Photoluminescence Dependance of 2-Bromo-3-aminobenzo[de]anthracene-7-one on Solvent Polarity for Potential Applications in Color-Tunable Optoelectronics
Abstract
1. Introduction
2. Results and Discussions
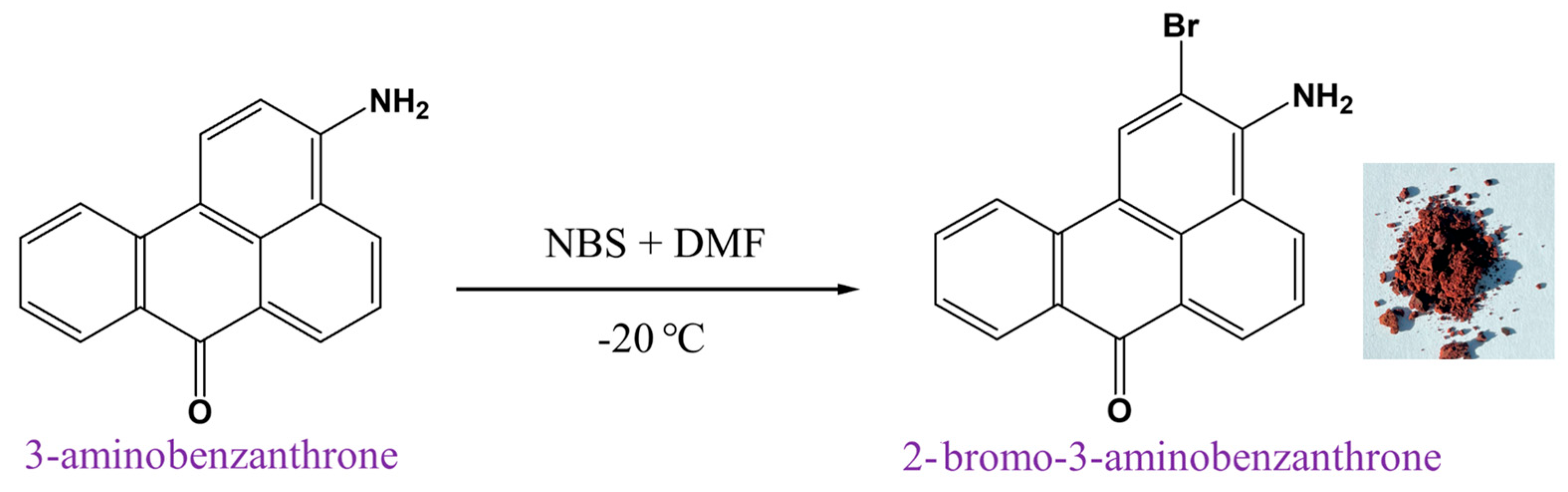
2.1. Synthesis of 2-Bromo-3-aminobenzo[de]anthracene-7-one
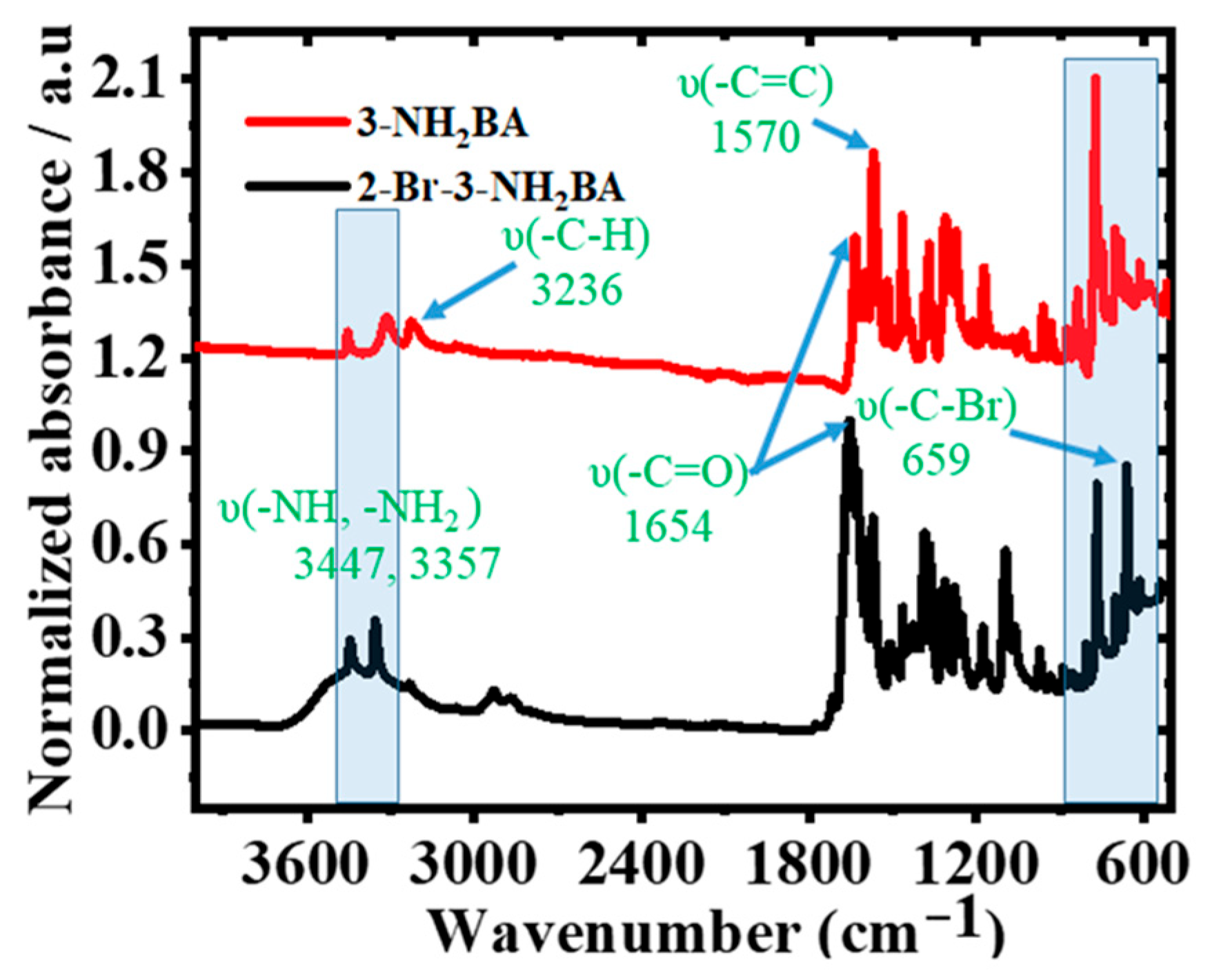
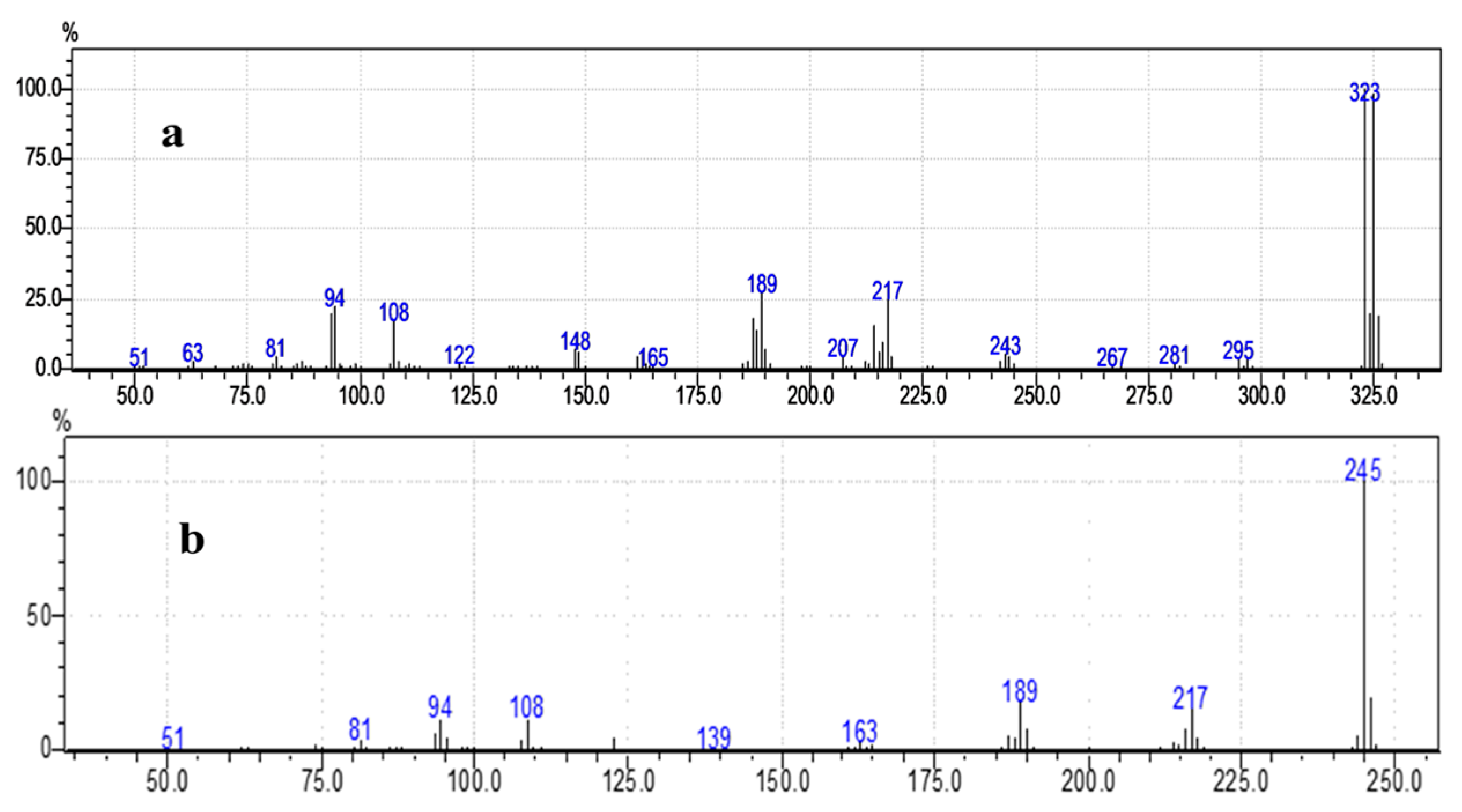
2.2. Structural and Elemental Properties of 3-NH2BA and 2-Br-3-NH2BA
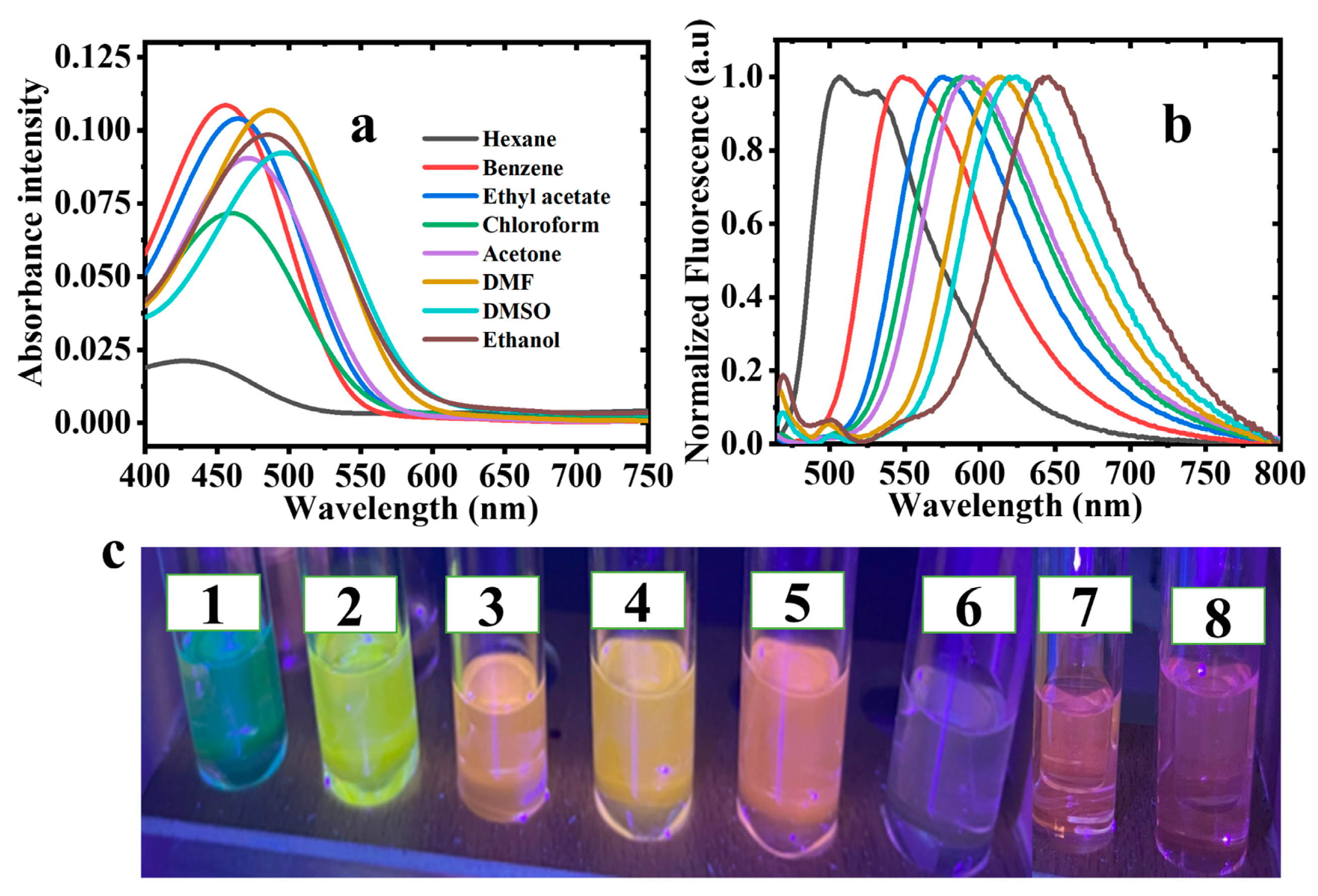
2.3. Effect of Solvents on the Photo-Physical Properties of 2-Bromo-3-aminobenzanthrone
2.4. Full Width Half Maxima (FWHM)
2.5. Effect of Solvent Polarity on Stokes Shift (∆ύ) of 2-Br-3-NH2BA
2.6. Fluorescence Quantum Yield (ϕ)
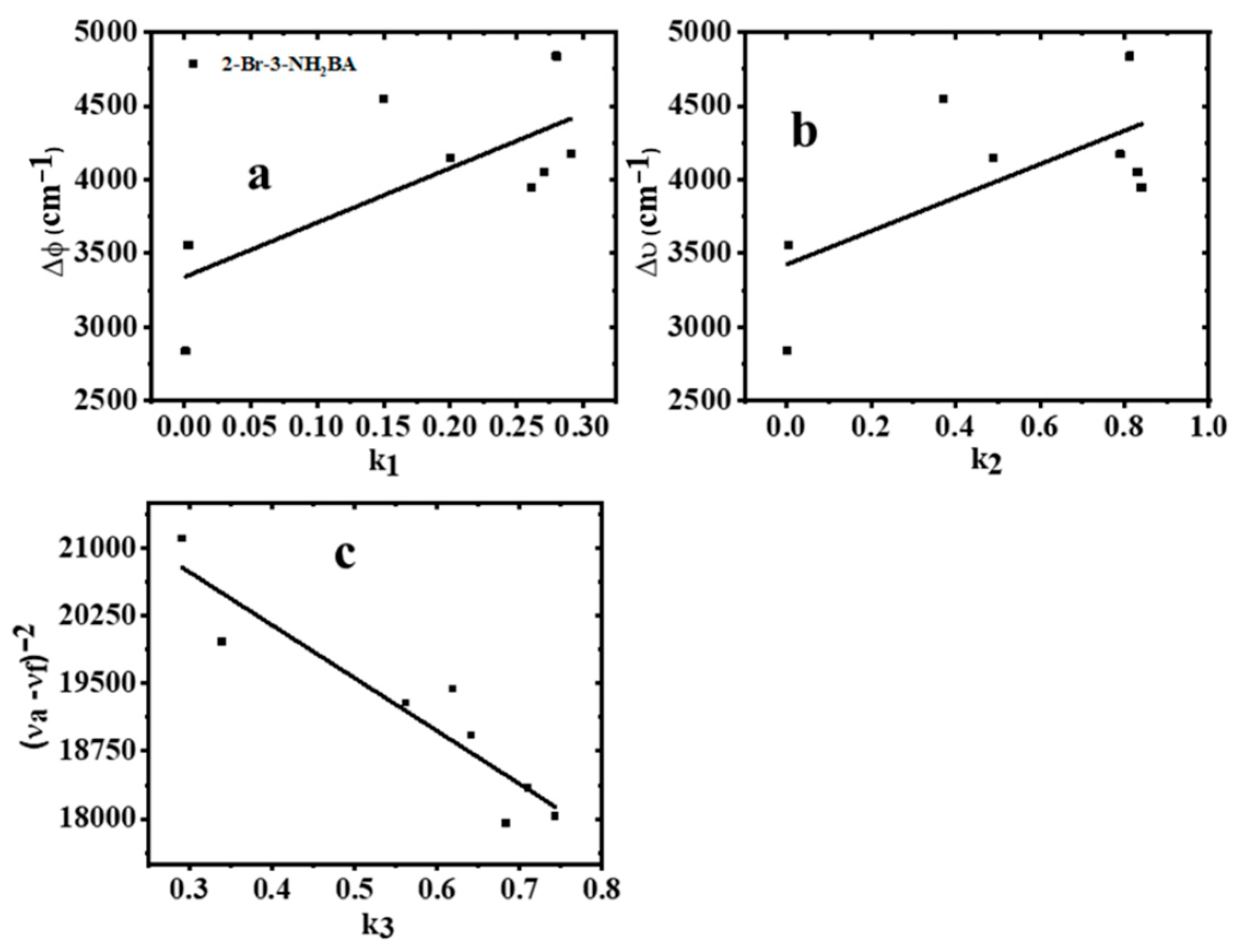
2.7. Estimation of 2-Br-3-NH2BA Dipole Moments
2.8. Effect of Solvent Polarity on Molar Extinction (ε) Coefficient
2.9. Effect of Solvents on the Optical Band Gap of 2-Br-3-NH2BA
3. Materials and Methods
3.1. Materials and Basic Measurements
3.2. Synthesis of 2-Bromo-3-aminobenzanthrone
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.Y.; Wang, Y.K.; Zhou, J.G.; He, W.; Dong, X.H.; Zhang, C.; Shi, X.-B.; Lia, L.-S.; Fung, M.-K. Efficient red organic LEDs via the combination of an exciplex host and micro-cavity. Mater. Chem. Front. 2022, 6, 1623–1629. [Google Scholar] [CrossRef]
- Xiao, X.M.; Zhu, L.S.; Guan, Y.; Hua, J.; Wang, H.M.; Dong, H.; Wang, J. Highly efficient all-phosphorescent white organic light-emitting diodes with low efficiency roll-off and stable-color by managing triplet excitons in emissive layer. Acta Phys. Sin. 2020, 69, 047202. [Google Scholar] [CrossRef]
- Chandra, V.K.; Chandra, B.P.; Jha, P. Organic Light—Emitting Diodes and their Applications. Defect Diffus. Forum 2014, 357, 29–93. [Google Scholar] [CrossRef]
- Banerjee, S.; Singh, P.; Purkayastha, P.; Kumar Ghosh, S. Evolution of Organic Light Emitting Diode (OLED) Materials and their Impact on Display Technology. Chem. Asian J. 2025, 4, e202401291. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Mittal, P.; Negi, S. Recent advancements over a decade for organic light-emitting diodes: From structural diversity, role of layers, colour emission, material classification, performance improvement, fabrication to applications. Bull. Mater. Sci. 2022, 45, 109. [Google Scholar] [CrossRef]
- Huang, Y. Recent research of materials for emissive layer of OLED. J. Phys. Conf. Ser. 2023, 2608, 012012. [Google Scholar] [CrossRef]
- Yang, H. Emerging Materials for High-Efficiency Blue Organic Light-Emitting Diodes (OLEDs). Highl. Sci. Eng. Technol. 2024, 16, 144–149. [Google Scholar] [CrossRef]
- Nayak, D.; Choudhary, R.B.; Nayak, D.; Choudhary, R.B. Conducting Polymer-Based Emissive Layer on Efficiency of OLEDs. In Light-Emitting Diodes and Photodetectors-Advances and Future Directions; Intechopen: London, UK, 2021. [Google Scholar]
- Reineke, S.; Cheng, G.; Su, S.J.; Yin, X.; He, Y.; Wang, X.; Wang, J. Recent Advances in Thermally Activated Delayed Fluorescent Polymer—Molecular Designing Strategies. Front. Chem. 2020, 8, 546192. [Google Scholar] [CrossRef]
- Li, X.; Xie, Y.; Li, Z. Diversity of Luminescent Metal Complexes in OLEDs: Beyond Traditional Precious Metals. Chem. Asian J. 2021, 16, 2817–2829. [Google Scholar] [CrossRef]
- Liu, S.; Xie, W.; Lee, C.S. Organic light-emitting diodes, what’s next? Next Nanotechnol. 2023, 1, 100003. [Google Scholar] [CrossRef]
- Jiang, J.; Lee, J.Y. Degradation mechanisms and lifetime extending strategy of phosphorescent and thermally activated delayed-fluorescence organic light-emitting diodes. Mater. Today 2023, 68, 204–233. [Google Scholar] [CrossRef]
- Chen, M.; Chen, Y.; Li, Y.; Lin, Y.; Wu, Y. Efficient orange and red thermally activated delayed fluorescence materials based on 1,8-naphthalimide derivatives. RSC Adv. 2024, 14, 6494–6500. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, Y.; Qiao, X.; Yang, D.; Sun, Q.; Dai, Y.; Zhu, X.; Ma, D. Achieving efficient and stable blue organic light-emitting diodes via suppressing triplet–polaron annihilation processes. Org. Electron. 2024, 125, 106983. [Google Scholar] [CrossRef]
- Patel, M.B.; Trivedi, V.A.; Dabhi, R.C.; Maru, J.J.; Patel, Z.M. Synthetic Development of 3-Functionalized Benzanthrone as a Fluorophore for Various Uses. Mini Rev. Org. Chem. 2024, 22, e18756298330235. [Google Scholar] [CrossRef]
- Grabchev, I.; Bojinov, V.; Moneva, I. The synthesis and application of fluorescent dyes based on 3-amino benzanthrone. Dyes Pigm. 2001, 48, 143–150. [Google Scholar] [CrossRef]
- Altaf, Y.; Ullah, S.; Khan, F.A.; Maalik, A.; Rubab, S.L.; Hashmi, M.A. Finding New Precursors for Light Harvesting Materials: A Computational Study of the Fluorescence Potential of Benzanthrone Dyes. ACS Omega 2021, 6, 32334–32341. [Google Scholar] [CrossRef]
- Maļeckis, A.; Romanovska-Dzalbe, E. Recent progress of benzanthrone chemistry: A condensed review. Chem. Pap. 2025, 79, 3463–3473. [Google Scholar] [CrossRef]
- Kapusta, P.; Machalický, O.; Hrdina, R.; Neprašs, M.; Zimmt, M.B.; Fidler, V. Photophysics of 3-substituted benzanthrones: Substituent and solvent control of intersystem crossing. J. Phys. Chem. A 2003, 107, 9740–9746. [Google Scholar] [CrossRef]
- Li, B.; Xiao, T.; Gu, F.L.; Jiang, J.; Jia, C. Torsion Angles between Donor and Acceptor Moieties as a Descriptor for Designing Nonlinear Optics and Thermally Activated Delayed Fluorescence Materials. J. Phys. Chem. A 2023, 127, 7274–7283. [Google Scholar] [CrossRef]
- Deng, Y.; Shi, Y.; Li, L.; Tang, R.; Zhou, Z.; Xiong, S.; Li, W.; Liu, J.; Huang, Y. Molecular modification: A promising strategy for the design of donor-acceptor-type organic polymers photocatalyst. Appl. Catal. B Environ. 2024, 352, 124043. [Google Scholar] [CrossRef]
- Luponosov, Y.N.; Min, J.; Solodukhin, A.N.; Kozlov, O.V.; Obrezkova, M.A.; Peregudova, S.M.; Ameri, T.; Chvalun, S.N.; Pshenichnikov, M.S.; Brabec, C.J.; et al. Effects of electron-withdrawing group and electron-donating core combinations on physical properties and photovoltaic performance in D-π-A star-shaped small molecules. Org. Electron. 2016, 32, 157–168. [Google Scholar] [CrossRef]
- Eisenberg, J.B.; Lee, K.; Yuan, X.; Schmidt, J.R.; Choi, K.S. The Impact of Electron Donating and Withdrawing Groups on Electrochemical Hydrogenolysis and Hydrogenation of Carbonyl Compounds. J. Am. Chem. Soc. 2024, 146, 15309–15319. [Google Scholar] [CrossRef] [PubMed]
- Orlova, N.; Nikolajeva, I.; Pučkins, A.; Belyakov, S.; Kirilova, E. Heterocyclic Schiff Bases of 3-Aminobenzanthrone and Their Reduced Analogues: Synthesis, Properties and Spectroscopy. Molecules 2021, 26, 2570. [Google Scholar] [CrossRef] [PubMed]
- Gonta, S.; Utinans, M.; Kirilov, G.; Belyakov, S.; Ivanova, I.; Fleisher, M.; Savenkov, V.; Kirilova, E. Fluorescent substituted amidines of benzanthrone: Synthesis, spectroscopy and quantum chemical calculations. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 101, 325–334. [Google Scholar] [CrossRef]
- Takamura-Enya, T.; Kawanishi, M.; Yagi, T.; Hisamatsu, Y. Structural Identification of DNA Adducts Derived from 3-Nitrobenzanthrone, a Potent Carcinogen Present in the Atmosphere. Chem. Asian J. 2007, 2, 1174–1185. [Google Scholar] [CrossRef]
- Mitchell, R.H.; Lai, Y.H.; Williams, R.V. N-Bromosuccinimide-Dimethylformamide: A Mild, Selective Nuclear Monobromination Reagent for Reactive Aromatic Compounds. J. Org. Chem. 1979, 44, 4733–4735. [Google Scholar] [CrossRef]
- Kharaka, Y.K.; Hanor, J.S. Deep Fluids in Sedimentary Basins. In Treatise on Geochemistry, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 471–515. [Google Scholar]
- Kirilova, E.M.; Puckins, A.I.; Romanovska, E.; Fleisher, M.; Belyakov, S.V. Novel amidine derivatives of benzanthrone: Effect of bromine atom on the spectral parameters. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 202, 41–49. [Google Scholar] [CrossRef]
- Arslan, B.S. Effect of electron donor groups on the performance of benzothiadiazole dyes with simple structures for dye-sensitized solar cells. J. Photochem. Photobiol. A Chem. 2024, 449, 115392. [Google Scholar] [CrossRef]
- Maļeckis, A.; Cvetinska, M.; Griškjāns, E.; Kirilova, E. Exploring Dual Solvatochromic Traits in Novel Fluorescent Benzanthrone Ethynyl Derivatives. J. Solut. Chem. 2024, 53, 1074–1088. [Google Scholar] [CrossRef]
- Shivraj; Siddlingeshwar, B.; Kirilova, E.M.; Belyakov, S.V.; Divakar, D.D.; Alkheraif, A.A. Photophysical properties of benzanthrone derivatives: Effect of substituent, solvent polarity and hydrogen bonding. Photochem. Photobiol. Sci. 2018, 17, 453–464. [Google Scholar] [CrossRef]
- Kirilova, E.; Yanichev, A.; Puckins, A.; Fleisher, M.; Belyakov, S. Experimental and Theoretical Study on Structure and Spectroscopic Properties of 2-Bromo-3-N-(N′,N′dimethylformamidino)benzanthrone. Luminescence 2018, 33, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Rubenina, I.; Gavarane, I.; Kirilova, E.; Mezaraupe, L.; Kirjusina, M. Comparison of the Benzanthrone Luminophores: They Are Not Equal for Rapid Examination of Parafasciolopsis fasciolaemorpha (Trematoda: Digenea). Biomolecules 2021, 11, 598. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Sun, M.; Xue, S.; Yang, W. Recent progress of blue fluorescent organic light-emitting diodes with narrow full width at half maximum. Dye. Pigment 2023, 208, 110799. [Google Scholar] [CrossRef]
- Sun, H.; Xu, S.; Chen, Z.; Liu, F.; Zong, S.; Wang, Z.; Wang, C. Solvent-free and dispersant-free synthesis of narrow full width at half maximum carbon dots via sole precursor of p-phenylenediamine. Mater. Res. Bull. 2023, 159, 112092. [Google Scholar] [CrossRef]
- Ha, J.M.; Hur, S.H.; Pathak, A.; Jeong, J.E.; Woo, H.Y. Recent advances in organic luminescent materials with narrowband emission. NPG Asia Mater. 2021, 13, 53. [Google Scholar] [CrossRef]
- Shivaleela, B.; Shivraj, G.G.; Mayadevi, K.; Mahantesh, B.; Hanagodimath, S.M. Effect of solvent on fluorescence and absorption spectra of fluorescent coumarin derivative: Estimation of ground and excited state dipole moments. Mater. Today Proc. 2022, 68, 564–572. [Google Scholar] [CrossRef]
- Siddlingeshwar, B.S.; Thomas, A.; Kirilova, E.M.; Divakar, D.D.; Alkheraif, A.A. Experimental and theoretical insights on the effect of solvent polarity on the photophysical properties of a benzanthrone dye. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 218, 221–228. [Google Scholar]
- Huo, X.; Shen, H.; Liu, R.; Shao, J. Solvent Effects on Fluorescence Properties of Carbon Dots: Implications for Multicolor Imaging. ACS Omega 2021, 6, 26499–26508. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, X. Microfluidic and lab-on-chip technologies for biosensors. In Biosensors Based on Nanomaterials and Nanodevices, 1st ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 443–478. [Google Scholar]
- Williams, A.T.R.; Winfield, S.A.; Miller, J.N. Relative fluorescence quantum yields using a computer-controlled luminescence spectrometer. Analyst 1983, 108, 1067–1071. [Google Scholar] [CrossRef]
- Grabowski, Z.R.; Rotkiewicz, K.; Rettig, W. Structural Changes Accompanying Intramolecular Electron Transfer: Focus on Twisted Intramolecular Charge-Transfer States and Structures. Chem. Rev. 2003, 103, 3899–4031. [Google Scholar] [CrossRef]
- Valeur, B.; Berberan-Santos, M.N. Molecular Fluorescence: Principles and Applications, 2nd ed.; Wiley-VCH: Hoboken, NJ, USA, 2013. [Google Scholar]
- Mataga, N.; Kaifu, Y.; Koizumi, M. The Solvent Effect on Fluorescence Spectrum, Change of Solute-Solvent Interaction during the Lifetime of Excited Solute Molecule. Bull. Chem. Soc. Jpn. 1955, 28, 690–691. [Google Scholar] [CrossRef]
- Husain, M.M.; Sindhu, R.; Tandon, H.C. Determination of excited singlet-state dipole moments of hydroxy and methoxy coumarins using solvatochromic method. Eur. J. Chem. 2012, 3, 75–80. [Google Scholar] [CrossRef]
- Bilot, L.; Kawski, A. Zur Theorie des Einflusses von Lösungsmitteln auf die Elektronenspektren der Moleküle. Z. Naturforschung A 1962, 17, 621–627. [Google Scholar] [CrossRef]
- Edward, J.T. Molecular volumes and the Stokes-Einstein equation. J. Chem. Educ. 1970, 47, 261. [Google Scholar] [CrossRef]
- Xia, B.; Wu, H.; Rui, J.; Tang, H.; Wang, L.; Cao, D. 7,16-Dihydro-14H-diindolo[3,2-c:2′,3′-h]phenothiazine-based organic dyes with high molar extinction coefficient for efficient dye-sensitized solar cells. Dye. Pigment 2024, 223, 111923. [Google Scholar] [CrossRef]
- Casasanta, G.; Falcini, F.; Garra, R. Beer–Lambert law in photochemistry: A new approach. J. Photochem. Photobiol. A Chem. 2022, 432, 114086. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Zang, L.; Liu, R.; Holman, M.W.; Nguyen, K.T.; Adams, D.M. A single-molecule probe based on intramolecular electron transfer. J. Am. Chem. Soc. 2002, 124, 10640–10641. [Google Scholar] [CrossRef]
- Han, Y.; Yu, J.; Yang, Y.; Zhang, H.; Wang, Z.; Hu, J. Nonequilibrium bandgap modification in porphyrin-based metal-organic frameworks revealed by transient absorption spectroscopy. Appl. Phys. Lett. 2024, 125, 111901. [Google Scholar] [CrossRef]
- Majid, A.; Kiran, S.; Sandhu, Q.u.A.; Khan, S.; Khan, S. The effects of polar solvents on structural, electronic, and optical properties of organic dyes. Int. J. Quantum Chem. 2022, 122, 26876. [Google Scholar] [CrossRef]
- An, B.; Feng, S.; Wen, K.; Wu, W.; Yuan, H.; Zhu, Q.; Guo, X.; Zhang, J. Theoretical insights into the ultrafast excited-state intramolecular proton transfer (ESIPT) mechanism in a series of amide-based NH⋯N hydrogen-bonding compounds. Org. Electron. 2017, 45, 1–8. [Google Scholar] [CrossRef]
- Vallés-Pelarda, M.; Gualdrón-Reyes, A.F.; Felip-León, C.; Angulo-Pachón, C.A.; Agouram, S.; Muñoz-Sanjosé, V.; Miravet, J.F.; Galindo, F.; Mora-Seró, I. High Optical Performance of Cyan-Emissive CsPbBr3 Perovskite Quantum Dots Embedded in Molecular Organogels. Adv. Opt. Mater. 2021, 9, 2001786. [Google Scholar] [CrossRef]
- Yoon, S.B.; Hwang, S.; Kim, Y.; Kim, B.G.; Na, H.B. Preparation of Water-Dispersible Perovskite-Quantum Dots for Biomedical Applications. Korean J. Chem. Eng. 2024, 41, 3345–3357. [Google Scholar] [CrossRef]
- Lombardo, M.E.; Benetti, D.; La Carrubba, V.; Rosei, F. Heteroatom-Doping for Carbon Dots: An Efficient Strategy to Improve Their Optoelectronic Properties. ECS Meet. Abstr. 2020, MA2020-01, 1087. [Google Scholar]
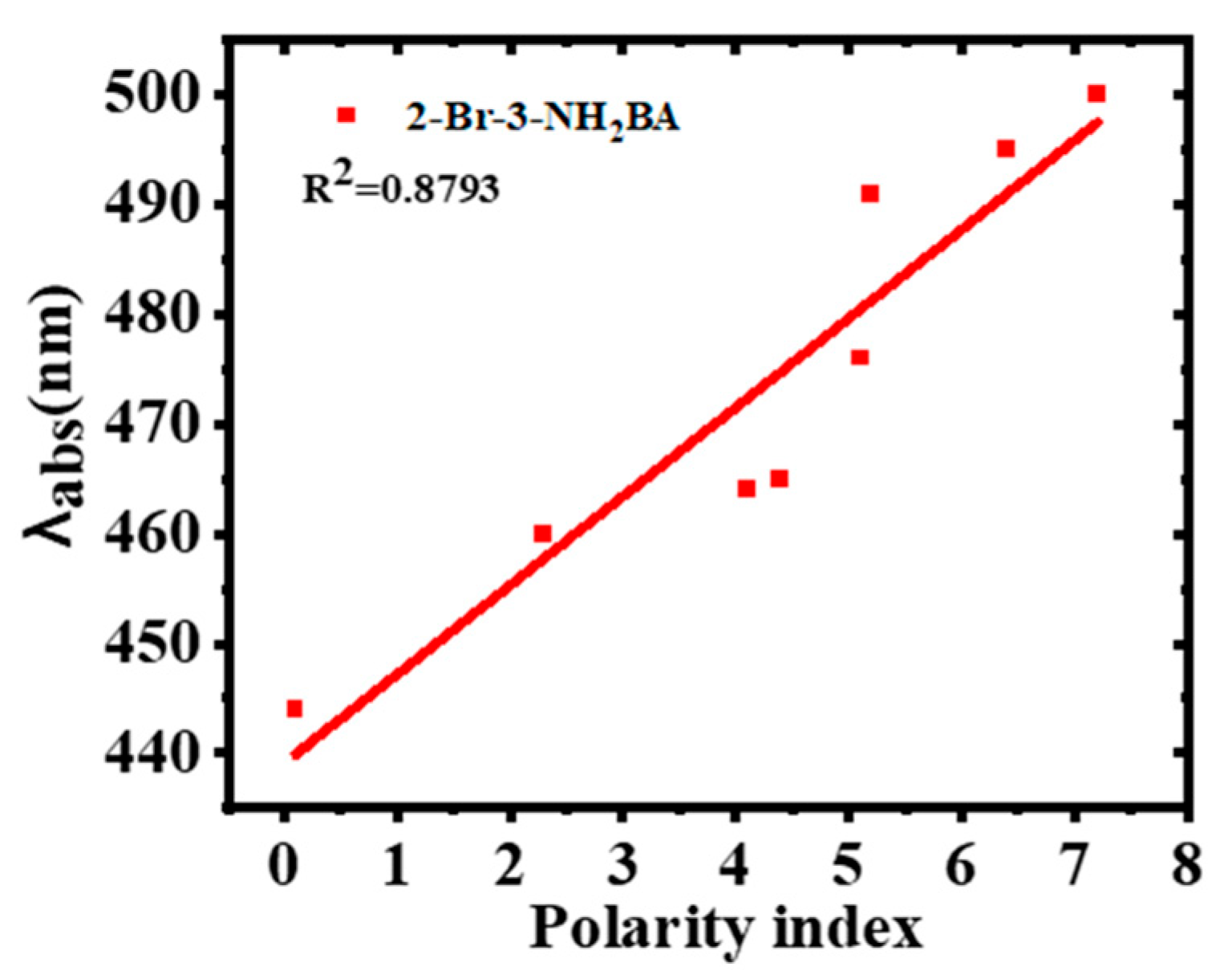
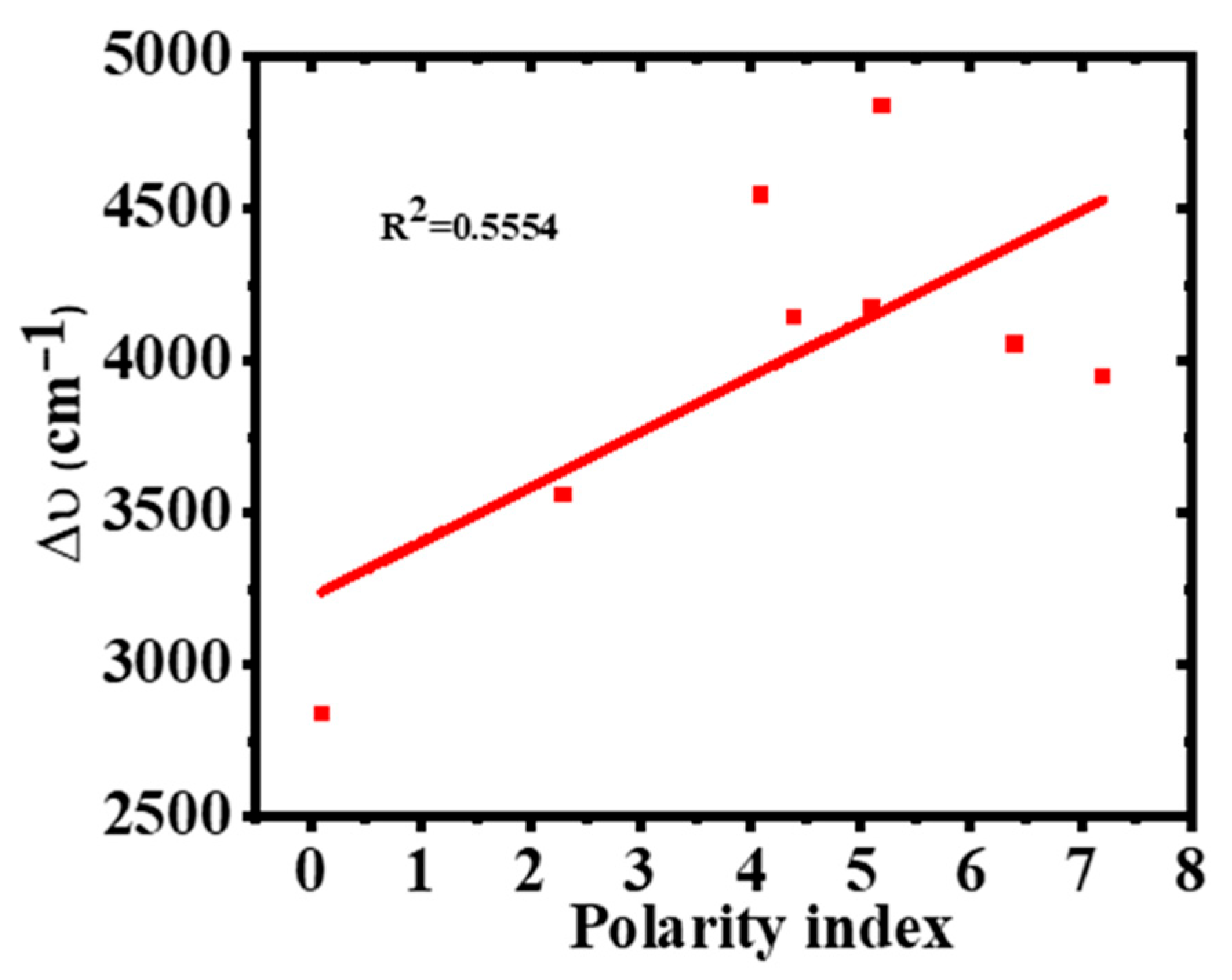
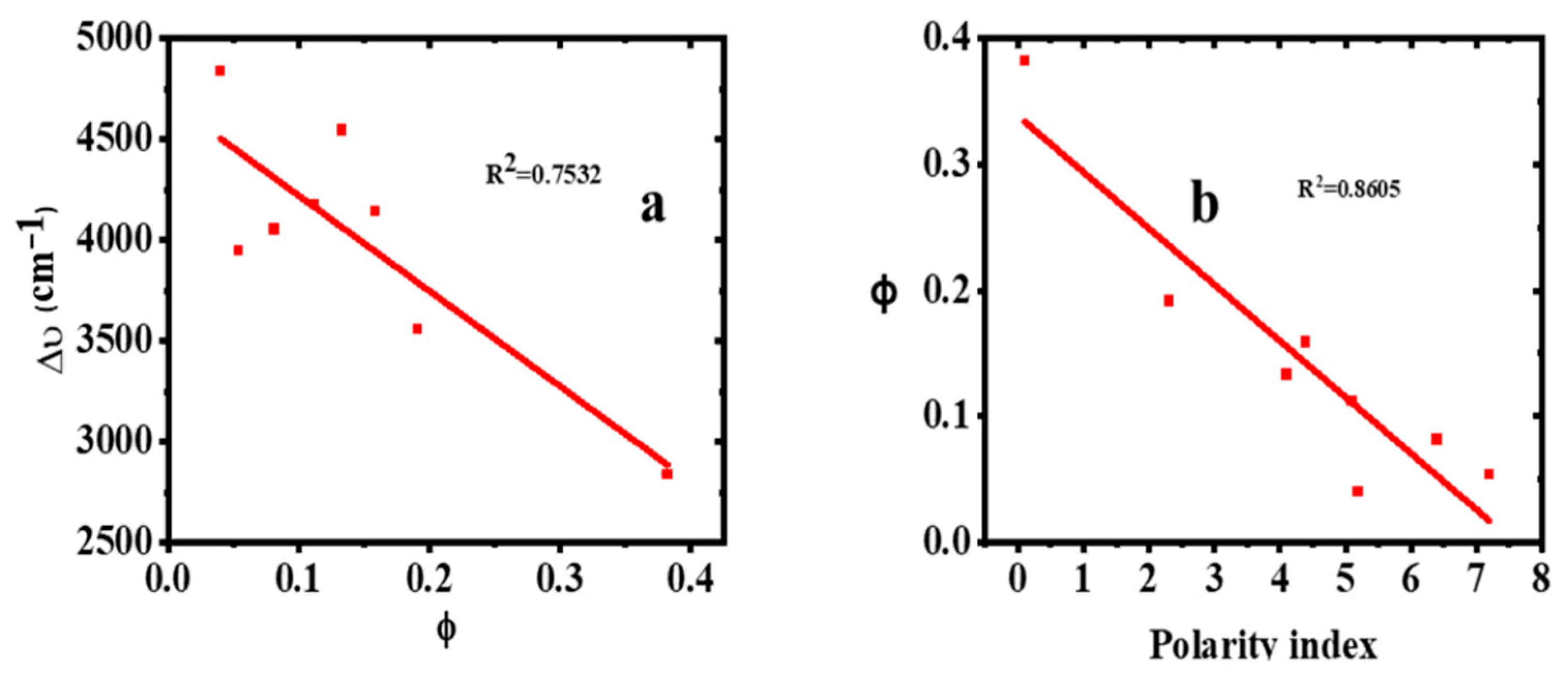
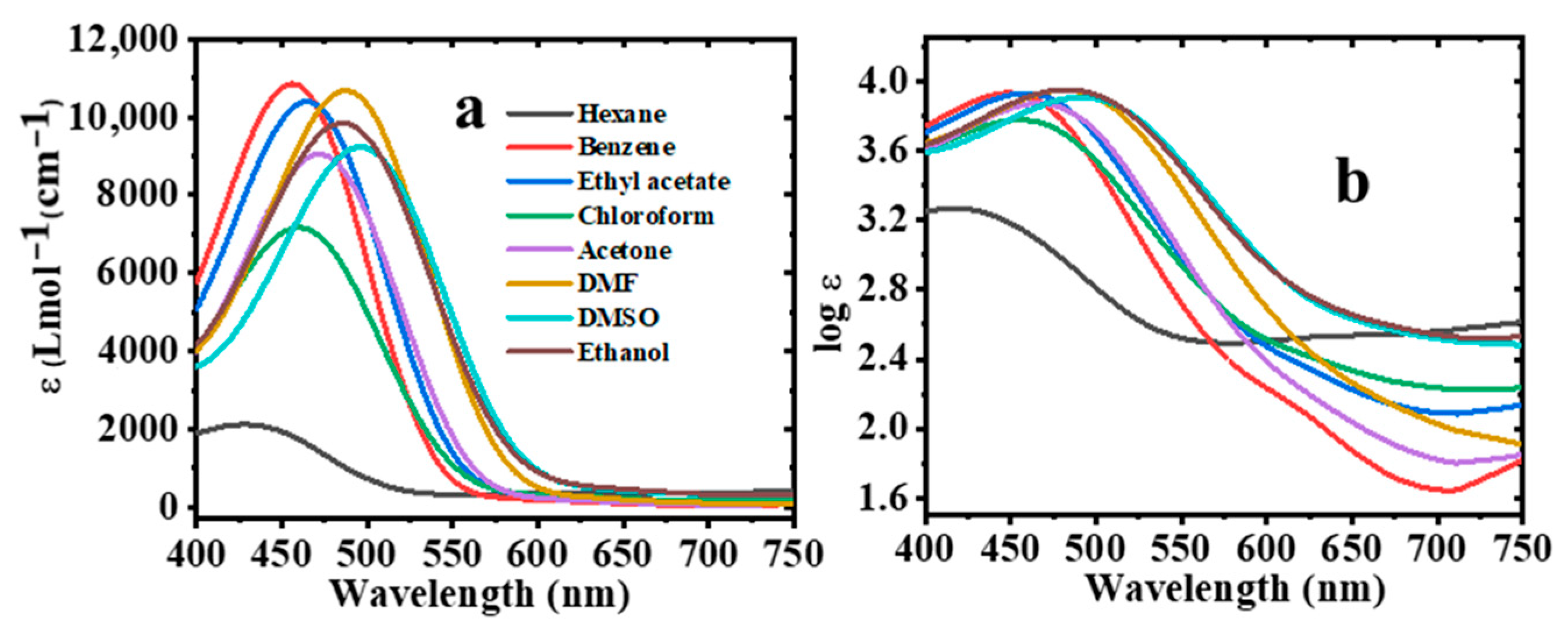
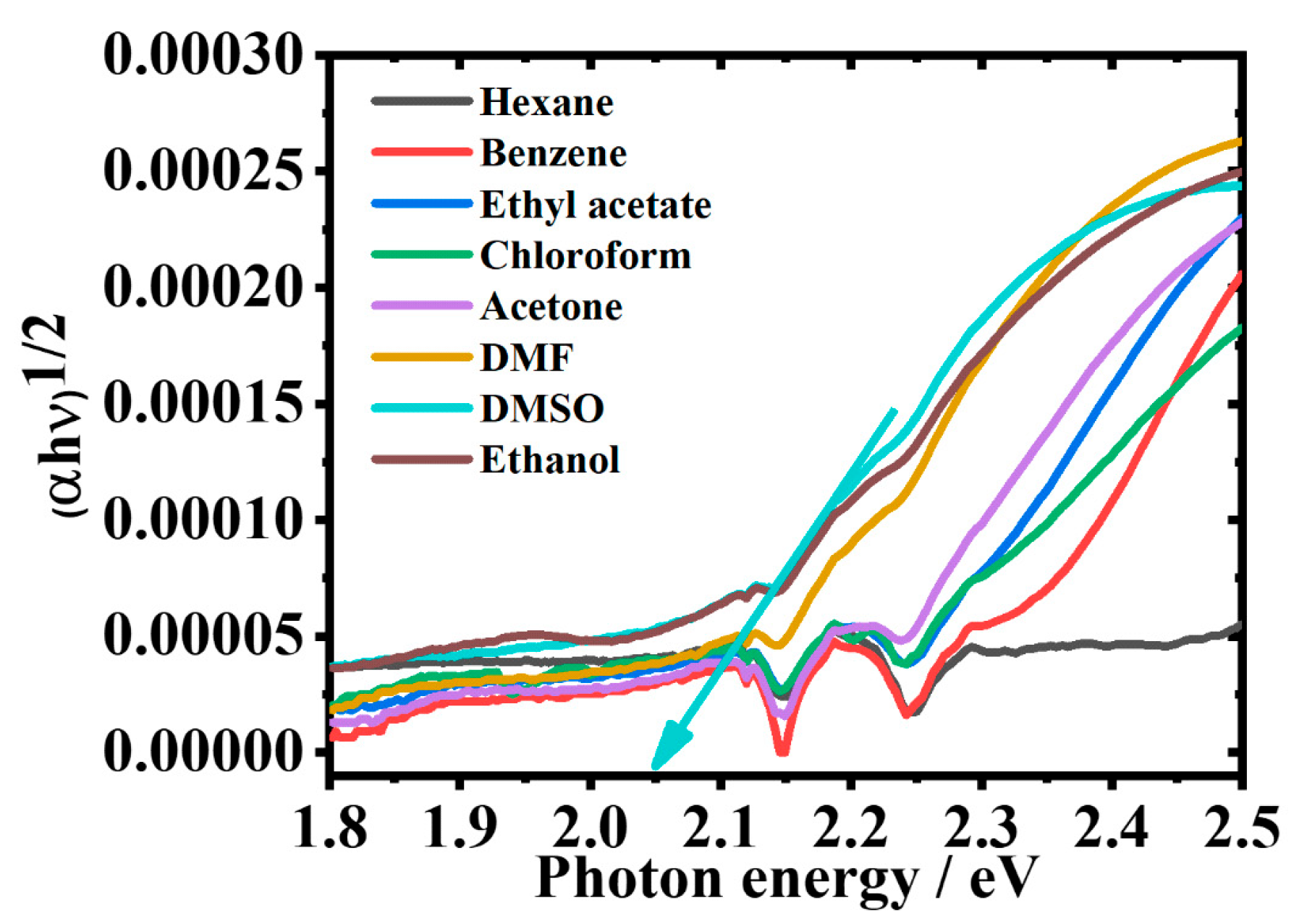
| Solvent | λabs (nm) | log ε | λems (nm) | PI | ∆ύ (cm−1) | FWHM (nm) | ϕ |
|---|---|---|---|---|---|---|---|
| Hexane | 444 | 3.415 | 508 | 0.1 | 2837.48 | 86 | 0.382160861 |
| Benzene | 460 | 4.110 | 550 | 2.3 | 3557.31 | 89 | 0.191602503 |
| Chloroform | 464 | 3.915 | 588 | 4.1 | 4544.92 | 91 | 0.133138514 |
| Ethyl acetate | 465 | 4.077 | 576 | 4.4 | 4144.27 | 92 | 0.158607877 |
| Acetone | 476 | 4.012 | 594 | 5.1 | 4173.39 | 94 | 0.112330369 |
| Ethanol | 491 | 4.040 | 644 | 5.2 | 4838.65 | 95 | 0.040469667 |
| DMF | 491 | 4.082 | 613 | 6.4 | 4053.39 | 97 | 0.081548666 |
| DMSO | 500 | 4.015 | 623 | 7.2 | 3948.64 | 98 | 0.054056066 |
| Solvent | k1 | k2 | k3 | ||
|---|---|---|---|---|---|
| Hexane | 1.375 | 1.89 | 0.001 | 0.0015 | 0.290818 |
| Benzene | 1.501 | 2.274 | 0.003 | 0.0045 | 0.3391 |
| Chloroform | 1.443 | 4.807 | 0.15 | 0.3722 | 0.562419 |
| EtOAc | 1.372 | 6.02 | 0.2 | 0.4893 | 0.619282 |
| Aceton | 1.359 | 20.7 | 0.291 | 0.7903 | 0.6419 |
| Ethanol | 1.361 | 24.55 | 0.28 | 0.8131 | 0.6841 |
| DMF | 1.446 | 36.71 | 0.271 | 0.8307 | 0.7105 |
| DMSO | 1.478 | 46.6 | 0.261 | 0.8407 | 0.7432 |
| 2-Br-3-NH2BA | ||||||
| Slopes | 3699 | 1135 | −5860 | 5.89 | 8.72 | 2.83 |
| R2 | 0.571 | 0.553 | 0.867 |
| Solvent | Hexane | Benzene | Chloroform | Ethyl Acetate | Acetone | Ethanol | DMF | DMSO |
|---|---|---|---|---|---|---|---|---|
| Band gap (eV) | 2.204 | 2.198 | 2.175 | 2.161 | 2.148 | 1.967 | 2.051 | 2.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karungani, E.; Kirilova, E.; Avotina, L.; Puckins, A.; Osipovs, S.; Ochodo, T.; Airo, M.; Otieno, F. Photoluminescence Dependance of 2-Bromo-3-aminobenzo[de]anthracene-7-one on Solvent Polarity for Potential Applications in Color-Tunable Optoelectronics. Molecules 2025, 30, 2677. https://doi.org/10.3390/molecules30132677
Karungani E, Kirilova E, Avotina L, Puckins A, Osipovs S, Ochodo T, Airo M, Otieno F. Photoluminescence Dependance of 2-Bromo-3-aminobenzo[de]anthracene-7-one on Solvent Polarity for Potential Applications in Color-Tunable Optoelectronics. Molecules. 2025; 30(13):2677. https://doi.org/10.3390/molecules30132677
Chicago/Turabian StyleKarungani, Emmanuel, Elena Kirilova, Liga Avotina, Aleksandrs Puckins, Sergejs Osipovs, Titus Ochodo, Mildred Airo, and Francis Otieno. 2025. "Photoluminescence Dependance of 2-Bromo-3-aminobenzo[de]anthracene-7-one on Solvent Polarity for Potential Applications in Color-Tunable Optoelectronics" Molecules 30, no. 13: 2677. https://doi.org/10.3390/molecules30132677
APA StyleKarungani, E., Kirilova, E., Avotina, L., Puckins, A., Osipovs, S., Ochodo, T., Airo, M., & Otieno, F. (2025). Photoluminescence Dependance of 2-Bromo-3-aminobenzo[de]anthracene-7-one on Solvent Polarity for Potential Applications in Color-Tunable Optoelectronics. Molecules, 30(13), 2677. https://doi.org/10.3390/molecules30132677








