Exploring the Synthesis, Anti-Inflammatory and Anti-Tumor Potential of 4-Maleimidylphenyl-Hydrazide Derivatives
Abstract
1. Introduction
2. Results and Discussion
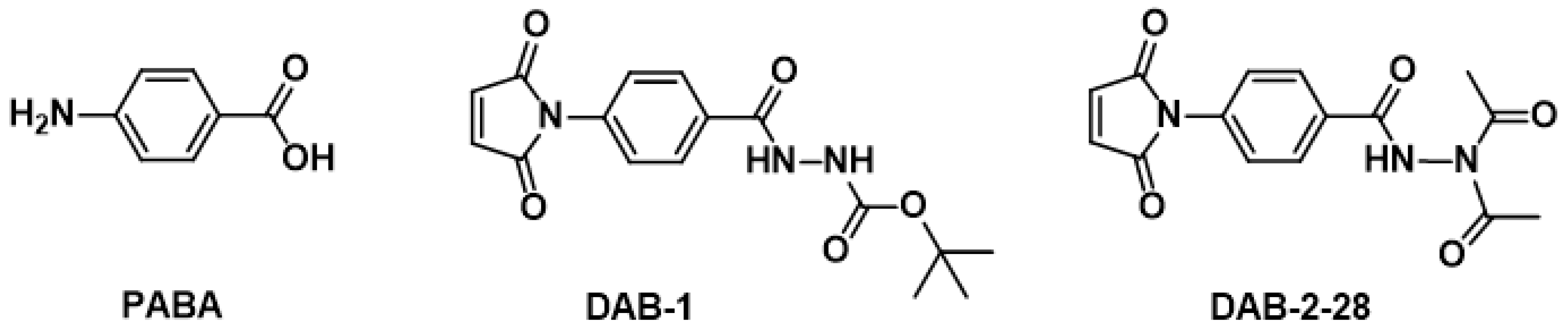
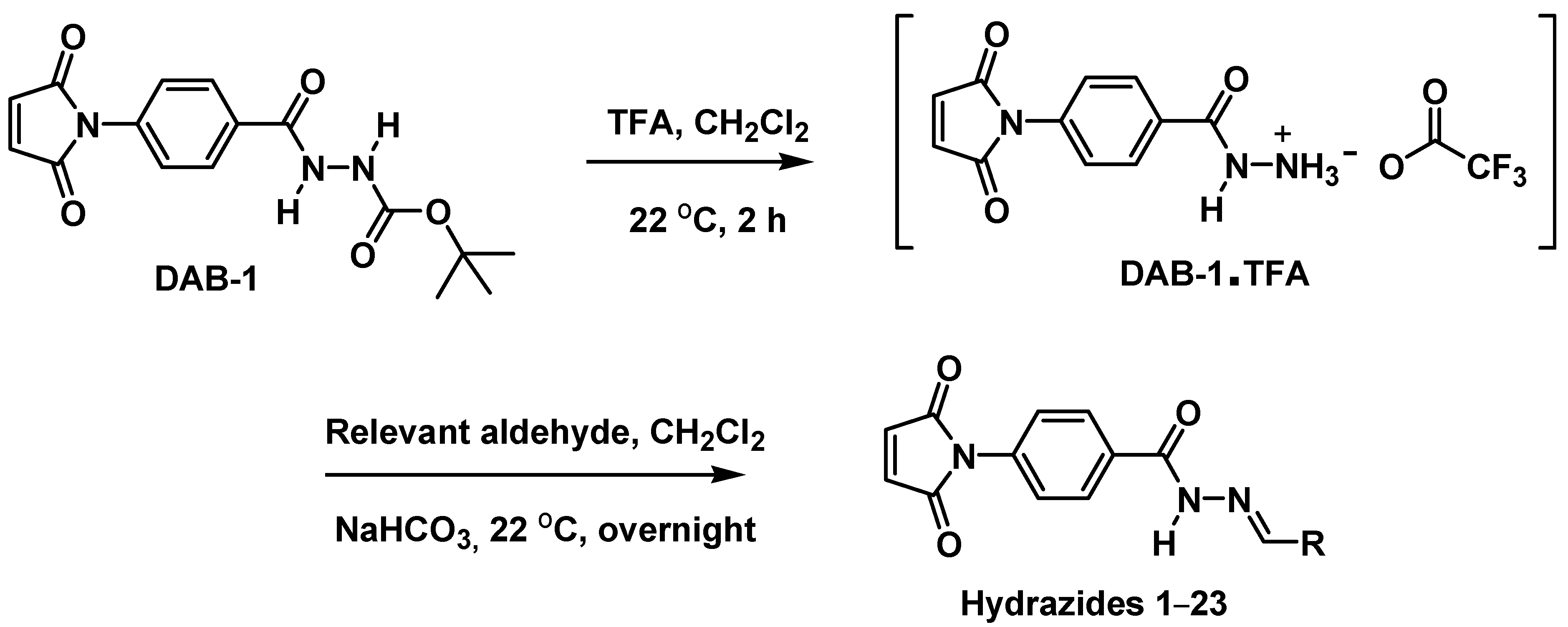
2.1. Design and Chemistry
2.2. Biological Results
2.2.1. Antiproliferative Activity on Macrophage Cell Lines
2.2.2. Evaluation of Anti-Inflammatory Properties of the Structural Components of DAB-1 and Its Derivatives 1–23
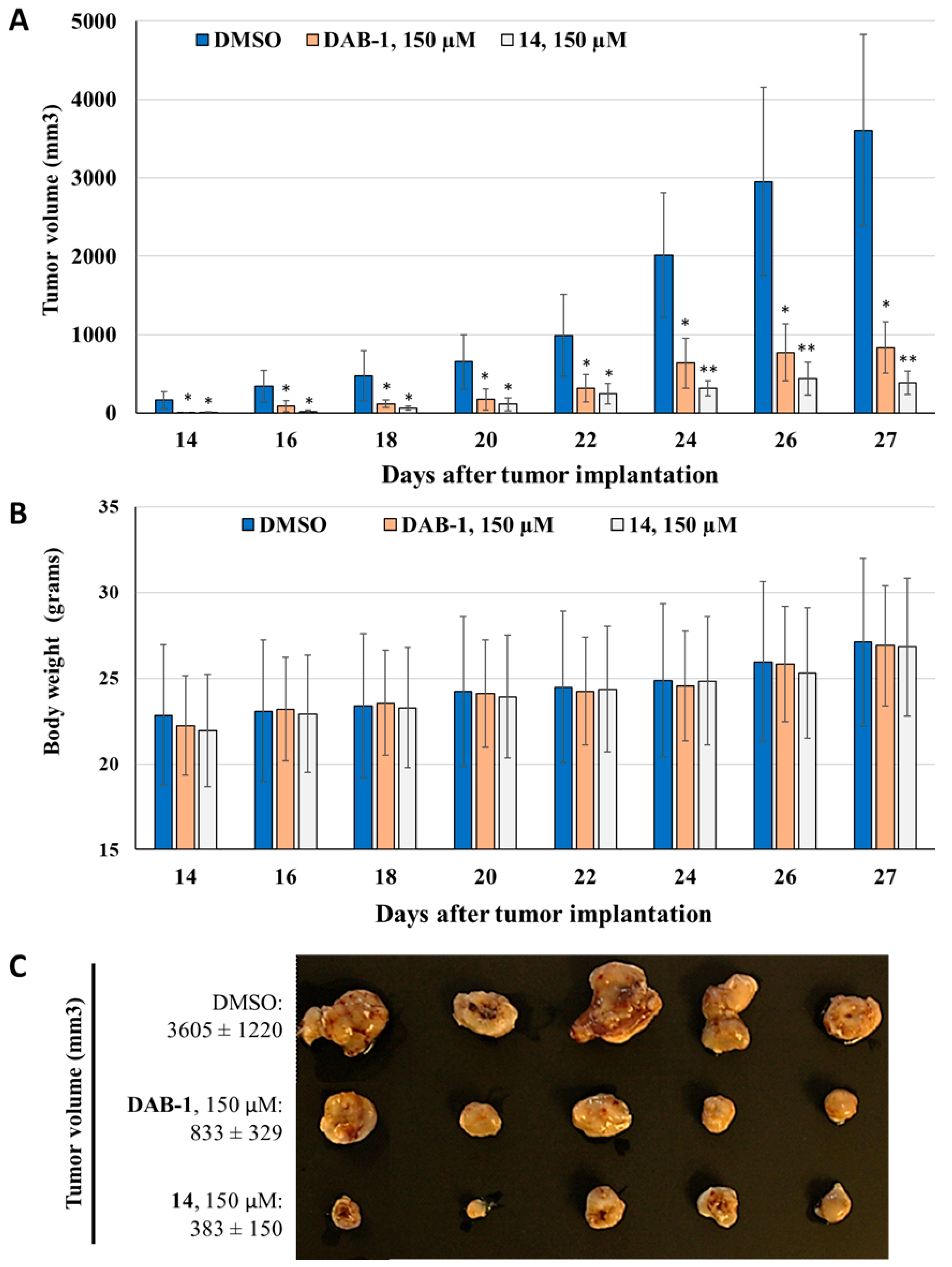
2.2.3. In Vivo Evaluation of Anti-Inflammatory and Anti-Tumoral Properties of Hydrazide Derivative 14
3. Materials and Methods
3.1. Biological Methods
3.1.1. Evaluation of NO Production and Cell Viability/Proliferation
3.1.2. Acute Inflammation Study in Mice
3.1.3. Growth Kinetics and Treatment of Murine Bladder Tumors
3.1.4. Statistical Analyses
3.2. Chemistry
3.2.1. Synthesis of 4-Maleimidylphenyl-Hydrazide Molecules
Synthesis of Tert-Butyl 2-(4-(2,5-Dioxo-2,5-Dihydro-1H-Pyrrol-1-yl)Benzoyl)Hydrazinecarboxylate (DAB-1)
Synthesis of N-Benzylidene-4-(2,5-Dioxo-2,5-Dihydro-1H-Pyrrol-1-yl)Benzohydrazide (1)
Synthesis of 4-(2,5-Dioxo-2,5-Dihydro-1H-Pyrrol-1-yl)-N-(3-Phenyallylidene)Benzohydrazide (2)
Synthesis of 4-(2,5-Dioxo-2,5-Dihydro-1H-Pyrrol-1-yl)-N-(2-Methylbenzylidene)Benzohydrazide (3)
Synthesis of 4-(2,5-Dioxo-2,5-Dihydro-1H-Pyrrol-1-yl)-N-(3-Methylbenzylidene)Benzohydrazide (4)
Synthesis of 4-(2,5-Dioxo-2,5-Dihydro-1H-Pyrrol-1-yl)-N-(4-Methylbenzylidene)Benzohydrazide (5)
Synthesis of 4-(2,5-Dioxo-2,5-Dihydro-1H-Pyrrol-1-yl)-N-(2-Hydroxybenzylidene)Benzohydrazide (6)
Synthesis of 4-(2,5-Dioxo-2,5-Dihydro-1H-Pyrrol-1-yl)-N-(3-Hydroxybenzylidene)Benzohydrazide (7)
Synthesis of 4-(2,5-Dioxo-2,5-Dihydro-1H-Pyrrol-1-yl)-N-(4-Hydroxybenzylidene)Benzohydrazide (8)
Synthesis of 4-(2,5-Dioxo-2,5-Dihydro-1H-Pyrrol-1-yl)-N-(2-Methoxybenzylidene)Benzohydrazide (9)
Synthesis of 4-(2,5-Dioxo-2,5-Dihydro-1H-Pyrrol-1-yl)-N-(3-Methoxybenzylidene)Benzohydrazide (10)
Synthesis of 4-(2,5-Dioxo-2,5-Dihydro-1H-Pyrrol-1-yl)-N-(2-Nitrobenzylidene)Benzohydrazide (11)
Synthesis of 4-(2,5-Dioxo-2,5-Dihydro-1H-Pyrrol-1-yl)-N-(3-Nitrobenzylidene)Benzohydrazide (12)
Synthesis of 4-(2,5-Dioxo-2,5-Dihydro-1H-Pyrrol-1-yl)-N-(4-Bromobenzylidene)Benzohydrazide (13)
Synthesis of 4-(2,5-Dioxo-2,5-Dihydro-1H-Pyrrol-1-yl)-N-(2-Hydroxy-3-Methoxybenzylidene)Benzohydrazide (14)
Synthesis of 4-(2,5-Dioxo-2,5-Dihydro-1H-Pyrrol-1-yl)-N-(3-Hydroxy-4-Methoxybenzelidene)Benzohydrazide (15)
Synthesis of 4-(2,5-Dioxo-2,5-Dihydro-1H-Pyrrol-1-yl)-N-(4-Hydroxy-3-Methoxybenzelidene)Benzohydrazide (16)
Synthesis of 4-(2,5-Dioxo-2,5-Dihydro-1H-Pyrrol-1-yl)-N-(Furan-2-Ylmethylene)Benzohydrazide (17)
Synthesis of 4-(2,5-Dioxo-2,5-Dihydro-1H-Pyrrol-1-yl)-N-(Furan-3-Ylmethylene)Benzohydrazide (18)
Synthesis of 4-(2,5-Dioxo-2,5-Dihydro-1H-Pyrrol-1-yl)-N-(5-Methylfuran-2-Ylmethylene)Benzohydrazide (19)
Synthesis of 4-(2,5-Dioxo-2,5-Dihydro-1H-Pyrrol-1-yl)-N-(5-Nitrofuran-2-Ylmethylene)Benzohydrazide (20)
Synthesis of 4-(2,5-Dioxo-2,5-Dihydro-1H-Pyrrol-1-yl)-N-(5-(Hydroxymethyl)Furan-2-Ylmethylene)Benzohydrazide (21)
Synthesis of 4-(2,5-Dioxo-2,5-Dihydro-1H-Pyrrol-1-yl)-N-(5-(4-Nitrophenyl)Furan-2-Ylmethylene)Benzohydrazide (22)
Synthesis of 4-(2,5-Dioxo-2,5-Dihydro-1H-Pyrrol-1-yl)-N-(Pyridin-3-Ylmethylene)Benzohydrazide (23)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BCa | Bladder cancer |
| PABA | p-aminobenzoic acid |
| DMSO | Dimethyl sulfoxide |
| IR | Infrared spectroscopy |
| NMR | Nuclear magnetic spectroscopy |
| TFA | Trifluoroacetic acid |
| NO | Nitric oxide |
| PBS | Phosphate-buffered saline |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide |
| IP | Intraperitoneal |
References
- Eccles, R.L.; Carreno, G.; de la Rica, L.; Balmer, G.M.; Scott, D. Tackling Cancer through Global Team Science. Cancer Discov. 2025, 15, 673–677. [Google Scholar] [CrossRef]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Daneshmand, S.; Bazargani, S.T.; Cai, J.; Miranda, G.; Schuckman, A.K.; Djaladat, H. Postoperative Pain Management after Radical Cystectomy: Comparing Traditional versus Enhanced Recovery Protocol Pathway. J. Urol. 2015, 194, 1209–1213. [Google Scholar] [CrossRef]
- Zhang, Y. Understanding the Gender Disparity in Bladder Cancer Risk: The Impact of Sex Hormones and Liver on Bladder Susceptibility to Carcinogens. J. Environ. Sci. Health Part C 2013, 31, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Dobruch, J.; Oszczudłowski, M. Bladder Cancer: Current Challenges and Future Directions. Medicina 2021, 57, 749. [Google Scholar] [CrossRef]
- Shinagare, A.B.; Ramaiya, N.H.; Jagannathan, J.P.; Fennessy, F.M.; Taplin, M.-E.; Van den Abbeele, A.D. Metastatic Pattern of Bladder Cancer: Correlation With the Characteristics of the Primary Tumor. Am. J. Roentgenol. 2011, 196, 117–122. [Google Scholar] [CrossRef]
- Ripoll, J.; Ramos, M.; Montaño, J.; Pons, J.; Ameijide, A.; Franch, P. Cancer-specific survival by stage of bladder cancer and factors collected by Mallorca Cancer Registry associated to survival. BMC Cancer 2021, 21, 676. [Google Scholar]
- Zhu, Z.; Shen, Z.; Xu, C. Inflammatory Pathways as Promising Targets to Increase Chemotherapy Response in Bladder Cancer. Mediat. Inflamm. 2012, 2012, 528690. [Google Scholar] [CrossRef]
- Sui, X.; Lei, L.; Chen, L.; Xie, T.; Li, X. Inflammatory microenvironment in the initiation and progression of bladder cancer. Oncotarget 2017, 8, 93279–93294. [Google Scholar] [CrossRef]
- Fortuny, J.; Kogevinas, M.; Zens, M.S.; Schned, A.; Andrew, A.S.; Heaney, J.; Kelsey, K.T.; Karagas, M.R. Analgesic and anti-inflammatory drug use and risk of bladder cancer: A population based case control study. BMC Urol. 2007, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Sagone, A.L.; Husney, R.M.; Bruce Davis, W. Biotransformation of para-aminobenzoic acid and salicylic acid by PMN. Free Radic. Biol. Med. 1993, 14, 27–35. [Google Scholar] [CrossRef]
- Haroon, F.; Farwa, U.; Arif, M.; Raza, M.A.; Sandhu, Z.A.; El Oirdi, M.; Farhan, M.; Alhasawi, M.A.I. Novel Para-Aminobenzoic Acid Analogs and Their Potential Therapeutic Applications. Biomedicines 2023, 11, 2686. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Bansal, D.; Bajaj, K.; Sharma, S.; Archana; Srivastava, V.K. Synthesis of some newer derivatives of 2-amino benzoic acid as potent anti-inflammatory and analgesic agents. Bioorganic Med. Chem. 2003, 11, 5281–5291. [Google Scholar] [CrossRef] [PubMed]
- Krátký, M.; Konečná, K.; Janoušek, J.; Brablíková, M.; Janďourek, O.; Trejtnar, F.; Stolaříková, J.; Vinšová, J. 4-Aminobenzoic Acid Derivatives: Converting Folate Precursor to Antimicrobial and Cytotoxic Agents. Biomolecules 2019, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Hamelin-Morrissette, J.; Cloutier, S.; Girouard, J.; Belgorosky, D.; Eiján, A.M.; Legault, J.; Reyes-Moreno, C.; Bérubé, G. Identification of an anti-inflammatory derivative with anti-cancer potential: The impact of each of its structural components on inflammatory responses in macrophages and bladder cancer cells. Eur. J. Med. Chem. 2015, 96, 259–268. [Google Scholar] [CrossRef]
- Chanphai, P.; Cloutier, F.; Oufqir, Y.; Leclerc, M.F.; Eiján, A.M.; Reyes-Moreno, C.; Bérubé, G.; Tajmir-Riahi, H.A. Biomolecular study and conjugation of two para-aminobenzoic acid derivatives with serum proteins: Drug binding efficacy and protein structural analysis. J. Biomol. Struct. Dyn. 2020, 39, 79–90. [Google Scholar] [CrossRef]
- Berube, G.; Reyes-Moreno, C. Aminobenzoic Acid Derivatives for Use as Anti-Inflammatory Agents, Anti-Metastatic Agents and/or Anticancer Agents. WO2017/177316 A1, 19 October 2017. [Google Scholar]
- Oufqir, Y.; Fortin, L.; Girouard, J.; Cloutier, F.; Cloutier, M.; Leclerc, M.-F.; Belgorosky, D.; Eiján, A.M.; Bérubé, G.; Reyes-Moreno, C. Synthesis of new para-aminobenzoic acid derivatives, in vitro biological evaluation and preclinical validation of DAB-2-28 as a therapeutic option for the treatment of bladder cancer. Eur. J. Med. Chem. Rep. 2022, 6, 100069. [Google Scholar] [CrossRef]
- Cloutier, F.; Oufqir, Y.; Fortin, L.; Leclerc, M.-F.; Girouard, J.; Tajmir-Riahi, H.-A.; Reyes-Moreno, C.; Bérubé, G. Design of novel 4-maleimidylphenyl-hydrazide molecules displaying anti-inflammatory properties: Refining the chemical structure. Eur. J. Med. Chem. Rep. 2022, 5, 100064. [Google Scholar] [CrossRef]
- Narang, R.; Narasimhan, B.; Sharma, S. A Review on Biological Activities and Chemical Synthesis of Hydrazide Derivatives. Curr. Med. Chem. 2012, 19, 569–612. [Google Scholar] [CrossRef]
- Murugappan, S.; Dastari, S.; Jungare, K.; Barve, N.M.; Shankaraiah, N. Hydrazide-hydrazone/hydrazone as enabling linkers in anti-cancer drug discovery: A comprehensive review. J. Mol. Struct. 2024, 1307, 138012. [Google Scholar] [CrossRef]
- Facchin, B.M.; dos Reis, G.O.; Vieira, G.N.; Mohr, E.T.B.; da Rosa, J.S.; Kretzer, I.F.; Demarchi, I.G.; Dalmarco, E.M. Inflammatory biomarkers on an LPS-induced RAW 264.7 cell model: A systematic review and meta-analysis. Inflamm. Res. 2022, 71, 741–758. [Google Scholar] [CrossRef] [PubMed]
- Posadas, I.; Bucci, M.; Roviezzo, F.; Rossi, A.; Parente, L.; Sautebin, L.; Cirino, G. Expression of Concern: Carrageenan-induced mouse paw oedema is biphasic, age-weight dependent and displays differential nitric oxide cyclooxygenase-2 expression. Br. J. Pharmacol. 2009, 142, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Girouard, J.; Belgorosky, D.; Hamelin-Morrissette, J.; Boulanger, V.; D’Orio, E.; Ramla, D.; Perron, R.; Charpentier, L.; Van Themsche, C.; Eiján, A.M.; et al. Molecular therapy with derivatives of amino benzoic acid inhibits tumor growth and metastasis in murine models of bladder cancer through inhibition of TNFα/NFΚB and iNOS/NO pathways. Biochem. Pharmacol. 2020, 176, 113778. [Google Scholar] [CrossRef]
- Wiemer, B.D.D. Perrin and W. L. F. Armarego: Purification of Laboratory Chemicals. 3. Aufl. Oxford. Pergamon Press, 1988, 391 S. ISBN 0-08-034714-2. Acta Hydrochim. Hydrobiol. 1989, 17, 632. [Google Scholar] [CrossRef]
- Still, W.C.; Kahn, M.; Mitra, A. Rapid chromatographic technique for preparative separations with moderate resolution. J. Org. Chem. 1978, 43, 2923–2925. [Google Scholar] [CrossRef]

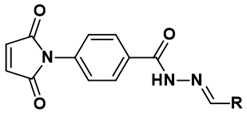 | |||||
|---|---|---|---|---|---|
| Compound | R | Yield (%) | Compound | R | Yield (%) |
| 1 | 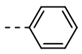 | 62 | 13 | 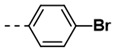 | 78 |
| 2 | 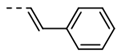 | 76 | 14 | 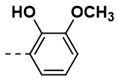 | 91 |
| 3 | 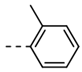 | 57 | 15 | 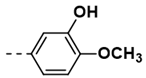 | 35 |
| 4 |  | 90 | 16 |  | 80 |
| 5 | 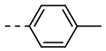 | 85 | 17 | 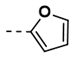 | 94 |
| 6 | 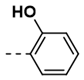 | 78 | 18 | 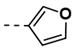 | 26 |
| 7 | 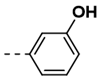 | 88 | 19 | 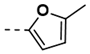 | 100 |
| 8 | 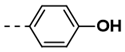 | 100 | 20 | 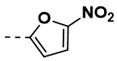 | 82 |
| 9 | 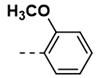 | 74 | 21 |  | 50 |
| 10 |  | 86 | 22 | 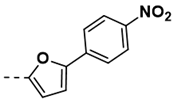 | 76 |
| 11 | 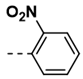 | 68 | 23 | 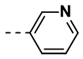 | 99 |
| 12 | 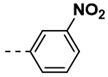 | 86 | |||
| Compound | % Viability | Compound | % Viability |
|---|---|---|---|
| DAB-1 | 99 ± 2 | 13 | 91 ± 3 |
| 1 | 46 ± 2 | 14 | 98 ± 1 |
| 2 | 42 ± 2 | 15 | 110 ± 8 |
| 3 | 107 ± 4 | 16 | 110 ± 7 |
| 4 | 88 ± 3 | 17 | 90 ± 7 |
| 5 | 102 ± 2 | 18 | 102 ± 2 |
| 6 | 40 ± 1 | 19 | 111 ± 4 |
| 7 | 101 ± 2 | 20 | 112 ± 3 |
| 8 | 105 ± 3 | 21 | 93 ± 3 |
| 9 | 100 ± 2 | 22 | 102 ± 2 |
| 10 | 72 ± 1 | 23 | 106 ± 7 |
| 11 | 96 ± 2 | ||
| 12 | 105 ± 4 |
| Compound | NO Concentration | Normalized NO | Normalized NO |
|---|---|---|---|
| (% of Control) | Production (%) | Inhibition (%) | |
| DAB-1 | 80 ± 2 | 81 | 19 |
| 1 | 19 ± 1 | 41 | 59 |
| 2 | 19 ± 2 | 45 | 55 |
| 3 | 100 ± 4 | 93 | 7 |
| 4 | 54 ± 3 | 61 | 39 |
| 5 | 62 ± 1 | 61 | 39 |
| 6 | 31 ± 2 | 78 | 22 |
| 7 | 56 ± 1 | 55 | 45 |
| 8 | 89 ± 6 | 85 | 15 |
| 9 | 80 ± 3 | 80 | 20 |
| 10 | 46 ± 4 | 64 | 36 |
| 11 | 62 ± 2 | 65 | 35 |
| 12 | 92 ± 1 | 97 | 3 |
| 13 | 94 ± 2 | 103 | −3 |
| 14 | <5% | <5% | >95% |
| 15 | 96 ± 6 | 87 | 13 |
| 16 | 78 ± 1 | 71 | 29 |
| 17 | 91 ± 4 | 101 | −1 |
| 18 | 23 ± 2 | 25 | 75 |
| 19 | 93 ± 3 | 84 | 16 |
| 20 | 84 ± 1 | 75 | 25 |
| 21 | 98 ± 3 | 105 | -5 |
| 22 | 53 ± 4 | 52 | 48 |
| 23 | 50 ± 2 | 47 | 53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cloutier, F.; Paquin, A.; Cloutier, M.; Oufqir, Y.; Fortin, L.; Girouard, J.; Tajmir-Riahi, H.-A.; Reyes-Moreno, C.; Bérubé, G. Exploring the Synthesis, Anti-Inflammatory and Anti-Tumor Potential of 4-Maleimidylphenyl-Hydrazide Derivatives. Molecules 2025, 30, 4035. https://doi.org/10.3390/molecules30204035
Cloutier F, Paquin A, Cloutier M, Oufqir Y, Fortin L, Girouard J, Tajmir-Riahi H-A, Reyes-Moreno C, Bérubé G. Exploring the Synthesis, Anti-Inflammatory and Anti-Tumor Potential of 4-Maleimidylphenyl-Hydrazide Derivatives. Molecules. 2025; 30(20):4035. https://doi.org/10.3390/molecules30204035
Chicago/Turabian StyleCloutier, Francis, Alexis Paquin, Maude Cloutier, Yassine Oufqir, Laurie Fortin, Julie Girouard, Heidar-Ali Tajmir-Riahi, Carlos Reyes-Moreno, and Gervais Bérubé. 2025. "Exploring the Synthesis, Anti-Inflammatory and Anti-Tumor Potential of 4-Maleimidylphenyl-Hydrazide Derivatives" Molecules 30, no. 20: 4035. https://doi.org/10.3390/molecules30204035
APA StyleCloutier, F., Paquin, A., Cloutier, M., Oufqir, Y., Fortin, L., Girouard, J., Tajmir-Riahi, H.-A., Reyes-Moreno, C., & Bérubé, G. (2025). Exploring the Synthesis, Anti-Inflammatory and Anti-Tumor Potential of 4-Maleimidylphenyl-Hydrazide Derivatives. Molecules, 30(20), 4035. https://doi.org/10.3390/molecules30204035









