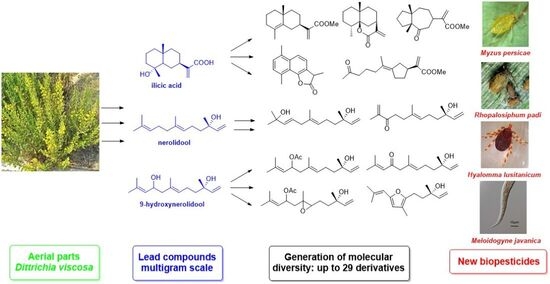
Major Components of Dittrichia viscosa (Asteraceae) as a Source of New Pesticides
Abstract
1. Introduction
2. Results
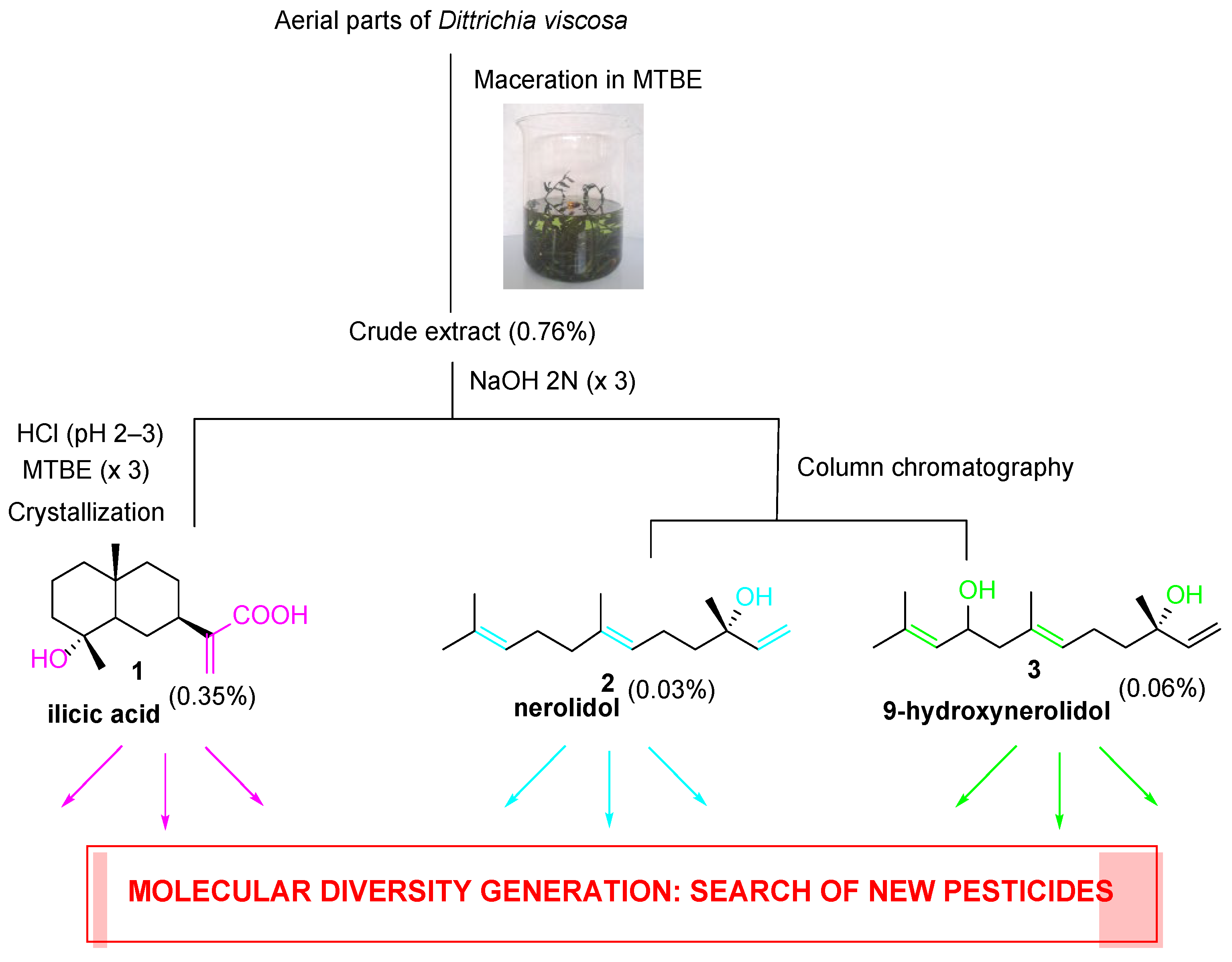
2.1. Extraction of Major Compounds from the Aerial Parts of D. viscosa
2.2. Ilicic Acid as a Source of Molecular Diversity
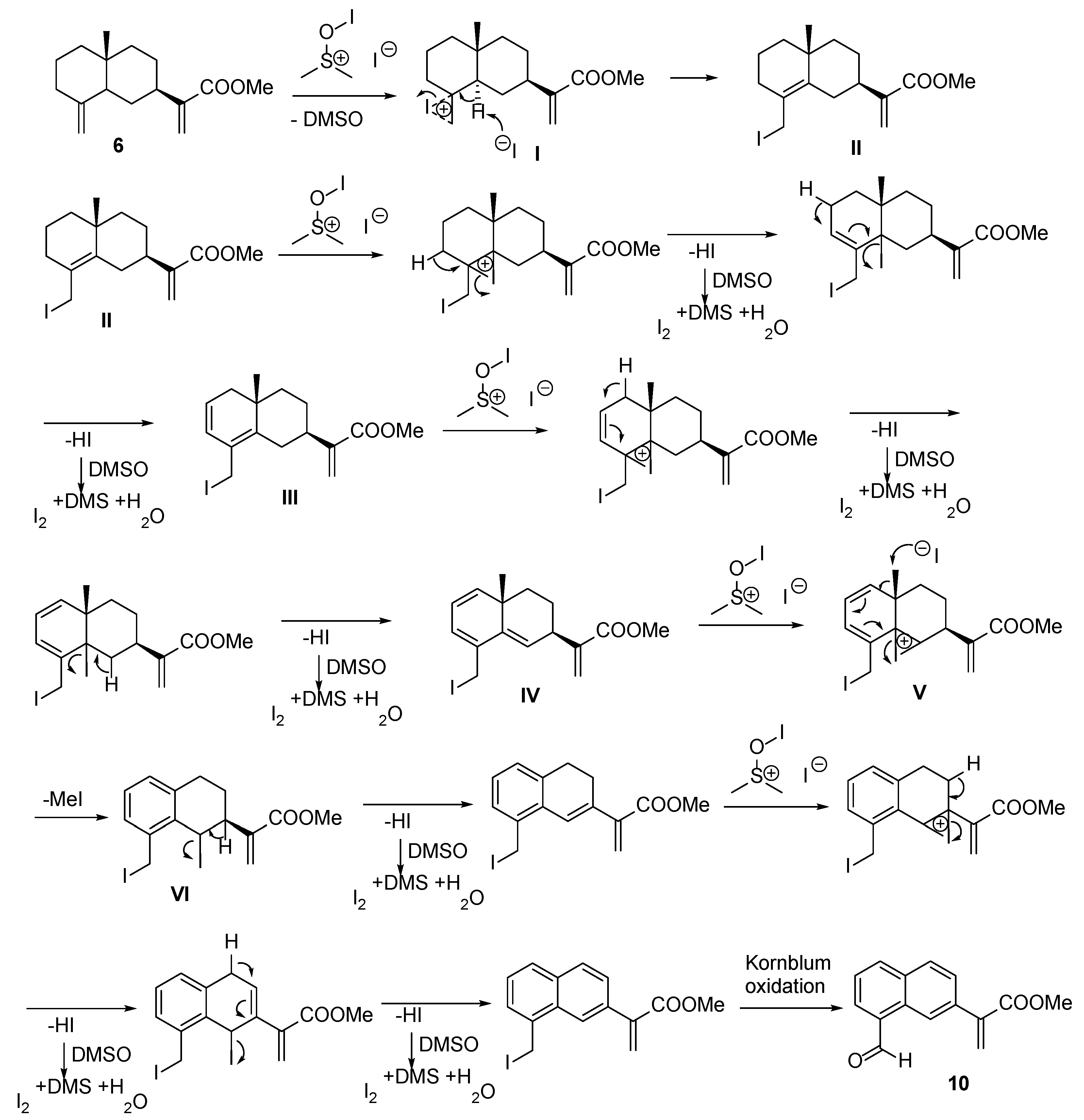
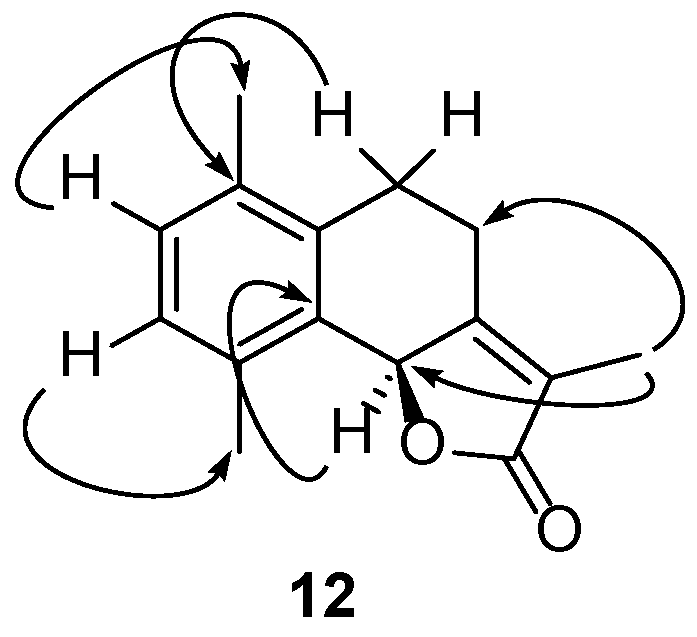
2.2.1. Esterification of Ilicic Acid—Reactions with Iodine
2.2.2. Reactions of γ-Costic Acid Methyl Ester (5) with Iodine
- Synthesis of 3-oxo-γ-costic acid methyl ester (19)
- Epoxidation of 5. Et2AlCl-mediated opening reactions of the corresponding epoxy derivatives
2.3. Nerolidol as a Source of Molecular Diversity
2.4. 9-Hydroxynerolidol as a Source of Molecular Diversity
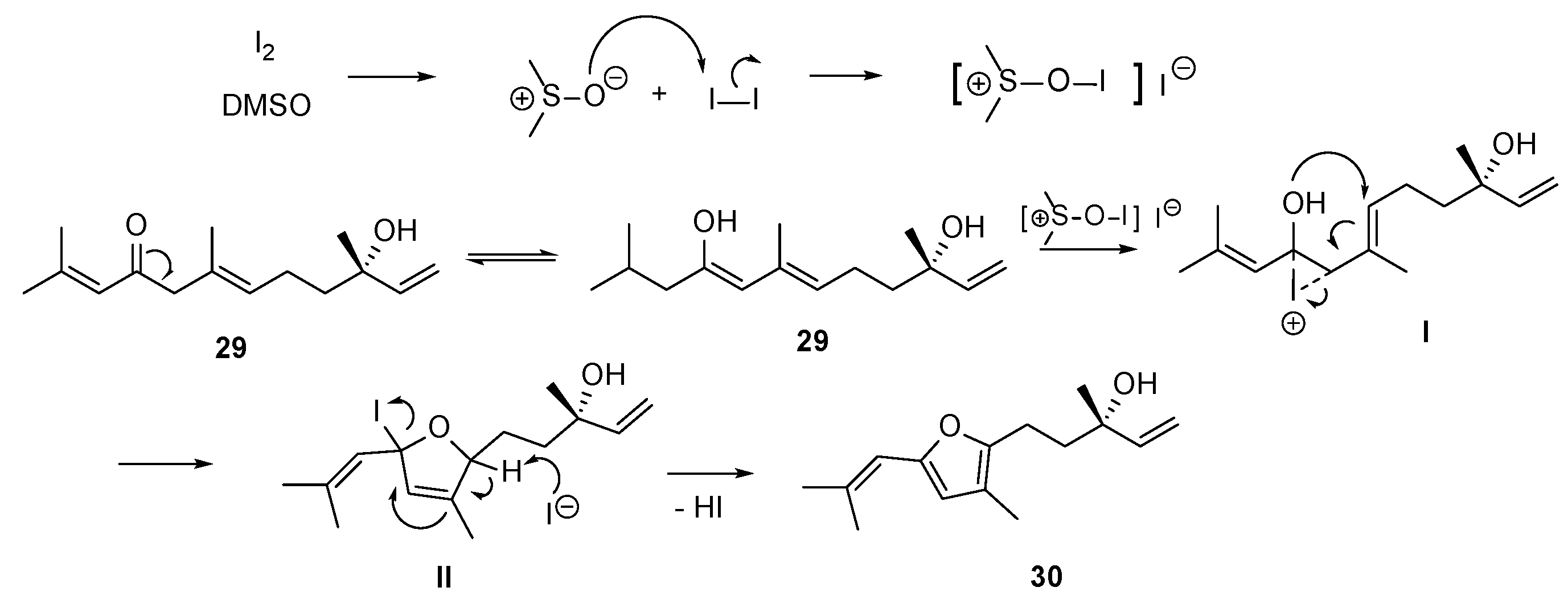
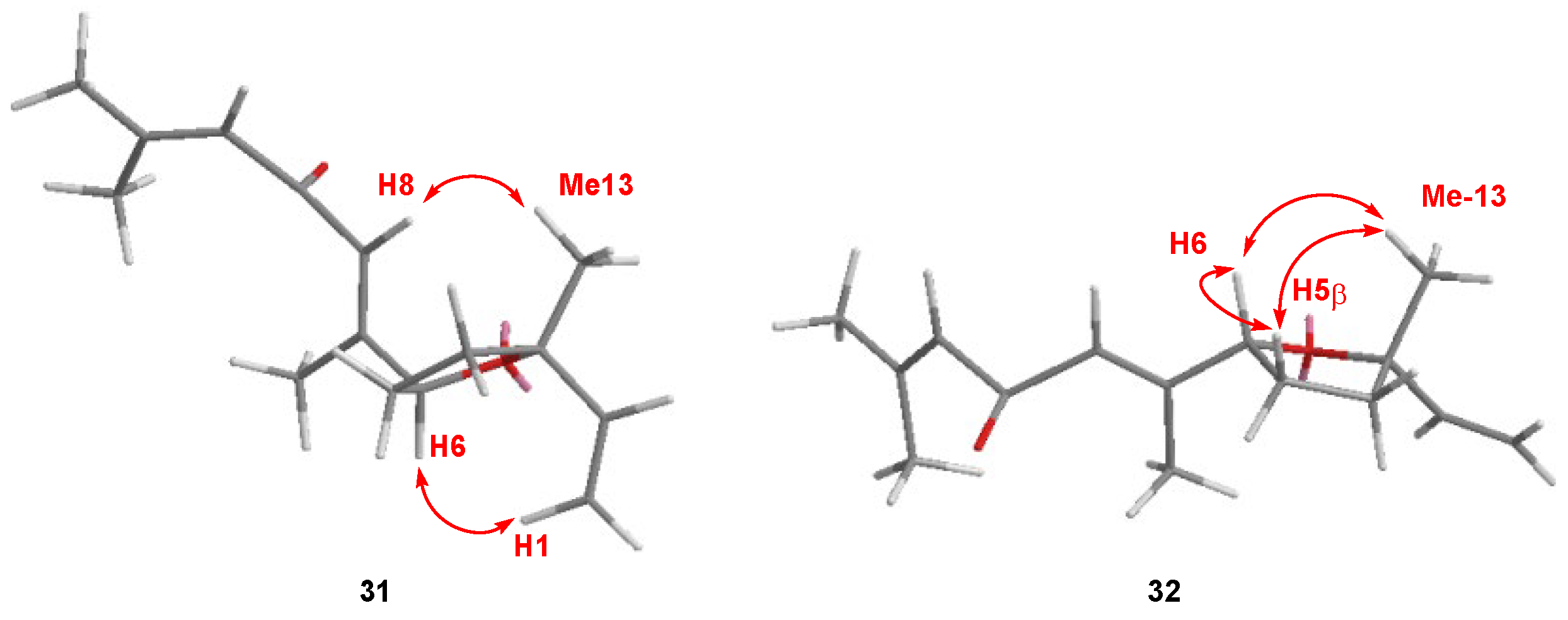
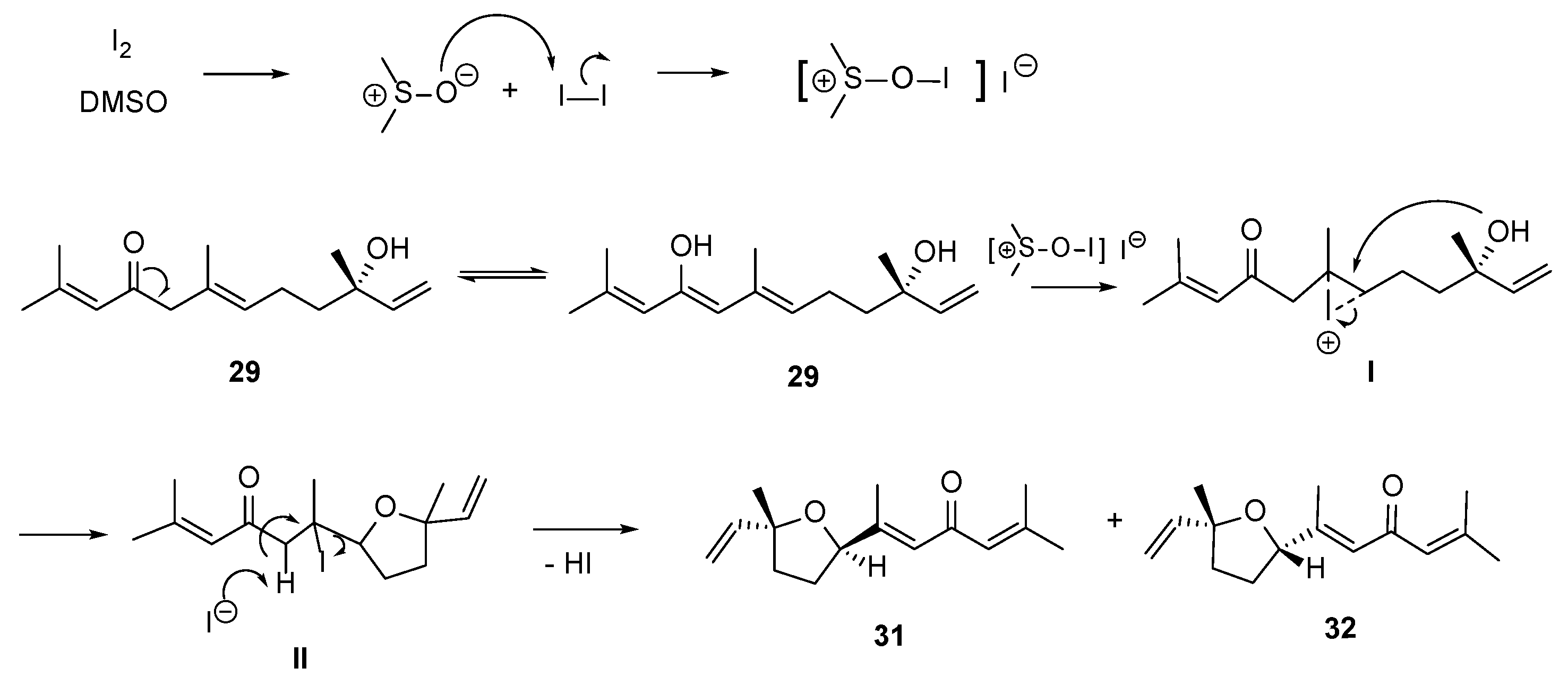
Synthesis of 9-Oxonerolidol (29)—Reaction of 29 with Iodine to Produce Diversity
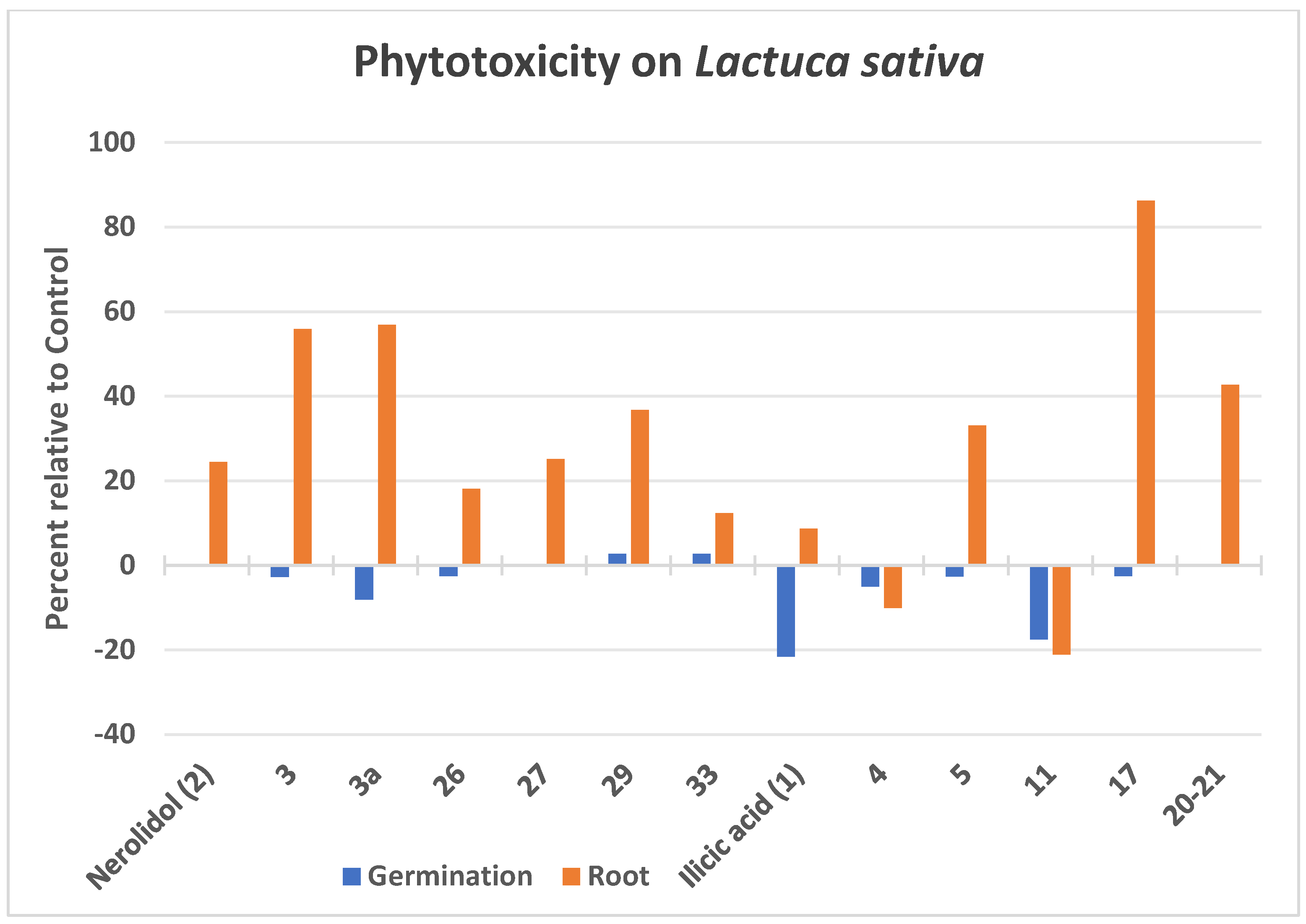
2.5. Plant Protection Effects
2.6. Ixodicidal Effects
3. Materials and Methods
3.1. Instruments and Chemicals
3.2. Plant Material, Extraction, and Isolation of Natural Products
3.3. Synthesis
3.3.1. Esterification of Ilicic Acid
3.3.2. Dehydration of Compound 4
3.3.3. Selective Dehydration of Compound 4
3.3.4. Reaction of Compound 4 with I2 and DMSO
3.3.5. Reaction of Compound 4 with I2 and Toluene: MeOH (16 h)
3.3.6. Reaction of Compound 4 with I2 and Toluene: MeOH (38 h)
3.3.7. Reaction of Compound 5 with I2 Using Toluene: MeOH (13 h)
3.3.8. Reaction of Compound 5 with I2 Using Toluene: MeOH (32 h)
3.3.9. Oxidation of Compound 5
3.3.10. Epoxidation of Compound 5
3.3.11. Reaction of Epoxides 20 and 21 with Et2AlCl
3.3.12. Reaction of Epoxides 20 and 21 with Me2AlCl
3.3.13. Acetylation of 2
3.3.14. Photooxygenation of Compound 2
3.3.15. Oxidation of Compound 26
3.3.16. Oxidation of Compound 3
3.3.17. Reaction of Compound 29 with I2 and DMSO
3.3.18. Acetylation of Compound 3
3.3.19. Epoxidation of Compound 3a
3.4. Antifeedant Bioassay
3.5. Nematicidal Bioassay
3.6. Phytotoxic Bioassay
3.7. Ixodicidal Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parolin, P.; Scotta, M.I.; Bresch, C. Biology of Dittrichia viscosa, a Mediterranean ruderal plant: A review. Phyton-Int. J. Exp. Bot. 2014, 83, 251–262. [Google Scholar] [CrossRef]
- Sladonja, B.; Poljuha, D.; Krapac, M.; Uzelac, M.; Mikulic-Petkovsek, M. Dittrichia viscosa: Native-non native invader. Diversity 2021, 13, 380. [Google Scholar] [CrossRef]
- Araniti, F.; Lupini, A.; Sunseri, F.; Abenavoli, M.R. Allelopatic Potential of Dittrichia viscosa (L.) W. Greuter Mediated by VOCs: A Physiological and Metabolomic Approach. PLoS ONE 2017, 12, e0170161. [Google Scholar] [CrossRef] [PubMed]
- Boccaccio, L.; Petacchi, R. Landscape effects on the complex of Bactrocera oleae parasitoids and implications for conservation biological control. Biocontrol 2009, 54, 607–616. [Google Scholar] [CrossRef]
- Barbafieri, M.; Dadea, C.; Tassi, E.; Bretzel, F.; Fanfani, L. Uptake of heavy metals by native species growing in a mining area in Sardenia, Italy: Discovering native flora for phytoremediation. Int. J. Phytoremediat. 2011, 13, 985–997. [Google Scholar] [CrossRef] [PubMed]
- Perez, C.; Martínez-Sánchez, M.; Martínez-López, S.; Bech, J.; Bolan, N. Distribution and bioaccumulation of arsenic and antimony in Dittrichia viscosa growing in mining-affected semiarid soils in southeast Spain. J. Geochem. Explor. 2012, 123, 128–135. [Google Scholar] [CrossRef]
- Grauso, L.; Cesarano, G.; Zotti, M.; Ranesi, M.; Sun, W.; Bonanomi, G.; Lanzotti, V. Exploring Dittrichia viscosa (L.) Greuter phytochemical diversity to explain its antimicrobial, nematicidal and insecticidal activity. Phytochem. Rev. 2020, 19, 659–689. [Google Scholar] [CrossRef]
- Ali-Shtayeh, M.S.; Yaghmour, R.M.; Faidi, Y.R.; Salem, K.; Al-Nuri, M.A. Antimicrobial activity of 20 plants used in folkloric medicine in the Palestinian area. J. Ethnopharmacol. 1998, 60, 265–271. [Google Scholar] [CrossRef]
- Jerada, R.; Er-Rakibi, A.; Hassani, A.C.; Benzeid, H.; El Ouardi, A.; Harhar, H.; Goh, B.J.; Yow, Y.Y.; Ser, H.L.; Bouyahya, A.; et al. A comprehensive review on ethnomedicinal uses, phytochemistry, toxicology, and pharmacological activities of Dittrichia viscosa (L.) Greuter. J. Tradit. Complement. Med. 2024, 14, 355–380. [Google Scholar] [CrossRef]
- Mamoci, E.; Cavoski, I.; Andrés, M.F.; González-Coloma, A. Chemical characterization of the aphid antifeedant extracts from Dittrichia viscosa and Ferula communis. Biochem. Syst. Ecol. 2012, 43, 101–107. [Google Scholar] [CrossRef]
- Perdikis, D.; Favas, C.; Lykouressis, D.; Fantinou, A. Ecological relationships between non-cultivated plants and insect predators in agroecosystems: The case of Dittrichia viscosa (Asteraceae) and Macrolophus melanotoma (Hemiptera: Miridae). Acta Ecol. 2007, 31, 299–306. [Google Scholar] [CrossRef]
- García, M.; Sosa, M.E.; Donadel, O.J.; Giordano, O.S.; Tonn, C.E. Allelochemical effects of eudesmane and eremophilane sesquiterpenes on Tribolium castaneum larvae. J. Chem. Ecol. 2003, 29, 175–187. [Google Scholar] [CrossRef]
- González-Coloma, A.; Guadaño, A.; Tonn, C.E.; Sosa, M.E. Antifeedant/insecticidal terpenes from Asteraceae and Labiatae species native to Argentinean semi-arid lands. Z. Naturforschung C J. Biosci. 2005, 60, 855–861. [Google Scholar] [CrossRef]
- Ghoneim, K.; Hamadah, K.; Selim, S.; Waheeb, H. Biopesticidal potential of Nerolidol, a sesquiterpene compound, and its drastic impact on growth and metamorphosis of the cotton leafworm Spodoptera littoralis (Lepidoptera: Noctuidae). Sch. Acad. J. Biosci. 2021, 9, 36–57. [Google Scholar] [CrossRef]
- El-Habashy, D.E.; Abdel Rasoul, M.A.; Abdelgaleil, S.A. Nematicidal activity of phytochemicals and their potential use for the control of Meloidogyne javanica infected eggplant in the greenhouse. Eur. J. Plant Pathol. 2020, 158, 381–390. [Google Scholar] [CrossRef]
- Domingo, V.; Prieto, C.; Silva, L.; Rodilla, J.M.; Quilez del Moral, J.F.; Barrero, A.F. Iodine, a mild reagent for the aromatization of terpenoids. J. Nat. Prod. 2016, 79, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Domingo, V.; Prieto, C.; Castillo, A.; Silva, L.; Quilez del Moral, J.F.; Barrero, A.F. Iodine-Promoted Metal-Free Aromatization: Synthesis of Biaryls, Oligo p-Phenylenes and A-Ring Modified Steroids. Adv. Synth. Catal. 2016, 357, 3359–3364. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Chen, C.H. Diversifolol, a novel rearranged eudesmane sesquiterpene from the leaves of Tithonia diversifolia. Chem. Pharm. Bull. 1997, 45, 1223–1224. [Google Scholar] [CrossRef]
- Tan, R.X.; Wang, W.Z.; Yang, L.; Wei, J.H. A new eudesmenoic acid from Artemisia phaeolepis. J. Nat. Prod. 1995, 58, 288–290. [Google Scholar] [CrossRef]
- Sanz, J.F.; Castellano, G.; Marco, J.A. Sesquiterpene lactones from Artemisia herba-alba. Phytochemistry 1990, 29, 541–545. [Google Scholar] [CrossRef]
- Barrero, A.F.; Herrador, M.M.; Arteaga, P.; Catalán, J.V. Ilicic Acid as a Natural Quiron for the Efficient Preparation of Bioactive α- and β-Eudesmol. Eur. J. Org. Chem. 2009, 2009, 3589–3594. [Google Scholar] [CrossRef]
- He, Q.; Hu, D.B.; Zhang, L.; Xia, M.Y.; Yan, H.; Li, X.N.; Luo, J.F.; Wang, Y.S.; Yang, J.H.; Wang, Y.H. Neuroprotective compounds from the resinous heartwood of Aquilaria sinensis. Phytochemistry 2021, 181, 112554. [Google Scholar] [CrossRef] [PubMed]
- Salihila, J.; Silva, L.; Perez del Pulgar, H.; Quílez Molina, A.; Gonzalez-Coloma, A.; Olmeda, S.; Quílez del Moral, J.F.; Barrero, A.F. One-Step Synthesis of Furan Rings from α-Isopropylidene Ketones Mediated by Iodine/DMSO: An Approach to Potent Bioactive Terpenes. J. Org. Chem. 2019, 84, 6886–6894. [Google Scholar] [CrossRef] [PubMed]
- Donadel, O.J.; Guerreiro, E.; María, A.O.; Wendel, G.; Enriz, R.D.; Giordano, O.S.; Tonn, C.E. Gastric cytoprotective activity of ilicic aldehyde: Structure–activity relationships. Bioorganic Med. Chem. Lett. 2005, 15, 3547–3550. [Google Scholar] [CrossRef] [PubMed]
- Zdero, C.; Bohlmann, F. Cis-eudesman-12,6-olides from Calostephane divaricata. Phytochem. 1989, 28, 1433–1439. [Google Scholar] [CrossRef]
- Hegazy, M.-E.F.; El-Beih, A.A.; Hamed, A.R.; El Aty, A.A.A.; Mohamed, N.S.; Paré, P.W. 3-Oxo-g-costic acid fungal-transformation generates eudesmane sesquiterpenes with in vitro tumor-inhibitory activity. Bioorganic Med. Chem. Lett. 2017, 27, 3825–3828. [Google Scholar] [CrossRef]
- Al-Dabbas, M.M.; Hashinaga, F.; Abdelgaleil, S.A.M.; Suganuma, T.; Akiyama, K.; Hayashi, H. Antibacterial activity of an eudesmane sesquiterpene isolated from common Varthemia, Varthemia iphionoides. J. Ethnopharmacol. 2005, 97, 237–240. [Google Scholar] [CrossRef]
- Quilez del Moral, J.F.; Domingo, V.; Pérez, Á.; Martinez Andrade, K.A.; Enríquez, L.; Jaraiz, M.; Barrero, A.F. Mimicking halimane synthases: Monitoring a cascade of cyclizations and rearrangements from epoxypolyprenes. J. Org. Chem. 2019, 84, 13764–13779. [Google Scholar] [CrossRef]
- Zaki, M.; Tebbaa, M.; Hiebel, M.A.; Benharref, A.; Akssira, M.; Berteina-Raboin, S. Acid-promoted opening of 4, 5-and 3, 4-epoxy eudesmane scaffolds from α-isocostic acid. Tetrahedron 2015, 71, 2035–2042. [Google Scholar] [CrossRef]
- Han, Z.-Z.; Zu, X.-P.; Wang, J.-X.; Li, H.-L.; Chen, B.-Y.; Liu, Q.-X.; Hu, X.-Q.; Yan, Z.-H.; Zhang, W.-D. Neomerane-type sesquiterpenoids from Valeriana officinalis var. latifolia. Tetrahedron 2014, 70, 962–966. [Google Scholar] [CrossRef]
- Zdero, C.; Bohlmann, F.; King, R.M. Eudesmane derivatives other constituents from Apalochlamys spectabilis Cassinia species. Phytochemistry 1990, 29, 3201–3206. [Google Scholar] [CrossRef]
- Ceccherelli, P.; Curini, M.; Marcotullio, M.C.; Rosati, O. Biogenetic-type transformation of 3-keto-4, 5-epoxy-eudesmanes: Synthesis of cyperanes, eremophilanes and spirovetivanes. Tetrahedron 1989, 45, 3809–3818. [Google Scholar] [CrossRef]
- Bohlmann, F.; Zdero, C.; King, R.M.; Robinson, H. Neue Sesquiterpenlactone und andere Inhaltsstoffe aus Stevia mercedensis und Stevia achalensis. Liebigs Ann. Chem. 1986, 1986, 799–813. [Google Scholar] [CrossRef]
- Li, W.; Zhang, W.; Liu, Z.; Song, H.; Wang, S.; Zhang, Y.; Zhan, C.; Liu, D.; Tian, Y.; Tang, M.; et al. Review of Recent Advances in Microbial Production and Applications of Nerolidol. J. Agric. Food Chem. 2025, 73, 5724–5747. [Google Scholar] [CrossRef] [PubMed]
- Barrero, A.F.; Herrador, M.M.; González Portero, A.; Arteaga Buron, P.; Arteaga, J.F.; Burillo Alquézar, J.; Díaz, C.E.; González-Coloma, A. Terpenes and polyacetylenes from cultivated Artemisia granatensis boiss (Royal chamomile) and their defensive properties. Phytochemistry 2013, 94, 192–197. [Google Scholar] [CrossRef]
- Zhou, M.-X.; Li, G.-H.; Sunc, B.; Xu, Y.-W.; Li, A.-L.; Li, Y.-R.; Ren, D.-M.; Wang, X.-N.; Wen, X.-S.; Lou, H.-X.; et al. Identification of novel Nrf2 activators from Cinnamomum chartophyllum H.W. Li and their potential application of preventing oxidative insults in human lung epithelial cells. Redox Biol. 2018, 14, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Valcárcel, F.; Olmeda, A.S.; González, M.G.; Andrés, M.F.; Navarro-Rocha, J.; González-Coloma, A. Acaricidal and Insect Antifeedant Effects of Essential Oils From Selected Aromatic Plants and Their Main Components. Front. Agron. 2021, 3, 662802. [Google Scholar] [CrossRef]
- González López, L.A.; Andres, M.F.; Quiñones, W.; Echeverri, F.; Gonzalez-Coloma, A. Potential of 2-Hydroxyacetophenone Derivatives and Simple Phenol’s for the control of Meloidogyne javanica. Nat. Prod. Commun. 2024, 19, 1934578X241227689. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Drenaggi, E.; Desneux, N.; Maggi, F. Phytol, (E)-nerolidol and spathulenol from Stevia rebaudiana leaf essential oil as effective and eco-friendly botanical insecticides against Metopolophium dirhodum. Ind. Crop. Prod. 2020, 155, 112844. [Google Scholar] [CrossRef]
- de Melo, J.P.R.; da Câmara, C.A.G.; de Moraes, M.M. Bioactivity of formulas containing essential oils from the family Myrtaceae for the management of deltamethrin-resistant Plutella xylostella (L.) (Lepidoptera: Plutellidae). Phytoparasitica 2023, 51, 305–321. [Google Scholar] [CrossRef]
- Dai, H.; Liu, B.; Yang, L.; Yao, Y.; Liu, M.; Xiao, W.; Li, S.; Ji, R.; Sun, Y. Investigating the Regulatory Mechanism of the Sesquiterpenol Nerolidol from a Plant on Juvenile Hormone-Related Genes in the Insect Spodoptera exigua. Int. J. Mol. Sci. 2023, 24, 13330. [Google Scholar] [CrossRef]
- Diksha, S.S.; Mahajan, E.; Sohal, S.K. Immunomodulatory, cyto-genotoxic, and growth regulatory effects of nerolidol on melon fruit fly, Zeugodacus cucurbitae (Coquillett) (Diptera: Tephritidae). Toxicon 2023, 233, 107248. [Google Scholar] [CrossRef] [PubMed]
- Haris, A.; Azeem, M.; Binyameen, M. Mosquito Repellent Potential of Carpesium abrotanoides Essential Oil and Its Main Components Against a Dengue Vector, Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2022, 59, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Wróblewska-Kurdyk, A.; Dancewicz, K.; Gliszczyńska, A.; Gabryś, B. New insight into the behaviour modifying activity of two natural sesquiterpenoids farnesol and nerolidol towards Myzus persicae (Sulzer) (Homoptera: Aphididae). Bull. Entomol. Res. 2020, 110, 249–258. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.M.C.; da Camara, C.A.G.; de Moraes, M.M.; de Melo, J.P.R.; dos Santos, R.B.; Neves, R.C.S. Insecticidal and acaricidal activity of essential oils rich in (E)-nerolidol from Melaleuca leucadendra occurring in the state of Pernambuco (Brazil) and effects on two important agricultural pests. J. Braz. Chem. Soc. 2020, 31, 813–820. [Google Scholar] [CrossRef]
- de Assis Lage, T.C.; Montanari, R.M.; Fernandes, S.A.; de Oliveira Monteiro, C.M.; de Oliveira Souza Senra, T.; Zeringota, V.; da Silva Matos, R.; Daemon, E. Chemical composition and acaricidal activity of the essential oil of Baccharis dracunculifolia De Candole (1836) and its constituents nerolidol and limonene on larvae and engorged females of Rhipicephalus microplus (Acari: Ixodidae). Exp. Parasitol. 2015, 148, 24–29. [Google Scholar] [CrossRef]
- Landi, M.; Misra, B.B.; Muto, A.; Bruno, L.; Araniti, F. Phytotoxicity, Morphological, and Metabolic Effects of the Sesquiterpenoid Nerolidol on Arabidopsis thaliana Seedling Roots. Plants 2020, 9, 1347. [Google Scholar] [CrossRef]
- Sosa, M.E.; Tonn, C.E.; Giordano, O.S. Insect antifeedant activity of clerodane diterpenoids. J. Nat. Prod. 1994, 57, 1262–1265. [Google Scholar] [CrossRef]
- Abu Irmaileh, B.E.; Al-Aboudi, A.M.F.; Abu Zarga, M.H.; Awwadi, F.; Haddad, S.F. Selective phytotoxic activity of 2,3,11β,13-tetrahydroaromaticin and ilicic acid isolated from Inula graveolens. Nat. Prod. Res. 2014, 29, 893–898. [Google Scholar] [CrossRef]
- Truzi, C.C.; Vieira, N.F.; De Souza, J.M.; De Bortoli, S.A. Artificial diets with different protein levels for rearing Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Insect Sci. 2021, 21, 2. [Google Scholar] [CrossRef]
- González-Coloma, A.; Andres, M.F.; Contreras, R.; Zúñiga, G.E.; Díaz, C.E. Sustainable production of insecticidal com-poundsfrom Persea indica. Plants 2022, 11, 418. [Google Scholar] [CrossRef]
- Morales-Sánchez, V.; Díaz, C.E.; Trujillo, E.; Olmeda, S.A.; Valcárcel, F.; Muñoz, R.; Andrés, M.F.; González-Coloma, A. Bioactive metabolites from the endophytic fungus Aspergillus sp. SPH2. J. Fungi 2021, 7, 109. [Google Scholar] [CrossRef]
- Andrés, M.F.; González-Coloma, A.; Sanz, J.; Burillo, J.; Sainz, P. Nematicidal activity of essential oils: A review. Phytochem. Rev. 2012, 11, 371–390. [Google Scholar] [CrossRef]
- Julio, L.F.; Burillo, J.; Giménez, C.; Cabrera, R.; Díaz, C.E.; Sanz, J.; González-Coloma, A. Chemical and biocidal characterization of two cultivated Artemisia absinthium populations with different domestication levels. Ind. Crops Prod. 2015, 76, 787–792. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]
- Püntener, W. Manual for Field Trials in Plant Protection, 2nd ed.; Agricultural Division, Ciba-Geigy: Basel, Switzerland, 1981. [Google Scholar]
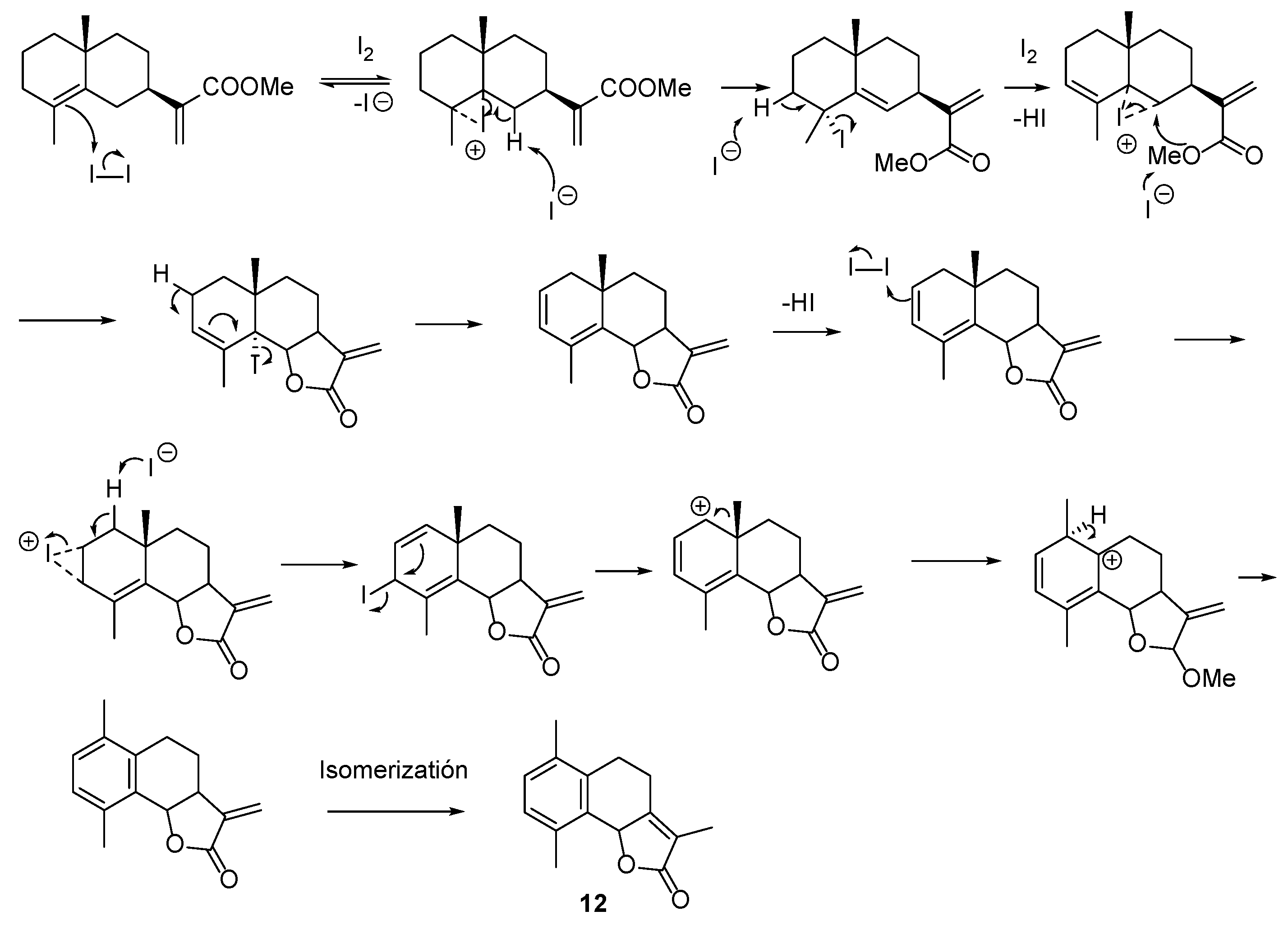
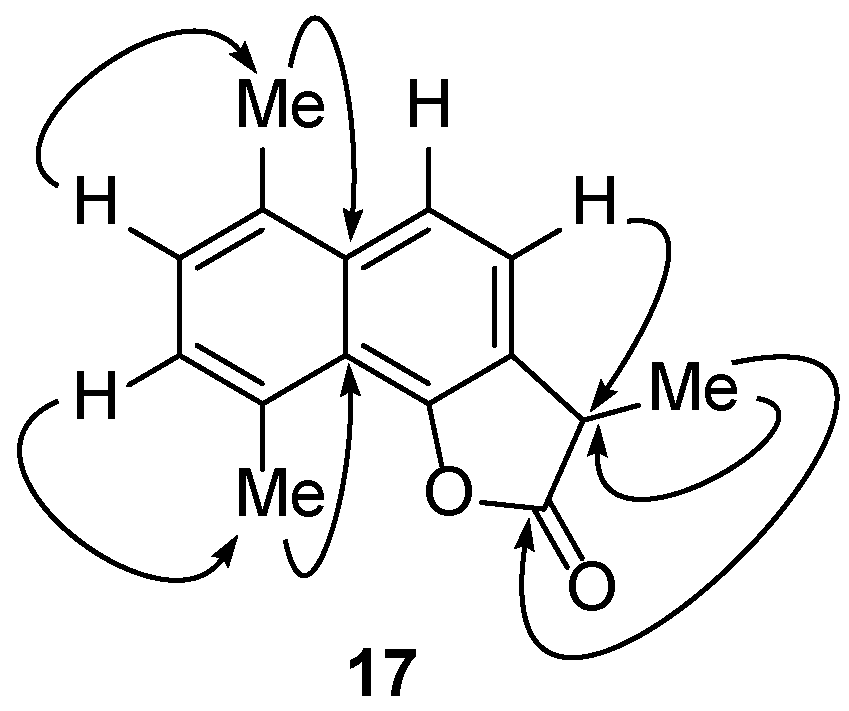

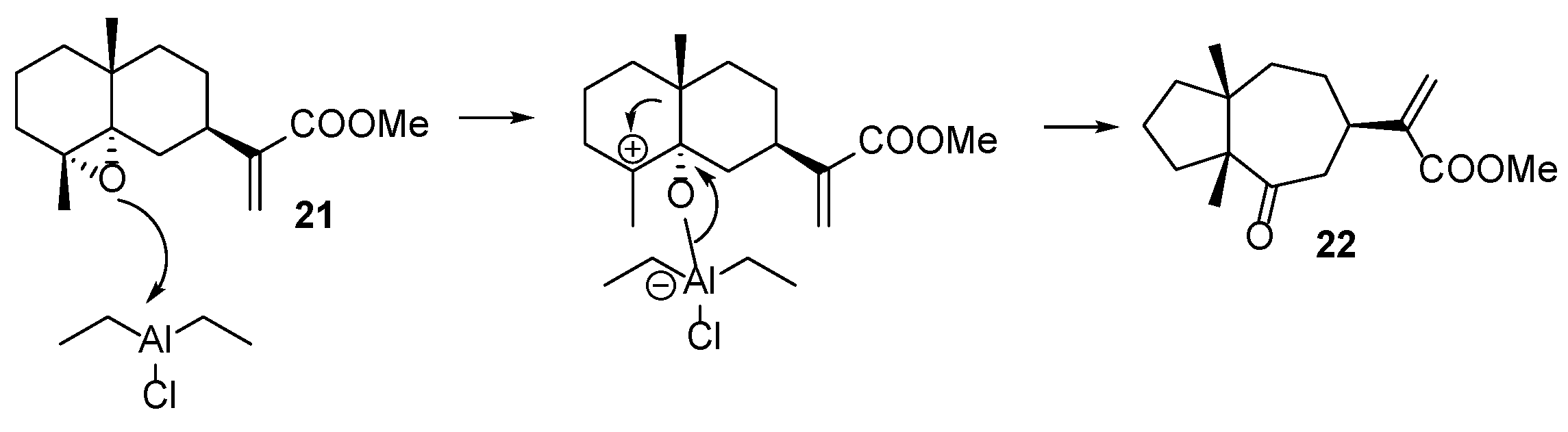
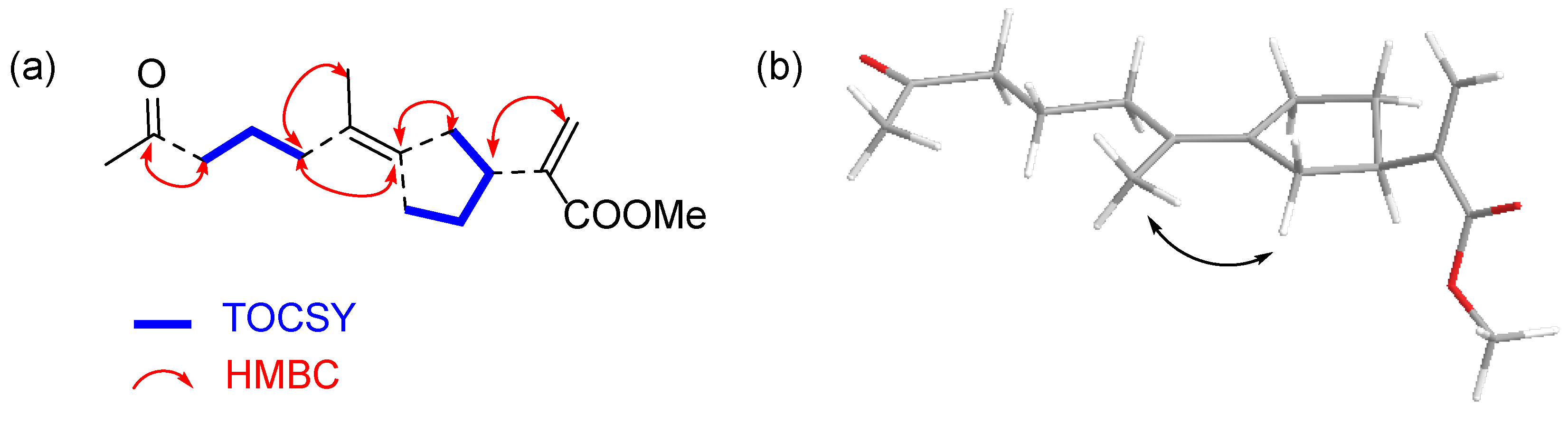
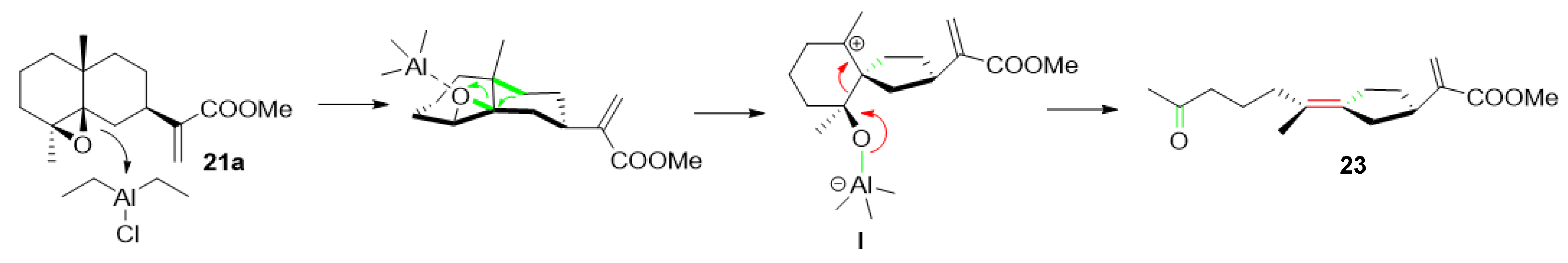
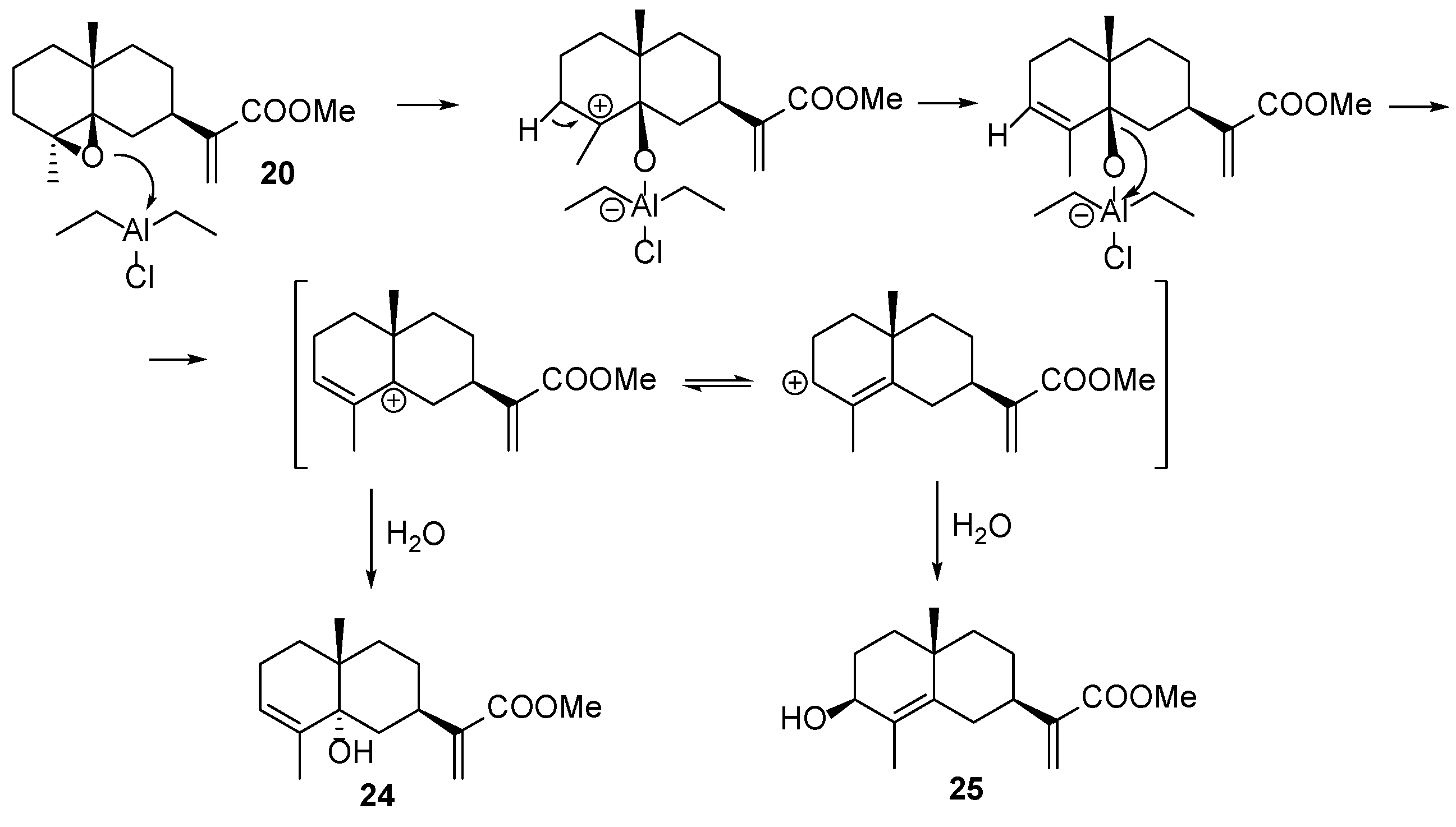
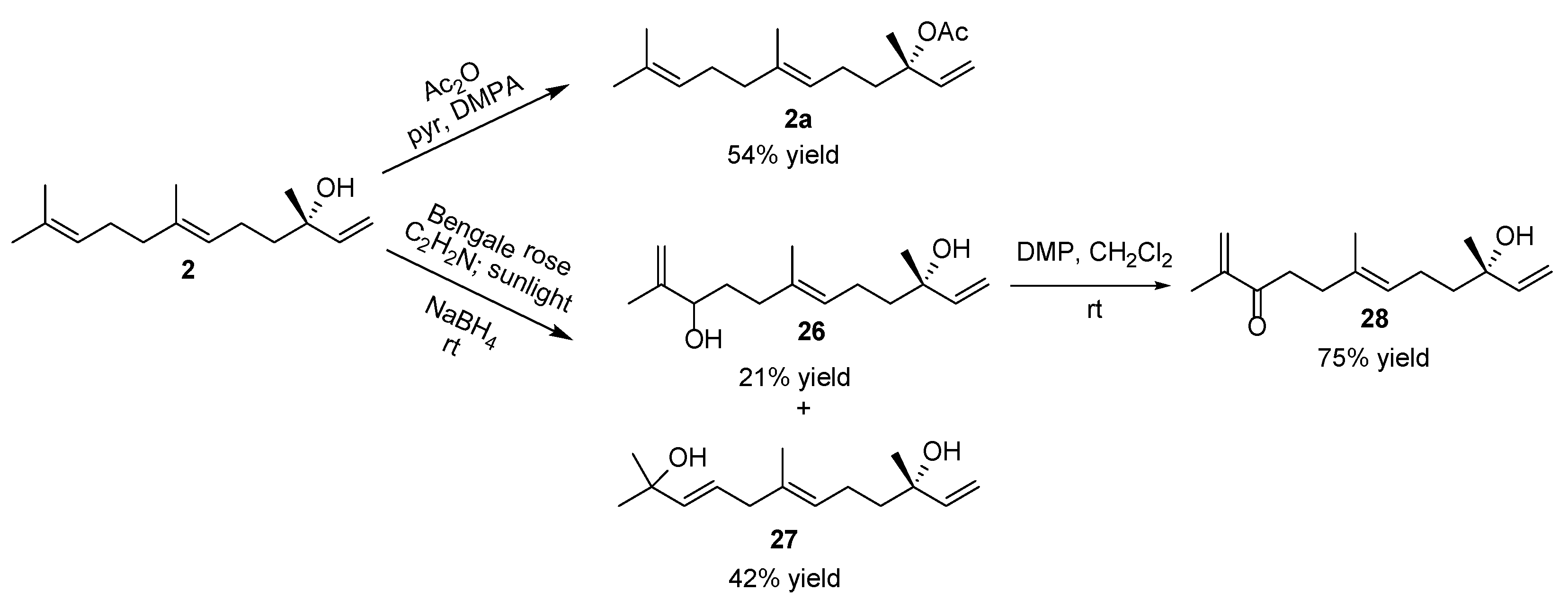


 | ||||
|---|---|---|---|---|
| Entry | I2 (Equiv) | Solvent | Time (h) | Compound (Yield) |
| entry 1 | 1 | benzene | 5 | 5 (85%) |
| entry 2 | 1 | toluene | 10 | 5 (48%), 6 (9%), 7 (18%) |
| entry 3 | 0.25 | toluene:DMSO (8:1) | 18 | 5 (37%), 6 (15%), 7 (8%), 8 (4%), 9 (4%), 10 (6%) |
| entry 4 | 2 | toluene:MeOH (9:1) | 6.5 | 5 (45%), 11 (6%), 12 (19%), 13 (9%) |
| entry 5 | 2 | toluene:MeOH (9:1) | 16 | 5 (40%), 11 (9%), 12 (19%), 13 (17%). |
| entry 6 | 2 | toluene:MeOH (9:1) | 38 | 5 (24%), 11 (10%), 14 (22%), 15(10%), 16 (7%), 17 (12%) |
 | ||||
|---|---|---|---|---|
| Entry | I2 (Equiv) | Solvent | Time (h) | Compound (Yield) |
| entry 1 | 2 | toluene:MeOH (9:1) | 13 | 11 (9%), 12 (25%), 15 (14%), 17 (10%) |
| entry 2 | 2 | toluene:MeOH (9:1) | 32 | 11 (6%), 12 (8%), 13 (7%), 18 (24%) |
 | ||||
| Entry | Reactant | Temperature | Time (h) | Compound (Yield) |
| entry 1 | Et2AlCl (2 equiv) | −60 °C | 6 | 20 (38%), 22 (39%), 23 (4%) |
| entry 2 | Me2AlCl (3 equiv) | −40 °C | 6 | 20 (2%), 22 (41%), 23 (2%), 24 (24%), 25 (12%) |
| Compound | μg/cm2 | S. littoralis | M. persicae | R. padi |
|---|---|---|---|---|
| %FI b (n = 6–10) | %SI b (n = 20) | |||
| Nerolidol (2) | 50 | 25.91 ± 16.44 | 60.94 ± 10.32 * | 54.02 ± 8.34 * |
| EC50 a | >50 | ≅50 | ≥50 | |
| 3 | 50 | 31.10 ± 9.70 | 29.21 ± 8.55 | 69.30 ± 6.20 * |
| EC50 | >50 | >50 | 25–50 | |
| 3a | 50 | 48.70 ± 15.02 | 56.80 ± 7.38 * | 88.32 ± 4.12 * |
| EC50 | >50 | ≥50 | 8.34 (6.3–11.1) | |
| 26 | 50 | 24.63 ± 14.52 | 83.55 ± 5.97 * | 67.08 ± 6.85 * |
| EC50 | >50 | 18.91 (12.71–28.12) | ≅50 | |
| 27 | 50 | 29.14 ± 15.85 | 100 * | 80.16 ± 5.36 * |
| EC50 | >50 | 9.97 (5.85–16.98) | 19.97 (15.66–25.47) | |
| 28 | 50 | 28.78 ± 15.35 | 70.87 ± 5.22 * | 78.49 ± 5.29 * |
| EC50 | >50 | 15.11 (8.05–28.37) | 11.95 (6.23–22.92) | |
| 29 | 50 | 50.19 ± 8.67 * | 66.10 ± 7.36 * | 76.37 ± 7.76 * |
| EC50 | ≥50 | ≅50 | 21.3 (16.2–25.3) | |
| 33 | 50 | 34.43 ± 14.66 | 23.70 ± 7.14 | 44.24 ± 6.88 |
| EC50 | >50 | >50 | >50 | |
| Ilicic acid (1) | 50 | 51.77 ± 9.50 * | 39.05 ± 8.16 | 27.34 ± 7.83 |
| EC50 | ≥50 | >50 | >50 | |
| 4 | 50 | 40.45 ± 12.96 | 51.01 ± 7.56 * | 96.05 ± 2.15 * |
| EC50 | >50 | ≥50 | 15.90 (13.04–19.26) | |
| 5 | 50 | nt | 61.62 ± 8.35 * | 92.32 ± 4.03 * |
| EC50 | ≅50 | 22.6 (20.3–25.1) | ||
| 11 | 50 | 76.09 ± 11.94 * | 54.34 ± 8.69 * | 89.72 ± 5.07 * |
| EC50 | 25.28 (17.96–35.57) | ≥50 | 11.11 (8.81–14.01) | |
| 17 | 50 | nt | 28.90 ± 8.27 | 34.18 ± 8.04 |
| EC50 | >50 | >50 | ||
| 20–21 | 50 | 41.70 ± 8.91 | 57.65 ± 8.49 * | 56.53 ± 8.03 * |
| EC50 | >50 | ≥50 | ≥50 | |
| Thymol c | 50 | 52.4 ± 10.1 * | 81.8 ± 7.7 * | 92.1 ± 2.6 * |
| EC50 | ≥50 | 7.6 (4.1–8.7) | 18.6 (4.1–23.3.5) | |
| Compound | Mortality (%) | Lethal Dose (ug/uL) a | |
|---|---|---|---|
| (1 ug/uL) | LD50 | LD90 | |
| Nerolidol (2) | 10.97 ± 2.01 | ||
| 3 | 3.63 ± 0.73 | ||
| 3a | 0.13 ± 0.71 | ||
| 26 | 17.41± 3.50 | ||
| 27 | 14.47 ± 2.87 | ||
| 28 | 100 ± 0 | MLC b = 0.5 | |
| 29 | 2.89 ± 0.97 | ||
| 33 | 10.97 ± 1.35 | ||
| Ilicic acid (1) | 19.74 ±1.80 | ||
| 4 | 61.11 ± 5.60 | ||
| 5 | 1.49 ± 1.21 | ||
| 11 | 93.24 ± 3.15 | 0.31 (0.29–0.33) | 0.59 (0.55–0.65) |
| 20–21 | 5.98 ± 2.57 | ||
| Thymol c | 100 ± 0 | 0.14 (0.13–0.14) | 0.22 (0.2 1–0.25) |
| Compound | % Mortality a (20 μg/μL) | Effective Concentration (μg/mg) b | |
|---|---|---|---|
| LD50 | LD90 | ||
| Nerolidol (2) | 10. 97 ± 2.01 | >20 | >20 |
| 3 | 100 ± 0 | 2.30 (2.07–2.58) | 3.44 (3.05–4.14) |
| 3a | 100 ± 0 | 2.58 (2.29–2.92) | 5.25 (4.62–6.21) |
| 29 | 100 ±0 | 0.62 (0.55–0.70) | 1.09 (0.97–1.26) |
| 33 | 100 ± 0 | Nc | MLD c = 5 |
| Ilicic acid (1) | 8 47 ± 4.61 | >20 | >20 |
| 4 | 25.8 ± 1.43 | >20 | >20 |
| 5 | 98.4 ± 0.6 | 1.71 (1.52–1.87) | 2.78 (2.54–3.15) |
| 11 | 100 ± 0 | 2.61 (2.39–2.88) | 3.69 (3.35–4.18) |
| 17 | 90.3 ± 3.8 | 2.36 (1.29–3.18) | 8.26 (6.96–10.49) |
| Thymol d | 100 ± 0 | 2.94 (2.08–3.54) | 6.16 (5.30–7.84) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segura-Navarro, M.J.; Quílez del Moral, J.F.; Andrés, M.F.; Valcárcel, F.; González-Coloma, A.; Molina Inzunza, D.O.; Barrero, A.F. Major Components of Dittrichia viscosa (Asteraceae) as a Source of New Pesticides. Molecules 2025, 30, 3950. https://doi.org/10.3390/molecules30193950
Segura-Navarro MJ, Quílez del Moral JF, Andrés MF, Valcárcel F, González-Coloma A, Molina Inzunza DO, Barrero AF. Major Components of Dittrichia viscosa (Asteraceae) as a Source of New Pesticides. Molecules. 2025; 30(19):3950. https://doi.org/10.3390/molecules30193950
Chicago/Turabian StyleSegura-Navarro, María José, José Francisco Quílez del Moral, María Fe Andrés, Félix Valcárcel, Azucena González-Coloma, Diego O. Molina Inzunza, and Alejandro F. Barrero. 2025. "Major Components of Dittrichia viscosa (Asteraceae) as a Source of New Pesticides" Molecules 30, no. 19: 3950. https://doi.org/10.3390/molecules30193950
APA StyleSegura-Navarro, M. J., Quílez del Moral, J. F., Andrés, M. F., Valcárcel, F., González-Coloma, A., Molina Inzunza, D. O., & Barrero, A. F. (2025). Major Components of Dittrichia viscosa (Asteraceae) as a Source of New Pesticides. Molecules, 30(19), 3950. https://doi.org/10.3390/molecules30193950