Proposal for Self-Degrading Power Cables Incorporating Graphitic Carbon Nitride to Address Electronic Waste Challenges and Evaluation of Decomposition Efficiencies
Abstract
1. Introduction
2. Results and Discussion
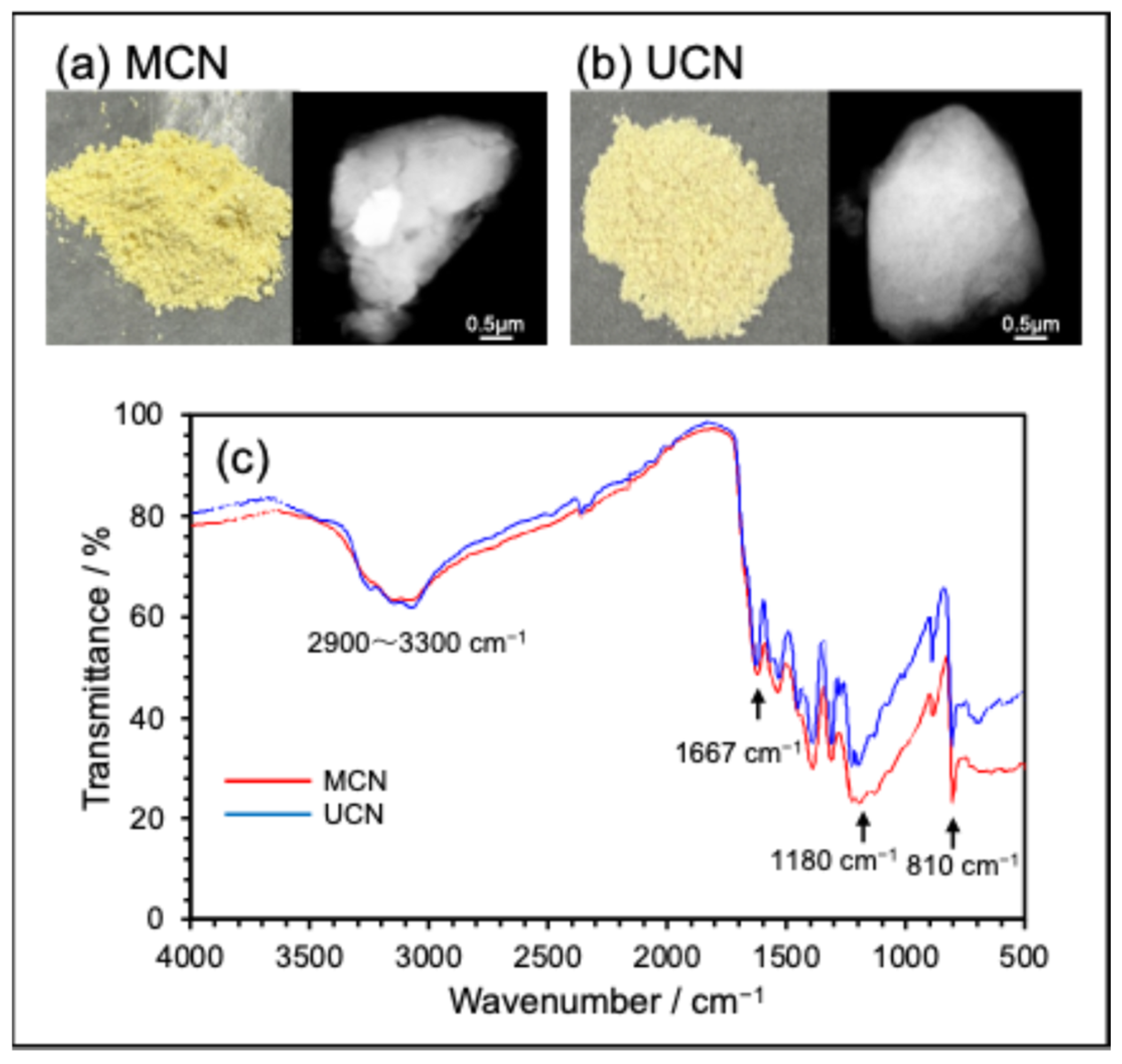
2.1. Synthesis and Physical Properties of MCN or UCN Powders
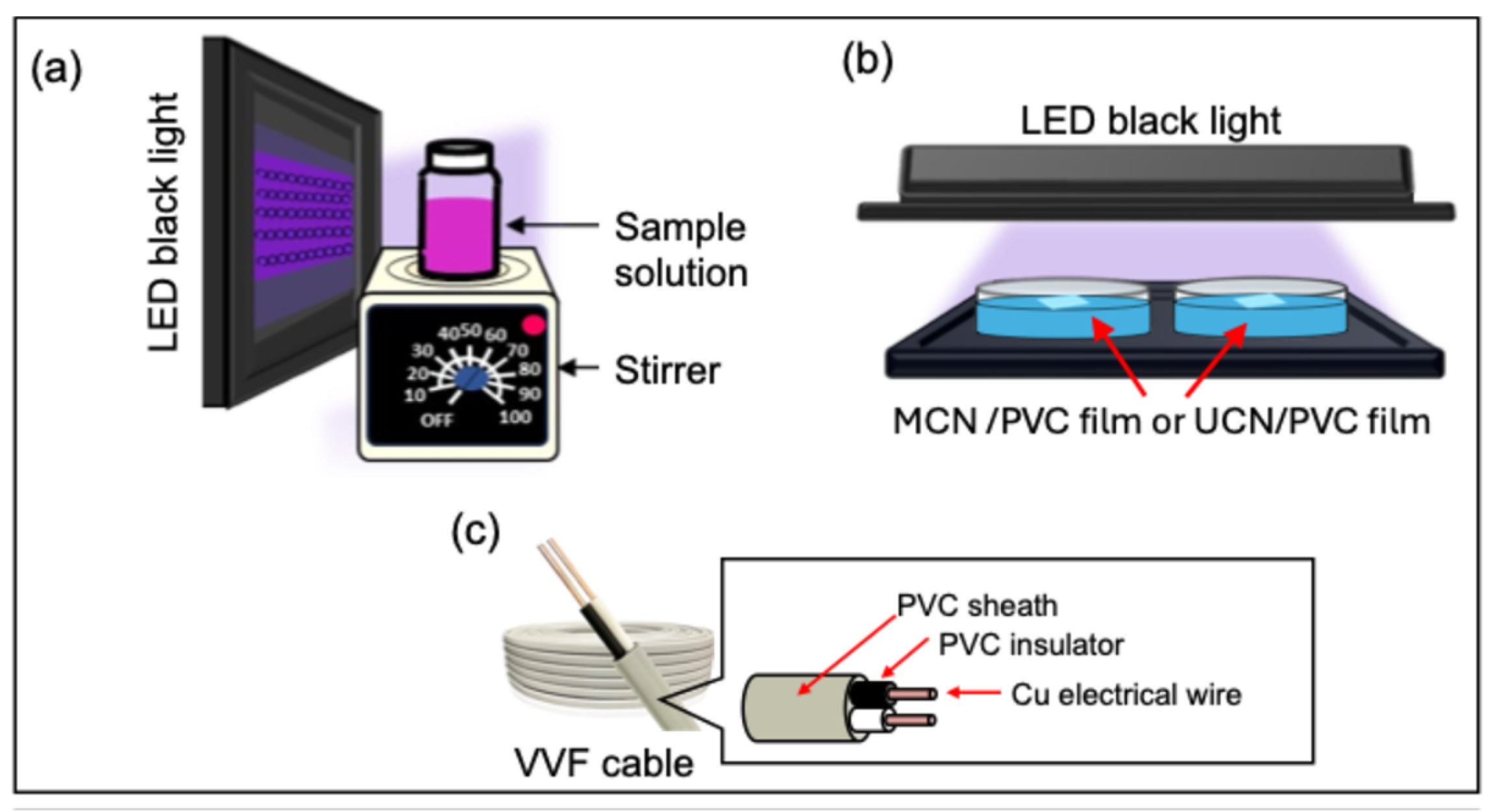
2.2. RhB Decolorization Experiment Using Powdered MCN or UCN
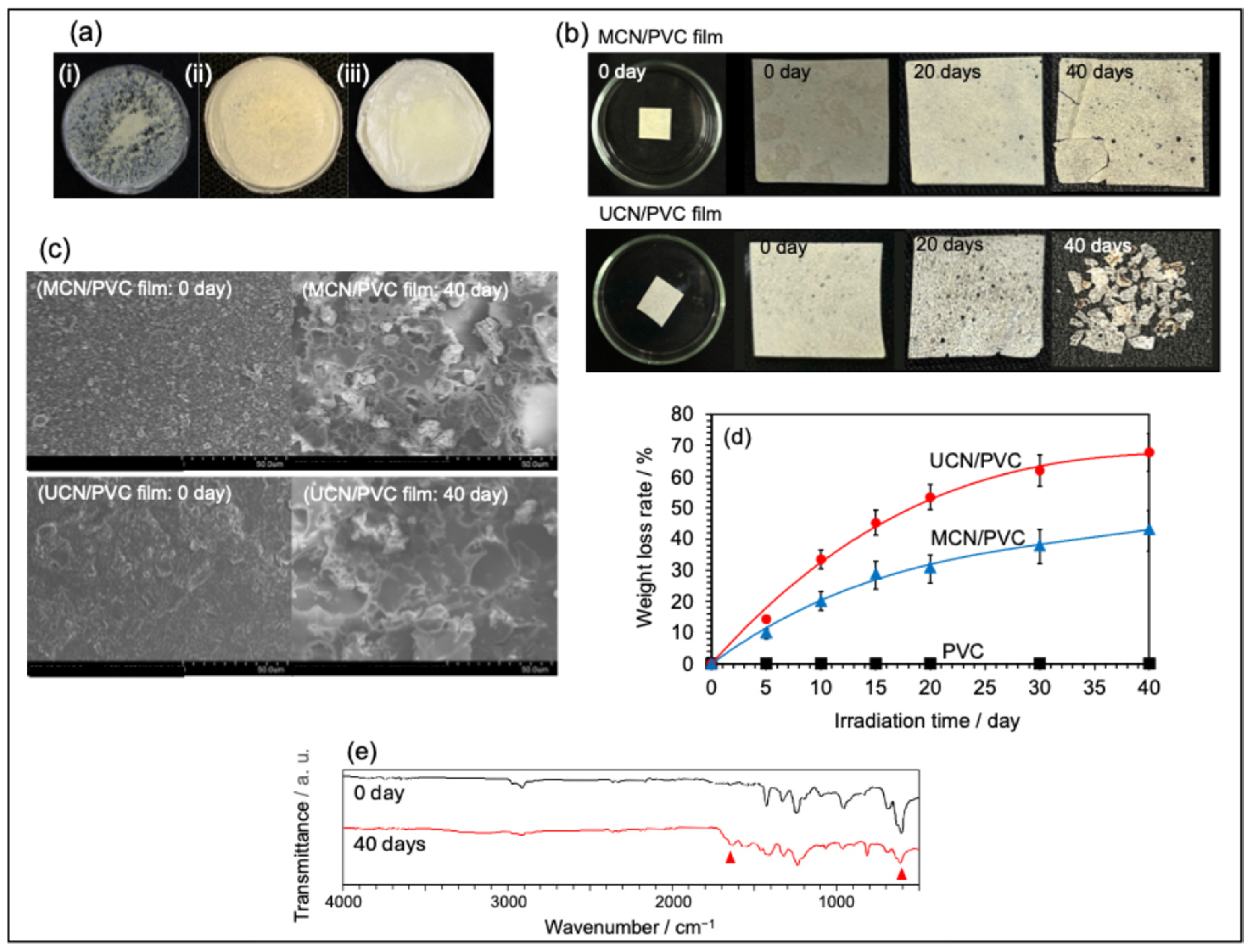
2.3. Preparation of MCN- or UCN-Blended PVC Films and Assessment of Their Degradation
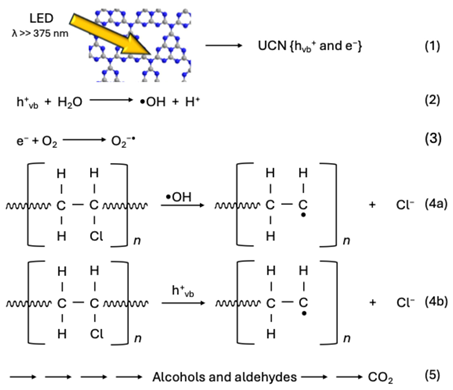
2.4. Proposed Decomposition Mechanism of UCN-Blended PVC Film
2.5. Material Strength of UCN/PVC Film
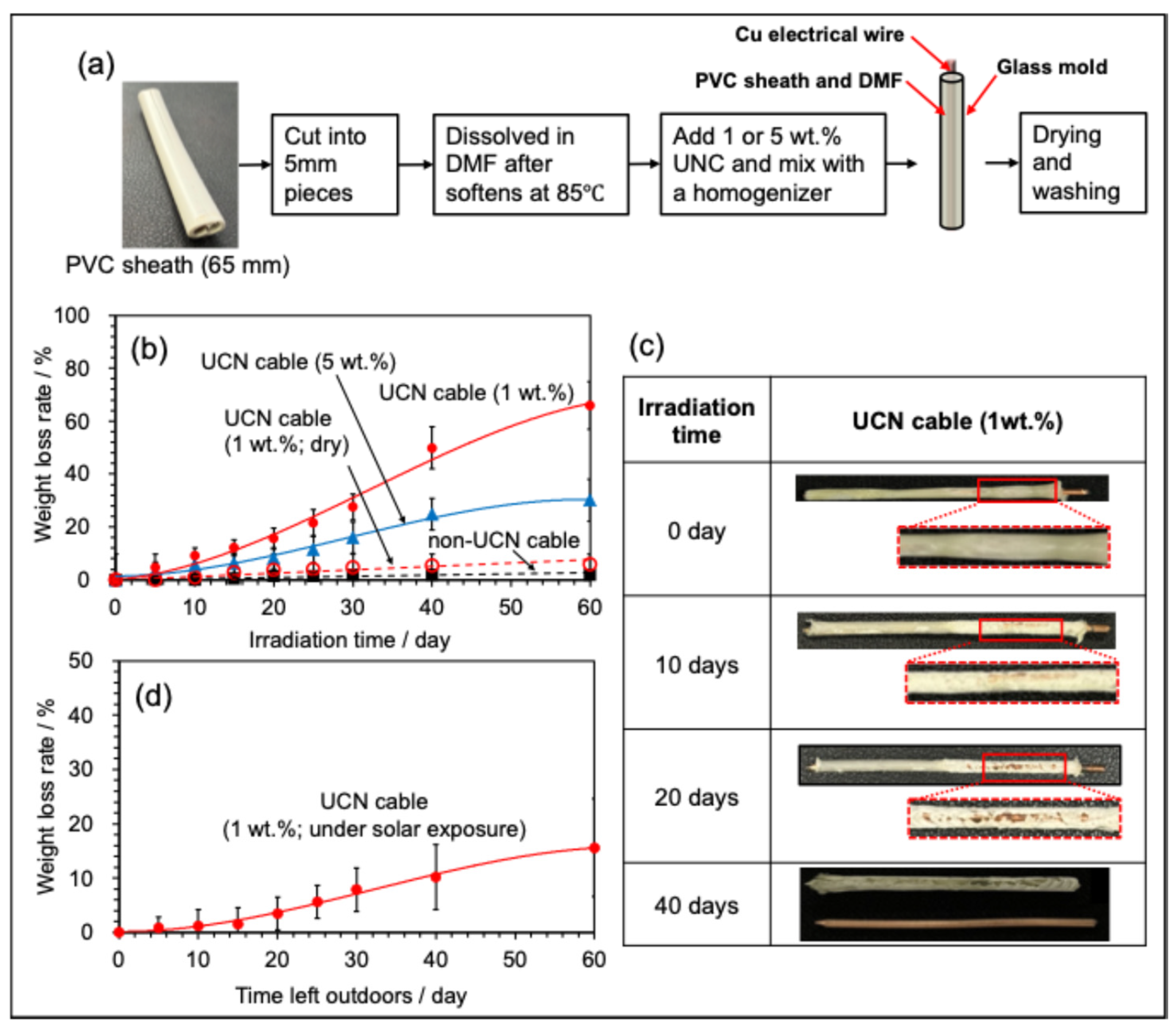
2.6. Fabrication and Disassembly Evaluation of MCN or UCN Mixed Wire and Cable
2.7. Material Strength of UCN Cable
3. Materials and Methods
3.1. Synthesis of MCN or UCN
3.2. Decolorization Experiment of RhB Using Powdered MCN or UCN
3.3. Degradation Experiments of MCN or UCN-Blended PVC Films
3.4. Decomposition Experiment of MCN or UCN Mixed Electric Cable
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Balde, C.P.; Kuehr, R.; Yamamoto, T.; McDonald, R.; D’Angelo, E.; Althaf, S.; Bel, G.; Deubzer, O.; Fernandez-Cubillo, E.; Forti, V.; et al. The Global E-waste Monitor 2024. Available online: https://ewastemonitor.info/the-global-e-waste-monitor-2024/ (accessed on 3 August 2025).
- Available online: https://www.env.go.jp/recycle/recycling/raremetals/conf_ruca/01/mat06.pdf (accessed on 3 August 2025). (In Japanese).
- Basel Convention. UNEP. Available online: https://www.basel.int/tabid/1275/Default.aspx (accessed on 3 August 2025).
- The Japanese Electric Wire & Cable Makers’ Association. Available online: https://www.jcma2.jp/toukei/index.html (accessed on 3 August 2025). (In Japanese).
- S&P Global. The Future of Copper: Will the Looming Supply Gap Short-Circuit the Energy Transition? 2022. Available online: https://cdn.ihsmarkit.com/www/pdf/0722/The-Future-of-Copper_Full-Report_14July2022.pdf (accessed on 3 August 2025).
- Çetin, A.; Erzengin, S.; Alp, F.B. Various combinations of flame retardants for poly(vinyl chloride). Open Chem. 2019, 17, 980–987. [Google Scholar] [CrossRef]
- Wędrychowicz, M.; Kurowiak, J.; Skrzekut, T.; Noga, P. Recycling of electrical cables—Current challenges and future prospects. Materials 2023, 16, 6632. [Google Scholar] [CrossRef] [PubMed]
- Horikoshi, S.; Hachisuga, N.; Serpone, N. Recycling of e-waste power cables using microwave-induced pyrolysis—Process characteristics and facile recovery of copper metal. RSC Adv. 2024, 14, 29955–29964. [Google Scholar] [CrossRef]
- Horikoshi, S.; Serpone, N.; Hisamatsu, Y.; Hidaka, H. Photocatalyzed degradation of polymers in aqueous semiconductor suspensions. 3. Photooxidation of a solid polymer: TiO2-blended poly(vinyl chloride) film. Environ. Sci. Technol. 1998, 32, 4010–4016. [Google Scholar] [CrossRef]
- United Nations Environment Assembly of the United Nations Environment Programme. Available online: https://www.env.go.jp/content/000045136.pdf (accessed on 3 August 2025).
- Dong, S.; Feng, J.; Fan, M.; Pi, L.; Hu, L.; Han, X.; Liu, M.; Sun, J.; Sun, J. Recent developments in heterogeneous photocatalytic water treatment using visible light-responsive photocatalysts: A review. RSC Adv. 2015, 5, 14610–14630. [Google Scholar] [CrossRef]
- Tang, S.-B.; Zhang, S.-Y.; Jiang, Y.-X.; Wang, Z.-X.; Li, K.; Luo, Y.-Y.; Long, B.; Su, J. Visible-light-induced photocatalytic degradation of polyvinyl chloride under normal temperature and pressure via uranyl photocatalyst. Ind. Eng. Chem. Res. 2025, 64, 7661–7669. [Google Scholar] [CrossRef]
- Kynman, A.E.; Christodoulou, S.; Ouellette, E.T.; Peterson, A.; Kelly, S.N.; Maron, L.; Arnold, P. Photocatalytic dechlorination of unactivated chlorocarbons including PVC using organolanthanide complexes. Chem. Commun. 2023, 59, 10924–10927. [Google Scholar] [CrossRef]
- Ran, J.; Talebian-Kiakalaieh, A.; Zhang, S.; Hashem, E.M.; Guo, M.; Qiao, S.-Z. Recent advancement on photocatalytic plastic upcycling. Chem. Sci. 2024, 15, 1611–1637. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Gou, N.; Yang, W.; Gao, S.; Li, Q. Incorporation of ultrathin porous metal-free graphite carbon nitride nanosheets in polyvinyl chloride for efficient photodegradation. J. Hazar. Mater. 2023, 447, 130795. [Google Scholar] [CrossRef]
- Kumar, S.; Karthikeyan, S.; Lee, A.F. g-C3N4-based nanomaterials for visible light-driven photocatalysis. J. Hazard. Catal. 2018, 8, 74. [Google Scholar] [CrossRef]
- Gupta, A.; Lakshmp, Y.N.; Manivannan, R.; Victoria, S.N. Visible range photocatalysts for solid phase photocatalytic degradation of polyethylene and polyvinyl chloride. J. Chil. Chem. Soc. 2017, 62, 3393–3398. [Google Scholar] [CrossRef]
- Cho, S.; Choi, W. Solid-phase photocatalytic degradation of PVC–TiO2 polymer composites. J. Photochem. Photobiol. A Chem. 2001, 143, 221–228. [Google Scholar] [CrossRef]
- Yang, C.; Gong, C.; Peng, T.; Deng, K.; Zan, L. High photocatalytic degradation activity of the polyvinyl chloride (PVC)-vitamin C (VC)-TiO2 nano-composite film. J. Hazard. Mater. 2010, 178, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Fa, W.; Gong, C.; Tian, L.; Peng, T.; Zan, L. Enhancement of photocatalytic degradation of poly(vinyl chloride) with perchlorinated iron (II) phthalocyanine modified nano-TiO2. J. Appl. Polym. Sci. 2011, 122, 1823–1828. [Google Scholar] [CrossRef]
- Yang, C.; Deng, K.; Peng, T.; Zan, L. Enhanced Solid-Phase Photocatalytic Degradation Activity of a Poly(vinyl chloride)-TiO2 Nanocomposite Film with Bismuth Oxyiodide. Chem. Eng. Technol. 2011, 34, 886–892. [Google Scholar] [CrossRef]
- Yang, C.; Tian, L.; Ye, L.; Peng, T. Enhancement of photocatalytic degradation activity of poly(vinyl chloride)-TiO2 nanocomposite film with polyoxometalate. J. Appl. Polym. Sci. 2010, 120, 2048–2053. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, T.; Zhang, D.; Shi, Z.; Shi, Z.; Zhang, X.; Li, C.; Wang, L.; Song, J.; Lin, Q. Enhanced photodegradability of PVC plastics film by codoping nano-graphite and TiO2. Polym. Degrad. Stab. 2020, 181, 109332. [Google Scholar] [CrossRef]
- Kaur, K.; Kaur, H. Visible light induced photocatalytic degradation of polyvinyl chloride from water using graphene oxide/nitrogen-doped TiO2 nanocomposite. J. Environ. Chem. Eng. 2025, 13, 115664. [Google Scholar] [CrossRef]
- She, X.; Wu, J.; Zhong, J.; Xu, H.; Yang, Y.; Vajta, R.; Lou, J.; Liu, Y.; Du, D.; Li, H.; et al. Oxygenated monolayer carbon nitride for excellent photocatalytic hydrogen evolution and external quantum efficiency. Nano Energy 2016, 27, 138–146. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, Y.; An, X.; Zhao, J.; Bao, Y.; Hou, L.A. Exfoliation method matters: The microstructure-dependent photoactivity of g-C3N4 nanosheets for water purification. J. Hazard. Mater. 2022, 424, 127424. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Sarngan, P.P.; Lakshmanan, A.; Sarkar, D. One-step synthesis of highly reactive g-C3N4. J. Mater. Sci. Mater. Electron. 2022, 33, 9116–9125. [Google Scholar] [CrossRef]
- Guo, Q.X.; Xie, Y.; Wang, X.J.; Lv, S.C.; Hou, T.; Liu, X.M. Characterization of well-crystallized graphitic carbon nitride nanocrystallites via a benzene-thermal route at low temperatures. Chem. Phys. Lett. 2003, 380, 84–87. [Google Scholar] [CrossRef]
- AEROXIDE® TIO2 P 25. Available online: https://www.coatino.com/products/PR_52043891 (accessed on 3 August 2025).
- Hu, Z.-Y.; Liu, T.; Yang, Y.-R.; An, A.K.; Liew, K.M.; Li, W.-W. Binder—Free immobilization of photocatalyst on membrane surface for efficient photocatalytic—H2O2 production and water decontamination. Nano-Micro Lett. 2025, 17, 301. [Google Scholar] [CrossRef]
- Akerdi, A.G.; Mohsenzadeh, M.; Mahmoudian, K.; Bahrami, S.H. A review on heterogeneous g—C3N4 for efficient treatment of contaminants: Fabrication, morphology control and environmental application. Inter. J. Environ. Sci. Techn. 2025, 22, 7317–7352. [Google Scholar] [CrossRef]
- The Japanese Electric Wire & Cable Makers’ Association (JCMA). Available online: https://kikakurui.com/c3/C3605-2002-01.html (accessed on 3 August 2025).
- Wang, Y.; Shen, S. Progress and prospects of non-metal doped graphitic carbon nitride for improved photocatalytic performances. Acta Phys. Chim. Sin. 2020, 36, 1905080. [Google Scholar] [CrossRef]

| Mass Concentration/% | ||||
|---|---|---|---|---|
| Surface of MCN/PVC Film | Surface of UCN/PVC Film | |||
| Element | Initial Film | After 40 Days | Initial Film | After 40 Days |
| C | 43.8 | 43.2 | 43.2 | 46.5 |
| N | 29.2 | 29.8 | 28.9 | 26.7 |
| Cl | 26.9 | 23.4 | 27.9 | 23.5 |
| O | 3.6 | 3.3 | ||
| Measurement Sample | Maximum Stress /MPa | Elastic Modulus/MPa | Elongation/%GL |
|---|---|---|---|
| UCN-containing PVC film | 40.3 | 1142.8 | 6.4 |
| PVC film (UCN-free) | 38.4 | 100.3 | 12.0 |
| Measurement Sample | Maximum Stress /MPa | Elastic Modulus/MPa | Elongation/%GL |
|---|---|---|---|
| UCN (1 wt.%)/PVC film | 1.1 | 0.4 | 595.6 |
| UCN (5 wt.%)/PVC film | 1.1 | 0.4 | 356.2 |
| PVC film (UCN-free) | 1.0 | 0.3 | 627.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horikoshi, S.; Hirota, K.; Serpone, N. Proposal for Self-Degrading Power Cables Incorporating Graphitic Carbon Nitride to Address Electronic Waste Challenges and Evaluation of Decomposition Efficiencies. Molecules 2025, 30, 3951. https://doi.org/10.3390/molecules30193951
Horikoshi S, Hirota K, Serpone N. Proposal for Self-Degrading Power Cables Incorporating Graphitic Carbon Nitride to Address Electronic Waste Challenges and Evaluation of Decomposition Efficiencies. Molecules. 2025; 30(19):3951. https://doi.org/10.3390/molecules30193951
Chicago/Turabian StyleHorikoshi, Satoshi, Kanon Hirota, and Nick Serpone. 2025. "Proposal for Self-Degrading Power Cables Incorporating Graphitic Carbon Nitride to Address Electronic Waste Challenges and Evaluation of Decomposition Efficiencies" Molecules 30, no. 19: 3951. https://doi.org/10.3390/molecules30193951
APA StyleHorikoshi, S., Hirota, K., & Serpone, N. (2025). Proposal for Self-Degrading Power Cables Incorporating Graphitic Carbon Nitride to Address Electronic Waste Challenges and Evaluation of Decomposition Efficiencies. Molecules, 30(19), 3951. https://doi.org/10.3390/molecules30193951







