Salidroside: A Potential Drug Candidate to Treat Rheumatoid Arthritis
Abstract
1. Introduction
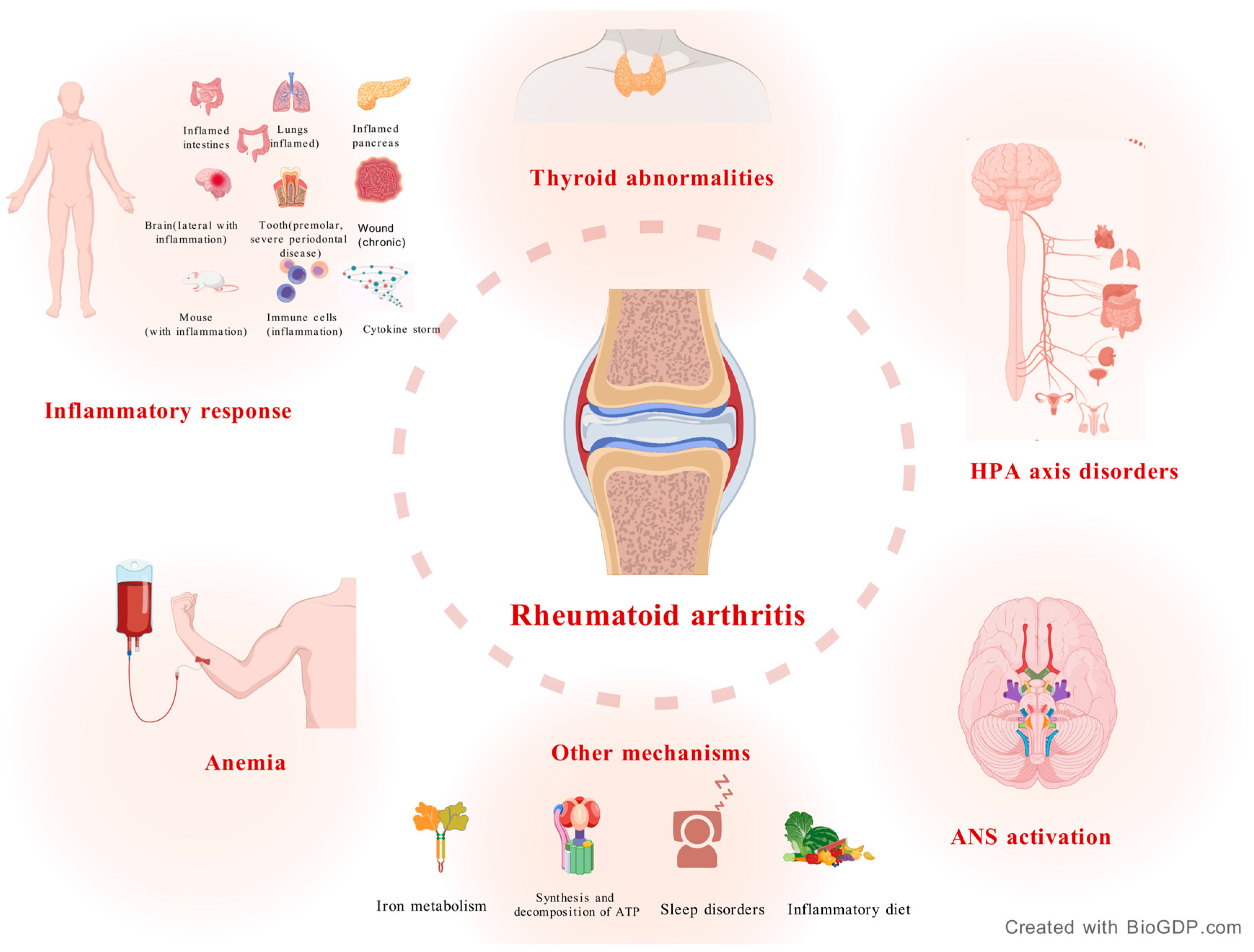
2. Mechanisms of RA
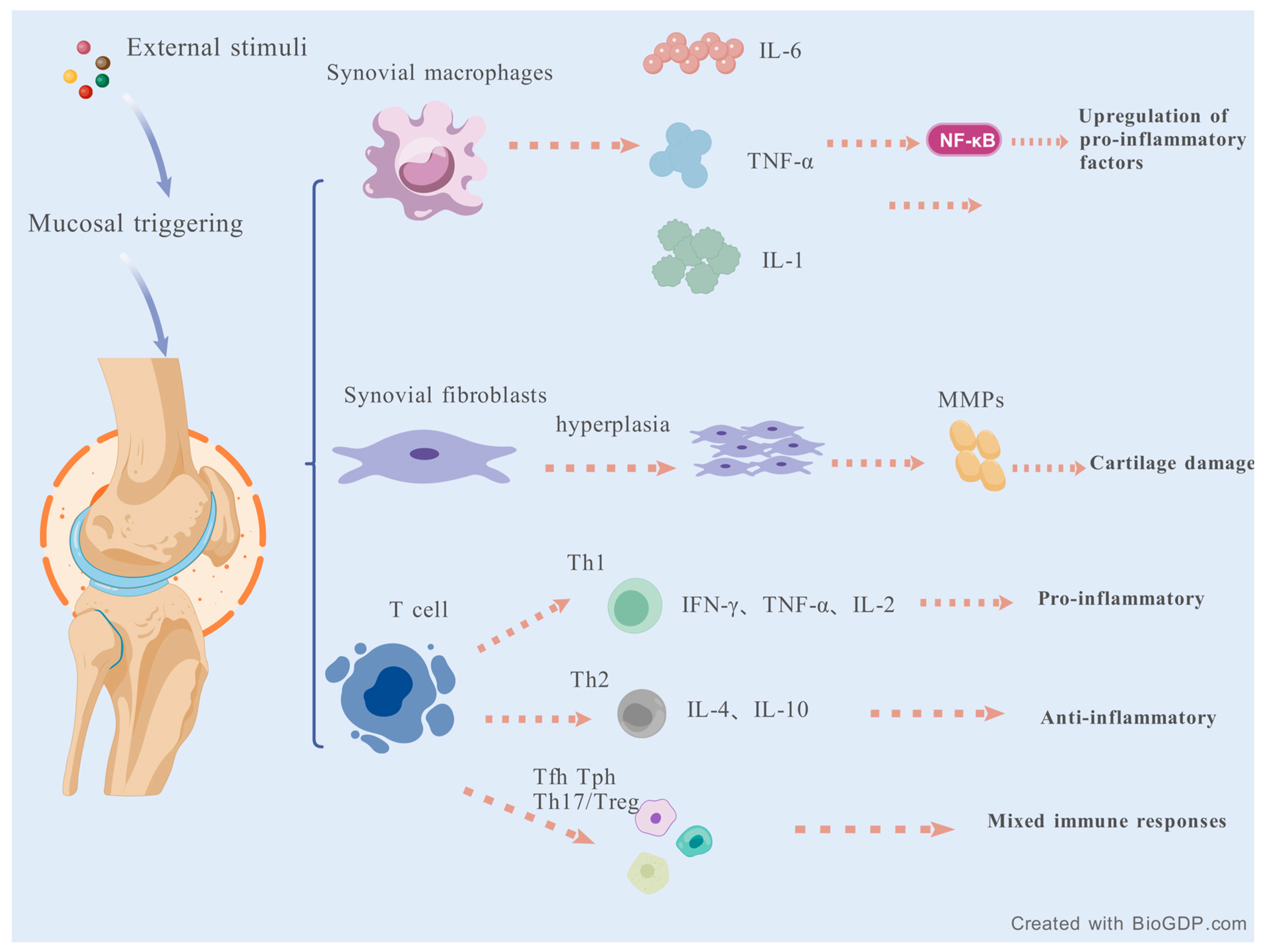
2.1. Inflammatory Response
2.2. Hypothalamic-Pituitary-Adrenal (HPA) Axis Disorders
2.3. Autonomic Nervous System (ANS) Activation
2.4. Anemia
2.5. Thyroid Abnormalities
2.6. Other Mechanisms
3. Current Research Progress of the RA Model
4. The Potential Pharmacological Mechanism of SAL in Anti-RA
4.1. Improve Exercise Endurance
4.2. Energy Metabolism Effects
4.2.1. Glycogen Storage Effects
4.2.2. Promote Lipid Metabolism
4.2.3. Repair Mitochondrial Dysfunction
4.3. Regulate Amino Acid Metabolism
4.4. Reduce Metabolite Accumulation
4.5. Antioxidant Activity
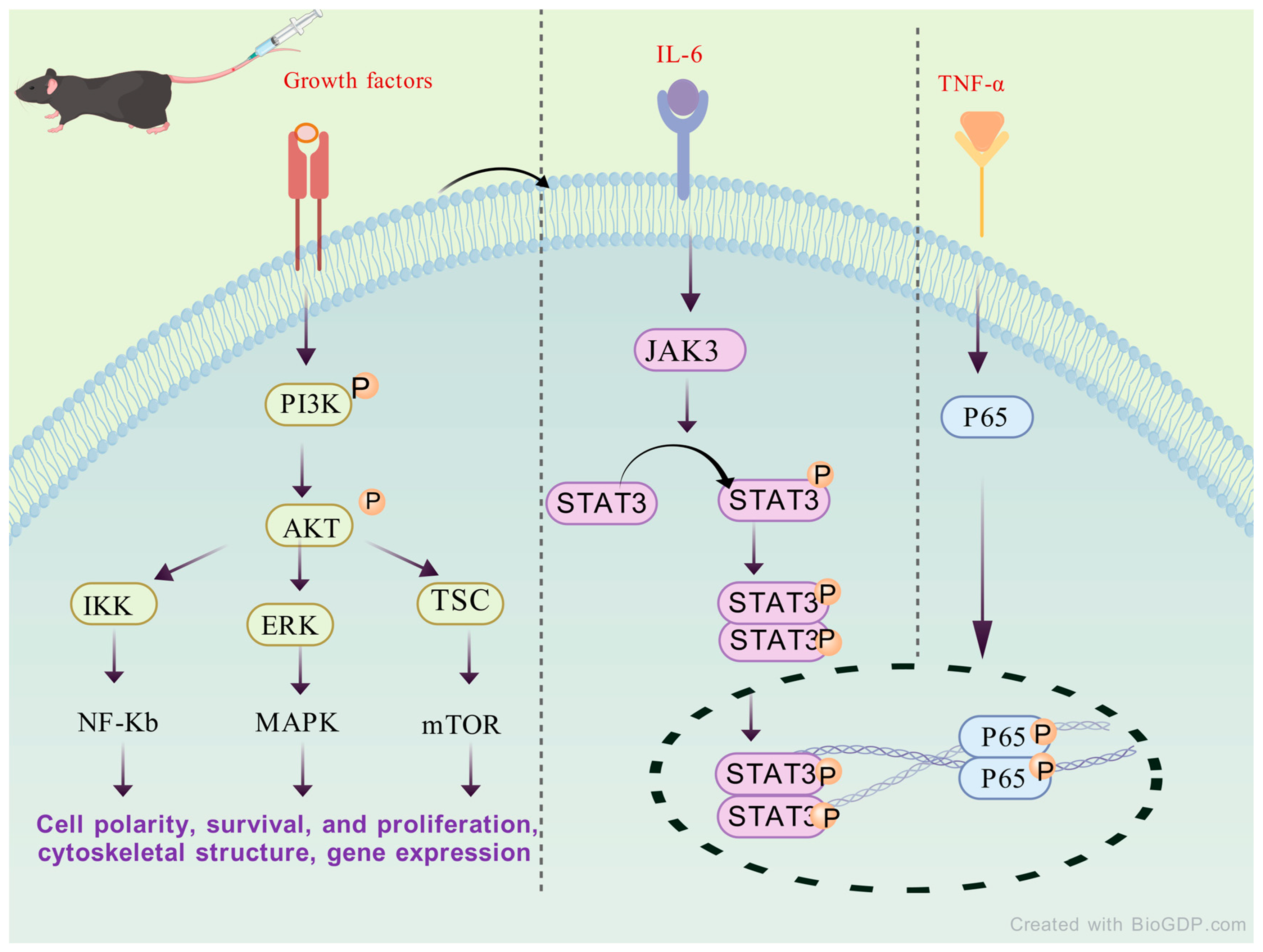
4.6. Regulate Immune System
4.7. Interfere with the Synthesis and Release of Neurotransmitters in the Brain
5. Pharmacokinetics, Safety and Toxicity
6. Conclusion and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- Wu, D.; Luo, Y.; Li, T.; Chen, Y.; Liu, F.; Wu, Y.; He, Y.; Sun, W.; Chen, J.; Li, Z.; et al. Systemic complications of rheumatoid arthritis: Focus on pathogenesis and treatment. Front. Immunol. 2022, 13, 1051082. [Google Scholar] [CrossRef]
- Singh, J.A. Treatment Guidelines in Rheumatoid Arthritis. Rheum. Dis. Clin. N. Am. 2022, 48, 679–689. [Google Scholar] [CrossRef]
- Tanaka, Y. Recent progress in treatments of rheumatoid arthritis: An overview of developments in biologics and small molecules, and remaining unmet needs. Rheumatology 2021, 60 (Suppl. S6), vi12–vi20. [Google Scholar] [CrossRef] [PubMed]
- Chuang, A.T.; Tsai, D.H.; Weng, M.Y.; Huang, H.K.; Lai, E.C. Effectiveness of JAK inhibitors in biologics-naïve patients with RA: A population-based study. Rheumatology 2025, 64, 4668–4677. [Google Scholar] [CrossRef] [PubMed]
- CTarjányi, O.; Olasz, K.; Rátky, F.; Sétáló, G.; Boldizsár, F. Proteasome Inhibitors: Potential in Rheumatoid Arthritis Therapy? Int. J. Mol. Sci. 2025, 26, 2943. [Google Scholar] [CrossRef] [PubMed]
- Küçükdeveci, A.A. Nonpharmacological treatment in established rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 2019, 33, 101482. [Google Scholar] [CrossRef]
- Forsyth, C.; Kouvari, M.; D’Cunha, N.M.; Panagiotakos, D.B.; Mellor, D.D.; Kellett, J.; Naumovski, N. The effects of the Mediterranean diet on rheumatoid arthritis prevention and treatment: A systematic review of human prospective studies. Rheumatol. Int. 2018, 38, 737–747. [Google Scholar] [CrossRef]
- Pu, W.L.; Zhang, M.Y.; Bai, R.Y.; Sun, L.K.; Li, W.H.; Yu, Y.L. Anti-inflammatory effects of Rhodiola rosea L.: A review. Biomed. Pharmacother. 2020, 121, 109552. [Google Scholar] [CrossRef]
- Xue, H.; Li, P.; Luo, Y.; Fan, L.; Liu, X.; Zhang, L.; Liu, J.; Wang, N.; He, J.; Sun, S.; et al. Salidroside stimulates the Sirt1/PGC-1α axis and ameliorates diabetic nephropathy in mice. Phytomedicine 2019, 54, 240–247. [Google Scholar] [CrossRef]
- Magani, S.K.J.; Mupparthi, S.D.; Gollapalli, B.P.; Nile, S.H.; Garg, M.; Monga, J.; Mocan, A.; Atanasov, A.G. Salidroside—Can it be a Multifunctional Drug? Curr. Drug Metab. 2020, 21, 512–524. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Brodacki, S.; Cieślicka, E.; Dobkowska, K.; Frasuńska, J.; Głuszko, P.; Paradowska-Gorycka, A. Salidroside: A Promising Agent in Bone Metabolism Modulation. Nutrients 2024, 16, 2387. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Q.; Lou, Y.; Zhou, J.; Tang, Q.; Li, M.; Gu, P.; Li, X.; Hao, Y.; Yang, Z.; et al. Salidroside attenuates neuroinflammation and improves functional recovery after spinal cord injury through microglia polarization regulation. J. Cell. Mol. Med. 2018, 22, 1148–1166. [Google Scholar] [CrossRef]
- Shand, G.; Fuller, D.T.; Lufkin, L.; Katz, P.; Lanata, C.M.; Criswell, L.A.; Jolly, M. A stronger association of depression with rheumatoid arthritis in presence of obesity and hypertriglyceridemia. Front. Epidemiol. 2023, 3, 1216497. [Google Scholar] [CrossRef]
- Spagnolo, P.; Lee, J.S.; Sverzellati, N.; Rossi, G.; Cottin, V. The Lung in Rheumatoid Arthritis: Focus on Interstitial Lung Disease. Arthritis Rheumatol. 2018, 70, 1544–1554. [Google Scholar] [CrossRef]
- Willemze, A.; Trouw, L.A.; Toes, R.E.; Huizinga, T.W. The influence of ACPA status and characteristics on the course of RA. Nat. Rev. Rheumatol. 2012, 8, 144–152. [Google Scholar] [CrossRef]
- Shi, H.; Wu, H.; Winkler, M.A.; Chen, Y.; Li, T.; Han, X.; Zhao, Y.; Sun, L.; Zhang, Y.; Li, Y.; et al. Perivascular adipose tissue in autoimmune rheumatic diseases. Pharmacol. Res. 2022, 182, 106354. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Wei, Y.; Zhu, Y.; Xie, Z.; Hai, Q.; Li, Z.; Qin, D. Gut microbiota and rheumatoid arthritis: From pathogenesis to novel therapeutic opportunities. Front. Immunol. 2022, 13, 1007165. [Google Scholar] [CrossRef] [PubMed]
- Kuis, W.; Heijnen, C.J. Rheumatoid arthritis and juvenile chronic arthritis: The role of the neuro-endocrine system. Clin. Exp. Rheumatol. 1994, 12 (Suppl. S10), S29–S34. [Google Scholar] [PubMed]
- Sattler, J.; Tu, J.; Stoner, S.; Lue, Y.; Swerdloff, R.S.; Wang, C.; Hama, S.; Yuen, K.C.J.; Haddad, G.H. Role of 11β-HSD type 1 in abnormal HPA axis activity during immune-mediated arthritis. Endocr. Connect. 2018, 7, 385–394. [Google Scholar] [CrossRef]
- D’Haens, G.; Eberhardson, M.; Cabrijan, Z.; Danese, S.; van den Wall Bake, K.; Rijk, M.; Löwenberg, M.; Ponsioen, C.; van der Woude, J.; Bergmans, P.; et al. Neuroimmune Modulation Through Vagus Nerve Stimulation Reduces Inflammatory Activity in Crohn’s Disease Patients: A Prospective Open-label Study. J. Crohns Colitis 2023, 17, 1897–1909. [Google Scholar] [CrossRef]
- Campbell, K.L.; Winters-Stone, K.M.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.S.; Matthews, C.E.; Ligibel, J.A.; Gerber, L.H.; et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- Heath, E.I.; Weise, A.; Vaishampayan, U.; Danforth, D.; Ungerleider, R.S.; Urata, Y. Phase Ia dose escalation study of OBP-801, a cyclic depsipeptide class I histone deacetylase inhibitor, in patients with advanced solid tumors. Investig. New Drugs 2022, 40, 300–307. [Google Scholar] [CrossRef]
- Hockenberry, M.J.; Hooke, M.C.; Gregurich, M.; McCarthy, K. Carnitine plasma levels and fatigue in children/adolescents receiving cisplatin, ifosfamide, or doxorubicin. J. Pediatr. Hematol. Oncol. 2009, 31, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.R.; Wolff, B.S.; Liwang, J.; Regan, J.; Alshawi, S.; Raheem, S.; Saligan, L.N. Cancer-related fatigue during combined treatment of androgen deprivation therapy and radiotherapy is associated with mitochondrial dysfunction. Int. J. Mol. Med. 2019, 45, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Agca, R.; Heslinga, S.C.; Rollefstad, S.; Heslinga, M.; McInnes, I.B.; Peters, M.J.L.; Kvien, T.K.; Dougados, M.; Radner, H.; Atzeni, F.; et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann. Rheum. Dis. 2017, 76, 17–28. [Google Scholar] [CrossRef]
- Boissier, M.C.; Assier, E.; Falgarone, G.; Bessis, N. Shifting the imbalance from Th1/Th2 to Th17/treg: The changing rheumatoid arthritis paradigm. Joint Bone Spine 2008, 75, 373–375. [Google Scholar] [CrossRef]
- Khalaf, W.; Al-Rubaie, H.A.; Shihab, S. Studying anemia of chronic disease and iron deficiency in patients with rheumatoid arthritis by iron status and circulating hepcidin. Hematol. Rep. 2019, 11, 7708. [Google Scholar] [CrossRef]
- Behl, T.; Upadhyay, T.; Singh, S.; Chigurupati, S.; Alsubayiel, A.M.; Mani, V.; Vargas-De-La-Cruz, C.; Uivarosan, D.; Bustea, C.; Sava, C.; et al. Polyphenols Targeting MAPK Mediated Oxidative Stress and Inflammation in Rheumatoid Arthritis. Molecules 2021, 26, 6570. [Google Scholar] [CrossRef]
- Furst, D.E. The risk of infections with biologic therapies for rheumatoid arthritis. Semin. Arthritis Rheum. 2010, 39, 327–346. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, Y.; Gao, Y.; Qi, D.; Zhao, L.; Zhao, L.; Liu, C.; Tao, T.; Zhou, C.; Sun, X.; et al. NR1D1 modulates synovial inflammation and bone destruction in rheumatoid arthritis. Cell Death Dis. 2020, 11, 129. [Google Scholar] [CrossRef]
- Deane, K.D.; Holers, V.M. The Natural History of Rheumatoid Arthritis. Clin. Ther. 2019, 41, 1256–1269. [Google Scholar] [CrossRef]
- Rivellese, F.; Pontarini, E.; Pitzalis, C. Tertiary Lymphoid Organs in Rheumatoid Arthritis. Curr. Top. Microbiol. Immunol. 2020, 426, 119–141. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Kwon, E.J.; Lee, J.J. Rheumatoid Arthritis: Pathogenic Roles of Diverse Immune Cells. Int. J. Mol. Sci. 2022, 23, 905. [Google Scholar] [CrossRef] [PubMed]
- Mateen, S.; Zafar, A.; Moin, S.; Khan, A.Q.; Zubair, S. Understanding the role of cytokines in the pathogenesis of rheumatoid arthritis. Clin. Chim. Acta 2016, 455, 161–171. [Google Scholar] [CrossRef]
- Pablos, J.L.; Cañete, J.D. Immunopathology of rheumatoid arthritis. Curr. Top. Med. Chem. 2013, 13, 705–711. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, Y.; Wu, R.; Ding, T.; Xue, H.; Gao, C.; Li, X.; Wang, C. New Insights From Single-Cell Sequencing Data: Synovial Fibroblasts and Synovial Macrophages in Rheumatoid Arthritis. Front. Immunol. 2021, 12, 709178. [Google Scholar] [CrossRef] [PubMed]
- Kurowska-Stolarska, M.; Alivernini, S. Synovial tissue macrophages in joint homeostasis, rheumatoid arthritis and disease remission. Nat. Rev. Rheumatol. 2022, 18, 384–397. [Google Scholar] [CrossRef]
- Siouti, E.; Andreakos, E. The many facets of macrophages in rheumatoid arthritis. Biochem. Pharmacol. 2019, 165, 152–169. [Google Scholar] [CrossRef]
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef]
- Wen, X.; Chen, X.; Liang, X.; Wu, J.; Li, X.; Wang, X.; Huang, W.; Lin, J. The small molecule NSM00191 specifically represses the TNF-α/NF-κB axis in foot and ankle rheumatoid arthritis. Int. J. Biol. Sci. 2018, 14, 1732–1744. [Google Scholar] [CrossRef]
- Zwerina, J.; Redlich, K.; Polzer, K.; Joosten, L.; Krönke, G.; Distler, J.; Hess, A.; Pundt, N.; Pap, T.; Hoffmann, O.; et al. TNF-induced structural joint damage is mediated by IL-1. Proc. Natl. Acad. Sci. USA 2007, 104, 11742–11747. [Google Scholar] [CrossRef]
- Yao, X.; Huang, J.; Zhong, H.; Shen, N.; Faggioni, R.; Fung, M.; Yao, Y. Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol. Ther. 2014, 141, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Srirangan, S.; Choy, E.H. The role of interleukin 6 in the pathophysiology of rheumatoid arthritis. Ther. Adv. Musculoskelet. Dis. 2010, 2, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Li, X.; Lin, A.; Larsson, S.C. Interleukins and rheumatoid arthritis: Bi-directional Mendelian randomization investigation. Semin. Arthritis Rheum. 2022, 53, 151958. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, D.; Chen, W.; Tao, Y.; Li, W.; Qi, J. Microenvironment-Activatable Probe for Precise NIR-II Monitoring and Synergistic Immunotherapy in Rheumatoid Arthritis. Adv. Mater. 2024, 36, 2409661. [Google Scholar] [CrossRef]
- Kinne, R.W.; Bräuer, R.; Stuhlmüller, B.; Palombo-Kinne, E.; Burmester, G.R. Macrophages in rheumatoid arthritis. Arthritis Res. 2000, 2, 189–202. [Google Scholar] [CrossRef]
- Dong, C. Cytokine Regulation and Function in T Cells. Annu. Rev. Immunol. 2021, 39, 51–76. [Google Scholar] [CrossRef]
- Wang, Z.; Zhuo, F.; Chu, P.; Yang, X.; Zhao, G. Germacrone alleviates collagen-induced arthritis via regulating Th1/Th2 balance and NF-κB activation. Biochem. Biophys. Res. Commun. 2019, 518, 560–564. [Google Scholar] [CrossRef]
- Ruschpler, P.; Stiehl, P. Shift in Th1 (IL-2 and IFN-gamma) and Th2 (IL-10 and IL-4) cytokine mRNA balance within two new histological main-types of rheumatoid-arthritis (RA). Cell. Mol. Biol. 2002, 48, 285–293. [Google Scholar]
- Cañete, J.D.; Martínez, S.E.; Farrés, J.; Sanmartí, R.; Blay, M.; Gómez, A.; Salvador, G.; Muñoz-Gómez, J. Differential Th1/Th2 cytokine patterns in chronic arthritis: Interferon gamma is highly expressed in synovium of rheumatoid arthritis compared with seronegative spondyloarthropathies. Ann. Rheum. Dis. 2000, 59, 263–268. [Google Scholar] [CrossRef]
- Niu, Y.; Dong, Q.; Li, R. Matrine regulates Th1/Th2 cytokine responses in rheumatoid arthritis by attenuating the NF-κB signaling. Cell Biol. Int. 2017, 41, 611–621. [Google Scholar] [CrossRef]
- Bao, Y.; Peng, J.; Yang, K.L.; Ma, Y.; Li, X.; Zou, Y.; Chen, H.; Zhou, P.; Liu, E.H.; Li, P.; et al. Therapeutic effects of Chinese medicine Di-Long (Pheretima vulgaris) on rheumatoid arthritis through inhibiting NF-κB activation and regulating Th1/Th2 balance. Biomed. Pharmacother. 2022, 147, 112643. [Google Scholar] [CrossRef]
- Lina, C.; Conghua, W.; Nan, L.; Ping, Z. Combined treatment of etanercept and MTX reverses Th1/Th2, Th17/Treg imbalance in patients with rheumatoid arthritis. J. Clin. Immunol. 2011, 31, 596–605. [Google Scholar] [CrossRef]
- Wang, J.; Conlon, D.; Rivellese, F.; Pereira, J.M.; Silverman, G.J.; Gregory, A.P.; Gravallese, E.M.; Weinblatt, M.E.; Shadick, N.A.; Su, L.; et al. Synovial Inflammatory Pathways Characterize Anti-TNF-Responsive Rheumatoid Arthritis Patients. Arthritis Rheumatol. 2022, 74, 1916–1927. [Google Scholar] [CrossRef] [PubMed]
- Rogozynski, N.P.; Dixon, B. The Th1/Th2 paradigm: A misrepresentation of helper T cell plasticity. Immunol. Lett. 2024, 268, 106870. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Niu, X. T follicular helper cells in autoimmune diseases. J. Autoimmun. 2023, 134, 102976. [Google Scholar] [CrossRef]
- Lu, J.; Wu, J.; Xia, X.; Peng, H.; Wang, S. Follicular helper T cells: Potential therapeutic targets in rheumatoid arthritis. Cell. Mol. Life Sci. 2021, 78, 5095–5106. [Google Scholar] [CrossRef] [PubMed]
- Yoshitomi, H. Peripheral helper T cells, mavericks of peripheral immune responses. Int. Immunol. 2024, 36, 9–16. [Google Scholar] [CrossRef]
- Weinand, K.; Sakaue, S.; Nathan, A.; Zhang, J.; Nasser, J.; Korsunsky, I.; Karjalainen, J.; Okada, Y.; Raychaudhuri, S. The chromatin landscape of pathogenic transcriptional cell states in rheumatoid arthritis. Nat. Commun. 2024, 15, 4650. [Google Scholar] [CrossRef] [PubMed]
- Fuite, J.; Vernon, S.D.; Broderick, G. Neuroendocrine and immune network re-modeling in chronic fatigue syndrome: An exploratory analysis. Genomics 2008, 92, 393–399. [Google Scholar] [CrossRef]
- Feldt-Rasmussen, U.; Effraimidis, G.; Klose, M. The hypothalamus-pituitary-thyroid (HPT)-axis and its role in physiology and pathophysiology of other hypothalamus-pituitary functions. Mol. Cell. Endocrinol. 2021, 525, 111173. [Google Scholar] [CrossRef] [PubMed]
- Spies, C.M.; Straub, R.H.; Cutolo, M.; Buttgereit, F. Circadian rhythms in rheumatology--a glucocorticoid perspective. Arthritis Res. Ther. 2014, 16 (Suppl. S2), S3. [Google Scholar] [CrossRef] [PubMed]
- Eijsbouts, A.M.; Murphy, E.P. The role of the hypothalamic-pituitary-adrenal axis in rheumatoid arthritis. Baillieres Best Pract. Res. Clin. Rheumatol. 1999, 13, 599–613. [Google Scholar] [CrossRef]
- Atzeni, F.; Straub, R.H.; Cutolo, M.; Sarzi-Puttini, P. Psoriatic arthritis: Clinical improvement and correlation with hormone axes in etanercept-treated patients. Ann. N. Y. Acad. Sci. 2010, 1193, 176–178. [Google Scholar] [CrossRef]
- Mravcova, M.; Chovanova, L.; Paulikova, L.; Lazurova, I. Genetics of neuroendocrine factors in rheumatoid arthritis. Horm. Metab. Res. 2015, 47, 411–417. [Google Scholar] [CrossRef]
- Chen, C.C.; Parker, C.R., Jr. Adrenal androgens and the immune system. Semin. Reprod. Med. 2004, 22, 369–377. [Google Scholar] [CrossRef]
- Imrich, R.; Rovenský, J. Hypothalamic-pituitary-adrenal axis in rheumatoid arthritis. Rheum. Dis. Clin. N. Am. 2010, 36, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Bach, E.; Møller, A.B.; Jørgensen, J.O.L.; Vendelbo, M.H.; Jessen, N.; Olesen, J.L.; Pedersen, S.B.; Møller, N. Intact pituitary function is decisive for the catabolic response to TNF-α: Studies of protein, glucose and fatty acid metabolism in hypopituitary and healthy subjects. J. Clin. Endocrinol. Metab. 2015, 100, 578–586. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abdullahi, A.; Wong, T.W.L.; Ng, S.S.M. Putative role of non-invasive vagus nerve stimulation in cancer pathology and immunotherapy: Can this be a hidden treasure, especially for the elderly? Cancer Med. 2023, 12, 19081–19090. [Google Scholar] [CrossRef]
- Cutolo, M.; Villaggio, B.; Foppiani, L.; Briata, M.; Sulli, A.; Pizzorni, C. The hypothalamic-pituitary-adrenal and gonadal axes in rheumatoid arthritis. Ann. N. Y. Acad. Sci. 2000, 917, 835–843. [Google Scholar] [CrossRef]
- Cutolo, M. Sex hormone adjuvant therapy in rheumatoid arthritis. Rheum. Dis. Clin. N. Am. 2000, 26, 881–895. [Google Scholar] [CrossRef]
- Koopman, F.A.; van Maanen, M.A.; Vervoordeldonk, M.J.; Tak, P.P. Balancing the autonomic nervous system to reduce inflammation in rheumatoid arthritis. J. Intern. Med. 2017, 282, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Syngle, V.; Syngle, A.; Garg, N.; Krishan, P.; Verma, I. Predictors of autonomic neuropathy in rheumatoid arthritis. Auton. Neurosci. 2016, 201, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Karemaker, J.M. An introduction into autonomic nervous function. Physiol. Meas. 2017, 38, R89–R118. [Google Scholar] [CrossRef]
- Mendiola, A.S.; Ryu, J.K.; Bardehle, S.; Meyer-Franke, A.; Ang, K.K.H.; Wilson, C.; Baeten, K.M.; Hanspers, K.; Merlini, M.; Thomas, S.; et al. Transcriptional profiling and therapeutic targeting of oxidative stress in neuroinflammation. Nat. Immunol. 2020, 21, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Louthrenoo, W.; Ruttanaumpawan, P.; Aramrattana, A.; Sukitawut, W. Cardiovascular autonomic nervous system dysfunction in patients with rheumatoid arthritis and systemic lupus erythematosus. QJM 1999, 92, 97–102. [Google Scholar] [CrossRef]
- Gullett, N.; Zajkowska, Z.; Walsh, A.; Harper, R.; Mondelli, V. Heart rate variability (HRV) as a way to understand associations between the autonomic nervous system (ANS) and affective states: A critical review of the literature. Int. J. Psychophysiol. 2023, 192, 35–42. [Google Scholar] [CrossRef]
- Vallerand, I.A.; Lewinson, R.T.; Frolkis, A.D.; Lowerison, M.W.; Kaplan, G.G.; Swain, M.G.; Patten, S.B.; Bulloch, A.G.M. Depression as a risk factor for the development of rheumatoid arthritis: A population-based cohort study. RMD Open 2018, 4, e000670. [Google Scholar] [CrossRef]
- Dhavale, H.S.; Gawande, S.; Bhagat, V.; Kalra, P.; Deo, S.S. Evaluation of efficacy and tolerability of dothiepin hydrochloride in the management of major depression in patients suffering from rheumatoid arthritis. J. Indian Med. Assoc. 2005, 103, 291–294. [Google Scholar]
- Hider, S.L.; Tanveer, W.; Brownfield, A.; Mattey, D.L.; Packham, J.C. Depression in RA patients treated with anti-TNF is common and under-recognized in the rheumatology clinic. Rheumatology 2009, 48, 1152–1154. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, D.; Salvadore, G.; Hsu, B.; Curran, M.; Casper, C.; Smolen, J.S. The effects of interleukin-6 neutralizing antibodies on symptoms of depressed mood and anhedonia in patients with rheumatoid arthritis and multicentric Castleman’s disease. Brain Behav. Immun. 2017, 66, 156–164. [Google Scholar] [CrossRef]
- Courties, A.; Berenbaum, F.; Sellam, J. Vagus nerve stimulation in musculoskeletal diseases. Joint Bone Spine 2021, 88, 105149. [Google Scholar] [CrossRef]
- Yan, Q.Q.; Sun, S.Y.; Tan, L.H.; Shi, Y.N.; Qiao, L.N.; Yang, Y.S. Effects of transcutaneous auricular vagus nerve stimulation on bone and cartilage destruction in rats with rheumatoid arthritis. Zhen Ci Yan Jiu 2022, 47, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.K.; Andersen, S.S.; Andersen, S.S.; Liboriussen, C.H.; Kristensen, S.; Jochumsen, M. Modulating Heart Rate Variability through Deep Breathing Exercises and Transcutaneous Auricular Vagus Nerve Stimulation: A Study in Healthy Participants and in Patients with Rheumatoid Arthritis or Systemic Lupus Erythematosus. Sensors 2022, 22, 7884. [Google Scholar] [CrossRef]
- Seca, S.; Kirch, S.; Cabrita, A.S.; Greten, H.J. Evaluation of the effect of acupuncture on hand pain, functional deficits and health-related quality of life in patients with rheumatoid arthritis—A study protocol for a multicenter, double-blind, randomized clinical trial. J. Integr. Med. 2016, 14, 219–227. [Google Scholar] [CrossRef]
- Hupin, D.; Sarajlic, P.; Venkateshvaran, A.; Börjesson, M.; Agewall, S.; Opava, C.H.; Brodin, N. Cardiovascular Autonomic Function Changes and Predictors During a 2-Year Physical Activity Program in Rheumatoid Arthritis: A PARA 2010 Substudy. Front. Med. 2021, 8, 788243. [Google Scholar] [CrossRef] [PubMed]
- Janse van Rensburg, D.C.; Ker, J.A.; Grant, C.C.; Fletcher, L. Effect of exercise on cardiac autonomic function in females with rheumatoid arthritis. Clin. Rheumatol. 2012, 31, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Ngô, T.L. Review of the effects of mindfulness meditation on mental and physical health and its mechanisms of action. Sante Mentale Au Quebec 2013, 38, 19–34. [Google Scholar] [CrossRef]
- Zautra, A.J.; Davis, M.C.; Reich, J.W.; Nicassario, P.; Tennen, H.; Finan, P.; Kratz, A. Comparison of cognitive behavioral and mindfulness meditation interventions on adaptation to rheumatoid arthritis for patients with and without history of recurrent depression. J. Consult. Clin. Psychol. 2008, 76, 408–421. [Google Scholar] [CrossRef]
- Bloxham, E.; Vagadia, V.; Scott, K.; Francis, G.; Saravanan, V.; Heycock, C.; Rynne, M.; Hamilton, J.; Kelly, C.A. Anaemia in rheumatoid arthritis: Can we afford to ignore it? Postgrad. Med. J. 2011, 87, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Liu, J.; Liu, S.; Wang, Y.; Wang, Y.; Li, J.; Wang, Y.; Zhang, X.; Li, Y.; Li, Z. Red Blood Cell-Related Parameters in Rheumatoid Arthritis: Clinical Value and Immunological Significance. J. Inflamm. Res. 2024, 17, 10641–10650. [Google Scholar] [CrossRef]
- Ford, J. Red blood cell morphology. Int. J. Lab. Hematol. 2013, 35, 351–357. [Google Scholar] [CrossRef]
- Mathru, M.; Solanki, D.R.; Woodson, L.C.; Funston, J.S.; Ozkan, A.K.; Roberts, P.R.; Lang, J.D.; Deyo, D.J. Splanchnic oxygen consumption is impaired during severe acute normovolemic anemia in anesthetized humans. Anesthesiology 2006, 105, 37–44. [Google Scholar] [CrossRef]
- Shah, J.; Farooq, A.; Zadran, S.; Kakar, Z.H.; Zarrar, M.; Bhatti, H. Prevalence of Anemia in Patients With Rheumatoid Arthritis Presenting at Multi-organization Tertiary Care Hospitals. Cureus 2024, 16, e72418. [Google Scholar] [CrossRef]
- Li, C.; Shi, X.; Chen, S.; Peng, X.; Zong, S. Novel mechanistic insights into the comorbidity of anemia and rheumatoid arthritis: Identification of therapeutic targets. Mol. Immunol. 2025, 180, 74–85. [Google Scholar] [CrossRef]
- Choudhury, C.; Sahib, A.; Karmakar, P.; Kar, S. Correlation of Serum Vitamin D and High-Density Lipoprotein (HDL) Cholesterol Levels With Disease Activity in Rheumatoid Arthritis: A Single-Center Experience From Eastern India. Cureus 2024, 16, e69333. [Google Scholar] [CrossRef]
- Vreugdenhil, G.; Wognum, A.W.; van Eijk, H.G.; Swaak, A.J. Anaemia in rheumatoid arthritis: The role of iron, vitamin B12, and folic acid deficiency, and erythropoietin responsiveness. Ann. Rheum. Dis. 1990, 49, 93–98. [Google Scholar] [CrossRef]
- Gluba-Brzózka, A.; Franczyk, B.; Olszewski, R.; Rysz, J. The Influence of Inflammation on Anemia in CKD Patients. Int. J. Mol. Sci. 2020, 21, 725. [Google Scholar] [CrossRef] [PubMed]
- Bertero, M.T.; Caligaris-Cappio, F. Anemia of chronic disorders in systemic autoimmune diseases. Haematologica 1997, 82, 375–381. [Google Scholar] [PubMed]
- Kheansaard, W.; Mas-Oo-Di, S.; Nilganuwong, S.; Tanyong, D.I. Interferon-gamma induced nitric oxide-mediated apoptosis of anemia of chronic disease in rheumatoid arthritis. Rheumatol. Int. 2013, 33, 151–156. [Google Scholar] [CrossRef]
- Weiss, G.; Ganz, T.; Goodnough, L.T. Anemia of inflammation. Blood 2019, 133, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Maury, C.P.; Andersson, L.C.; Teppo, A.M.; Partanen, S.; Juvonen, E. Mechanism of anaemia in rheumatoid arthritis: Demonstration of raised interleukin 1 beta concentrations in anaemic patients and of interleukin 1 mediated suppression of normal erythropoiesis and proliferation of human erythroleukaemia (HEL) cells in vitro. Ann. Rheum. Dis. 1988, 47, 972–978. [Google Scholar] [CrossRef]
- Stuart, C.M.; Jacob, C.; Varatharaj, A.; Grant, M.M.; Carden, M.J.; Spector, T.D.; Menni, C.; Berry, S.E.; Frost, G.S.; Chan, Q.; et al. Mild Systemic Inflammation Increases Erythrocyte Fragility. Int. J. Mol. Sci. 2024, 25, 7027. [Google Scholar] [CrossRef]
- Fulton, R.A. Megaloblastic anaemia and methotrexate treatment. Br. J. Dermatol. 1986, 114, 267–269. [Google Scholar] [CrossRef]
- Hurd, E.R.; Ziff, M. Studies on the anti-inflammatory action of 6-mercaptopurine. J. Exp. Med. 1968, 128, 785–800. [Google Scholar] [CrossRef] [PubMed]
- van Santen, S.; de Mast, Q.; Oosting, J.D.; van Ede, A.; Swinkels, D.W.; van der Ven, A.J. Hematologic parameters predicting a response to oral iron therapy in chronic inflammation. Haematologica 2014, 99, e171–e173. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.L.; Chan, F.K.; Lanas, A.; Peura, D.A.; Wilcox, C.M.; Fort, J.G.; Bello, A.E.; Huang, B.; Bauer, R.S.; Reasner, C.A.; et al. Haemoglobin decreases in NSAID users over time: An analysis of two large outcome trials. Aliment. Pharmacol. Ther. 2011, 34, 808–816. [Google Scholar] [CrossRef]
- Song, J.; Zhang, Y.; Li, A.; Wang, Y.; Chen, S.; Li, Y.; Wang, J. Prevalence of anemia in patients with rheumatoid arthritis and its association with dietary inflammatory index: A population-based study from NHANES 1999 to 2018. Medicine 2024, 103, e38471. [Google Scholar] [CrossRef]
- Staykova, N.D. Rheumatoid arthritis and thyroid abnormalities. Folia Med. 2007, 49, 5–12. [Google Scholar]
- Gu, P.; Pu, B.; Ma, Y.; Li, J.; Zhang, Y.; Wang, Y.; Zhang, Y.; Liu, Y.; Chen, Y.; Li, Z.; et al. Appraising the causal relationship between thyroid function and rheumatoid arthritis: A two-sample bidirectional Mendelian randomization study. Front. Immunol. 2023, 14, 1238757. [Google Scholar] [CrossRef]
- Lichtiger, A.; Fadaei, G.; Tagoe, C.E. Autoimmune thyroid disease and rheumatoid arthritis: Where the twain meet. Clin. Rheumatol. 2024, 43, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Lazúrová, I.; Jochmanová, I.; Benhatchi, K.; Sotak, S. Autoimmune thyroid disease and rheumatoid arthritis: Relationship and the role of genetics. Immunol. Res. 2014, 60, 193–200. [Google Scholar] [CrossRef]
- Rasmussen, A.K. Cytokine actions on the thyroid gland. Dan. Med. Bull. 2000, 47, 94–114. [Google Scholar] [PubMed]
- Zhai, Y.; Wang, B.; Han, W.; Yu, B.; Ci, J.; An, F. Correlation between systemic inflammatory response index and thyroid function: 2009–2012 NHANES results. Front. Endocrinol. 2023, 14, 1305386. [Google Scholar] [CrossRef]
- Liu, X.; Yuan, J.; Wang, X.; Li, J.; Zhang, Y.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Association between rheumatoid arthritis and autoimmune thyroid disease: Evidence from complementary genetic methods. Endocrine 2024, 84, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Shiroky, J.B.; Cohen, M.; Ballachey, M.L.; Neville, C. Thyroid dysfunction in rheumatoid arthritis: A controlled prospective survey. Ann. Rheum. Dis. 1993, 52, 454–456. [Google Scholar] [CrossRef]
- Silman, A.J.; Ollier, W.E.; Bubel, M.A. Autoimmune thyroid disease and thyroid autoantibodies in rheumatoid arthritis patients and their families. Br. J. Rheumatol. 1989, 28, 18–21. [Google Scholar] [CrossRef]
- Waldenlind, K.; Delcoigne, B.; Saevarsdottir, S.; Askling, J. Does autoimmune thyroid disease affect rheumatoid arthritis disease activity or response to methotrexate? RMD Open 2020, 6, e001282. [Google Scholar] [CrossRef]
- Lebiedziński, F.; Lisowska, K.A. Impact of Vitamin D on Immunopathology of Hashimoto’s Thyroiditis: From Theory to Practice. Nutrients 2023, 15, 3174. [Google Scholar] [CrossRef]
- Fedorchenko, Y.; Yessirkepov, M.; Doskaliuk, B.; Zaiats, L.; Mahmudov, K. Thyroid disease as a comorbidity in inflammatory rheumatic diseases. Rheumatol. Int. 2025, 45, 46. [Google Scholar] [CrossRef]
- Givian, A.; Azizan, A.; Jamshidi, A.; Mahmoudi, M.; Farhadi, E. Iron metabolism in rheumatic diseases. J. Transl. Autoimmun. 2025, 10, 100267. [Google Scholar] [CrossRef]
- Yang, Z.; Matteson, E.L.; Goronzy, J.J.; Weyand, C.M. T-cell metabolism in autoimmune disease. Arthritis Res. Ther. 2015, 17, 29. [Google Scholar] [CrossRef] [PubMed]
- Pal, Y.; Bandyopadhyay, N.; Pal, R.S.; Ahmed, S.; Bandopadhyay, S. Perspective and Potential of A2A and A3 Adenosine Receptors as Therapeutic Targets for the Treatment of Rheumatoid Arthritis. Curr. Pharm. Des. 2019, 25, 2859–2874. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, M.; Liu, S. Collagen-Induced Arthritis Models. Methods Mol. Biol. 2024, 2766, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, N.; Bhatt, L.K.; Prabhavalkar, K.S. Experimental animal models for rheumatoid arthritis. Immunopharmacol. Immunotoxicol. 2018, 40, 193–200. [Google Scholar] [CrossRef]
- Feng, Z.T.; Yang, T.; Hou, X.Q.; Yan, L.H.; Wang, G.C. Sinomenine mitigates collagen-induced arthritis mice by inhibiting angiogenesis. Biomed. Pharmacother. 2019, 113, 108759. [Google Scholar] [CrossRef]
- Ma, F.; Li, Z.; Liu, H.; Zhang, X.; Jiang, H.; Li, X.; Zhao, Y.; Wang, Y.; Chen, W.; Liu, X.; et al. Dietary-timing-induced gut microbiota diurnal oscillations modulate inflammatory rhythms in rheumatoid arthritis. Cell Metab. 2024, 36, 2367–2382.e5. [Google Scholar] [CrossRef]
- Lange, F.; Bajtner, E.; Rintisch, C.; Nandakumar, K.S.; Sack, U.; Holmdahl, R. Methotrexate ameliorates T cell dependent autoimmune arthritis and encephalomyelitis but not antibody induced or fibroblast induced arthritis. Ann. Rheum. Dis. 2005, 64, 599–605. [Google Scholar] [CrossRef]
- Khachigian, L.M. Collagen antibody-induced arthritis. Nat. Protoc. 2006, 1, 2512–2516. [Google Scholar] [CrossRef]
- Okano, T.; Ashida, H.; Komatsu, N.; Nakano, Y.; Yamaguchi, T.; Oda, Y.; Ohya, K.; Itoh, S.; Okada, Y.; Matsushita, K. Caspase-11 mediated inflammasome activation in macrophages by systemic infection of A. actinomycetemcomitans exacerbates arthritis. Int. J. Oral Sci. 2024, 16, 54. [Google Scholar] [CrossRef]
- Tu, J.; Chen, W.; Huang, W.; Li, J.; Zhang, Y.; Wang, Y.; Chen, S.; Li, Z. Positive feedback loop PU.1-IL9 in Th9 promotes rheumatoid arthritis development. Ann. Rheum. Dis. 2024, 83, 1707–1721. [Google Scholar] [CrossRef]
- Liu, S. Human Xenograft Model. Methods Mol. Biol. 2024, 2766, 9–15. [Google Scholar] [CrossRef]
- Kong, J.S.; Jeong, G.H.; Yoo, S.A. The use of animal models in rheumatoid arthritis research. Yeungnam Univ. J. Med. 2023, 40, 23–29. [Google Scholar] [CrossRef]
- Taneja, V.; Taneja, N.; Paisansinsup, T.; Behrens, M.; Griffiths, M.; Luthra, H.; David, C.S. CD4 and CD8 T cells in susceptibility/protection to collagen-induced arthritis in HLA-DQ8-transgenic mice: Implications for rheumatoid arthritis. J. Immunol. 2002, 168, 5867–5875. [Google Scholar] [CrossRef] [PubMed]
- Muruganandam, A.; Migliorini, F.; Jeyaraman, M.; Maffulli, N.; Udayakumar, P.D.; Jeyaraman, N. Molecular Mimicry Between Gut Microbiome and Rheumatoid Arthritis: Current Concepts. Med. Sci. 2024, 12, 72. [Google Scholar] [CrossRef]
- Lamba, A.; Taneja, V. Gut microbiota as a sensor of autoimmune response and treatment for rheumatoid arthritis. Immunol. Rev. 2024, 325, 90–106. [Google Scholar] [CrossRef]
- Skarpanska-Stejnborn, A.; Pilaczynska-Szczesniak, L.; Basta, P.; Deskur-Smielecka, E. The influence of supplementation with Rhodiola rosea L. extract on selected redox parameters in professional rowers. Int. J. Sport Nutr. Exerc. Metab. 2009, 19, 186–199. [Google Scholar] [CrossRef] [PubMed]
- De Bock, K.; Eijnde, B.O.; Ramaekers, M.; Hespel, P. Acute Rhodiola rosea intake can improve endurance exercise performance. Int. J. Sport Nutr. Exerc. Metab. 2004, 14, 298–307. [Google Scholar] [CrossRef]
- Huang, S.C.; Lee, F.T.; Kuo, T.Y.; Yang, J.H.; Chien, C.T. Attenuation of long-term Rhodiola rosea supplementation on exhaustive swimming-evoked oxidative stress in the rat. Chin. J. Physiol. 2009, 52, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Shen, F.; Wang, X.; Zhang, Y.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Reshaped Gut Microbial Composition and Functions Associated with the Antifatigue Effect of Salidroside in Exercise Mice. Mol. Nutr. Food Res. 2023, 67, e2300015. [Google Scholar] [CrossRef]
- Ni, J.; Li, Y.; Li, W.; Guo, R. Salidroside protects against foam cell formation and apoptosis, possibly via the MAPK and AKT signaling pathways. Lipids Health Dis. 2017, 16, 198. [Google Scholar] [CrossRef]
- Guo, W.; Huang, R.; Bian, J.; Zhang, Y.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Salidroside ameliorates macrophages lipid accumulation and atherosclerotic plaque by inhibiting Hif-1α-induced pyroptosis. Biochem. Biophys. Res. Commun. 2025, 742, 151104. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, L.; Zeng, Y.; Zhang, Y.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Salidroside alleviates cognitive impairment by inhibiting ferroptosis via activation of the Nrf2/GPX4 axis in SAMP8 mice. Phytomedicine 2023, 114, 154762. [Google Scholar] [CrossRef]
- Cui, Z.; Jin, N.; Amevor, F.K.; Du, X.; Zhang, Y.; Shu, G.; Zhao, X.; Tian, Y.; Zhu, Q. Dietary supplementation of salidroside alleviates liver lipid metabolism disorder and inflammatory response to promote hepatocyte regeneration via PI3K/AKT/Gsk3-β pathway. Poult. Sci. 2022, 101, 102034. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, D.; Xu, H.; Zhang, Y.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Salidroside Activates the AMP-Activated Protein Kinase Pathway to Suppress Nonalcoholic Steatohepatitis in Mice. Hepatology 2021, 74, 3056–3073. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, R.; Chen, Q.; Tian, Y.; Gao, L.; Lei, A. Salidroside improves porcine oocyte maturation and subsequent embryonic development by promoting lipid metabolism. Theriogenology 2022, 192, 89–96. [Google Scholar] [CrossRef]
- Wang, Y.F.; Chang, Y.Y.; Zhang, X.M.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Salidroside protects against osteoporosis in ovariectomized rats by inhibiting oxidative stress and promoting osteogenesis via Nrf2 activation. Phytomedicine 2022, 99, 154020. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.L.; Deng, X.L.; Wu, G.J.; Li, W.J.; Jin, S. Rhodiola and salidroside in the treatment of metabolic disorders. Mini-Rev. Med. Chem. 2019, 19, 1611–1626. [Google Scholar] [CrossRef]
- Ma, C.; Wang, J.; Hong, F.; Yang, S. Mitochondrial Dysfunction in Rheumatoid Arthritis. Biomolecules 2022, 12, 1216. [Google Scholar] [CrossRef]
- Wang, X.L.; Sun, R.X.; Li, D.X.; Zhang, Y.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Salidroside Regulates Mitochondrial Homeostasis After Polarization of RAW264.7 Macrophages. J. Cardiovasc. Pharmacol. 2023, 81, 85–92. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, M.; Zhang, Z.; Zhou, S. Salidroside induces mitochondrial dysfunction and ferroptosis to inhibit melanoma progression through reactive oxygen species production. Exp. Cell Res. 2024, 438, 114034. [Google Scholar] [CrossRef] [PubMed]
- Panfili, E.; Gerli, R.; Grohmann, U.; Pallotta, M.T. Amino Acid Metabolism in Rheumatoid Arthritis: Friend or Foe? Biomolecules 2020, 10, 1280. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Harms, A.C.; van Wijk, E.; Hankemeier, T.; van der Greef, J.; Wang, M. Role of amino acids in rheumatoid arthritis studied by metabolomics. Int. J. Rheum. Dis. 2019, 22, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Jia, F.; Wei, J.; Zhang, Y.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Salidroside protects against homocysteine-induced injury in human umbilical vein endothelial cells via the regulation of endoplasmic reticulum stress. Cardiovasc. Ther. 2017, 35, 33–39. [Google Scholar] [CrossRef]
- Chiang, H.M.; Chien, Y.C.; Wu, C.H.; Kuo, Y.H.; Wu, W.C.; Pan, Y.Y.; Su, Y.H.; Wen, K.C. Hydroalcoholic extract of Rhodiola rosea L. (Crassulaceae) and its hydrolysate inhibit melanogenesis in B16F0 cells by regulating the CREB/MITF/tyrosinase pathway. Food Chem. Toxicol. 2014, 65, 129–139. [Google Scholar] [CrossRef]
- Xue, F.; Guo, H.; Hu, Y.; Liu, R.; Huang, L.; Lv, Z.; Liu, T. Expression of Codon-Optimized Plant Glycosyltransferase UGT72B14 in Escherichia coli Enhances Salidroside Production. BioMed Res. Int. 2016, 2016, 9845927. [Google Scholar] [CrossRef]
- Xu, L.; Chang, C.; Jiang, P.; Zhang, Y.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Metabolomics in rheumatoid arthritis: Advances and review. Front. Immunol. 2022, 13, 961708. [Google Scholar] [CrossRef]
- Casanova-Vallve, N.; Constantin-Teodosiu, D.; Filer, A.; Hardy, R.S.; Greenhaff, P.L.; Chapman, V. Skeletal muscle dysregulation in rheumatoid arthritis: Metabolic and molecular markers in a rodent model and patients. PLoS ONE 2020, 15, e0235702. [Google Scholar] [CrossRef]
- Kim, S.; Miller, B.J.; Stefanek, M.E.; Miller, A.H. Inflammation-induced activation of the indoleamine 2,3-dioxygenase pathway: Relevance to cancer-related fatigue. Cancer 2015, 121, 2129–2136. [Google Scholar] [CrossRef]
- Qin, N.; Xie, H.; Zhao, A.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Effects of salidroside on exercise tolerance of mice under high altitude hypoxia environment. J. Zhejiang Univ. Med. Sci. 2022, 51, 397–404. [Google Scholar] [CrossRef]
- Smallwood, M.J.; Nissim, A.; Knight, A.R.; Whiteman, M.; Haigh, R.; Winyard, P.G. Oxidative stress in autoimmune rheumatic diseases. Free Radic. Biol. Med. 2018, 125, 3–14. [Google Scholar] [CrossRef]
- Tinsley, G.M.; Jagim, A.R.; Potter, G.D.M.; Garner, D.; Galpin, A.J. Rhodiola rosea as an adaptogen to enhance exercise performance: A review of the literature. Br. J. Nutr. 2024, 131, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Zhang, Y.; Jiang, S.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Salidroside intensifies mitochondrial function of CoCl2-damaged HT22 cells by stimulating PI3K-AKT-MAPK signaling pathway. Phytomedicine 2023, 109, 154568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Z.; Song, J.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Salidroside promotes healthy longevity by interfering with HSP90 activity. GeroScience 2024, 46, 1641–1655. [Google Scholar] [CrossRef]
- Chen, L.; Liu, P.; Feng, X.; Ma, C. Salidroside suppressing LPS-induced myocardial injury by inhibiting ROS-mediated PI3K/Akt/mTOR pathway in vitro and in vivo. J. Cell. Mol. Med. 2017, 21, 3178–3189. [Google Scholar] [CrossRef]
- Clanchy, F.I.L.; Williams, R.O. Ibudilast Inhibits Chemokine Expression in Rheumatoid Arthritis Synovial Fibroblasts and Exhibits Immunomodulatory Activity in Experimental Arthritis. Arthritis Rheumatol. 2019, 71, 703–711. [Google Scholar] [CrossRef]
- Maiuolo, J.; Muscoli, C.; Gliozzi, M.; Carresi, C.; Scarano, F.; Coppoletta, A.R.; Guarnieri, L.; Nusca, A.; Mollace, V. Endothelial Dysfunction and Extra-Articular Neurological Manifestations in Rheumatoid Arthritis. Biomolecules 2021, 11, 81. [Google Scholar] [CrossRef]
- Li, F.; Mao, Q.; Wang, J.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Salidroside inhibited cerebral ischemia/reperfusion-induced oxidative stress and apoptosis via Nrf2/Trx1 signaling pathway. Metab. Brain Dis. 2022, 37, 2965–2978. [Google Scholar] [CrossRef]
- Ju, I.J.; Tsai, B.C.; Kuo, W.W.; Ho, T.J.; Day, C.H.; Mahalakshmi, B.; Huang, C.Y. Rhodiola and Salidroside Attenuate Oxidative Stress-Triggered H9c2 Cardiomyoblast Apoptosis Through IGF1R-Induced ERK1/2 Activation. Environ. Toxicol. 2024, 39, 5150–5161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ge, H.; Yu, Y.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Dietary salidroside supplementation improves meat quality and antioxidant capacity and regulates lipid metabolism in broilers. Food Chem. 2024, 22, 101406. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.M.; Huang, Y.M.; Qin, Z.Q. Salidroside Exerts Beneficial Effect on Testicular Ischemia-Reperfusion Injury in Rats. Oxid. Med. Cell. Longev. 2022, 2022, 8069152. [Google Scholar] [CrossRef]
- Sun, Y.M.; Wang, X.Y.; Zhou, X.R.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Salidroside Ameliorates Radiation Damage by Reducing Mitochondrial Oxidative Stress in the Submandibular Gland. Antioxidants 2022, 11, 1414. [Google Scholar] [CrossRef]
- Gautam, S.; Kumar, R.; Kumar, U.; Kumar, S.; Luthra, K.; Dada, R. Yoga maintains Th17/Treg cell homeostasis and reduces the rate of T cell aging in rheumatoid arthritis: A randomized controlled trial. Sci. Rep. 2023, 13, 14924. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Tong, Y.; Wu, L.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Alteration of Gut Microbiota in Individuals at High-Risk for Rheumatoid Arthritis Associated With Disturbed Metabolome and the Initiation of Arthritis Through the Triggering of Mucosal Immunity Imbalance. Arthritis Rheumatol. 2023, 75, 1736–1748. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Feng, Y.; Zheng, X.; Li, Y.; Wang, J.; Chen, S.; Li, Z. M2-type exosomes nanoparticles for rheumatoid arthritis therapy via macrophage re-polarization. J. Control. Release 2022, 341, 16–30. [Google Scholar] [CrossRef]
- Guo, Q.; Mao, X.; Zhang, Y.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Guizhi-Shaoyao-Zhimu decoction attenuates rheumatoid arthritis partially by reversing inflammation-immune system imbalance. J. Transl. Med. 2016, 14, 165. [Google Scholar] [CrossRef]
- Zheng, Q.H.; Du, L.Y.; Zhao, Y.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Mechanism of Rhodiola rosea-Euonymus alatus drug pair against rheumatoid arthritis: Network pharmacology and experimental validation. Immun. Inflamm. Dis. 2023, 11, e1127. [Google Scholar] [CrossRef]
- Tang, L.; Guo, D.; Jia, D.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Exploring the therapeutic potential of “Tianyu” medicine pair in rheumatoid arthritis: An integrated study combining LC-MS/MS, bioinformatics, network pharmacology, and experimental validation. Front. Med. 2024, 11, 1475239. [Google Scholar] [CrossRef]
- Chen, T.; Li, S.; Lian, D.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Integrated Network Pharmacology and Experimental Approach to Investigate the Protective Effect of Jin Gu Lian Capsule on Rheumatoid Arthritis by Inhibiting Inflammation via IL-17/NF-κB Pathway. Drug Des. Devel. Ther. 2023, 17, 3723–3748. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, M.; Dai, Z.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Salidroside alleviates ulcerative colitis via inhibiting macrophage pyroptosis and repairing the dysbacteriosis-associated Th17/Treg imbalance. Phytother. Res. 2023, 37, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Mihai, I.R.; Rezus, C.; Burlui, M.A.; Cardoneanu, A.; Macovei, L.A.; Bratoiu, I.; Rezus, E. Autoimmune Liver Diseases and Rheumatoid Arthritis—Is There an Etiopathogenic Link? Int. J. Mol. Sci. 2024, 25, 3848. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Guo, Z.; Liao, Q.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Network Pharmacology and Machine Learning Reveal Salidroside’s Mechanisms in Idiopathic Pulmonary Fibrosis Treatment. J. Inflamm. Res. 2024, 17, 9453–9467. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Du, X.; Zhou, Y.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Drp1-mediated mitochondrial fission promotes pulmonary fibrosis progression through the regulation of lipid metabolic reprogramming by ROS/HIF-1α. Cell. Signal. 2024, 117, 111075. [Google Scholar] [CrossRef]
- Cai, L.; Li, Y.; Zhang, Q.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Salidroside protects rat liver against ischemia/reperfusion injury by regulating the GSK-3β/Nrf2-dependent antioxidant response and mitochondrial permeability transition. Eur. J. Pharmacol. 2017, 806, 32–42. [Google Scholar] [CrossRef]
- Wilson, A.; Yu, H.T.; Goodnough, L.T.; Nissenson, A.R. Prevalence and outcomes of anemia in rheumatoid arthritis: A systematic review of the literature. Am. J. Med. 2004, 116 (Suppl. S7A), 50S–57S. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, L.; Xiong, D.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Salidroside affects the Th17/Treg cell balance in aplastic anemia via the STAT3/HIF-1α/RORγt pathway. Redox Rep. 2023, 28, 2225868. [Google Scholar] [CrossRef]
- Li, X.; Sipple, J.; Pang, Q.; Du, W. Salidroside stimulates DNA repair enzyme Parp-1 activity in mouse HSC maintenance. Blood 2012, 119, 4162–4173. [Google Scholar] [CrossRef]
- Zhang, X.S.; Zhu, B.D.; Hung, X.Q.; Chen, Y.F. Effect of salidroside on bone marrow cell cycle and expression of apoptosis-related proteins in bone marrow cells of bone marrow depressed anemia mice. Sichuan Da Xue Xue Bao Yi Xue Ban 2005, 36, 820–823, 846. [Google Scholar]
- Zhu, L.; Wei, T.; Gao, J.; Chang, X.; He, H.; Miao, M.; Yan, T. Salidroside attenuates lipopolysaccharide (LPS) induced serum cytokines and depressive-like behavior in mice. Neurosci. Lett. 2015, 606, 1–6. [Google Scholar] [CrossRef]
- Doroszkiewicz, J.; Mroczko, J.; Winkel, I.; Mroczko, B. Metabolic and Immune System Dysregulation: Unraveling the Connections between Alzheimer’s Disease, Diabetes, Inflammatory Bowel Diseases, and Rheumatoid Arthritis. J. Clin. Med. 2024, 13, 5057. [Google Scholar] [CrossRef]
- Zhang, J.; Zhen, Y.F.; Pu Bu Ci, R.; Wang, W.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Salidroside attenuates beta amyloid-induced cognitive deficits via modulating oxidative stress and inflammatory mediators in rat hippocampus. Behav. Brain Res. 2013, 244, 70–81. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Wu, Y.; Li, T.; Wang, W. Salidroside shows anticonvulsant and neuroprotective effects by activating the Nrf2-ARE pathway in a pentylenetetrazol-kindling epileptic model. Brain Res. Bull. 2020, 164, 14–20. [Google Scholar] [CrossRef]
- Liang, L.; Ma, Z.; Dong, M.; Ma, J.; Jiang, A.; Sun, X. Protective effects of salidroside against isoflurane-induced cognitive impairment in rats. Hum. Exp. Toxicol. 2017, 36, 1295–1302. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Q.; Cheng, Q.; Ding, F. Protective effect of salidroside against H2O2-induced cell apoptosis in primary culture of rat hippocampal neurons. Mol. Cell. Biochem. 2009, 332, 85–93. [Google Scholar] [CrossRef]
- Li, X.; Ye, X.; Li, X.; Li, X.; Li, H.; Zhang, Z.; Lin, A.; Liu, K.; Chen, X.; Xu, R. Salidroside protects against MPP+-induced apoptosis in PC12 cells by inhibiting the NO pathway. Brain Res. 2011, 1382, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Wang, Z.; Ji, Y.; Yin, J.; Yang, W.H.; Ren, L.M. Effects of Salidroside on Tic Behavior of Tourette Syndrome Model Rats. Zhongguo Zhong Xi Yi Jie He Za Zhi 2016, 36, 90–93. [Google Scholar] [PubMed]
- Cruz-Pereira, J.S.; Rea, K.; Nolan, Y.M.; O’Leary, O.F.; Dinan, T.G.; Cryan, J.F. Depression’s Unholy Trinity: Dysregulated Stress, Immunity, and the Microbiome. Annu. Rev. Psychol. 2020, 71, 49–78. [Google Scholar] [CrossRef] [PubMed]
- Hassamal, S. Chronic stress, neuroinflammation, and depression: An overview of pathophysiological mechanisms and emerging anti-inflammatories. Front. Psychiatry 2023, 14, 1130989. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Jung, J.; Lee, S.M.; Lee, M.J.; Kim, J.E.; Lee, J.E.; Hahm, D.H.; Shim, I. Lipidomics reveals that acupuncture modulates the lipid metabolism and inflammatory interaction in a mouse model of depression. Brain Behav. Immun. 2021, 94, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Ren, T.; Xiong, Q.; Lin, Z.; Lin, X.; Lin, G. Salidroside alleviates diet-induced obesity and insulin resistance by activating Nrf2/ARE pathway and enhancing the thermogenesis of adipose tissues. Food Sci. Nutr. 2023, 11, 4735–4744. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xu, T.; Zhou, F.; Wang, Y.; Wang, J.; Chen, S.; Li, Z. Neuroprotective Effects of Four Phenylethanoid Glycosides on H2O2-Induced Apoptosis on PC12 Cells via the Nrf2/ARE Pathway. Int. J. Mol. Sci. 2018, 19, 1135. [Google Scholar] [CrossRef] [PubMed]
- Veenema, A.H.; Koolhaas, J.M.; de Kloet, E.R. Basal and stress-induced differences in HPA axis, 5-HT responsiveness, and hippocampal cell proliferation in two mouse lines. Ann. N. Y. Acad. Sci. 2004, 1018, 255–265. [Google Scholar] [CrossRef]
- Ao, W.; Gao, W.; Li, T. Research progress on the mechanism of antidepressant effect of salidroside. Int. Immunopharmacol. 2025, 162, 115205. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Lin, L.; Wang, Y.; Wang, J.; Chen, S.; Li, Z. Pharmacokinetics, tissue distribution, and excretion of salidroside in rats. Planta Med. 2013, 79, 1429–1433. [Google Scholar] [CrossRef]
- Guo, N.; Hu, Z.; Fan, X.; Zheng, J.; Zhang, Z.; Shang, L.; Wang, C.; Li, Y. Simultaneous determination of salidroside and its aglycone metabolite p-tyrosol in rat plasma by liquid chromatography-tandem mass spectrometry. Molecules 2012, 17, 4733–4754. [Google Scholar] [CrossRef]
- He, Y.-X.; Liu, X.-D.; Wang, X.-T.; Liu, X.; Wang, G.-J.; Xie, L. Sodium-dependent Glucose Transporter Was Involved in Salidroside Absorption in Intestine of Rats. Chin. J. Nat. Med. 2009, 7, 444–448. [Google Scholar] [CrossRef]
- Mao, Y.; Zhang, X.; Zhang, X.; Lu, G. Development of an HPLC method for the determination of salidroside in beagle dog plasma after administration of salidroside injection: Application to a pharmacokinetics study. J. Sep. Sci. 2007, 30, 3218–3222. [Google Scholar] [CrossRef]
- Gan, Z.; Fang, X.; Yin, C.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Extraction, purification, structural characterization, and bioactivities of the genus Rhodiola L. polysaccharides: A review. Int. J. Biol. Macromol. 2024, 276, 133614. [Google Scholar] [CrossRef]
- Zhu, J.; Wan, X.; Zhu, Y.; Ma, X.; Zheng, Y.; Zhang, T. Evaluation of salidroside in vitro and in vivo genotoxicity. Drug Chem. Toxicol. 2010, 33, 220–226. [Google Scholar] [CrossRef]
- Zdanowski, R.; Skopińska-Różewska, E.; Wilczak, J.; Borecka, A.; Lewicka, A.; Lewicki, S. Different effects of feeding pregnant and lactating mice Rhodiola kirilowii aqueous and hydro-alcoholic extracts on their serum angiogenic activity and content of selected polyphenols. Cent. Eur. J. Immunol. 2017, 42, 17–23. [Google Scholar] [CrossRef]
- Wang, X.; Tang, Y.; Xie, N.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Salidroside, a phenyl ethanol glycoside from Rhodiola crenulata, orchestrates hypoxic mitochondrial dynamics homeostasis by stimulating Sirt1/p53/Drp1 signaling. J. Ethnopharmacol. 2022, 293, 115278. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Jakstas, V.; Kopustinskiene, D.M. Phenolic Compounds of Rhodiola rosea L. as the Potential Alternative Therapy in the Treatment of Chronic Diseases. Int. J. Mol. Sci. 2023, 24, 12293. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, J.; Zeng, A.; Song, L. Pharmacological functions of salidroside in renal diseases: Facts and perspectives. Front. Pharmacol. 2023, 14, 1309598. [Google Scholar] [CrossRef]
- Liu, H.; Tian, Y.; Zhou, Y.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Multi-modular engineering of Saccharomyces cerevisiae for high-titre production of tyrosol and salidroside. Microb. Biotechnol. 2021, 14, 2605–2616. [Google Scholar] [CrossRef]
- Di Felice, G.; Colombo, P. Nanoparticle-allergen complexes for allergen immunotherapy. Int. J. Nanomedicine 2017, 12, 4493–4504. [Google Scholar] [CrossRef]
- Jiang, S.; Li, H.; Zhang, L.; Li, Y.; Wang, J.; Chen, S.; Li, Z. Generic Diagramming Platform (GDP): A comprehensive database of high-quality biomedical graphics. Nucleic Acids Res. 2025, 53, D1670–D1676. [Google Scholar] [CrossRef]

| smDMARDs | tsDMARDs | PIs | SAL | |
|---|---|---|---|---|
| Chemical nature | Artificially synthesized small molecules | Biological macromolecules (antibodies) | Artificially synthesized peptide small molecules | Small molecules of natural plant origin |
| Mode of operation | Inhibit a single kinase or enzyme | Neutralize single cytokines | Inhibit the intracellular proteasome complex | Regulate the core signal hub (NF-κB, Nrf2) |
| Advantage | Low cost, exact efficacy, basic drug | High potency, strong specificity, oral convenience, quick action | Can induce proinflammatory cell apoptosis | Multiple benefits (anti-inflammatory + antioxidant), high safety potential, natural source |
| Disadvantage | Non-specific and with many side effects | Immunogenicity, high cost, and low selectivity | High toxicity (neuropathy, cytopenia | Slow onset, high dose, and complex mechanism |
| Whole Body System | Specific Mechanism |
|---|---|
| Endocrine system | Thyroid abnormalities [19] |
| Central nervous system | HPA axis function, neuroinflammation, and the gut microbiota–gut–brain axis [20] |
| Peripheral nervous system | Stimulation of the vagus nerve via inflammation [21] |
| Metabolic function | Decreased aerobic exercise [22] Metabolite accumulation [23] Decreased free energy of ATP breakdown [24] Mitochondrial dysfunction disorders [25] |
| Immune system | Changes in inflammatory factor levels [26] Th1/Th2 cell imbalance [27] |
| Depression | Overexpression of the main iron regulatory hormone hepcidin [28] |
| Oxidative stress | Oxidative stress [29] |
| Others | Viral infection; circadian rhythm disorder [30] |
| Major Biological Effects | Mechanism | In Vivo/Vitro Models |
|---|---|---|
| Improve exercise endurance | Inhibit the production of O2(-)* in blood and liver. Increase the plasma concentrations of malondialdehyde. Upward adjustment of the expressions of Mn-superoxide dismutase, Cu/Zn-superoxide dismutase, and catalase | Acute intake of Rhodiola rosea experiment Swimming-induced oxidative stress experiment in rats |
| Energy metabolism effects | ||
| (1) Glycogen storage effects | Enhance endurance and fight fatigue (mitochondrial function) Inhibit foam cell formation (anti-atherosclerosis) | Rat weight-bearing experiment Macrophage induction program |
| (2) Promote lipid metabolism | Enhances mitochondrial metabolism, iron metabolism, lipid metabolism, and redox metabolism in the brains Inhibit the generation of ROS and lipid accumulation through PI3K/AKT/Gsk3-β Activate AMPK signal transduction Galactose metabolism and fatty acid metabolism | Fatty liver model in laying hens Lipid accumulation model of primary hepatocytes |
| (3) Repair mitochondrial dysfunction | Anti-polarization of M1 macrophages and protection of mitochondrial function Inhibit the growth, proliferation, migration and metabolism of melanoma | Macrophage cell lines of mice or humans Human melanoma cell lines |
| Regulate amino acid metabolism | CREB/MITF/tyrosinase pathway Bip, CHOP, p-PERK, p-IRE1α | CIA mouse model Cytotoxicity model of HUVECs induced by hcy |
| Reduce metabolite accumulation | Regulating homeostasis dysregulation Reduce oxidative stress damage Reduce oxidative stress, reduce MDA, H2O2 and lactate levels in liver and muscle tissue | CIA Model Mouse exhaustion experiment |
| Antioxidant activity | Directly bind to and inhibit HSP90 Target and inhibit the iNOS/COX-2 and NF-κB pathways, and activate the PI3K/Akt/mTOR pathway Reduce infarct area, inhibit apoptosis (cleaved caspase-3, Bax/Bcl-2), lower MDA, and upregulate SOD, CAT, Nrf2, and Trx1. Activate the IGF1R and p-ERK1/2 signaling pathways | Lps-induced injury model H9C2 cardiomyocytes MCAO rat model (cerebral artery occlusion) PC12 cells (OGD/R model) Testicular ischemia-reperfusion model |
| Regulate immune system | Inhibit the PI3K/AKT signaling pathway Regulate the HDAC1-HSP90AA1-NFKB2-IKBK-TNF-α axis IL-17/nf-kappa B GSK-3β/nrf2 STAT3/HIF-1α/RORγt pathway Regulate the cell cycle and the expression of downstream genes | CIA rat model TNF-α -induced HFLS-RA model Mouse model of colitis Hepatocyte death and apoptosis model |
| Interfere with the synthesis and release of neurotransmitters in the brain | Increase the expression of BDNF/TrkB and reduce the levels of NE and 5-HT Inhibit NADPH oxidase-mediated oxidative stress, suppress NF-κB activation, and downregulate inflammatory factors such as COX-2 and iNOS Reduce apoptosis and mitochondrial membrane potential collapse, and inhibit the accumulation of NO, nNOS, iNOS, ROS and intracellular Ca2+. Activating the Nrf2/ARE pathway for antioxidant effects, inhibiting the P2X7/NF-κB/NLRP3 axis for anti-inflammatory effects, reducing IL-6 and TNF-α, enhancing monoaminergic neurotransmission (5-HT, NE, DA), and stimulating hippocampal neurogenesis (through the SIRT1/PGC-1α pathway). Activate the Nrf2/ARE pathway as an antioxidant and inhibit the P2X7/NF-κB/NLRP3 axis Inhibit the activity of caspase-3 and antagonize the production of NO and NOS. | Lps-induced inflammation model Aβ1-40-induced rat model A cognitive impairment model induced by isoflurane MPP+-induced PC12 cell injury model A rat tic model induced by IDPN Chronic stress model |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Jiang, S.; Liu, M.; Wang, M.; Han, B.; Zhang, N.; Liao, Y.; Xiang, Y.; Liu, J.; Sun, H. Salidroside: A Potential Drug Candidate to Treat Rheumatoid Arthritis. Molecules 2025, 30, 3865. https://doi.org/10.3390/molecules30193865
Guo J, Jiang S, Liu M, Wang M, Han B, Zhang N, Liao Y, Xiang Y, Liu J, Sun H. Salidroside: A Potential Drug Candidate to Treat Rheumatoid Arthritis. Molecules. 2025; 30(19):3865. https://doi.org/10.3390/molecules30193865
Chicago/Turabian StyleGuo, Jiaying, Shan Jiang, Mei Liu, Min Wang, Beibei Han, Ning Zhang, Yumei Liao, Yinhong Xiang, Jianxin Liu, and Huifeng Sun. 2025. "Salidroside: A Potential Drug Candidate to Treat Rheumatoid Arthritis" Molecules 30, no. 19: 3865. https://doi.org/10.3390/molecules30193865
APA StyleGuo, J., Jiang, S., Liu, M., Wang, M., Han, B., Zhang, N., Liao, Y., Xiang, Y., Liu, J., & Sun, H. (2025). Salidroside: A Potential Drug Candidate to Treat Rheumatoid Arthritis. Molecules, 30(19), 3865. https://doi.org/10.3390/molecules30193865







