Upcycling of Whole Pisco Grape Pomace: Influence of Emerging Extractions on Antioxidant Potential and Functional Quality of the Lipophilic Fractions
Abstract
1. Introduction
2. Results and Discussion
2.1. Influence of Different Extraction Techniques on Total Extraction Yield
2.2. Indicators of Quality for Pisco Grape Pomace Lipophilic Fractions
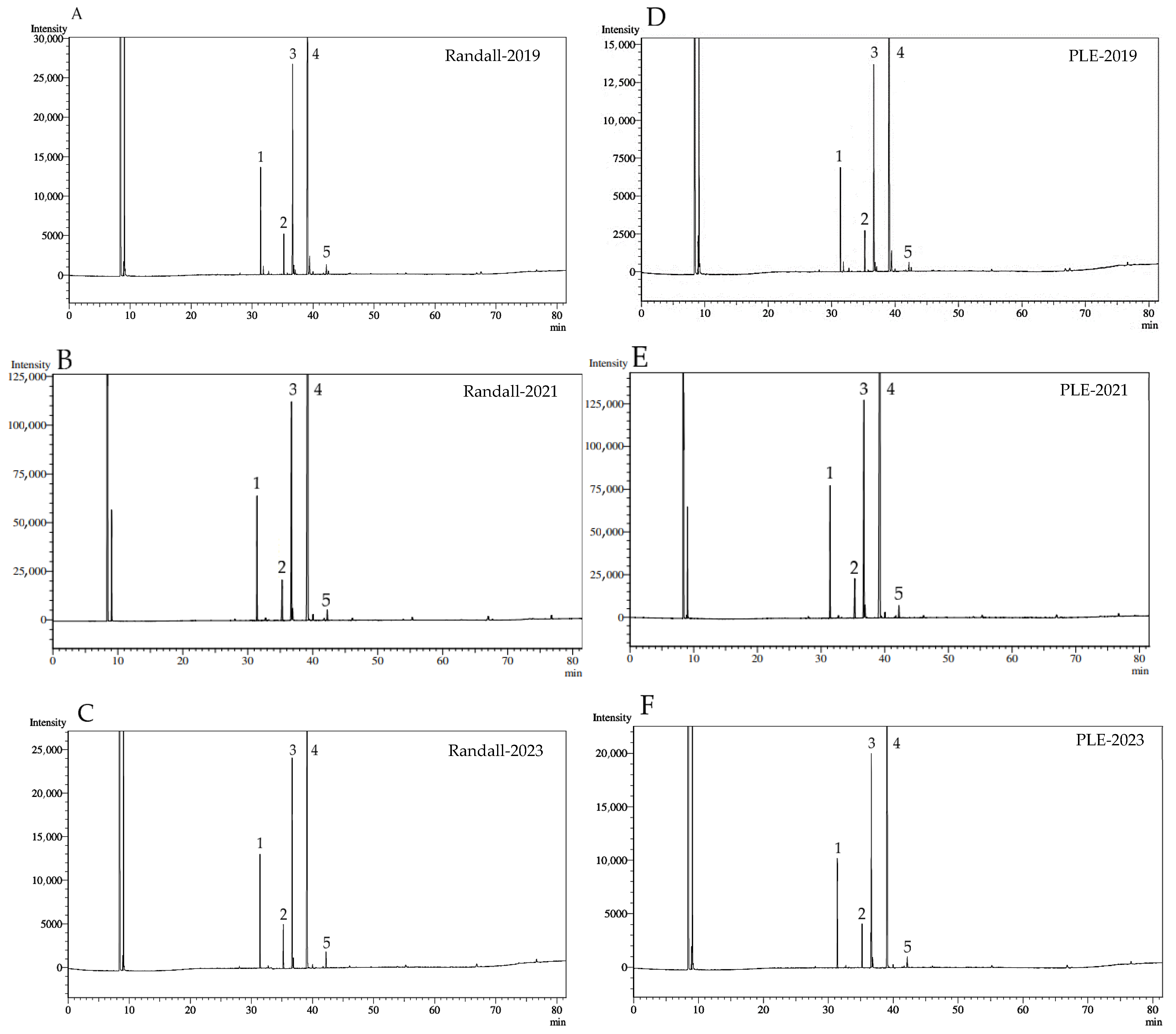
2.3. Fatty Acid Profile and Functional Quality Indicators
2.4. α-Tocopherol Content
2.5. Total Polyphenols Content (TPC)
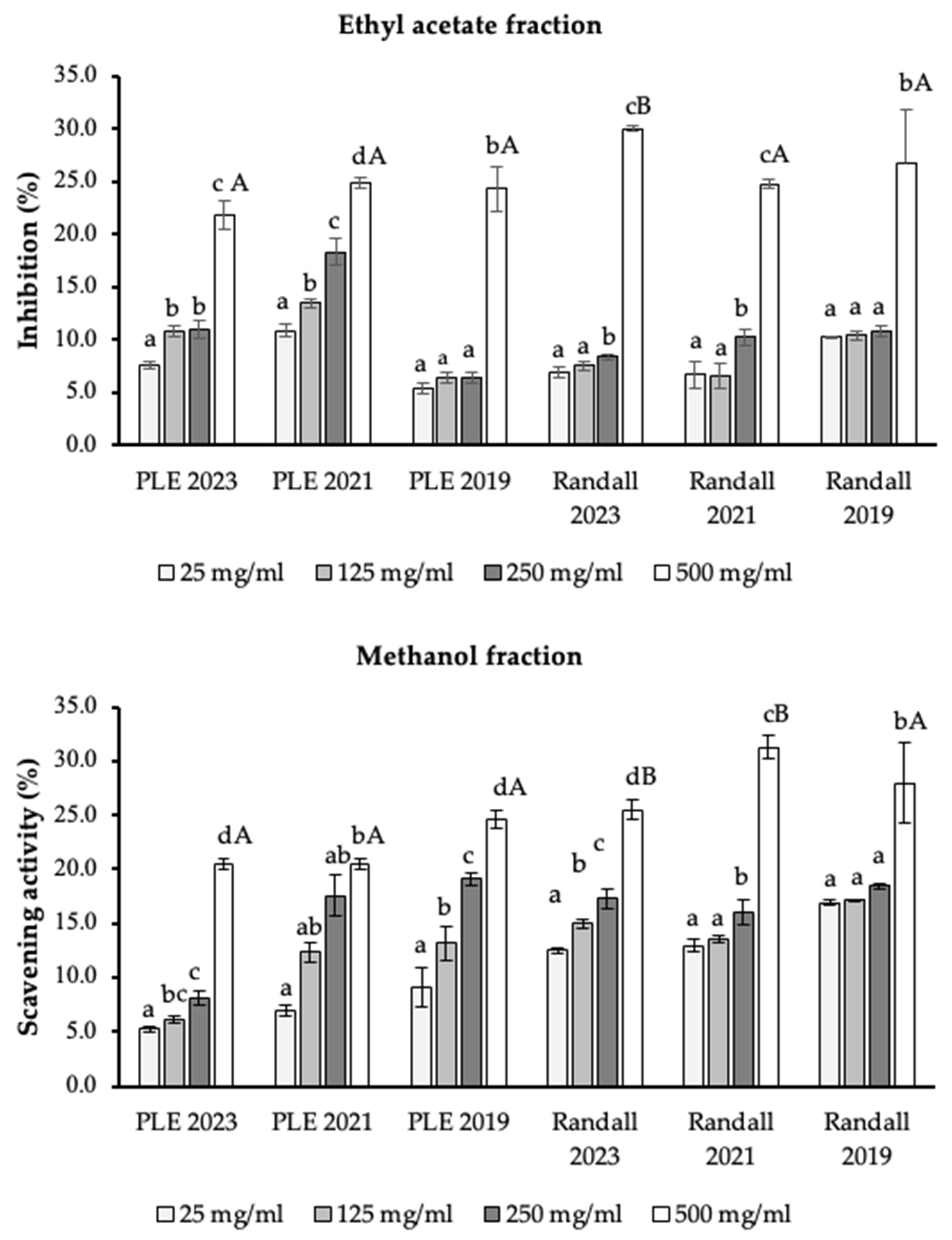
2.6. Antioxidant Potential
3. Materials and Methods
3.1. Plant Material
3.2. Extraction Techniques
3.2.1. Randall Extraction Method
3.2.2. Pressurized Liquid Extraction (PLE) Method
3.2.3. Extraction Yield
3.3. Chemical and Antioxidant Characterization of Lipophilic Fractions from Pisco Grape Pomaces
3.3.1. Indicators of Quality
3.3.2. Fatty Acid Profile
3.3.3. Indicators of the Functional Quality
3.3.4. Quantification of α-Tocopherol Content
3.3.5. Total Polyphenol Content (TPC)
3.4. Antioxidant Potential of Lipophilic Fractions from Pisco Grape Pomaces
3.4.1. DPPH Assay
3.4.2. ORAC Assay
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OIV. Análisis Anual Del Sector Vitivinícola Mundial En 2021; Organización Internacional de la Viña y el Vino: Dijon, France, 2021. [Google Scholar]
- Salgado, M.M.M.; Ortega, B.R.; Janssens, M.; Fincheira, P. Grape Pomace Compost as a Source of Organic Matter: Evolution of Quality Parameters to Evaluate Maturity and Stability. J. Clean. Prod. 2019, 216, 56–63. [Google Scholar] [CrossRef]
- Bustamante, M.A.; Moral, R.; Paredes, C.; Pérez-Espinosa, A.; Moreno-Caselles, J.; Pérez-Murcia, M.D. Agrochemical Characterisation of the Solid By-Products and Residues from the Winery and Distillery Industry. Waste Manag. 2008, 28, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Poblete, J.; Quispe-Fuentes, I.; Aranda, M.; Vega-Gálvez, A. Application of Vacuum and Convective Drying Processes for the Valorization of Pisco Grape Pomace to Enhance the Retention of Its Bioactive Compounds. Waste Biomass Valorization 2023, 15, 3093–3107. [Google Scholar] [CrossRef]
- Rodríguez-Ramos, F.; Cañas-Sarazúa, R.; Briones-Labarca, V. Pisco Grape Pomace: Iron/Copper Speciation and Antioxidant Properties, towards Their Comprehensive Utilization. Food Biosci. 2022, 47, 101781. [Google Scholar] [CrossRef]
- Cortes, L.; Pérez-Won, M.; Lemus-Mondaca, R.; Giovagnoli-Vicuña, C.; Uribe, E. Quality Properties and Mathematical Modeling of Vinasse Films Obtained under Different Conditions. J. Food Process. Preserv. 2020, 44, e14477. [Google Scholar] [CrossRef]
- Carmona-Jiménez, Y.; Igartuburu, J.M.; Guillén-Sánchez, D.A.; García-Moreno, M.V. Fatty Acid and Tocopherol Composition of Pomace and Seed Oil from Five Grape Varieties Southern Spain. Molecules 2022, 27, 6980. [Google Scholar] [CrossRef]
- Di Pietro Fernandes, C.; Figueiredo Santana, L.; dos Santos, J.R.; Stéphanie Fernandes, D.; Aiko Hiane, P.; Pott, A.; de Cássia Freitas, K.; Bogo, D.; Aragão do Nascimento, V.; de Oliveira Filiú, W.F.; et al. Nutraceutical Potential of Grape (Vitis vinifera L.) Seed Oil in Oxidative Stress, Inflammation, Obesity and Metabolic. Molecules 2023, 28, 7811. [Google Scholar] [CrossRef]
- Oppedisano, F.; Macrì, R.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Bosco, F.; Nucera, S.; Zito, M.C.; Guarnieri, L.; et al. The Anti-Inflammatory and Antioxidant Properties of n-3 PUFAs: Their Role in Cardiovascular Protection. Biomedicines 2020, 8, 306. [Google Scholar] [CrossRef]
- Prado-Acebo, I.; Cubero-Cardoso, J.; Lu-Chau, T.A.; Eibes, G. Integral Multi-Valorization of Agro-Industrial Wastes: A Review. Waste Manag. 2024, 183, 42–52. [Google Scholar] [CrossRef]
- Tellez-Robles, D.; López-Cortez, M. del S.; Santoyo-Tepole, F.; Rosales-Martínez, P.; García Ochoa, F.; Hernández-Botello, M.T.; Salgado-Cruz, M. la P. Optimization of the Extraction of Bioactive Compounds from Cabernet Sauvignon Grape Pomace from Querétaro, Mexico, Using MSPD. Separations 2024, 11, 13. [Google Scholar] [CrossRef]
- Chakka, A.K.; Babu, A.S. Bioactive Compounds of Winery By-Products: Extraction Techniques and Their Potential Health Benefits. Appl. Food Res. 2022, 2, 100058. [Google Scholar] [CrossRef]
- Garavaglia, J.; Markoski, M.M.; Oliveira, A.; Marcadenti, A. Grape Seed Oil Compounds: Biological and Chemical Actions for Health. Nutr. Metab. Insights 2016, 9, 59–64. [Google Scholar] [CrossRef]
- Gitea, M.A.; Bungau, S.G.; Gitea, D.; Pasca, B.M.; Purza, A.L.; Radu, A.F. Evaluation of the Phytochemistry–Therapeutic Activity Relationship for Grape Seeds Oil. Life 2023, 13, 178. [Google Scholar] [CrossRef] [PubMed]
- Ben Mohamed, H.; Duba, K.S.; Fiori, L.; Abdelgawed, H.; Tlili, I.; Tounekti, T.; Zrig, A. Bioactive Compounds and Antioxidant Activities of Different Grape (Vitis vinifera L.) Seed Oils Extracted by Supercritical CO2 and Organic Solvent. LWT 2016, 74, 557–562. [Google Scholar] [CrossRef]
- Sabir, A.; Unver, A.; Kara, Z. The Fatty Acid and Tocopherol Constituents of the Seed Oil Extracted from 21 Grape Varieties (Vitis Spp.). J. Sci. Food Agric. 2012, 92, 1982–1987. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Pérez, R.M.; Almena, A.; Ramírez-Márquez, C.; Bonilla-Petriciolet, A.; Martín, M. Techno-Economic and Environmental Comparison of Processes for the Production of Grape Oil. J. Clean. Prod. 2024, 441, 141041. [Google Scholar] [CrossRef]
- Dimić, I.; Teslić, N.; Putnik, P.; Kovačević, D.B.; Zeković, Z.; Šojić, B.; Mrkonjić, Ž.; Čolović, D.; Montesano, D.; Pavlić, B. Innovative and Conventional Valorizations of Grape Seeds from Winery By-Products as Sustainable Source of Lipophilic Antioxidants. Antioxidants 2020, 9, 568. [Google Scholar] [CrossRef]
- Lopes de Menezes, M.; Johann, G.; Diório, A.; Pereira, N.C.; da Silva, E.A. Phenomenological Determination of Mass Transfer Parameters of Oil Extraction from Grape Biomass Waste. J. Clean. Prod. 2018, 176, 130–139. [Google Scholar] [CrossRef]
- Kyriakoudi, A.; Radojčić Redovniković, I.; Vidović, S.; Radošević, K.; Andreou, T.; Mourtzinos, I.; Cvjetko Bubalo, M. Coupling Deep Eutectic Solvents with Innovative Extraction Techniques towards Plant Derived Bioactive Compositions. RSC Sustain. 2024, 2, 1675–1691. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Ora, A.; Häkkinen, S.T.; Ritala, A.; Räisänen, R.; Kallioinen-Mänttäri, M.; Melin, K. Innovative Extraction Technologies of Bioactive Compounds from Plant By-Products for Textile Colorants and Antimicrobial Agents. Biomass Convers. Biorefinery 2024, 14, 24973–25002. [Google Scholar] [CrossRef]
- Fiori, L.; Lavelli, V.; Duba, K.S.; Sri Harsha, P.S.C.; Mohamed, H.B.; Guella, G. Supercritical CO2 Extraction of Oil from Seeds of Six Grape Cultivars: Modeling of Mass Transfer Kinetics and Evaluation of Lipid Profiles and Tocol Contents. J. Supercrit. Fluids 2014, 94, 71–80. [Google Scholar] [CrossRef]
- Jin, Q.; Neilson, A.P.; Stewart, A.C.; O’Keefe, S.F.; Kim, Y.T.; McGuire, M.; Wilder, G.; Huang, H. Integrated Approach for the Valorization of Red Grape Pomace: Production of Oil, Polyphenols, and Acetone-Butanol-Ethanol. ACS Sustain. Chem. Eng. 2018, 6, 16279–16286. [Google Scholar] [CrossRef]
- Miklav, A.; Barp, L.; Lucci, P.; Moret, S. Pressurized Liquid Extraction for the Determination of Bioactive Compounds in Plants with Emphasis on Phenolics. Trends Anal. Chem. 2024, 173, 117620. [Google Scholar] [CrossRef]
- Barp, L.; Višnjevec, A.M.; Moret, S. Pressurized Liquid Extraction: A Powerful Tool to Implement Extraction and Purification of Food Contaminants. Foods 2023, 12, 2017. [Google Scholar] [CrossRef] [PubMed]
- Perez-Vazquez, A.; Carpena, M.; Barciela, P.; Cassani, L.; Simal-Gandara, J.; Prieto, M.A. Pressurized Liquid Extraction for the Recovery of Bioactive Compounds from Seaweeds for Food Industry Application: A Review. Antioxidants 2023, 12, 612. [Google Scholar] [CrossRef] [PubMed]
- Zwingelstein, M.; Draye, M.; Besombes, J.L.; Piot, C.; Chatel, G. Viticultural Wood Waste as a Source of Polyphenols of Interest: Opportunities and Perspectives through Conventional and Emerging Extraction Methods. Waste Manag. 2020, 102, 782–794. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound Assisted Extraction of Food and Natural Products. Mechanisms, Techniques, Combinations, Protocols and Applications. A Review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for Extraction and Isolation of Natural Products: A Comprehensive Review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef]
- Poblete, J.; Aranda, M.; Quispe-Fuentes, I. Efficient Conditions of Enzyme-Assisted Extractions and Pressurized Liquids for Recovering Polyphenols with Antioxidant Capacity from Pisco Grape Pomace as a Sustainable Strategy. Molecules 2025, 30, 2977. [Google Scholar] [CrossRef]
- Da Silva, L.C.; Viganó, J.; Sanches, V.L.; De Souza Mesquita, L.M.; Pizani, R.; Rostagno, M.A. Simultaneous Extraction and Analysis of Apple Pomace by Gradient Pressurized Liquid Extraction Coupled In-Line with Solid-Phase Extraction and on-Line with HPLC. Food Chem. 2023, 407, 135117. [Google Scholar] [CrossRef]
- Alvarez-Rivera, G.; Bueno, M.; Ballesteros-Vivas, D.; Mendiola, J.; Ibañez, E. Chapter 13—Pressurized Liquid Extraction. In Liquid-Phase Extraction Handbooks in Separation Science; Elsevier: Amsterdam, The Netherlands, 2020; pp. 375–398. [Google Scholar]
- Freitas, L.D.S.; Dariva, C.; Jacques, R.A.; Caramão, E.B. Effect of Experimental Parameters in the Pressurized Liquid Extraction of Brazilian Grape Seed Oil. Sep. Purif. Technol. 2013, 116, 313–318. [Google Scholar] [CrossRef]
- Fahim, M.A.; Alsahhaf, T.A.; Elkilani, A. Chapter 3—Thermophysical Properties of Petroleum Fractions and Crude Oils. In Fundamentals of Petroleum Refining; Elsevier: Amsterdam, The Netherlands, 2010; ISBN 978-0-444-52785-1. [Google Scholar]
- Flores, M.; Avendaño, V.; Bravo, J.; Valdés, C.; Forero-Doria, O.; Quitral, V.; Vilcanqui, Y.; Ortiz-Viedma, J. Edible Oil Parameters during Deterioration Processes. Int. J. Food Sci. 2021, 2021, 7105170. [Google Scholar] [CrossRef] [PubMed]
- Herculano, L.S.; Lukasievicz, G.V.B.; Sehn, E.; Torquato, A.S.; Belançon, M.P.; Savi, E.; Kimura, N.M.; Malacarne, L.C.; Baesso, M.L.; Astrath, N.G.C. The Correlation of Physicochemical Properties of Edible Vegetable Oils by Chemometric Analysis of Spectroscopic Data. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 245, 118877. [Google Scholar] [CrossRef] [PubMed]
- Vieira, D.S.; Menezes, M.; Gonçalves, G.; Mukai, H.; Lenzi, E.K.; Pereira, N.C.; Fernandes, P.R.G. Temperature Dependence of Refractive Index and of Electrical Impedance of Grape Seed (Vitis vinifera, Vitis labrusca) Oils Extracted by Soxhlet and Mechanical Pressing. Grasas Aceites 2015, 66, e083. [Google Scholar] [CrossRef]
- Zahir, E.; Saeed, R.; Hameed, M.A.; Yousuf, A. Study of Physicochemical Properties of Edible Oil and Evaluation of Frying Oil Quality by Fourier Transform-Infrared (FT-IR) Spectroscopy. Arab. J. Chem. 2017, 10, S3870–S3876. [Google Scholar] [CrossRef]
- Tapia, M.S.; Alzamora, S.M.; Chirife, J. Effects of Water Activity (Aw) on Microbial Stability as a Hurdle in Food Preservation. In Water Activity in Foods: Fundamentals and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2020. [Google Scholar]
- Karel, M. Lipid Oxidation, Secondary Reactions, and Water Activity of Foods. In Autoxidation in Food and Biological Systems; Imic, M.G., Karel, M., Eds.; Springer: Boston, MA, USA, 1980; ISBN 978-1-4757-9353-6. [Google Scholar]
- Meral, R.; Kına, E.; Ceylan, Z. Low-Calorie Cookies Enhanced with Fish Oil-Based Nano-Ingredients for Health-Conscious Consumers. ACS Omega 2024, 9, 39159–39169. [Google Scholar] [CrossRef]
- Tilami, S.K.; Kouřimská, L. Assessment of the Nutritional Quality of Plant Lipids Using Atherogenicity and Thrombogenicity Indices. Nutrients 2022, 14, 3795. [Google Scholar] [CrossRef]
- Modi, Z.; Dubey, K.; Salunke, P. Characterization of Fatty Acids and Nutritional Health Indicators of Ghee (Butteroil) Manufactured from Bovine Colostrum and Sweet Cream. Dairy 2025, 6, 2. [Google Scholar] [CrossRef]
- Gharby, S.; Asbbane, A.; Nid Ahmed, M.; Gagour, J.; Hallouch, O.; Oubannin, S.; Bijla, L.; Goh, K.W.; Bouyahya, A.; Ibourki, M. Vegetable Oil Oxidation: Mechanisms, Impacts on Quality, and Approaches to Enhance Shelf Life. Food Chem. X 2025, 28, 102541. [Google Scholar] [CrossRef]
- Symoniuk, E.; Ksibi, N.; Wroniak, M.; Lefek, M.; Ratusz, K. Oxidative Stability Analysis of Selected Oils from Unconventional Raw Materials Using Rancimat Apparatus. Appl. Sci. 2022, 12, 10355. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- NIH Office of Dietary Supplements, Vitamin E—Health Professional Fact Sheet. Available online: https://ods.od.nih.gov/factsheets/VitaminE-HealthProfessional/ (accessed on 8 May 2024).
- Sabliov, C.M.; Fronczek, C.F.; Astete, C.; Khachaturyan, M. Effects of Temperature and UV Light on Degradation of A-Tocopherol in Free and Dissolved Form Effects of Temperature and UV Light on Degradation of α-Tocopherol in Free and Dissolved Form. J. Am. Oil Chem. Soc. 2009, 86, 895–902. [Google Scholar] [CrossRef]
- Kmiecik, D.; Fedko, M.; Siger, A.; Kulczynski, B. Degradation of Tocopherol Molecules and Its Impact on the Polymerization of Triacylglycerols During. Molecules 2019, 24, 4555. [Google Scholar] [CrossRef] [PubMed]
- Siddiq, M.; Iezzoni, A.; Khan, A.; Breen, P.; Sebolt, A.M.; Dolan, K.D.; Ravi, R.; Iezzoni, A. Characterization of New Tart Cherry (Prunus Cerasus L.): Selections Based on Fruit Quality, Total Anthocyanins, and Antioxidant Capacity. Int. J. Food Prop. 2011, 14, 471–480. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Athanasiadis, V.; Kotsou, K.; Mantiniotou, M.; Kalompatsios, D.; Makrygiannis, I.; Bozinou, E.; Lalas, S.I. Optimization of Pressurized Liquid Extraction (PLE) Parameters for Extraction of Bioactive Compounds from Moringa oleifera Leaves and Bioactivity Assessment. Int. J. Mol. Sci. 2024, 25, 4628. [Google Scholar] [CrossRef]
- La, J.; Kim, M.J.; Lee, J. Evaluation of Solvent Effects on the DPPH Reactivity for Determining the Antioxidant Activity in Oil Matrix. Food Sci. Biotechnol. 2021, 20, 367–375. [Google Scholar] [CrossRef]
- Fernandes, L.; Casal, S.; Cruz, R.; Alberto, J.; Ramalhosa, E. Seed Oils of Ten Traditional Portuguese Grape Varieties with Interesting Chemical and Antioxidant Properties. Food Res. Int. 2013, 50, 161–166. [Google Scholar] [CrossRef]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bachchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for Hydrophilic and Lipophilic Antioxidant Capacity (Oxygen Radical Absorbance Capacity (ORAC FL)) of Plasma and Other Biological and Food Samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef]
- Grajeda-Iglesias, C.; Salas, E.; Barouh, N.; Baréa, B.; Panya, A.; Figueroa-espinoza, M.C. Antioxidant Activity of Protocatechuates Evaluated by DPPH, ORAC, and CAT Methods. Food Chem. 2016, 194, 749–757. [Google Scholar] [CrossRef]
- A.O.A.C. Index of Refraction of Oils and Fats, 7th ed.; 921.08; AOAC: Rockville, MD, USA, 2000. [Google Scholar]
- Quispe-Fuentes, I.; Vega-Gálvez, A.; Aranda, M.; Poblete, J.; Pasten, A.; Bilbao-Sainz, C.; Wood, D.; McHugh, T.; Delporte, C. Effects of Drying Processes on Composition, Microstructure and Health Aspects from Maqui Berries. J. Food Sci. Technol. 2020, 57, 2241–2250. [Google Scholar] [CrossRef]
- Bail, S.; Stuebiger, G.; Krist, S.; Unterweger, H.; Buchbauer, G. Characterisation of Various Grape Seed Oils by Volatile Compounds, Triacylglycerol Composition, Total Phenols and Antioxidant Capacity. Food Chem. 2008, 108, 1122–1132. [Google Scholar] [CrossRef]
- Espín, J.C.; Soler-Rivas, C.; Wichers, H.J. Characterization of the Total Free Radical Scavenger Capacity of Vegetable Oils and Oil Fractions Using 2,2-Diphenyl-1-Picrylhydrazyl Radical. J. Agric. Food Chem. 2000, 48, 648–656. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Hogan, S.; Chung, H.; Welbaum, G.E.; Zhou, K. Inhibitory Effect of Raspberries on Starch Digestive Enzyme and Their Antioxidant Properties and Phenolic Composition. Food Chem. 2010, 119, 592–599. [Google Scholar] [CrossRef]
| Harvest | Randall (%) | PLE (%) |
|---|---|---|
| 2019 | 9.12 ± 1.47 aA | 15.20 ± 1.33 bB |
| 2021 | 9.58 ± 0.85 aA | 13.42 ± 0.90 bAB |
| 2023 | 9.03 ± 1.49 aA | 11.79 ± 1.16 aA |
| Extraction Method | Randall | PLE | |
|---|---|---|---|
| Refractive index * | 2019 | 1.4561 ± 0.0054 a | 1.4560 ± 0.0023 a |
| 2021 | 1.4484 ± 0.0240 a | 1.4634 ± 0.0012 a | |
| 2023 | 1.4620 ± 0.0036 a | 1.4569 ± 0.0035 a | |
| 2019 | 21.70 ± 2.94 a | 14.51 ± 0.29 b | |
| Peroxide value (PV) | 2021 | 27.24 ± 4.18 a | 22.97 ± 1.96 b |
| 2023 | 28.95 ± 1.43 a | 21.66 ± 0.86 b | |
| Water activity *(aw) | 2019 | 0.3741 ± 0.010 a | 0.4229 ± 0.018 b |
| 2021 | 0.4357 ± 0.053 a | 0.4278 ± 0.053 a | |
| 2023 | 0.4034 ± 0.005 a | 0.3966 ± 0.004 a | |
|
Fatty Acid/
Extraction Method and Harvest | Palmitic (C16:0) | Stearic (C18:0) | Oleic (C18:1n9c) | Linoleic (C18:2n6c) | Eicosenoic (C20:1) | Saturated Fatty Acids (S) | Monounsaturated Fatty Acids | Polyunsaturated Fatty Acids | Unsaturated Fatty Acids (U) | Ratio S/U |
|---|---|---|---|---|---|---|---|---|---|---|
| PLE-2019 | 8.64 ± 0.05 d | 3.81 ± 0.02 c | 20.90 ± 0.06 c | 65.63 ± 0.12 a | 1.02 ± 0.12 a | 12.45 ± 0.06 a | 21.92 ± 0.06 a | 65.63 ± 0.12 a | 87.55 ± 0.06 a | 0.14 ± 0.00 a |
| Randall-2019 | 8.58 ± 0.09 d | 3.79 ± 0.01 c | 21.01 ± 0.04 c | 65.66 ± 0.18 a | 0.95 ± 0.16 a | 12.38 ± 0.10 a | 21.96 ± 0.12 a | 65.66 ± 0.18 a | 87.62 ± 0.10 a | 0.14 ± 0.00 a |
| PLE-2021 | 7.59 ± 0.05 ab | 3.58 ± 0.03 a | 18.87 ± 0.09 b | 69.03 ± 0.15 bc | 0.94 ± 0.01 a | 11.17 ± 0.07 b | 19.85 ± 0.08 b | 69.03 ± 0.15 a | 88.88 ± 0.15 a | 0.13 ± 0.00 a |
| Randall-2021 | 7.39 ± 0.04 a | 3.64 ± 0.01 b | 18.78 ± 0.01 b | 69.36 ± 0.07 c | 0.84 ± 0.01 a | 11.02 ± 0.02 a | 19.63 ± 0.01 a | 69.36 ± 0.07 b | 88.99 ± 0.07 a | 0.12 ± 0.00 a |
| PLE-2023 | 7.81 ± 0.16 b | 3.55 ± 0.03 ab | 17.81 ± 0.07 a | 69.21 ± 0.21 b | 1.62 ± 0.11 b | 11.36 ± 0.18 a | 19.43 ± 0.05 a | 69.21 ± 0.21 a | 88.64 ± 0.18 a | 0.13 ±0.00 a |
| Randall-2023 | 8.15 ± 0.39 c | 3.59 ± 0.06 a | 18.18 ± 0.55 a | 68.62 ± 0.52 c | 1.43 ± 0.35 b | 11.74 ± 0.45 a | 19.60 ± 0.31 a | 68.62 ± 0.52 a | 88.23 ± 0.40 a | 0.13 ± 0.00 a |
| Extraction Method and Harvest | AI | TI | H/H | COX Value | USFA/SFA | PUFA/SFA |
|---|---|---|---|---|---|---|
| PLE-2019 | 0.099 ± 0.000 a | 0.285 ± 0.001 a | 10.018 ± 0.073 a | 6.760 ± 0.012 a | 7.031 ± 0.040 a | 5.270 ± 0.035 a |
| Randall-2019 | 0.098 ± 0.008 a | 0.282 ± 0.003 a | 10.097 ± 0.124 a | 6.763 ± 0.019 a | 7.081 ± 0.066 a | 5.306 ± 0.056 a |
| PLE-2021 | 0.085 ± 0.001 b | 0.251 ± 0.002 b | 11.580 ± 0.088 a | 7.100 ± 0.014 a | 7.954 ± 0.056 a | 6.166 ± 0.039 a |
| Randall-2021 | 0.083 ± 0.000 a | 0.248 ± 0.001 a | 11.931 ± 0.071 b | 7.139 ± 0.006 b | 8.071 ± 0.043 b | 6.281 ± 0.026 b |
| PLE-2023 | 0.092 ± 0.002 a | 0.266 ± 0.011 a | 10.670 ± 0.619 a | 7.089 ± 0.019 a | 7.522 ± 0.327 a | 6.851 ± 0.264 a |
| Randall-2023 | 0.088 ± 0.002 a | 0.256 ± 0.004 a | 11.149 ± 0.272 a | 7.131 ± 0.021 a | 7.805 ± 0.136 a | 6.094 ± 0.113 a |
| Harvest | Randall α-Tocopherol (μg/g) | PLE α-Tocopherol (μg/g) |
|---|---|---|
| 2019 | 180.53 ± 6.49 a | 86.63 ± 15.97 b |
| 2021 | 644.68 ± 12.49 b | 156.02 ± 1.39 a |
| 2023 | 330.88 ± 3.18 b | 172.92 ± 11.45 a |
| Harvest | Randall | PLE |
|---|---|---|
| 2019 | 0.61 ± 0.05 a | 0.85 ± 0.05 b |
| 2021 | 0.62 ± 0.23 a | 0.58 ± 0.07 a |
| 2023 | 0.71 ± 0.07 a | 0.80 ± 0.13 a |
| Extraction Method | Harvest | Acetone | Ethyl Acetate | n-Hexane |
|---|---|---|---|---|
| Randall | 2019 | 27.08 ± 2.56 a | 48.83 ± 2.03 b | 74.61 ± 3.27 ab |
| 2021 | 39.70 ± 3.20 b | 73.68 ± 1.21 c | 75.28 ± 3.93 b | |
| 2023 | 35.70 ± 0.92 b | 36.79 ± 2.58 a | 68.56 ± 1.99 a | |
| PLE | 2019 | 68.61 ± 4.67 a | 73.59 ± 4.92 a | 89.37 ± 2.08 a |
| 2021 | 119.10 ± 5.38 b | 120.92 ± 4.88 b | 111.28 ± 4.38 b | |
| 2023 | 65.89 ± 3.41 a | 80.58 ± 1.91 a | 119.55 ± 2.87 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quispe-Fuentes, I.; Rodríguez-Ramos, F.; Poblete, J.; Leyton-Valle, I.; Uribe, E. Upcycling of Whole Pisco Grape Pomace: Influence of Emerging Extractions on Antioxidant Potential and Functional Quality of the Lipophilic Fractions. Molecules 2025, 30, 3776. https://doi.org/10.3390/molecules30183776
Quispe-Fuentes I, Rodríguez-Ramos F, Poblete J, Leyton-Valle I, Uribe E. Upcycling of Whole Pisco Grape Pomace: Influence of Emerging Extractions on Antioxidant Potential and Functional Quality of the Lipophilic Fractions. Molecules. 2025; 30(18):3776. https://doi.org/10.3390/molecules30183776
Chicago/Turabian StyleQuispe-Fuentes, Issis, Fátima Rodríguez-Ramos, Jacqueline Poblete, Iván Leyton-Valle, and Elsa Uribe. 2025. "Upcycling of Whole Pisco Grape Pomace: Influence of Emerging Extractions on Antioxidant Potential and Functional Quality of the Lipophilic Fractions" Molecules 30, no. 18: 3776. https://doi.org/10.3390/molecules30183776
APA StyleQuispe-Fuentes, I., Rodríguez-Ramos, F., Poblete, J., Leyton-Valle, I., & Uribe, E. (2025). Upcycling of Whole Pisco Grape Pomace: Influence of Emerging Extractions on Antioxidant Potential and Functional Quality of the Lipophilic Fractions. Molecules, 30(18), 3776. https://doi.org/10.3390/molecules30183776









