the harm that comes from too much glucose. Oleiferasaponin A1 prevented the damage caused by excessive glucose and increased insulin expression in RIN-m5f cells [21]. It was discovered that the polysaccharide CCP raises the relative glucose consumption rate in HepG2 cells in a dose-dependent manner within the range of 0.125–0.500 mg/mL [50]. The fruit hull also contained substantial amounts of two polysaccharides, CFPB and CFPA-3, which were isolated and showed dose-dependent inhibition of α-glucosidase activity with IC50 values of 11.80 and 10.95 μg/mL, respectively [81].
In streptozotocin-induced diabetic mice, both crude and purified polysaccharides at a dose of 200 mg/kg/d significantly alleviated various symptoms, reduced the levels of MDA, and enhanced the activities of glutathione peroxidase (GSH-Px), catalase (CAT), and superoxide dismutase (SOD) [53]. Further animal experiments indicated that administration of polysaccharides SCP-1 and SCP-2 at a dose of 400 mg/(kg·d) decreased plasma glucose levels in streptozotocin-induced diabetic mice by 46.83% and 33.00%, respectively. These polysaccharides also elevated the activities of GSH-Px, CAT, and SOD levels, while reducing MDA content in hyperglycemic mice [55].
3.5. Anti-Inflammatory Activity
Excessive free radicals, which are created when the body’s metabolism and biochemical reactions are out of balance, can harm biological macromolecules like DNA, proteins, and mitochondria as well as cell structures. This can result in a number of diseases, including cardiovascular and inflammatory conditions. Thus, controlling genes to limit the overproduction of free radicals is crucial for apoptosis and cell division as well as preserving human health [82].
According to pharmacological research, phenolic substances, certain triterpenoids, and C. oleifera seed extract (camellia oil) all have strong anti-inflammatory properties [83]. Significant analgesic and anti-inflammatory properties are exhibited by the diflavonoids that were extracted from the shells of C. oleifera fruits. The findings of the mouse writhing experiments with hot plates and acetic acid suggested that this diflavonone compound could significantly lower serum MDA levels, boost SOD and GSH-Px activities, and dose-dependently reduce rat foot swelling caused by carrageenan and mouse ear inflammation caused by croton oil [41]. The ethanol extract of C. oleifera seeds effectively inhibits Drosophila enteritis. The chemicals with comparatively high antienteritis activity were identified by ultra-high performance liquid chromatography-tandem mass spectrometry as bruceine B, miltirone, 8-geranyloxypsoralen, cedrelone, wighteone, kaempferitrin, and kaempferol-3-O-rutinoside [84].
The hydrolyzed sasanquasaponins extracted from the defatted seeds of C. oleifera exhibited significant anti-inflammatory and analgesic effects. These compounds effectively alleviated carrageenan-induced paw edema in rats and soybean oil-induced ear inflammation in mice. Furthermore, they were found to reduce serum MDA levels while simultaneously increasing the levels of SOD and GSH-Px. Additionally, these saponins demonstrated the ability to inhibit the expression of pro-inflammatory cytokines, including interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and prostaglandin E2 (PGE2) [85].
3.6. Neuroprotective Activity
A progressive, late-onset condition known as neurodegeneration is typified by loss or degeneration of neurons as well as cognitive and motor deficits. Its fundamental mechanics are yet not fully understood. Oxidative stress has been the subject of numerous investigations, as it plays a part in the series of events that culminate in neurodegenerative pathology [86].
Camellia oil contains unsaturated fatty acids, squalene, polyphenols, and other bioactive compounds. Its unsaturated fatty acids, such as oleic acid, can reduce the production of Aβ in the brain tissue of Alzheimer’s disease (AD) mice and inhibit the deposition of amyloid plaques caused by abnormal accumulation of Aβ [87]. It has been demonstrated that camellia oil regulates the metabolism of aspartic acid, increases the level of N-acetyl-L-aspartic acid in mice, enhances the brain’s energy supply, and alleviates the symptoms of AD [88]. A previous report demonstrated that camellia oil may reverse AD-related brain pathology by alleviating memory impairments, enhancing learning ability, increasing antioxidant activity, modulating the expression of immune-related cytokines, promoting autophagy, and improving the composition of gut microbiota in rats treated with aluminum chloride. These findings suggest that camellia oil may mitigate the pathophysiological progression of AD through mechanisms involving the microbiome-gut–brain axis [89]. Camellia oil may ameliorate Aβ25–35-induced memory impairment in mice by modulating immune cell activity and neuroinflammation through the PPARs signaling pathway, which in turn influences gut microbiota composition and lipid metabolism [90].
The sapogenin from sasanqua saponin hydrolysis and its amination derivative increase dopamine levels in the substantia nigra and striatum, increase the number of tyrosine hydroxylase-positive cells, reduce neuroinflammation and behavioral deficits, and protect against Parkinson’s disease in MPTP-treated mice. These compounds appear to shield dopaminergic neurons via anti-neuroinflammatory effects and dopamine receptor activation, with aminated derivatives showing greater efficacy [29]. Treatment with oleiferasaponin A1 at concentrations of 5, 25, and 125 μM significantly increased cell viability, indicating its cytoprotective effect against H2O2-induced damage [22].
While increasing superoxide dismutase activity, tyrosine hydroxylase expression, and dopamine and acetylcholine levels in a dose-dependent way, iron-sapogenin nanoparticles can ameliorate behavioral problems and lower malondialdehyde levels in the mouse brain. Iron-sapogenin nanoparticles have much better therapeutic benefits than sapogenin by itself [91]. Through the coordination of zinc with sapogenin, the nanoparticle promotes electron transport among atoms, increasing DPPH radical scavenging activity. Zinc-sapogenin administered intraperitoneally has been shown to improve antioxidant capacity, raise dopamine and acetylcholine levels in the brain, and reduce behavioral abnormalities and neuronal damage in mice induced by rotenone neurotoxicity [92].
3.7. Antimicrobial Activity
The health of people, animals, and the environment is seriously threatened by the global spread of antimicrobial resistance (AMR). Antimicrobial resistance has been accelerated by the widespread usage and incorrect application of antimicrobial drugs, despite the fact that they have revolutionized modern medicine [93]. As a result, natural antibacterial agents derived from plants have drawn more interest as possible substitutes.
Camelliasides A and B, as well as a saponin mixture containing camelliasaponin B1, demonstrate the ability to reduce Rhizoctonia solani Kühn AG-4 infection in cabbage seedlings and inhibit the growth of the pathogen on potato dextrose agar plates [21]. Two compounds from C. oleifera fruit hull, epicatechin-(5,6-bc)-4β-(p-hydroxyphenyl)-dihydro-2(3H)-pyranone and 2-O-(3,4-dihydroxybenzoyl)-2,4,6-trihydroxyphenylmthylacetate, show good anti-respiratory syncytial virus activity. The IC50 values are 9.67 ± 0.68 and 21.53 ± 2.54, respectively [41].
From camellia oil, Akihisa et al. extracted seven triterpenoids (1–7) and assessed their capacity to prevent Raji cells from producing Epstein–Barr virus early antigens (EBV-EA) in response to stimulation. With IC50 values ranging from 277 to 420 mol TPA/32 pmol TPA, compounds 5–7 showed notable inhibitory efficacy against EBV-EA induction [94]. The minimum inhibitory concentration (MIC) values of tea oil extracted using organic solvents against Escherichia coli, Staphylococcus aureus, and yeast were all 100 mg/mL, with inhibitory effects increasing as the concentration increased. For pressed tea oil, the MIC value against E. coli was 100 mg/mL, while those against S. aureus and yeast were 200 mg/mL. Inhibition by pressed tea oil also exhibited a concentration-dependent effect. In contrast, no antimicrobial activity was detected against Penicillium, green mold, or Bacillus subtilis [95]. It has been reported that four samples of C. oleifera seed oil at different refining stages, as well as their methanol extracts, have certain inhibitory effects on E. coli, S. aureus, Pseudomonas aeruginosa, and Candida albicans. The primary antibacterial active constituents are speculated to include catechin derivatives, chlorogenic acid, puerarin, carotene, α-tocopherol, and 3-p-coumaryl quinic acid [96].
3.8. Other Activity
The components of C. oleifera fruit also have the functions of regulating intestinal flora, protecting gastric mucosa, immunomodulation, and so on.
Jin et al. [57] obtained two polysaccharides (CCPA and CCPB) from tea seed cakes. Animal experiments demonstrated that these polysaccharides could modulate gut microbiota composition and enhance microbial diversity.
The phagocytic activity of primary peritoneal macrophages and RAW 264.7 macrophages was markedly increased by camellia oil. It also decreased the generation of nitric oxide (NO) in BALB/c mice and RAW 264.7 cells. Additionally, camellia oil significantly increased the production of IL-10 by primary splenocytes.
In addition to increasing the mRNA expression of heme oxygenase-1 (HO-1), GSH-Px, and SOD, treating Int-407 cells with 50–75 μg/mL of camellia oil also increased the secretion of vascular endothelial growth factor (VEGF) and PGE2, strengthening the mucosal defense against gastrointestinal oxidative injury. Additionally, pretreatment with camellia oil (2 mL/kg/d) before ketoprofen administration (50 mg/kg/d) in Sprague Dawley rats inhibited the expression of the cyclooxygenase-2 (COX-2) protein, decreased the production of NO and IL-6, restored compromised antioxidant systems, and lessened oxidative damage in the gastrointestinal mucosa [97].
Camellia oil significantly enhanced phagocytic activity in both RAW 264.7 macrophages and primary peritoneal macrophages. Additionally, it reduced NO production in RAW 264.7 cells and BALB/c mice. Moreover, camellia oil markedly stimulated IL-10 production in primary splenocytes. These findings suggest that camellia oil, which is rich in oleic acid, can potently promote the CD19+-mediated humoral immune response [98].
4. Conclusions
C. oleifera, a Chinese endemic woody oil crop cultivated for over 2000 years, yields seed oil rich in unsaturated fatty acids. As a traditional medicinal plant, it demonstrates anti-inflammatory, antioxidant, and lipid-regulating properties, showing promise for pharmaceutical and functional product development. China currently has a cultivation area of approximately 30,000 m2 for C. oleifera, with an annual production of one million tons of Camellia oleifera seeds and 270,000 tons of oil. As a pure, natural, woody, edible, and healthy vegetable oil (Figure 7), tea oil is promoted by the Chinese government and is the first plant-based edible oil recommended by the Food and Agriculture Organization of the United Nations.
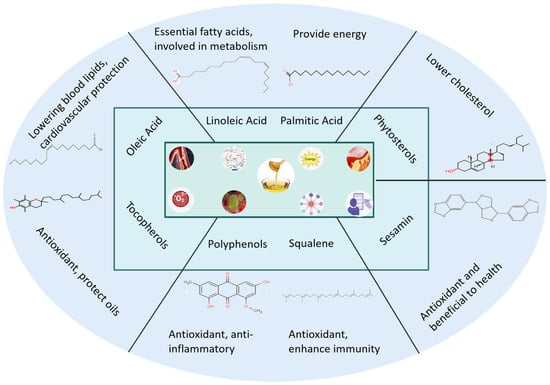
Figure 7.
The main components and their pharmacological activities of camellia oil.
The utilization of seed oil is currently the main focus of research and development activities on this plant, although the utilization of by-products like fruit shells and cake meal is still in its infancy. There is a serious risk of environmental contamination because these processing leftovers are often discarded as waste after manufacturing. To solve this problem, the primary phytochemical components isolated from C. oleifera fruits and their pharmacological effects were covered in this review. In terms of the number of isolated compounds, triterpene saponins are the most numerous, followed by flavonoids and polysaccharides. In the meantime, C. oleifera’s constituents have outstanding pharmacological properties and can serve as lead compounds for the creation of novel medications. The primary focus of current research on the therapeutic regulation of these substances is their pharmacological effects and underlying mechanisms. Future studies should prioritize clinical application research to bridge the gap between preclinical findings and therapeutic practice. In addition, some evidence suggests that C. oleifera’s bioactive constituents may modulate gut microbiota composition. As compound efficacy is intrinsically linked to bioavailability and host-microenvironment interactions, systematic investigations into their microbiota-modulating effects are warranted.
In conclusion, as mentioned above, it offers valuable insights for further research on the health benefits of this plant and suggests new potential approaches for the large-scale utilization of C. oleifera in disease prevention and treatment.
Author Contributions
B.X.: writing—original draft, software. A.-N.D.: investigation, formal analysis. T.-Z.L.: formal analysis. P.-H.W. and B.-R.Z.: investigation, data curation, resources. K.C.: writing—review and editing, supervision, conceptualization. L.S.: writing—review and editing, supervision, conceptualization. All authors discussed and commented on the manuscript and finally approved it for submission. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Foundation of Camellia oleifera Industry Research Institute construction project of Shangrao Normal University (Grant No. 2023002), Open Bidding for Selecting the Best Candidates Project of Yanshan (Grant No. 202203), and the Project of the Liaoning Provincial Department of Education (JYTMS20231276).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
Author Bo-Rong Zhu was employed by the company Jiangxi Xin Zhongye Camellia Industry. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Kong, Q.; Chen, T.; Wang, H.; Zheng, S.; Wang, H.; Liang, H.; Zhou, L.; Yang, H.; Jiang, X.; Ding, C.; et al. Variation of Camellia oleifera fruit traits and nutritional constituents in seed oil during development and post-harvest. Sci. Hortic. 2025, 339, 113903. [Google Scholar] [CrossRef]
- Luan, F.; Zeng, J.S.; Yang, Y.; He, X.R.; Wang, B.J.; Gao, Y.B.; Nan, Z. Recent advances in Camellia oleifera Abel a review of nutritional constituents, biofunctional properties and potential industrial applications. J. Funct. Foods 2020, 75, 104242. [Google Scholar] [CrossRef]
- Jiang, N.; Xu, Z.; Lu, S.; Xia, X.; Li, M.; Miao, Y.; Xiang, X. Advances in valorization of Camellia oleifera Abel. Seed cake: A review on the bioactive components, health benefits, extraction methods, and potential food applications. Food Res. Int. 2025, 208, 116134. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tan, Y.; Ma, G.; Bai, X.; Cao, J. Advances in research and application of Camellia oil. J. Chin. Cereals Oils Assoc. 2017, 32, 191–196. [Google Scholar]
- Wang, L.Q.; Xu, Q.L.; Dong, L.M.; Zhang, Q.; Tan, J.W. Chemical constituents from the fruit shell of Camellia oleifera. J. Trop. Subtrop. Bot. 2017, 25, 81–86. [Google Scholar]
- Kuo, P.C.; Lin, T.C.; Yang, C.W.; Lin, C.L.; Chen, G.F.; Huang, J.W. Bioactive saponin from tea seed pomace with inhibitory effects against Rhizoctonia solani. J. Agric. Food Chem. 2010, 58, 8618–8622. [Google Scholar] [CrossRef]
- Hu, J.L.; Nie, S.P.; Huang, D.F.; Li, C.; Xie, M.Y. Extraction of saponin from Camellia oleifera cake and evaluation of its antioxidant activity. Int. J. Food Sci. Tech. 2012, 47, 1676–1687. [Google Scholar] [CrossRef]
- Zhang, X.F.; Han, Y.Y.; Di, T.M.; Gao, L.P.; Xia, T. Triterpene saponins from tea seed pomace (Camellia oleifera Abel) and their cytotoxic activity on MCF-7 cells in vitro. Nat. Prod. Res. 2019, 35, 2730–2733. [Google Scholar] [CrossRef]
- Jian, H.L.; Liao, X.X.; Zhu, L.W.; Zhang, W.M.; Jiang, J.X. Synergism and foaming properties in binary mixtures of a biosurfactant derived from Camellia oleifera Abel and synthetic surfactants. J. Colloid. Interf. Sci. 2011, 359, 487–492. [Google Scholar] [CrossRef]
- Chen, Y.F.; Yang, C.H.; Chang, M.S.; Ciou, Y.P.; Huang, Y.C. Foam properties and detergent abilities of the saponins from Camellia oleifera. Int. J. Mol. Sci. 2010, 11, 4417–4425. [Google Scholar] [CrossRef]
- Zhang, L.X.; He, Y.F.; Zhu, Y.J.; Liu, Y.T.; Wang, X.C. Camellia oleifera shell as an alternative feedstock for furfural production using a high surface acidity solid acid catalyst. Bioresour. Technol. 2018, 249, 536–541. [Google Scholar] [CrossRef]
- Jung, K.; Yeh, W.J.; Huang, W.C.; Yang, H.Y. Camellia oleifera seed extract mildly ameliorates carbon tetrachloride-induced hepatotoxicity in rats by suppressing inflammation. J. Food Sci. 2019, 84, 1586–1591. [Google Scholar]
- Yang, Y.; Cui, J.; Zhang, J.; Jiang, J.; Chen, X.; Shan, K. Advancements in extraction and sustainable applications of Camellia oleifera: A comprehensive review. Food Chem. 2025, 488, 144940. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Deng, S.; Fan, B.; Xu, D.; Du, G.; Li, X. Agricultural waste of Camellia oleifera fruit shell extract with potassium iodide as a novel synergistic composite inhibitor for the corrosion of steel in methylsulfonic acid solution. Ind. Crop. Prod. 2024, 222, 119834. [Google Scholar] [CrossRef]
- State Pharmacopoeia Commission of PR China. Pharmacopoeia of the People’s Republic of China; Chemical Industry Press: Beijing, China, 2020; Volume 1, p. 429. [Google Scholar]
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef]
- Zuo, J.; Zhu, Y.; Yang, N.; Xu, D.; Zhao, J. Pharmacological mechanisms and pharmacokinetic analysis of anti-tumor components in Chinese herbal medicine. Chinese J. Anal. Chem. 2025, 53, 100590. [Google Scholar]
- Chen, J. Essential role of medicine and food homology in health and wellness. Chin. Herb. Med. 2023, 15, 347–348. [Google Scholar] [CrossRef]
- Aoyama, S. Camellia Saponin. Yakugaku Zasshi. 1930, 50, 378–379. [Google Scholar] [CrossRef][Green Version]
- Chen, J.; Wu, H.; Liau, B.; Chang, C.J.; Jong, T.; Wu, L. Identification and evaluation of antioxidants defatted Camellia oleifera seeds by isopropanol salting-out pretreatment. Food Chem. 2010, 121, 1246–1254. [Google Scholar] [CrossRef]
- Di, T.M.; Yang, S.L.; Du, F.Y.; Zhao, L.; Xia, T.; Zhang, X.F. Cytotoxic and hypoglycemic activity of triterpenoid saponins from Camellia oleifera Abel. seed pomace. Molecules 2017, 22, 1562. [Google Scholar] [CrossRef]
- Zhang, X.F.; Han, Y.Y.; Bao, G.H.; Ling, T.J.; Zhang, L.; Gao, L.P.; Xia, T. A new saponin from tea seed pomace (Camellia oleifera Abel) and its protective effect on PC12 cells. Molecules 2012, 17, 11721–11728. [Google Scholar] [CrossRef] [PubMed]
- Di, T.M.; Yang, S.L.; Du, F.Y.; Zhao, L.; Li, X.H.; Xia, T.; Zhang, X.F. Oleiferasaponin A2, a novel saponin from Camellia oleifera Abel. seeds, inhibits lipid accumulation of HepG2 cells through regulating fatty acid metabolism. Molecules 2018, 23, 3296. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, C.Z.; Ye, J.Z.; Chen, H.X. New triterpene saponins from the seed cake of Camellia Oleifera and their cytotoxic activity. Phytochem. Lett. 2014, 8, 46–51. [Google Scholar] [CrossRef]
- Zong, J.F.; Wang, R.; Bao, G.; Ling, T.; Zhang, L.; Zhang, X.; Hou, R. Novel triterpenoid saponins from residual seed cake of Camellia oleifera Abel. show anti-proliferative activity against tumor cells. Fitoterapia 2015, 104, 7–13. [Google Scholar] [CrossRef]
- Zong, J.F.; Peng, Y.; Bao, G.; Hou, R.; Wan, X. Two new oleanane-type saponins with anti-proliferative activity from Camellia oleifera Abel. seed cake. Molecules 2016, 21, 188. [Google Scholar] [CrossRef]
- Zong, J.F.; Wang, D.; Jiao, W.; Zhang, L.; Bao, G.; Ho, C.; Hou, R.; Wan, X. Oleiferasaponin C6 from the seeds of Camellia oleifera Abel.: A novel compound inhibits proliferation through inducing cell-cycle arrest and apoptosis on human cancer cell lines in vitro. Rsc. Advances 2016, 6, 91386–91393. [Google Scholar] [CrossRef]
- Fu, H.Z.; Wan, K.H.; Yan, Q.W.; Zhou, G.P.; Feng, T.T.; Dai, M.; Zhong, R.J. Cytotoxic triterpenoid saponins from the defatted seeds of Camellia oleifera Abel. J. Asian Nat. Prod. Res. 2018, 20, 412–422. [Google Scholar] [CrossRef]
- Ye, Y.; Fang, F.; Li, Y. Isolation of the sapogenin from defatted seeds of Camellia oleifera and its neuroprotective effects on dopaminergic neurons. J. Agric. Food. Chem. 2014, 62, 6175–6182. [Google Scholar] [CrossRef]
- Xiong, L.; Fu, H.Z.; Yan, Q.W. A new triterpene saponin from defatted seeds of Camellia oleifera. Chin. Tradit. Herb. Drugs 2017, 48, 4375–4380. [Google Scholar]
- Wu, Z.; Tan, X.; Zhou, J.; Yuan, J.; Yang, G.; Li, Z.; Long, H.; Yi, Y.; Lv, C.; Zeng, C.; et al. Discovery of new triterpenoids extracted from Camellia oleifera seed cake and the molecular mechanism underlying their antitumor activity. Antioxidants 2023, 12, 7. [Google Scholar] [CrossRef]
- Zong, J.F.; Guo, X.X.; Zou, K.K.; Cui, C.J.; Hu, Z.H.; Hou, R.Y. Isolation and identification of triterpenoid saponins with antiproliferative and hemolytic activities from Camellia oleifera Abel seeds. Phytochemistry 2025, 235, 114476. [Google Scholar] [CrossRef]
- Chen, S.P.; Huang, Y.; Wu, L.; Wu, J. Chemical constituents from fruit shells of Camellia oleifera. Biol. Chem. Eng. 2017, 3, 21–23. [Google Scholar]
- Fang, Y.D.; Liu, L.Q.; Li, H. Study on pentacyclic triterpene alcohol and tetracyclic triterpene alcohol in the unsaponifiable of Camellia seed oil. J. Chin. Cereal Oil Ass. 1999, 14, 18–22, 26. [Google Scholar]
- Ai, J.X.; Zheng, D.J.; Wu, X. Progress in germplasm resources, chemical composition and pharmacological activities of Camellia oleifera. Food Drug 2025, 27, 17–26. [Google Scholar]
- Luo, Y.M.; Li, B.; Xie, Y.H. Research on the chemical components of Camellia oleifera. Chin. Traditi. Herbal. Drugs 2003, 34, 117–118. [Google Scholar]
- Wang, Y.; Fei, X.; Lu, K.; Yao, X.; Guo, S.; Wang, K. Inhibitory effect of Aspergillus flavus and component analysis of methanol extraction from camellia seed cake. Trans. Chin. Soc. Agric. Eng. 2019, 35, 322–329. [Google Scholar]
- Cheng, H.Y. Study on the Chemical Constituents of Camellia oleifera Abel. Master’s Thesis, Jinan University, Guangzhou, China, 2022. [Google Scholar]
- Ye, Y.; Guo, Y.; Luo, Y.T.; Wang, Y.F. Isolation and free radical scavenging activities of a novel biflavonoid from the shells of Camellia oleifera Abel. Fitoterapia 2012, 83, 1585–1589. [Google Scholar] [CrossRef]
- Ye, Y.; Guo, Y.; Luo, Y.T. Anti-inflammatory and analgesic activities of a novel biflavonoid from shells of Camellia oleifera. Int. J. Mol. Sci. 2012, 13, 12401–12411. [Google Scholar] [CrossRef]
- Shu, Q.; Wu, Y.Y.; Tang, Y.J.; Yuan, Y.F.; Wang, M.; Zhou, G.X. Chemical constituents from hull of Camellia oleifera Able. Nat. Prod. Res. Dev. 2025, 37, 1243–1251. [Google Scholar]
- Jiao, B.; Xu, C.; Li, Q.; Qin, J.; Luo, Y.; Yang, W. Chemical constituents of the flavonoids from Camellia oleifera and their anti-inflammatory activities in vitro. Chin. Tradit. Pat. Med. 2019, 41, 327–333. [Google Scholar]
- Luo, Y.; Li, B. Studies on chemical constituents of Camellia oleifera Abel. Chem. J. Int. 2003, 5, 20–26. [Google Scholar]
- Zhu, W.F.; Wang, C.L.; Ye, F.; Sun, H.P.; Ma, C.Y.; Liu, W.Y.; Feng, F.; Abe, M.; Akihisa, T.; Zhang, J. Chemical constituents of the seed cake of Camellia oleifera and their antioxidant and antimelanogenic activities. Chem. Biodivers. 2018, 15, e1800137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.D.; Nie, S.P.; Xie, M.Y.; Hu, J.L. Antioxidant and antibacterial capabilities of phenolic compounds and organic acids from Camellia oleifera cake. Food Sci. Biotechnol. 2020, 29, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.F.; Chen, L.; Li, J.; Liang, L.; Fan, Y.; Qiu, L.; Deng, Z. Two kaempferol glycosides separated from Camellia oleifera meal by high-speed countercurrent chromatography and their possible application for antioxidant. J. Food Sci. 2019, 84, 2805–2811. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.J. Structure and growth of plant cell walls. Nat. Rev. Mol. Cell Biol. 2024, 25, 340–358. [Google Scholar] [CrossRef]
- Sha, M.; Li, X.; Liu, Y.; Tian, H.; Liang, X.; Li, X.; Gao, W. Comparative chemical characters of Pseudostellaria heterophylla from geographical origins of China. Chin. Herb. Med. 2023, 15, 439–446. [Google Scholar] [CrossRef]
- Zhang, S.; Li, X. Hypoglycemic activity in vitro of polysaccharides from Camellia oleifera Abel. seed cake. Int. J. Biol. Macromol. 2018, 115, 811–819. [Google Scholar] [CrossRef]
- Feng, S.; Zhang, J.; Luo, X.; Xu, Z.; Liu, K.; Chen, T.; Zhou, L.; Ding, C. Green extraction of polysaccharides from Camellia oleifera fruit shell using tailor-made deep eutectic solvents. Int. J. Biol. Macromol. 2023, 253, 127286. [Google Scholar] [CrossRef]
- Zhang, L.M.; Yang, T.T.; Hu, J.N.; Liu, Z.G.; Deng, Z.Y. Ultrasonic-assisted extraction and antioxidant activity of polysaccharides from seed cake of Camellia oleifera Abel. Food Sci. 2013, 34, 36–40. [Google Scholar]
- Shen, S.; Cheng, H.; Li, X.; Li, T.; Yuan, M.; Zhou, Y.; Ding, C. Effects of extraction methods on antioxidant activities of polysaccharides from camellia seed cake. Eur. Food Res. Technol. 2014, 238, 1015–1021. [Google Scholar] [CrossRef]
- Xu, B.Y. Extraction, Separation, Structure Analysis and Hypoglycemic Activity of Polysaccharide from Camellia Seed Meal. Master’s Thesis, Hefei University of Technology, Hefei, China, 2016. [Google Scholar]
- Xu, Z.; Li, X.; Feng, S.; Liu, J.; Zhou, L.; Yuan, M.; Ding, C. Characteristics and bioactivities of different molecular weight polysaccharides from camellia seed cake. Int. J. Biol. Macromol. 2016, 91, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.S.; Guo, Y.H.; Xu, B.Y.; Wang, H.X.; Yuan, C.X. Physicochemical properties of polysaccharides separated from Camellia oleifera Abel seed cake and its hypoglycemic activity on streptozotocin-induced diabetic mice. Int. J. Biol. Macromol. 2019, 125, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.H. Separation, Purification, Physicochemical Properties and Antioxidant Activity of Polysaccharides Separated from Camellia oleifera Abel Seed Cake. Master’s Thesis, Hefei University of Technology, Hefei, China, 2019. [Google Scholar]
- Jin, R.S.; Shan, Q.; Yuan, C.X. Structural characterization of camellia seed meal polysaccharide and its effect on intestinal microflora. Farm. Prod. Process. 2022, 543, 7–13. [Google Scholar]
- Guo, X.; Zhao, J.Y.; Zhao, B.; Zhang, G.C. Optimization of ultrasonic-assisted extraction process and antioxidant activity of polysaccharides from oil-tea camellia seed hull. China Oils Fats 2024, 49, 104–109, 117. [Google Scholar]
- Cheng, H.Y.; Xu, T.Q.; Hu, Y.L.; Shu, Q.; Xu, W.; Fan, C.L.; Zhou, G.X. Two new aryltetralin-type lignans from Camellia oleifera husk. Nat. Prod. Res. 2024, 38, 2264–2271. [Google Scholar] [CrossRef]
- Lee, C.P.; Yen, G.C. Antioxidant activity and bioactive compounds of tea seed (Camellia oleifera Abel.) oil. J. Agr. Food Chem. 2006, 54, 779–784. [Google Scholar] [CrossRef]
- Xiao, Y.P.; Zhang, B.; Deng, D.W.; Luo, J.X.; Bai, X. Separation and analysis of unsaponifiable matters in camellia seed oil. J. Nanchang Univ. Eng. Technol. 2015, 37, 16–19. [Google Scholar]
- Luo, Y.; Wei, Z.; Liu, J.; Yang, X.; Du, C.; Liu, Y.; Xue, Y.; Wang, X.; Zheng, Z.; Duan, Z. Chemometric assessment of quality biomarkers in Camellia oleifera seed oils in the leading production regions of China. J. Food Compos. Anal. 2025, 146, 107949. [Google Scholar] [CrossRef]
- Gao, S.; Wang, B.F.; Liu, F.D.; Zhao, J.R.; Yuan, J.; Xiao, S.X.; Masabni, J.; Zou, F.; Yuan, D.Y. Variation in fruit morphology and seed oil fatty acid composition of Camellia oleifera collected from diverse regions in southern China. Horticulturae 2022, 8, 818. [Google Scholar] [CrossRef]
- Cao, M.; Tang, Y.; Luo, Y.; Gu, F.; Zhu, Y.; Liu, X.; Yan, C.; Hu, W.; Wang, S.; Chao, X.; et al. Natural compounds modulating mitophagy: Implications for cancer therapy. Cancer. Lett. 2024, 582, 216500. [Google Scholar] [CrossRef]
- Wang, D.X.; Huo, R.W.; Cui, C.J.; Gao, Q.; Zong, J.F.; Wang, Y.J.; Sun, Y.; Hou, R.Y. Anticancer activity and mechanism of total saponins from the residual seed cake of Camellia oleifera Abel. in hepatoma-22 tumor-bearing mice. Food Funct. 2019, 10, 2480–2490. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, S.; Chen, S.; Zuo, Y.; Mu, X.; Zhou, H.; Yao, Y.; Peng, X.; Li, W. UPLC-Q-TOF/MS-based serum metabolomics revealed anti-tumor mechanism of ethanol-soluble acidic components from Camellia oleifera cake and synergy with PD-1 inhibitor. Food Biosci. 2025, 65, 106005. [Google Scholar] [CrossRef]
- Li, T.T.; Zhang, H.; Wu, C. Screening of antioxidant and antitumor activities of major ingredients from defatted Camellia oleifera seeds. Food Sci. Biotechnol. 2014, 23, 873–880. [Google Scholar] [CrossRef]
- Jin, X.; Ning, Y. Antioxidant and antitumor activities of the polysaccharide from seed cake of Camellia oleifera Abel. Int. J. Biol. Macromol. 2012, 51, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Kavyani, B.; Ahmadi, S.; Nabizadeh, E.; Abdi, M. Anti-oxidative activity of probiotics; focused on cardiovascular disease, cancer, aging, and obesity. Microb. Pathog. 2024, 196, 107001. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Kang, J.; Guo, W.; Wang, F.; Guo, M.; Feng, S.; Zhou, W.; Li, J.; Tahir, A.T.; Wang, S.; et al. An optimal medicinal and edible Chinese herbal formula attenuates particulate matter-induced lung injury through its anti-oxidative, anti-inflammatory and anti-apoptosis activities. Chin. Herb. Med. 2023, 15, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Tu, P.S.; Tung, Y.T.; Lee, W.T.; Yen, G.C. Protective effect of camellia oil (Camellia oleifera Abel.) against ethanol-induced acute oxidative injury of the gastric mucosa in mice. J. Agric. Food Chem. 2017, 65, 4932–4941. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, Y.; Xie, J.; Yu, Q.; Lu, H.; Zhong, J.; Chen, Y. Camellia oleifera seeds cake: Polyphenol profile and in vitro antioxidant activities as determined by different harvest periods. Food Biosci. 2023, 55, 103081. [Google Scholar] [CrossRef]
- Wei, M.; Hu, Y.; Zou, W.; Li, Y.; Cao, Y.; Li, S.; Huang, J.; Xing, L.; Huang, B.; Wang, X. Physicochemical property and antioxidant activity of polysaccharide from the seed cakes of Camellia oleifera Abel. Food Sci. Nutr. 2022, 10, 1667–1682. [Google Scholar] [CrossRef]
- Yao, L.; Huang, Q.; Wang, H.; Feng, T.; Yu, C.; Xie, K.; Liu, H.; Song, S.; Shao, L.; Sun, M. Microbial hydrolysis of Camellia seed cake with Bacillus subtilis: Fermentation process optimization and bioactivity assessment of the hydrolysates. Biocatal. Agr. Biotech. 2025, 66, 103579. [Google Scholar] [CrossRef]
- Nechchadi, H.; Boulbaroud, S.; Nadir, Y.; Benhssaine, K.; Berrougui, H.; Alem, C.; Ramchoun, M. Hypolipidemic activity of phytochemical combinations: A mechanistic review of preclinical and clinical studies. Food Chem. 2024, 459, 140264. [Google Scholar] [CrossRef]
- Yang, H.Y.; Yeh, W.J.; Ko, J.; Chen, J.R. Camellia oleifera seed extract attenuated abdominal and hepatic fat accumulation in rats fed a high-fat diet. Appl. Physiol. Nutr. Metab. 2019, 44, 320–325. [Google Scholar] [CrossRef]
- Shi, H.; He, X.E.; Ding, R.H.; Wang, W.L.; Huang, Q.; You, J. Effect of Camellia polyphenols on blood lipid reduction and antioxidation in SD rats. Food Res. Dev. 2021, 42, 28–34. [Google Scholar]
- Wang, J.; Zhang, Y.H.; Liu, Y.H.; Zhang, Y.; Xu, Q.; Zhang, X.S.; Yu, X.M.; Zhang, R.X.; Xue, C.Y. Effects of teaseed oil on triglyceride and weight in hypertriglyceridemic subjects. J. Hyg. Res. 2014, 43, 92–95. [Google Scholar]
- Bumrungpert, A.; Pavadhgul, P.; Kalpravidh, R.W. Camellia oil-enriched diet attenuates oxidative stress and inflammatory markers in hypercholesterolemic subjects. J. Med. Food 2016, 19, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Tan, D.H.; Li, B.; Wang, Y.Q.; Shi, L. Gypenoside ameliorates insulin resistance and hyperglycemia via the AMPK-mediated signaling pathways in the liver of type 2 diabetes mellitus mice. Food Sci. Hum. Well. 2022, 11, 1347–1354. [Google Scholar] [CrossRef]
- Zhang, S.; Li, X.Z. Inhibition of α-glucosidase by polysaccharides from the fruit hull of Camellia oleifera Abel. Carbohy. Polym. 2015, 115, 38–43. [Google Scholar] [CrossRef]
- Chandimali, N.; Bak, S.G.; Park, E.H.; Lim, H.J.; Won, Y.S.; Kim, E.K.; Park, S.I.; Lee, S.J. Free radicals and their impact on health and antioxidant defenses: A review. Cell Death Discov. 2025, 24, 19. [Google Scholar] [CrossRef]
- Duan, Y.; Zhou, Y.H.; Li, S.X.; Li, J. Advances in phytochemical constituents and antibacterial and anti-inflammatory activity of Camellia oleifera Abel. Nat. Prod. Res. Dev. 2021, 33, 1603–1615. [Google Scholar]
- Lin, X.; Li, Y.; Zhan, M.; Fu, X.J.; Zhong, H.Y.; Yao, W.; Liu, C. Metabolite composition and anti-inflammatory activity of ethanol extract of Camellia oleifera seed. Food Sci. 2023, 44, 304–311. [Google Scholar]
- Ye, Y.; Xing, H.T.; Chen, X.L. Anti-inflammatory and analgesic activities of the hydrolyzed sasanquasaponins from the defatted seeds of Camellia oleifera. Arch. Pharm. Res. 2013, 36, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Dharmalingam, K. Herbal remedies for Alzheimer’s disease: Neuroprotective mechanisms and cognitive enhancement potential. Digital Chin. Med. 2025, 8, 183. [Google Scholar]
- Amtul, Z.; Westaway, D.; Cechetto, D.F.; Rozamhel, R.F. Oleic acid ameliorates amyloidosis in cellular and mouse models of Alzheimer’s disease. Brain Pathol. 2011, 21, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhou, N.; Chen, L.; Liu, T.; Guo, P.; Zhang, B.; Zhang, Z.; Zeng, M.; Xiong, W.; Zheng, X.; et al. Urine metabolomics analysis of the intervention effect of camellia oil in mice with Alzheimer’s disease. Food Sci. 2024, 45, 114–121. [Google Scholar]
- Weng, M.H.; Chen, S.Y.; Li, Z.Y.; Yen, G.C. Camellia oil alleviates the progression of Alzheimer’s disease in aluminum chloride-treated rats. Free Radic. Biol. Med. 2020, 152, 411–421. [Google Scholar] [CrossRef]
- Guo, P.; Zeng, M.; Cao, B.; Liu, M.; Zhang, Y.; Jia, J.; Zhang, Q.; Zhang, B.; Wang, R.; Xiong, W.; et al. Camellia oil improves Aβ25–35-induced memory impairment by regulating the composition of the gut microbiota and lipid metabolism in mice. J. Funct. Foods 2022, 96, 105214. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, C.; Zhao, J.; Ye, Y. Synthesis and neuroprotective effects of the complex nanoparticles of iron and sapogenin isolated from the defatted seeds of Camellia oleifera. Pharm. Biol. 2017, 55, 428–434. [Google Scholar] [CrossRef]
- Yang, Q.; Fang, F.; Li, Y.; Ye, Y. Neuroprotective effects of the nanoparticles of Zinc sapogenin from seeds of Camellia oleifera. J. Nanosci. Nanotechnol. 2017, 17, 2394–2400. [Google Scholar] [CrossRef]
- James, R.; Hardefeldt, L.Y.; Ierano, C.; Charani, E.; Dowson, L.; Elkins, S.; Thursky, K. Antimicrobial stewardship from a One Health perspective. Nat. Rev. Microbiol. 2025, 9, 10. [Google Scholar] [CrossRef]
- Akihisa, T.; Tokuda, H.; Ukiya, M.; Suzuki, T.; Enjo, F.; Koike, K.; Nikaido, T.; Nishino, H. 3-Epicabraleahydroxylactone and other triterpenoids from camellia oil and their inhibitory effects on Epstein–Barr virus activation. Chem. Pharm. Bull. 2004, 52, 153–156. [Google Scholar] [CrossRef]
- Yang, C.; Wang, W.J.; Tang, W.L.; Chen, H. Comparison of antibacterial activity of tea oil extracted by two different methods. J. Gannan Norm. Univ. 2021, 3, 121–124. [Google Scholar]
- Duan, Y.; Huang, D.; Zhou, Y.H.; Yang, F.M.; Zhou, T.T.; Li, S.X.; Li, J. Effect of refining process on chemical components and antibacterial activity of solvent extracted oil-tea camellia seed oil. China Oils Fats 2022, 47, 45–51. [Google Scholar]
- Cheng, Y.T.; Wu, S.L.; Ho, C.Y.; Huang, S.M.; Cheng, C.L.; Yen, G.C. Beneficial effects of camellia oil (Camellia oleifera Abel.) on ketoprofen-induced gastrointestinal mucosal damage through upregulation of HO-1 and VEGF. J. Agric. Food Chem. 2014, 62, 642–650. [Google Scholar] [CrossRef]
- Lin, C.Y.; Chen, S.Y.; Lee, W.T.; Yen, G.C. Immunomodulatory effect of camellia oil (Camellia oleifera Abel.) on CD19+ B cells enrichment and IL-10 production in BALB/c mice. J. Funct. Foods 2022, 88, 104863. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).