SABRE Ir-IMes Catalysis for the Masses †
Abstract
1. Introduction
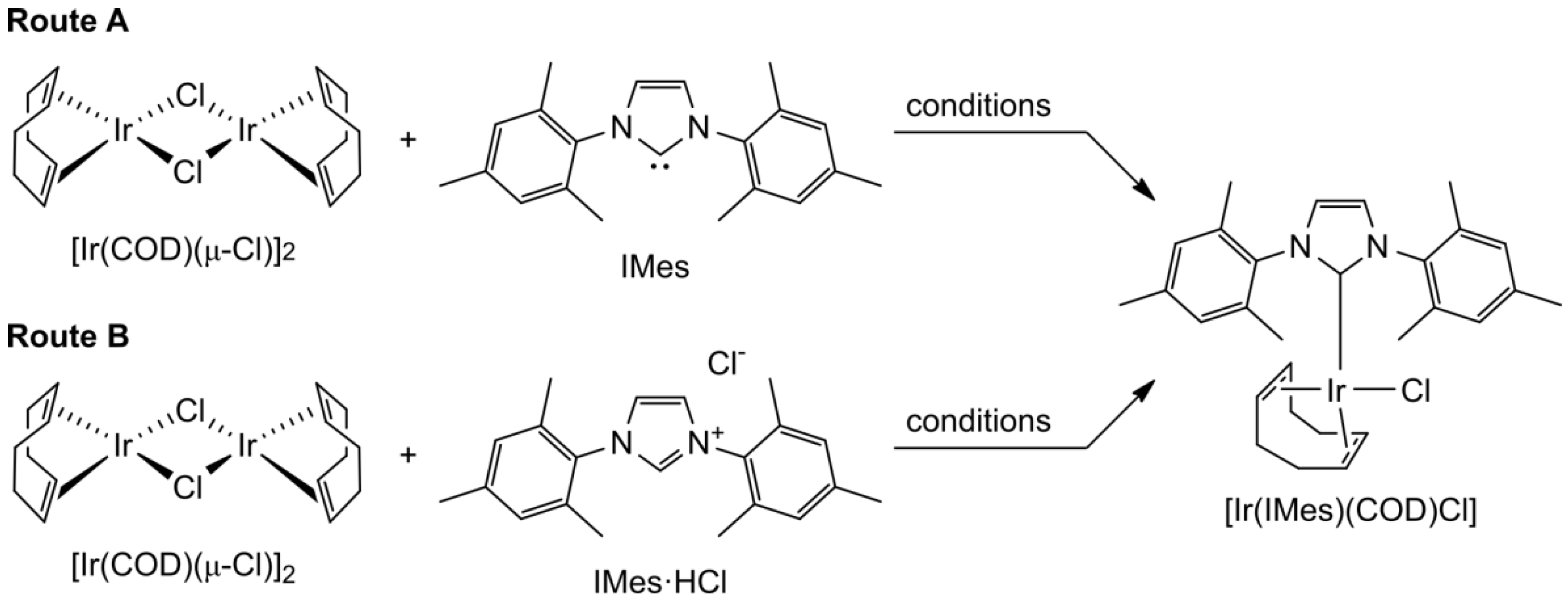
| Route | Conditions | Yield (%) | Reference |
|---|---|---|---|
| A | THF, RT, inert atmosphere | 52 | [47] |
| A | benzene, RT, inert atmosphere | 93 | [48] |
| B | tBuOK, THF, RT, inert atmosphere | 64 | [45] |
| B | K2CO3, acetone, 60 °C, air | 67 | [51] |
| A | benzene, RT, air | 82 | [52] |
| B | K2CO3, acetone, 60 °C, air | 85 | [52] |
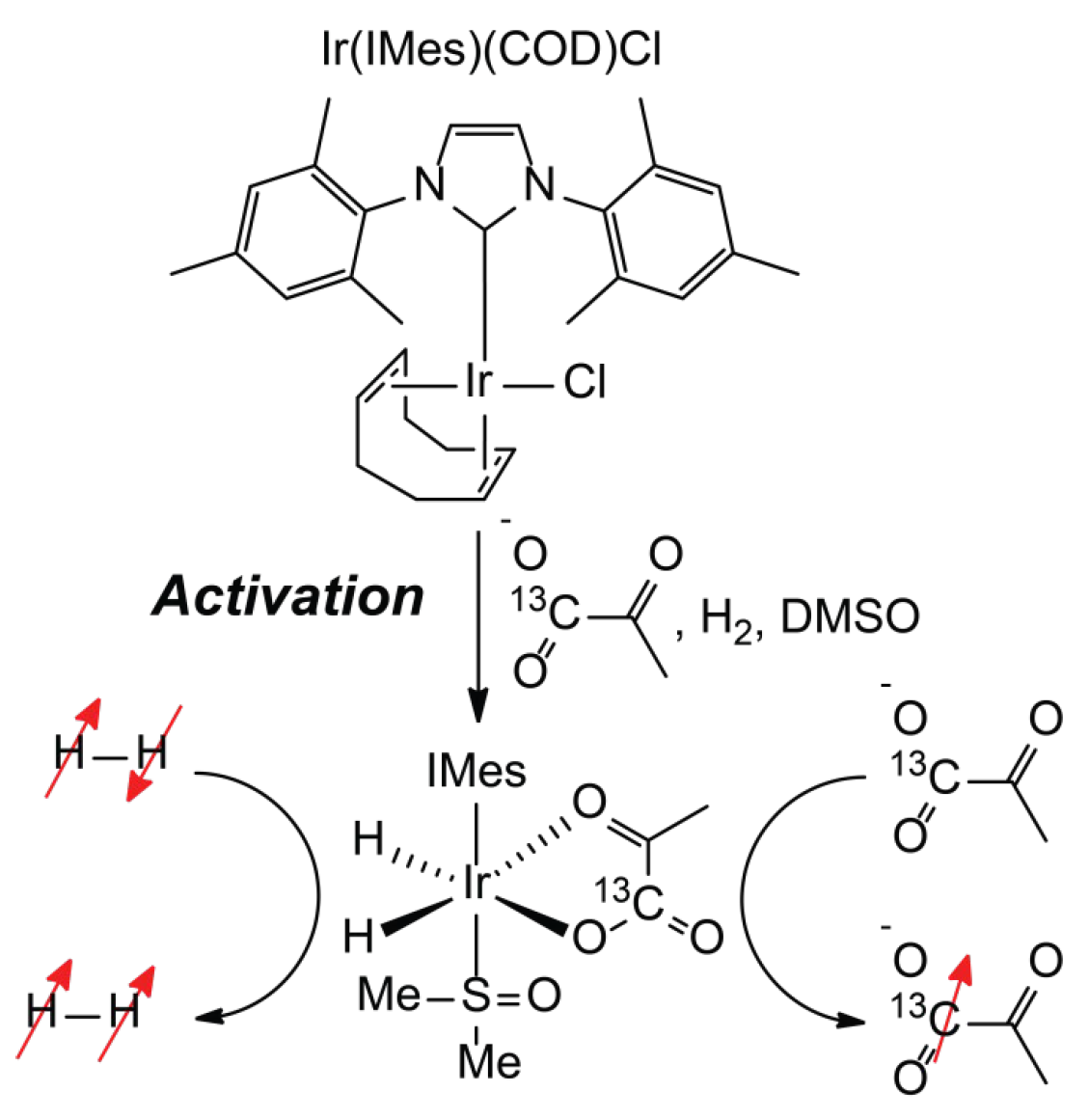
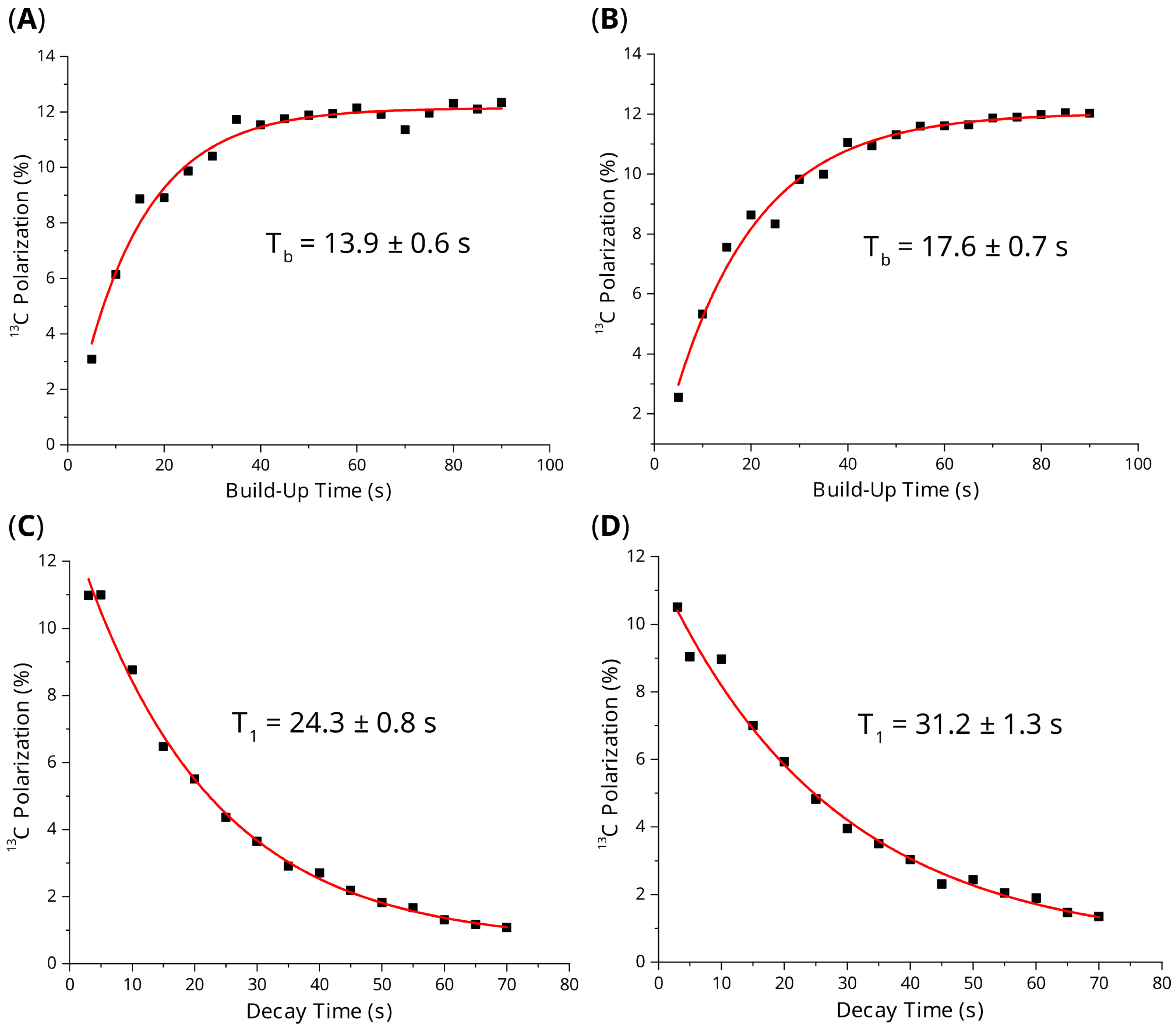
2. Results and Discussion
3. Materials and Methods
3.1. General Synthesis Procedure
3.2. SABRE-SHEATH Hyperpolarization Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eills, J.; Budker, D.; Cavagnero, S.; Chekmenev, E.Y.; Elliott, S.J.; Jannin, S.; Lesage, A.; Matysik, J.; Meersmann, T.; Prisner, T.; et al. Spin Hyperpolarization in Modern Magnetic Resonance. Chem. Rev. 2023, 123, 1417–1551. [Google Scholar] [CrossRef]
- Chaumeil, M.M.; Bankson, J.A.; Brindle, K.M.; Epstein, S.; Gallagher, F.A.; Grashei, M.; Guglielmetti, C.; Kaggie, J.D.; Keshari, K.R.; Knecht, S.; et al. New Horizons in Hyperpolarized 13C MRI. Mol. Imaging Biol. 2024, 26, 222–232. [Google Scholar] [CrossRef]
- Stewart, N.J.; Matsumoto, S. Biomedical Applications of the Dynamic Nuclear Polarization and Parahydrogen Induced Polarization Techniques for Hyperpolarized 13C MR Imaging. Magn. Reson. Med. Sci. 2021, 20, 1–17. [Google Scholar] [CrossRef]
- Ribay, V.; Praud, C.; Letertre, M.P.M.; Dumez, J.-N.; Giraudeau, P. Hyperpolarized NMR Metabolomics. Curr. Opin. Chem. Biol. 2023, 74, 102307. [Google Scholar] [CrossRef]
- Silva Terra, A.I.; Taylor, D.A.; Halse, M.E. Hyperpolarised Benchtop NMR Spectroscopy for Analytical Applications. Prog. Nucl. Magn. Reson. Spectrosc. 2024, 144–145, 153–178. [Google Scholar] [CrossRef]
- Negroni, M.; Kurzbach, D. Missing Pieces in Structure Puzzles: How Hyperpolarized NMR Spectroscopy Can Complement Structural Biology and Biochemistry. ChemBioChem 2023, 24, e202200703. [Google Scholar] [CrossRef]
- Kurhanewicz, J.; Vigneron, D.B.; Ardenkjaer-Larsen, J.H.; Bankson, J.A.; Brindle, K.; Cunningham, C.H.; Gallagher, F.A.; Keshari, K.R.; Kjaer, A.; Laustsen, C.; et al. Hyperpolarized 13C MRI: Path to Clinical Translation in Oncology. Neoplasia 2019, 21, 1–16. [Google Scholar] [CrossRef]
- Larson, P.E.Z.; Bernard, J.M.L.; Bankson, J.A.; Bøgh, N.; Bok, R.A.; Chen, A.P.; Cunningham, C.H.; Gordon, J.W.; Hövener, J.; Laustsen, C.; et al. Current Methods for Hyperpolarized [1-13C]Pyruvate MRI Human Studies. Magn. Reson. Med. 2024, 91, 2204–2228. [Google Scholar] [CrossRef]
- Vaeggemose, M.; Schulte, R.F.; Laustsen, C. Comprehensive Literature Review of Hyperpolarized Carbon-13 MRI: The Road to Clinical Application. Metabolites 2021, 11, 219. [Google Scholar] [CrossRef]
- Comment, A. Dissolution DNP for in Vivo Preclinical Studies. J. Magn. Reson. 2016, 264, 39–48. [Google Scholar] [CrossRef]
- Gutte, H.; Hansen, A.E.; Johannesen, H.H.; Clemmensen, A.E.; Ardenkjær-Larsen, J.H.; Nielsen, C.H.; Kjær, A. The Use of Dynamic Nuclear Polarization 13C-Pyruvate MRS in Cancer. Am. J. Nucl. Med. Mol. Imaging 2015, 5, 548–560. [Google Scholar]
- Timm, K.N.; Miller, J.J.; Henry, J.A.; Tyler, D.J. Cardiac Applications of Hyperpolarised Magnetic Resonance. Prog. Nucl. Magn. Reson. Spectrosc. 2018, 106–107, 66–87. [Google Scholar] [CrossRef]
- Li, Y.; Vigneron, D.B.; Xu, D. Current Human Brain Applications and Challenges of Dynamic Hyperpolarized Carbon-13 Labeled Pyruvate MR Metabolic Imaging. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4225–4235. [Google Scholar] [CrossRef]
- Pedersen, M.; Ursprung, S.; Jensen, J.D.; Jespersen, B.; Gallagher, F.; Laustsen, C. Hyperpolarised 13C-MRI Metabolic and Functional Imaging: An Emerging Renal MR Diagnostic Modality. Magn. Reson. Mater. Phys. Biol. Med. 2020, 33, 23–32. [Google Scholar] [CrossRef]
- Nelson, S.J.; Kurhanewicz, J.; Vigneron, D.B.; Larson, P.E.Z.; Harzstark, A.L.; Ferrone, M.; van Criekinge, M.; Chang, J.W.; Bok, R.; Park, I.; et al. Metabolic Imaging of Patients with Prostate Cancer Using Hyperpolarized [1-13C]Pyruvate. Sci. Transl. Med. 2013, 5, 198ra108. [Google Scholar] [CrossRef]
- Ardenkjær-Larsen, J.H.; Fridlund, B.; Gram, A.; Hansson, G.; Hansson, L.; Lerche, M.H.; Servin, R.; Thaning, M.; Golman, K. Increase in Signal-to-Noise Ratio of >10,000 Times in Liquid-State NMR. Proc. Natl. Acad. Sci. USA 2003, 100, 10158–10163. [Google Scholar] [CrossRef]
- Pinon, A.C.; Capozzi, A.; Ardenkjær-Larsen, J.H. Hyperpolarization via Dissolution Dynamic Nuclear Polarization: New Technological and Methodological Advances. Magn. Reson. Mater. Phys. Biol. Med. 2021, 34, 5–23. [Google Scholar] [CrossRef]
- Elliott, S.J.; Stern, Q.; Ceillier, M.; El Daraï, T.; Cousin, S.F.; Cala, O.; Jannin, S. Practical Dissolution Dynamic Nuclear Polarization. Prog. Nucl. Magn. Reson. Spectrosc. 2021, 126–127, 59–100. [Google Scholar] [CrossRef]
- Bowers, C.R.; Weitekamp, D.P. Parahydrogen and Synthesis Allow Dramatically Enhanced Nuclear Alignment. J. Am. Chem. Soc. 1987, 109, 5541–5542. [Google Scholar] [CrossRef]
- Eisenschmid, T.C.; Kirss, R.U.; Deutsch, P.P.; Hommeltoft, S.I.; Eisenberg, R.; Bargon, J.; Lawler, R.G.; Balch, A.L. Para Hydrogen Induced Polarization in Hydrogenation Reactions. J. Am. Chem. Soc. 1987, 109, 8089–8091. [Google Scholar] [CrossRef]
- Green, R.A.; Adams, R.W.; Duckett, S.B.; Mewis, R.E.; Williamson, D.C.; Green, G.G.R. The Theory and Practice of Hyperpolarization in Magnetic Resonance Using Parahydrogen. Prog. Nucl. Magn. Reson. Spectrosc. 2012, 67, 1–48. [Google Scholar] [CrossRef]
- Salnikov, O.G.; Chukanov, N.V.; Pravdivtsev, A.N.; Burueva, D.B.; Sviyazov, S.V.; Them, K.; Hövener, J.; Koptyug, I.V. Heteronuclear Parahydrogen-Induced Hyperpolarization via Side Arm Hydrogenation. ChemPhysChem 2025, 26, e202401119. [Google Scholar] [CrossRef]
- Adams, R.W.; Aguilar, J.A.; Atkinson, K.D.; Cowley, M.J.; Elliott, P.I.P.; Duckett, S.B.; Green, G.G.R.; Khazal, I.G.; López-Serrano, J.; Williamson, D.C. Reversible Interactions with Para-Hydrogen Enhance NMR Sensitivity by Polarization Transfer. Science 2009, 323, 1708–1711. [Google Scholar] [CrossRef]
- Salnikov, O.G.; Burueva, D.B.; Skovpin, I.V.; Koptyug, I.V. Parahydrogen-Based NMR Signal Amplification by Reversible Exchange (SABRE): Recent Advances and Applications. Mendeleev Commun. 2023, 33, 583–596. [Google Scholar] [CrossRef]
- Rayner, P.J.; Duckett, S.B. Signal Amplification by Reversible Exchange (SABRE): From Discovery to Diagnosis. Angew. Chem. Int. Ed. 2018, 57, 6742–6753. [Google Scholar] [CrossRef]
- Schmidt, A.B.; Chekmenev, E.Y.; de Maissin, H.; Groß, P.R.; Petersen, S.; Nagel, L.; Schilling, F.; Schwartz, I.; Reinheckel, T.; Hövener, J.-B.; et al. Signal Amplification by Reversible Exchange and Its Translation to Hyperpolarized Magnetic Resonance Imaging in Biomedicine. Anal. Sens. 2024, 4, e202400039. [Google Scholar] [CrossRef]
- Nantogma, S.; Joalland, B.; Wilkens, K.; Chekmenev, E.Y. Clinical-Scale Production of Nearly Pure (> 98.5%) Parahydrogen and Quantification by Benchtop NMR Spectroscopy. Anal. Chem. 2021, 93, 3594–3601. [Google Scholar] [CrossRef]
- Hövener, J.B.; Bär, S.; Leupold, J.; Jenne, K.; Leibfritz, D.; Hennig, J.; Duckett, S.B.; von Elverfeldt, D. A Continuous-Flow, High-Throughput, High-Pressure Parahydrogen Converter for Hyperpolarization in a Clinical Setting. NMR Biomed. 2013, 26, 124–131. [Google Scholar] [CrossRef]
- Du, Y.; Zhou, R.; Ferrer, M.-J.; Chen, M.; Graham, J.; Malphurs, B.; Labbe, G.; Huang, W.; Bowers, C.R. An Inexpensive Apparatus for up to 97% Continuous-Flow Parahydrogen Enrichment Using Liquid Helium. J. Magn. Reson. 2020, 321, 106869. [Google Scholar] [CrossRef]
- Rayner, P.J.; Burns, M.J.; Olaru, A.M.; Norcott, P.; Fekete, M.; Green, G.G.R.; Highton, L.A.R.; Mewis, R.E.; Duckett, S.B. Delivering Strong 1H Nuclear Hyperpolarization Levels and Long Magnetic Lifetimes through Signal Amplification by Reversible Exchange. Proc. Natl. Acad. Sci. USA 2017, 114, E3188–E3194. [Google Scholar] [CrossRef]
- Rayner, P.J.; Norcott, P.; Appleby, K.M.; Iali, W.; John, R.O.; Hart, S.J.; Whitwood, A.C.; Duckett, S.B. Fine-Tuning the Efficiency of Para-Hydrogen-Induced Hyperpolarization by Rational N-Heterocyclic Carbene Design. Nat. Commun. 2018, 9, 4251. [Google Scholar] [CrossRef] [PubMed]
- Salnikov, O.G.; Chukanov, N.V.; Svyatova, A.; Trofimov, I.A.; Kabir, M.S.H.; Gelovani, J.G.; Kovtunov, K.V.; Koptyug, I.V.; Chekmenev, E.Y. 15N NMR Hyperpolarization of Radiosensitizing Antibiotic Nimorazole by Reversible Parahydrogen Exchange in Microtesla Magnetic Fields. Angew. Chem. Int. Ed. 2021, 60, 2406–2413. [Google Scholar] [CrossRef]
- Chapman, B.; Joalland, B.; Meersman, C.; Ettedgui, J.; Swenson, R.E.; Krishna, M.C.; Nikolaou, P.; Kovtunov, K.V.; Salnikov, O.G.; Koptyug, I.V.; et al. Low-Cost High-Pressure Clinical-Scale 50% Parahydrogen Generator Using Liquid Nitrogen at 77 K. Anal. Chem. 2021, 93, 8476–8483. [Google Scholar] [CrossRef] [PubMed]
- Ellermann, F.; Pravdivtsev, A.; Hövener, J.-B. Open-Source, Partially 3D-Printed, High-Pressure (50-Bar) Liquid-Nitrogen-Cooled Parahydrogen Generator. Magn. Reson. 2021, 2, 49–62. [Google Scholar] [CrossRef]
- Wagner, S. Conversion Rate of Para-Hydrogen to Ortho-Hydrogen by Oxygen: Implications for PHIP Gas Storage and Utilization. Magn. Reson. Mater. Phys. Biol. Med. 2014, 27, 195–199. [Google Scholar] [CrossRef]
- Bowers, C.R.; Weitekamp, D.P. Transformation of Symmetrization Order to Nuclear-Spin Magnetization by Chemical Reaction and Nuclear Magnetic Resonance. Phys. Rev. Lett. 1986, 57, 2645–2648. [Google Scholar] [CrossRef]
- Barskiy, D.A.; Knecht, S.; Yurkovskaya, A.V.; Ivanov, K.L. SABRE: Chemical Kinetics and Spin Dynamics of the Formation of Hyperpolarization. Prog. Nucl. Magn. Reson. Spectrosc. 2019, 114–115, 33–70. [Google Scholar] [CrossRef]
- Wibbels, G.L.; Oladun, C.; O’Hara, T.Y.; Adelabu, I.; Robinson, J.E.; Ahmed, F.; Bender, Z.T.; Samoilenko, A.; Gyesi, J.; Kovtunova, L.M.; et al. Parahydrogen-Based Hyperpolarization for the Masses at Millitesla Fields. Magnetochemistry, 2025; accepted. [Google Scholar]
- Cowley, M.J.; Adams, R.W.; Atkinson, K.D.; Cockett, M.C.R.; Duckett, S.B.; Green, G.G.R.; Lohman, J.A.B.; Kerssebaum, R.; Kilgour, D.; Mewis, R.E. Iridium N-Heterocyclic Carbene Complexes as Efficient Catalysts for Magnetization Transfer from Para-Hydrogen. J. Am. Chem. Soc. 2011, 133, 6134–6137. [Google Scholar] [CrossRef] [PubMed]
- Semenova, O.; Richardson, P.M.; Parrott, A.J.; Nordon, A.; Halse, M.E.; Duckett, S.B. Reaction Monitoring Using SABRE-Hyperpolarized Benchtop (1 T) NMR Spectroscopy. Anal. Chem. 2019, 91, 6695–6701. [Google Scholar] [CrossRef]
- Kircher, R.; Xu, J.; van Dyke, E.; Mazlumian, R.; Budker, D.; Barskiy, D.A. Benchtop 1H NMR Study of Hyperpolarized Charged and Neutral Ir-IMes Dihydride Complexes. Organometallics 2025, 44, 1251–1256. [Google Scholar] [CrossRef]
- Colell, J.F.P.; Logan, A.W.J.; Zhou, Z.; Lindale, J.R.; Laasner, R.; Shchepin, R.V.; Chekmenev, E.Y.; Blum, V.; Warren, W.S.; Malcolmson, S.J.; et al. Rational Ligand Choice Extends the SABRE Substrate Scope. Chem. Commun. 2020, 56, 9336–9339. [Google Scholar] [CrossRef] [PubMed]
- Pham, P.; Hilty, C. Tunable Iridium Catalyst Designs with Bidentate N-Heterocyclic Carbene Ligands for SABRE Hyperpolarization of Sterically Hindered Substrates. Chem. Commun. 2020, 56, 15466–15469. [Google Scholar] [CrossRef]
- van Weerdenburg, B.J.A.; Glöggler, S.; Eshuis, N.; Engwerda, A.H.J.T.; Smits, J.M.M.; de Gelder, R.; Appelt, S.; Wymenga, S.S.; Tessari, M.; Feiters, M.C.; et al. Ligand Effects of NHC-Iridium Catalysts for Signal Amplification by Reversible Exchange (SABRE). Chem. Commun. 2013, 49, 7388–7390. [Google Scholar] [CrossRef]
- Lloyd, L.S.; Asghar, A.; Burns, M.J.; Charlton, A.; Coombes, S.; Cowley, M.J.; Dear, G.J.; Duckett, S.B.; Genov, G.R.; Green, G.G.R.; et al. Hyperpolarisation through Reversible Interactions with Parahydrogen. Catal. Sci. Technol. 2014, 4, 3544–3554. [Google Scholar] [CrossRef]
- Hadjiali, S.; Savka, R.; Plaumann, M.; Bommerich, U.; Bothe, S.; Gutmann, T.; Ratajczyk, T.; Bernarding, J.; Limbach, H.H.; Plenio, H.; et al. Substituent Influences on the NMR Signal Amplification of Ir Complexes with Heterocyclic Carbene Ligands. Appl. Magn. Reson. 2019, 50, 895–902. [Google Scholar] [CrossRef]
- Vázquez-Serrano, L.D.; Owens, B.T.; Buriak, J.M. Catalytic Olefin Hydrogenation Using N-Heterocyclic Carbene-Phosphine Complexes of Iridium. Chem. Commun. 2002, 21, 2518–2519. [Google Scholar] [CrossRef]
- Kownacki, I.; Kubicki, M.; Szubert, K.; Marciniec, B. Synthesis, Structure and Catalytic Activity of the First Iridium(I) Siloxide versus Chloride Complexes with 1,3-Mesitylimidazolin-2-Ylidene Ligand. J. Organomet. Chem. 2008, 693, 321–328. [Google Scholar] [CrossRef]
- Atkinson, K.D.; Cowley, M.J.; Elliott, P.I.P.; Duckett, S.B.; Green, G.G.R.; López-Serrano, J.; Whitwood, A.C. Spontaneous Transfer of Parahydrogen Derived Spin Order to Pyridine at Low Magnetic Field. J. Am. Chem. Soc. 2009, 131, 13362–13368. [Google Scholar] [CrossRef]
- Pravdivtsev, A.N.; Ivanov, K.L.; Yurkovskaya, A.V.; Petrov, P.A.; Limbach, H.-H.; Kaptein, R.; Vieth, H.-M. Spin Polarization Transfer Mechanisms of SABRE: A Magnetic Field Dependent Study. J. Magn. Reson. 2015, 261, 73–82. [Google Scholar] [CrossRef]
- Savka, R.; Plenio, H. Facile Synthesis of [(NHC)MX(Cod)] and [(NHC)MCl(CO)2] (M = Rh, Ir; X = Cl, I) Complexes. Dalton Trans. 2015, 44, 891–893. [Google Scholar] [CrossRef]
- Blanchard, J.W.; Ripka, B.; Suslick, B.A.; Gelevski, D.; Wu, T.; Münnemann, K.; Barskiy, D.A.; Budker, D. Towards Large-Scale Steady-State Enhanced Nuclear Magnetization with in Situ Detection. Magn. Reson. Chem. 2021, 59, 1208–1215. [Google Scholar] [CrossRef]
- Theis, T.; Truong, M.L.; Coffey, A.M.; Shchepin, R.V.; Waddell, K.W.; Shi, F.; Goodson, B.M.; Warren, W.S.; Chekmenev, E.Y. Microtesla SABRE Enables 10% Nitrogen-15 Nuclear Spin Polarization. J. Am. Chem. Soc. 2015, 137, 1404–1407. [Google Scholar] [CrossRef]
- Golman, K.; in ’t Zandt, R.; Thaning, M. Real-Time Metabolic Imaging. Proc. Natl. Acad. Sci. USA 2006, 103, 11270–11275. [Google Scholar] [CrossRef] [PubMed]
- Golman, K.; in’t Zandt, R.; Lerche, M.; Pehrson, R.; Ardenkjaer-Larsen, J.H. Metabolic Imaging by Hyperpolarized 13C Magnetic Resonance Imaging for In Vivo Tumor Diagnosis. Cancer Res. 2006, 66, 10855–10860. [Google Scholar] [CrossRef]
- Christensen, D.B.; Skre, I.S.; Ardenkjær-Larsen, J.H.; Karlsson, M.; Lerche, M.H. Developing Hyperpolarized Metabolic Contrast Agents at High Field dDNP for Large Animal Research. J. Magn. Reson. Open 2025, 22, 100184. [Google Scholar] [CrossRef]
- Cavallari, E.; Carrera, C.; Aime, S.; Reineri, F. Studies to Enhance the Hyperpolarization Level in PHIP-SAH-Produced C13-Pyruvate. J. Magn. Reson. 2018, 289, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Mamone, S.; Jagtap, A.P.; Korchak, S.; Ding, Y.; Sternkopf, S.; Glöggler, S. A Field-Independent Method for the Rapid Generation of Hyperpolarized [1-13C]Pyruvate in Clean Water Solutions for Biomedical Applications. Angew. Chem. Int. Ed. 2022, 61, e202206298. [Google Scholar] [CrossRef] [PubMed]
- Stevanato, G.; Ding, Y.; Mamone, S.; Jagtap, A.P.; Korchak, S.; Glöggler, S. Real-Time Pyruvate Chemical Conversion Monitoring Enabled by PHIP. J. Am. Chem. Soc. 2023, 145, 5864–5871. [Google Scholar] [CrossRef]
- Nagel, L.; Gierse, M.; Gottwald, W.; Ahmadova, Z.; Grashei, M.; Wolff, P.; Josten, F.; Karaali, S.; Müller, C.A.; Lucas, S.; et al. Parahydrogen-Polarized [1-13C]Pyruvate for Reliable and Fast Preclinical Metabolic Magnetic Resonance Imaging. Adv. Sci. 2023, 10, 2303441. [Google Scholar] [CrossRef]
- Hune, T.; Mamone, S.; Schroeder, H.; Jagtap, A.P.; Sternkopf, S.; Stevanato, G.; Korchak, S.; Fokken, C.; Müller, C.A.; Schmidt, A.B.; et al. Metabolic Tumor Imaging with Rapidly Signal-Enhanced 1-13C-Pyruvate-d3. ChemPhysChem 2023, 24, e202200615. [Google Scholar] [CrossRef]
- Iali, W.; Roy, S.S.; Tickner, B.J.; Ahwal, F.; Kennerley, A.J.; Duckett, S.B. Hyperpolarising Pyruvate through Signal Amplification by Reversible Exchange (SABRE). Angew. Chem. Int. Ed. 2019, 58, 10271–10275. [Google Scholar] [CrossRef]
- TomHon, P.; Abdulmojeed, M.; Adelabu, I.; Nantogma, S.; Kabir, M.S.H.; Lehmkuhl, S.; Chekmenev, E.Y.; Theis, T. Temperature Cycling Enables Efficient 13C SABRE-SHEATH Hyperpolarization and Imaging of [1-13C]-Pyruvate. J. Am. Chem. Soc. 2022, 144, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Ettedgui, J.; Blackman, B.; Raju, N.; Kotler, S.A.; Chekmenev, E.Y.; Goodson, B.M.; Merkle, H.; Woodroofe, C.C.; LeClair, C.A.; Krishna, M.C.; et al. Perfluorinated Iridium Catalyst for Signal Amplification by Reversible Exchange Provides Metal-Free Aqueous Hyperpolarized [1-13C]-Pyruvate. J. Am. Chem. Soc. 2024, 146, 946–953. [Google Scholar] [CrossRef]
- Adelabu, I.; TomHon, P.; Kabir, M.S.H.; Nantogma, S.; Abdulmojeed, M.; Mandzhieva, I.; Ettedgui, J.; Swenson, R.E.; Krishna, M.C.; Theis, T.; et al. Order-Unity 13C Nuclear Polarization of [1-13C]Pyruvate in Seconds and the Interplay of Water and SABRE Enhancement. ChemPhysChem 2022, 23, 131–136. [Google Scholar] [CrossRef]
- de Maissin, H.; Groß, P.R.; Mohiuddin, O.; Weigt, M.; Nagel, L.; Herzog, M.; Wang, Z.; Willing, R.; Reichardt, W.; Pichotka, M.; et al. In Vivo Metabolic Imaging of [1-13C]Pyruvate-d3 Hyperpolarized By Reversible Exchange With Parahydrogen. Angew. Chem. Int. Ed. 2023, 62, e202306654. [Google Scholar] [CrossRef] [PubMed]
- Ettedgui, J.; Yamamoto, K.; Blackman, B.; Koyasu, N.; Raju, N.; Vasalatiy, O.; Merkle, H.; Chekmenev, E.Y.; Goodson, B.M.; Krishna, M.C.; et al. In Vivo Metabolic Sensing of Hyperpolarized [1-13C]Pyruvate in Mice Using a Recyclable Perfluorinated Iridium Signal Amplification by Reversible Exchange Catalyst. Angew. Chem. Int. Ed. 2024, 63, e202407349. [Google Scholar] [CrossRef]
- Petersen, S.; Nagel, L.; Groß, P.R.; de Maissin, H.; Willing, R.; Heß, L.; Mitschke, J.; Klemm, N.; Treiber, J.; Müller, C.A.; et al. In Vivo Molecular Imaging of Breast Cancer Metabolic Heterogeneity Using [1-13C]Pyruvate-d3 Hyperpolarized by Reversible Exchange with Parahydrogen. Theranostics 2025, 15, 3714–3723. [Google Scholar] [CrossRef]
- McBride, S.J.; Pike, M.; Curran, E.; Zavriyev, A.; Adebesin, B.; Tucker, L.; Harzan, J.M.; Senanayake, I.M.; Abdulmojeed, M.; Theiss, F.; et al. Scalable Hyperpolarized MRI Enabled by Ace-SABRE of [1-13C]Pyruvate. Angew. Chem. Int. Ed. 2025, 64, e202501231. [Google Scholar] [CrossRef]
- Nantogma, S.; Chowdhury, M.R.H.; Kabir, M.S.H.; Adelabu, I.; Joshi, S.M.; Samoilenko, A.; de Maissin, H.; Schmidt, A.B.; Nikolaou, P.; Chekmenev, Y.A.; et al. MATRESHCA: Microtesla Apparatus for Transfer of Resonance Enhancement of Spin Hyperpolarization via Chemical Exchange and Addition. Anal. Chem. 2024, 96, 4171–4179. [Google Scholar] [CrossRef]
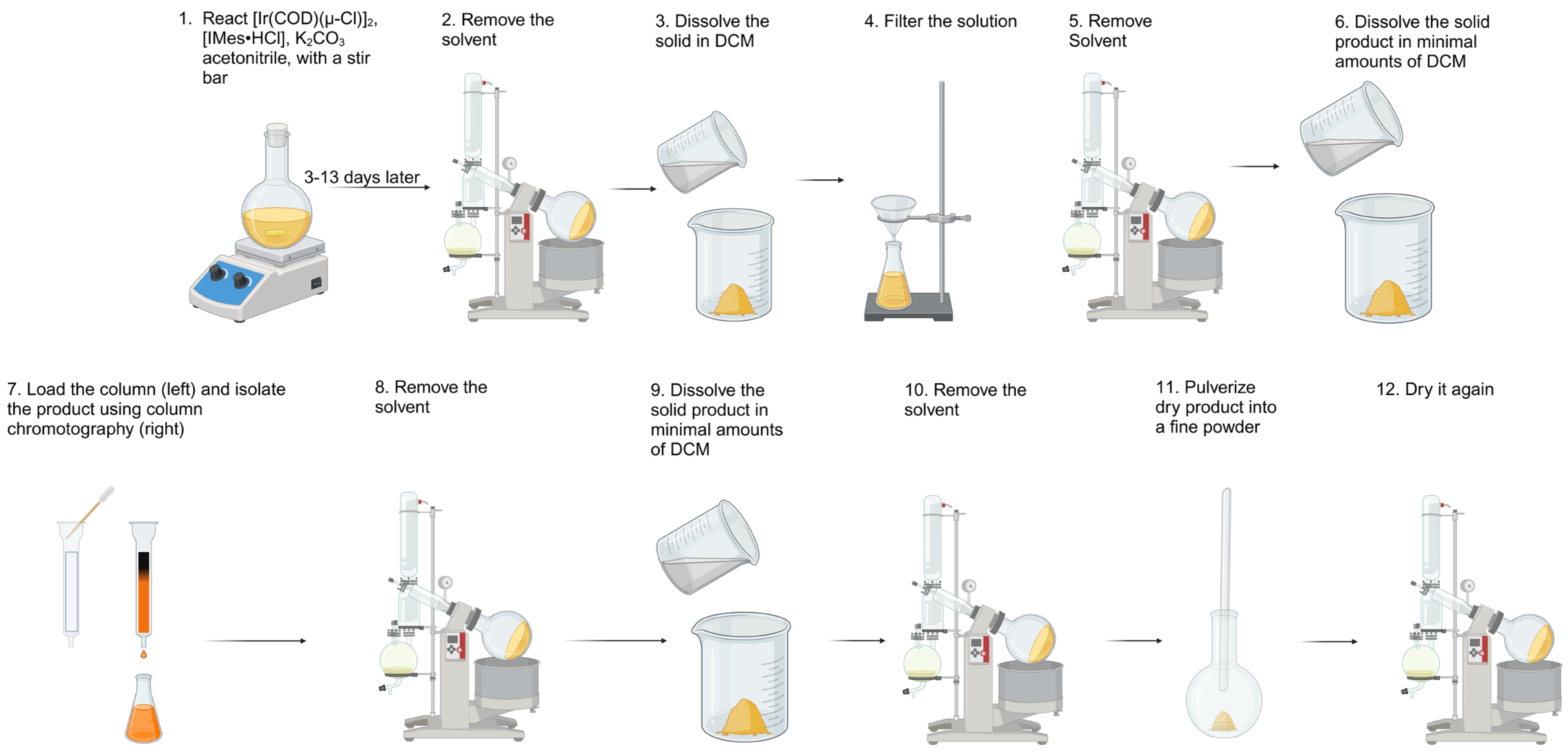
| Entry | Solvent | Reaction Time (Days) | Yield (%) | Modifications to the General Procedure |
|---|---|---|---|---|
| 1 | Acetone | ~0.8 | 0 | ~56 °C (acetone reflux) |
| 2 | Acetone | 8 | 78 | None |
| 3 | Acetone | 11 | 81 | None |
| 4 | Acetone | 3 | 63 | ×2 concentrations compared to the general procedure |
| 5 | Acetonitrile | 3 | 63 | None |
| 6 | Acetonitrile | 8 | 67 | 1.0 g of extra [Ir(COD)(μ-Cl)]2 added after 7 days |
| 7 | Acetonitrile | 13 | 70 | 1.2 g of extra [Ir(COD)(μ-Cl)]2 added after 12 days |
| 8 | Acetonitrile | 3 | 59 | ×4 concentrations compared to the general procedure |
| 9 | Acetonitrile | 4 | 57 | ×4 concentrations compared to the general procedure; acetonitrile used for column solvent |
| 10 | Acetonitrile | 8 | 45 | no argon flushing |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, I.; Terkildsen, N.; Bender, Z.; Abdurraheem, A.; Nantogma, S.; Samoilenko, A.; Gyesi, J.; Kovtunova, L.M.; Salnikov, O.G.; Koptyug, I.V.; et al. SABRE Ir-IMes Catalysis for the Masses. Molecules 2025, 30, 3837. https://doi.org/10.3390/molecules30183837
Smith I, Terkildsen N, Bender Z, Abdurraheem A, Nantogma S, Samoilenko A, Gyesi J, Kovtunova LM, Salnikov OG, Koptyug IV, et al. SABRE Ir-IMes Catalysis for the Masses. Molecules. 2025; 30(18):3837. https://doi.org/10.3390/molecules30183837
Chicago/Turabian StyleSmith, Izabelle, Noah Terkildsen, Zachary Bender, Abubakar Abdurraheem, Shiraz Nantogma, Anna Samoilenko, Joseph Gyesi, Larisa M. Kovtunova, Oleg G. Salnikov, Igor V. Koptyug, and et al. 2025. "SABRE Ir-IMes Catalysis for the Masses" Molecules 30, no. 18: 3837. https://doi.org/10.3390/molecules30183837
APA StyleSmith, I., Terkildsen, N., Bender, Z., Abdurraheem, A., Nantogma, S., Samoilenko, A., Gyesi, J., Kovtunova, L. M., Salnikov, O. G., Koptyug, I. V., Kircher, R., Barskiy, D. A., Chekmenev, E. Y., & Shchepin, R. V. (2025). SABRE Ir-IMes Catalysis for the Masses. Molecules, 30(18), 3837. https://doi.org/10.3390/molecules30183837








