Brassinin Induces H2S Signals and Improves Vascular Smooth Muscle Cell Functions
Abstract
1. Introduction
2. Results
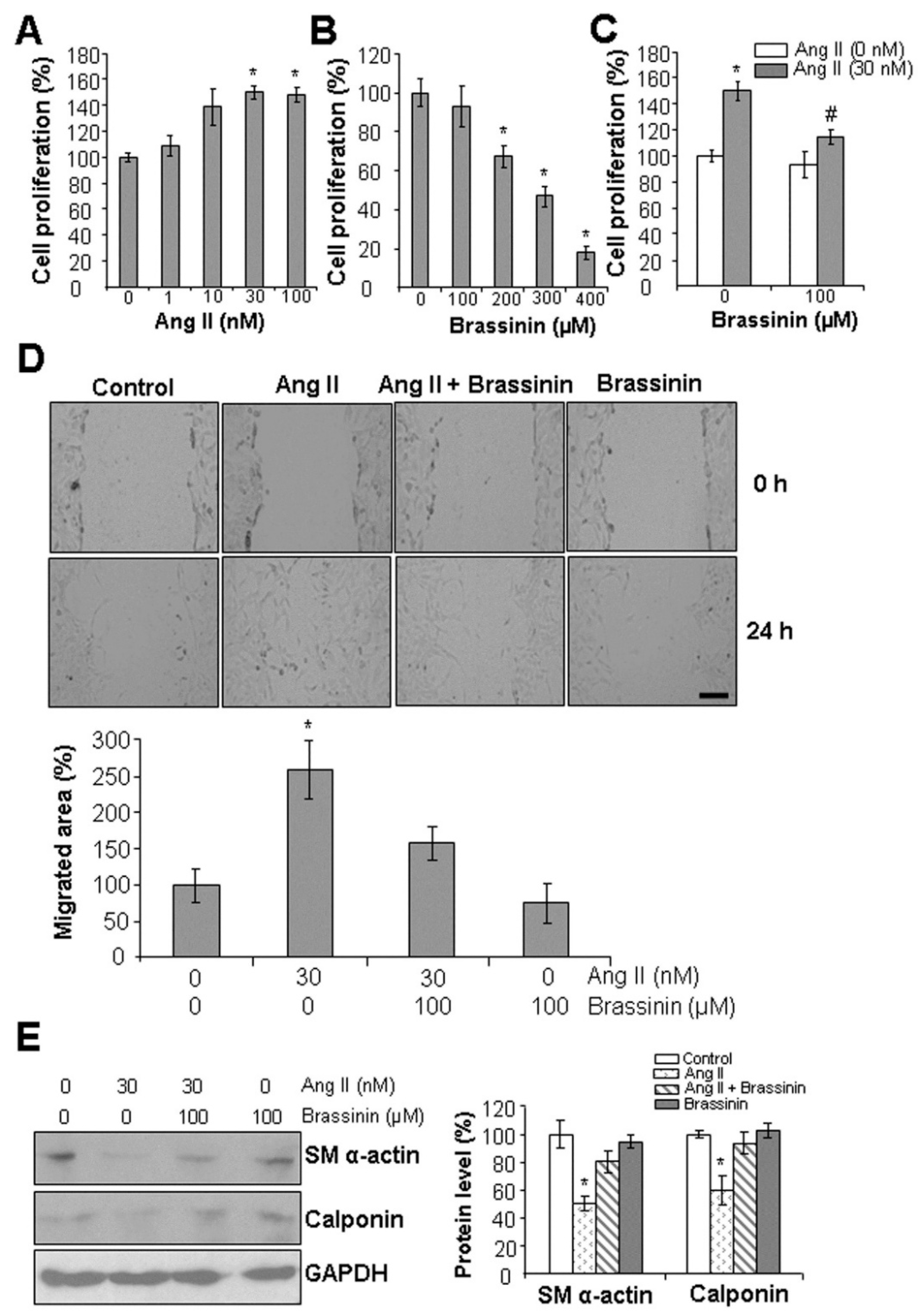
2.1. Brassinin Inhibits Ang II-Induced SMC Phenotypic Changes
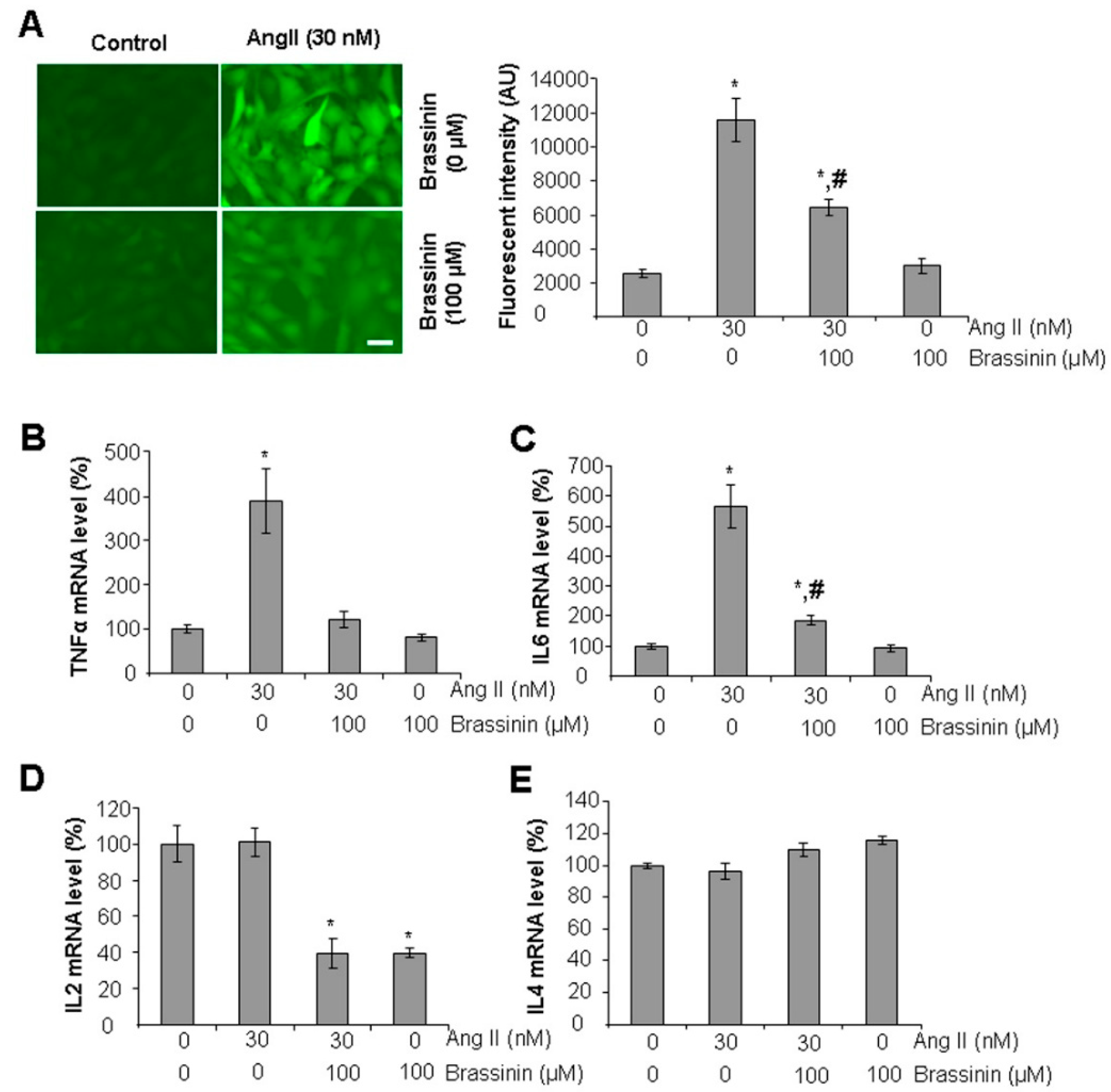
2.2. Brassinin Attenuates Ang II-Induced Oxidative Stress and Inflammatory Gene Expressions
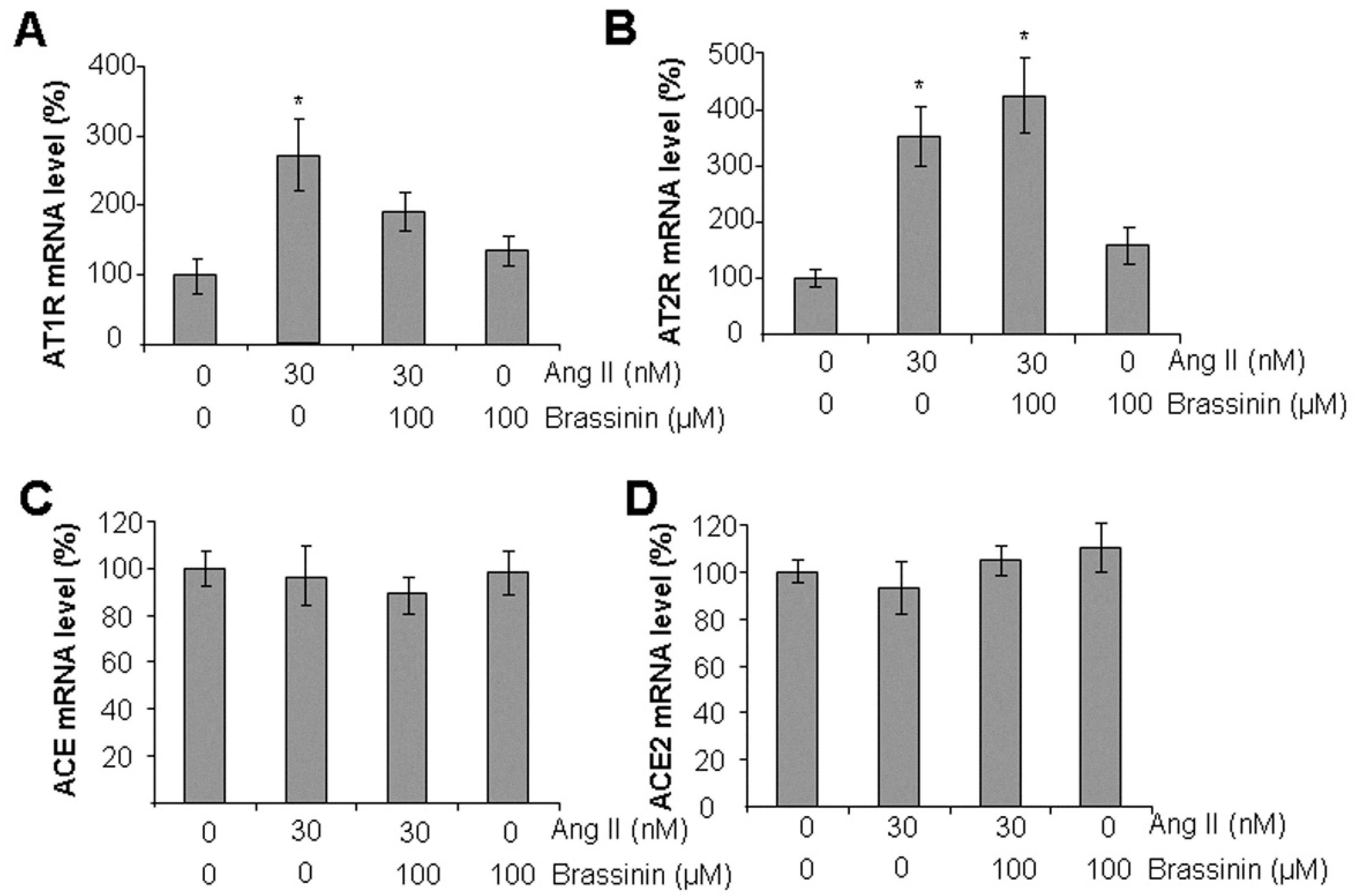
2.3. Brassinin Inhibited Ang II-Induced AT1R Gene Expression
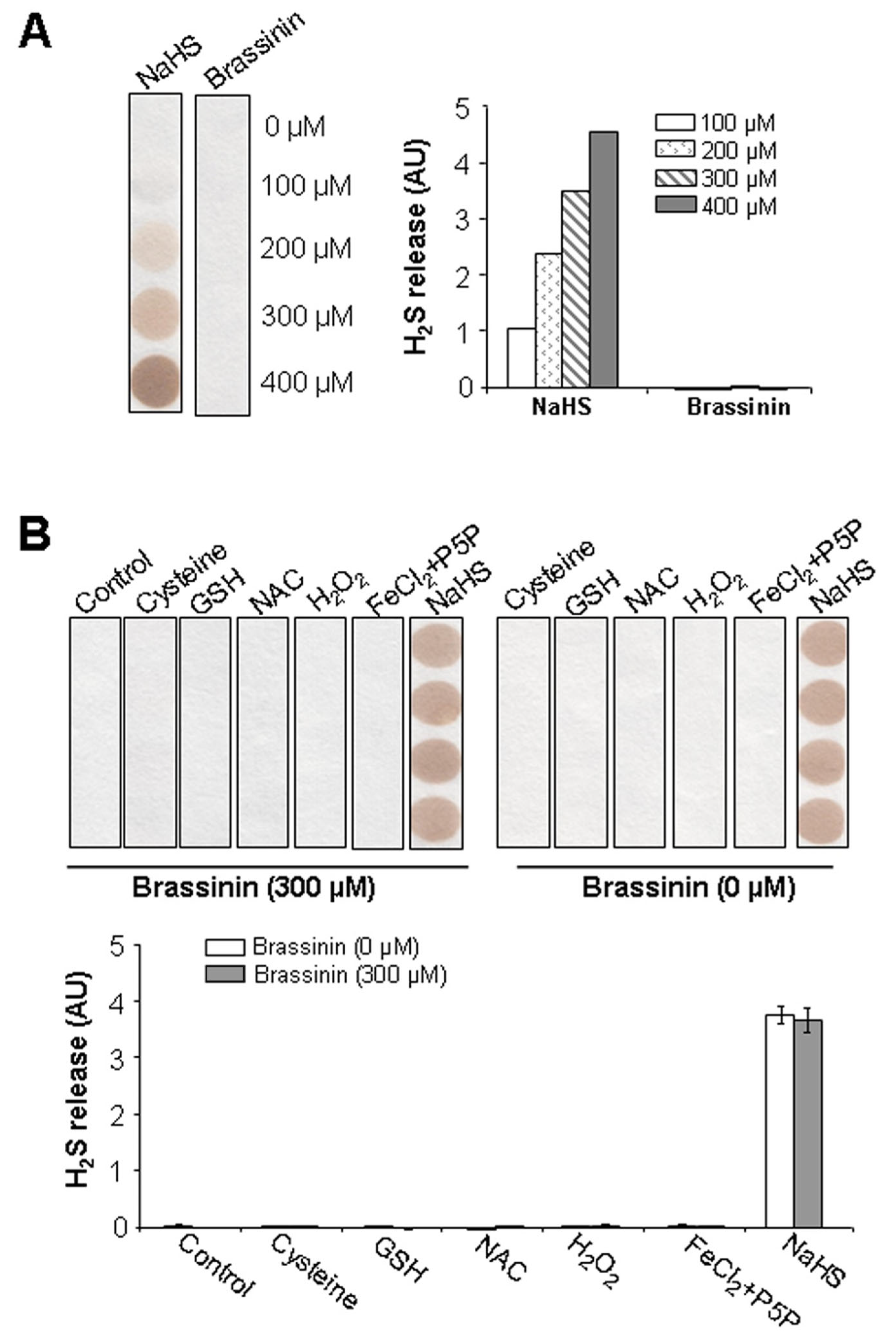
2.4. Brassinin Does Not Release H2S Naturally
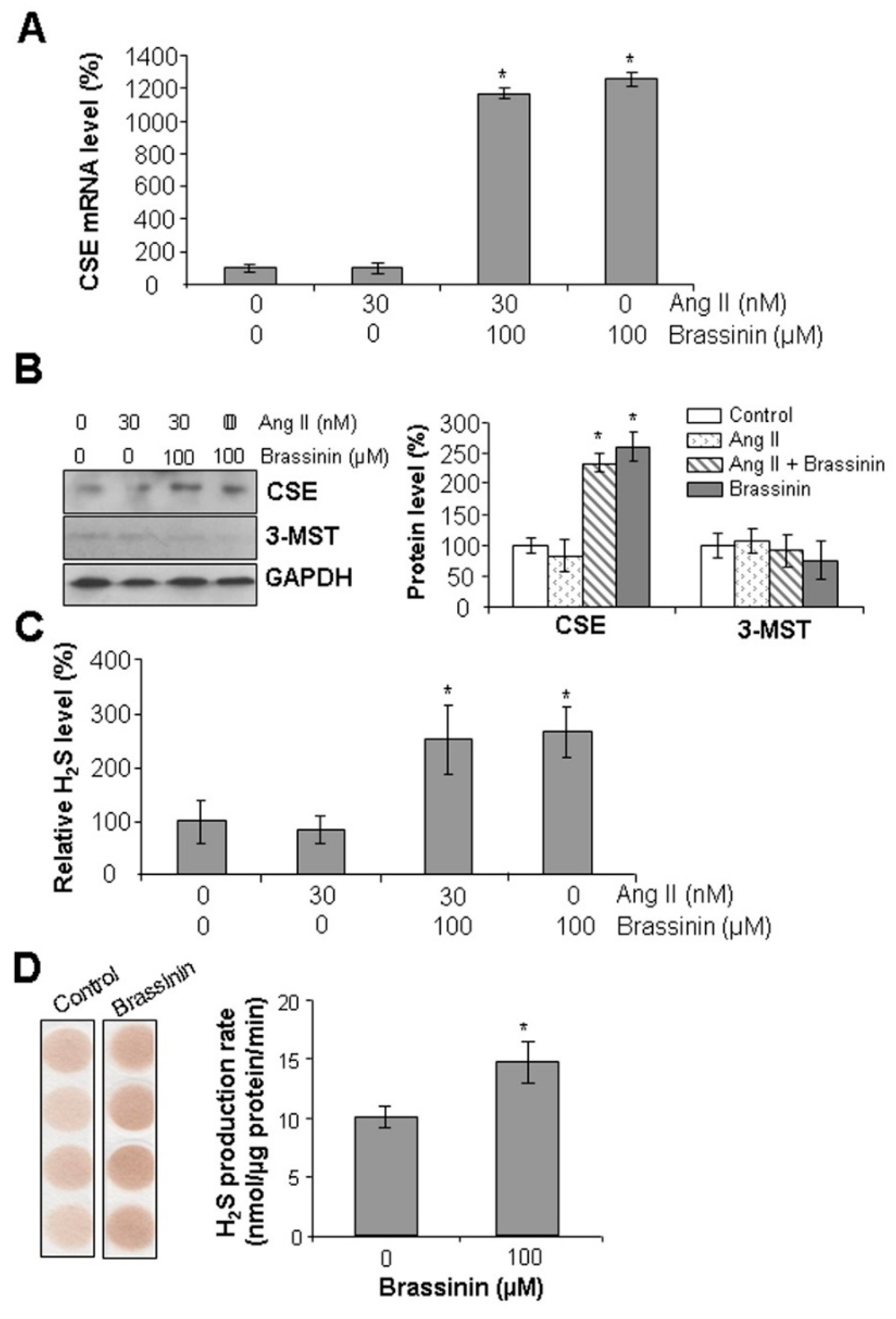
2.5. Brassinin Induces CSE Expression and H2S Synthesis in SMCs
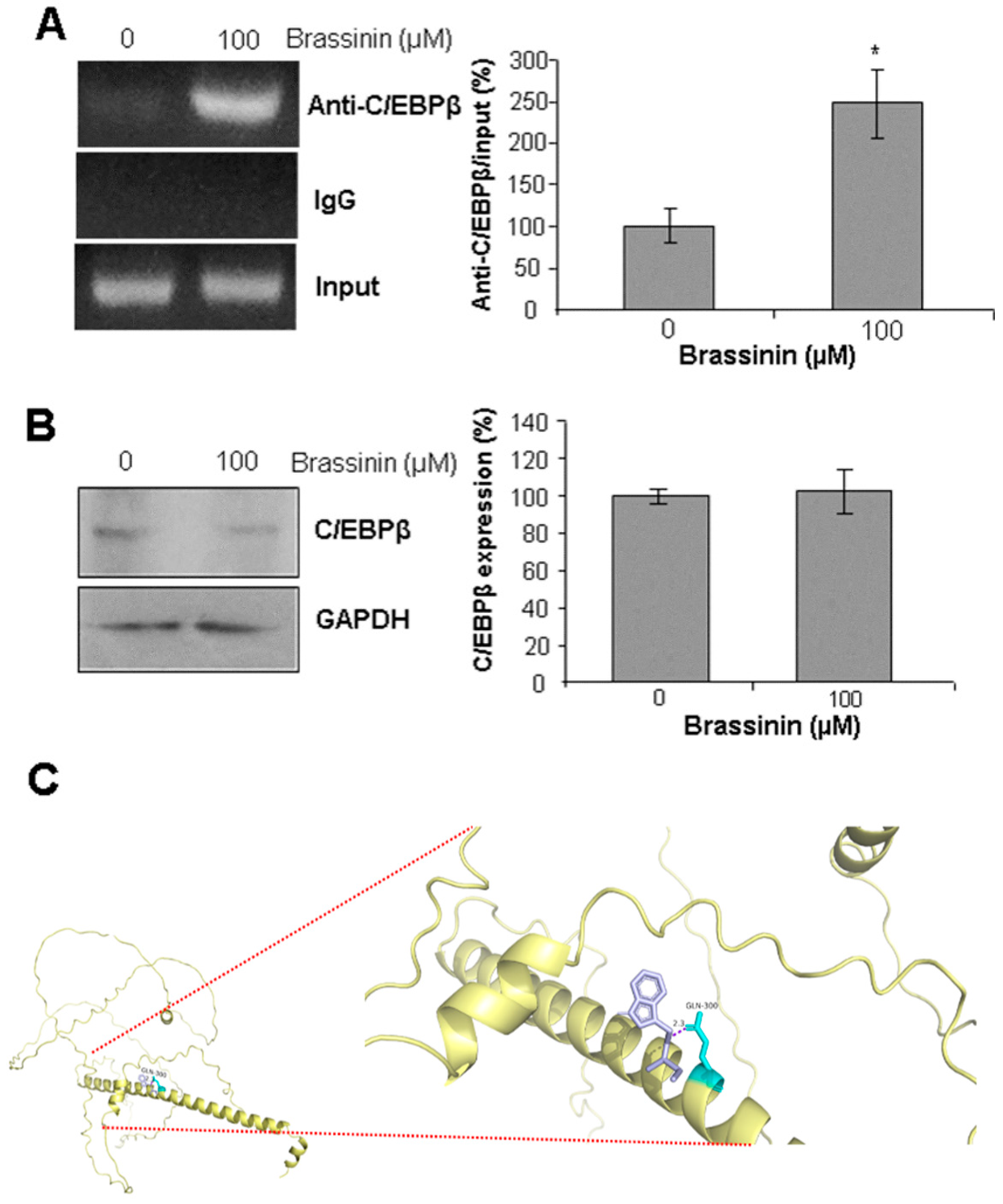
2.6. Brassinin Stimulates C/EBPβ Binding to CSE Gene Promoter
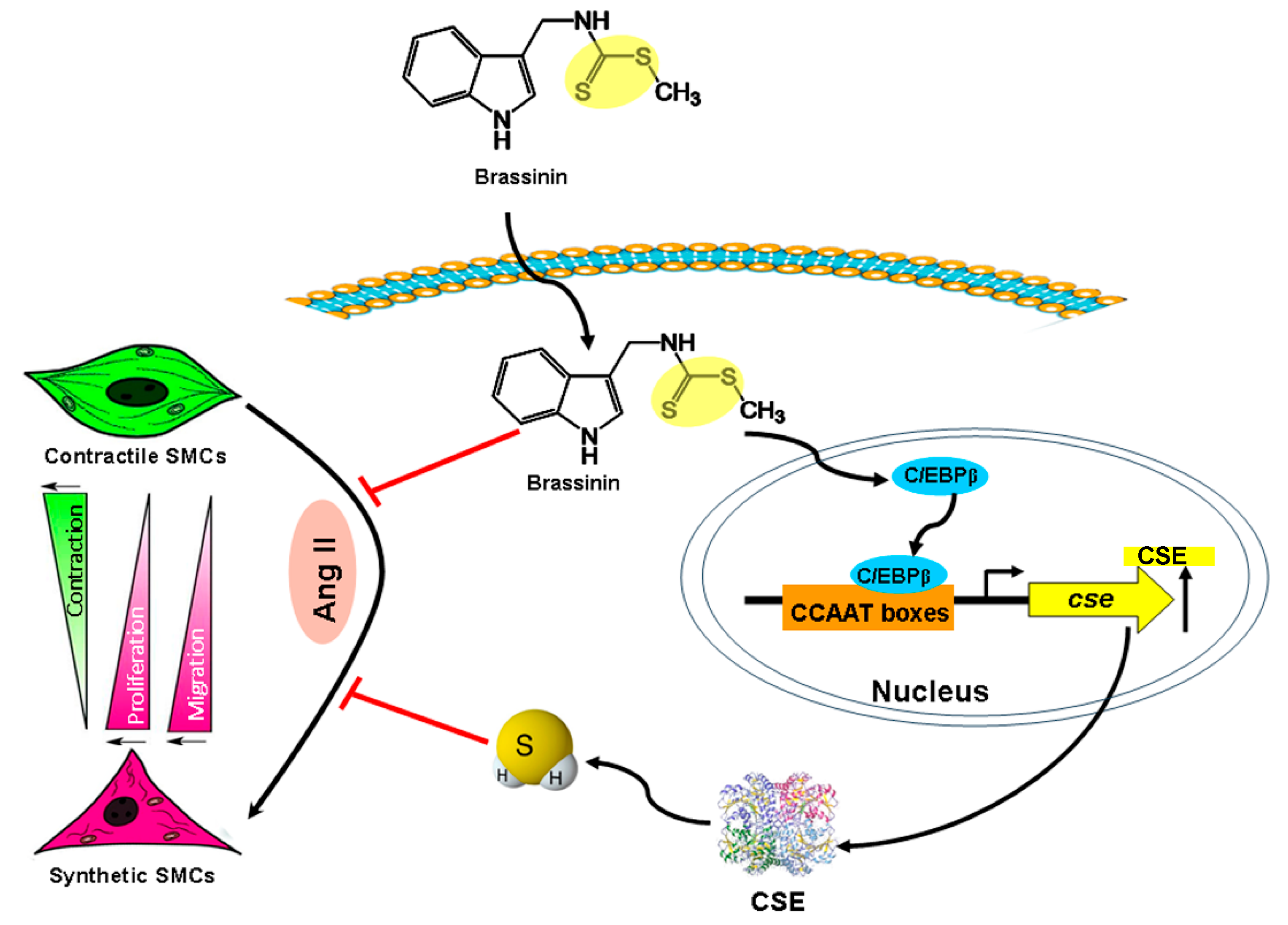
3. Discussion
4. Materials and Methods
4.1. The Reagents
4.2. Cell Culture
4.3. H2S Detection
4.4. Cell Proliferation
4.5. Cell Migration
4.6. Oxidative Stress Detection
4.7. Real-Time PCR
4.8. Western Blotting
4.9. Chromatin Immunoprecipitation (ChIP)
4.10. Molecular Docking
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jarad, S.; Gill, G.; Amadi, P.; Gu, H.M.; Zhang, D.W. VSMCs in atherosclerosis: Implications on the role of inflammation and extracellular matrix remodelling. Pharmacol. Res. 2025, 218, 107833. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, L.; Liu, D.; Huang, F.; Peng, Q.; Lu, J.; Zhou, J.; Zheng, S.; Liu, X. Vascular smooth muscle cells: A therapeutic target in atherosclerosis. Rev. Cardiovasc. Med. 2025, 26, 28240. [Google Scholar] [CrossRef]
- Grootaert, M.O.J.; Moulis, M.; Roth, L.; Martinet, W.; Vindis, C.; Bennett, M.R.; De Meyer, G.R.Y. Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis. Cardiovasc. Res. 2018, 114, 622–634. [Google Scholar] [CrossRef]
- Chen, S.; Chen, H.; Zhong, Y.; Ge, Y.; Li, C.; Qiao, Z.; Zhu, J. Insulin-like growth factor-binding protein 3 inhibits angiotensin II-induced aortic smooth muscle cell phenotypic switch and matrix metalloproteinase expression. Exp. Physiol. 2020, 105, 1827–1839. [Google Scholar] [CrossRef]
- Fatima, N.; Patel, S.N.; Hussain, T. Angiotensin II type 2 receptor: A target for protection against hypertension, metabolic dysfunction, and organ remodeling. Hypertension 2021, 77, 1845–1856. [Google Scholar] [CrossRef] [PubMed]
- St Paul, A.; Corbett, C.B.; Okune, R.; Autieri, M.V. Angiotensin II, hypercholesterolemia, and vascular smooth muscle cells: A perfect trio for vascular pathology. Int. J. Mol. Sci. 2020, 21, 4525. [Google Scholar] [CrossRef]
- Munteanu, C.; Popescu, C.; Vlădulescu-Trandafir, A.I.; Onose, G. Signaling paradigms of H2S-induced vasodilation: A comprehensive review. Antioxidants 2024, 13, 1158. [Google Scholar] [CrossRef]
- Islam, K.N.; Nguyen, I.D.; Islam, R.; Pirzadah, H.; Malik, H. Roles of hydrogen sulfide (H2S) as a potential therapeutic agent in cardiovascular diseases: A narrative review. Cureus 2024, 16, e64913. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Minkler, P.; Grove, D.; Wang, R.; Willard, B.; Dweik, R.; Hine, C. Non-enzymatic hydrogen sulfide production from cysteine in blood is catalyzed by iron and vitamin B6. Commun. Biol. 2019, 2, 194. [Google Scholar] [CrossRef]
- Powell, C.R.; Dillon, K.M.; Matson, J.B. A review of hydrogen sulfide (H2S) donors: Chemistry and potential therapeutic applications. Biochem. Pharmacol. 2018, 149, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Predmore, B.L.; Kondo, K.; Bhushan, S.; Zlatopolsky, M.A.; King, A.L.; Aragon, J.P.; Grinsfelder, D.B.; Condit, M.E.; Lefer, D.J. The polysulfide diallyl trisulfide protects the ischemic myocardium by preservation of endogenous hydrogen sulfide and increasing nitric oxide bioavailability. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H2410–H2418. [Google Scholar] [CrossRef]
- Peng, W.; Guo, W.; Zheng, Y. Recent development of hydrogen sulfide therapeutics in the treatment of cardiovascular diseases. Curr. Top. Med. Chem. 2021, 21, 2230–2242. [Google Scholar] [CrossRef]
- Bechelli, C.; Macabrey, D.; Deglise, S.; Allagnat, F. Clinical potential of hydrogen sulfide in peripheral arterial disease. Int. J. Mol. Sci. 2023, 24, 9955. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Y.; Rivett, A.; Li, H.; Wu, L.; Wang, R.; Yang, G. Deficiency of cystathionine gamma-lyase promotes aortic elastolysis and medial degeneration in aged mice. J. Mol. Cell Cardiol. 2022, 171, 30–44. [Google Scholar] [CrossRef]
- Armah, C.N.; Derdemezis, C.; Traka, M.H.; Dainty, J.R.; Doleman, J.F.; Saha, S.; Leung, W.; Potter, J.F.; Lovegrove, J.A.; Mithen, R.F. Diet rich in high glucoraphanin broccoli reduces plasma LDL cholesterol: Evidence from randomised controlled trials. Mol. Nutr. Food Res. 2015, 59, 918–926. [Google Scholar] [CrossRef]
- Jakše, B.; Jakše, B.; Pinter, S.; Jug, B.; Godnov, U.; Pajek, J.; Fidler Mis, N. Dietary intakes and cardiovascular health of healthy adults in short-, medium-, and long-term whole-food plant-based lifestyle program. Nutrients 2019, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- Martelli, A.; Testai, L.; Citi, V.; Marino, A.; Bellagambi, F.; Ghimenti, G.; Breschi, M.; Calderone, V. Pharmacological characterization of the vascular effects of aryl isothiocyanates: Is hydrogen sulfide the real player? Vascul Pharmacol. 2014, 60, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Benavides, G.A.; Squadrito, G.L.; Mills, R.W.; Patel, H.D.; Isbell, T.S.; Patel, R.P.; Darley-Usmar, V.M.; Doeller, J.E.; Kraus, D.W. Hydrogen sulfide mediates the vasoactivity of garlic. Proc. Natl. Acad. Sci. USA 2007, 104, 17977–17982. [Google Scholar] [CrossRef]
- Mehta, R.G.; Liu, J.; Constantinou, A.; Thomas, C.F.; Hawthorne, M.; You, M.; Gerhäuser, C.; Pezzuto, J.M.; Moon, R.C.; Moriarty, R.M. Cancer chemopreventive activity of brassinin, a phytoalexin from cabbage. Carcinogenesis 1995, 16, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Han, B.H.; Yoon, J.J.; Choi, E.S.; Jeong, D.H.; Lee, Y.J.; Kang, D.G.; Lee, H.S. Inhibitory effect of brassinin on TNF-α-induced vascular inflammation in human umbilical vein endothelial cells. Mol. Med. Rep. 2017, 16, 6890–6895. [Google Scholar] [CrossRef]
- Kello, M.; Drutovic, D.; Chripkova, M.; Pilatova, M.; Budovska, M.; Kulikova, L.; Urdzik, P.; Mojzis, J. ROS-dependent antiproliferative effect of brassinin derivative homobrassinin in human colorectal cancer Caco2 cells. Molecules 2014, 19, 10877–10897. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Hwang, J.; Choi, H.S. Brassinin, a brassica-derived phytochemical, regulates monocyte-to-macrophage differentiation and inflammatory responses in human monocytes and murine macrophages. J. Pharm. Pharmacol. 2020, 72, 1245–1255. [Google Scholar] [CrossRef]
- Lee, M.K.; Ryu, H.; Jeong, H.H.; Lee, B. Brassinin abundant in Brassicaceae suppresses melanogenesis through dual mechanisms of tyrosinase inhibition. Foods 2022, 12, 121. [Google Scholar] [CrossRef]
- Yang, G.; Ju, Y.; Fu, M.; Zhang, Y.; Pei, Y.; Racine, M.; Baath, S.; Merritt, T.J.S.; Wang, R.; Wu, L. Cystathionine gamma-lyase/hydrogen sulfide system is essential for adipogenesis and fat mass accumulation in mice. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Park, J.H.; Kim, K.D.; Nam, D.; Shim, B.S.; Kim, S.H.; Ahn, K.S.; Choi, S.H.; Ahn, K.S. Brassinin induces apoptosis in PC-3 human prostate cancer cells through the suppression of PI3K/Akt/mTOR/S6K1 signaling cascades. Phytother. Res. 2014, 28, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Izutani, Y.; Yogosawa, S.; Sowa, Y.; Sakai, T. Brassinin induces G1 phase arrest through increase of p21 and p27 by inhibition of the phosphatidylinositol 3-kinase signaling pathway in human colon cancer cells. Int. J. Oncol. 2012, 40, 816–824. [Google Scholar]
- Wen, S.Y.; Gao, R.R.; Chen, Y.Y.; Wang, Y.J.; Wang, X.T.; Liu, H.X. Brassinin from Brassica campestris L. inhibits colorectal cancer by inducing p62/NRF2/GPX4-regulated ferroptosis. Animal Model. Exp. Med. 2025, 8, 1155–1165. [Google Scholar] [CrossRef]
- Sellam, A.; Iacomi-Vasilescu, B.; Hudhomme, P.; Simoneau, P. In vitro antifungal activity of brassinin, camalexin and two isothiocyanates against the crucifer pathogens Alternaria brassicicola and Alternaria brassicae. Plant Pathol. 2007, 56, 296–301. [Google Scholar] [CrossRef]
- Kang, B.; Kim, C.Y.; Hwang, J.; Suh, H.J.; Choi, H.S. Brassinin, a phytoalexin in cruciferous vegetables, suppresses obesity-induced inflammatory responses through the Nrf2-HO-1 signaling pathway in an adipocyte-macrophage co-culture system. Phytother. Res. 2019, 33, 1426–1437. [Google Scholar] [CrossRef]
- Yang, M.H.; Jung, Y.Y.; Um, J.Y.; Sethi, G.; Ahn, K.S. Brassinin alleviates cancer cachexia by suppressing diverse inflammatory mechanisms in mice. MedComm 2024, 5, e558. [Google Scholar] [CrossRef]
- Dunbar, K.L.; Scharf, D.H.; Litomska, A.; Hertweck, C. Enzymatic carbon-sulfur bond formation in natural product biosynthesis. Chem. Rev. 2017, 117, 5521–5577. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yang, X.; Lu, Y.; Liang, D.; Huang, D. Isothiocyanates as H2S donors triggered by cysteine: Reaction mechanism and structure and activity relationship. Org. Lett. 2019, 21, 5977–5980. [Google Scholar] [CrossRef]
- Parfenova, H.; Liu, J.; Hoover, D.T.; Fedinec, A.L. Vasodilator effects of sulforaphane in cerebral circulation: A critical role of endogenously produced hydrogen sulfide and arteriolar smooth muscle KATP and BK channels in the brain. J. Cereb. Blood Flow. Metab. 2020, 40, 1987–1996. [Google Scholar] [CrossRef]
- Shuang, T.; Fu, M.; Yang, G.; Huang, Y.; Qian, Z.; Wu, L.; Wang, R. Interaction among estrogen, IGF-1, and H2S on smooth muscle cell proliferation. J. Endocrinol. 2021, 248, 17–30. [Google Scholar] [CrossRef]
- Zhou, Y.; Mazur, F.; Liang, K.; Chandrawati, R. Sensitivity and selectivity analysis of fluorescent probes for hydrogen sulfide detection. Chem. Asian J. 2022, 17, e202101399. [Google Scholar] [CrossRef] [PubMed]
- Harvey, F.; Aromokunola, B.; Montaut, S.; Yang, G. The antioxidant properties of glucosinolates in cardiac cells are independent of H2S signaling. Int. J. Mol. Sci. 2024, 25, 696. [Google Scholar] [CrossRef] [PubMed]
| Primers | Sequences |
|---|---|
| CSE | Forward: 5′-ATCAGCACCCAGAGCCAAGG-3′ Reverse: 5′-AGCGATCACACCACAGACCAAG-3′ |
| TNFα | Forward: 5′-CCCACACCGTCAGCCGATTT-3′ Reverse: 5′-GTCTAAGTACTTGGGCAGATT-3′ |
| IL2 | Forward: 5′-CGCAGAGGTCCAAGTTCATC-3′ Reverse: 5′-AACTCCCCAGGATGCTCAC-3′ |
| IL4 | Forward: 5′-CGAGCTCACTCTCTGTGGTG-3′ Reverse: 5′-TGAACGAGGTCACAGGAGAA-3′ |
| IL6 | Forward: 5′-ACCAGAGGAAATTTTCAATAGGC-3′ Reverse: 5′-TGGGAGTGGTATCCTCTGTGA-3′ |
| ACE | Forward: 5′-ACCCCACCCTCTCGCTACAACTTC-3′ Reverse: 5′-GACTTCGCCATTCCGCTGATT-3′ |
| ACE2 | Forward: 5′- TGGCGCACGGGGAAAGA-3′ Reverse: 5′- GCTGAAGGGCCTGTAGTTGACG-3′ |
| AT1R | Forward: 5′-CCCCGAGGCAAGCAGAATAGGTG-3′ Reverse: 5′-GCCCGTGAGAGCAGCTGGTTGAAG-3′ |
| AT2R | Forward: 5′-AGTCCGCCTTTAATTGCTCACAC-3′ Reverse: 5′-CCAAACAAGGGGAACTACATAAGA-3′ |
| GAPDH | Forward: 5′-TCTCCTGCGACTTCAACAGC-3′ Reverse: 5′-GGTGCACGAACTTTATTGATGGT-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fergani, J.; Han, X.; Jin, Z.; Pei, Y.; Montaut, S.; Yang, G. Brassinin Induces H2S Signals and Improves Vascular Smooth Muscle Cell Functions. Molecules 2025, 30, 3775. https://doi.org/10.3390/molecules30183775
Fergani J, Han X, Jin Z, Pei Y, Montaut S, Yang G. Brassinin Induces H2S Signals and Improves Vascular Smooth Muscle Cell Functions. Molecules. 2025; 30(18):3775. https://doi.org/10.3390/molecules30183775
Chicago/Turabian StyleFergani, Jazmin, Xiaoli Han, Zhuping Jin, Yanxi Pei, Sabine Montaut, and Guangdong Yang. 2025. "Brassinin Induces H2S Signals and Improves Vascular Smooth Muscle Cell Functions" Molecules 30, no. 18: 3775. https://doi.org/10.3390/molecules30183775
APA StyleFergani, J., Han, X., Jin, Z., Pei, Y., Montaut, S., & Yang, G. (2025). Brassinin Induces H2S Signals and Improves Vascular Smooth Muscle Cell Functions. Molecules, 30(18), 3775. https://doi.org/10.3390/molecules30183775










