Phytochemical Profiling of Ferula varia Extract and Its Antibiofilm Activity Against Streptococcus mutans
Abstract
1. Introduction
2. Results
2.1. Chemical Composition
2.2. Antibacterial Activity of Ferula varia 70% Ethanol Extract on S. mutans
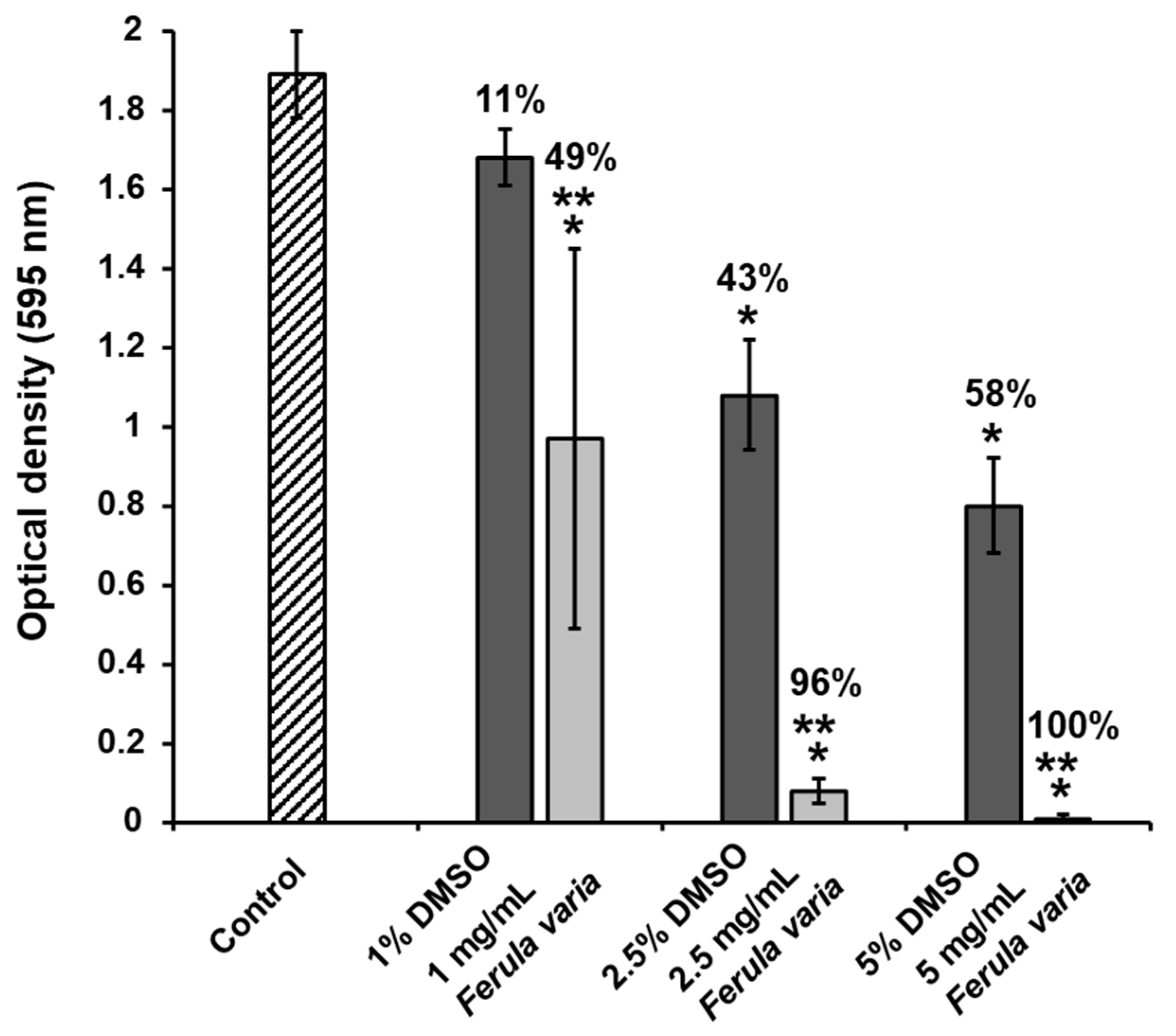
2.3. Biofilm Inhibition
3. Discussion
3.1. Chemical Characterization of Ferula varia 70% Ethanol Extract
3.2. Inhibition of Streptococcus mutans Biofilm Formation
4. Materials and Methods
4.1. Plant Material
4.2. Chemicals and Solvents
4.3. Preparation of the Ferula varia 70% Ethanol Extract (FVE)
4.4. Determination of Total Phenolic Content
4.5. Determination of Total Flavonoid Content
4.6. LC-UV-ESI-MS/MS Analysis
4.7. Bacterial Strain and Culture Conditions
4.8. Microdilution Test for Determining the Minimum Inhibitory Concentration (MIC)
4.9. Biofilm Formation Assay
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oral Health. Available online: https://www.who.int/news-room/fact-sheets/detail/oral-health (accessed on 3 September 2025).
- Atazhanova, G.A.; Levaya, Y.K.; Badekova, K.Z.; Ishmuratova, M.Y.; Smagulov, M.K.; Ospanova, Z.O.; Smagulova, E.M. Inhibition of the Biofilm Formation of Plant Streptococcus mutans. Pharmaceuticals 2024, 17, 1613. [Google Scholar] [CrossRef]
- Atazhanova, G.A.; Badekova, K.Z.; Ivasenko, S.A.; Kacergius, T.; Levaya, Y.K.; Kurmantaeva, G.K.; Ishmuratova, M.Y.; Smagulov, M.K. Influence of Essential Oils on the Formation of Streptococcus mutans Biofilms. Res. J. Pharm. Technol. 2022, 15, 4959–4966. [Google Scholar] [CrossRef]
- Sowmya, K.P.; Surenthar, M.; Lekha Sree, V. Effective Treatment of Oral Microbial Infections and Biofilm Using Flavonoid Rutin—An In Vitro Study. J. Oral Biol. Craniofacial Res. 2025, 15, 541–547. [Google Scholar] [CrossRef]
- Takenaka, S.; Sotozono, M.; Ohkura, N.; Noiri, Y. Evidence on the Use of Mouthwash for the Control of Supragingival Biofilm and Its Potential Adverse Effects. Antibiotics 2022, 11, 727. [Google Scholar] [CrossRef]
- Goto, I.; Saga, S.; Ichitani, M.; Kimijima, M.; Narisawa, N. Investigation of Components in Roasted Green Tea That Inhibit Streptococcus mutans Biofilm Formation. Foods 2023, 12, 2502. [Google Scholar] [CrossRef]
- Hirasawa, M.; Takada, K.; Otake, S. Inhibition of Acid Production in Dental Plaque Bacteria by Green Tea Catechins. Caries Res. 2006, 40, 265–270. [Google Scholar] [CrossRef]
- Galvão, L.C.d.C.; Furletti, V.F.; Bersan, S.M.F.; da Cunha, M.G.; Ruiz, A.L.T.G.; de Carvalho, J.E.; Sartoratto, A.; Rehder, V.L.G.; Figueira, G.M.; Teixeira Duarte, M.C.; et al. Antimicrobial Activity of Essential Oils against Streptococcus mutans and Their Antiproliferative Effects. Evid.-Based Complement. Altern. Med. ECAM 2012, 2012, 751435. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.E.; Aldahasi, R.M.; Rahman, I.; Shami, A.; Alotaibi, M.; BinShabaib, M.S.; ALHarthi, S.S.; Aabed, K. The Antimicrobial Activity of Tea Tree Oil (Melaleuca alternifolia) and Its Metal Nanoparticles in Oral Bacteria. PeerJ 2024, 12, e17241. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, B.; Rashidipour, M.; Marzban, A.; Soroush, S.; Azadpour, M.; Delfani, S.; Ramak, P. Mentha Piperita Essential Oils Loaded in a Chitosan Nanogel with Inhibitory Effect on Biofilm Formation against S. Mutans on the Dental Surface. Carbohydr. Polym. 2019, 212, 142–149. [Google Scholar] [CrossRef]
- Alshehri, F.A. The Use of Mouthwash Containing Essential Oils (LISTERINE®) to Improve Oral Health: A Systematic Review. Saudi Dent. J. 2018, 30, 2–6. [Google Scholar] [CrossRef]
- Decker, E.-M.; Maier, G.; Axmann, D.; Brecx, M.; von Ohle, C. Effect of Xylitol/Chlorhexidine versus Xylitol or Chlorhexidine as Single Rinses on Initial Biofilm Formation of Cariogenic Streptococci. Quintessence Int. Berl. Ger. 1985 2008, 39, 17–22. [Google Scholar]
- Sokolov, P.D. Plant Resources of the USSR: Flowering Plants, Their Chemical Composition, Use: Families Caprifoliaceae—Plantaginaceae; Science, Leningrad Branch: St. Petersburg, Russia, 1990; ISBN 978-5-02-026634-6. (In Russian) [Google Scholar]
- Suzuki, K.; Okasaka, M.; Kashiwada, Y.; Takaishi, Y.; Honda, G.; Ito, M.; Takeda, Y.; Kodzhimatov, O.K.; Ashurmetov, O.; Sekiya, M.; et al. Sesquiterpene Lactones from the Roots of Ferula varia and Their Cytotoxic Activity. J. Nat. Prod. 2007, 70, 1915–1918. [Google Scholar] [CrossRef] [PubMed]
- Khosnutdinova, T.S.; Gemejiyeva, N.G.; Karzhaubekova, Z.Z.; Sultanova, N.A. Coumarins of Genus Ferula L. (Apiaceae Lindl.). Eurasian Chem.-Technol. J. 2023, 25, 39–56. [Google Scholar] [CrossRef]
- Kang, X.; Wu, L.; Zhao, C.; Zhang, C.; Wang, Q. A new sesquiterpene coumarins from Ferula bungeana Kitagawa. Nat. Prod. Res. 2025, 39, 4112–4116. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Q.; Wang, H.; Shi, L.; Wang, G.; Zhao, Y.; Fan, C.; Si, J. Sesquiterpenes and Sesquiterpene Derivatives from Ferula: Their Chemical Structures, Biosynthetic Pathways, and Biological Properties. Antioxidants 2023, 13, 7. [Google Scholar] [CrossRef]
- Nouioura, G.; El fadili, M.; El Barnossi, A.; Loukili, E.H.; Laaroussi, H.; Bouhrim, M.; Giesy, J.P.; Aboul-Soud, M.A.M.; Al-Sheikh, Y.A.; Lyoussi, B.; et al. Comprehensive Analysis of Different Solvent Extracts of Ferula communis L. Fruit Reveals Phenolic Compounds and Their Biological Properties via In Vitro and in Silico Assays. Sci. Rep. 2024, 14, 8325. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, G.; Ma, S. Research Progress of Ferula ferulaeoides: A Review. Molecules 2023, 28, 3579. [Google Scholar] [CrossRef]
- Turdiyeva, Z.A.; Ishmuratova, M.Y.; Atazhanova, G.A.; Ramazanova, A. HistochemicalAnalysis of The Aerial Part of Ferula Songarica Growing in the Territory of theKaraganda Region (Central Kazakhstan). Res. J. Pharm. Technol. 2023, 16, 5079–5084. [Google Scholar] [CrossRef]
- Abbaszadegan, A.; Gholami, A.; Mirhadi, H.; Saliminasab, M.; Kazemi, A.; Moein, M.R. Antimicrobial and Cytotoxic Activity of Ferula gummosa Plant Essential Oil Compared to NaOCl and CHX: A Preliminary In Vitro Study. Restor. Dent. Endod. 2015, 40, 50–57. [Google Scholar] [CrossRef]
- Salehi, M.; Naghavi, M.R.; Bahmankar, M. A Review of Ferula Species: Biochemical Characteristics, Pharmaceutical and Industrial Applications, and Suggestions for Biotechnologists. Ind. Crops Prod. 2019, 139, 111511. [Google Scholar] [CrossRef]
- Boghrati, Z.; Iranshahi, M. Ferula species: A rich source of antimicrobial compounds. J. Herb. Med. 2019, 16, 100244. [Google Scholar] [CrossRef]
- Kurimoto, S.; Suzuki, K.; Okasaka, M.; Kashiwada, Y.; Kodzhimatov, O.K.; Takaishi, Y. Sesquiterpene Lactone Glycosides from the Roots of Ferula varia. Chem. Pharm. Bull. 2012, 60, 913–919. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maksumova, N.S.; Yuldashev, P.K.; Botirov, E. Luteolin Glucosides from Ferula varia. Chem. Nat. Compd. 1994, 30, 627–628. [Google Scholar] [CrossRef]
- Kotenko, L.D.; Mamatkhanova, M.A.; Khalilov, R.M.; Mamatkhanov, A.U.; Sotimov, G.B. Standardization of Ferula variable herb. Chem. Plant Raw Mater. 2009, 4, 151–154. (In Russian) [Google Scholar]
- Suriyaprom, S.; Ngamsaard, P.; Intachaisri, V.; Cheepchirasuk, N.; Panya, A.; Kaewkod, T.; Tragoolpua, Y. Inhibition of Oral Pathogenic Bacteria, Suppression of Bacterial Adhesion and Invasion on Human Squamous Carcinoma Cell Line (HSC-4 Cells), and Antioxidant Activity of Plant Extracts from Acanthaceae Family. Plants 2024, 13, 2622. [Google Scholar] [CrossRef]
- Mohammadnezhad, P.; Valdés, A.; Cifuentes, A. Optimization and Chemical Characterization of Extracts Obtained from Ferula persica var. Latisecta Aerial Parts and Roots and Their Neuroprotective Evaluation. Nutrients 2024, 16, 4210. [Google Scholar] [CrossRef]
- Khayat, M.T.; Alharbi, M.; Ghazawi, K.F.; Mohamed, G.A.; Ibrahim, S.R.M. Ferula sinkiangensis (Chou-AWei, Chinese Ferula): Traditional Uses, Phytoconstituents, Biosynthesis, and Pharmacological Activities. Plants 2023, 12, 902. [Google Scholar] [CrossRef] [PubMed]
- Şeker, M.E.; Erdoğan, A. Phenolic and Carotenoid Composition of Rhododendron Luteum Sweet and Ferula communis L. Subsp. Communis Flowers. Front. Life Sci. Relat. Technol. 2023, 4, 37–42. [Google Scholar] [CrossRef]
- Zengin, G.; Uysal, A.; Diuzheva, A.; Gunes, E.; Jekő, J.; Cziáky, Z.; Picot-Allain, C.M.N.; Mahomoodally, M.F. Characterization of Phytochemical Components of Ferula halophila Extracts Using HPLC-MS/MS and Their Pharmacological Potentials: A Multi-Functional Insight. J. Pharm. Biomed. Anal. 2018, 160, 374–382. [Google Scholar] [CrossRef]
- Niazmand, R.; Razavizadeh, B.M. Ferula asafoetida: Chemical composition, thermal behavior, antioxidant and antimicrobial activities of leaf and gum hydroalcoholic extracts. J. Food. Sci. Technol. 2021, 58, 2148–2159. [Google Scholar] [CrossRef]
- Rahali, F.Z.; Lamine, M.; Rebey, I.B.; Wannes, W.A.; Hammami, M.; Selmi, S.; Mliki, A.; Sellami, I.H. Biochemical characterization of fennel (Ferula communis L.) different parts through their essential oils, fatty acids and phenolics. Acta Sci. Pol. Hortorum Cultus 2021, 20, 3–14. [Google Scholar] [CrossRef]
- Beladjal, H.; Bouhadi, D.; Belkhodja, H. HPLC-DAD Analysis and Antioxidant Potential of Ferula Assa Foetida Resin Ethanol Extract. Trop. J. Nat. Prod. Res. 2024, 8, 6906–6910. [Google Scholar] [CrossRef]
- Ed-Dahmani, I.; El Fadili, M.; Nouioura, G.; Kandsi, F.; Atki, Y.E.; Abuelizz, H.A.; Conte, R.; Zahra Lafdil, F.; Taleb, A.; Abdellaoui, A.; et al. Ferula communis Leaf Extract: Antioxidant Capacity, UHPLC-MS/MS Analysis, and In Vivo and In Silico Toxicity Investigations. Front. Chem. 2024, 12, 1485463. [Google Scholar] [CrossRef]
- Boukhary, R.M.K.; Omeiche, Z.; Hijazi, M.A.; Assi, A.W.; Houri, T.; Al Jawiche, L.; Dirani, M.; Abdel Nabi, R.; Tabbara, M.; El-Lakany, A.; et al. Review on chemical constituents and biological activities of genus Ferula. BAU J.-Health Wellbeing 2024, 6, 4. [Google Scholar] [CrossRef]
- Kahraman, Ç.; Baysal, İ.; Çankaya, İ.İ.; Göger, F.; Kırımer, N.; Akdemir, Z.Ş. Acetylcholinesterase inhibitory activities and LC-MS analysis of theantioxidant Ferula caspica M. Bieb. and F. halophila Peşmen extracts. J. Res. Pharm. 2019, 23, 543–551. [Google Scholar] [CrossRef]
- Nguyen, V.; Taine, E.G.; Meng, D.; Cui, T.; Tan, W. Chlorogenic Acid: A Systematic Review on the Biological Functions, Mechanistic Actions, and Therapeutic Potentials. Nutrients 2024, 16, 924. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.J.; You, H.J.; Park, M.S.; Li, Z.; Choe, D.; Johnston, T.V.; Ku, S.; Ji, G.E. Microbial Biocatalysis of Quercetin-3-Glucoside and Isorhamnetin-3-Glucoside in Salicornia Herbacea and Their Contribution to Improved Anti-Inflammatory Activity. RSC Adv. 2020, 10, 5339–5350. [Google Scholar] [CrossRef]
- Habibi, Z.; Salehi, P.; Yousefi, M.; Hejazi, Y.; Laleh, A.; Mozaffarian, V.; Masoudi, S.; Rustaiyan, A. Chemical Composition and Antimicrobial Activity of the Essential Oils of Ferula Latisecta and Mozaffariania Insignis from Iran. Chem. Nat. Compd. 2006, 42, 689–692. [Google Scholar] [CrossRef]
- Nazari, Z.E.; Iranshahi, M. Biologically Active Sesquiterpene Coumarins from Ferula Species. Phytother. Res. PTR 2011, 25, 315–323. [Google Scholar] [CrossRef]
- Sonigra, P.; Meena, M. Metabolic Profile, Bioactivities, and Variations in the Chemical Constituents of Essential Oils of the Ferula Genus (Apiaceae). Front. Pharmacol. 2020, 11, 608649. [Google Scholar] [CrossRef] [PubMed]
- Satorov, S.; Mavlonazarova, S.; Yusufi, S.; Dushenkov, V. Total Polyphenol Content, Antioxidant Potential, Antibacterial and Antifungal Properties of Ferula L. Species Growing in Tajikistan. J. Drug Alcohol Res. 2024, 13, 236424. [Google Scholar] [CrossRef]
- Kashket, S.; Paolino, V.J.; Lewis, D.A.; van Houte, J. In-Vitro Inhibition of Glucosyltransferase from the Dental Plaque Bacterium Streptococcus mutans by Common Beverages and Food Extracts. Arch. Oral Biol. 1985, 30, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xue, J.; Li, J.; Zou, L.; Hao, Y.; Zhou, X.; Li, W. Effects of Galla Chinensis on Inhibition of Demineralization of Regular Bovine Enamel or Enamel Disposed of Organic Matrix. Arch. Oral Biol. 2009, 54, 817–822. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, K.; Zheng, S.; Wang, Y.; Ren, Q.; Li, H.; Ding, L.; Li, W.; Zhang, L. Antibacterial Effect of Caffeic Acid Phenethyl Ester on Cariogenic Bacteria and Streptococcus mutans Biofilms. Antimicrob. Agents Chemother. 2020, 64, e00251-20. [Google Scholar] [CrossRef]
- Liu, P.; Wang, L.; Song, Y.; Pei, H.; Cao, X. Virtual Screening of Inhibitors of Streptococcus mutans Biofilm from Lonicera japonica flos and Activity Validation. ACS Med. Chem. Lett. 2024, 15, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Li, J.; Li, J.; Tang, R.; Liu, L.; Shi, J.; Huang, Q.; Yang, H. Inhibition of Gallic Acid on the Growth and Biofilm Formation of Escherichia coli and Streptococcus mutans. J. Food Sci. 2015, 80, M1299–M1305. [Google Scholar] [CrossRef]
- Zeng, Y.; Nikikova, A.; Abdelsalam, H.; Li, J.; Xiao, J. Activity of Quercetin and Kaemferol against Streptococcus mutans Biofilm. Arch. Oral Biol. 2019, 98, 9–16. [Google Scholar] [CrossRef]
- Koo, H.; Rosalen, P.L.; Cury, J.A.; Park, Y.K.; Bowen, W.H. Effects of Compounds Found in Propolis on Streptococcus mutans Growth and on Glucosyltransferase Activity. Antimicrob. Agents Chemother. 2002, 46, 1302–1309. [Google Scholar] [CrossRef]
- Rudin, L.; Roth, N.; Kneubühler, J.; Dubey, B.N.; Bornstein, M.M.; Shyp, V. Inhibitory Effect of Natural Flavone Luteolin on Streptococcus mutans Biofilm Formation. Microbiol. Spectr. 2023, 11, e0522322. [Google Scholar] [CrossRef] [PubMed]
- Levaya, Y.K.; Zholdasbaev, M.E.; Atazhanova, G.A.; Akhmetova, S.B.; Ishmuratova, M.Y. Antibacterial activity of ultrasonic extracts of Salvia stepposa growing in Kazakhstan. Bull. Karaganda Univ. Biol. Med. Geogr. Ser. 2021, 1, 45–49. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Siddiqui, N.; Rauf, A.; Latif, A.; Mahmood, Z. Spectrophotometric Determination of the Total Phenolic Content, Spectral and Fluorescence Study of the Herbal Unani Drug Gul-e-Zoofa (Nepeta bracteata Benth). J. Taibah Univ. Med. Sci. 2017, 12, 360–363. [Google Scholar] [CrossRef]
- Ivasenko, S.; Orazbayeva, P.; Skalicka–Wozniak, K.; Ludwiczuk, A.; Marchenko, A.; Ishmuratova, M.; Poleszak, E.; Korona-Glowniak, I.; Akhmetova, S.; Karilkhan, I.; et al. Antimicrobial activity of ultrasonic extracts of two chemotypes of Thymus serpyllum L. of Central Kazakhstan and their polyphenolic profiles. Open Access Maced. J. Med. Sci. 2021, 9, 61–67. [Google Scholar] [CrossRef]
- Kacergius, T.; Abu-Lafi, S.; Kirkliauskiene, A.; Gabe, V.; Adawi, A.; Rayan, M.; Qutob, M.; Stukas, R.; Utkus, A.; Zeidan, M.; et al. Inhibitory Capacity of Rhus coriaria L. Extract and Its Major Component Methyl Gallate on Streptococcus mutans Biofilm Formation by Optical Profilometry: Potential Applications for Oral Health. Mol. Med. Rep. 2017, 16, 949–956. [Google Scholar] [CrossRef] [PubMed]
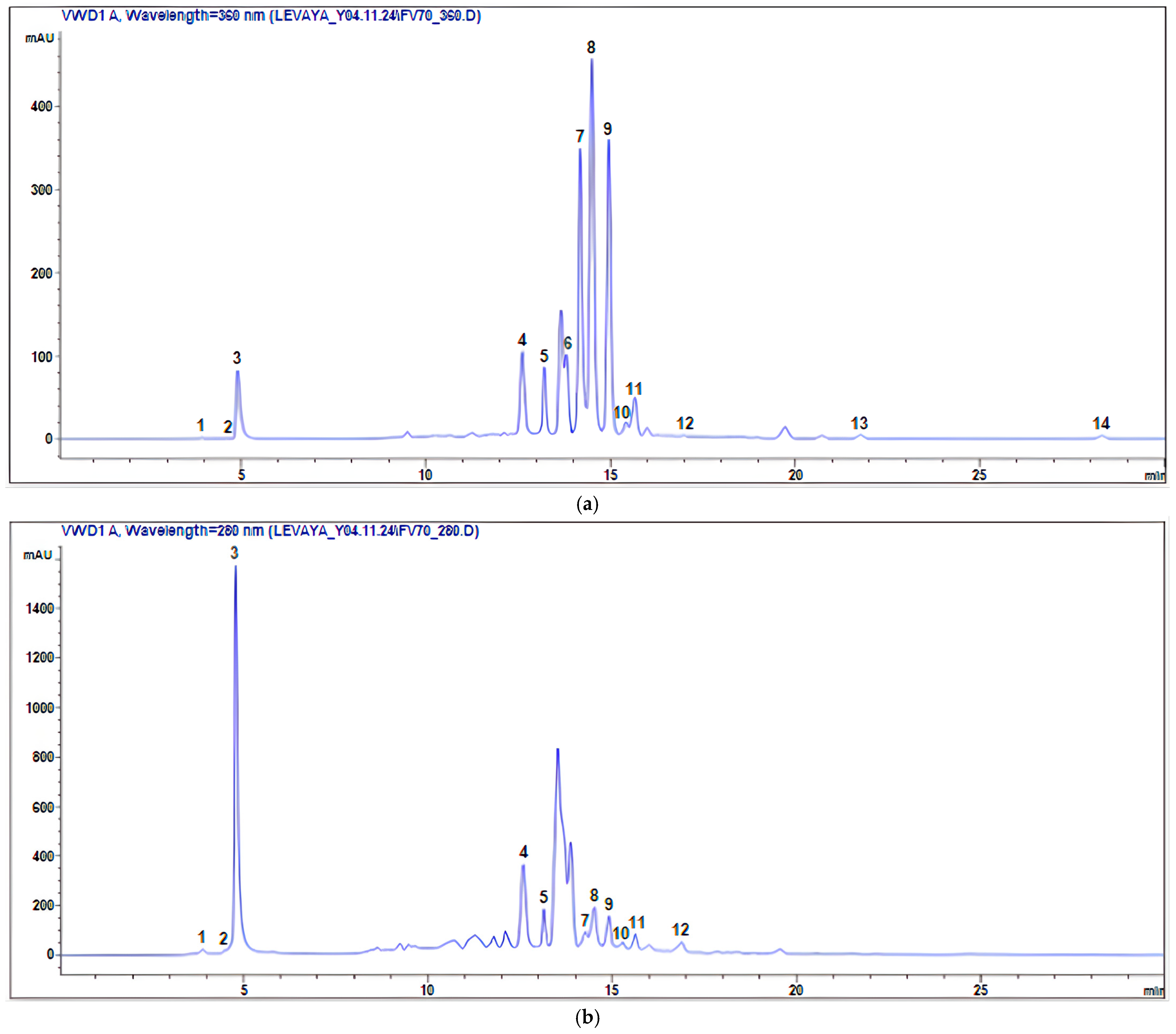
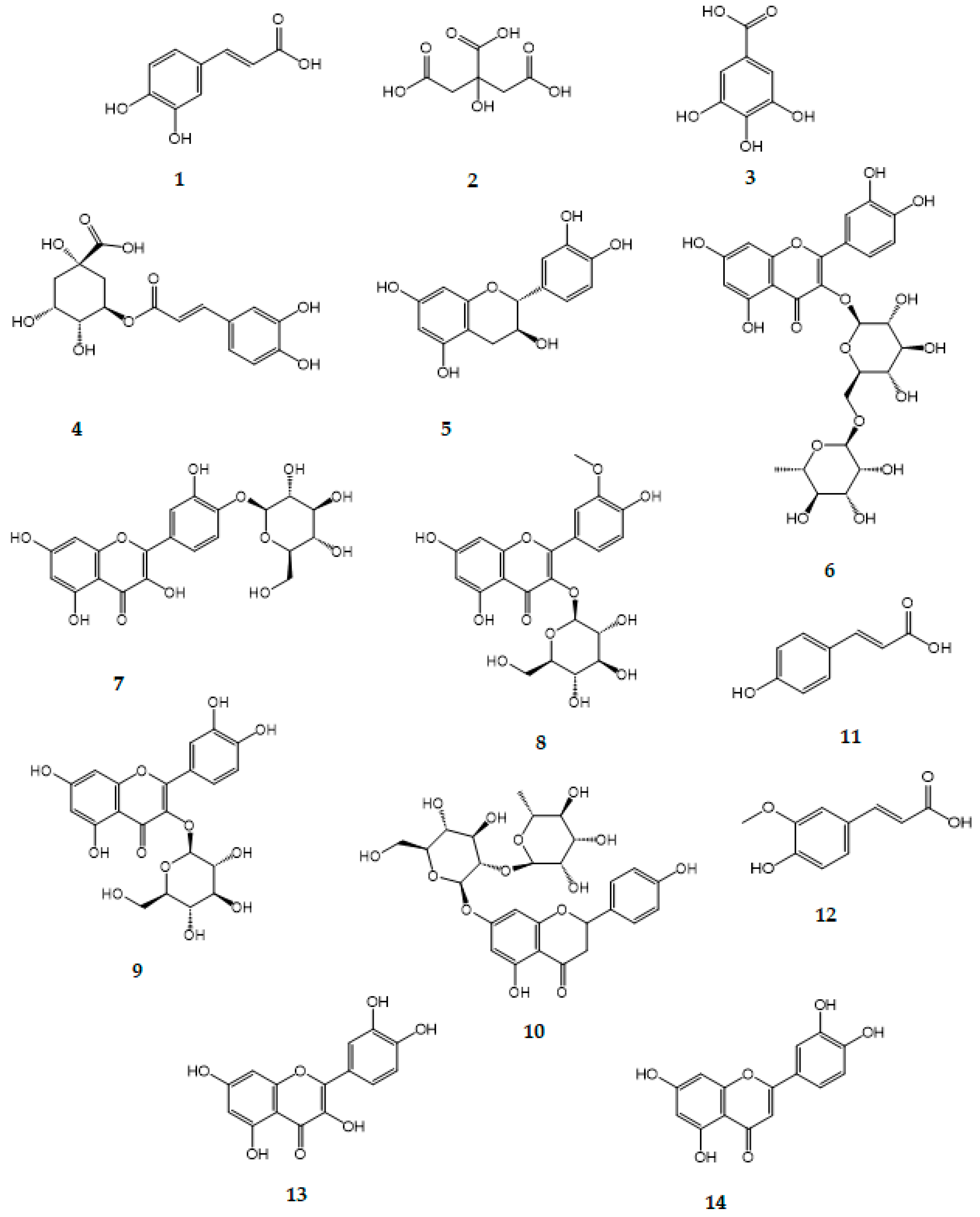
| Peak/№ | RT | M − H− | Compound Name | Quantitative Content (mg/g Extract) | Reference |
|---|---|---|---|---|---|
| 1 | 3.878 | 179 | Caffeic acid | 1.98 ± 0.14 | [18,28,29] |
| 2 | 4.820 | 191 | Citric acid | 1.42 ± 0.12 | [18,30,31] |
| 3 | 4.944 | 169 | Gallic acid | 24.01 ± 0.36 | [30,32,33] |
| 4 | 12.671 | 353 | Chlorogenic acid | 69.45 ± 1.12 | [8,30,31,33,34] |
| 5 | 13.325 | 289 | Catechin | 3.63 ± 0.16 | [18,33,35] |
| 6 | 13.962 | 609 | Rutin (Quercetin-3-O-rutinoside) | 1.92 ± 0.16 | [18,28,29] |
| 7 | 14.262 | 463 | Spiraeoside (Quercetin-4′-O-β-D-glucopyranoside) | 5.72 ± 0.24 | [35] |
| 8 | 14.566 | 477 | Isorhamnetin-3-O-β-D-glucopyranoside | 9.05 ± 0.32 | [35] |
| 9 | 14.967 | 463 | Isoquercitrin (Quercetin-3-O-β-D-glucopyranoside) | 10.59 ± 0.30 | [18,31] |
| 10 | 15.293 | 579 | Naringin (Naringenin-7-rhamnosidoglucoside) | 0.29 ± 0.06 | [18,36] |
| 11 | 15.792 | 163 | p-coumaric acid | 1.40 ± 0.12 | [18,30,32,34] |
| 12 | 16.998 | 193 | Ferulic acid | 1.12 ± 0.14 | [18,28,29,30,31,32,33,34] |
| 13 | 21.894 | 301 | Quercetin | 0.19 ± 0.16 | [28,31,33,36,37] |
| 14 | 28.354 | 285 | Luteolin | 0.09 ± 0.04 | [30,35] |
| Compound | MIC, mg/mL |
|---|---|
| F. varia extract | 25 mg/mL |
| CHX | 4.5 µg/mL |
| DMSO | 50% (v/v) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smagulov, M.K.; Levaya, Y.K.; Badekova, K.Z.; Ivasenko, S.A.; Atazhanova, G.A.; Gabe, V.; Ishmuratova, M.Y.; Kacergius, T. Phytochemical Profiling of Ferula varia Extract and Its Antibiofilm Activity Against Streptococcus mutans. Molecules 2025, 30, 4178. https://doi.org/10.3390/molecules30214178
Smagulov MK, Levaya YK, Badekova KZ, Ivasenko SA, Atazhanova GA, Gabe V, Ishmuratova MY, Kacergius T. Phytochemical Profiling of Ferula varia Extract and Its Antibiofilm Activity Against Streptococcus mutans. Molecules. 2025; 30(21):4178. https://doi.org/10.3390/molecules30214178
Chicago/Turabian StyleSmagulov, Marlen K., Yana K. Levaya, Karakoz Zh. Badekova, Svetlana A. Ivasenko, Gayane A. Atazhanova, Vika Gabe, Margarita Yu. Ishmuratova, and Tomas Kacergius. 2025. "Phytochemical Profiling of Ferula varia Extract and Its Antibiofilm Activity Against Streptococcus mutans" Molecules 30, no. 21: 4178. https://doi.org/10.3390/molecules30214178
APA StyleSmagulov, M. K., Levaya, Y. K., Badekova, K. Z., Ivasenko, S. A., Atazhanova, G. A., Gabe, V., Ishmuratova, M. Y., & Kacergius, T. (2025). Phytochemical Profiling of Ferula varia Extract and Its Antibiofilm Activity Against Streptococcus mutans. Molecules, 30(21), 4178. https://doi.org/10.3390/molecules30214178








