Theoretical Study of the Influence of K20N Glycosylation on the Dynamic Behavior of Im7 Protein
Abstract
1. Introduction
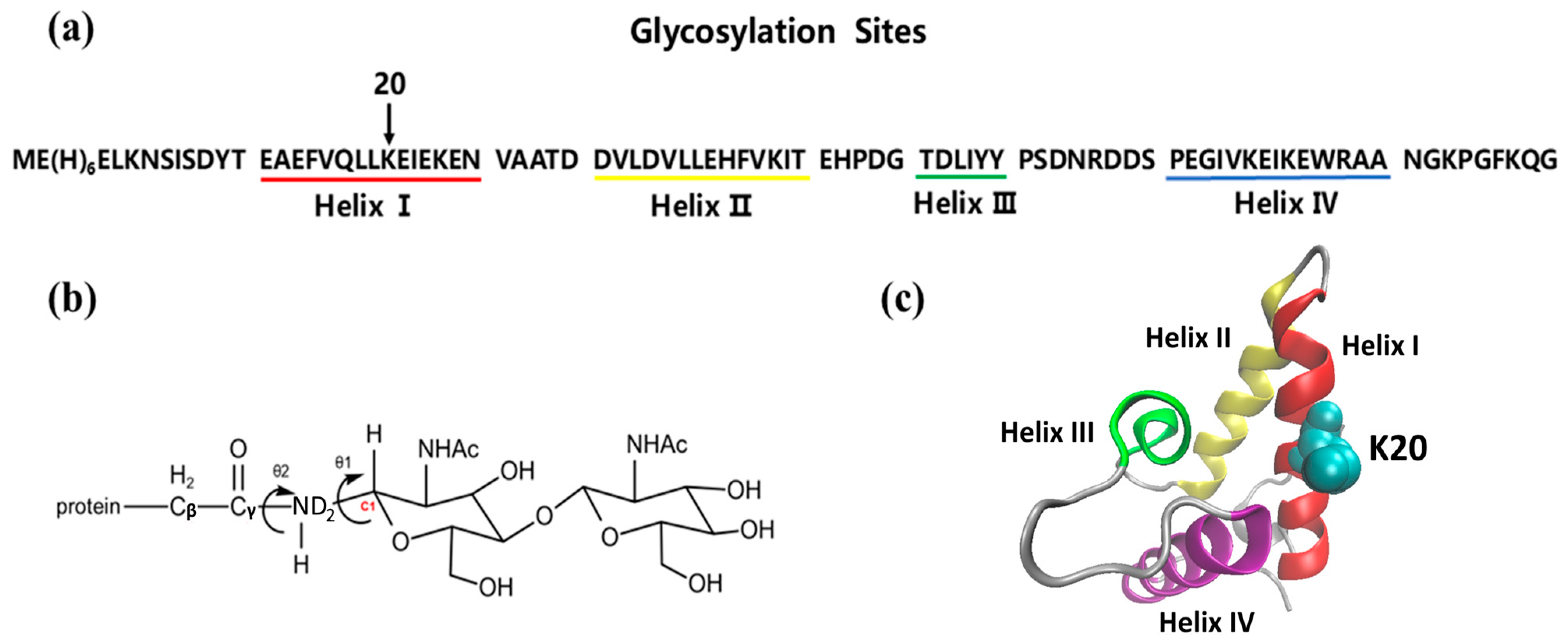
2. Simulation Details
2.1. Carbohydrate Building
2.2. Glycoprotein Construction
2.3. Urea Solvent Box Preparation
2.4. Simulation Protocol
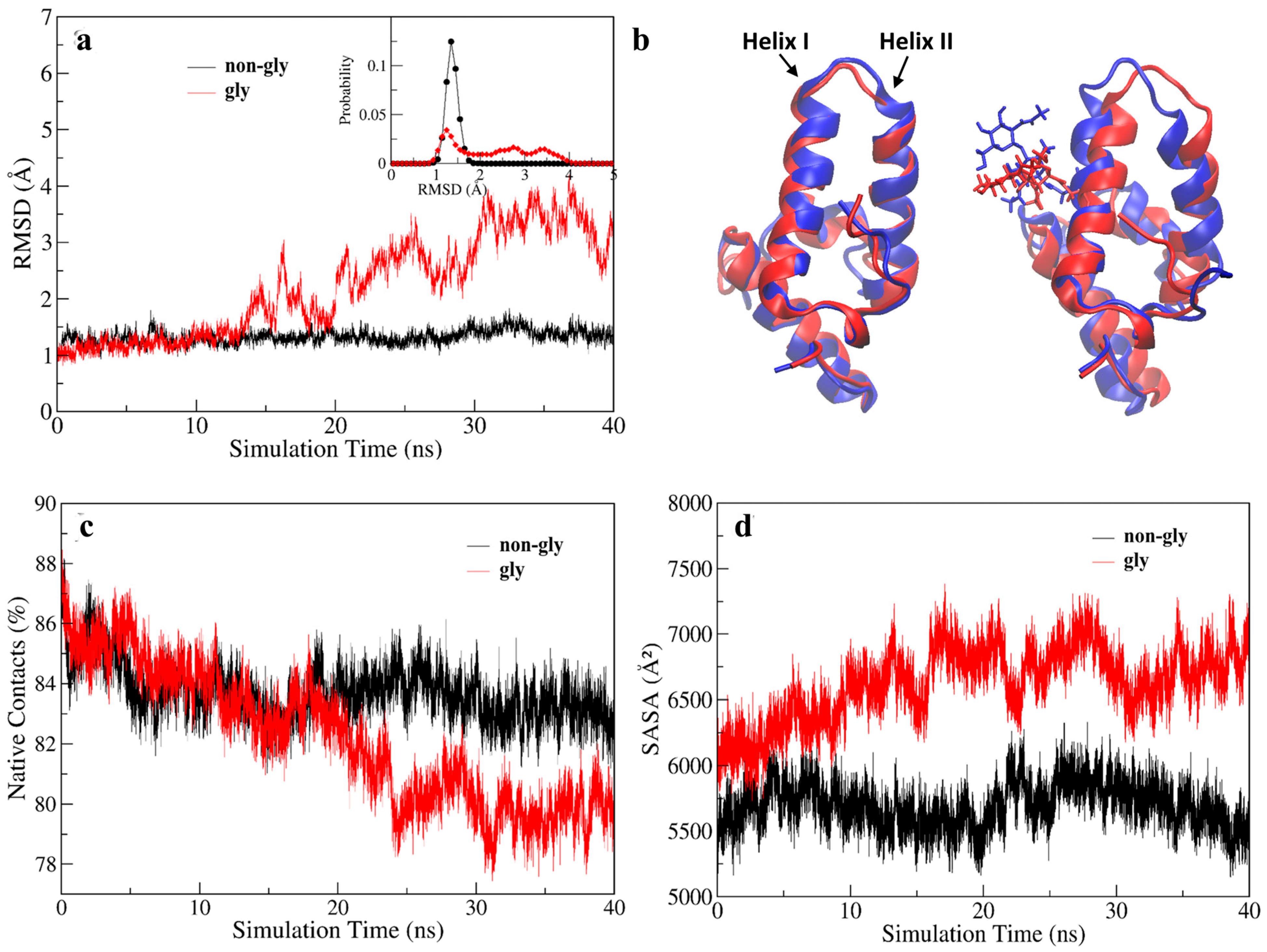
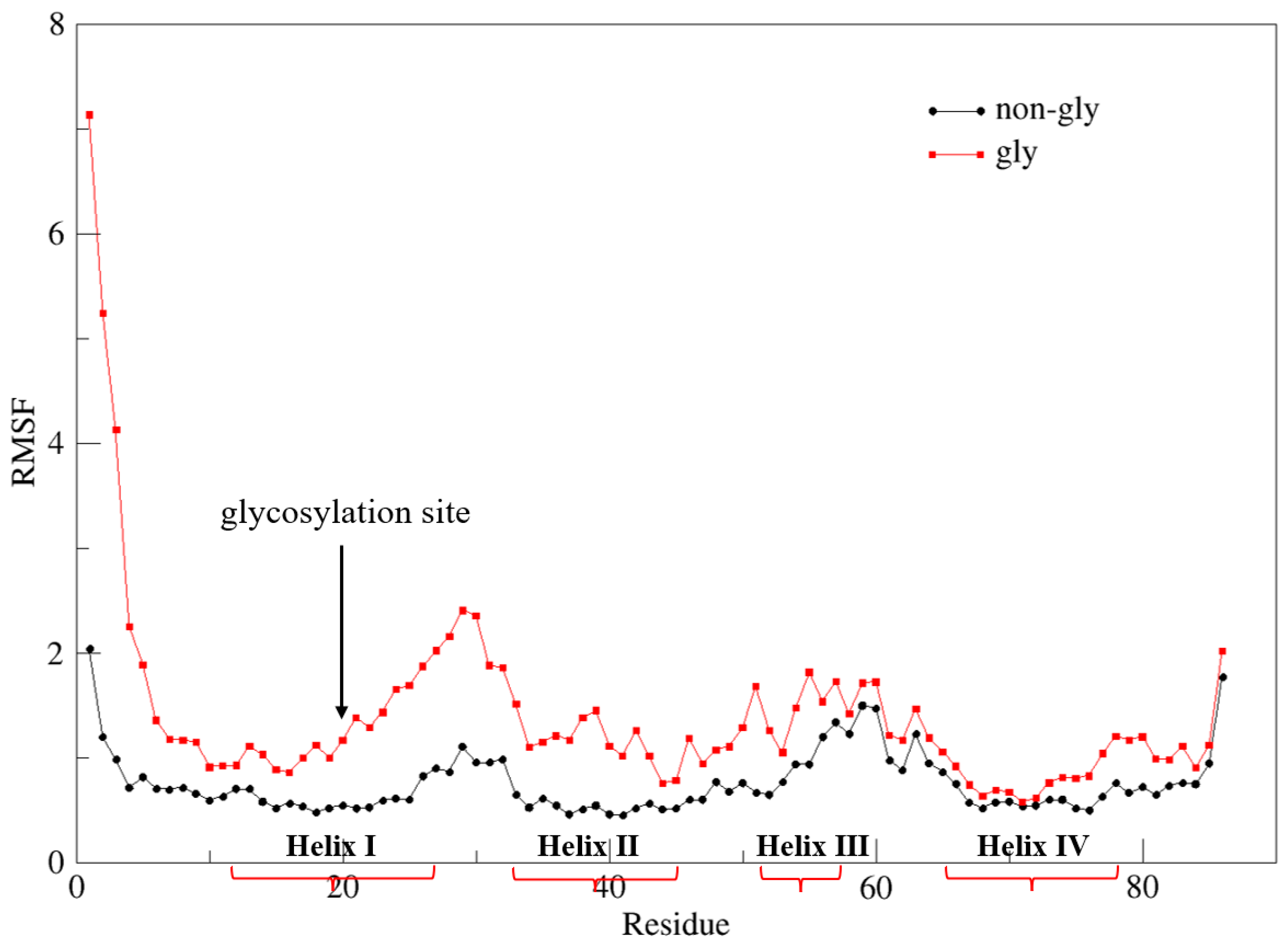
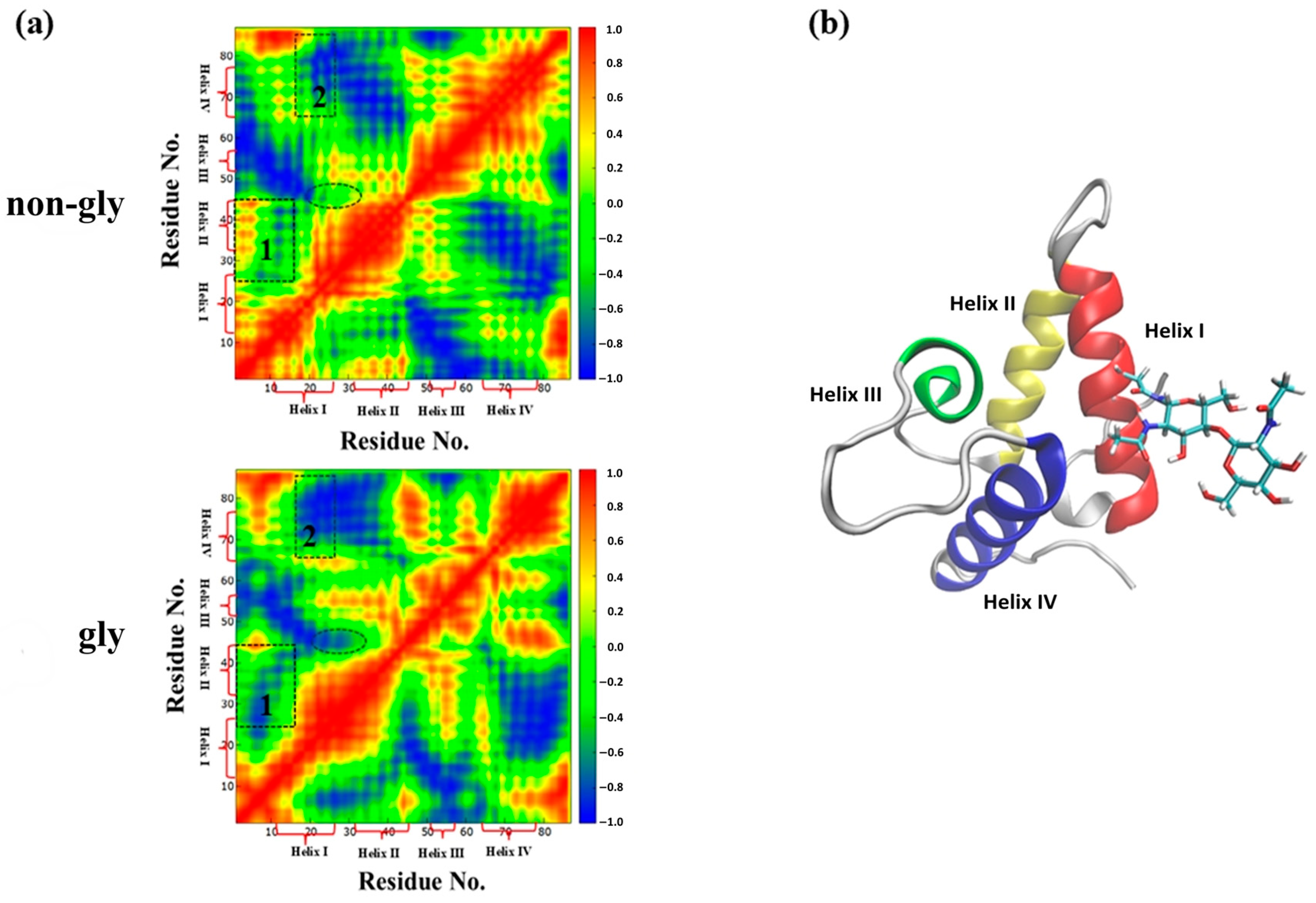
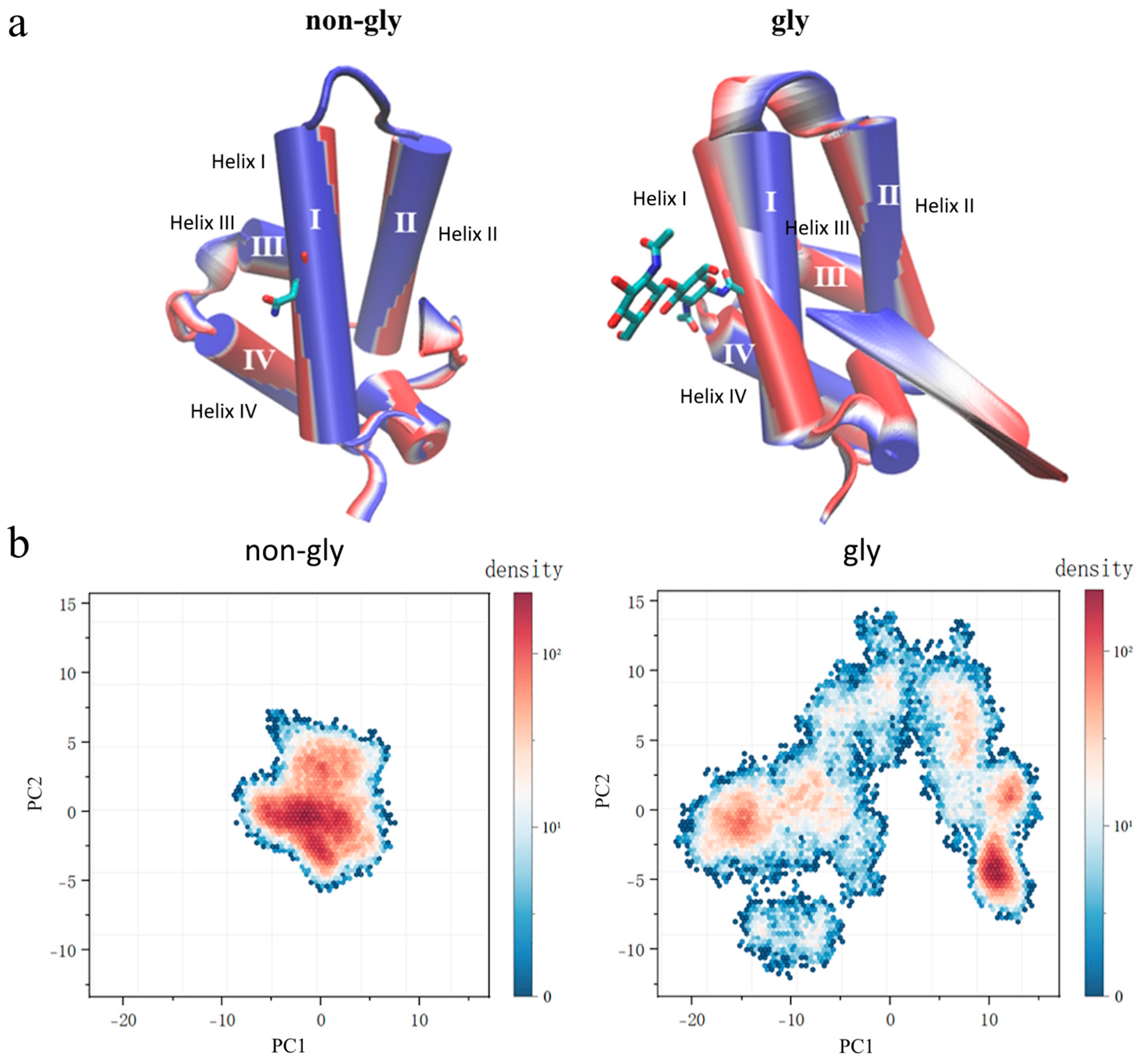
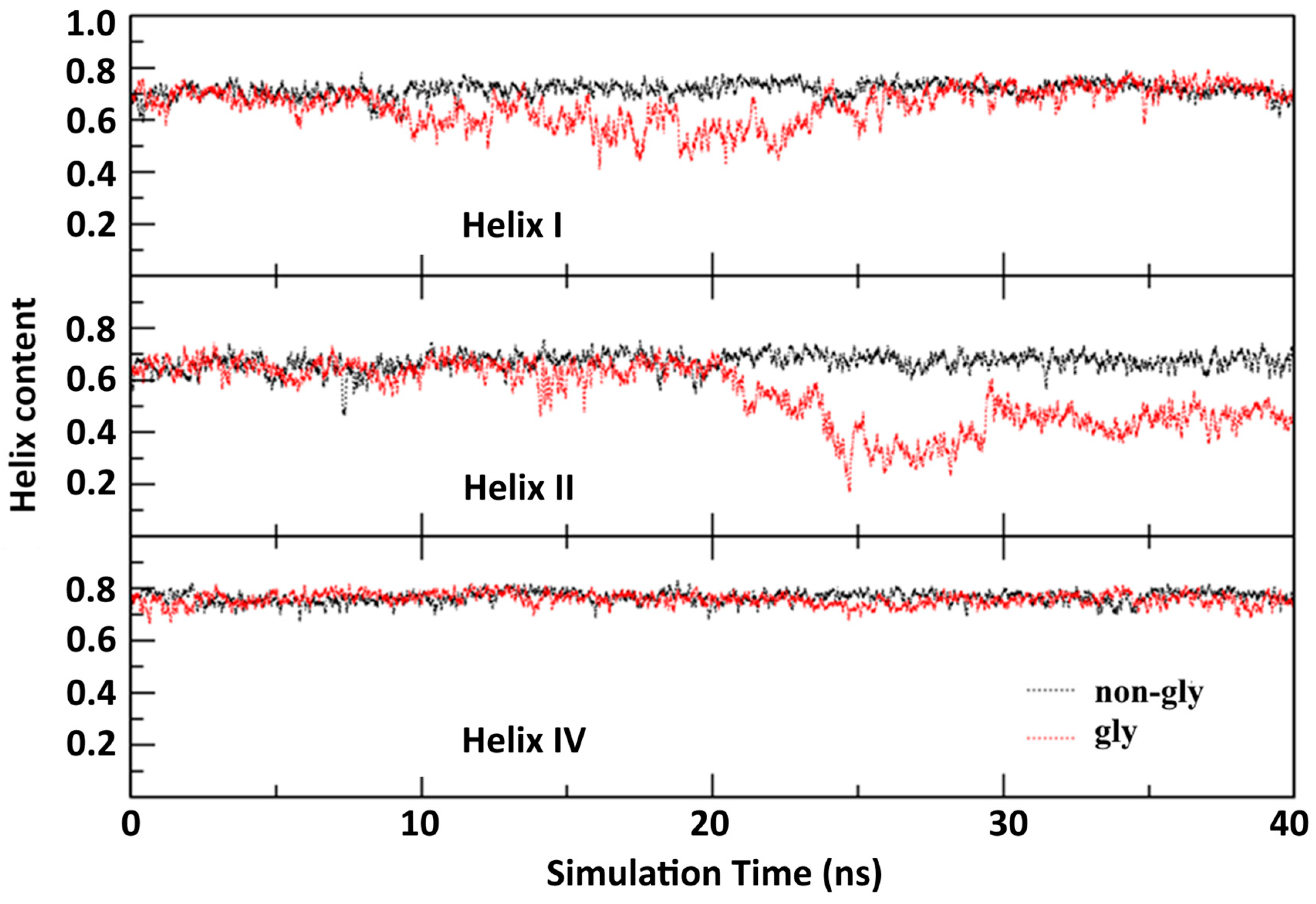
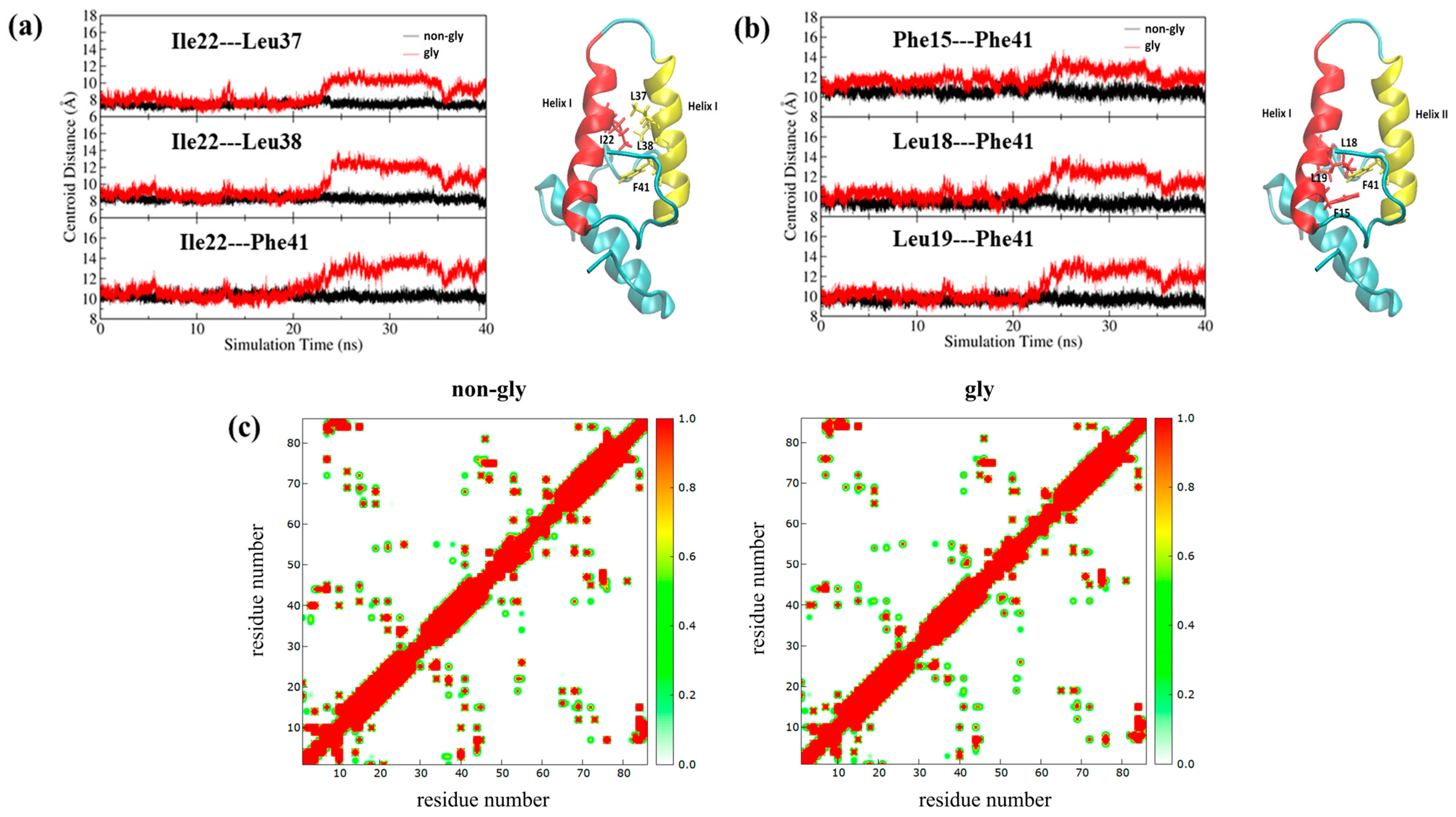
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scheper, A.F.; Schofield, J.; Bohara, R.; Ritter, T.; Pandit, A. Understanding glycosylation: Regulation through the metabolic flux of precursor pathways. Biotechnol. Adv. 2023, 67, 108184. [Google Scholar] [CrossRef]
- Freeze, H.H.; Chong, J.X.; Bamshad, M.J.; Ng, B.G. Solving glycosylation disorders: Fundamental approaches reveal complicated pathways. Am. J. Hum. Genet. 2014, 94, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Altmann, F. The role of protein glycosylation in allergy. Int. Arch. Allergy Immunol. 2007, 142, 99–115. [Google Scholar] [CrossRef]
- Rodrigues, J.G.; Balmaña, M.; Macedo, J.A.; Poças, J.; Fernandes, Â.; De-Freitas-Junior, J.C.M.; Pinho, S.S.; Gomes, J.; Magalhães, A.; Gomes, C.; et al. Glycosylation in cancer: Selected roles in tumour progression, immune modulation and metastasis. Cell. Immunol. 2018, 333, 46–57. [Google Scholar] [CrossRef]
- Veillon, L.; Fakih, C.; Abou-El-Hassan, H.; Kobeissy, F.; Mechref, Y. Glycosylation changes in brain cancer. ACS Chem. Neurosci. 2018, 9, 51–72. [Google Scholar] [CrossRef]
- Ondruskova, N.; Cechova, A.; Hansikova, H.; Honzik, T.; Jaeken, J. Congenital disorders of glycosylation: Still “hot” in 2020. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129751. [Google Scholar] [CrossRef]
- Bause, E. Structural requirements of N-glycosylation of proteins. Studies with proline peptides as conformational probes. Biochem. J. 1983, 209, 331–336. [Google Scholar] [CrossRef]
- Van den Steen, P.; Rudd, P.M.; Dwek, R.A.; Opdenakker, G. Concepts and principles of O-linked glycosylation. Crit. Rev. Biochem. Mol. Biol. 1998, 33, 151–208. [Google Scholar] [CrossRef]
- Mohanty, S.; Chaudhary, B.P.; Zoetewey, D. Structural insight into the mechanism of N-linked glycosylation by oligosaccharyl transferase. Biomolecules 2020, 10, 624. [Google Scholar] [CrossRef] [PubMed]
- Bause, E.; Legler, G. The role of the hydroxy amino acid in the triplet sequence Asn-Xaa-Thr (Ser) for the N-glycosylation step during glycoprotein biosynthesis. Biochem. J. 1981, 195, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ma, B.; Wang, Y.; Cui, Q.; Yao, L.; Li, Y.; Chen, B.; Feng, Y.; Tan, Z. Structural insight into why S-linked glycosylation cannot adequately mimic the role of natural O-glycosylation. Int. J. Biol. Macromol. 2023, 253, 126649. [Google Scholar] [CrossRef]
- Dror, R.O.; Dirks, R.M.; Grossman, J.; Xu, H.; Shaw, D.E. Biomolecular simulation: A computational microscope for molecular biology. Annu. Rev. Biophys. 2012, 41, 429–452. [Google Scholar] [CrossRef]
- Lilkova, E.; Petkov, P.; Ilieva, N.; Krachmarova, E.; Nacheva, G.; Litov, L. Molecular modeling of the effects of glycosylation on the structure and dynamics of human interferon-gamma. J. Mol. Model. 2019, 25, 127. [Google Scholar] [CrossRef] [PubMed]
- Weiß, R.G.; Losfeld, M.-E.; Aebi, M.; Riniker, S. N-glycosylation enhances conformational flexibility of protein disulfide isomerase revealed by microsecond molecular dynamics and Markov state modeling. J. Phys. Chem. B 2021, 125, 9467–9479. [Google Scholar] [CrossRef] [PubMed]
- Friel, C.T.; Smith, D.A.; Vendruscolo, M.; Gsponer, J.; E Radford, S. The mechanism of folding of Im7 reveals competition between functional and kinetic evolutionary constraints. Nat. Struct. Mol. Biol. 2009, 16, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Gsponer, J.; Hopearuoho, H.; Whittaker, S.B.-M.; Spence, G.R.; Moore, G.R.; Paci, E.; Radford, S.E.; Vendruscolo, M. Determination of an ensemble of structures representing the intermediate state of the bacterial immunity protein Im7. Proc. Natl. Acad. Sci. USA 2006, 103, 99–104. [Google Scholar] [CrossRef]
- Wang, F.; Cazzolli, G.; Wintrode, P.; Faccioli, P. Folding mechanism of proteins Im7 and Im9: Insight from all-atom simulations in implicit and explicit solvent. J. Phys. Chem. B 2016, 120, 9297–9307. [Google Scholar] [CrossRef]
- Hackenberger, C.P.R.; Friel, C.T.; Radford, S.E.; Imperiali, B. Semisynthesis of a glycosylated Im7 analogue for protein folding studies. J. Am. Chem. Soc. 2005, 127, 12882–12889. [Google Scholar] [CrossRef]
- Chen, M.M.; Bartlett, A.I.; Nerenberg, P.S.; Friel, C.T.; Hackenberger, C.P.R.; Stultz, C.M.; Radford, S.E.; Imperiali, B. Perturbing the folding energy landscape of the bacterial immunity protein Im7 by site-specific N-linked glycosylation. Proc. Natl. Acad. Sci. USA 2010, 107, 2252. [Google Scholar] [CrossRef]
- Dennis, C.A.; Videler, H.; Pauptit, R.A.; Wallis, R.; James, R.; Moore, G.R.; Kleanthous, C. A structural comparison of the colicin immunity proteins Im7 and Im9 gives new insights into the molecular determinants of immunity-protein specificity. Biochem. J. 1998, 333, 183–191. [Google Scholar] [CrossRef]
- Merrifield, R.B. Solid phase peptide synthesis. I. The synthesis of a tetrapeptide. J. Am. Chem. Soc. 1963, 85, 2149–2154. [Google Scholar] [CrossRef]
- Muttenthaler, M.; King, G.F.; Adams, D.J.; Alewood, P.F. Trends in peptide drug discovery. Nat. Rev. Drug Discov. 2021, 20, 309–325. [Google Scholar] [CrossRef]
- Schrödinger Release 2025-3: Maestro; Schrödinger, LLC: New York, NY, USA, 2025.
- Xue, Y.; Nestor, G. Determination of Amide cis/trans Isomers in N-Acetyl-d-glucosamine: Tailored NMR Analysis of the N-Acetyl Group Conformation. ChemBioChem 2022, 23, e202200338. [Google Scholar] [CrossRef]
- Case, D.A.; Darden, T.A.; Cheatham, T.E., III; Simmerling, C.L.; Wang, J.; Duke, R.E.; Luo, R.; Crowley, M.; Walker, R.C.; Zhang, W.; et al. Amber 10; University of California: San Francisco, CA, USA, 2008. [Google Scholar]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Zhang, D.; Lazim, R. Application of conventional molecular dynamics simulation in evaluating the stability of apomyoglobin in urea solution. Sci. Rep. 2017, 7, 44651. [Google Scholar] [CrossRef] [PubMed]
- Makarov, D.M.; Egorov, G.I. Density and volumetric properties of the aqueous solutions of urea at temperatures from T = (278 to 333) K and pressures up to 100 Mpa. J. Chem. Thermodyn. 2018, 120, 164–173. [Google Scholar] [CrossRef]
- Pastor, R.W.; Brooks, B.R.; Szabo, A. An analysis of the accuracy of Langevin and molecular dynamics algorithm. Mol. Phys. 1988, 65, 1409–1419. [Google Scholar] [CrossRef]
- Uberuaga, B.P.; Anghel, M.; Voter, A.F. Synchronization of trajectories in canonical molecular-dynamics simulations: Observation, explanation, and exploitation. J. Chem. Phys. 2004, 120, 6363–6374. [Google Scholar] [CrossRef]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general AMBER force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef]
- Özpinar, G.A.; Peukert, W.; Clark, T. An improved generalized AMBER force field (GAFF) for urea. J. Mol. Model. 2010, 16, 1427–1440. [Google Scholar] [CrossRef] [PubMed]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N• log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089. [Google Scholar] [CrossRef]
- Ryckaert, J.P.; Ciccotti, G.; Berendsen, H.J.C. Numerical integration of the cartesian equations of motion of a system with constraints: Molecular dynamics of n-alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. ff14SB: Improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef]
- Kirschner, K.N.; Yongye, A.B.; Tschampel, S.M.; González-Outeiriño, J.; Daniels, C.R.; Foley, B.L.; Woods, R.J. GLYCAM06: A generalizable biomolecular force field. Carbohydrates. J. Comput. Chem. 2008, 29, 622–655. [Google Scholar]
- Mallepogu, V.; Sankaran, K.R.; Pasala, C.; Bandi, L.R.; Maram, R.; Amineni, U.M.; Meriga, B. Ursolic acid regulates key EMT transcription factors, induces cell cycle arrest and apoptosis in MDA-MB-231 and MCF-7 breast cancer cells, an in-vitro and in silico studies. J. Cell. Biochem. 2023, 124, 1900–1918. [Google Scholar] [CrossRef]
- Pace, C.N.; Shirley, B.A.; McNutt, M.; Gajiwala, K. Forces contributing to the conformational stability of proteins. FASEB J. 1996, 10, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Tcherkasskaya, O.; Bychkova, V.E.; Uversky, V.N.; Gronenborn, A.M. Multisite fluorescence in proteins with multiple tryptophan residues. J. Biol. Chem. 2000, 275, 36285–36294. [Google Scholar] [CrossRef]
- Glandières, J.M.; Twist, C.; Haouz, A.; Zentz, C.; Alpert, B. Resolved fluorescence of the two tryptophan residues in horse apomyoglobin. Photochem. Photobiol. 2000, 71, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Twist, C.; Royer, C.; Alpert, B. Effect of solvent diffusion on the apomyoglobin-water interface. Biochemistry 2002, 41, 10343–10350. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Beresneva, O.; Rosario, R.; Roder, H. Microsecond folding dynamics of apomyoglobin at acidic pH. J. Phys. Chem. B 2012, 116, 7014–7025. [Google Scholar] [CrossRef]
- Lapidus, L.J.; Yao, S.; McGarrity, K.S.; Hertzog, D.E.; Tubman, E.; Bakajin, O. Protein hydrophobic collapse and early folding steps observed in a microfluidic mixer. Biophys. J. 2007, 93, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Brun, L.; Isom, D.G.; Velu, P.; García-Moreno, B.; Royer, C.A. Hydration of the folding transition state ensemble of a protein. Biochemistry 2006, 45, 3473–3480. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Zhu, W.; Li, G. A computational analysis of binding modes and conformation changes of MDM2 induced by p53 and inhibitor bindings. J. Comput.-Aided Mol. Des. 2013, 27, 965–974. [Google Scholar] [CrossRef]
- Chen, J.; Liang, Z.; Wang, W.; Yi, C.; Zhang, S.; Zhang, Q. Revealing origin of decrease in potency of Darunavir and Amprenavir against HIV-2 relative to HIV-1 protease by molecular dynamics simulations. Sci. Rep. 2014, 4, 6872. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.; Zhu, T.; Zhang, Q.; Zhang, J.Z. A comparative insight into Amprenavir resistance of mutations V32I, G48V, I50V, I54V, and I84V in HIV-1 protease based on thermodynamic integration and MM-PBSA methods. J. Chem. Inf. Model. 2015, 55, 1903–1913. [Google Scholar] [CrossRef]
- Lou, H.; Cukier, R.I. Molecular dynamics of apo-adenylate kinase: A principal component analysis. J. Phys. Chem. B 2006, 110, 12796–12808. [Google Scholar] [CrossRef]
- Maisuradze, G.G.; Liwo, A.; Scheraga, H.A. Principal component analysis for protein folding dynamics. J. Mol. Biol. 2009, 385, 312–329. [Google Scholar] [CrossRef]
- Roychowdhury, T.; McNutt, S.W.; Pasala, C.; Nguyen, H.T.; Thornton, D.T.; Sharma, S.; Botticelli, L.; Digwal, C.S.; Joshi, S.; Yang, N.; et al. Phosphorylation-driven epichaperome assembly is a regulator of cellular adaptability and proliferation. Nat. Commun. 2024, 15, 8912–8939. [Google Scholar] [CrossRef] [PubMed]
- Mongan, J. Interactive essential dynamics. J. Comput.-Aided Mol. Des. 2004, 18, 433–436. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD—Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Wang, P.; Cheng, G.; Zhang, D. Theoretical Study of the Influence of K20N Glycosylation on the Dynamic Behavior of Im7 Protein. Molecules 2025, 30, 3939. https://doi.org/10.3390/molecules30193939
Wang J, Wang P, Cheng G, Zhang D. Theoretical Study of the Influence of K20N Glycosylation on the Dynamic Behavior of Im7 Protein. Molecules. 2025; 30(19):3939. https://doi.org/10.3390/molecules30193939
Chicago/Turabian StyleWang, Jianqiang, Panpan Wang, Guojie Cheng, and Dawei Zhang. 2025. "Theoretical Study of the Influence of K20N Glycosylation on the Dynamic Behavior of Im7 Protein" Molecules 30, no. 19: 3939. https://doi.org/10.3390/molecules30193939
APA StyleWang, J., Wang, P., Cheng, G., & Zhang, D. (2025). Theoretical Study of the Influence of K20N Glycosylation on the Dynamic Behavior of Im7 Protein. Molecules, 30(19), 3939. https://doi.org/10.3390/molecules30193939




