Fate of Tebuconazole and Trifloxystrobin in Edible Rose Petals: Storage Stability and Human Health Risk Assessment
Abstract
1. Introduction
2. Results and Discussion
2.1. Method Validation
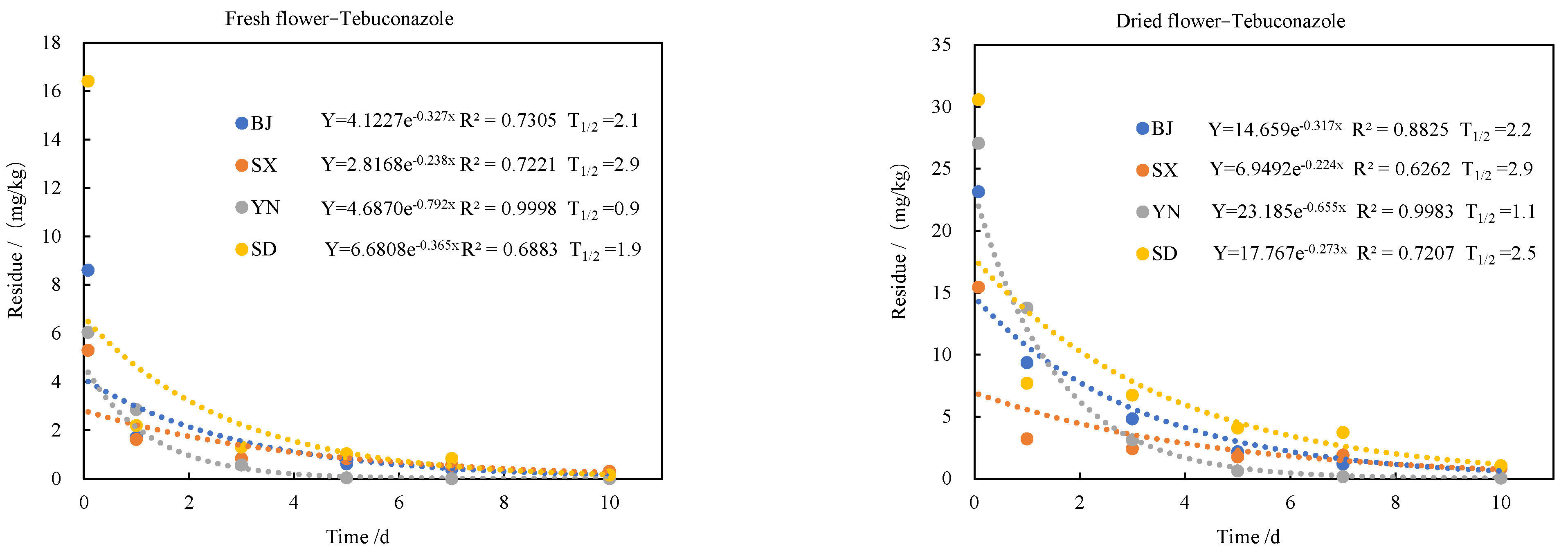
2.2. Dissipation Dynamics of the Fungicides
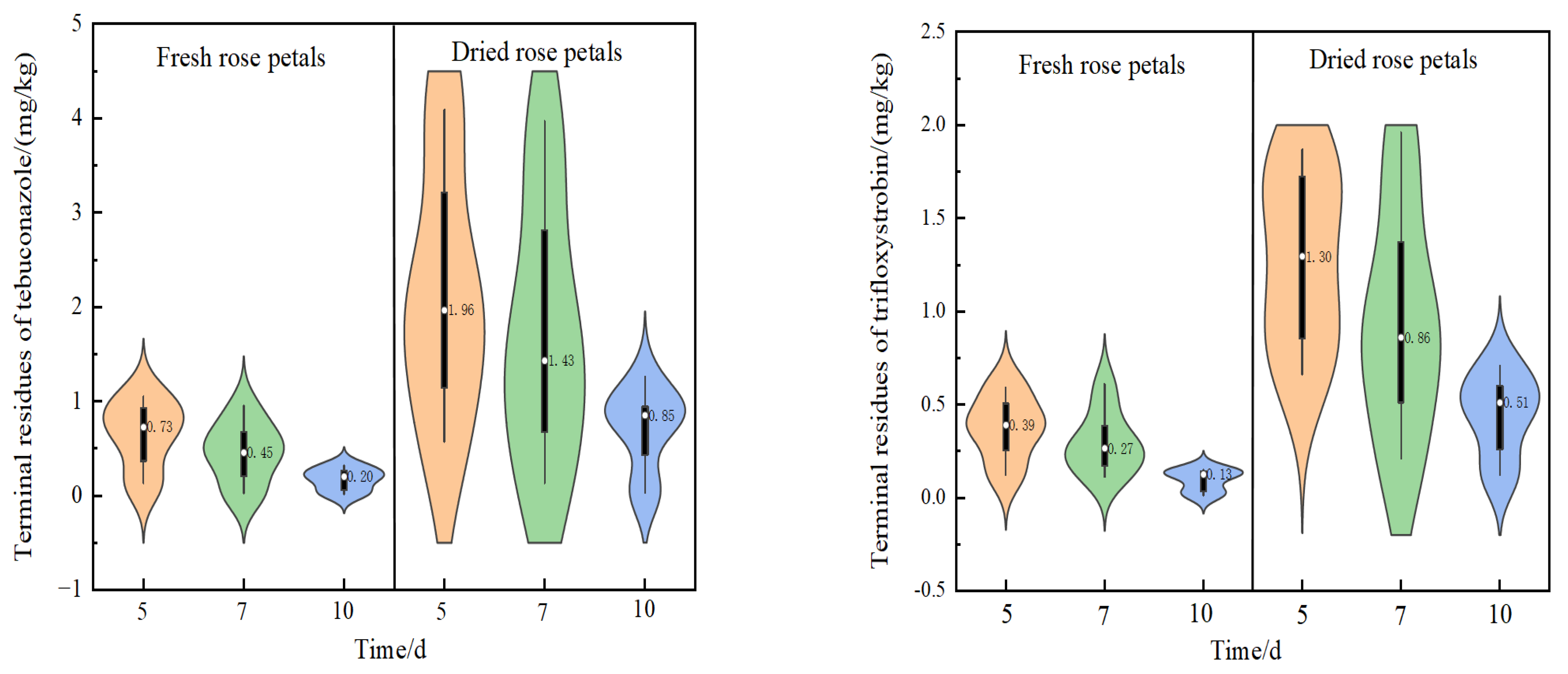
2.3. Terminal Residues of the Fungicides
2.4. Assessment of the Storage Stability of the Fungicides
2.5. Effect of Brewing on the Fungicide Residues
2.6. Dietary Risk Assessment
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Field Trials and Drying Process
3.3. Storage Stability Test
3.4. Brewing Process
3.5. Analytical Method
3.5.1. Extraction and Purification
3.5.2. UHPLC–MS/MS Analysis
3.6. Data Processing Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chetia, I.; Vijayakumar, A.; Badwaik, L.S. Edible flowers’ flavor, safety and their utilization as functional ingredients: A review. J. Food Sci. Technol.-Mysore 2025, 62, 11–23. [Google Scholar] [CrossRef]
- Devecchi, A.; Demasi, S.; Saba, F.; Rosato, R.; Gambino, R.; Ponzo, V.; De Francesco, A.; Massarenti, P.; Bo, S.; Scariot, V. Compositional Characteristics and Antioxidant Activity of Edible Rose Flowers and Their Effect on Phenolic Urinary Excretion. Pol. J. Food Nutr. Sci. 2021, 71, 383–392. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Wang, M.L.; Xing, S.; Zhang, C. Flavonoids of Rosa rugosa Thunb. inhibit tumor proliferation and metastasis in human hepatocellular carcinoma HepG2 cells. Food Sci. Hum. Wellness 2022, 11, 374–382. [Google Scholar] [CrossRef]
- Różyło, R.; Amarowicz, R.; Janiak, M.A.; Domin, M.; Różyło, I.; Rząd, K.; Matwijczuk, A.; Rusinek, R.; Gancarz, M. Micronized rose petal powder: A valuable edible floral food ingredient containing bioactive compounds. Molecules 2024, 29, 4899. [Google Scholar] [CrossRef]
- Mrázková, M.; Sumczynski, D.; Orsavová, J. Non-Traditional Muesli Mixtures Supplemented by Edible Flowers: Analysis of Nutritional Composition, Phenolic acids, Flavonoids and Anthocyanins. Plant Foods Hum. Nutr. 2021, 76, 371–376. [Google Scholar] [CrossRef]
- Jiang, L. Advancements and prospects in China’s edible rose industry: Breeding, cultivation, and processing. Environ. Dev. Sustain. 2024, 1–22. [Google Scholar] [CrossRef]
- Dong, B.Z. A comprehensive review on toxicological mechanisms and transformation products of tebuconazole: Insights on pesticide management. Sci. Total Environ. 2024, 908, 168205. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Lee, A.Y.; Chang, S.-H.; Jeong, S.-H.; Park, K.-H.; Paik, M.-K.; Cho, N.-J.; Kim, J.-E.; Cho, M.-H. Trifloxystrobin induces tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis in HaCaT, human keratinocyte cells. Drug Chem. Toxicol. 2017, 40, 67–73. [Google Scholar]
- Li, H.; Cao, F.; Zhao, F.; Yang, Y.; Teng, M.; Wang, C.; Qiu, L. Developmental toxicity, oxidative stress and immunotoxicity induced by three strobilurins (pyraclostrobin, trifloxystrobin and picoxystrobin) in zebrafish embryos. Chemosphere 2018, 207, 781–790. [Google Scholar] [CrossRef]
- Wang, S.W.; Liu, Y.P.; Zhu, M.S. Safe application of 75% trifloxystrobin-tebuconazole as water-dispersible granules in paddy based on residue and dietary risk assessment. Molecules 2024, 29, 271. [Google Scholar] [CrossRef]
- Feng, Y.; Qi, X.; Wang, X.; Liang, L.; Zuo, B. Residue dissipation and dietary risk assessment of trifloxystrobin, trifloxystrobin acid, and tebuconazole in wheat under field conditions. Int. J. Environ. Anal. Chem. 2022, 102, 1598–1612. [Google Scholar] [CrossRef]
- Pallavi, M.; Naik, R.H.; Ratnamma; Nidoni, U.; Bheemanna, M.; Pramesh, D. Simultaneous determination, dissipation and decontamination of fungicides applied on cabbage using LC-MS/MS. Food Chem. 2021, 355, 129523. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Yang, Y.; Pang, N.; Hu, J. Residue dissipation and risk assessment of tebuconazole, thiophanatemethyl and its metabolite in table grape by liquid chromatography-tandem mass spectrometry. Food Chem. 2018, 260, 66–72. [Google Scholar] [CrossRef]
- Tripathy, V.; Sharma, K.K.; Mohapatra, S.; Siddamallaiah, L.; Matadha, N.Y.; Patil, C.S.; Saindane, Y.S.; Deore, B.; Rao, C.S.; Parmar, K.D.; et al. Persistence evaluation of fluopyram plus tebuconazole residues on mango and pomegranate and their risk assessment. Environ. Sci. Pollut. Res. 2022, 29, 33180–33190. [Google Scholar] [CrossRef]
- Gormez, E.; Odabas, E.; Golge, O.; González-Curbelo, M.Á.; Kabak, B. Assessment of pesticide contamination in pomegranates: A multivariate approach and health risk evaluation. Food Chem. Toxicol. 2025, 200, 115123. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.; Guan, S.; Liang, J.; Fang, L.; Ding, R.; Wang, J.; Li, T.; Dong, Z.; Wu, X.; Zheng, Y. Dissipation, metabolism, accumulation, processing and risk assessment of fluopyram and trifloxystrobin in cucumbers and cowpeas from cultivation to consumption. Foods 2023, 12, 2009. [Google Scholar] [CrossRef] [PubMed]
- Litoriya, N.S.; Patel, J.H.; Thakor, P.M.; Chauhan, N.R.; Chawla, S.; Shah, P.G. Behavior of trifloxystrobin and propineb as combination product in tomato and their risk assessment for human health. Biomed. Chromatogr. 2023, 37, e5676. [Google Scholar] [CrossRef]
- Katna, S.; Banshtu, T.; Sharma, A.; Dubey, J.K.; Sharma, S.; Devi, N.; Kumar, A.; Singh, S. Residual behaviour and dietary risk assessment of combination product of trifloxystrobin and propineb in/on tomato, chilli and apple. Accredit. Qual. Assur. 2025, 30, 591–604. [Google Scholar] [CrossRef]
- FuFu, Y.; Wang, Q.; Zhang, L.; Ling, S.; Jia, H.; Wu, Y. Dissipation, occurrence, and risk assessment of 12 pesticides in Dendrobium officinale Kimura et Migo. Ecotoxicol. Environ. Saf. 2021, 222, 112495. [Google Scholar] [CrossRef]
- NY/T 788—2018; Guideline for Pesticide Residue Trials in Crops. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China; China Agriculture Press: Beijing, China, 2018.
- Paramasivam, M.; Selvi, C.; Deepa, M.; Jayaprakash, S.A.; Chandrasekaran, S. Simultaneous determination of tebuconazole, trifloxystrobin, and its metabolite trifloxystrobin acid residues in gherkin under field conditions. J. Sep. Sci. 2015, 38, 958–964. [Google Scholar] [CrossRef]
- Mohapatra, S. Comparison of the residue persistence of trifloxystrobin (25%) + tebuconazole (50%) on gherkin and soil at two locations. Environ. Monit. Assess. 2015, 187, 769. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.K.; Tripathy, V.; Rao, C.S.; Bhushan, V.S.; Reddy, K.N.; Jyot, G.; Sahoo, S.K.; Singh, B.; Mandal, K.; Banerjee, H.; et al. Persistence, dissipation, and risk assessment of a combination formulation of trifloxystrobin and tebuconazole fungicides in/on tomato. Regul. Toxicol. Pharmacol. 2019, 108, 104468. [Google Scholar] [CrossRef]
- Malhat, F.; Saber, E.-S.; El-Salam, S.A.; Anagnostopoulos, C.; Ahmed, M.T. Dissipation behavior of the fungicide tebuconazole in strawberries using liquid chromatograph tandem mass spectrometry (LC-MS/MS), a dryland ecosystem-based study. Int. J. Environ. Anal. Chem. 2022, 102, 7394–7408. [Google Scholar] [CrossRef]
- Chen, G.; Liu, F.; Zhang, X.; Dong, J.; Qiao, Y.; Zhang, R.; Liao, H. Dissipation rates, residue distribution and dietary risk assessment of isoprothiolane and tebuconazole in paddy field using UPLC-MS/MS. Int. J. Environ. Anal. Chem. 2022, 102, 5200–5212. [Google Scholar] [CrossRef]
- Zhao, J.; Tan, Z.; Wen, Y.; Fan, S.; Gao, Y.; Liu, C. Residues, dissipation and risk assessment of triazole fungicide tebuconazole in green onion (Allium fistulosum L.). Int. J. Environ. Anal. Chem. 2022, 102, 3833–3840. [Google Scholar] [CrossRef]
- Wu, J.; Lin, S.; Huang, J.; Tang, S.; Zhou, J.; Chen, H.; Cheng, D.; Zhang, Z. Dissipation and residue of tebuconazole in banana and dietary intake risk assessment for various populations. Int. J. Environ. Anal. Chem. 2024, 104, 1223–1233. [Google Scholar] [CrossRef]
- Bhandari, G.; Pandey, A.; Arif, S.; Singh, S.P. Farm to fork strategy for evaluating the qualitative and quantitative health risks to the Nepalese population associated with pesticide residues in apple and banana. Food Control 2024, 163, 110512. [Google Scholar] [CrossRef]
- Fang, Q.; Wu, R.; Hu, G.; Lai, A.; Wu, K.; Zhang, L.; Feng, J.; Cao, H. Dissipation behavior, residue distribution and risk assessment of three fungicides in pears. J. Sci. Food Agric. 2020, 100, 1757–1763. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Hu, K.; Li, X.; Liu, C.; Xu, Y.; Zhang, Z.; Wu, X. Dissipation Dynamics and Dietary Risk Assessment of Four Fungicides as Preservatives in Pear. Agriculture 2022, 12, 630. [Google Scholar] [CrossRef]
- You, X.; Li, Y.; Wang, X.; Xu, J.; Zheng, X.; Sui, C. Residue analysis and risk assessment of tebuconazole in jujube (Ziziphus jujuba Mill). Biomed. Chromatogr. 2017, 31, e3914. [Google Scholar] [CrossRef]
- Sharma, N.; Mandal, K.; Sharma, S. Dissipation and risk assessment of fluopyram and trifloxystrobin on onion by GC-MS/MS. Environ. Sci. Pollut. Res. 2022, 29, 80612–80623. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Ding, J.; Luo, Y.; Zhang, H.; Yu, Y.; Yao, H.; Zhu, Z.; Chadwick, D.R.; Jones, D.; Chen, J.; et al. The dissipation, processing factors, metabolites, and risk assessment of pesticides in honeysuckle from field to table. J. Hazard. Mater. 2022, 431, 128589. [Google Scholar] [CrossRef]
- Xu, F.; Xu, D.; Du, G.; Guo, Z.; Zha, X.; Chen, L. Residue analysis, dissipation patterns of chlorfenapyr, diafenthiuron and their corresponding metabolites in tea trees, and dietary intake risk assessment. J. Sci. Food Agric. 2022, 102, 5826–5836. [Google Scholar] [CrossRef]
- Wu, Y.; An, Q.; Hao, X.; Li, D.; Zhou, C.; Zhang, J.; Wei, X.; Pan, C. Dissipative behavior, residual pattern, and risk assessment of four pesticides and their metabolites during tea cultivation, processing and infusion. Pest Manag. Sci. 2022, 78, 2883–2894. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Feng, Y.; Zhang, A.; Qi, X.; Ma, X.; Pan, J.; Han, J.; Liang, L. Residue behaviors, processing factors and transfer rates of pesticides and metabolites in rose from cultivation to consumption. J. Hazard. Mater. 2023, 442, 130042. [Google Scholar] [CrossRef] [PubMed]
- GB 2763-2021; National Food Safety Standard: Maximum Residue Limits for Pesticides in Food. National Health Commission of the People’s Republic of China: Beijing, China; Ministry of Agriculture and Rural Affairs: Beijing, China; State Admin-istration for Market Regulation: Beijing, China; Standards Press of China: Beijing, China, 2021.
- GB 2763.1-2022; National Food Safety Standard: Maximum Residue Limits for 112 Pesticides Including Sodium 2,4-D Butylate in Food. National Health Commission of the People’s Republic of China: Beijing, China; Ministry of Agriculture and Rural Affairs: Beijing, China; State Administration for Market Regulation: Beijing, China; Standards Press of China: Beijing, China, 2022.
- Codex Alimentarius Commission. Pesticide Residues in Food Online Database. 2023. Available online: https://www.fao.org/fao-who-codexalimentarius/codex-texts/dbs/pestres/pesticides/en/ (accessed on 12 June 2025).
- United States Environmental Protection Agency. Tolerances and Exemptions for Pesticide Chemical Residues. 2023. Available online: https://www.ecfr.gov/current/title-40/chapter-I/subchapter-E/part-180/subpart-C (accessed on 12 June 2025).
- Australian Pesticides and Veterinary Medicines Authority. Agricultural and Veterinary Chemicals Code Instrument No. 4 (MRL Standard) 2012. 2023. Available online: https://www.legislation.gov.au/F2012L02501/latest/text (accessed on 12 June 2025).
- Ministry of Food and Drug Safety, Korea. Pesticide Maximum Residue Limits Database. 2023. Available online: http://www.foodsafetykorea.go.kr/residue/prd/mrls/list.do (accessed on 12 June 2025).
- European Commission. EU Pesticides Database. 2023. Available online: https://food.ec.europa.eu/plants/pesticides/eu-pesticides-database_en (accessed on 12 June 2025).
- Japan Food Chemical Research Foundation. Maximum Residue Limits of Agricultural Chemicals in Foods. 2023. Available online: http://db.ffcr.or.jp/front/ (accessed on 12 June 2025).
- NY/T 3094—2017; Guideline for Storage Stability Testing of Pesticide Resi-Dues in Plant-Derived Agricultural Products. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China; China Agriculture Press: Beijing, China, 2017.

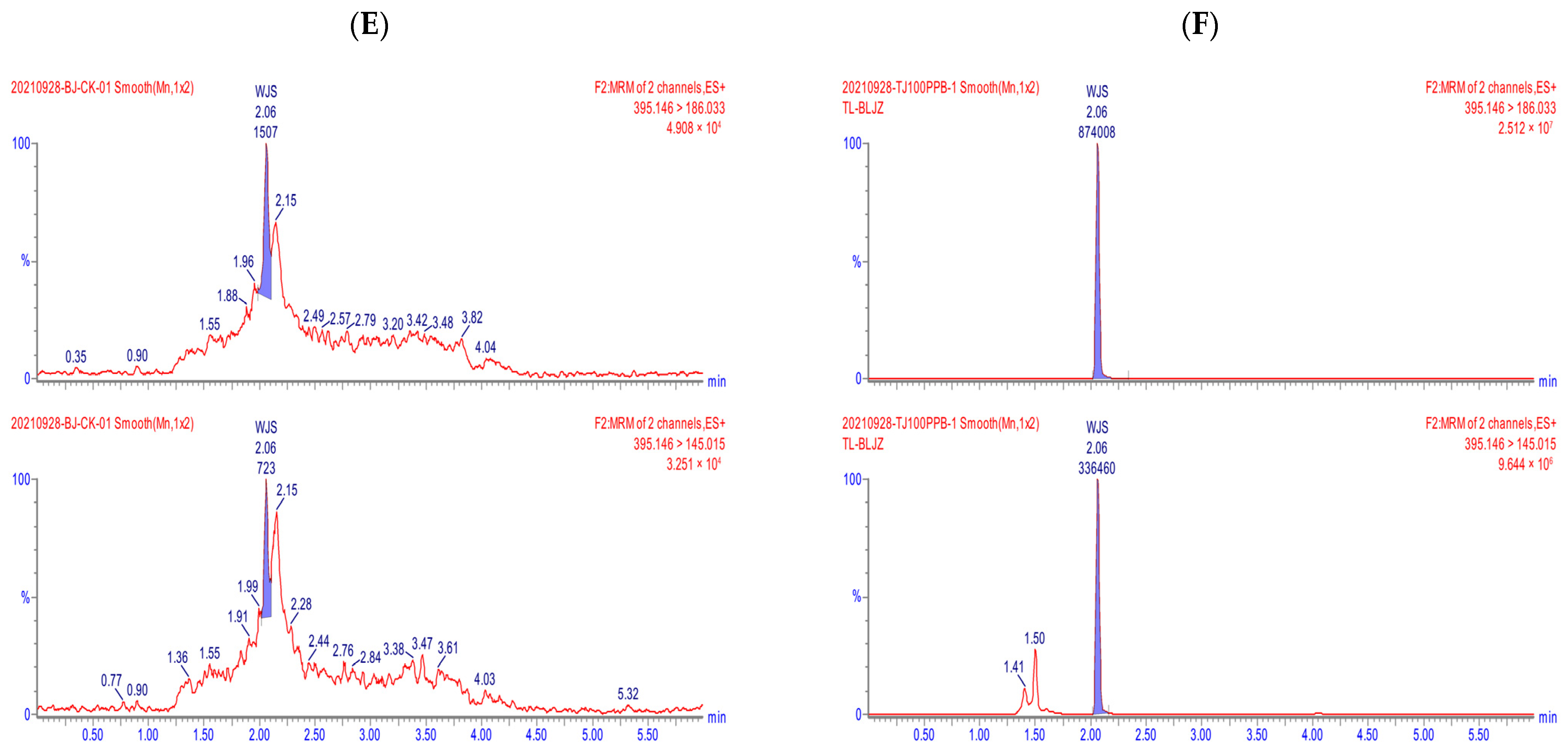

| Compound | Storage Time | Fresh Rose Petals | Dried Rose Petals | ||||
|---|---|---|---|---|---|---|---|
| (Months) | Concentration (mg/kg) | DR (%) | QC Recovery (%) | Concentration (mg/kg) | DR (%) | QC Recovery (%) | |
| Tebuconazole | 0 | 1.00 ± 0.06 | 0.5 | 101.8 | 0.99 ± 0.05 | 1.4 | 89.7 |
| 1 | 1.12 ± 0.07 | −11.2 | 107.0 | 0.99 ± 0.05 | 1.6 | 100.4 | |
| 2 | 0.92 ± 0.05 | 7.7 | 88.6 | 1.01 ± 0.05 | −0.9 | 99.8 | |
| 3 | 1.01 ± 0.06 | −1.4 | 87.8 | 0.97 ± 0.05 | 3.4 | 93.0 | |
| 6 | 1.00 ± 0.06 | 0.7 | 100.6 | 0.94 ± 0.05 | 6.2 | 99.6 | |
| 12 | 1.04 ± 0.06 | −9.2 | 103.5 | 1.09 ± 0.06 | −8.9 | 108.2 | |
| Trifloxystrobin | 0 | 1.05 ± 0.06 | −4.3 | 103.9 | 1.03 ± 0.05 | −2.5 | 98.4 |
| 1 | 0.96 ± 0.05 | 3.8 | 96.3 | 0.95 ± 0.05 | 8.1 | 96.0 | |
| 2 | 0.94 ± 0.05 | 6.2 | 102.7 | 0.99 ± 0.05 | 1.4 | 99.8 | |
| 3 | 0.94 ± 0.05 | 6.6 | 102.0 | 1.05 ± 0.06 | −8.1 | 106.7 | |
| 6 | 1.05 ± 0.06 | −4.6 | 103.9 | 1.00 ± 0.05 | 0.4 | 107.6 | |
| 12 | 0.91 ± 0.05 | 8.7 | 98.3 | 1.09 ± 0.06 | −8.3 | 104.2 | |
| CGA321113 | 0 | 1.06 ± 0.06 | −5.8 | 101.2 | 1.07 ± 0.06 | −6.9 | 94.0 |
| 1 | 1.12 ± 0.06 | −12.1 | 105.0 | 0.98 ± 0.05 | 2.0 | 90.5 | |
| 2 | 1.02 ± 0.05 | −1.4 | 91.4 | 1.00 ± 0.05 | 0.7 | 100.5 | |
| 3 | 1.08 ± 0.06 | −7.6 | 107.9 | 0.97 ± 0.05 | 2.9 | 99.9 | |
| 6 | 1.07 ± 0.06 | −6.6 | 108.7 | 1.00 ± 0.05 | 0.6 | 104.0 | |
| 12 | 0.87 ± 0.05 | 13.0 | 108.9 | 1.11 ± 0.06 | −10.9 | 84.7 | |
| Brewing Time (min) | Concentration of Rose Petals (mg/kg) | |||||
|---|---|---|---|---|---|---|
| Tebuconazole | Trifloxystrobin | CGA321113 | ||||
| Fresh | Dried | Fresh | Dried | Fresh | Dried | |
| 1 | 13.10 ± 0.78 | 23.10 ± 1.39 | 7.35 ± 0.44 | 11.20 ± 0.67 | 0.20 ± 0.012 | 0.34 ± 0.020 |
| 2 | 12.50 ± 0.75 | 21.50 ± 1.29 | 7.10 ± 0.43 | 10.50 ± 0.63 | 0.19 ± 0.011 | 0.32 ± 0.019 |
| 5 | 11.80 ± 0.71 | 19.20 ± 1.15 | 6.60 ± 0.40 | 9.30 ± 0.56 | 0.17 ± 0.010 | 0.30 ± 0.018 |
| 10 | 11.00 ± 0.66 | 16.80 ± 1.01 | 6.10 ± 0.37 | 8.20 ± 0.49 | 0.15 ± 0.009 | 0.28 ± 0.017 |
| 30 | 10.20 ± 0.61 | 13.90 ± 0.83 | 5.75 ± 0.35 | 6.80 ± 0.41 | 0.14 ± 0.008 | 0.27 ± 0.016 |
| Compound | Crop | MRLs (mg/kg) | ||||||
|---|---|---|---|---|---|---|---|---|
| China [37,38] | CAC [39] | USA [40] | Australia [41] | Korea [42] | EU [43] | Japan [44] | ||
| Tebuconazole | Sanchi | 15.00 | / | / | / | / | / | / |
| Celery | 15.00 | 5.00 | 5.00 | 5.00 | 5.00 | 0.50 | 5.00 | |
| Chinese cabbage | 7.00 | / | / | / | 2.00 | 0.02 | 3.00 | |
| Banana | 3.00 | 1.50 | 0.05 | 0.20 | 0.05 | 1.50 | 2.00 | |
| Strawberry | 2.00 | / | / | 2.00 | 0.50 | 15.00 | 2.00 | |
| Tomato | 2.00 | 0.70 | 1.30 | 0.50 | 1.00 | 0.90 | 1.00 | |
| Citrus | 2.00 | / | 1.00 | 0.20 | 3.00 | 5.00 | 5.00 | |
| Bitter melon | 2.00 | 0.15 | 0.40 | 0.50 | 0.20 | 0.15 | 0.20 | |
| Pepper | 2.00 | / | 1.30 | 0.50 | 3.00 | 0.05 | 1.00 | |
| Cotton | 2.00 | 2.00 | 2.00 | 1.00 | / | 2.00 | 2.00 | |
| Apple | 2.00 | 1.00 | 1.00 | 0.01 | 1.00 | 0.30 | 1.00 | |
| Grape | 2.00 | 6.00 | 6.00 | 5.00 | 5.00 | 0.50 | 10.00 | |
| Peach | 2.00 | 2.00 | 1.00 | 0.01 | 1.00 | 0.60 | 2.00 | |
| Sunflower | 2.00 | 0.10 | 0.10 | 0.10 | / | 0.02 | 0.10 | |
| Cucumber | 1.00 | 0.20 | 0.40 | 0.50 | 0.20 | 0.60 | 0.20 | |
| Radish | 1.00 | / | 0.70 | 0.50 | 0.30 | 0.02 | 0.10 | |
| Welsh onion | 0.50 | 0.15 | 1.30 | 2.00 | 3.00 | 2.00 | 0.20 | |
| Pear | 0.50 | 1.00 | 1.00 | 0.01 | 1.00 | 0.30 | 5.00 | |
| Rice | 0.50 | 1.50 | / | 0.20 | 0.20 | 1.50 | 0.05 | |
| Ginseng | 0.40 | 0.40 | / | / | 0.50 | / | / | |
| Loquat | 0.20 | / | 1.00 | 0.01 | / | 0.50 | / | |
| Garlic | 0.10 | 0.10 | 0.20 | 0.20 | 0.10 | 0.10 | 0.20 | |
| Peanut | 0.10 | 0.15 | / | 0.10 | 0.05 | 0.15 | 0.05 | |
| Watermelon | 0.10 | / | 1.00 | / | / | 0.15 | 0.20 | |
| Onion | 0.10 | / | 0.20 | / | / | 0.15 | 0.20 | |
| Pecan | 0.05 | 0.05 | / | 0.05 | 0.05 | 0.05 | 0.05 | |
| Sorghum | 0.05 | / | / | / | 7.00 | / | / | |
| Mango | 0.05 | 0.05 | 1.00 | 0.01 | 0.70 | 0.10 | 0.10 | |
| Wheat | 0.05 | 0.15 | 3.00 | 0.20 | 0.05 | 0.30 | 2.00 | |
| Winter jujube | / | / | / | / | 5.00 | / | / | |
| Goji berry | / | / | / | / | / | 0.50 | / | |
| Ginger | / | / | / | / | / | / | 0.20 | |
| Cowpea | / | / | 0.10 | 0.50 | 0.50 | 0.20 | 3.00 | |
| Honeysuckle | / | / | / | / | / | / | / | |
| Potato | 0.10 | / | / | / | 0.05 | 0.02 | 0.10 | |
| Dwarf lilyturf | / | / | / | / | / | / | / | |
| Pomegranate | / | / | / | / | / | 0.02 | / | |
| Edible rose | / | / | / | / | / | 15.00 | / | |
| Dendrobium officinale | / | / | / | / | / | / | / | |
| Tobacco | / | / | / | / | 0.05 | / | / | |
| Red bayberry | / | / | 1.00 | / | / | / | / | |
| Maize | / | 0.60 | 0.50 | 0.70 | 0.50 | 0.60 | 0.60 | |
| Corydalis | / | / | / | / | / | / | / | |
| Trifloxystrobin | Grape | 3.00 | 3.00 | 2.00 | 0.50 | 3.00 | 0.50 | 5.00 |
| Strawberry | 1.00 | 1.00 | 2.00 | 2.00 | 0.70 | 0.05 | 1.00 | |
| Tomato | 0.70 | 0.70 | 0.50 | 0.70 | 2.00 | 0.70 | 0.70 | |
| Apple | 0.70 | 3.00 | 5.00 | 0.70 | / | 0.70 | 3.00 | |
| Eggplant | 0.70 | 0.70 | 0.50 | / | 0.70 | 0.70 | 0.70 | |
| Mandarin orange | 0.50 | 0.50 | 0.60 | 2.00 | 0.50 | 0.50 | 3.00 | |
| Cucumber | 0.30 | / | 0.50 | 0.10 | 0.50 | 3.00 | 0.70 | |
| Watermelon | 0.20 | / | 2.00 | / | 0.50 | 0.30 | 0.30 | |
| Wheat | 0.20 | 0.20 | 0.05 | / | 0.15 | 0.30 | 0.20 | |
| Banana | 0.10 | 0.05 | 0.10 | 0.50 | 0.05 | 0.70 | 0.50 | |
| Onion | 0.05 | / | 0.04 | / | 0.05 | 0.01 | 0.70 | |
| Maize | 0.02 | / | 0.05 | / | 0.02 | 0.02 | 0.05 | |
| Garlic | / | / | 0.04 | / | 0.50 | 0.01 | 0.05 | |
| Daylily | / | / | / | / | / | 0.01 | / | |
| Cowpea | / | / | 1.50 | / | / | 0.20 | / | |
| Coffee | / | / | 0.02 | / | 0.01 | 0.70 | 0.05 | |
| Bitter melon | / | / | 0.50 | / | 1.00 | 0.30 | 0.30 | |
| Pepper | / | 0.30 | 0.50 | 0.50 | 2.00 | 0.40 | 0.50 | |
| Lychee | / | / | / | / | / | 0.01 | / | |
| Potato | / | 0.02 | 0.10 | / | 0.02 | 0.02 | 0.04 | |
| Mango | / | 3.00 | 0.70 | / | 1.50 | 3.00 | 0.70 | |
| Kiwifruit | / | 0.70 | 0.50 | / | 1.00 | / | / | |
| Loquat | / | 0.70 | 2.00 | / | / | 3.00 | 0.70 | |
| Ginseng | / | 0.03 | / | / | 0.10 | 0.40 | / | |
| Sanchi | / | / | / | / | / | / | / | |
| Pecan | / | 0.02 | / | / | / | / | 0.04 | |
| Edible rose | / | / | / | / | / | 15.00 | / | |
| Rice | / | 5.00 | 3.50 | / | / | 5.00 | 2.00 | |
| Peach | / | 3.00 | 0.50 | 5.00 | / | 3.00 | 0.30 | |
| Melon | / | / | 0.50 | / | 1.00 | 0.30 | 0.30 | |
| Sunflower | / | / | / | / | / | 0.01 | / | |
| Red bayberry | / | / | 2.00 | / | / | / | / | |
| Cherry | / | 3.00 | 2.00 | / | 0.50 | / | 3.00 | |
| Jujube | / | 0.70 | 2.00 | / | 3.00 | / | / | |
| Food Classification | Fi (kg) | Tebuconazole | Trifloxystrobin | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Reference Limits (mg/kg) | Sources | NEDI (mg) | RQ (%) | Reference Limits (mg/kg) | Sources | NEDI (mg) | RQ (%) | ||
| Rice and its products | 0.2399 | 0.5 | China | 0.12 | 0.1 | China | 0.0240 | ||
| Flour and its products | 0.1385 | 0.05 | China | 0.0069 | 0.2 | China | 0.0277 | ||
| Other cereals | 0.0233 | 0.05 | China | 0.0012 | 0.02 | China | 0.0005 | ||
| Tubers | 0.0495 | 0.1 | China | 0.005 | 0.2 | China | 0.0099 | ||
| Darker vegetables | 0.0915 | 15 | China | 1.3725 | 0.7 | China | 0.0641 | ||
| Light vegetables | 0.1837 | 7 | China | 1.2859 | 0.7 | China | 0.1286 | ||
| Fruits | 0.0457 | 3 | China | 0.1371 | 3 | China | 0.1371 | ||
| Nuts | 0.0039 | 0.05 | China | 0.0002 | 0.02 | CAC | 0.0001 | ||
| Vegetable oil | 0.0327 | 2 | China | 0.0654 | 0.02 | China | 0.0007 | ||
| Salt | 0.0120 | 15 | EU | 0.18 | 15 | EU | 0.1800 | ||
| Soy sauce | 0.0090 | 15 | China | 0.135 | 0.03 | CAC | 0.0003 | ||
| Total | 1.0286 | 3.3091 | 175.1 | 0.5728 | 22.7 | ||||
| Location | GPS | Soil Properties | Climatic | Plant Height (m) | BBCH | |||
|---|---|---|---|---|---|---|---|---|
| pH | OMC (%) | CEC (cmol/kg) | Mean Temp (°C) | Rainfall (mm) | ||||
| Beijing | 39.991° N, 116.008° E | 7.8 | 2.3 | 24.1 | 24.2 | 0 | 1.0–1.5 | 65 |
| Shanxi | 36.734° N, 103.265° E | 8.4 | 1.4 | 23.9 | 20.5 | 5 | 1.5–2.0 | 67 |
| Yunnan | 25.361° N, 103.495° E | 6.9 | 3.9 | 11.9 | 20.7 | 35 | 1.2–1.8 | 66 |
| Shandong | 36.251° N, 116.350° E | 6.5 | 2.0 | 9.3 | 25.4 | 2 | 0.5–1.2 | 61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, X.; Zhang, J.; Tao, Y.; Chen, L.; Yu, P.; Jing, J.; Zhao, E.; Zheng, Y.; He, M. Fate of Tebuconazole and Trifloxystrobin in Edible Rose Petals: Storage Stability and Human Health Risk Assessment. Molecules 2025, 30, 3938. https://doi.org/10.3390/molecules30193938
Qin X, Zhang J, Tao Y, Chen L, Yu P, Jing J, Zhao E, Zheng Y, He M. Fate of Tebuconazole and Trifloxystrobin in Edible Rose Petals: Storage Stability and Human Health Risk Assessment. Molecules. 2025; 30(19):3938. https://doi.org/10.3390/molecules30193938
Chicago/Turabian StyleQin, Xiaotong, Jinwei Zhang, Yan Tao, Li Chen, Pingzhong Yu, Junjie Jing, Ercheng Zhao, Yongquan Zheng, and Min He. 2025. "Fate of Tebuconazole and Trifloxystrobin in Edible Rose Petals: Storage Stability and Human Health Risk Assessment" Molecules 30, no. 19: 3938. https://doi.org/10.3390/molecules30193938
APA StyleQin, X., Zhang, J., Tao, Y., Chen, L., Yu, P., Jing, J., Zhao, E., Zheng, Y., & He, M. (2025). Fate of Tebuconazole and Trifloxystrobin in Edible Rose Petals: Storage Stability and Human Health Risk Assessment. Molecules, 30(19), 3938. https://doi.org/10.3390/molecules30193938






