Iridoids from Himatanthus sucuuba Modulate Feeding Behavior of Lutzomyia longipalpis: Integrated Experimental and Computational Approaches
Abstract
1. Introduction
2. Results
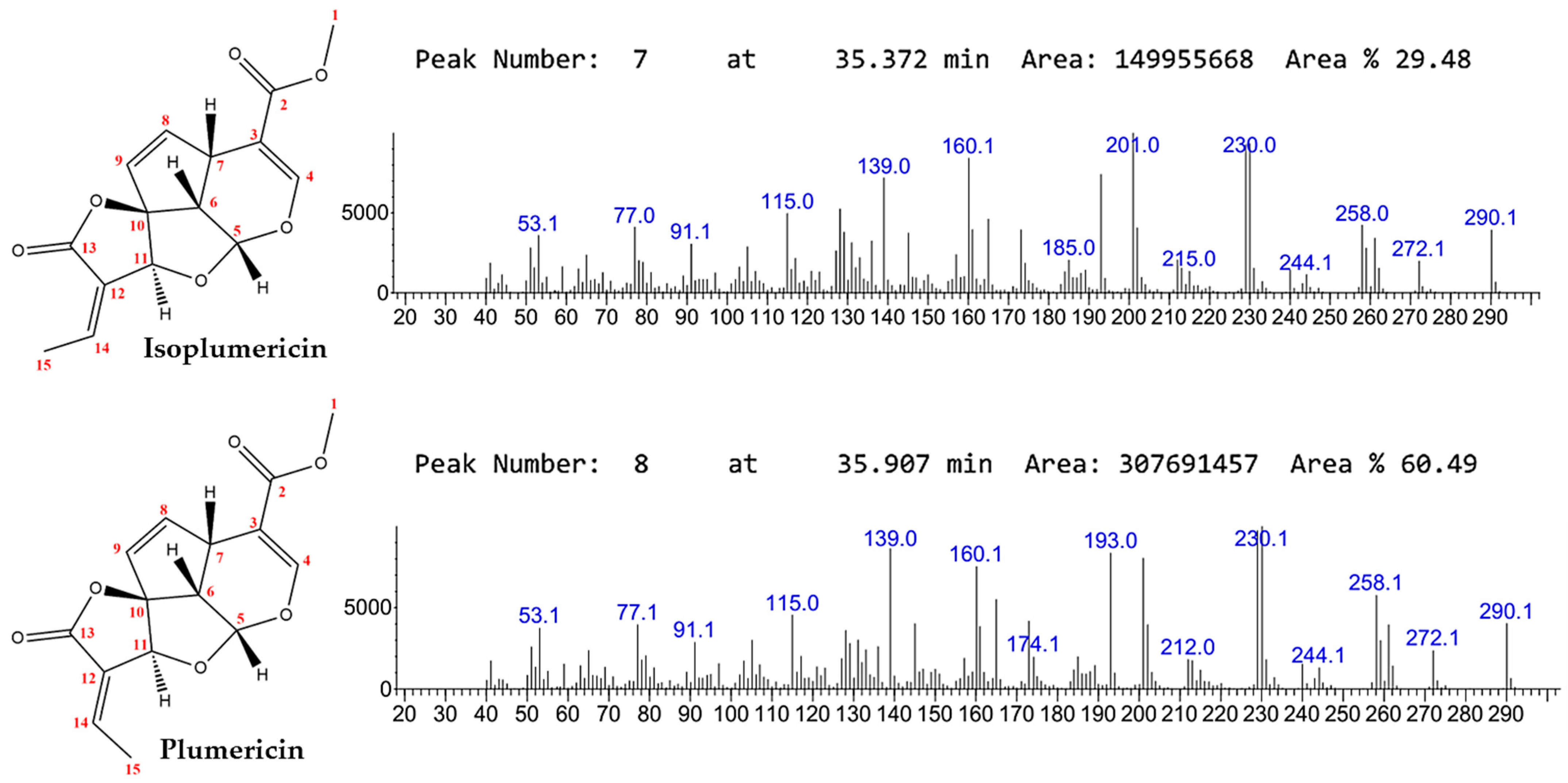
2.1. Preparation of Crude Extract and Isolation of Iridoids
2.2. Survival Studies
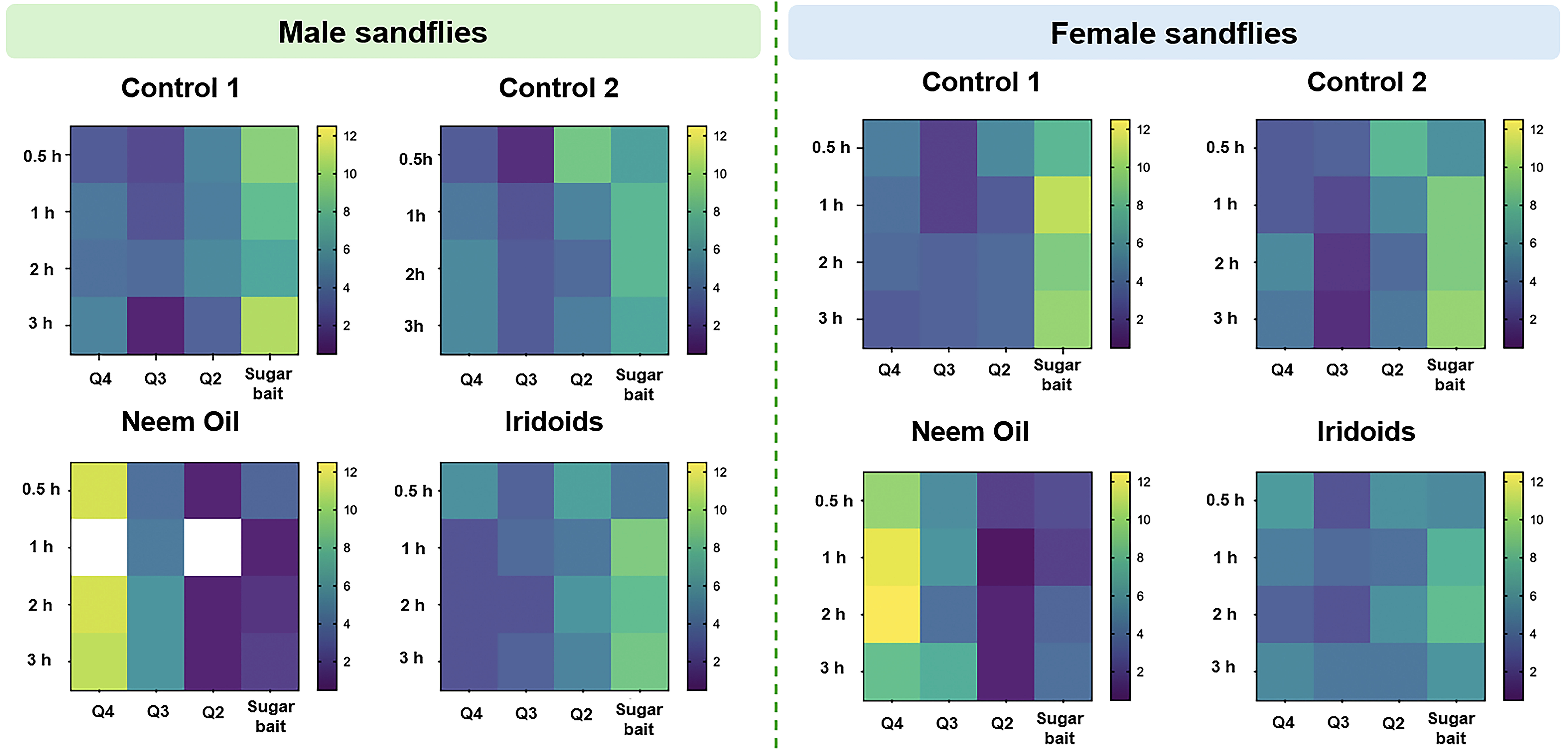
2.3. Repellency Studies
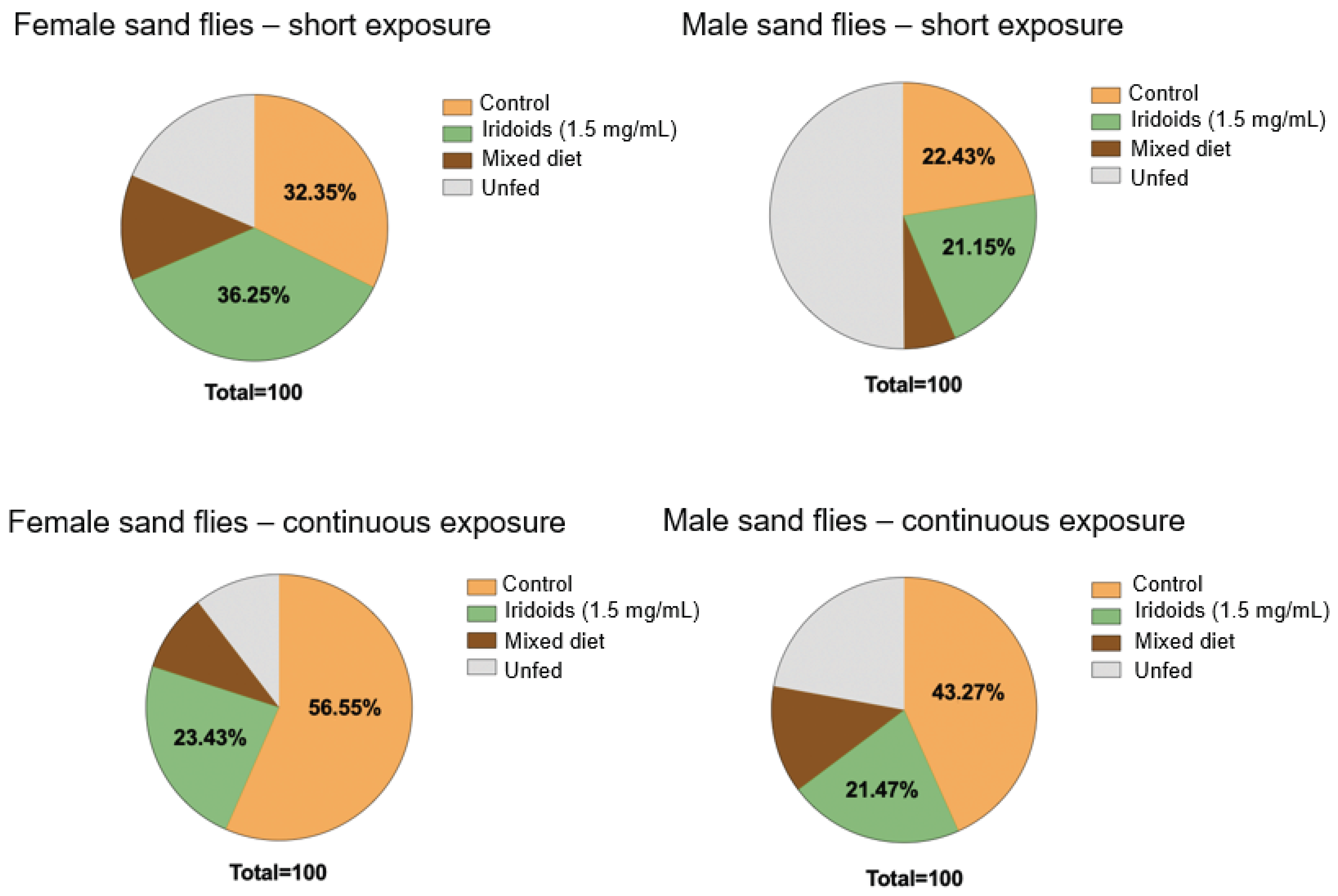
2.4. Diet Preference Detection
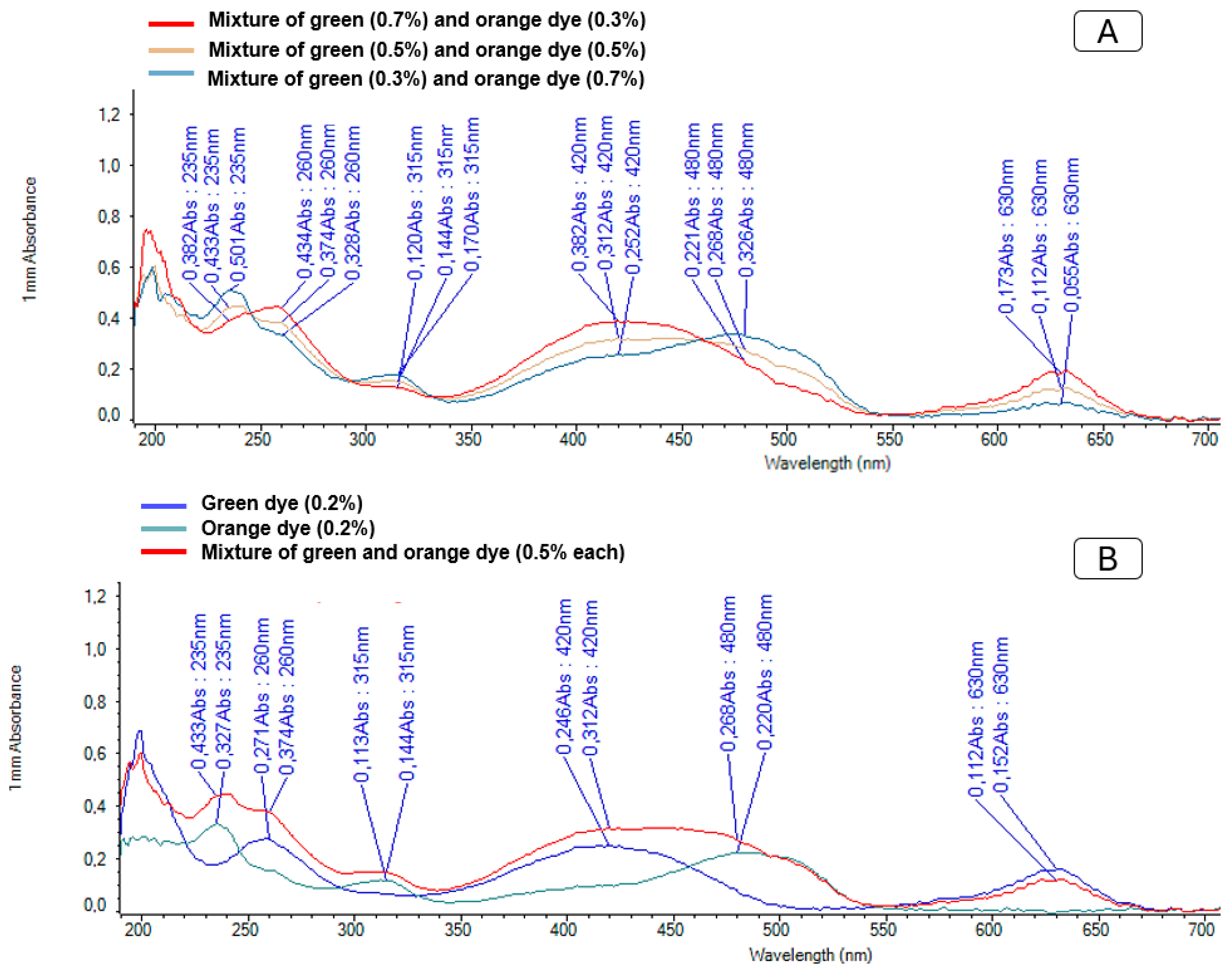
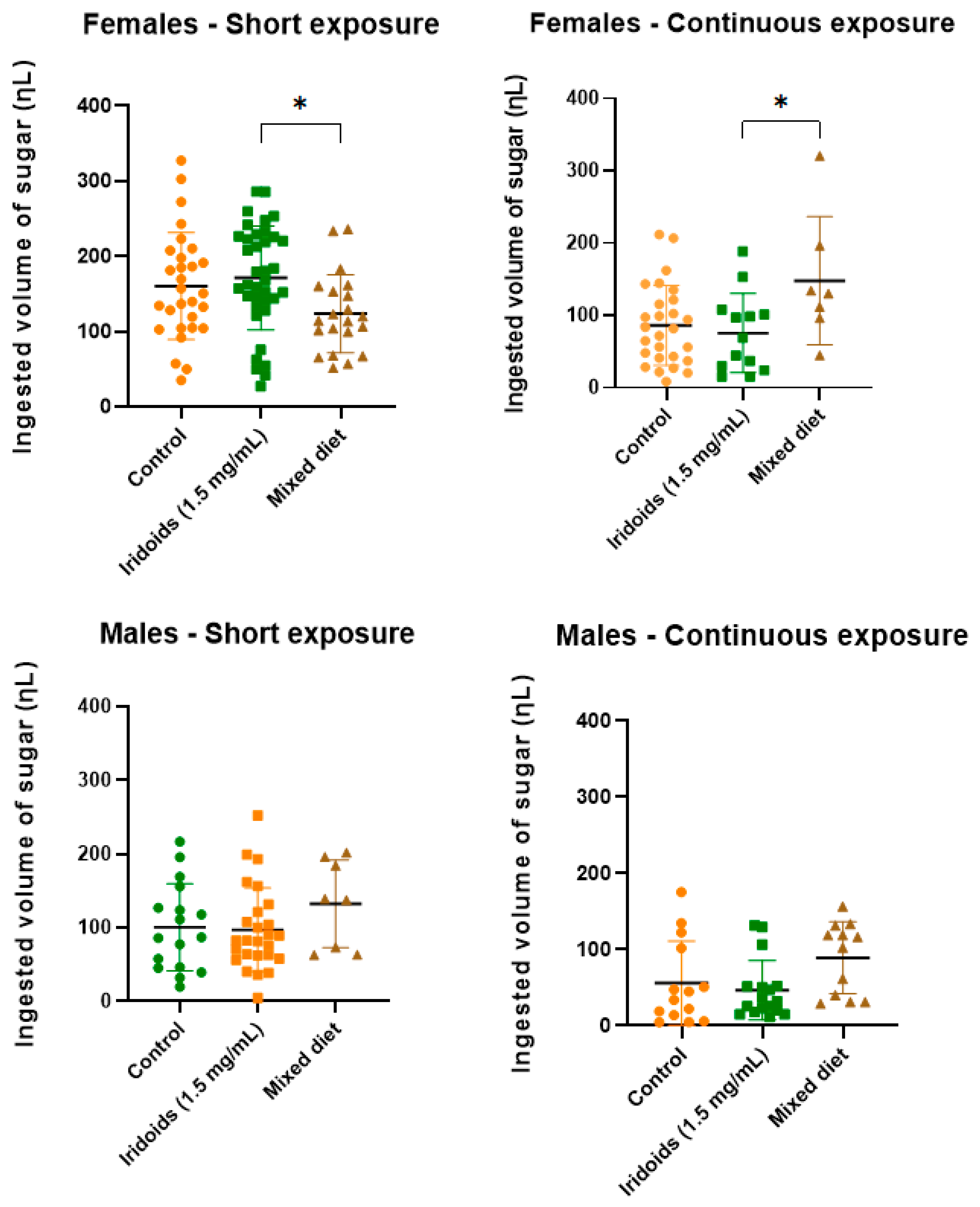
2.5. Quantification of Sugar Intake
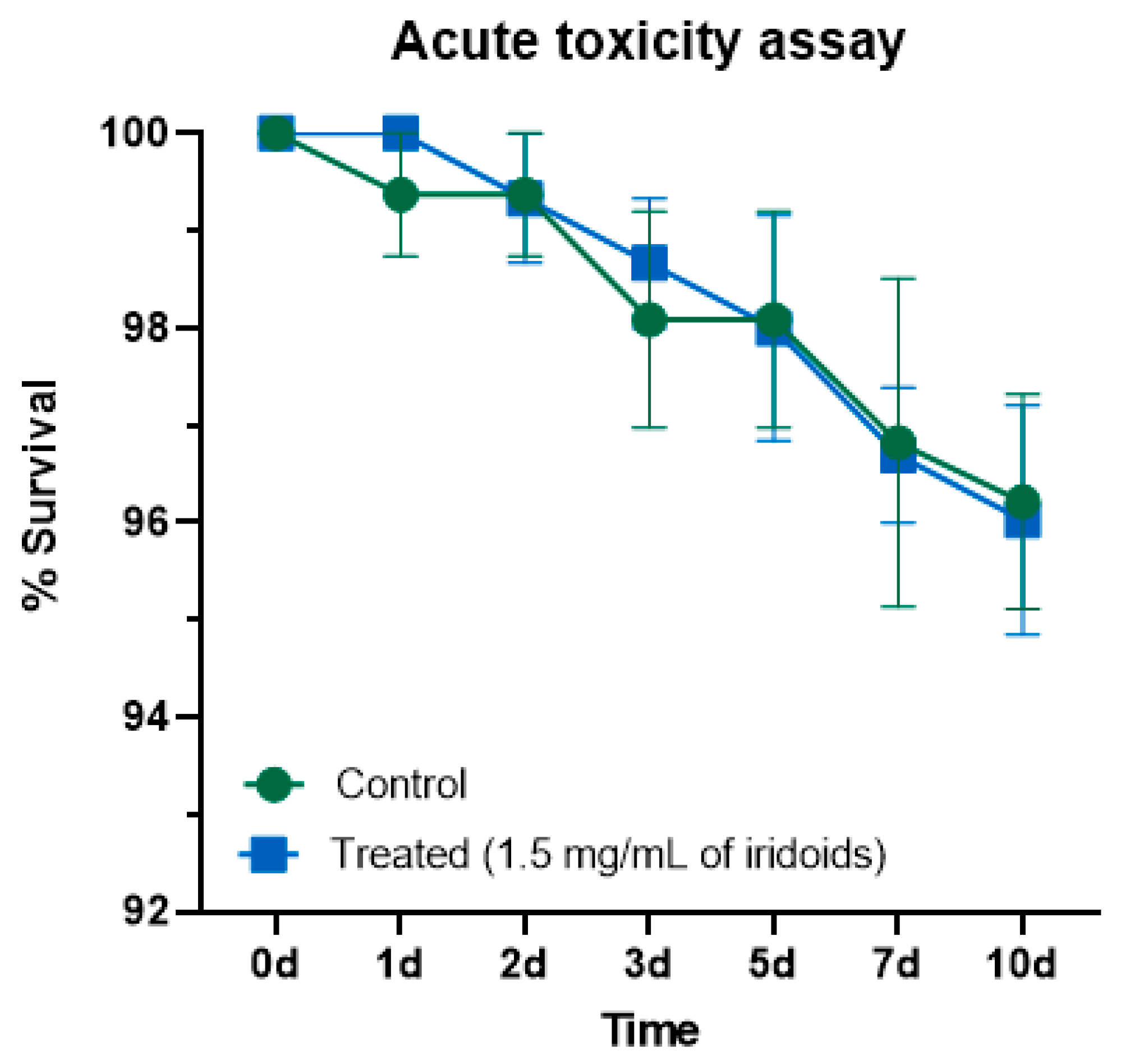
2.6. Acute Toxicity in Non-Target Organism: Drosophila melanogaster
2.7. Homology Modeling, Molecular Docking, and Molecular Dynamics Simulation
2.7.1. Homology Modeling and Quality Assessment
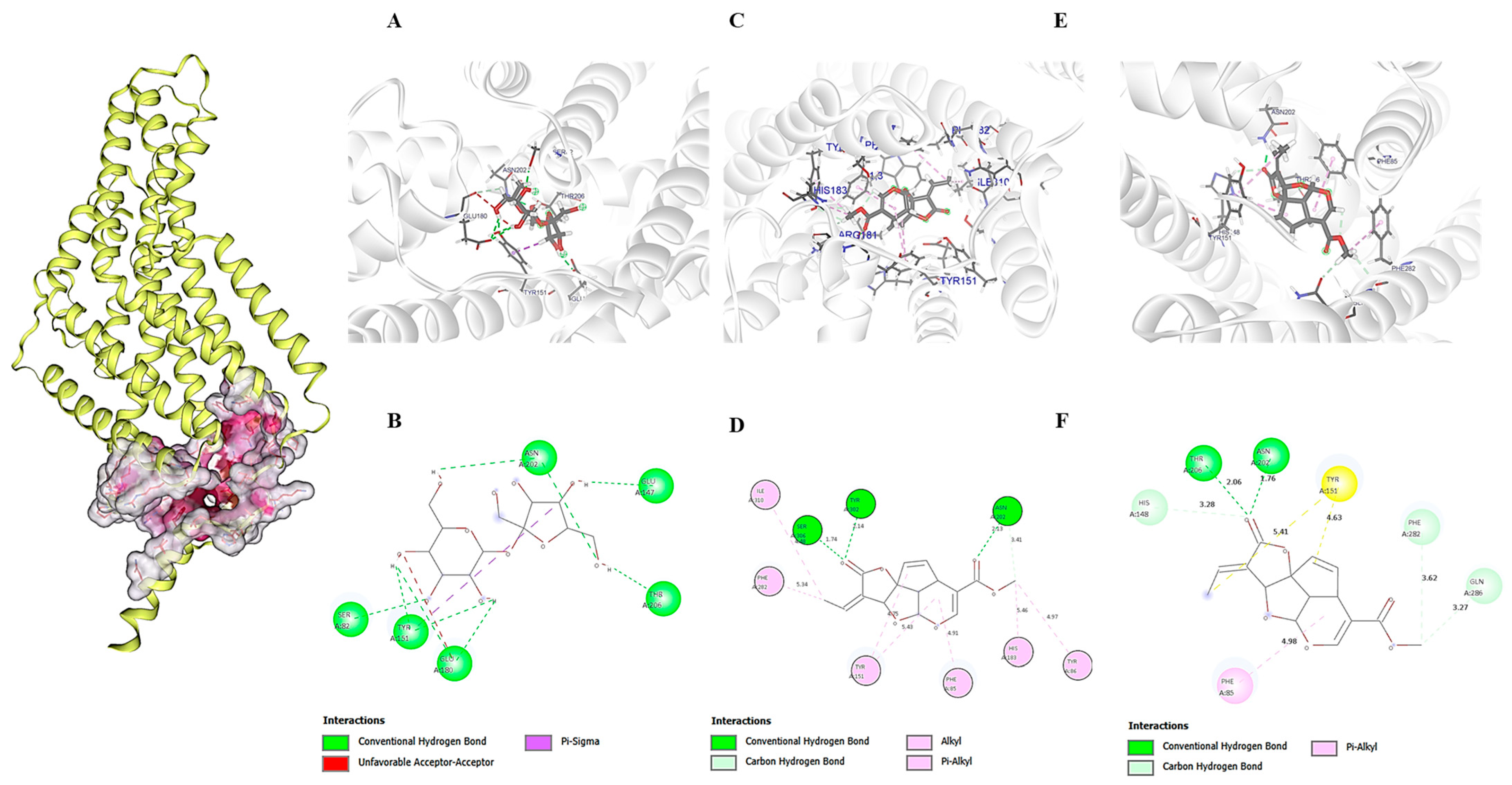
2.7.2. Molecular Docking—Gustatory Receptor A0A1B0CHD5
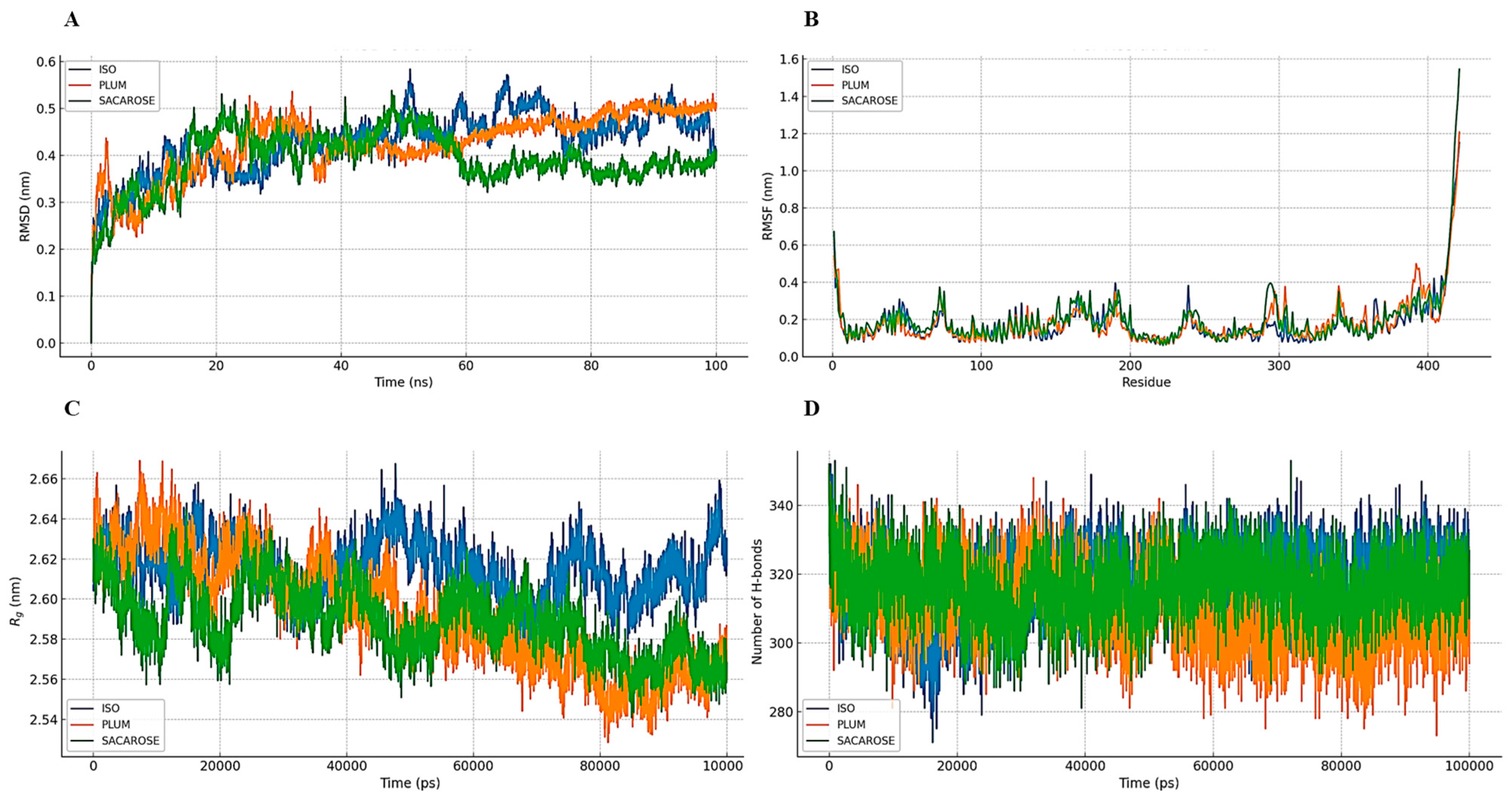
2.7.3. Simulation of the Molecular Dynamics of Ligand–GR Complexes
3. Discussion
3.1. Characterization and Biological Activity of Isolated Iridoids
3.2. Effects on Longevity and Feeding Behavior of L. longipalpis
3.3. Evaluation of the Repellent Potential of Iridoids
3.4. Diet Preference Studies: Temporal Exposure and Choice Behavior
3.5. Quantifying Sugar Intake: Dietary Patterns and Physiological Implications
3.6. Safety Assessment in Non-Target Organisms: Drosophila melanogaster Model
3.7. Homology Modeling, Molecular Docking, and Molecular Dynamics Simulation
4. Materials and Methods
4.1. Plant Material and Crude Extract Preparation
4.2. Fractionation of the Mixture Isoplumericin and Plumericin from the Dichloromethane Extract
4.3. Identification and Characterization of the Iridoids Isoplumericin and Plumericin
4.3.1. Gas Chromatography-Mass Spectrometry (GC-MS)
4.3.2. Nuclear Magnetic Resonance (NMR)
4.4. In Vivo Experiments
4.4.1. Insects
4.4.2. Lutzomyia longipalpis Longevity Curves
4.4.3. Repellency Tests on Sand Flies
4.4.4. Sugar Intake Experiments
- Short exposure (3 h)
- Continuous exposure (48 h)
4.4.5. Detection and Quantification of Sugars from Sugar Intake Experiments
4.4.6. Non-Target Organism Toxicity
4.5. In Silico Analysis
4.5.1. Sequence Retrieval and Homology Modeling
4.5.2. Model Quality Assessment
4.5.3. Selection and Construction of 3D Ligand Structures
4.5.4. Validation of Docking Protocol
4.5.5. Molecular Docking
4.5.6. Analysis of Intermolecular Interactions and Figure Construction
4.5.7. Molecular Dynamic Simulation
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CL | Cutaneous leishmaniasis |
| MCL | Mucocutaneous leishmaniasis |
| VL | Visceral leishmaniasis |
| DMSO | Dimethyl sulfoxide |
| GC-MS | Gas Chromatograph-Mass Spectrometer |
| GR | Gustatory Receptor |
| NMR | Nuclear Magnetic Resonance |
| RMSD | Root Mean Square Deviation |
| RMSF | Root Mean Square Fluctuation |
| SR | Sugar receptor |
Appendix A
References
- CDC. Centers for Disease Control and Prevention. CDC Yellow Book 2026. Available online: https://www.cdc.gov/yellow-book/hcp/travel-associated-infections-diseases/leishmaniasis.html#:~:text = Leishmaniasis%20is%20caused%20by%20obligate,form%20varies%20by%20Leishmania%20species (accessed on 8 August 2025).
- World Health Organization (WHO). Leishmaniasis. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 31 January 2025).
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef]
- Akhoundi, M.; Kuhls, K.; Cannet, A.; Votýpka, J.; Marty, P.; Delaunay, P.; Sereno, D. A Historical Overview of the Classification, Evolution, and Dispersion of Leishmania Parasites and Sand flies. PLoS Negl. Trop. Dis. 2016, 10, e0004349. [Google Scholar] [CrossRef]
- Maroli, M.; Feliciangeli, M.D.; Bichaud, L.; Charrel, R.N.; Gradoni, L. Phlebotomine Sand Flies and the Spreading of Leishmaniases. Med. Vet. Entomol. 2013, 27, 123–147. [Google Scholar] [CrossRef]
- Shimabukuro, P.H.F.; de Andrade, A.J.; Galati, E.A.B. Checklist of American Sand Flies (Diptera: Psychodidae). ZooKeys 2017, 660, 67–106. [Google Scholar] [CrossRef]
- Rego, F.D.; Soares, R.P. Lutzomyia longipalpis: An Update on This Sand Fly Vector. An. Acad. Bras. Ciênc. 2021, 93, e20200254. [Google Scholar] [CrossRef]
- Sousa-Paula, L.C.; Otranto, D.; Dantas-Torres, F. Lutzomyia longipalpis (Sand Fly). Trends Parasitol. 2020, 36, 796–797. [Google Scholar] [CrossRef]
- Salomón, O.D. Lutzomyia longipalpis, Gone with the Wind and Other Variables. Neotrop. Entomol. 2021, 50, 161–171. [Google Scholar] [CrossRef]
- Alexandre, J.; Sadlova, J.; Lestinova, T.; Vojtkova, B.; Jancarova, M.; Podesvova, L.; Volf, P. Experimental Infections and Co-Infections with Leishmania braziliensis and Leishmania infantum in Two Sand Fly Species, Lutzomyia migonei and Lutzomyia longipalpis. Sci. Rep. 2020, 10, 3566. [Google Scholar] [CrossRef]
- Carvalho-Silva, R.; Ribeiro-da-Silva, R.C.; Cruz, L.N.P.D.; Oliveira, M.D.S.; Amoedo, P.M.; Rebêlo, J.M.M.; Pinheiro, V.C.S. Predominance of Leishmania (Leishmania) amazonensis DNA in Lutzomyia longipalpis Sand Flies from Northeastern Brazil. Rev. Inst. Med. Trop. São Paulo 2022, 64, e32. [Google Scholar] [CrossRef]
- Cecílio, P.; Pires, A.C.A.; Valenzuela, J.G.; Pimenta, P.F.; Cordeiro-da-Silva, A.; Secundino, N.F.; Oliveira, F. Exploring Lutzomyia longipalpis Vector Competence for Leishmania major. J. Infect. Dis. 2020, 222, 1199–1203. [Google Scholar] [CrossRef]
- Rogers, M.E.; Chance, M.L.; Bates, P.A. Role of Promastigote Secretory Gel in the Transmission of Leishmania mexicana by Lutzomyia longipalpis. Parasitology 2002, 124, 495–507. [Google Scholar] [CrossRef]
- Volf, P.; Pecková, J. Sand Flies and Leishmania: Specific versus Permissive Vectors. Trends Parasitol. 2007, 23, 91–99. [Google Scholar] [CrossRef]
- Sunter, J.; Gull, K. Shape, Form, Function and Leishmania Pathogenicity. Open Biol. 2017, 7, 170165. [Google Scholar] [CrossRef]
- Foster, W.A. Mosquito Sugar Feeding and Reproductive Energetics. Annu. Rev. Entomol. 1995, 40, 443–474. [Google Scholar] [CrossRef]
- Qualls, W.A.; Müller, G.C.; Khallaayoune, K.; Revay, E.E.; Zhioua, E.; Kravchenko, V.D.; Beier, J.C. Control of Sand Flies with Attractive Toxic Sugar Baits and Potential Impact on Non-Target Organisms in Morocco. Parasites Vectors 2015, 8, 87. [Google Scholar] [CrossRef]
- Mascari, T.M.; Foil, L.D. Insecticide-Treated Sugar Baits for Control of Phlebotomine Sand Flies. J. Am. Mosq. Control Assoc. 2010, 26, 398–402. [Google Scholar] [CrossRef]
- Castro, D.P.; Genta, F.A.; Rogers, M.E. Exploiting Nectar and Blood Feeding Cues to Optimise Attractive Targeted Sugar Baits against a Sand Fly Vector of Leishmaniasis. bioRxiv 2025. [Google Scholar] [CrossRef]
- McDermott, E.G.; Morris, E.K.; Garver, L.S. Sodium Ascorbate as a Potential Toxicant in Attractive Sugar Baits for Mosquito and Sand Fly Control. J. Med. Entomol. 2019, 56, 1359–1367. [Google Scholar] [CrossRef]
- Müller, G.C.; Schlein, Y. Attractive Sugar Baits for the Control of Phlebotomus papatasi. J. Vector Ecol. 2011, 36, S64–S70. [Google Scholar] [CrossRef]
- Hopkins, M.J.G. Flora da Reserva Ducke, Amazonas, Brasil. Rodriguésia 2005, 56, 9–25. [Google Scholar] [CrossRef]
- Woodson, R.E. Studies in the Apocynaceae. VII. An Evaluation of the Genera Plumeria and Himatanthus. Ann. Mo. Bot. Gard. 1938, 25, 189–224. [Google Scholar] [CrossRef]
- Soares, F.P.; Cavalcante, L.F.; Romero, N.R.; Bandeira, M.A. Himatanthus (Apocynaceae): A Review. Pharmacogn. Rev. 2016, 10, 6–10. [Google Scholar] [CrossRef]
- Ferreira, C.D.S.; Piedade, M.T.F.; Bonates, L.C. Germinação de Sementes e Sobrevivência de Plântulas de Himatanthus sucuuba em Resposta ao Alagamento. Acta Amazon. 2006, 36, 413–418. [Google Scholar] [CrossRef]
- Tropicos.org. Missouri Botanical Garden. Available online: https://tropicos.org/name/1801500 (accessed on 18 August 2025).
- Amaral, A.C.F.; Silva, J.R.A.; Ferreira, J.L.P.; Pinheiro, M.L.B. Monograph of Himatanthus sucuuba, a Plant of Amazonian Folk Medicine. Pharmacogn. Rev. 2007, 1, 305–313. [Google Scholar]
- Soares, F.P.; Fraga, A.F.; Neves, J.P.O.; Romero, N.R.; Bandeira, M.A.M. Etnofarmacologia de Himatanthus drasticus (Janaguba). Rev. Bras. Plantas Med. 2015, 17, 900–908. [Google Scholar] [CrossRef]
- Miranda, A.L.P.; Silva, J.R.A.; Rezende, C.M.; Neves, J.S.; Parrini, S.C.; Pinheiro, M.L.; Pinto, A.C. Anti-inflammatory and analgesic activities of the latex containing triterpenes from Himatanthus sucuuba. Planta Med. 2000, 66, 284–286. [Google Scholar] [CrossRef]
- Castillo, D.; Arevalo, J.; Herrera, F.; Ruiz, C.; Rojas, R.; Rengifo, E.; Vaisberg, A.; Lock, O.; Lemesre, J.-L.; Gornitzka, H.; et al. Spirolactone Iridoids Might Be Responsible for the Antileishmanial Activity of a Peruvian Traditional Remedy Made with Himatanthus sucuuba. J. Ethnopharmacol. 2007, 112, 410–414. [Google Scholar] [CrossRef]
- Soares, D.C.; Andrade, A.L.; Delorenzi, J.C.; Silva, J.R.A.; Freire-de-Lima, L.; Falcão, C.A.; Saraiva, E.M. Leishmanicidal activity of Himatanthus sucuuba latex against Leishmania amazonensis. Parasitol. Int. 2010, 59, 173–177. [Google Scholar] [CrossRef]
- Silva, J.R.A.; Rezende, C.M.; Pinto, A.C.F.; Amaral, A.C.F. Cytotoxicity and antibacterial studies of iridoids and phenolic compounds isolated from the latex of Himatanthus sucuuba. Afr. J. Biotechnol. 2010, 9, 7357–7360. [Google Scholar]
- Filho, V.C.; Meyre-Silva, C.; Niero, R.; Bolda Mariano, L.N.; Gomes do Nascimento, F.; Vicente Farias, I.; Malheiros, A. Evaluation of Antileishmanial Activity of Selected Brazilian Plants and Identification of the Active Principles. Evid.-Based Complement. Alternat. Med. 2013, 2013, 265025. [Google Scholar]
- Sharma, U.; Singh, D.; Kumar, P.; Dobhal, M.P.; Singh, S. Antiparasitic Activity of Plumericin and Isoplumericin against Leishmania donovani. Indian J. Med. Res. 2011, 134, 709–716. [Google Scholar] [CrossRef]
- Ramos, A.S.; Silva, J.R.A.; Oliveira, A.A.; Mpalantinos, M.A.; Basso, S.L.; Ferreira, J.L.P.; Amaral, A.C.F. Fingerprint by Gas Chromatography-Mass Spectrometry of Two Himatanthus Species of Brazilian North Region. Chem. Nat. Compd. 2015, 51, 1149–1151. [Google Scholar] [CrossRef]
- Elsässer, B.; Krohn, K.; Nadeem Akhtar, M.; Flörke, U.; Kouam, S.F.; Kuigoua, M.G.; Ngadjui, B.T.; Abegaz, B.M.; Antus, S.; Kurtán, T. Revision of the Absolute Configuration of Plumericin and Isoplumericin from Plumeria rubra. Chem. Biodivers. 2005, 2, 799–808. [Google Scholar] [CrossRef]
- Silva, J.R.A.; Rezende, C.M.; Pinto, A.C.; Pinheiro, M.L.; Cordeiro, M.C.; Tamborini, E.; Bolzani, V.D.S. Ésteres triterpênicos de Himatanthus sucuuba (Spruce) Woodson. Quím. Nova 1998, 21, 702–704. [Google Scholar] [CrossRef]
- Sobolev, O.V.; Afonine, P.V.; Moriarty, N.W.; Hekkelman, M.L.; Joosten, R.P.; Perrakis, A.; Adams, P.D. A global Ramachandran score identifies protein structures with unlikely stereochemistry. Structure 2020, 28, 1249–1258. [Google Scholar] [CrossRef]
- Park, S.W.; Lee, B.H.; Song, S.H.; Kim, M.K. Revisiting the Ramachandran plot based on statistical analysis of static and dynamic characteristics of protein structures. J. Struct. Biol. 2023, 215, 107939. [Google Scholar] [CrossRef]
- Benkert, P.; Biasini, M.; Schwede, T. Toward the Estimation of the Absolute Quality of Individual Protein Structure Models. Bioinformatics 2011, 27, 343–350. [Google Scholar] [CrossRef]
- Kupchan, S.M.; Morris, R. Isolation and Structural Elucidation of Allamandin, an Antileukemic Iridoid Lactone from Allamanda cathartica. J. Org. Chem. 1974, 39, 2477–2482. [Google Scholar] [CrossRef]
- Ferreira, T.N.; Pita-Pereira, D.; Costa, S.G.; Brazil, R.P.; Moraes, C.S.; Díaz-Albiter, H.M.; Genta, F.A. Transmission Blocking Sugar Baits for the Control of Leishmania Development inside Sand Flies Using Environmentally Friendly β-Glycosides. Parasites Vectors 2018, 11, 661. [Google Scholar] [CrossRef]
- Abbasi, I.; Queiroz, A.T.L.; Kirstein, O.D.; Warburg, A. Plant-feeding phlebotomine sand flies, vectors of leishmaniasis, prefer Cannabis sativa. Proc. Natl. Acad. Sci. USA 2018, 115, 11790–11795. [Google Scholar] [CrossRef]
- Cameron, M.M.; Pessoa, F.A.C.; Vasconcelos, A.W.; Ward, R.D. Sugar Meal Sources for the Phlebotomine Sandfly Lutzomyia longipalpis in Ceará State, Brazil. Med. Vet. Entomol. 1995, 9, 263–272. [Google Scholar] [CrossRef]
- Amane, M.; Echchakery, M.; Dardouna, Z.; Hafidi, M.; Boussaâ, S. Repellent and Insecticidal Activities of Vegetal Material against Sand Fly Populations (Diptera: Psychodidae): Systematic Review and Meta-Analysis. Sci. Afr. 2023, 20, e01561. [Google Scholar] [CrossRef]
- Pugliese, M.; Gaglio, G.; Passantino, A.; Brianti, E.; Napoli, E. Natural Products against Sand Fly Vectors of Leishmaniosis: A Systematic Review. Vet. Sci. 2021, 8, 150. [Google Scholar] [CrossRef]
- Sharma, V.P.; Dhiman, R.C. Neem Oil as a Sand Fly Repellent. J. Am. Mosq. Control Assoc. 1993, 9, 364–366. [Google Scholar]
- Maciel, M.V.; Morais, S.M.; Bevilaqua, C.M.; Silva, R.A.; Barros, R.S.; Sousa, R.N.; Souza-Neto, M.A. In Vitro Insecticidal Activity of Neem Seed Oil against Lutzomyia longipalpis. Rev. Bras. Parasitol. Vet. 2010, 19, 7–11. [Google Scholar] [CrossRef]
- Ready, P.D. Biology of Phlebotomine Sand Flies as Vectors of Disease Agents. Annu. Rev. Entomol. 2013, 58, 227–250. [Google Scholar] [CrossRef]
- Uenoyama, R.; Miyazaki, T.; Adachi, M.; Nishikawa, T.; Hurst, J.L.; Miyazaki, M. Domestic Cat Damage to Plant Leaves Containing Iridoids Enhances Chemical Repellency to Pests. iScience 2022, 25, 104460. [Google Scholar] [CrossRef]
- Hao, H.; Sun, J.; Dai, J. Dose-dependent behavioral response of the mosquito Aedes albopictus to floral odorous compounds. J. Insect Sci. 2013, 13, 127. [Google Scholar] [CrossRef]
- Ferreira, T.N.; Brazil, R.P.; McDowell, M.A.; Cunha-Júnior, E.F.; Costa, P.R.R.; Netto, C.D.; Genta, F.A. Effects of Anti-Leishmania Compounds on the Behaviour of Lutzomyia longipalpis. Pest Manag. Sci. 2022, 78, 2792–2805. [Google Scholar] [CrossRef]
- Gaglio, G.; Nali, E.; Arfuso, F.; Abbate, J.M.; Giannetto, S.; Brianti, E. Do Different LED Colours Influence Sand Fly Collection by Light Trap in the Mediterranean? Biomed Res. Int. 2018, 2018, 7569483. [Google Scholar] [CrossRef]
- Mellor, H.E.; Hamilton, J.G.C.; Anderson, M. Spectral Sensitivity in the Eyes of Male and Female Lutzomyia longipalpis. Med. Vet. Entomol. 1996, 10, 371–374. [Google Scholar] [CrossRef]
- Muñoz, I.J.; Schilman, P.E.; Barrozo, R.B. Impact of Alkaloids on Food Consumption, Metabolism and Survival in a Blood-Sucking Insect. Sci. Rep. 2020, 10, 9443. [Google Scholar] [CrossRef]
- Prokopy, R.J.; Roitberg, B.D. Joining and avoidance behavior in nonsocial insects. Entomol. Exp. App. 2001, 100, 1–13. [Google Scholar]
- Tang, Y.; Ward, R.D. Sugar Feeding and Fluid Destination Control in Lutzomyia longipalpis. Med. Vet. Entomol. 1998, 12, 13–19. [Google Scholar] [CrossRef]
- Demir, E. Drosophila as a Model for Assessing Nanopesticide Toxicity. Nanotoxicology 2020, 14, 1271–1279. [Google Scholar] [CrossRef]
- Alaraby, M.; Annangi, B.; Marcos, R.; Hernández, A. Drosophila melanogaster as a Suitable In Vivo Model to Determine Potential Side Effects of Nanomaterials: A Review. J. Toxicol. Environ. Health Part B 2016, 19, 65–104. [Google Scholar] [CrossRef] [PubMed]
- Holsopple, J.M.; Smoot, S.R.; Popodi, E.M.; Colburne, J.K.; Shaw, J.R.; Oliver, B.; Tennessen, J.M. Assessment of Chemical Toxicity in Adult Drosophila melanogaster. J. Vis. Exp. 2023, 193, e62713. [Google Scholar] [CrossRef] [PubMed]
- Giansanti, M.G.; Frappaolo, A.; Piergentili, R. Drosophila melanogaster: How and Why It Became a Model Organism. Int. J. Mol. Sci. 2025, 26, 7485. [Google Scholar] [CrossRef]
- Haller, S.; Meissle, M.; Romeis, J. Establishing Drosophila melanogaster to Assess the Non-Target Effects of Gut-Active Insecticidal Compounds. Ecotoxicology 2016, 25, 1794–1804. [Google Scholar] [CrossRef]
- Tolwinski, N.S. Introduction: Drosophila—A Model System for Developmental Biology. J. Dev. Biol. 2017, 5, 9. [Google Scholar] [CrossRef]
- Khallaayoune, K.; Qualls, W.A.; Revay, E.E.; Allan, S.A.; Arheart, K.L.; Kravchenko, V.D.; Müller, G.C. Attractive Toxic Sugar Baits: Control of Mosquitoes with Dinotefuran and Potential Impacts on Non-Target Organisms in Morocco. Environ. Entomol. 2013, 42, 1040–1045. [Google Scholar] [CrossRef]
- Gomes, J.V.; Patturayil, N.; Thapliyal, S.; Amiri, S.M.; Galan, J.J.; Brudner, J.; Galan, J.J. Structural Basis of Fructose Binding and Activation in a Ligand-Gated Ion Channel. Nature 2024, 629, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Ruedenauer, F.A.; Parreño, M.A.; Kadow, I.C.G.; Spaethe, J.; Leonhardt, S.D. The ecology of nutrient sensation and perception in insects. Trends Ecol. Evol. 2023, 38, 994–1004. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, R.; Li, L.; Wang, B.; Gao, Z.; Liu, F.; Chen, Y.; Tian, Y.; Li, B.; Chen, Q. Structure Basis for Sugar Specificity of Gustatory Receptors in Insects. Cell Discov. 2024, 10, 716. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Schwede, T. SWISS-MODEL: Homology Modelling of Protein Structures and Complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed]
- Dweck, H.K.M.; Carlson, J.R. Molecular Logic and Evolution of Bitter Taste in Drosophila. Curr. Biol. 2020, 30, 17–30. [Google Scholar] [CrossRef]
- Sargsyan, K.; Grauffel, C.; Lim, C. How Molecular Size Impacts RMSD Applications in Molecular Dynamics Simulations. J. Chem. Theory Comput. 2017, 13, 1518–1524. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Fox, J.D.; Robyt, J.F. Miniaturization of Three Carbohydrate Analyses Using a Microsample Plate Reader. Anal. Biochem. 1991, 195, 93–96. [Google Scholar] [CrossRef]
- Biasini, M.; Bienert, S.; Waterhouse, A.; Bertoni, M.; Bordoli, L.; Studer, G.; Tiwari, S.; Schwede, T. SWISS-MODEL: Modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014, 42, W252–W258. [Google Scholar] [CrossRef]
- Zhang, Y. I-TASSER server for protein 3D structure prediction. BMC Bioinform. 2008, 9, 40. [Google Scholar] [CrossRef]
- Powell, H.R.; Islam, S.A.; David, A.; Sternberg, M.J.E. Phyre2.2: A Community Resource for Template-based Protein Structure Prediction. J. Mol. Biol. 2025, 437, 168960. [Google Scholar] [CrossRef]
- Laskowski, R.A.; MacArthur, M.W.; Moss, D.S.; Thornton, J.M. PROCHECK—a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 1993, 26, 283–291. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Y.; Gan, J.; Xiao, Z.X.; Cao, Y. FitDock: Protein-ligand docking by template fitting. Brief. Bioinform. 2022, 23, bbac087. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, X.; Gan, J.; Chen, S.; Xiao, Z.-X.; Cao, Y. CB-Dock2: Improved Protein-Ligand Blind Docking by Integrating Cavity Detection, Docking and Homologous Template Fitting. Nucleic Acids Res. 2022, 50, W159–W164. [Google Scholar] [CrossRef]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef]

| Comparative | % Amino Acids in Ramachandran Plot Regions (PROCHECK) | PROSA | |||||
|---|---|---|---|---|---|---|---|
| Favorable | Allowed | Gen. allowed | Nonallowed | Qmean6 | Z-QMEAN | Z-score | |
| SWISS-MODEL | 95.4 | 4.6 | 0.0 | 0.0 | 0.74 | −1.66 | −5.8 |
| I-TASSER | 80.3 | 11.6 | 6.3 | 1.8 | 0.42 | −12.61 | −4.09 |
| Phyre2.2 | 94.9 | 4.9 | 0.3 | 0.0 | 0.73 | −3.00 | −5.91 |
| Ligand | ChemLP Score | Key Binding Residues | Redocking RMSD (Å) |
|---|---|---|---|
| Isoplumericin | 47.75 | Phe85, His183, Tyr86, Tyr151, Ile310, Ser306, Tyr302, Phe282, Asn202 | 0.132 |
| Plumericin | 49.08 | His148, Phe85, Thr206, Asn202, Tyr151, Phe282, Gln286 | 0.051 |
| Sucrose | 57.96 | Asn202, Glu147, Thr206, Ser82, Tyr151, Glu180 | 0.627 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, M.M.H.; da Cruz, J.D.; Silva, M.A.M.; Costa-Latgé, S.G.; Gomes, B.; Genta, F.A.; Silva, J.R.A.; Amaral, A.C.F. Iridoids from Himatanthus sucuuba Modulate Feeding Behavior of Lutzomyia longipalpis: Integrated Experimental and Computational Approaches. Molecules 2025, 30, 3937. https://doi.org/10.3390/molecules30193937
Almeida MMH, da Cruz JD, Silva MAM, Costa-Latgé SG, Gomes B, Genta FA, Silva JRA, Amaral ACF. Iridoids from Himatanthus sucuuba Modulate Feeding Behavior of Lutzomyia longipalpis: Integrated Experimental and Computational Approaches. Molecules. 2025; 30(19):3937. https://doi.org/10.3390/molecules30193937
Chicago/Turabian StyleAlmeida, Maíra M. H., Jefferson D. da Cruz, Maria Athana M. Silva, Samara G. Costa-Latgé, Bruno Gomes, Fernando A. Genta, Jefferson R. A. Silva, and Ana Claudia F. Amaral. 2025. "Iridoids from Himatanthus sucuuba Modulate Feeding Behavior of Lutzomyia longipalpis: Integrated Experimental and Computational Approaches" Molecules 30, no. 19: 3937. https://doi.org/10.3390/molecules30193937
APA StyleAlmeida, M. M. H., da Cruz, J. D., Silva, M. A. M., Costa-Latgé, S. G., Gomes, B., Genta, F. A., Silva, J. R. A., & Amaral, A. C. F. (2025). Iridoids from Himatanthus sucuuba Modulate Feeding Behavior of Lutzomyia longipalpis: Integrated Experimental and Computational Approaches. Molecules, 30(19), 3937. https://doi.org/10.3390/molecules30193937







