Solid-Phase Synthesis for Constructing Thiazolotriazinone-Based Compounds Library
Abstract
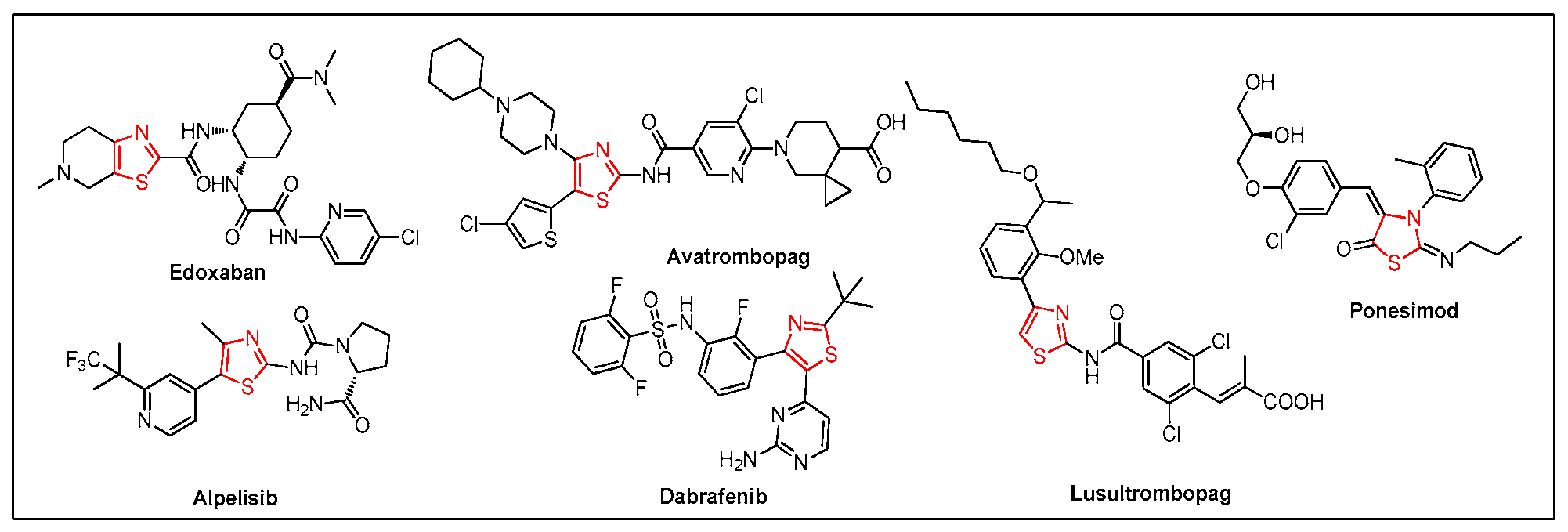
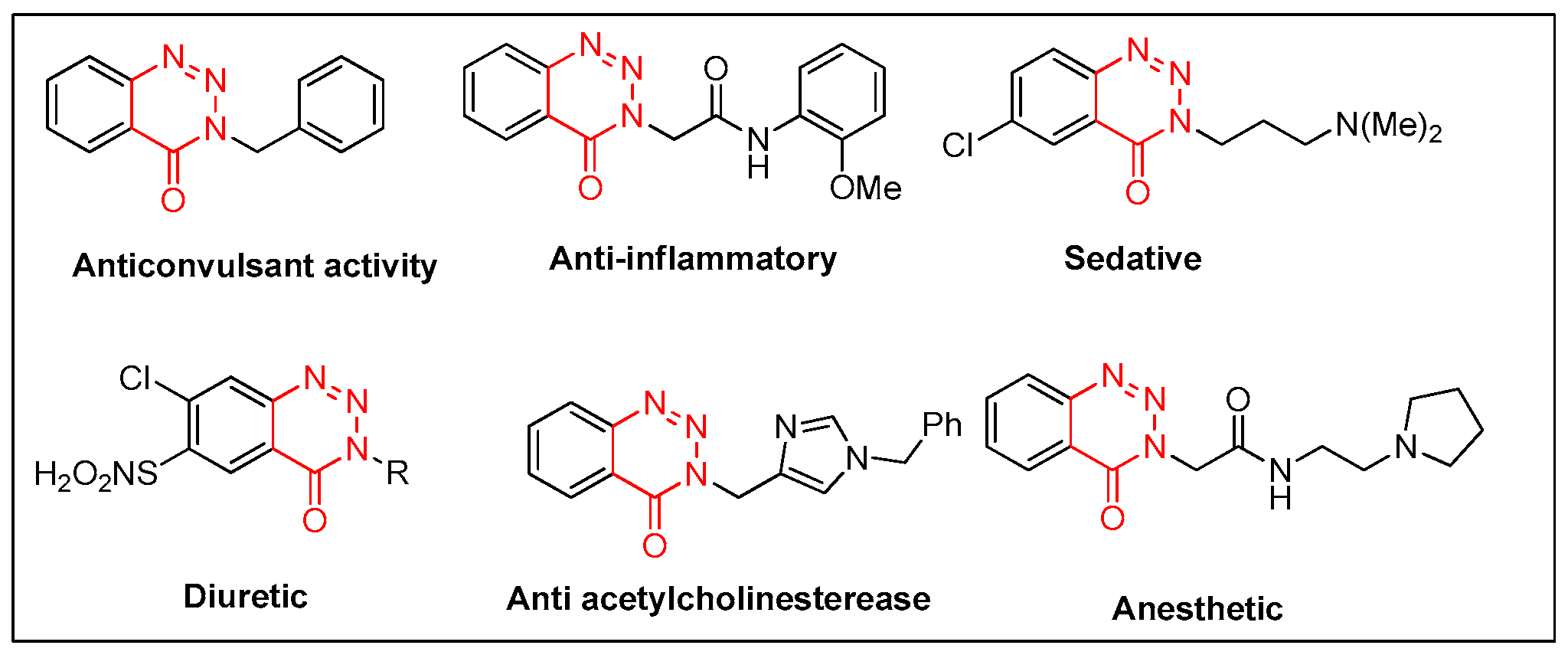
1. Introduction
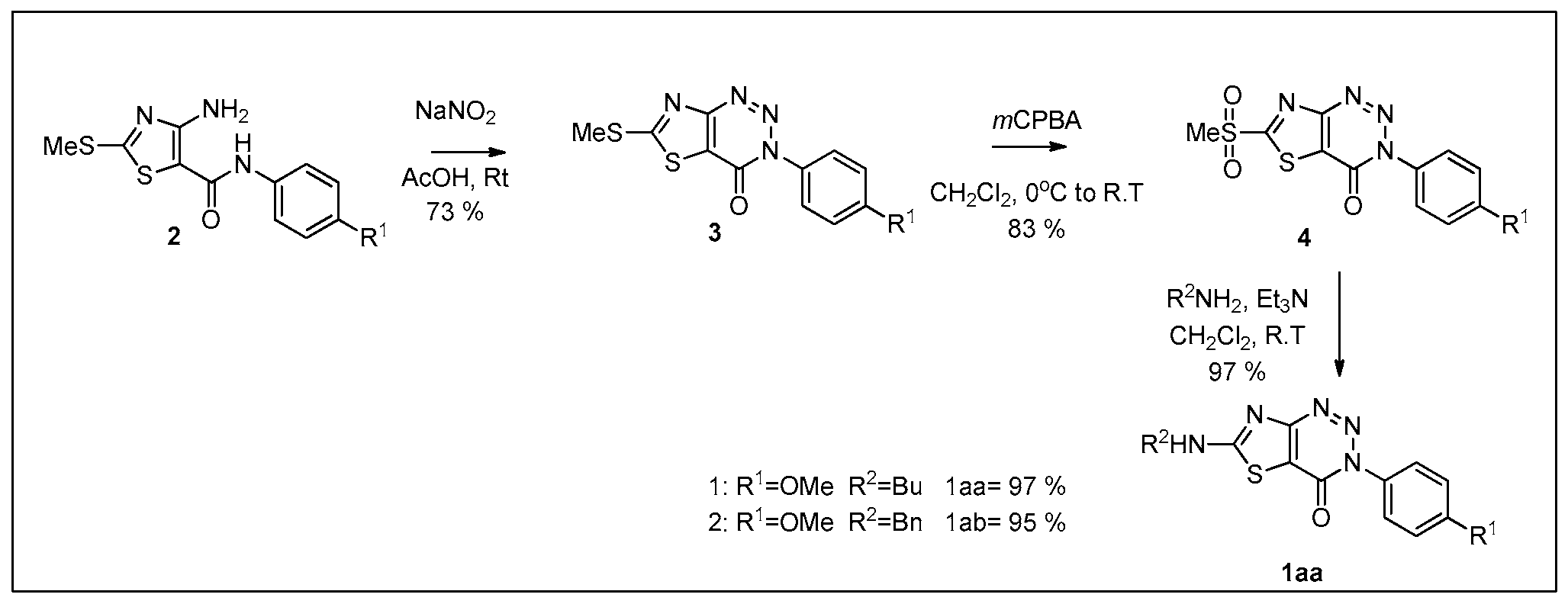
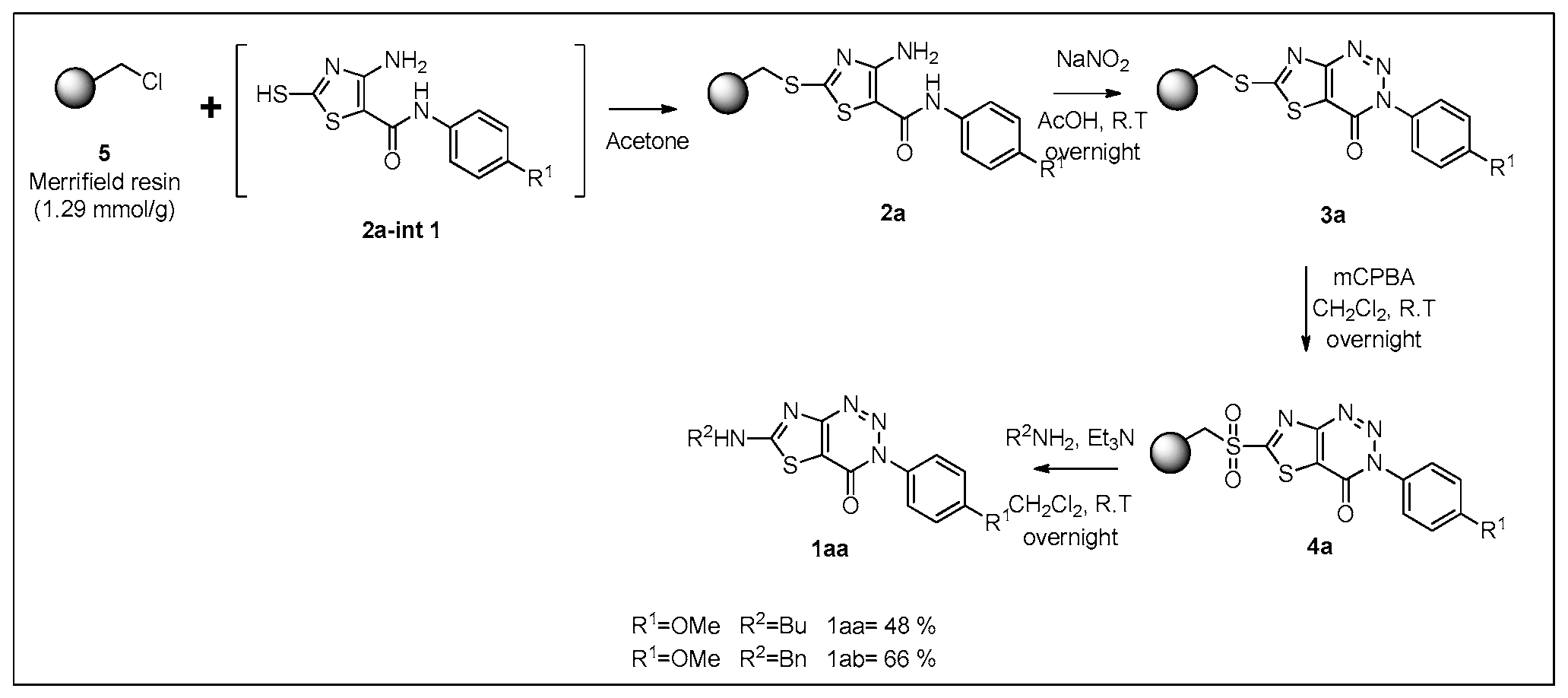
2. Results
3. Materials and Methods
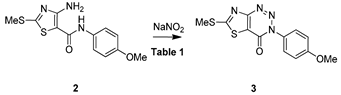
3.1. 4-Amino-N-(4-methoxyphenyl)-2-(methylthio) thiazole-5-carboxamide (2)
3.2. 3-(4-Methoxyphenyl)-6-(methylthio) thiazolo [4,5-d] [1,2,3] triazin-4(3H)-one (3)
3.3. 3-(4-Methoxyphenyl)-6-(methylsulfonyl) thiazolo [4,5-d] [1,2,3] triazin-4(3H)-one (4)
3.4. 6-(Butylamino)-3-(4-methoxyphenyl) thiazolo [4,5-d] [1,2,3] triazin-4(3H)-one (1aa)
3.5. Preparation of 4-Amino-N-(substituted) thiazole-5-carboxamide Resin 11a
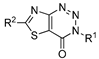
3.6. Preparation of 3-Substituted-thiazolo [4,5-d] [1,2,3] triazin-4(3H)-one Resin 3a
3.7. Preparation of Sulfonyl 3-substituted-thiazolo [4,5-d] [1,2,3] triazin-4(3H)-one Resin 4a
3.8. Preparation of Thiazolo [4,5-d] [1,2,3] triazin-4(3H)-one 1aa
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Marshall, C.M.; Federice, J.G.; Bell, C.N.; Cox, P.B.; Njardarson, J.T. An update on the nitrogen heterocycle compositions and properties of US FDA-approved pharmaceuticals (2013–2023). J. Med. Chem. 2024, 67, 11622–11655. [Google Scholar] [CrossRef] [PubMed]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.-X.; Wang, Y.-T.; Zhang, S.-N.; Li, Y.; Chen, X.-B.; Wang, S.-Q.; Liu, H.-M. Application and synthesis of thiazole ring in clinically approved drugs. Eur. J. Med. Chem. 2023, 250, 115172. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.H.; Hogg, K.; Weitz, J.I. Overview of the new oral anticoagulants: Opportunities and challenges. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Maan, R.; de Knegt, R.J.; Veldt, B.J. Management of thrombocytopenia in chronic liver disease: Focus on pharmacotherapeutic strategies. Drugs 2015, 75, 1981–1992. [Google Scholar] [CrossRef]
- Furet, P.; Guagnano, V.; Fairhurst, R.A.; Imbach-Weese, P.; Bruce, I.; Knapp, M.; Fritsch, C.; Blasco, F.; Blanz, J.; Aichholz, R. Discovery of NVP-BYL719 a potent and selective phosphatidylinositol-3 kinase alpha inhibitor selected for clinical evaluation. Bioorg. Med. Chem. Lett. 2013, 23, 3741–3748. [Google Scholar] [CrossRef]
- Lee, S.; Lee, D.; Song, K.-S.; Liu, K.-H.; Gong, Y.-D.; Lee, T. Parallel synthesis of 2, 4, 5-trisubstituted thiophene-3-carbonitrile derivatives on traceless solid support. Tetrahedron 2014, 70, 9183–9190. [Google Scholar] [CrossRef]
- Moon, J.; Kim, S.; Hua, S.; Lee, H.; Kim, J.; Lee, T. Synthesis of a Natural Product-Based 5 H-Thiazolo [5′, 4′: 5, 6] pyrido [2, 3-b] indole Derivative via Solid-Phase Synthesis. J. Org. Chem. 2025, 90, 3078–3086. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Lee, H.; Kim, J.; Hua, S.; Yoon, G.; Lee, H.; Seo, S.; Joo, Y.; Yim, H.-S.; Lee, T. Synthesis of thiazolo [4, 5-d] pyrimidine derivatives based on purine via solid-phase synthesis. Org. Biomol. Chem. 2025, 23, 7165–7171. [Google Scholar] [CrossRef] [PubMed]
- El Rayes, S.M.; Ali, I.A.I.; Fathalla, W.; Mahmoud, M.A.A. Synthesis and Biological Activities of Some New Benzotriazinone Derivatives Based on Molecular Docking; Promising HepG2 Liver Carcinoma Inhibitors. ACS Omega 2020, 5, 6781–6791. [Google Scholar] [CrossRef] [PubMed]
- Komet, M.J. Microwave synthesis and anticonvulsant activity of new 3-benzyl-1,2,3-benzotriazin-4(3H)-ones. J. Heterocycl. Chem. 1997, 34, 1391–1393. [Google Scholar] [CrossRef]
- Hosseininezhad, S.; Ramazani, A. Thiazole ring-the antimicrobial, anti-inflammatory, and anticancer active scaffold. Arab. J. Chem. 2023, 16, 105234. [Google Scholar] [CrossRef]
- Takwale, A.D.; Kim, E.Y.; Jang, Y.; Lee, D.H.; Kim, S.; Choi, Y.; Kim, J.H.; Lee, D.Y.; Kim, Y.; Lee, S.M.; et al. Structure-activity relationship analysis of novel GSPT1 degraders based on benzotriazinone scaffold and its antitumor effect on xenograft mouse model. Bioorg. Chem. 2022, 127, 105923. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Sun, Q.; Jiao, W.; Tang, J. Preparation of Thiazolopyrimidinone Compound and Their Medical Use. CN201510299946.7, 4 January 2017. [Google Scholar]
- McGrory, R.; Faggyas, R.J.; Sutherland, A. One-pot synthesis of N-substituted benzannulated triazoles via stable arene diazonium salts. Org. Biomol. Chem. 2021, 19, 6127–6140. [Google Scholar] [CrossRef] [PubMed]
- Barak, D.S.; Mukhopadhyay, S.; Dahatonde, D.J.; Batra, S. NaNO2/I2 as an alternative reagent for the synthesis of 1,2,3-benzotriazin-4(3H)-ones from 2-aminobenzamides. Tetrahedron Lett. 2019, 60, 248–251. [Google Scholar] [CrossRef]
- Yan, Y.; Li, H.; Niu, B.; Zhu, C.; Chen, T.; Liu, Y. Mild and efficient TBAI-catalyzed synthesis of 1,2,3-benzotriazine-4-(3 H)-ones from tert-butyl nitrite and 2-aminobenzamides under acid-free conditions. Tetrahedron Lett. 2016, 57, 4170–4173. [Google Scholar] [CrossRef]


 | ||||||
|---|---|---|---|---|---|---|
| Entry a | N-Source (3 eq) | Reagent (1 eq) | Acid (3 eq) | Solvent | T °C/t h | Yield (%) b |
| 1 | NaNO2 | - | - | AcOH/H2O (2:1) | Rt/2 h | 70 |
| 2 | NaNO2 | - | - | AcOH | Rt/1.5 h | 85 |
| 3 | NaNO2 | - | - | HCOOH | Rt/2 h | 61 |
| 4 | NaNO2 | - | - | MeCN | Rt/2 h | N.R c |
| 5 | NaNO2 | - | HCl | MeCN | Rt/5 h | N.R c |
| 6 | NaNO2 | - | HCl | EtOH | Rt/5 h | N.R c |
| 7 | NaNO2 | - | HCl | MeOH | Rt/5 h | N.R c |
| 8 | NaNO2 | - | HCl | THF | Rt/5 h | N.R c |
| 9 | NaNO2 | - | HCl | n-BuOH | Rt/5 h | N.R c |
| 10 | NaNO2 | - | HCl | Acetone | Rt/5 h | N.R c |
| 11 | NaNO2 | NIS | - | MeCN | 80 °C/5 h | N.R c |
| 12 | NaNO2 | I2 | - | MeCN | 80 °C/5 h | N.R c |
| 13 | t-BuONO | - | - | AcOH | Rt/1.5 h | 80 |
| 14 | NH4OAc | - | - | AcOH | Rt/2 h | NR c |
 | |||||||
|---|---|---|---|---|---|---|---|
| Entry a | R1 | R2 | Yield (%) b | Entry a | R1 | R2 | Yield (%) b |
| 1 | a | a | 48% | 21 | c | a | 70% |
| 2 | a | b | 66% | 22 | c | b | 79% |
| 3 | a | c | 75% | 23 | c | c | 87% |
| 4 | a | d | 56% | 24 | c | d | 69% |
| 5 | a | e | 61% | 25 | c | e | 65% |
| 6 | a | f | 48% | 26 | c | f | 55% |
| 7 | a | g | 53% | 27 | c | g | 73% |
| 8 | a | h | 55% | 28 | c | h | 56% |
| 9 | a | i | 60% | 29 | c | i | 45% |
| 10 | a | g | 61% | 30 | c | g | 67% |
| 11 | b | a | 75% | 31 | d | a | 33% |
| 12 | b | b | 71% | 32 | d | b | 42% |
| 13 | b | c | 48% | 33 | d | c | 39% |
| 14 | b | d | 79% | 34 | d | d | 27% |
| 15 | b | e | 86% | 35 | d | e | 22% |
| 16 | b | f | 53% | 36 | d | f | 54% |
| 17 | b | g | 74% | 37 | d | g | 47% |
| 18 | b | h | 51% | 38 | d | h | 34% |
| 19 | b | i | 30% c | 39 | d | i | 38% |
| 20 | b | g | 64% | 40 | d | g | 33% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hua, S.; Moon, J.; Kim, Y.; Baek, D.J.; Lee, T. Solid-Phase Synthesis for Constructing Thiazolotriazinone-Based Compounds Library. Molecules 2025, 30, 3838. https://doi.org/10.3390/molecules30183838
Hua S, Moon J, Kim Y, Baek DJ, Lee T. Solid-Phase Synthesis for Constructing Thiazolotriazinone-Based Compounds Library. Molecules. 2025; 30(18):3838. https://doi.org/10.3390/molecules30183838
Chicago/Turabian StyleHua, Shuanghui, Jimin Moon, Youngbeom Kim, Dong Jae Baek, and Taeho Lee. 2025. "Solid-Phase Synthesis for Constructing Thiazolotriazinone-Based Compounds Library" Molecules 30, no. 18: 3838. https://doi.org/10.3390/molecules30183838
APA StyleHua, S., Moon, J., Kim, Y., Baek, D. J., & Lee, T. (2025). Solid-Phase Synthesis for Constructing Thiazolotriazinone-Based Compounds Library. Molecules, 30(18), 3838. https://doi.org/10.3390/molecules30183838







