Hans Paulsen: Contributions to the Investigations of Glycoprotein Biosynthesis
Abstract
1. Introduction

2. Glycosyltransferases
3. Measurements of Glycosyltransferase Activities
4. Biological Significance of Glycoproteins
5. Glycoprotein Biosynthesis
6. N-Glycans
7. Early Pathways of N-Glycosylation at ER Membranes
8. Transfer of N-Glycans from Dolichol to Protein
9. Golgi Reactions
10. Synthesis of N-Glycan Antennae by GlcNAc-Transferases
11. GnT I-MGAT1
12. GlcNAc-Transferases (MGAT2-6)
13. Modifications of N-Glycan Antennae
14. Inhibition of Glycosyltransferases
15. Mucin Glycoproteins
16. Role of Mucins
17. Glycosyltransferases That Assemble Mucin O-Glycans
18. Elongation of O-Glycans
19. Synthesis of Glycopeptides
20. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Heyns, K.; Paulsen, H. Oxidative transformation of carbohydrates. VIII. Catalytic oxidation of meso-inositol to scyllo-meso-inosose. Chem. Berichte Chem. Eur. 1953, 86, 833–840. [Google Scholar] [CrossRef]
- Heyns, K.; Paulsen, H.; Rudiger, G.; Weyer, J. Configuration and conformation selectivity in catalytic oxidation with oxygen on platinum catalysts. Fortschritte Der Chem. Forsch. 1969, 11, 285–374. [Google Scholar]
- Paulsen, H.; Todt, K. Monosaccharides with a nitrogen-containing ring. XV. Nuclear magnetic resonance spectroscopic studies of hindered rotation of monosaccharides with a nitrogen-containing ring. Chem. Berichte Chem. Eur. 1967, 100, 3397–3404. [Google Scholar] [CrossRef]
- Paulsen, H.; Hayauchi, Y.; Sinnwell, V. Monosaccharides containing nitrogen in the ring. XXXVII. Synthesis of 1,5-dideoxy-1,5-imino-D-galactitol. Chem. Berichte Chem. Eur. 1980, 113, 2601–2608. [Google Scholar] [CrossRef]
- Paulsen, H.; Brockhausen, I. From imino sugars to cancer glycoproteins. Glycoconj. J. 2001, 18, 867–870. [Google Scholar] [CrossRef] [PubMed]
- Luger, P.; Vangehr, K.; Bock, K.; Paulsen, H. Conformational analysis. Part XXIII. Determination of the conformation of 2-acetamido-1,4,6-tri-O-acetyl-2-deoxy-3-O-(2-acetamido-3,4,6-tri-O-acetyl-2-deoxy-α-D-galactopyranosyl)-2-D-galactopyranose by x-ray structural analysis, NOE measurements and HSEA calculations. Carbohydr. Res. 1983, 117, 23–38. [Google Scholar] [CrossRef]
- Paulsen, H.; Behre, H.; Herold, C.-P. Acyloxonium ion rearrangement in carbohydrate chemistry. Fortschritte Der Chem. Forsch. 1970, 14, 472–525. [Google Scholar]
- Paulsen, H. Cyclic acyloxonium ions in carbohydrate chemistry. Adv. Carbohydr. Chem. 1971, 26, 127–195. [Google Scholar] [CrossRef]
- Thiem, J.; Rasch, D.; Paulsen, H. Phosphorus-containing carbohydrates, XV. Perkow reaction with α-acyloxy keto sugars for the synthesis of enol phosphates and their reactions. Chem. Berichte Chem. Eur. 1976, 109, 3588–3597. [Google Scholar] [CrossRef]
- Schuster, O.; Klich, G.; Sinnwell, V.; Kränz, H.; Paulsen, H.; Meyer, B. “Wave-type” structure of a synthetic hexaglycosylated decapeptide: A part of the extracellular domain of human glycophorin A. J. Biomol. NMR 1999, 14, 33–45. [Google Scholar] [CrossRef]
- Depmeier, W.; Jarchov, O.H.; Stadler, P.; Sinnwell, V.; Paulsen, H. Conformation of L-streptose reducing sugars as crystals and in solution. Carbohydr. Res. 1974, 34, 214–218. [Google Scholar] [CrossRef]
- Strumpel, M.; Schmidt, H.-J.; Luger, P.; Paulsen, H. X-ray analysis of 3-O-(6-O-acetyl-2,4-diazido-3-O-benzyl-2,4-dideoxy-α-d-glucopyranosyl)-1,6-anhydro-2,4-diazido-2,4-dideoxy-β-d-glucopyranose, a disaccharide having an unusual α-d-(1→3) linkage. Carbohydr. Res. 1984, 125, 185–201. [Google Scholar] [CrossRef]
- Paulsen, H.; Reck, F.; Brockhausen, I. Synthese von modifizierten Oligosacchariden der N-Glycoproteine als Substrate für N-Acetylglucosaminyltransferase I [Synthesis of modified oligosaccharides of the N-glycoprotein as substrate for N-acetylglucosaminyltransferase I]. Carbohydr. Res. 1992, 236, 39–71. [Google Scholar] [CrossRef] [PubMed]
- Brockhausen, I.; Möller, G.; Yang, J.M.; Khan, S.H.; Matta, K.L.; Paulsen, H.; Grey, A.A.; Shah, R.N.; Schachter, H. Control of glycoprotein synthesis. Characterization of (1→4)-N-acetyl-beta-D-glucosaminyltransferases acting on the alpha-D-(1→3)- and alpha-D-(1→6)-linked arms of N-linked oligosaccharides. Carbohydr. Res. 1992, 236, 281–299. [Google Scholar] [CrossRef]
- Bai, L.; Li, H. Protein N-glycosylation and O-mannosylation are catalyzed by two evolutionarily related GT-C glycosyltransferases. Curr. Opin. Struct. Biol. 2021, 68, 66–73. [Google Scholar] [CrossRef]
- Breton, C.; Fournel-Gigleux, S.; Palcic, M.M. Recent structures, evolution and mechanisms of glycosyltransferases. Curr. Opin. Struct. Biol. 2012, 22, 540–549. [Google Scholar] [CrossRef]
- Möller, G.; Reck, F.; Paulsen, H.; Kaur, K.J.; Sarkar, M.; Schachter, H.; Brockhausen, I. Control of glycoprotein synthesis: Substrate specificity of rat liver UDP-GlcNAc:Man alpha 3R beta 2-N-acetylglucosaminyltransferase I using synthetic substrate analogues. Glycoconj. J. 1992, 9, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Brockhausen, I.; Benn, M.; Bhat, S.; Marone, S.; Riley, J.G.; Montoya-Peleaz, P.; Vlahakis, J.Z.; Paulsen, H.; Schutzbach, J.S.; Szarek, W.A. UDP-Gal: GlcNAc-R beta1,4-galactosyltransferase--a target enzyme for drug design. Acceptor specificity and inhibition of the enzyme. Glycoconj. J. 2006, 23, 525–541. [Google Scholar] [CrossRef]
- Ramasamy, V.; Ramakrishnan, B.; Boeggeman, E.; Ratner, D.M.; Seeberger, P.H.; Qasba, P.K. Oligosaccharide preferences of beta1,4-galactosyltransferase-I: Crystal structures of Met340His mutant of human beta1,4-galactosyltransferase-I with a pentasaccharide and trisaccharides of the N-glycan moiety. J. Mol. Biol. 2005, 353, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Dohi, T.; Yuyama, Y.; Natori, Y.; Smith, P.L.; Lowe, J.B.; Oshima, M. Detection of N-acetylgalactosaminyltransferase mRNA which determines expression of Sda blood group carbohydrate structure in human gastrointestinal mucosa and cancer. Int. J. Cancer 1996, 67, 626–631. [Google Scholar] [CrossRef]
- Cull, J.; Pink, R.C.; Samuel, P.; Brooks, S.A. Myriad mechanisms: Factors regulating the synthesis of aberrant mucin-type O-glycosylation found on cancer cells. Glycobiology 2025, 35, cwaf023. [Google Scholar] [CrossRef] [PubMed]
- Munkley, J. Aberrant Sialylation in Cancer: Therapeutic Opportunities. Cancers 2022, 14, 4248. [Google Scholar] [CrossRef]
- Brockhausen, I.; Argueso, P. Mucin-type O-glycans: Biosynthesis and functions. In Comprehensive Glycoscience, 2nd ed.; Barchi, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 233–252. [Google Scholar] [CrossRef]
- Venturi, G.; Gomes Ferreira, I.; Pucci, M.; Ferracin, M.; Malagolini, N.; Chiricolo, M.; Dall’Olio, F. Impact of sialyltransferase ST6GAL1 overexpression on different colon cancer cell types. Glycobiology 2019, 29, 684–695. [Google Scholar] [CrossRef]
- Lin, W.R.; Yeh, C.T. GALNT14: An Emerging Marker Capable of Predicting Therapeutic Outcomes in Multiple Cancers. Int. J. Mol. Sci. 2020, 21, 1491. [Google Scholar] [CrossRef]
- Sun, H.; Chang, J.; Ye, M.; Weng, W.; Zhang, M.; Ni, S.; Tan, C.; Huang, D.; Wang, L.; Du, X.; et al. GCNT4 is Associated with Prognosis and Suppress Cell Proliferation in Gastric Cancer. Oncol. Targets Ther. 2020, 13, 8601–8613. [Google Scholar] [CrossRef]
- Burchell, J.; Poulsom, R.; Hanby, A.; Whitehouse, C.; Cooper, L.; Clausen, H.; Miles, D.; Taylor-Papadimitriou, J. An alpha2,3 sialyltransferase (ST3Gal I) is elevated in primary breast carcinomas. Glycobiology 1999, 9, 1307–1311. [Google Scholar] [CrossRef] [PubMed]
- Marcos, N.T.; Pinho, S.; Grandela, C.; Cruz, A.; Samyn-Petit, B.; Harduin-Lepers, A.; Almeida, R.; Silva, F.; Morais, V.; Costa, J.; et al. Role of the human ST6GalNAc-I and ST6GalNAc-II in the synthesis of the cancer-associated sialyl-Tn antigen. Cancer Res. 2004, 6, 7050–7057. [Google Scholar] [CrossRef]
- González-Vallinas, M.; Vargas, T.; Moreno-Rubio, J.; Molina, S.; Herranz, J.; Cejas, P.; Burgos, E.; Aguayo, C.; Custodio, A.; Reglero, G.; et al. Clinical relevance of the differential expression of the glycosyltransferase gene GCNT3 in colon cancer. Eur. J. Cancer 2015, 51, 1–8. [Google Scholar] [CrossRef]
- Kurosaka, A.; Nakajima, H.; Funakoshi, I.; Matsuyama, M.; Nagayo, T.; Yamashina, I. Structures of the major oligosaccharides from a human rectal adenocarcinoma glycoprotein. J. Biol. Chem. 1983, 258, 11594–11598. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Chachadi, V.B.; Cheng, P.W.; Brockhausen, I. Glycosylation potential of human prostate cancer cell lines. Glycoconj. J. 2012, 29, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Schachter, H. Complex N-glycans: The story of the “yellow brick road”. Glycoconj. J. 2014, 31, 1–5. [Google Scholar] [CrossRef]
- Unligil, U.M.; Zhou, S.; Yuwaraj, S.; Sarkar, M.; Schachter, H.; Rini, J.M. X-ray crystal structure of rabbit N-acetylglucosaminyltransferase I: Catalytic mechanism and a new protein superfamily. EMBO J. 2000, 19, 5269–5280. [Google Scholar] [CrossRef]
- Sharma, C.B.; Lehle, L.; Tanner, W. N-Glycosylation of yeast proteins. Characterization of the solubilized oligosaccharyl transferase. Eur. J. Biochem. 1981, 116, 101–108. [Google Scholar] [CrossRef]
- Yoo, J.; Mashalidis, E.H.; Kuk, A.C.Y.; Yamamoto, K.; Kaeser, B.; Ichikawa, S.; Lee, S.Y. GlcNAc-1-P-transferase-tunicamycin complex structure reveals basis for inhibition of N-glycosylation. Nature Struct. Mol. Biol. 2018, 25, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Timal, S.; Hoischen, A.; Lehle, L.; Adamowicz, M.; Huijben, K.; Sykut-Cegielska, J.; Paprocka, J.; Jamroz, E.; van Spronsen, F.J.; Körner, C.; et al. Gene identification in the congenital disorders of glycosylation type I by whole-exome sequencing. Human Mol. Gen. 2012, 21, 4151–4161. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.W.; Schutzbach, J.S. Activation of dolichyl-phospho-mannose synthase by phospholipids. Eur. J. Biochem. 1985, 153, 41–48. [Google Scholar] [CrossRef]
- Reznik, N.; Fass, D. Disulfide bond formation and redox regulation in the Golgi apparatus. FEBS Lett. 2022, 596, 2859–2872. [Google Scholar] [CrossRef]
- Stanley, P.; Narasimhan, S.; Siminovitch, L.; Schachter, H. Chinese hamster ovary cells selected for resistance to the cytotoxicity of phytohemagglutinin are deficient in a UDP-N-acetylglucosamine--glycoprotein N-acetylglucosaminyltransferase activity. Proc. Nat. Acad. Sci. USA 1975, 72, 3323–3327. [Google Scholar] [CrossRef]
- Paulsen, H.; Heume, M.; Nürnberger, H. Synthese der verzweigten Nonasaccharid-Sequenz der “bisected” Struktur von N-Glycoproteinen [Synthesis of a highly branched nonasaccharide sequence of the “bisected”structure of N-glycoproteins]. Carbohydr. Res. 1990, 200, 127–166. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, H.; Peters, T.; Sinnwell, V.; Meyer, B. Konformationsanalyse modifizierter Tetrasaccharidsequenzen vom Typ der N-Glycoproteine--zum Problem der alpha-(1----6)-glycosidischen Bindung [Conformation analysis of modified tetrasaccharide sequences of the N-glycoprotein type--problem of the alpha-(1 to 6)-glycosidic bond]. Carbohydr. Res. 1987, 165, 251–266. [Google Scholar] [CrossRef]
- Reck, F.; Springer, M.; Meinjohanns, E.; Paulsen, H.; Brockhausen, I.; Schachter, H. Synthetic substrate analogues for UDP-GlcNAc: Man alpha 1-3R beta 1-2-N-acetylglucosaminyltransferase I. Substrate specificity and inhibitors for the enzyme. Glycoconj. J. 1995, 12, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Reck, F.; Springer, M.; Paulsen, H.; Brockhausen, I.; Sarkar, M.; Schachter, H. Synthesis of tetrasaccharide analogues of the N-glycan substrate of beta-(1→2)-N-acetylglucosaminyltransferase II using trisaccharide precursors and recombinant beta-(1→2)-N-acetylglucosaminyltransferase I. Carbohydr. Res. 1994, 259, 93–101. [Google Scholar] [CrossRef]
- Reck, F.; Meinjohanns, E.; Springer, M.; Wilkens, R.; Van Dorst, J.A.; Paulsen, H.; Möller, G.; Brockhausen, I.; Schachter, H. Synthetic substrate analogues for UDP-GlcNAc: Man alpha 1-6R beta(1-2)-N-acetylglucosaminyltransferase II. Substrate specificity and inhibitors for the enzyme. Glycoconj. J. 1994, 11, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, H.; Springer, M.; Reck, F.; Brockhausen, I.; Schachter, H. Synthese von modifizierten Tetrasacchariden als analoge Akzeptor-Inhibitoren der N-Acetylglucosaminyltransferase II [Synthesis of modified tetrasaccharides as analog acceptor-inhibitors of N-acetylglucosaminyltransferase II]. Carbohydr. Res. 1995, 275, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Kadirvelraj, R.; Yang, J.Y.; Sanders, J.H.; Liu, L.; Ramiah, A.; Prabhakar, P.K.; Boons, G.J.; Wood, Z.A.; Moremen, K.W. Human N-acetylglucosaminyltransferase II substrate recognition uses a modular architecture that includes a convergent exosite. Proc. Nat. Acad. Sci. USA 2018, 115, 4637–4642. [Google Scholar] [CrossRef]
- de-Souza-Ferreira, M.; Ferreira, É.E.; de-Freitas-Junior, J.C.M. Aberrant N-glycosylation in cancer: MGAT5 and β1,6-GlcNAc branched N-glycans as critical regulators of tumor development and progression. Cell. Oncol. 2023, 46, 481–501. [Google Scholar] [CrossRef]
- Brockhausen, I.; Reck, F.; Kuhns, W.; Khan, S.; Matta, K.L.; Meinjohanns, E.; Paulsen, H.; Shah, R.N.; Baker, M.A.; Schachter, H. Substrate specificity and inhibition of UDP-GlcNAc:GlcNAc beta 1-2Man alpha 1-6R beta 1,6-N-acetylglucosaminyltransferase V using synthetic substrate analogues. Glycoconj. J. 1995, 12, 371–379. [Google Scholar] [CrossRef]
- Khan, S.H.; Crawley, S.C.; Kanie, O.; Hindsgaul, O. A trisaccharide acceptor analog for N-acetylglucosaminyltransferase V which binds to the enzyme but sterically precludes the transfer reaction. J. Biol. Chem. 1993, 268, 2468–2473. [Google Scholar] [CrossRef] [PubMed]
- Kanie, O.; Crawley, S.C.; Palcic, M.M.; Hindsgaul, O. Acceptor-substrate recognition by N-acetylglucosaminyltransferase-V: Critical role of the 4”-hydroxyl group in beta-D-GlcpNAc-(1→2)-alpha-D-Manp(1→6)-beta-D-Glcp-OR. Carbohydr. Res. 1993, 243, 139–164. [Google Scholar] [CrossRef]
- Kanie, O.; Crawley, S.C.; Palcic, M.M.; Hindsgaul, O. Key involvement of all three GlcNAc hydroxyl groups in the recognition of beta-D-GlcpNAc-(1→2)-alpha-D-Manp-(1→6)-beta-D-Glcp-OR by N-acetylglucosaminyltransferase-V. Bioorg. Med. Chem. 1994, 2, 1231–1241. [Google Scholar] [CrossRef]
- Nagae, M.; Kizuka, Y.; Mihara, E.; Kitago, Y.; Hanashima, S.; Ito, Y.; Takagi, J.; Taniguchi, N.; Yamaguchi, Y. Structure and mechanism of cancer-associated N-acetylglucosaminyltransferase-V. Nat. Commun. 2018, 9, 3380. [Google Scholar] [CrossRef]
- Osuka, R.F.; Nagae, M.; Ohuchi, A.; Ohno, S.; Yamaguchi, Y.; Kizuka, Y. The cancer-associated glycosyltransferase GnT-V (MGAT5) recognizes the N-glycan core via residues outside its catalytic pocket. FEBS Lett. 2023, 597, 3102–3113. [Google Scholar] [CrossRef]
- Osuka, R.F.; Yamasaki, T.; Kizuka, Y. Structure and function of N-acetylglucosaminyltransferase V (GnT-V). Biochim. Biophys. Acta Gen. Subj. 2024, 1868, 130709. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, H.; Peters, T.; Sinnwell, V.; Heume, M.; Meyer, B. Konformationsanalyse der verzweigten Pentasaccharid-sequenz der “bisected” Struktur von N-Glycoproteinen [Conformational analysis of the double pentasaccharide sequence of the “bisected” structure ov N-glycoproteins]. Carbohydr. Res. 1986, 156, 87–106. [Google Scholar] [CrossRef]
- Brockhausen, I.; Hull, E.; Hindsgaul, O.; Schachter, H.; Shah, R.N.; Michnick, S.W.; Carver, J.P. Control of glycoprotein synthesis. Detection and characterization of a novel branching enzyme from hen oviduct, UDP-N-acetylglucosamine:GlcNAc beta 1-6 (GlcNAc beta 1-2)Man alpha-R (GlcNAc to Man) beta-4-N-acetylglucosaminyltransferase VI. J. Biol. Chem. 1989, 264, 11211–11221. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, Y.; Taguchi, T.; Honke, K.; Korekane, H.; Watanabe, H.; Tano, Y.; Dohmae, N.; Takio, K.; Horii, A.; Taniguchi, N. Molecular cloning and expression of cDNA encoding chicken UDP-N-acetyl-D-glucosamine (GlcNAc): GlcNAcbeta 1-6(GlcNAcbeta 1-2)- manalpha 1-R[GlcNAc to man]beta 1,4N-acetylglucosaminyltransferase VI. J. Biol. Chem. 2000, 275, 36029–36034. [Google Scholar] [CrossRef]
- Dalle Vedove, E.; Costabile, G.; Merkel, O.M. Mannose and Mannose-6-Phosphate Receptor-Targeted Drug Delivery Systems and Their Application in Cancer Therapy. Adv. Healthc. Mater. 2018, 7, e1701398. [Google Scholar] [CrossRef]
- Brockhausen, I.; Lehotay, M.; Yang, J.M.; Qin, W.; Young, D.; Lucien, J.; Coles, J.; Paulsen, H. Glycoprotein biosynthesis in porcine aortic endothelial cells and changes in the apoptotic cell population. Glycobiology 2002, 12, 33–45. [Google Scholar] [CrossRef]
- Kuhn, B.; Benz, J.; Greif, M.; Engel, A.M.; Sobek, H.; Rudolph, M.G. The structure of human α-2,6-sialyltransferase reveals the binding mode of complex glycans. Acta Crystal. Sect. D Biol. Crystallogr. 2013, 69 Pt 9, 1826–1838. [Google Scholar] [CrossRef] [PubMed]
- Qasba, P.K.; Ramakrishnan, B.; Boeggeman, E. Structure and function of beta -1,4-galactosyltransferase. Curr. Drug Targets 2008, 9, 292–309. [Google Scholar] [CrossRef]
- Inaba, R.; Vujakovic, S.; Bergstrom, K. The gut mucus network: A dynamic liaison between microbes and the immune system. Sem. Immunol. 2023, 69, 101807. [Google Scholar] [CrossRef] [PubMed]
- Luis, A.S.; Hansson, G.C. Intestinal mucus and their glycans: A habitat for thriving microbiota. Cell Host Microbe 2023, 31, 1087–1100. [Google Scholar] [CrossRef]
- Wagner, K.W.; Punnoose, E.A.; Januario, T.; Lawrence, D.A.; Pitti, R.M.; Lancaster, K.; Lee, D.; von Goetz, M.; Yee, S.F.; Totpal, K.; et al. Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nature Med. 2007, 13, 1070–1077. [Google Scholar] [CrossRef]
- Hounsell, E.F.; Lawson, A.M.; Feeney, J.; Gooi, H.C.; Pickering, N.J.; Stoll, M.S.; Lui, S.C.; Feizi, T. Structural analysis of the O-glycosidically linked core-region oligosaccharides of human meconium glycoproteins which express oncofoetal antigens. Eur. J. Biochem. 1985, 148, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Pollex-Krüger, A.; Meyer, B.; Stuike-Prill, R.; Sinnwell, V.; Matta, K.L.; Brockhausen, I. Preferred conformations and dynamics of five core structures of mucin type O-glycans determined by NMR spectroscopy and force field calculations. Glycoconj. J. 1993, 10, 365–380. [Google Scholar] [CrossRef]
- Müller, S.; Alving, K.; Peter-Katalinic, J.; Zachara, N.; Gooley, A.A.; Hanisch, F.G. High density O-glycosylation on tandem repeat peptide from secretory MUC1 of T47D breast cancer cells. J. Biol. Chem. 1999, 274, 18165–18172. [Google Scholar] [CrossRef] [PubMed]
- Bennett, E.P.; Hassan, H.; Hollingsworth, M.A.; Clausen, H. A novel human UDP-N-acetyl-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase, GalNAc-T7, with specificity for partial GalNAc-glycosylated acceptor substrates. FEBS Lett. 1999, 460, 226–230. [Google Scholar] [CrossRef]
- Kato, K.; Hansen, L.; Clausen, H. Polypeptide N-acetylgalactosaminyltransferase-Associated Phenotypes in Mammals. Molecules 2021, 26, 5504. [Google Scholar] [CrossRef]
- Ju, T.; Cummings, R.D. Protein glycosylation: Chaperone mutation in Tn syndrome. Nature 2005, 437, 1252. [Google Scholar] [CrossRef]
- Patsos, G.; Hebbe-Viton, V.; Robbe-Masselot, C. O-glycan inhibitors generate aryl-glycans, induce apoptosis and lead to growth inhibition in colorectal cancer cell lines. Glycobiology 2009, 19, 382–398. [Google Scholar] [CrossRef] [PubMed]
- Brockhausen, I.; Möller, G.; Pollex-Krüger, A.; Rutz, V.; Paulsen, H.; Matta, K.L. Control of O-glycan synthesis: Specificity and inhibition of O-glycan core 1 UDP-galactose:N-acetylgalactosamine-alpha-R beta 3-galactosyltransferase from rat liver. Biochem. Cell Biol. 1992, 70, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.C.; Ong, E.; Fukuda, M. Molecular cloning and expression of a novel beta-1,6-N-acetylglucosaminyltransferase that forms core 2, core 4, and I branches. J. Biol. Chem. 1999, 274, 3215–3221. [Google Scholar] [CrossRef] [PubMed]
- Kuhns, W.; Rutz, V.; Paulsen, H.; Matta, K.L.; Baker, M.A.; Barner, M.; Granovsky, M.; Brockhausen, I. Processing O-glycan core 1, Gal beta 1-3GalNAc alpha-R. Specificities of core 2, UDP-GlcNAc: Gal beta 1-3 GalNAc-R(GlcNAc to GalNAc) beta 6-N-acetylglucosaminyltransferase and CMP-sialic acid: Gal beta 1-3GalNAc-R alpha 3-sialyltransferase. Glycoconj. J. 1993, 10, 381–394. [Google Scholar] [CrossRef]
- Mardahl, M.; Schröter, M.F.; Engelbert, D.; Pink, M.; Sperandio, M.; Hamann, A.; Syrbe, U. Core 2 ß1,6-N-acetylglucosaminyltransferase-I, crucial for P-selectin ligand expression is controlled by a distal enhancer regulated by STAT4 and T-bet in CD4+ T helper cells 1. Mol. Immun. 2016, 77, 132–140. [Google Scholar] [CrossRef]
- Schwientek, T.; Yeh, J.C.; Levery, S.B.; Keck, B.; Merkx, G.; van Kessel, A.G.; Fukuda, M.; Clausen, H. Control of O-glycan branch formation. Molecular cloning and characterization of a novel thymus-associated core 2 beta1, 6-n-acetylglucosaminyltransferase. J. Biol. Chem. 2000, 275, 11106–11113. [Google Scholar] [CrossRef]
- Vavasseur, F.; Yang, J.M.; Dole, K.; Paulsen, H.; Brockhausen, I. Synthesis of O-glycan core 3: Characterization of UDP-GlcNAc: GalNAc-R beta 3-N-acetyl-glucosaminyltransferase activity from colonic mucosal tissues and lack of the activity in human cancer cell lines. Glycobiology 1995, 5, 351–357. [Google Scholar] [CrossRef]
- Huang, M.C.; Chen, H.Y.; Huang, H.C.; Huang, J.; Liang, J.-T.; Shen, T.-L.; Lin, N.-Y.; Ho, C.-C.; Hsu, S.-M. C2GnT-M is downregulated in colorectal cancer and its re-expression causes growth inhibition of colon cancer cells. Oncogene 2006, 25, 3267–3276. [Google Scholar] [CrossRef]
- Pak, J.E.; Arnoux, P.; Zhou, S.; Sivarajah, P.; Satkunarajah, M.; Xing, X.; Rini, J.M. X-ray crystal structure of leukocyte type core 2 beta1,6-N-acetylglucosaminyltransferase. Evidence for a convergence of metal ion-independent glycosyltransferase mechanism. J. Biol. Chem. 2006, 281, 26693–26701. [Google Scholar] [CrossRef]
- Pak, J.E.; Satkunarajah, M.; Seetharaman, J.; Rini, J.M. Structural and mechanistic characterization of leukocyte-type core 2 β1,6-N-acetylglucosaminyltransferase: A metal-ion-independent GT-A glycosyltransferase. J. Mol. Biol. 2011, 414, 798–811. [Google Scholar] [CrossRef]
- Kurosaka, A.; Funakoshi, I.; Matsuyama, M.; Nagayo, T.; Yamashina, I. UDP-GalNAc:GalNAc-mucin alpha-N-acetylgalactosamine transferase activity in human intestinal cancerous tissues. FEBS Lett. 1985, 190, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.C.; Hiraoka, N.; Petryniak, B.; Nakayama, J.; Ellies, L.G.; Rabuka, D.; Hindsgaul, O.; Marth, J.D.; Lowe, J.B.; Fukuda, M. Novel sulfated lymphocyte homing receptors and their control by a Core1 extension beta 1,3-N-acetylglucosaminyltransferase. Cell 2001, 105, 957–969. [Google Scholar] [CrossRef]
- Nakayama, J.; Yeh, J.C.; Misra, A.K.; Ito, S.; Katsuyama, T.; Fukuda, M. Expression cloning of a human alpha1, 4-N-acetylglucosaminyltransferase that forms GlcNAcalpha1→4Galbeta→R, a glycan specifically expressed in the gastric gland mucous cell-type mucin. Proc. Nat. Acad. Sci. USA 1999, 96, 8991–8996. [Google Scholar] [CrossRef] [PubMed]
- Yuki, A.; Fujii, C.; Yamanoi, K.; Matoba, H.; Harumiya, S.; Kawakubo, M.; Nakayama, J. Glycosylation of MUC6 by α1,4-linked N-acetylglucosamine enhances suppression of pancreatic cancer malignancy. Cancer Sci. 2022, 113, 576–586. [Google Scholar] [CrossRef]
- Datta, A.K. Comparative sequence analysis in the sialyltransferase protein family: Analysis of motifs. Curr. Drug Targets 2009, 10, 483–498. [Google Scholar] [CrossRef] [PubMed]
- Moremen, K.W.; Ramiah, A.; Stuart, M.; Steel, J.; Meng, L.; Forouhar, F.; Moniz, H.A.; Gahlay, G.; Gao, Z.; Chapla, D.; et al. Expression system for structural and functional studies of human glycosylation enzymes. Nature Chem. Biol. 2018, 14, 156–162. [Google Scholar] [CrossRef]
- Grewal, R.K.; Shaikh, A.R.; Gorle, S.; Kaur, M.; Videira, P.A.; Cavallo, L.; Chawla, M. Structural Insights in Mammalian Sialyltransferases and Fucosyltransferases: We Have Come a Long Way, but It Is Still a Long Way Down. Molecules 2021, 26, 5203. [Google Scholar] [CrossRef] [PubMed]
- Rao, F.V.; Rich, J.R.; Rakić, B.; Buddai, S.; Schwartz, M.F.; Johnson, K.; Bowe, C.; Wakarchuk, W.W.; Defrees, S.; Withers, S.G.; et al. Structural insight into mammalian sialyltransferases. Nature Struct. Mol. Biol. 2009, 16, 1186–1188. [Google Scholar] [CrossRef]
- Meldal, M.; Bielfeldt, T.; Peters, S.; Jensen, K.J.; Paulsen, H.; Bock, K. Susceptibility of glycans to beta-elimination in Fmoc-based O-glycopeptide synthesis. Int. J. Peptide Protein Res. 1994, 43, 529–536. [Google Scholar] [CrossRef]
- Paulsen, H.; Peters, S.; Bielfeldt, T.; Meldal, M.; Bock, K. Synthesis of the glycosyl amino acids N alpha-Fmoc-Ser[Ac4-beta-D-Galp-(1→3)-Ac2-alpha-D-GalN3p]-OPfp and N alpha-Fmoc-Thr[Ac4-beta-D-Galp-(1→3)-Ac2-alpha-D-GalN3p]-OPfp and the application in the solid-phase peptide synthesis of multiply glycosylated mucin peptides with Tn and T antigenic structures. Carbohydr. Res. 1995, 268, 17–34. [Google Scholar] [CrossRef]
- Peters, S.; Bielfeldt, T.; Meldal, M.; Bock, K.; Paulsen, H. Multiple-column solid-phase glycopeptide synthesis. J. Chem. Soc. Perkin Trans. 1992, 1, 1163–1171. [Google Scholar] [CrossRef]
- Brockhausen, I.; Möller, G.; Merz, G.; Adermann, K.; Paulsen, H. Control of mucin synthesis: The peptide portion of synthetic O-glycopeptide substrates influences the activity of O-glycan core 1 UDPgalactose:N-acetyl-alpha-galactosaminyl-R beta 3-galactosyltransferase. Biochemistry 1990, 29, 10206–10212. [Google Scholar] [CrossRef] [PubMed]
- Brockhausen, I.; Toki, D.; Brockhausen, J.; Peters, S.; Bielfeldt, T.; Kleen, A.; Paulsen, H.; Meldal, M.; Hagen, F.; Tabak, L.A. Specificity of O-glycosylation by bovine colostrum UDP-GalNAc: Polypeptide alpha-N-acetylgalactosaminyltransferase using synthetic glycopeptide substrates. Glycoconj. J. 1996, 13, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Granovsky, M.; Bielfeldt, T.; Peters, S.; Paulsen, H.; Meldal, M.; Brockhausen, J.; Brockhausen, I. UDPgalactose:glycoprotein-N-acetyl-D-galactosamine 3-beta-D-galactosyltransferase activity synthesizing O-glycan core 1 is controlled by the amino acid sequence and glycosylation of glycopeptide substrates. Eur. J. Biochem. 1994, 221, 1039–1046. [Google Scholar] [CrossRef]
- Brockhausen, I.; Dowler, T.; Paulsen, H. Site directed processing: Role of amino acid sequences and glycosylation of acceptor glycopeptides in the assembly of extended mucin type O-glycan core 2. Biochim. Biophys. Acta 2009, 1790, 1244–1257. [Google Scholar] [CrossRef] [PubMed]
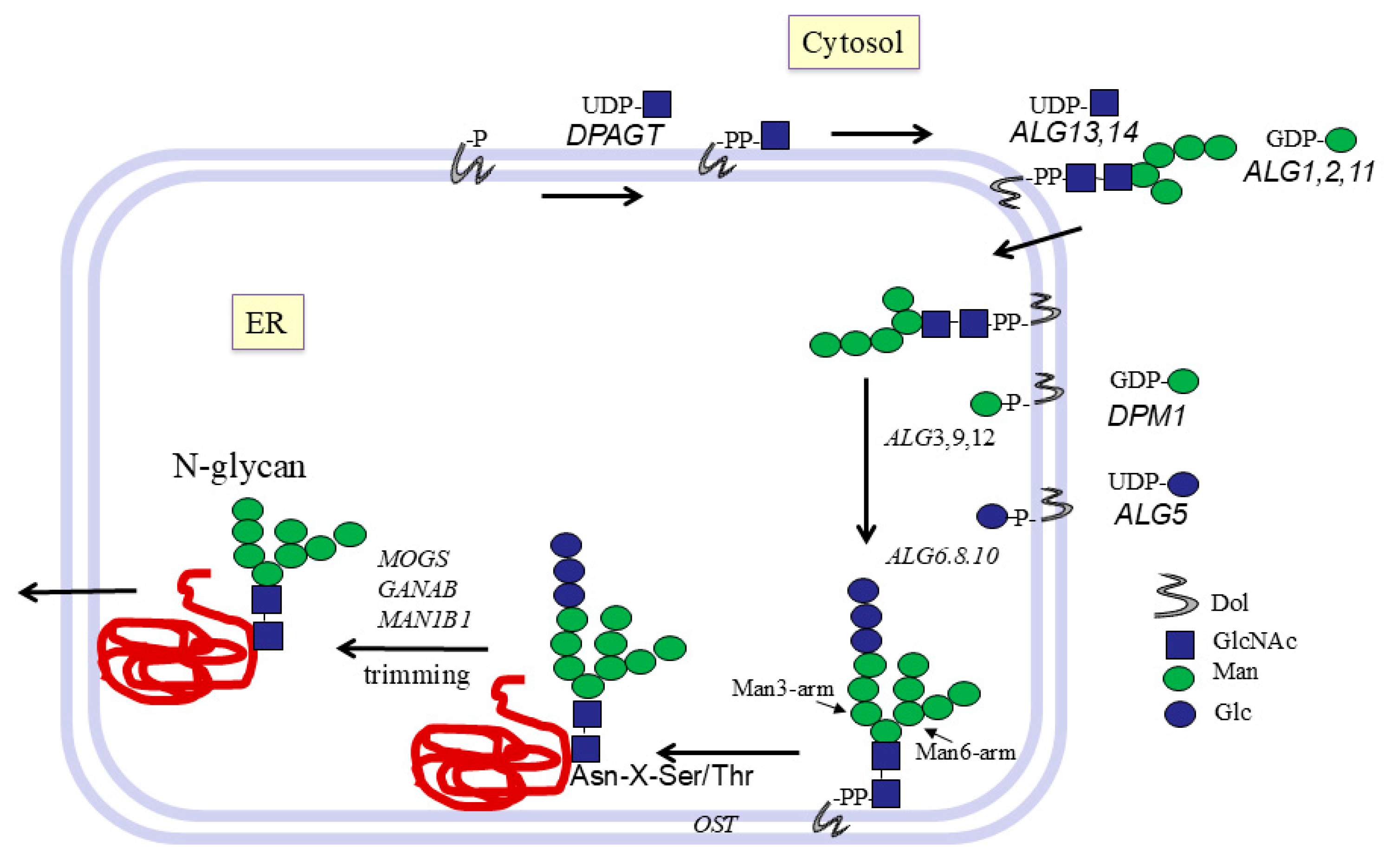
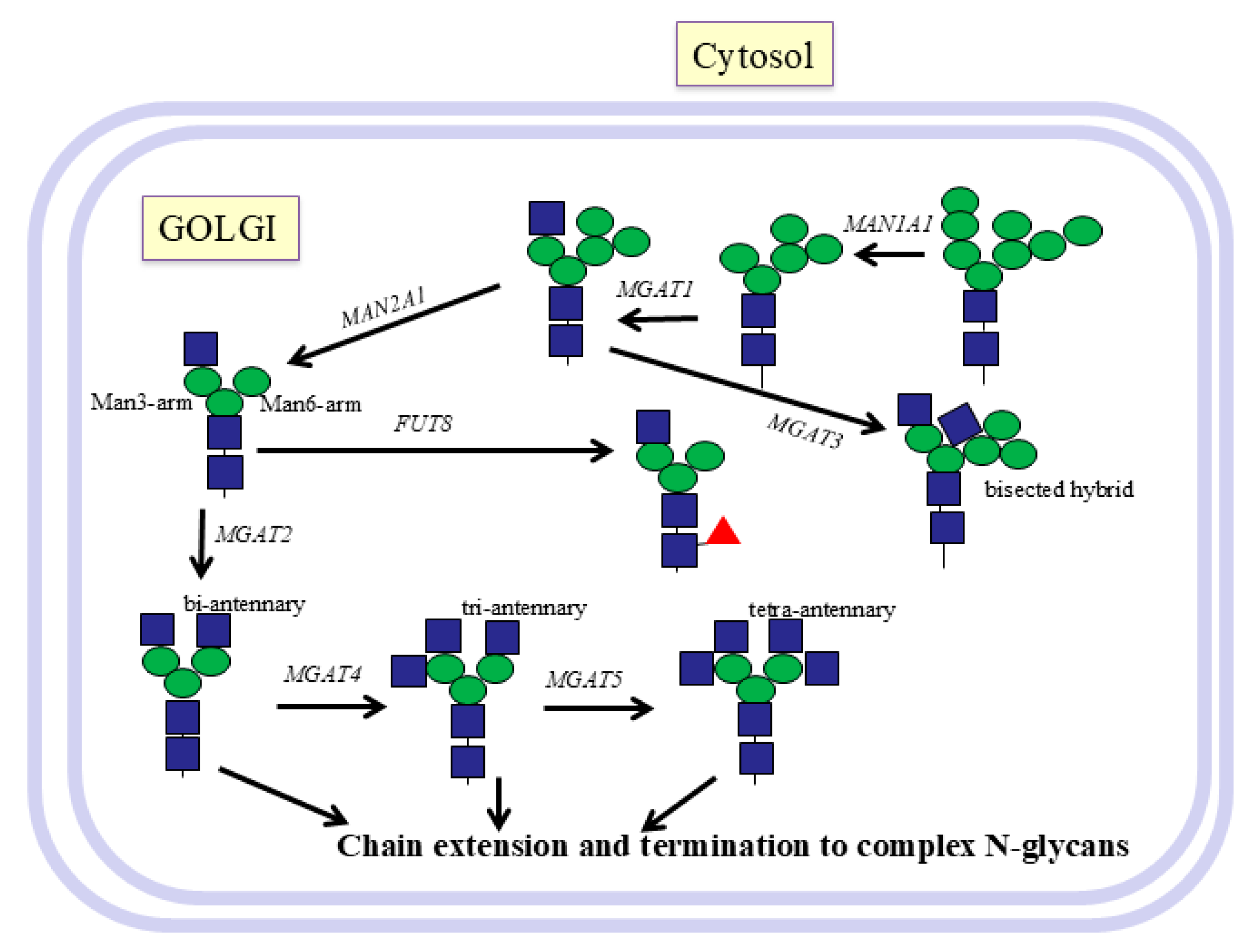
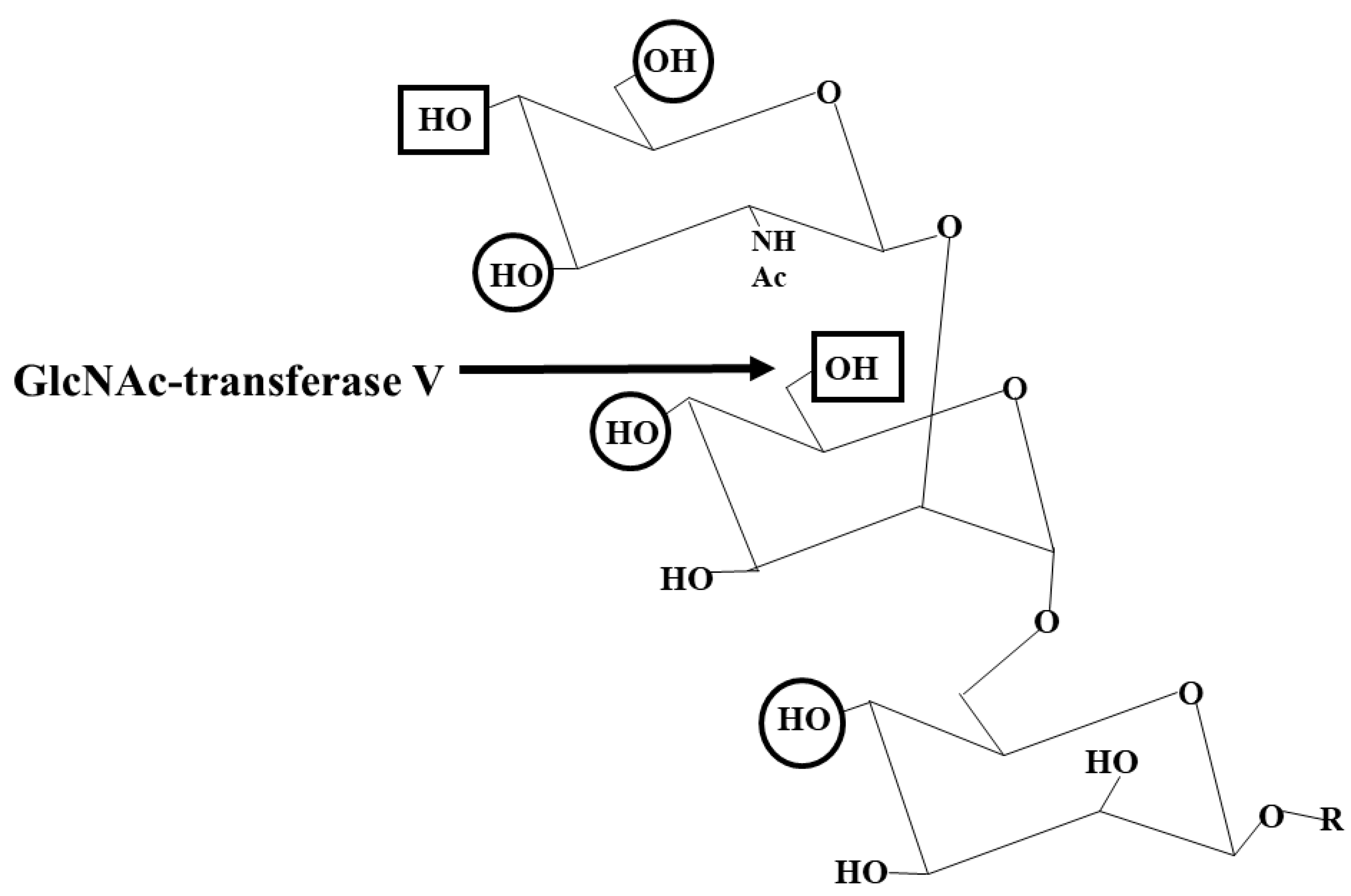
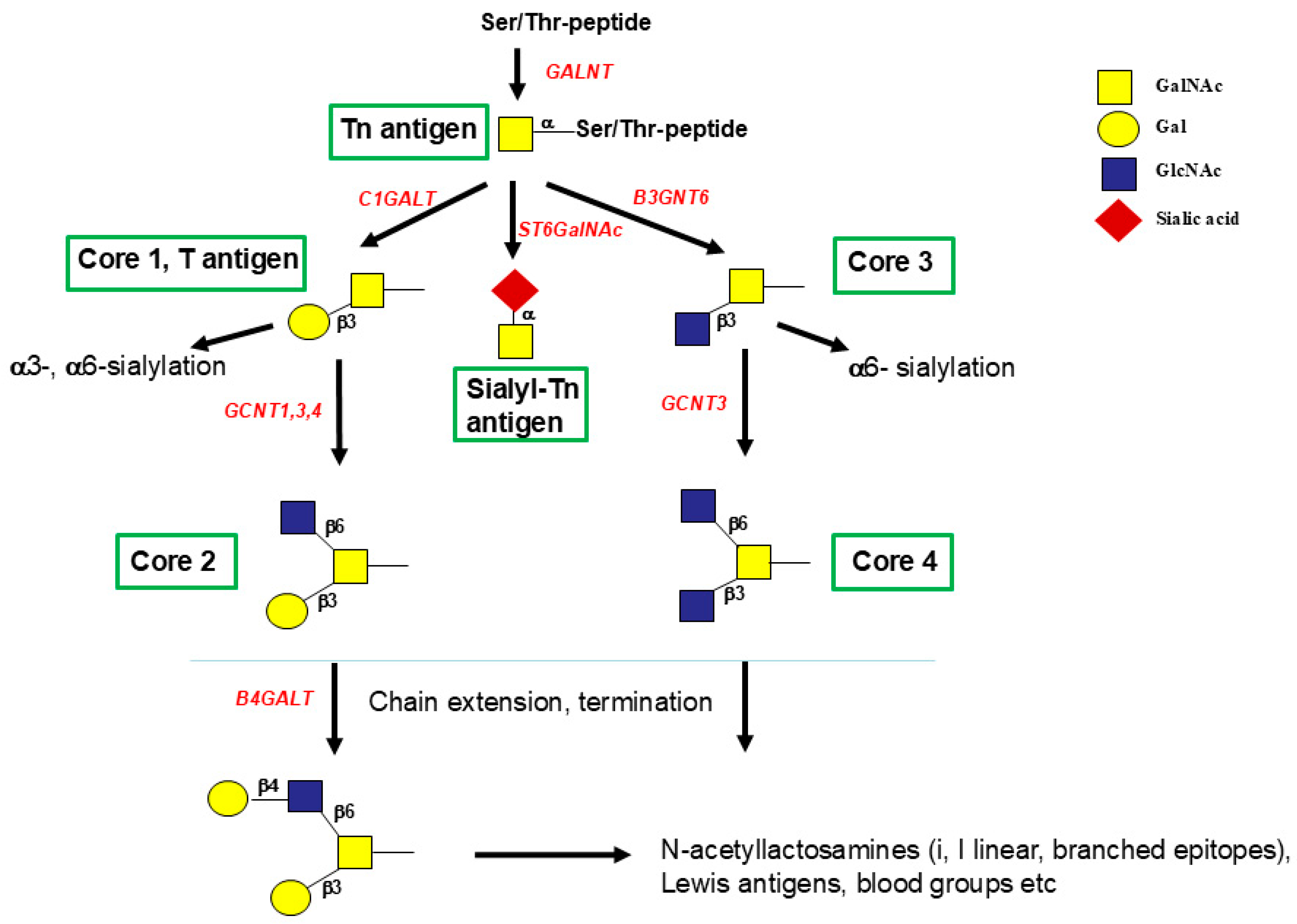

| Enzyme Name | Uniprot | CAZy GT | Donor | Product |
|---|---|---|---|---|
| ER: N-glycan biosynthesis | ||||
| *GlcNAc-P-T DPAGT | Q9H3H5 | - | UDP-GlcNAc | GlcNAc-PP-Dol |
| β4GlcNAcT ALG13/14 | Q9NP73 | 1 | UDP-GlcNAc | GlcNAc2-PP-Dol |
| Q96F25 | ||||
| DPM1 | O60762 | 2 | GDP-Man | Manβ-P-Dol |
| β4ManT ALG1 | Q9BT22 | 33 | GDP-Man | Man-GlcNAc2-PP-Dol |
| α3/6ManT ALG2 | Q9H553 | 4 | GDP-Man | Man3-GlcNAc2-PP-Dol |
| α2ManT ALG11 | Q2TAA5 | 4 | GDP-Man | Man5-GlcNAc2-PP-Dol |
| α3ManT ALG3 | Q92685 | 58 | Dol-P-Man | Man7-GlcNAc2-PP-Dol |
| α2ManT ALG9 | Q9H6U8 | 22 | Dol-P-Man | Man9-GlcNAc2-PP-Dol |
| α2ManT ALG12 | Q9BV10 | 22 | Dol-P-Man | Man9-GlcNAc2-PP-Dol |
| DolPGlc synthase ALG5 | Q9Y673 | 2 | UDP-Glc | Glcβ-P-Dol |
| α3GlcT ALG6 | Q9Y672 | 57 | Dol-P-Glc | Glc-Man9-GlcNAc2-Dol |
| α3GlcT ALG8 | Q9BVK2 | 57 | Dol-P-Glc | Glc2-Man9-GlcNAc2-Dol |
| α2GlcT ALG10 | Q5BKT4 | 59 | Dol-P-Glc | Glc3-Man9-GlcNAc2-Dol |
| OST complex | - | - | Glycan-Dol | Glycan-Asn |
| α2Glc MOGS | Q13724 | GH63 | Glc2-Man9-GlcNAc2-Asn | |
| α3Glc GANAB | Q14697 | GH31 | Man9-GlcNAc2-Asn | |
| α2Man MAN1B1 | Q9UKM7 | GH47 | Man8-GlcNAc2-Asn | |
| Golgi: N-glycan processing | ||||
| α2Man MAN1A1 | P33908 | GH47 | Man5GlcNAc2-Asn | |
| *β2GnT I MGAT1 | P26572 | 13 | UDP-GlcNAc | GlcNAcβ2Man5GlcNAc2- |
| α3,6Man MAN2A1 | Q16706 | GH38 | GlcNAcβ2-Man3GlcNAc2- | |
| *β2GnT II MGAT2 | Q10469 | 16 | UDP-GlcNAc | GlcNAcβ22Man3GlcNAc2- |
| β4GnT III MGAT3 | Q09327 | 17 | UDP-GlcNAc | bisecting GlcNAcβ4Manβ- |
| β4GnT IV MGAT4A | Q9UM21 | 54 | UDP-GlcNAc | GlcNAcβ3Man3GlcNAc2- |
| *β6GnT V MGAT5 | Q09328 | 18 | UDP-GlcNAc | GlcNAcβ4Man3GlcNAc2- |
| *GNPTAB | Q3T906 | - | UDP-GlcNAc | GlcNAc-6P-Man |
| GNPTG complex | Q9UJJ9 | - | UDP-GlcNAc | GlcNAc-6P-Man |
| *α6FucT FUT8 | Q9BYC5 | 23 | GDP-Fuc | N-glycan core Fuc |
| *α6SiaT ST6Gal1 | P15907 | 29 | CMP-Sia | complex N-glycans |
| α8SiaT ST8SIA2 | Q92186 | 29 | CMP-Sia | Siaα2-8Sia- |
| *α8SiaT ST8SIA3 | O43173 | 29 | CMP-Sia | Siaα2-8Sia- |
| α8SiaT ST8SIA4 | Q92187 | 29 | CMP-Sia | Siaα2-8Sia- |
| Golgi: O-glycan biosynthesis | ||||
| *GALNT1 | Q10472 | 27 | UDP-GalNAc | O-glycan initiation |
| *β3GalT C1GALT1 | Q9NS00 | 31 | UDP-Gal | core 1 |
| *β6GnT GCNT1 | Q02742 | 14 | UDP-GlcNAc | core 2 |
| β6GnT GCNT3 | O95395 | 14 | UDP-GlcNAc | core 2/4 |
| β6GnT GCNT4 | Q9P109 | 14 | UDP-GlcNAc | core2 |
| β3GnT B3GNT3 | Q9Y2A9 | 14 | UDP-GlcNAc | core 1,2 elongation |
| β3GnT B3GNT6 | Q6ZMB0 | 31 | UDP-GlcNAc | core 3 |
| α4GnT A4GNT | Q9UNA3 | 32 | UDP-GlcNAc | GlcNAcα4-Gal of core 2 |
| *α3SiaT ST3Gal1 | Q11201 | 29 | CMP-Sia | Sia-core 1 |
| α6SiaT ST6GalNAc1 | K7EMB6 | 29 | CMP-Sia | Sia-GalNAc-Thr |
| *α6SiaT ST6GalNAc2 | Q9UJ37 | 29 | CMP-Sia | Sia-GalNAc/Gal-GalNAc-Thr |
| α6SiaT ST6GalNAc3 | Q8NDV1 | 29 | CMP-Sia | Sia-Gal-(Sia)-GalNAc) |
| α6SiaT ST6GalNAc4 | Q9H4F1 | 29 | CMP-Sia | Sia-Gal (Sia)-GalNAc |
| Golgi: N- and O-glycan chain extension and termination | ||||
| *iβ3GnT B3GNT2 | Q9NY97 | 31 | UDP-GlcNAc | i antigen |
| β3GnT B3GN8 | Q7Z7M8 | 31 | UDP-GlcNAc | i antigen |
| β6GnT GCNT2 | Q8N0V5 | 14 | UDP-GlcNAc | I antigen |
| β4GalNAcT B4GALNT3 | Q6L9W6 | 7 | UDP-GalNAc | Lac-diNAc |
| *α3GalNAcT, GTA, BGAT | P16442 | 6 | UDP-GalNAc | Blood group A |
| V5ZDP0 | ||||
| β4GalNAcT B4GALNT2 | Q8NHY0 | 12 | UDP-GalNAc | Sda antigen |
| *α3GalT GTB | V9GWR7 | 6 | UDP-Gal | Blood group B |
| *β4GalT B4GALT1 | P15291 | 7 | UDP-Gal | GlcNAc extension |
| β4GalT B4GALT2 | O60909 | 7 | UDP-Gal | GlcNAc extension |
| β4GalT B4GALT3 | O60512 | 7 | UDP-Gal | GlcNAc extension |
| *β3GalT B3GALT5 | Q9Y2C3 | 31 | UDP-Gal | GlcNAc extension |
| α2FucT FUT1 | P19526 | 11 | GDP-Fuc | Blood group H/O |
| α2FucT FUT2 | Q10981 | 11 | GDP-Fuc | Blood group H/O |
| α3/4FucT FUT3 | P21217 | 10 | GDP-Fuc | Lewis antigens |
| α3FucT FUT4 | P22083 | 10 | GDP-Fuc | Lewis antigens, type 2 chains |
| α3/4FucT FUT5 | Q11128 | 10 | GDP-Fuc | Lewis antigens |
| α3FucT FUT7 | Q11130 | 10 | GDP-Fuc | sialyl-Lewis |
| *α3FucT FUT9 | Q9Y231 | 10 | GDP-Fuc | Lewis antigens |
| α3SiaT ST3Gal3 | Q11203 | 29 | CMP-Sia | Sia-Gal-GlcNAc- |
| α3SiaT ST3Gal4 | Q11206 | 29 | CMP-Sia | Sia-core 1/Gal-GlcNAc |
| α3SiaT ST3Gal6 | Q9Y274 | 29 | CMP-Sia | Sia-Gal-GlcNAc |
| α8SiaT ST8SIA6 | P61647 | 29 | CMP-Sia | Siaα2-8Sia |
| Epitope | Structure | Alteration in Cancer |
|---|---|---|
| Tn antigen | GalNAcα-Ser/Thr- | Increased |
| Sialyl-Tn antigen | Siaα2-6GalNAcα- | Increased |
| Core 1, T antigen | Galβ1-3GalNAcα- | Increased |
| Sialyl-T antigen | Siaα2-3Galβ1-3GalNAcα- | Increased |
| Core 2 | GlcNAcβ1-6(Galβ1-3)GalNAcα- | Variable |
| Core 3 | GlcNAcβ1-3GalNAcα- | Decreased |
| Core 4 | GlcNAcβ1-6(GlcNAcβ1-3)GalNAcα- | Decreased |
| Core 5 | GalNAcα1-3GalNAcα- | Increased |
| Core 6 | GlcNAcβ1-6GalNAcα- | |
| Core 7 | GalNAcα1-6GalNAc- | |
| Core 8 | Galα1-3GalNAc- | |
| Sialyl-Lewis x | Siaα2-3Galβ1-4(Fucα1-3)GlcNAcβ- | Increased |
| Sda/Cad | GalNAcβ1-4(Siaα2-3)Galβ- | Decreased |
| i antigen | Galβ1-4GlcNAcβ1-3Galβ1- | Variable |
| I antigen | Galβ1-4GlcNAcβ1-6 | |
| (Galβ1-4GlcNAcβ1-3)Galβ1- | ||
| LacdiNAc | GalNAcβ1-4GlcNAcβ1-3- | |
| Sialyl-LacNAc | Siaα2,6Galβ1-4GlcNAc | Increased |
| Blood group O(H) | Fucα1-2Galβ- | |
| Blood group A | GalNAcα1-3(Fucα1-2)Galβ- | |
| Blood group B | Galα1-3(Fucα1-2)Galβ- | |
| Complex N-glycans | Highly branched N-glycans | Increased GnT V |
| Sialylated termini | Sialylated N-glycans | Increased ST6Gal1 |
| Enzyme | Inhibitors |
|---|---|
| GnT I | Manα6(Manα3)4-O-methyl-Manβ4GlcNAc |
| Manα6(6-O-methyl-Manα3)Manβ-oct | |
| Manα6(6-O-4,5-epoxy-pentyl-Manα3)Manβ-oct | |
| Manα6(6-O-4,4-azo-pentyl-Manα3)Manβ-oct | |
| GnT II | 2-deoxy-Manα6(GlcNAcβ2Manα3)Manβ-oct |
| GlcNAcβ2Manα3Manβ-oct | |
| 3-O(4,4 azo)pentylManα6(GlcNAcβ2Manα3)Manβ-oct | |
| 2-deoxy-Manα6(GlcNAcβ2Manα3)Manβ-oct | |
| GnT V | GlcNAcβ2(6-deoxy)Manα6Glcβ-O-R |
| GlcNAcβ2(4-O-methyl)Manα6Glcβ-O-R | |
| GlcNAcβ2(6-deoxy, 4-O-methyl)Manα6 Glcβ-O-R | |
| GlcNAcβ2(4,6-di-O-methyl)Manα6Glcβ-pnp | |
| O-glycan synthesis | GalNAcα-aryl |
| C1GALT1 | 6-O-(4,4-azo)pentyl-GalNAcα-Bn + UV |
| GCNT1 | Galβ3GalNAcα-pnp + UV |
| Galβ3(6-deoxy) GalNAcα-Bn | |
| β4GalT | GlcNAcβ-naphthyl derivatives |
| 1-thio-N-butyryl-GlcNAcβ-2-naphthyl |
| Different Series with Examples of Glycopeptides | Variants Synthesized |
|---|---|
| AcTPPP | |
| 1. APTGalNAcα SGalNAcα SS | Thr/Ser, position of GalNAc |
| 2. APTGalβ3GalNAcα SSS | Thr/Ser, position of core 1 |
| 3. Ac-TGalNAcα P-t-Bu | protected N/C-terminal protection |
| 4. APTGalNAcα-SSSTKKT | peptide length |
| 5. Ac-VTGalNAcα P-NH2 | peptide length, N/C-terminal protection |
| 6. Ac-PTTTGalNAcα PIST-NH2 | amino acids, position of GalNAc |
| 7. Ac-PTPTGTQTPTTGalNAcα TPITTTTTVTPT-NH2 | number of GalNAc |
| 8. AHGVTSGalβ3GalNAcα APDTRPAPGSTAP TGalβ3GalNAcα A | amino acids, position and number of GalNAc, core 1 |
| 9. PTTTPITTTG | amino acid sequence |
| 10. Ac-PSGalNAcα SGalNAcα SGalNAcα PIST-NH2 | amino acids, number, Ser/Thr position of glycans |
| 11. TTT GlcNAcβ3GalNAcα VTPT GlcNAcβ3GalNAcα PTG | number and position of GalNAc, core 3 |
| 12. TETTSHSTGalNAcα PG | number and position of GalNAc, core 3, length of peptide |
| 13. TTTGalβ3GalNAcα VTPT Galβ3GalNAcα PTG | position and number of core 1 |
| 14.TTTVTPTPTGlcNAcβ6(Galβ3)GalNAcαG | position and number of core 1, 2 |
| 15. TTT GlcNAcβ6(Galβ3)GalNAcα VTPTGalβ3GalNAcα PTG | position of core 1, 2 |
| 16. TTTGlcNAcβ6(GlcNAcβ3)GalNAcα VTP TGlcNAcβ6(GlcNAcβ3)GalNAcα PTG | position and number of core 4 |
| 17. TTTVTPTGlcNAcβ6GalNAcα PTG | position and number of core 6 |
| 18. TTTGlcNAcβ6(GlcNAcβ3)GalNAcα VTPTGlcNAcβ6GalNAcα PTG | position and number of core 4, 6 |
| 19. Ac-PTGalNAcα TGalβ3GalNAcα TGalβ3GalNAcα PIST-NH2 | position and number of GalNAc and core 1 |
| 20. Ac-PTTTGalβ3GalNAcα PIST-NH2 | position of core 1α or β, amino acids |
| 21. TETTSHSTGalβ3GalNAcα PG | position and number of core 1, 2, 4, 6 |
| 22. Ac-ELSTGalNAcα T GalNAcα3GalNAcα GPG- NH2 | amino acids, number and position of GalNAc, core 5, 7 |
| 23. Ac-ELATGalNAcα VGPG-NH2 | amino acids, GalNAc, core 5, 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brockhausen, I. Hans Paulsen: Contributions to the Investigations of Glycoprotein Biosynthesis. Molecules 2025, 30, 3735. https://doi.org/10.3390/molecules30183735
Brockhausen I. Hans Paulsen: Contributions to the Investigations of Glycoprotein Biosynthesis. Molecules. 2025; 30(18):3735. https://doi.org/10.3390/molecules30183735
Chicago/Turabian StyleBrockhausen, Inka. 2025. "Hans Paulsen: Contributions to the Investigations of Glycoprotein Biosynthesis" Molecules 30, no. 18: 3735. https://doi.org/10.3390/molecules30183735
APA StyleBrockhausen, I. (2025). Hans Paulsen: Contributions to the Investigations of Glycoprotein Biosynthesis. Molecules, 30(18), 3735. https://doi.org/10.3390/molecules30183735





