Anti-Atrophic Effects of Dichotomine B from Stellaria dichotoma During Starvation-Induced Skeletal Muscle Atrophy
Abstract
1. Introduction
2. Results
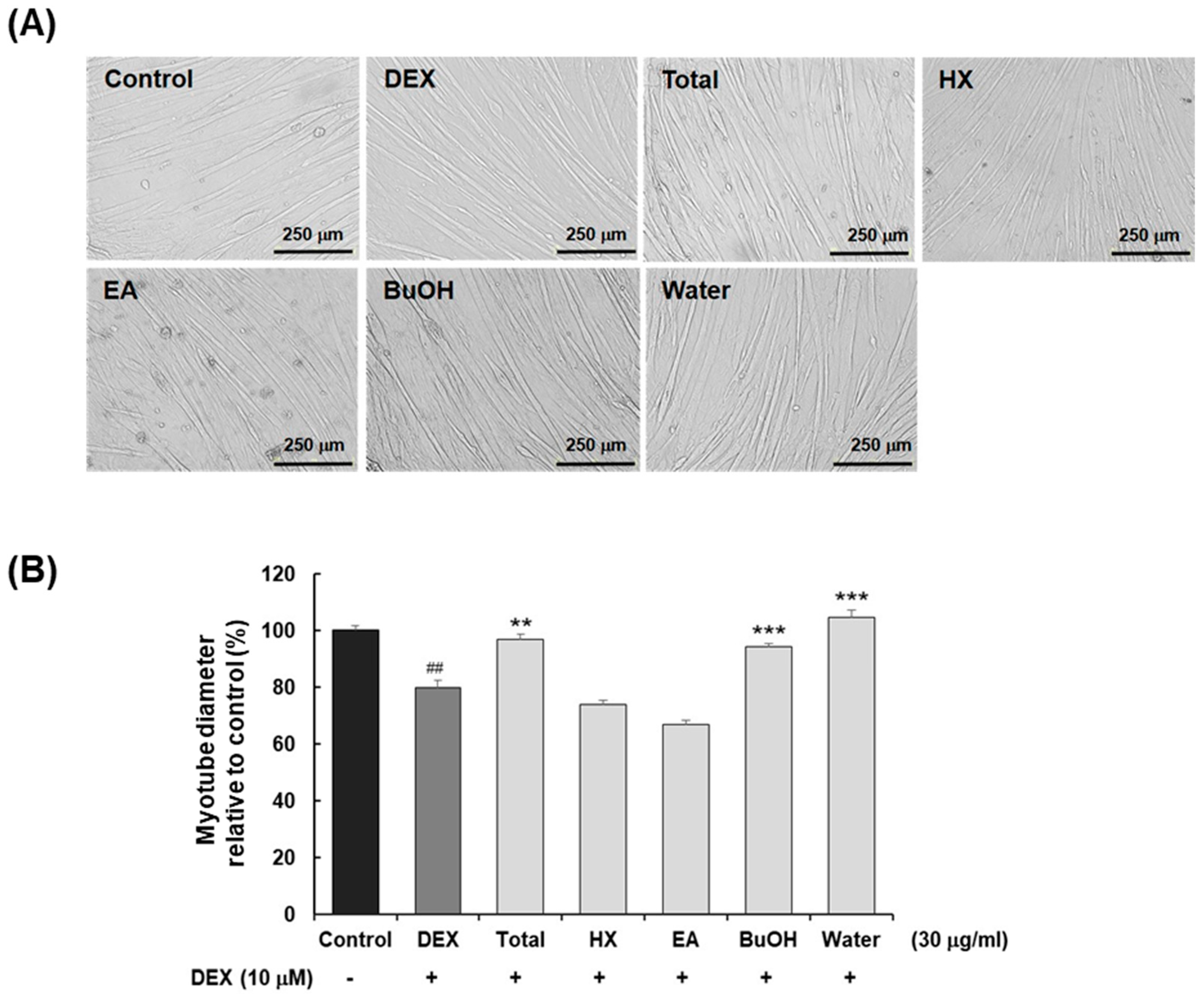
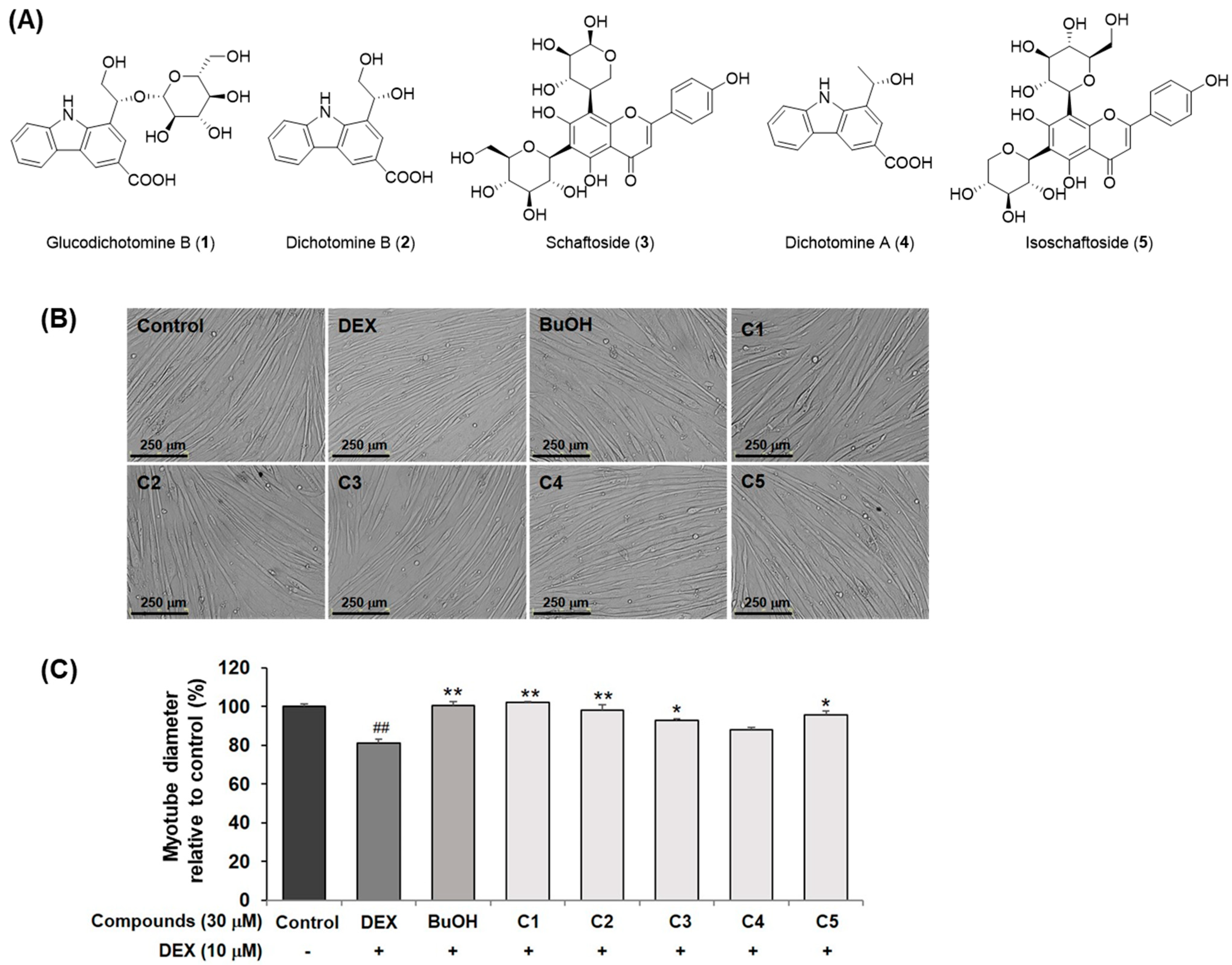
2.1. Bioactivity-Guided Isolation of Compounds 1–5 Using Dexamethasone-Induced C2C12 Myotube Atrophy Model
2.2. Dichotomine B Ameliorates Dexamethasone-Induced Skeletal Muscle Atrophy by Inhibiting Protein Degradation
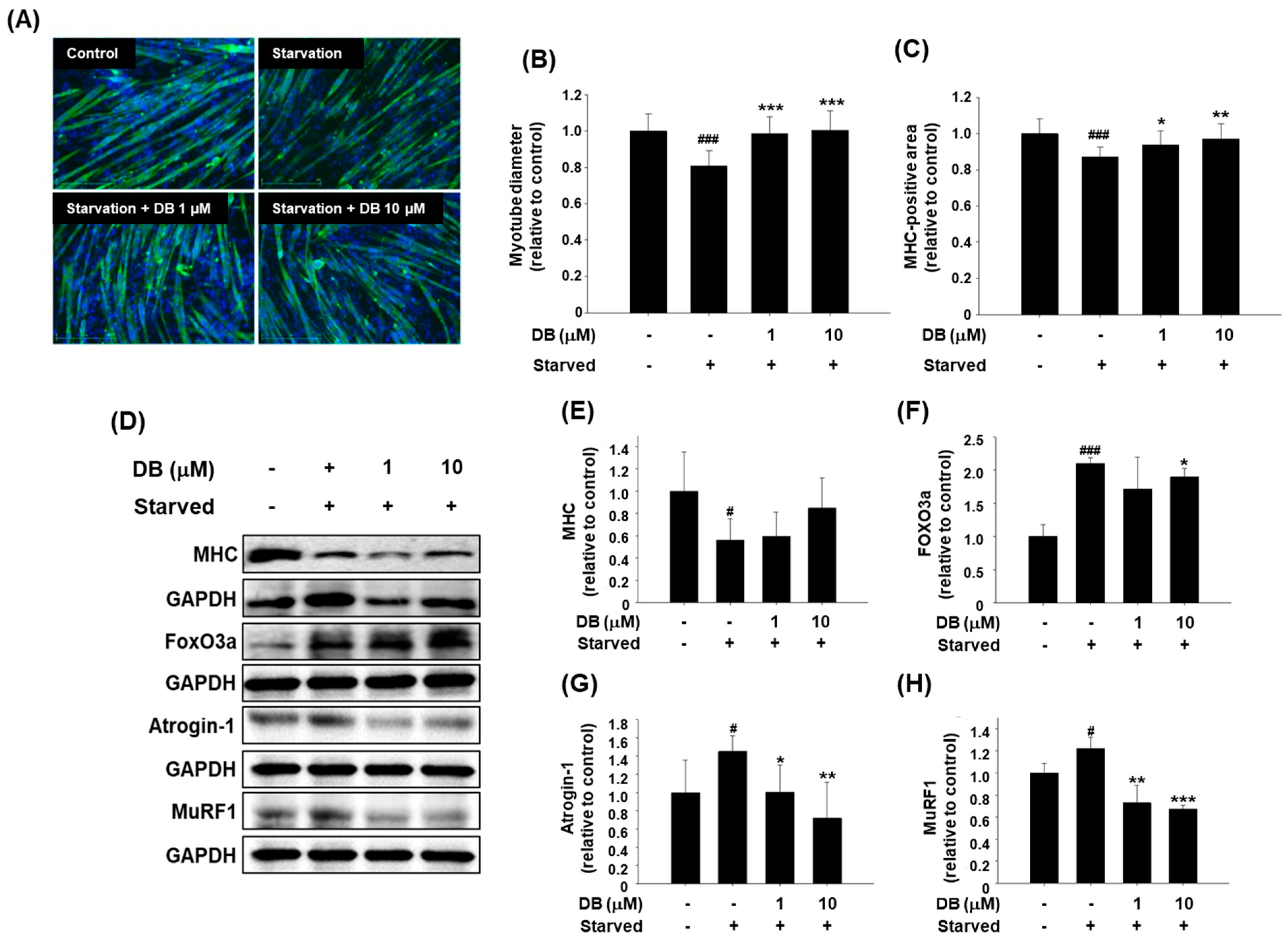
2.3. Dichotomine B Maintains Myotube Thickness in Serum-Free in Vitro Conditions
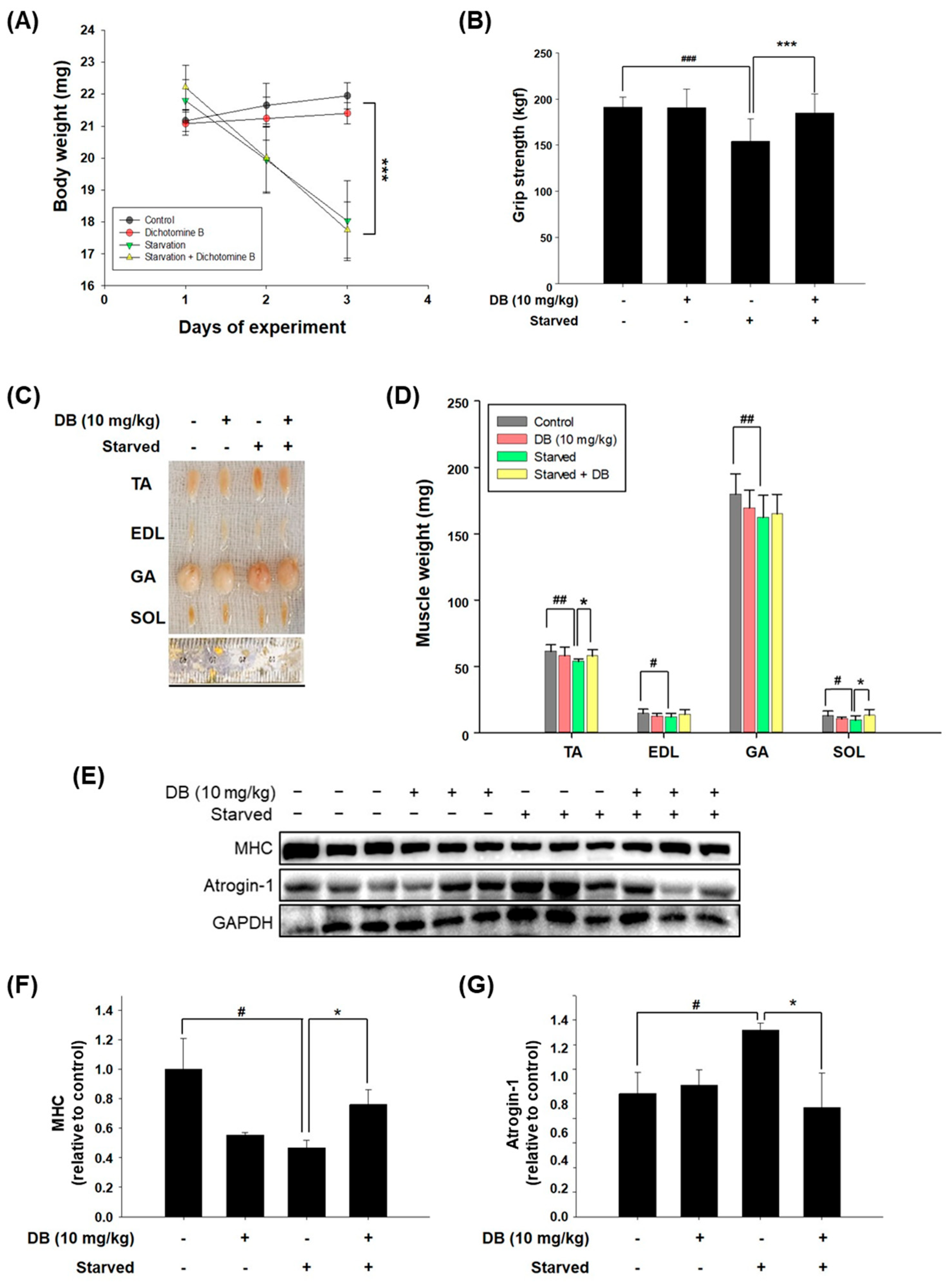
2.4. Dichotomine B Preserves Muscle Mass and Function in Starved Mice
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Extraction and Isolation of Compounds 1–5
4.3. C2C12 Cell Culture and Differentiation
4.4. Myotube Treatments and Starvation
4.5. Cell Viability Test
4.6. Western Blot Analysis
4.7. Myosin Heavy Chain Immunofluorescence Staining
4.8. In Vivo Starvation Model
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, Y.; Feng, J.; Yu, Y.; Ling, M.; Wang, X. Advances in sarcopenia: Mechanisms, therapeutic targets, and intervention strategies. Arch. Pharm. Res. 2024, 47, 301–324. [Google Scholar] [CrossRef]
- Sergeeva, X.V.; Lvova, I.D.; Sharlo, K.A. Disuse-induced muscle fatigue: Facts and assumptions. Int. J. Mol. Sci. 2024, 25, 4984. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wang, S.; Liu, S.; Wang, Q.; Che, X.; Wu, G. Frontiers in sarcopenia: Advancements in diagnostics, molecular mechanisms, and therapeutic strategies. Mol. Asp. Med. 2024, 97, 101270. [Google Scholar] [CrossRef]
- Grima-Terrén, M.; Campanario, S.; Ramírez-Pardo, I.; Cisneros, A.; Hong, X.; Perdiguero, E.; Serrano, A.L.; Isern, J.; Muñoz-Cánoves, P. Muscle aging and sarcopenia: The pathology, etiology, and most promising therapeutic targets. Mol. Asp. Med. 2024, 100, 101319. [Google Scholar] [CrossRef]
- Beaudart, C.; Demonceau, C.; Reginster, J.Y.; Locquet, M.; Cesari, M.; Cruz Jentoft, A.J.; Bruyère, O. Sarcopenia and health-related quality of life: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2023, 14, 1228–1243. [Google Scholar] [CrossRef] [PubMed]
- Damluji, A.A.; Alfaraidhy, M.; AlHajri, N.; Rohant, N.N.; Kumar, M.; Al Malouf, C.; Bahrainy, S.; Ji Kwak, M.; Batchelor, W.B.; Forman, D.E.; et al. Sarcopenia and cardiovascular diseases. Circulation 2023, 147, 1534–1553. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Tripathy, D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 2009, 32, S157–S163. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, R.; Dieterich, W.; Natarajan, A.; Schwappacher, R.; Reljic, D.; Herrmann, H.J.; Neurath, M.F.; Zopf, Y. Influence of amino acids and exercise on muscle protein turnover, particularly in cancer cachexia. Cancers 2024, 16, 1921. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, C.; Dai, C.; Zhang, Y.; Wang, K.; Gao, Z.; Chen, X.; Yang, X.; Sun, H.; Yao, X.; et al. Nutritional strategies for muscle atrophy: Current evidence and underlying mechanisms. Mol. Nutr. Food Res. 2024, 68, e2300347. [Google Scholar] [CrossRef]
- Feng, L.T.; Chen, Z.N.; Bian, H. Skeletal muscle: Molecular structure, myogenesis, biological functions, and diseases. MedComm 2024, 5, e659. [Google Scholar] [CrossRef]
- Yoshida, T.; Delafontaine, P. Mechanisms of IGF-1-mediated regulation of skeletal muscle hypertrophy and atrophy. Cells 2020, 9, 1970. [Google Scholar] [CrossRef]
- Ahmad, S.S.; Chun, H.J.; Ahmad, K.; Shaikh, S.; Lim, J.H.; Ali, S.; Han, S.S.; Hur, S.J.; Sohn, J.H.; Lee, E.J.; et al. The roles of growth factors and hormones in the regulation of muscle satellite cells for cultured meat production. J. Anim. Sci. Technol. 2023, 65, 16–31. [Google Scholar] [CrossRef]
- Zhang, S.; Zeng, X.; Ren, M.; Mao, X.; Qiao, S. Novel metabolic and physiological functions of branched chain amino acids: A review. J. Anim. Sci. Biotechnol. 2017, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Nakao, R.; Abe, T.; Yamamoto, S.; Oishi, K. Ketogenic diet induces skeletal muscle atrophy via reducing muscle protein synthesis and possibly activating proteolysis in mice. Sci. Rep. 2019, 9, 19652. [Google Scholar] [CrossRef] [PubMed]
- Permpoon, U.; Moon, J.; Kim, C.Y.; Nam, T.-g. Glucocorticoid-mediated skeletal muscle atrophy: Molecular mechanisms and potential therapeutic targets. Int. J. Mol. Sci. 2025, 26, 7616. [Google Scholar] [CrossRef]
- Baida, G.; Bhalla, P.; Kirsanov, K.; Lesovaya, E.; Yakubovskaya, M.; Yuen, K.; Guo, S.; Lavker, R.M.; Readhead, B.; Dudley, J.T.; et al. REDD 1 functions at the crossroads between the therapeutic and adverse effects of topical glucocorticoids. EMBO Mol. Med. 2015, 7, 42–58. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.S.; Zhang, P.; Chen, X.P.; Liu, W.M. Ubiquitin-proteasome pathway in skeletal muscle atrophy. Front. Physiol. 2023, 14, 1289537. [Google Scholar] [CrossRef]
- Foletta, V.C.; White, L.J.; Larsen, A.E.; Léger, B.; Russell, A.P. The role and regulation of MAFbx/atrogin-1 and MuRF1 in skeletal muscle atrophy. Pflug. Arch. 2011, 461, 325–335. [Google Scholar] [CrossRef]
- Wada, S.; Kato, Y.; Okutsu, M.; Miyaki, S.; Suzuki, K.; Yan, Z.; Schiaffino, S.; Asahara, H.; Ushida, T.; Akimoto, T. Translational suppression of atrophic regulators by microRNA-23a integrates resistance to skeletal muscle atrophy. J. Biol. Chem. 2011, 286, 38456–38465. [Google Scholar] [CrossRef]
- Hudson, M.B.; Woodworth-Hobbs, M.E.; Gooch, J.L.; Russ Price, S. Calcineurin-NFAT signaling regulates atrogin-1 and MuRF1 via microRNA-23a (miR-23a) during muscle atrophy. Kidney Res. Clin. Pract. 2012, 31, A92. [Google Scholar] [CrossRef][Green Version]
- Zheng, B.; Ohkawa, S.; Li, H.; Roberts-Wilson, T.K.; Price, S.R. FOXO3a mediates signaling crosstalk that coordinates ubiquitin and atrogin-1/MAFbx expression during glucocorticoid-induced skeletal muscle atrophy. FASEB J. 2010, 24, 2660–2669. [Google Scholar] [CrossRef]
- Waddell, D.S.; Baehr, L.M.; Van Den Brandt, J.; Johnsen, S.A.; Reichardt, H.M.; Furlow, J.D.; Bodine, S.C. The glucocorticoid receptor and FOXO1 synergistically activate the skeletal muscle atrophy-associated MuRF1 gene. Am. J. Physiol. Endocrinol. Metab. 2008, 295, e785-97. [Google Scholar] [CrossRef]
- Xu, D.; Shan, B.; Lee, B.-H.; Zhu, K.; Zhang, T.; Sun, H.; Liu, M.; Shi, L.; Liang, W.; Qian, L.; et al. Phosphorylation and activation of ubiquitin-specific protease-14 by Akt regulates the ubiquitin-proteasome system. eLife 2015, 4, e10510. [Google Scholar] [CrossRef] [PubMed]
- Gellhaus, B.; Böker, K.O.; Schilling, A.F.; Saul, D. Therapeutic consequences of targeting the IGF-1/PI3K/AKT/FOXO3 axis in sarcopenia: A narrative review. Cells 2023, 12, 2787. [Google Scholar] [CrossRef] [PubMed]
- Domingo-Fernández, D.; Gadiya, Y.; Preto, A.J.; Krettler, C.A.; Mubeen, S.; Allen, A.; Healey, D.; Colluru, V. Natural Products Have Increased Rates of Clinical Trial Success throughout the Drug Development Process. J. Nat. Prod. 2024, 87, 1844–1851. [Google Scholar] [CrossRef]
- Bae, S.-J.; Choi, J.-W.; Park, B.-J.; Lee, J.; Jo, E.-K.; Lee, Y.-H.; Kim, S.-B.; Yuk, J.-M. Protective effects of a traditional herbal extract from Stellaria dichotoma var. lanceolata against Mycobacterium abscessus infections. PLoS ONE 2018, 13, e0207696. [Google Scholar] [CrossRef]
- Dong, L.; Zhou, X.; Ma, J.; Zhou, H.; Fu, X. Chemical constituents of Stellaria dichotoma var. lanceolata and their anti-inflammatory effect on lipopolysaccharide-stimulated RAW 264.7 cells. Chem. Nat. Compd. 2021, 57, 158–162. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Fan, Y.; Yang, B.; Huang, C. Radix Stellariae extract prevents high-fat-diet-induced obesity in C57BL/6 mice by accelerating energy metabolism. Peer J. 2017, 5, e3305. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Kuo, P.-C.; Chan, H.-H.; Kuo, I.J.; Lin, F.-W.; Su, C.-R.; Yang, M.-L.; Li, D.-T.; Wu, T.-S. β-Carboline alkaloids from Stellaria dichotoma var lanceolata and their anti-inflammatory activity. J. Nat. Prod. 2010, 73, 1993–1998. [Google Scholar] [CrossRef]
- Yu, F.; Li, S.; Li, B.; Zhan, P.; Wang, X.; Yang, J. Recent research progress of β-carbolines as privileged scaffold in the discovery of anticancer agent (2019–2024). Bioorg. Med. Chem. 2025, 129, 118325. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Ma, F.; Cao, Y.; Bai, M.; Gao, X.; Yang, Z.; Xu, Y.; Yan, Y. Bis(7)-harmine derivatives as potential multi-target anti-Alzheimer agents. Front. Chem. 2025, 13, 1545908. [Google Scholar] [CrossRef]
- Zhou, X.; Quan, H.; Zang, L.; Dong, L. Dichotomine B attenuates neuroinflammatory responses by regulating TLR4/MyD88-mTOR signaling pathway in BV2 cells. Neurochem. Res. 2023, 48, 2451–2462. [Google Scholar] [CrossRef]
- Long, T.; Chen, X.; Zhang, Y.; Zhou, Y.-J.; He, Y.-N.; Zhu, Y.-F.; Fu, H.-J.; Yu, L.; Yu, C.-L.; Law, B.Y.-K.; et al. Protective effects of Radix Stellariae extract against Alzheimer’s disease via autophagy activation in Caenorhabditis elegans and cellular models. Biomed. Pharmacother. 2023, 165, 115261. [Google Scholar] [CrossRef]
- Cho, C.H.; Chae, S.H.; Thi, N.H.L.; Um, S.H.; Lee, S.; Yu, J.S.; Kang, K.S.; Kim, K.H. Lambertianic Acid from Platycladus orientalis inhibits muscle atrophy in dexamethasone-induced C2C12 muscle atrophy cells. Plants 2025, 14, 1357. [Google Scholar] [CrossRef]
- Vincent, A.; Stange, K.; Louveau, I.; Röntgen, M.; Dessauge, F. Myogenic and adipogenic potential of porcine muscle satellite cells isolated by flow cytometry. Differentiation 2025, 144, 100871. [Google Scholar] [CrossRef]
- Bensaid, S.; Fabre, C.; Yahya Rajaei, A.; Claeyssen, C.; Daussin, F.N.; Cieniewski-Bernard, C. Multi-therapeutic strategy targeting Akt-mTOR and FoxO1 pathway to counteract skeletal muscle atrophy consecutive to hypoxia. Am. J. Physiol. Cell Physiol. 2025, 328, 2057–2069. [Google Scholar] [CrossRef]
- Paolini, A.; Omairi, S.; Mitchell, R.; Vaughan, D.; Matsakas, A.; Vaiyapuri, S.; Ricketts, T.; Rubinsztein, D.C.; Patel, K. Attenuation of autophagy impacts on muscle fibre development, starvation induced stress and fibre regeneration following acute injury. Sci. Rep. 2021, 11, 24532. [Google Scholar] [CrossRef]
- Vendelbo, M.H.; Møller, A.B.; Christensen, B.; Nellemann, B.; Clasen, B.F.F.; Nair, K.S.; Jørgensen, J.O.L.; Jessen, N.; Møller, N. Fasting increases human skeletal muscle net phenylalanine release and this is associated with decreased mTOR signaling. PLoS ONE 2014, 9, 102031. [Google Scholar] [CrossRef]
- Layman, D.K. Impacts of protein quantity and distribution on body composition. Front. Nutr. 2024, 11, 1388986. [Google Scholar] [CrossRef] [PubMed]
- McKendry, J.; Coletta, G.; Nunes, E.A.; Lim, C.; Phillips, S.M. Mitigating disuse-induced skeletal muscle atrophy in ageing: Resistance exercise as a critical countermeasure. Exp. Physiol. 2024, 109, 1650–1662. [Google Scholar] [CrossRef]
- Ren, Y.; Gao, Y.B.; Yu, D.X.; Huang, H.B. Beta-hydroxy-beta-methyl butyrate supplementation in critically ill patients: A systematic review and meta-analysis of randomized controlled trials. Front. Nutr. 2025, 12, 1505797. [Google Scholar] [CrossRef] [PubMed]
- Ebert, S.M.; Nicolas, C.S.; Schreiber, P.; Lopez, J.G.; Taylor, A.T.; Judge, A.R.; Judge, S.M.; Rasmussen, B.B.; Talley, J.J.; Rème, C.A.; et al. Ursolic acid induces beneficial changes in skeletal muscle mRNA expression and increases exercise participation and performance in dogs with age-related muscle atrophy. Animals 2024, 14, 186. [Google Scholar] [CrossRef]
- Morikawa, T.; Sun, B.; Matsuda, H.; Wu, L.J.; Harima, S.; Yoshikawa, M. Bioactive constituents from Chinese natural medicines. XIV. New glycosides of β-carboline-type alkaloid, neolignan, and phenylpropanoid from Stellaria dichotoma L. var. lanceolata and their antiallergic activities. Chem. Pharm. Bull. 2004, 52, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Simirgiotis, M.J.; Schmeda-Hirschmann, G.; Bórquez, J.; Kennelly, E.J. The Passiflora tripartita (Banana Passion) fruit: A source of bioactive flavonoid C-glycosides isolated by HSCCC and characterized by HPLC–DAD–ESI/MS/MS. Molecules 2013, 18, 1672–1692. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-Y.; Permpoon, U.; Lee, J.-h.; Kim, J.H.; Kim, H.M.; Jeon, J.-S.; Nam, T.-g.; Kim, C.Y. Anti-Atrophic Effects of Dichotomine B from Stellaria dichotoma During Starvation-Induced Skeletal Muscle Atrophy. Molecules 2025, 30, 3839. https://doi.org/10.3390/molecules30183839
Kim J-Y, Permpoon U, Lee J-h, Kim JH, Kim HM, Jeon J-S, Nam T-g, Kim CY. Anti-Atrophic Effects of Dichotomine B from Stellaria dichotoma During Starvation-Induced Skeletal Muscle Atrophy. Molecules. 2025; 30(18):3839. https://doi.org/10.3390/molecules30183839
Chicago/Turabian StyleKim, Jae-Yong, Uttapol Permpoon, Ju-hee Lee, Ji Hoon Kim, Hye Mi Kim, Je-Seung Jeon, Tae-gyu Nam, and Chul Young Kim. 2025. "Anti-Atrophic Effects of Dichotomine B from Stellaria dichotoma During Starvation-Induced Skeletal Muscle Atrophy" Molecules 30, no. 18: 3839. https://doi.org/10.3390/molecules30183839
APA StyleKim, J.-Y., Permpoon, U., Lee, J.-h., Kim, J. H., Kim, H. M., Jeon, J.-S., Nam, T.-g., & Kim, C. Y. (2025). Anti-Atrophic Effects of Dichotomine B from Stellaria dichotoma During Starvation-Induced Skeletal Muscle Atrophy. Molecules, 30(18), 3839. https://doi.org/10.3390/molecules30183839






