Acid Yellow 9 Azo Dye Gets the Blues: An Optical Spectroscopy and DFT Study of Unusual Photochemistry in Multilayer Films with PAH and Chitosan
Abstract
1. Introduction
2. Results
2.1. Multilayer Film Characterization
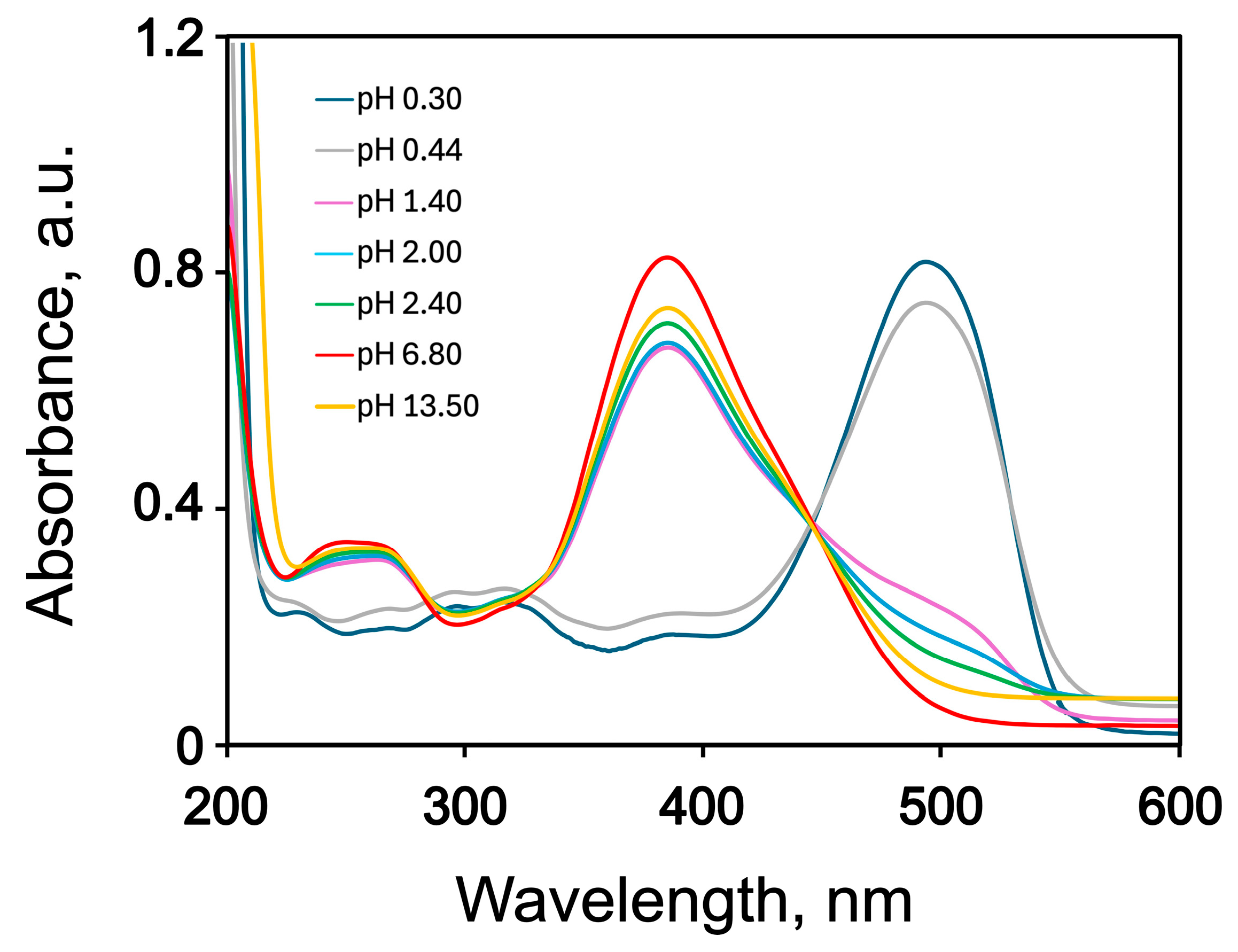
2.2. UV–Vis Spectra of AY9 Solutions and Multilayer Films
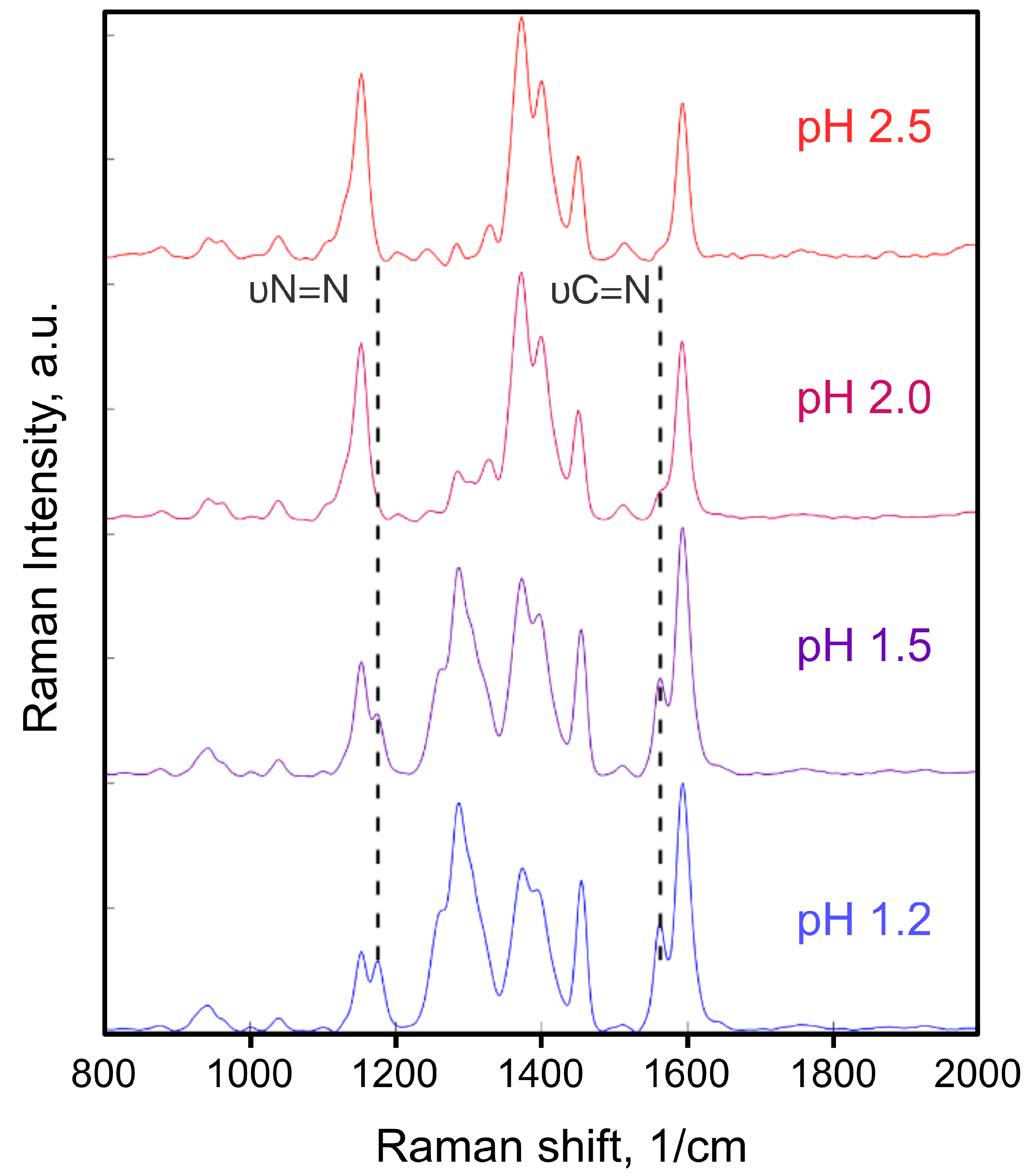
2.3. DFT and TD-DFT Calculations
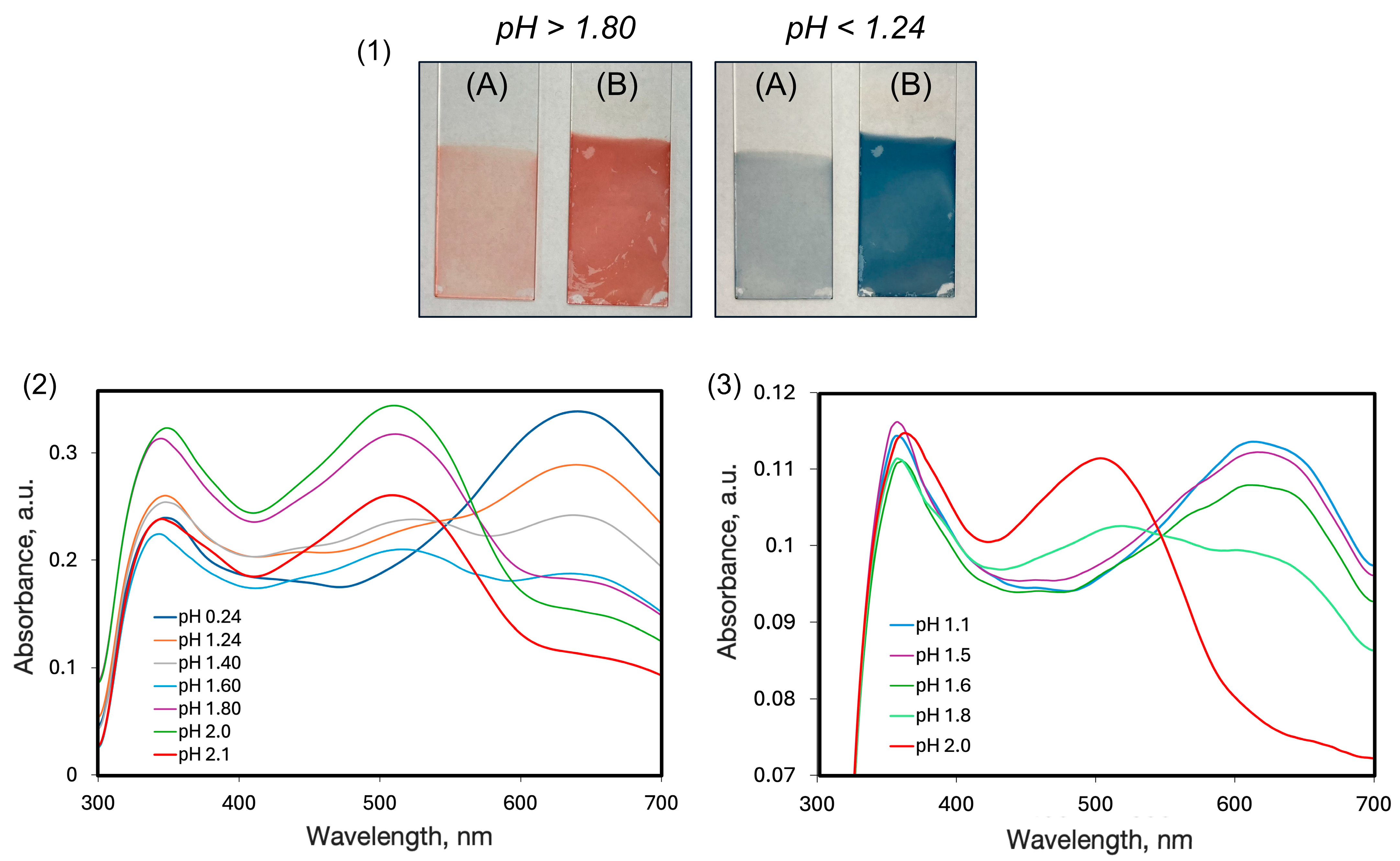
2.4. Effect of the Counter-Polymer
3. Discussion
4. Materials and Methods
4.1. LbL Assembly of Thin Films
4.2. Raman Spectroscopy
4.3. UV–Visible (UV–Vis) Spectroscopy
4.4. pH Measurements
4.5. Photo-Response of the Multilayer Films
4.6. Computational Chemistry
4.7. Data Processing and Visualization, Computational Chemistry Software
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PAH | Poly(allylamine hydrochloride) |
| AY9 | Acid Yellow 9 |
| LbL | Layer-by-layer |
| UV–Vis | Ultraviolet–visible |
| DFT | Density functional theory |
| TD-DFT | Time-dependent density functional theory |
| DW | Distilled water |
| PAA | Poly(acrylic acid) |
References
- Mitscherlich, E. Ueber Die Zusammensetzung Des Nitrobenzids Und Sulfobenzids. Ann. Der Pharm. 1834, 12, 305–311. [Google Scholar] [CrossRef]
- Bandara, H.M.D.; Burdette, S.C. Photoisomerization in Different Classes of Azobenzene. Chem. Soc. Rev. 2012, 41, 1809–1825. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Hillel, C.; Edwards, K.; Borchers, T.H.; Mermut, O.; Pietro, W.J.; Barrett, C.J. Azo Dye Polyelectrolyte Multilayer Films Reversibly Re-Soluble with Visible Light. Front. Mater. 2024, 11, 1334863. [Google Scholar] [CrossRef]
- Edwards, K.E.K.; Kim, M.; Borchers, T.H.; Barrett, C.J. Controlled Disassembly of Azobenzene Cellulose-Based Thin Films Using Visible Light. Mater. Adv. 2022, 3, 6222–6230. [Google Scholar] [CrossRef]
- Kortekaas, L.; Simke, J.; Arndt, N.B.; Böckmann, M.; Doltsinis, N.L. Bart Jan Ravoo Acid-Catalysed Liquid-To-Solid Transitioning of Arylazoisoxazole Photoswitches. Chem. Sci. 2021, 12, 11338–11346. [Google Scholar] [CrossRef]
- Rickhoff, J.; Arndt, N.B.; Böckmann, M.; Doltsinis, N.L.; Ravoo, B.J.; Kortekaas, L. Reversible, Red-Shifted Photoisomerization in Protonated Azobenzenes. J. Org. Chem. 2022, 87, 10605–10612. [Google Scholar] [CrossRef]
- Garcia-Amorós, J.; Sánchez-Ferrer, A.; Massad, W.A.; Nonell, S.; Velasco, D. Kinetic Study of the Fast Thermal Cis-To-Trans Isomerisation of Para-, Ortho- and Polyhydroxyazobenzenes. Phys. Chem. Chem. Phys. 2010, 12, 13238. [Google Scholar] [CrossRef]
- Merino, E.; Ribagorda, M. Control over Molecular Motion Using the Cis–Trans Photoisomerization of the Azo Group. Beilstein J. Org. Chem. 2012, 8, 1071–1090. [Google Scholar] [CrossRef]
- Gao, M.; Kwaria, D.; Norikane, Y.; Yue, Y. Visible-Light-Switchable Azobenzenes: Molecular Design, Supramolecular Systems, and Applications. Nat. Sci. 2022, 3, e220020. [Google Scholar] [CrossRef]
- Cruickshank, D.L.; Hendon, C.H.; Verbeek, M.J.R.; Walsh, A.; Wilson, C.C. Polymorphism of the Azobenzene Dye Compound Methyl Yellow. CrystEngComm 2016, 18, 3456–3461. [Google Scholar] [CrossRef]
- Gardner, H.C.; Kennedy, A.R.; McCarney, K.M.; Staunton, E.; Stewart, H.; Teat, S.J. Structures of Five Salt Forms of Disulfonated Monoazo Dyes. Acta Crystallogr. Sect. C Struct. Chem. 2020, 76, 972–981. [Google Scholar] [CrossRef]
- Honda, A.; Kakihara, S.; Kawai, M.; Takahashi, T.; Miyamura, K. Cold Crystallization and Polymorphism Triggered by the Mobility of the Phenyl Group in Alkyl Azo Dye Molecules. Cryst. Growth Des. 2021, 21, 6223–6229. [Google Scholar] [CrossRef]
- Yang, Y.; Tian, H.; Napolitano, S.; Zuo, B. Crystallization in Thin Films of Polymer Glasses: The Role of Free Surfaces, Solid Interfaces and Their Competition. Prog. Polym. Sci. 2023, 144, 101725. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, X.; He, Y.; Guo, B.; Xu, J. In Situ Monitoring and Tuning Multilayer Stacking of Polymer Lamellar Crystals in Solution with Aggregation-Induced Emission. Nano Lett. 2024, 24, 4717–4724. [Google Scholar] [CrossRef]
- Carr, J.M.; Langhe, D.S.; Ponting, M.T.; Hiltner, A.; Baer, E. Confined Crystallization in Polymer Nanolayered Films: A Review. J. Mater. Res. 2012, 27, 1326–1350. [Google Scholar] [CrossRef]
- Begum, S.; Cianci, M.; Durbeej, B.; Falklöf, O.; Hädener, A.; Helliwell, J.R.; Helliwell, M.; Regan, A.C.; Watt, C.I.F. On the Origin and Variation of Colors in Lobster Carapace. Phys. Chem. Chem. Phys. 2015, 17, 16723–16732. [Google Scholar] [CrossRef] [PubMed]
- Hunt, D.M.; Wilkie, S.E.; Bowmaker, J.K.; Poopalasundaram, S. Vision in the Ultraviolet. Cell. Mol. Life Sci. 2001, 58, 1583–1598. [Google Scholar] [CrossRef]
- Van Hazel, I.; Sabouhanian, A.; Day, L.; Endler, J.A.; Chang, B.S. Functional Characterization of Spectral Tuning Mechanisms in the Great Bowerbird Short-Wavelength Sensitive Visual Pigment (SWS1), and the Origins of UV/Violet Vision in Passerines and Parrots. BMC Evol. Biol. 2013, 13, 250. [Google Scholar] [CrossRef]
- Stockman, A.; Sharpe, L.T.; Merbs, S.L.; Nathans, J. Spectral Sensitivities of Human Cone Visual Pigments Determined in Vivo and in Vitro. Methods Enzymol. 2000, 316, 626–650. [Google Scholar] [PubMed]
- Matthias Broser Far-Red Absorbing Rhodopsins, Insights from Heterodimeric Rhodopsin-Cyclases. Front. Mol. Biosci. 2022, 8, 806–922.
- Burke, S.E.; Barrett, C.J. Acid−Base Equilibria of Weak Polyelectrolytes in Multilayer Thin Films. Langmuir 2003, 19, 3297–3303. [Google Scholar] [CrossRef]
- You, K.; Kwon, O.; Kim, D. Effects of the Protonation and the Polar Solvation on the Molecular Properties of Methyl Orange: A Density Functional Theory Study. Bull. Korean Chem. Soc. 2023, 44, 523–527. [Google Scholar] [CrossRef]
- Zheng, D.; Yuan, X.-A.; MA, J. Rationalization of PH-Dependent Absorption Spectrum of O-Methyl Red in Aqueous Solutions: TD-DFT Calculation and Experiment Study. Acta Phys. -Chim. Sin. 2016, 32, 290–300. [Google Scholar] [CrossRef]
- Pirone, D.; Bandeira, N.A.G.; Tylkowski, B.; Boswell, E.; Labeque, R.; Garcia Valls, R.; Giamberini, M. Contrasting Photo-Switching Rates in Azobenzene Derivatives: How the Nature of the Substituent Plays a Role. Polymers 2020, 12, E1019. [Google Scholar] [CrossRef] [PubMed]
- Gelabert, R.; Moreno, M.; Lluch, J.M. Predicting the Electronic Absorption Band Shape of Azobenzene Photoswitches. Int. J. Mol. Sci. 2023, 24, 25. [Google Scholar] [CrossRef]
- Peach, M.J.G.; Benfield, P.; Helgaker, T.; Tozer, D.J. Excitation Energies in Density Functional Theory: An Evaluation and a Diagnostic Test. J. Chem. Phys. 2008, 128, 044118. [Google Scholar] [CrossRef]
- Tomasi, J.; Menucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. ChemInform 2005, 36, 628. [Google Scholar] [CrossRef]
- Sharma, A.; Bekir, M.; Lomadze, N.; Santer, S. Photo-Isomerization Kinetics of Azobenzene Containing Surfactant Conjugated with Polyelectrolyte. Molecules 2020, 26, 19. [Google Scholar] [CrossRef]
- Liu, X.-M.; Jin, X.-Y.; Zhang, Z.-X.; Wang, J.; Bai, F.-Q. Theoretical Study on the Reaction Mechanism of the Thermal Cis–Trans Isomerization of Fluorine-Substituted Azobenzene Derivatives. RSC Adv. 2018, 8, 11580–11588. [Google Scholar] [CrossRef]
- Lee, H.; Machida, K.; Kuwae, A.; Saito, Y. Resonance Raman Spectra and Structure of P-Aminoazobenzene Dyes in Diprotonated Form. J. Mol. Struct. 1980, 68, 51–57. [Google Scholar] [CrossRef]
- Trotter, P.J. Azo Dye Tautomeric Structures Determined by Laser-Raman Spectroscopy. Appl. Spectroscopy 1977, 31, 30–35. [Google Scholar] [CrossRef]
- Cembran, A.; Bernardi, F.; Garavelli, M.; Gagliardi, L.; Orlandi, G. On the Mechanism of the Cis−Trans Isomerization in the Lowest Electronic States of Azobenzene: S0, S1, and T1. J. Am. Chem. Soc. 2004, 126, 3234–3243. [Google Scholar] [CrossRef]
- Sahanawaz, M.; Maity, M.L.; Goswami, K.G.; Sar, P.; De, P.; Bandyopadhyay, S. Sequence Effects on the Thermal Cis–Trans Isomerization of Side-Chain Stearate-Containing Azobenzene Polymers. J. Phys. Org. Chem. 2024, 37, e4599. [Google Scholar] [CrossRef]
- Wegermann, C.A.; Cesar, J.; Drechsel, S.M. Fábio Souza Nunes Semi-Empirical ZINDO/S Description of the Electronic Structure and the Spectral Features of Methyl Orange and Its Products of Oxidation. A Study of Relationship between Molecular Geometry and Spectroscopic Properties. Dye. Pigment. 2013, 99, 839–849. [Google Scholar] [CrossRef]
- Jacquemin, D.; Planchat, A.; Adamo, C. Benedetta Mennucci TD-DFT Assessment of Functionals for Optical 0–0 Transitions in Solvated Dyes. J. Chem. Theory Comput. 2012, 8, 2359–2372. [Google Scholar] [CrossRef]
- Casida, M.E. Time-Dependent Density-Functional Theory for Molecules and Molecular Solids. J. Mol. Struct. THEOCHEM 2009, 914, 318. [Google Scholar] [CrossRef]
- Marques, M.A.L.; Maitra, N.T.; Nogueira, F.; Gross, E.K.U.; Rubio, A. Fundamentals of Time-Dependent Density Functional Theory; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2012. [Google Scholar]
- Appel, H. Oscillator Strengths from Time-Dependent Density Functional Theory. Master’s Thesis, The State University of New Jersey, New Brunswick, NJ, USA, 1999. [Google Scholar]
- How Do I Generate Natural Transition Orbitals? 2016. Available online: https://gaussian.com/faq4 (accessed on 10 October 2023).
- Spange, S.; Mayerhöfer, T.G. The Negative Solvatochromism of Reichardt‘s Dye B30—A Complementary Study. ChemPhysChem 2022, 23, e202200100. [Google Scholar] [CrossRef]
- Kuntze, K.; Viljakka, J.; Virkki, M.; Huang, C.-Y.D.; Hecht, S. Arri Priimagi Red-Light Photoswitching of Indigos in Polymer Thin Films. Chem. Sci. 2023, 14, 2482–2488. [Google Scholar] [CrossRef]
- Tang, Z.; Chang, C.; Bao, F.; Tian, L.; Liu, H.; Wang, M.; Zhu, C.; Xu, J. Feasibility of Predicting Static Dielectric Constants of Polymer Materials: A Density Functional Theory Method. Polymers 2021, 13, 284. [Google Scholar] [CrossRef] [PubMed]
- Hensel, R.C.; Pereira-da-Silva, M.A.; Riul, A.; Rodrigues, V. Dielectric Permittivity and Surface Charge Density in Layer-By-Layer Poly(Diallyldimethylammonium Chloride)/Poly(Styrenesulfonate) Nanostructured Films: Implications for Biosensing. ACS Appl. Nano Mater. 2020, 3, 1749–1754. [Google Scholar] [CrossRef]
- Durstock, M.F.; Rubner, M.F. Dielectric Properties of Polyelectrolyte Multilayers. Langmuir 2001, 17, 7865–7872. [Google Scholar] [CrossRef]
- Yin, L.; He, Y.; Guo, W.; Wang, S.; He, J.; Wang, T. Low Dielectric Constant Ultrathin Polyimide Films Alternated by Poly(Amic Acid) Salt/Poly(Allylamine Hydrochloride)/Imogolite through Layer by Layer Deposition. Adv. Compos. Hybrid Mater. 2023, 6, 223. [Google Scholar] [CrossRef]
- Smallwood, I.M. Handbook of Organic Solvent Properties. Elsevier: New York, NY, USA; Toronto, ON, Canada; London, UK; Sydney, Australia; Auckland, New Zealand, 1996. [Google Scholar]
- Alizadeh, K.; Rezaei, B.; Maddah, B. Spectrophotometric Determination of Aqueous Acidity Constants of Three Azo Dyes. Open Chem. 2010, 8, 392–395. [Google Scholar] [CrossRef]
- Ferreira, Q.; Gomes, S.; Ribeiro, P.Q.; Jones, N.C.; Hoffmann, S.V.; Mason, N.J.; Oliveira, O.N.; Oliveira, O.N. Determination of Degree of Ionization of Poly(Allylamine Hydrochloride) (PAH) and Poly [1-[4-(3-Carboxy-4 Hydroxyphenylazo)Benzene Sulfonamido]-1,2-Ethanediyl, Sodium Salt] (PAZO) in Layer-By-Layer Films Using Vacuum Photoabsorption Spectroscopy. Langmuir 2012, 29, 448–455. [Google Scholar] [CrossRef]
- Pliego Jr, J.R.; Riveros, J.M. Gibbs Energy of Solvation of Organic Ions in Aqueous and Dimethyl Sulfoxide Solutions. Phys. Chem. Chem. Phys. 2002, 4, 1622–1627. [Google Scholar] [CrossRef]
- Perevozchikova, P.S.; Chernikova, E.Y.; Fedorov, Y.V.; Fedorova, O.A. Quinolino [1, 2-a] Quinolinium Bromide and Isoquinolino [2, 1-a] Quinolinium Bromide Derivatives as DNA Ligands and Photocytotoxic Agents. J. Photochem. Photobiol. A Chem. 2025, 461, 116163. [Google Scholar] [CrossRef]
- Saifutiarova, A.E.; Fedorov, Y.V.; Maurel, F.; Gulakova, E.N.; Karnoukhova, V.A.; Fedorova, O.A. Highly Regioselective and Stereoselective Photodimerization of Azine-Containing Stilbenes in Neat Condition: An Efficient Synthesis of Novel Cyclobutanes with Heterocyclic Substituents. J. Photochem. Photobiol. A Chem. 2022, 427, 113804. [Google Scholar] [CrossRef]
- Dong, M.; Babalhavaeji, A.; Samanta, S.; Beharry, A.A.; Woolley, G.A. Red-Shifting Azobenzene Photoswitches for in Vivo Use. Acc. Chem. Res. 2015, 48, 2662–2670. [Google Scholar] [CrossRef]
- Dantas, D.; Ribeiro, A.I.; Carvalho, F.; Gil-Martins, E.; Silva, R.; Remião, F.; Zille, A.; Cerqueira, F.; Pinto, E.; Dias, A.M. Red-Shifted and PH-Responsive Imidazole-Based Azo Dyes with Potent Antimicrobial Activity. Chem. Commun. 2023, 19, 2791–2794. [Google Scholar] [CrossRef]
- Chen, H.; Chen, W.; Lin, Y.; Xie, Y.; Liu, S.H.; Yin, J. Visible and Near-Infrared Light Activated Azo Dyes. Chin. Chem. Lett. 2021, 32, 2359–2368. [Google Scholar] [CrossRef]
- Swanson, N.; Sabat, R.G. Inscription and Analysis of Non-Uniform Diffraction Gratings in Azobenzene Molecular Glass Thin Films. Opt. Express 2018, 26, 7876. [Google Scholar] [CrossRef] [PubMed]
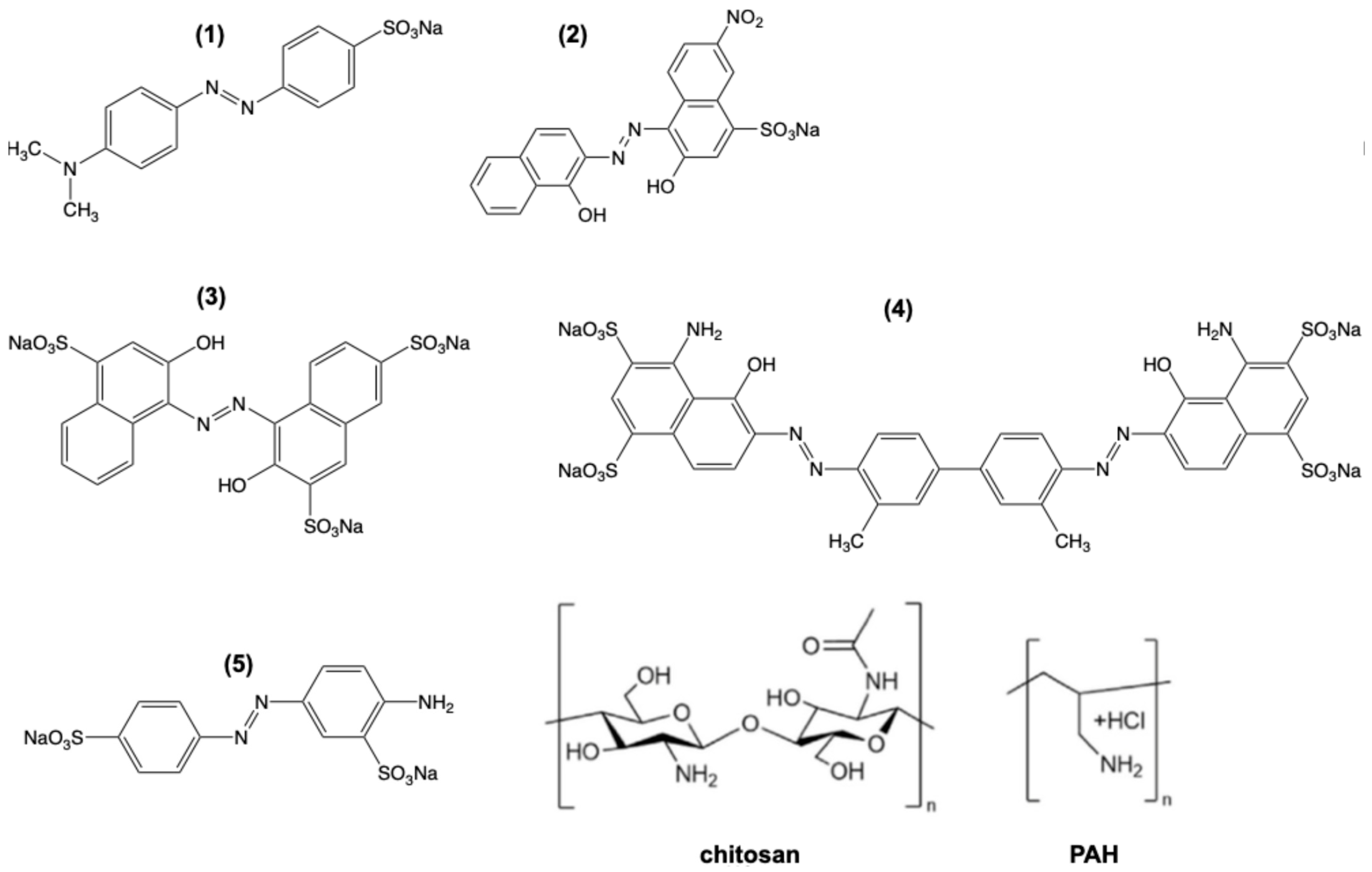
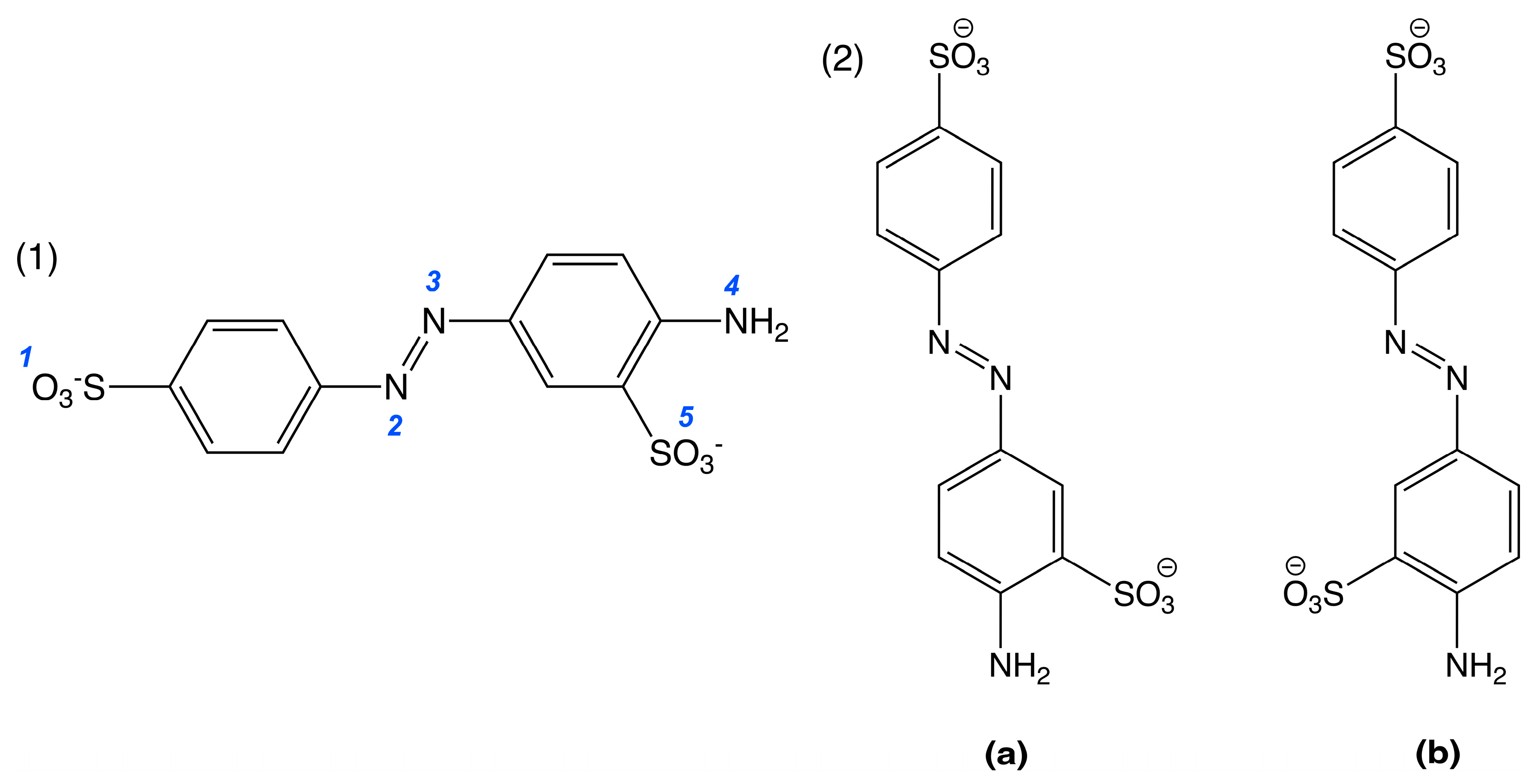

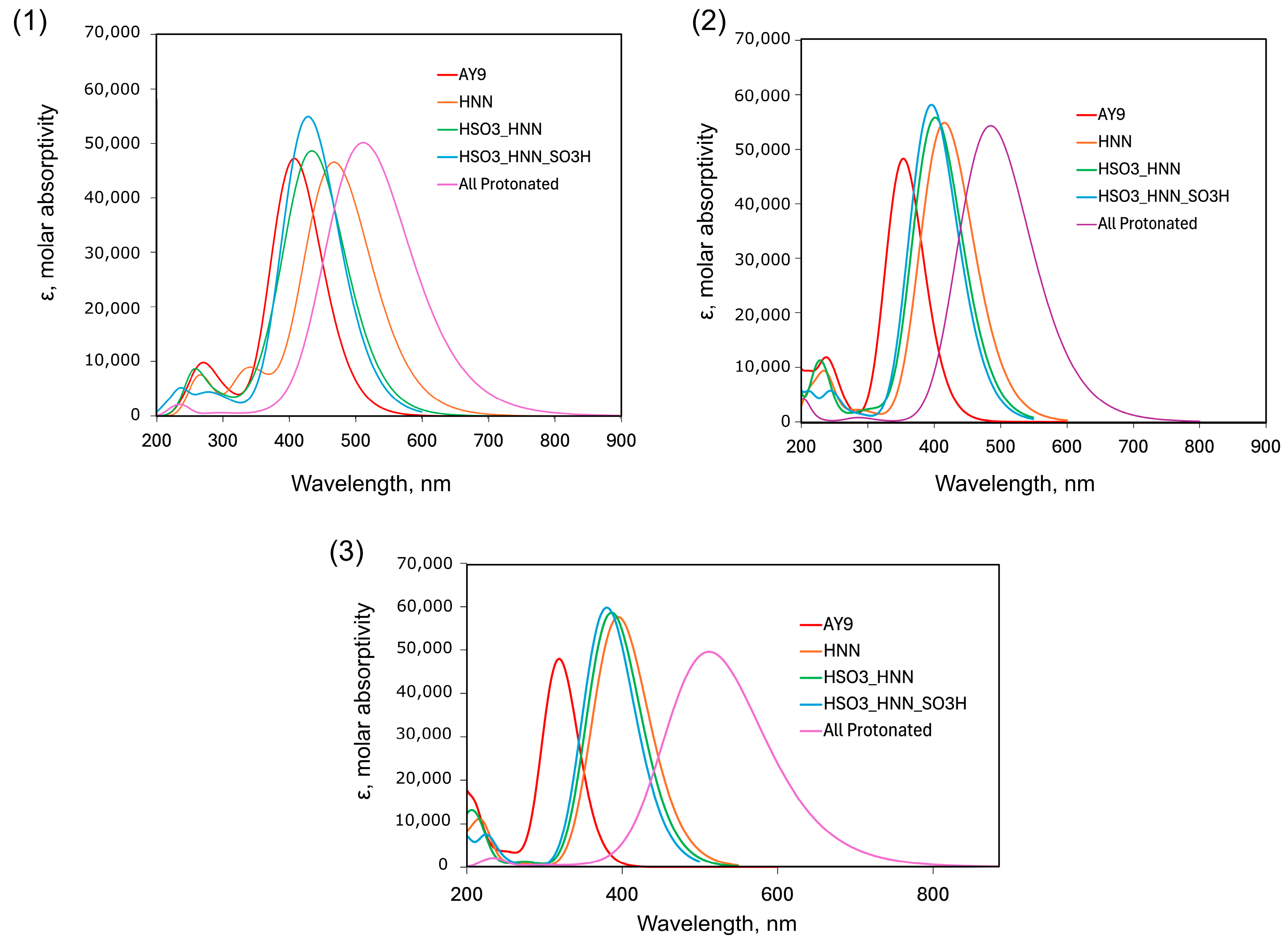
| Protonated State | Total Charge | Functional | ||
|---|---|---|---|---|
| B3LYP | CAM-B3LYP | LC-ωHPBE | ||
| SO3_NN_NH2_SO3 (a) | −2 | 0 | 0 | 0 |
| SO3_NN_NH2_SO3 (b) | 0.0001 | 0.0001 | 0.0004 | |
| SO3_ HNN_NH2_SO3 (a) | −1 | −0.4412 | −0435 | −0.4331 |
| SO3_HNN_NH2_SO3 (b) | −0.4417 | −0.4349 | −0.4325 | |
| HSO3_ HNN _NH2_SO3 (a) | 0 | −0.8743 | −0.8648 | −0.8636 |
| HSO3_ HNN _NH2_SO3 (b) | −0.8737 | −0.8648 | −0.8642 | |
| HSO3_HNN_NH2_SO3H (a) | +1 | −1.2935 | −1.2817 | −1.2825 |
| HSO3_HNN_NH2_SO3H (b) | −1.2915 | −1.2800 | −1.2807 | |
| HSO3_HNNH_NH2H_SO3H (a) | +3 | −2.0223 | −1.9997 | −1.9990 |
| HSO3_HNNH_NH2H_SO3H (b) | −2.0229 | −2.0010 | −1.9995 | |
| Protonated State | Excited State | Functional | ||
|---|---|---|---|---|
| B3LYP | CAM-B3LYP | LC-ωHPBE | ||
| SO3_NN_NH2_SO3 | S1 | 2.66 (0.0000) | 2.85 (0.0001) | 2.92 (0.0001) |
| S2 | 3.04 (1.1516) | 3.51 (0.0210) | 3.89 (1.1832) | |
| Sn | 4.53 (0.1680)/S8 | 5.28 (0.1996)/S7 | 7.27 (0.3175)/S18 | |
| SO3_HNN_NH2_SO3 | S1 | 2.54 (0.0004) | 2.98 (1.3539) | 3.14 (1.4213) |
| S2 | 2.65 (1.1334) | 4.09 (0.0242) | 4.46 (0.0029) | |
| Sn | 3.65 (0.1447)/S8 | 5.20 (0.1869)/S9 | 6.95 (0.3695)/S17 | |
| HSO3_ HNN _NH2_SO3 | S1 | 2.60 (0.0000) | 3.09 (1.3784) | 3.21 (1.4477) |
| S2 | 2.83 (1.1281) | 3.98 (0.0009) | 4.44 (0.0053) | |
| Sn | 3.28 (0.1963)/S3 | 6.68 (0.2263)/S20 | 7.14 (0.4329)/S16 | |
| HSO3_HNN_NH2_SO3H | S1 | 2.89 (1.3353) | 3.13 (1.4363) | 3.26 (1.4763) |
| S2 | 3.42 (0.0290) | 4.32 (0.0224) | 4.42 (0.0025) | |
| Sn | 5.26 (0.0746)/S14 | 6.80 (0.2640)/S17 | 7.20 (0.3345)/S13 | |
| HSO3_HNNH_NH2H_SO3H | S1 | 2.03 (0.0072) | 2.43 (0.0408) | 2.67 (1.2842) |
| S2 | 2.36 (0.2236) | 2.56 (1.3002) | 2.84 (0.0638) | |
| Sn | 2.38 (0.5720)/S3 | 6.06 (0.0388)/S17 | 6.98 (0.1485)/S20 | |
| Polymer, Conditions of LbL Fabrication | Deprotonated (Red Colour) | Protonated (Blue Colour) | ||
|---|---|---|---|---|
| Absorption Peaks | Shift | Absorption Peaks | Shift | |
| PAH, DW | 512 348 | 125 86 | 642 350 | 255 88 |
| PAH, NaCl | 500 364 | 113 102 | 630 358 | 243 96 |
| CS, DW | 505 364 | 118 102 | 620 357 | 233 95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Borchers, T.H.; Lin, M.; Barrett, C.J. Acid Yellow 9 Azo Dye Gets the Blues: An Optical Spectroscopy and DFT Study of Unusual Photochemistry in Multilayer Films with PAH and Chitosan. Molecules 2025, 30, 3850. https://doi.org/10.3390/molecules30193850
Kim M, Borchers TH, Lin M, Barrett CJ. Acid Yellow 9 Azo Dye Gets the Blues: An Optical Spectroscopy and DFT Study of Unusual Photochemistry in Multilayer Films with PAH and Chitosan. Molecules. 2025; 30(19):3850. https://doi.org/10.3390/molecules30193850
Chicago/Turabian StyleKim, Mikhail, Tristan H. Borchers, Monica Lin, and Christopher J. Barrett. 2025. "Acid Yellow 9 Azo Dye Gets the Blues: An Optical Spectroscopy and DFT Study of Unusual Photochemistry in Multilayer Films with PAH and Chitosan" Molecules 30, no. 19: 3850. https://doi.org/10.3390/molecules30193850
APA StyleKim, M., Borchers, T. H., Lin, M., & Barrett, C. J. (2025). Acid Yellow 9 Azo Dye Gets the Blues: An Optical Spectroscopy and DFT Study of Unusual Photochemistry in Multilayer Films with PAH and Chitosan. Molecules, 30(19), 3850. https://doi.org/10.3390/molecules30193850






