N-Myristoyltransferase Inhibition in Parasitic Pathogens: Insights from Computer-Aided Drug Design
Abstract
1. Introduction
2. An Overview of Leishmaniasis, HAT, and Malaria
2.1. Leishmaniasis
2.2. Human African Trypanosomiasis (HAT)
2.3. Malaria
3. Targeting NMT: Insights in Drug Design
3.1. Structure and Functions of NMT
3.2. Structure-Based Drug Design (SBDD) to Discover NMT Inhibitors
4. NMT Inhibitors Against Parasitic Diseases Identified by Computational Methods
4.1. NMT Inhibitors Against Leishmaniasis
4.1.1. Chromone Analogs
4.1.2. Peptidomimetics
4.1.3. Thienopyrimidine, Piperidinylindole, and Aminoacylpyrrolidine
4.1.4. Pyran-Acrylate Analogs
4.1.5. Pyrrole Analogs
4.1.6. Natural Compounds
4.2. NMT Inhibitors Against Plasmodium sp.
4.2.1. Pyrazole Analogs
4.2.2. Piperidine Analogs
4.2.3. Piperazine, Steroid, and Thiazolidine Derivatives
4.2.4. Benzothiophene Analogs
4.2.5. Quinoline Analogs
4.2.6. Oxadiazole Analogs
4.3. NMT Inhibition Against Human African Trypanosomiasis (HAT)
4.3.1. Pyrazole Analogs
4.3.2. Thiazolidin and Benzoxazine Analogs
5. Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACTs | Artemisinin-Based Combination Therapies |
| ADMET | Absorption, Distribution, Metabolism, Excretion, and Toxicity |
| AI | Artificial Intelligence |
| CADD | Computer-Aided Drug Design |
| CD | Chagas Disease |
| CNS | Central Nervous System |
| DALYs | Disability-Adjusted Life Years |
| HAT | Human African Trypanosomiasis |
| HsNMT | Homo sapiens N-myristoyltransferase |
| LBDD | Ligand-Based Drug Design |
| LdNMT | Leishmania donovani N-myristoyltransferase |
| LELP | Ligand Efficiency-Dependent Lipophilicity |
| LmNMT | Leishmania major N-myristoyltransferase |
| LPG | Lipophosphoglycan |
| MCL | Mucocutaneous Leishmaniasis |
| MyrCoA | Myristoyl-CoA |
| NMT | N-myristoyltransferase |
| NPOs | Non-Profit Organizations |
| NTDs | Neglected Tropical Diseases |
| PDB | Protein Data Bank |
| PfNMT | Plasmodium falciparum N-myristoyltransferase |
| PvNMT | Plasmodium vivax N-myristoyltransferase |
| QSAR | Quantitative Structure–Activity Relationship |
| Rg | Radius of Gyration |
| RMSD | Root Mean Square Deviation |
| RMSF | Root Mean Square Fluctuation |
| ROCs | Receiver Operating Characteristic |
| SBDD | Structure-Based Drug Design |
| SI | Selectivity Index |
| SSG | Sodium Stibogluconate |
| TbNMT | Trypanosoma brucei N-myristoyltransferase |
| VL | Visceral Leishmaniasis |
| VSAs | Variant Surface Antigens |
| WHO | World Health Organization |
References
- Global Report on Neglected Tropical Diseases 2024: Executive Summary; WHO: Geneva, Switzerland, 2024; ISBN 9789240091535.
- Nascimento, I.; Albino, S.; Menezes, K.; Cavalcanti, M.; Oliveira, M.; Mali, S.; Moura, R. Targeting SmCB1: Perspectives and Insights to Design Antischistosomal Drugs. Curr. Med. Chem. 2023, 31, 2264–2284. [Google Scholar] [CrossRef] [PubMed]
- Utzinger, J.; Becker, S.L.; Knopp, S.; Blum, J.; Neumayr, A.L.; Keiser, J.; Hatz, C.F. Neglected Tropical Diseases: Diagnosis, Clinical Management, Treatment and Control. Swiss. Med. Wkly. 2012, 142, w13727. [Google Scholar] [CrossRef] [PubMed]
- WHO|World Health Organization. Available online: https://www.who.int/neglected_diseases/diseases/en/ (accessed on 22 June 2020).
- dos Santos Nascimento, I.J.; de Aquino, T.M.; da Silva-Júnior, E.F. Cruzain and Rhodesain Inhibitors: Last Decade of Advances in Seeking for New Compounds Against American and African Trypanosomiases. Curr. Top. Med. Chem. 2021, 21, 1871–1899. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Nascimento, I.J.; da Silva Rodrigues, É.E.; da Silva, M.F.; de Araújo-Júnior, J.X.; de Moura, R.O. Advances in Computational Methods to Discover New NS2B-NS3 Inhibitors Useful Against Dengue and Zika Viruses. Curr. Top. Med. Chem. 2022, 22, 2435–2462. [Google Scholar] [CrossRef]
- dos Santos Nascimento, I.J.; Santos, M.B.; Marinho, W.P.D.J.; de Moura, R.O. Insights to Design New Drugs against Human African Trypanosomiasis Targeting Rhodesain Using Covalent Docking, Molecular Dynamics Simulations, and MM-PBSA Calculations. Curr. Comput. Aided Drug Des. 2024, 20, 67–82. [Google Scholar] [CrossRef] [PubMed]
- White, N.J. Anaemia and Malaria. Malar. J. 2018, 17, 371. [Google Scholar] [CrossRef]
- Carlton, J.M.; Sina, B.J.; Adams, J.H. Why Is Plasmodium Vivax a Neglected Tropical Disease? PLoS Negl. Trop. Dis. 2011, 5, e1160. [Google Scholar] [CrossRef]
- WHO. World Malaria Report 2021 [Internet]; WHO: Geneva, Switzerland, 2021; ISBN 9789240040496.
- World Health Organization. Ending the Neglect to Attain the Sustainable Development Goals; WHO (World Health Organization): Geneva, Switzerland, 2020; pp. 2–4.
- Reed, S.L.; McKerrow, J.H. Why Funding for Neglected Tropical Diseases Should Be a Global Priority. Clin. Infect. Dis. 2018, 67, 323–326. [Google Scholar] [CrossRef]
- Tidman, R.; Abela-Ridder, B.; De Castañeda, R.R. The Impact of Climate Change on Neglected Tropical Diseases: A Systematic Review. Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 147–168. [Google Scholar] [CrossRef]
- Barrett, M.P.; Vincent, I.M.; Burchmore, R.J.; Kazibwe, A.J.; Matovu, E. Drug Resistance in Human African Trypanosomiasis. Future Microbiol. 2011, 6, 1037–1047. [Google Scholar] [CrossRef]
- Peeling, R.W.; Boeras, D.I.; Nkengasong, J. Re-Imagining the Future of Diagnosis of Neglected Tropical Diseases. Comput. Struct. Biotechnol. J. 2017, 15, 271–274. [Google Scholar] [CrossRef]
- dos Santos Nascimento, I.J.; de Cavalcanti, M.A.T.; de Moura, R.O. Exploring N-Myristoyltransferase as a Promising Drug Target against Parasitic Neglected Tropical Diseases. Eur. J. Med. Chem. 2023, 258, 115550. [Google Scholar] [CrossRef]
- Bairoch, A. The ENZYME Database in 2000. Nucleic Acids Res. 2000, 28, 304–305. [Google Scholar] [CrossRef] [PubMed]
- Patrick, G.L. Transferases and Their Inhibition. In Antimalarial Agents; Elsevier: Amsterdam, The Netherlands, 2020; pp. 433–458. [Google Scholar]
- Toraskar, M.; Prasad, K.; Kadam, V. N-Myristoyltransferase: A Novel Target. Mini-Rev. Med. Chem. 2008, 8, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Tate, E.W.; Bell, A.S.; Rackham, M.D.; Wright, M.H. N- Myristoyltransferase as a Potential Drug Target in Malaria and Leishmaniasis. Parasitology 2014, 141, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Kosciuk, T.; Lin, H. N-Myristoyltransferase as a Glycine and Lysine Myristoyltransferase in Cancer, Immunity, and Infections. ACS Chem. Biol. 2020, 15, 1747–1758. [Google Scholar] [CrossRef]
- Vemula, D.; Jayasurya, P.; Sushmitha, V.; Kumar, Y.N.; Bhandari, V. CADD, AI and ML in Drug Discovery: A Comprehensive Review. Eur. J. Pharm. Sci. 2022, 181, 106324. [Google Scholar] [CrossRef]
- dos Santos Nascimento, I.J.; da Silva Santos-Júnior, P.F.; de Araújo-Júnior, J.X.; da Silva-Júnior, E.F. Strategies in Medicinal Chemistry to Discover New Hit Compounds against Ebola Virus: Challenges and Perspectives in Drug Discovery. Mini-Rev. Med. Chem. 2022, 22, 2896–2924. [Google Scholar] [CrossRef]
- Arya, P.K.; Barik, K.; Singh, A.K.; Kumar, A. Databases and Web Resources for Neglected Tropical Disease Research. J. Appl. Pharm. Sci. 2023, 13, 43–54. [Google Scholar] [CrossRef]
- dos Santos Nascimento, I.J.; da Silva-Júnior, E.F. TNF-α Inhibitors from Natural Compounds: An Overview, CADD Approaches, and Their Exploration for Anti-Inflammatory Agents. Comb. Chem. High Throughput Screen 2021, 25, 2317–2340. [Google Scholar] [CrossRef]
- Lee, J.W.; Maria-Solano, M.A.; Vu, T.N.L.; Yoon, S.; Choi, S. Big Data and Artificial Intelligence (AI) Methodologies for Computer-Aided Drug Design (CADD). Biochem. Soc. Trans. 2022, 50, 241–252. [Google Scholar] [CrossRef]
- dos Santos Nascimento, I.J.; de Aquino, T.M.; da Silva Júnior, E.F. Computer-Aided Drug Design of Anti-Inflammatory Agents Targeting Microsomal Prostaglandin E2 Synthase-1 (MPGES-1). Curr. Med. Chem. 2022, 29, 5397–5419. [Google Scholar] [CrossRef]
- Mann, S.; Frasca, K.; Scherrer, S.; Henao-Martínez, A.F.; Newman, S.; Ramanan, P.; Suarez, J.A. A Review of Leishmaniasis: Current Knowledge and Future Directions. Curr. Trop. Med. Rep. 2021, 8, 121–132. [Google Scholar] [CrossRef]
- Stuart, K.; Brun, R.; Croft, S.; Fairlamb, A.; Gürtler, R.E.; McKerrow, J.; Reed, S.; Tarleton, R. Kinetoplastids: Related Protozoan Pathogens, Different Diseases. J. Clin. Investig. 2008, 118, 1301–1310. [Google Scholar] [CrossRef]
- Cecílio, P.; Cordeiro-da-Silva, A.; Oliveira, F. Sand Flies: Basic Information on the Vectors of Leishmaniasis and Their Interactions with Leishmania Parasites. Commun. Biol. 2022, 5, 305. [Google Scholar] [CrossRef] [PubMed]
- Mathison, B.A.; Bradley, B.T. Review of the Clinical Presentation, Pathology, Diagnosis, and Treatment of Leishmaniasis. Lab. Med. 2023, 54, 363–371. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Rios, É.; Albino, S.L.; Olimpio de Moura, R.; Nascimento, I.J. dos S. Targeting Cysteine Protease B to Discover Antileishmanial Drugs: Directions and Advances. Eur. J. Med. Chem. 2025, 289, 117500. [Google Scholar] [CrossRef]
- Okwor, I.; Uzonna, J. Social and Economic Burden of Human Leishmaniasis. Am. J. Trop. Med. Hyg. 2016, 94, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Leishmaniasis. Available online: https://www.who.int/health-topics/leishmaniasis#tab=tab_1 (accessed on 22 June 2020).
- Minodier, P.; Parola, P. Cutaneous Leishmaniasis Treatment. Travel. Med. Infect. Dis. 2007, 5, 150–158. [Google Scholar] [CrossRef]
- Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J.; Arenas, R. Leishmaniasis: A Review. F1000Research 2017, 6, 750. [Google Scholar] [CrossRef]
- Chakravarty, J.; Sundar, S. Drug Resistance in Leishmaniasis. J. Glob. Infect. Dis. 2010, 2, 167. [Google Scholar] [CrossRef]
- de Aquino, T.M.; França, P.H.B.; Rodrigues, É.E.E.S.; Nascimento, I.J.S.; Santos-Júnior, P.F.S.; Aquino, P.G.V.; Santos, M.S.; Queiroz, A.C.; Araújo, M.V.; Alexandre-Moreira, M.S.; et al. Synthesis, Antileishmanial Activity and in Silico Studies of Aminoguanidine Hydrazones (AGH) and Thiosemicarbazones (TSC) Against Leishmania chagasi Amastigotes. Med. Chem. 2022, 18, 151–169. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Nascimento, I.J.; Santana Gomes, J.N.; de Oliveira Viana, J.; de Medeiros e Silva, Y.M.S.; Barbosa, E.G.; de Moura, R.O. The Power of Molecular Dynamics Simulations and Their Applications to Discover Cysteine Protease Inhibitors. Mini-Rev. Med. Chem. 2023, 23, 1125–1146. [Google Scholar] [CrossRef] [PubMed]
- Human African Trypanosomiasis (Sleeping Sickness). Available online: https://www.who.int/health-topics/human-african-trypanosomiasis#tab=tab_1 (accessed on 27 October 2020).
- Jacobs, R.T.; Nare, B.; Phillips, M.A. State of the Art in African Trypanosome Drug Discovery. Curr. Top. Med. Chem. 2011, 11, 1255–1274. [Google Scholar] [CrossRef] [PubMed]
- Gunaratne, R.S.; Sajid, M.; Ling, I.T.; Tripathi, R.; Pachebat, J.A.; Holder, A.A. Characterization of N-Myristoyltransferase from Plasmodium falciparum. Biochem. J. 2000, 348 Pt 2, 459–463. [Google Scholar] [CrossRef]
- Bottieau, E.; Clerinx, J. Human African Trypanosomiasis: Progress and Stagnation. Infect. Dis. Clin. N. Am. 2019, 33, 61–77. [Google Scholar] [CrossRef]
- Dickie, E.A.; Giordani, F.; Gould, M.K.; Mäser, P.; Burri, C.; Mottram, J.C.; Rao, S.P.S.; Barrett, M.P. New Drugs for Human African Trypanosomiasis: A Twenty First Century Success Story. Trop. Med. Infect. Dis. 2020, 5, 29. [Google Scholar] [CrossRef]
- Álvarez-Rodríguez, A.; Jin, B.-K.; Radwanska, M.; Magez, S. Recent Progress in Diagnosis and Treatment of Human African Trypanosomiasis Has Made the Elimination of This Disease a Realistic Target by 2030. Front. Med. 2022, 9, 1037094. [Google Scholar] [CrossRef]
- Menkin-Smith, L.; Winders, W.T. Plasmodium Vivax Malaria; StatPearls: St. Petersburg, FL, USA, 2024. [Google Scholar]
- World Malaria Report 2024. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2024 (accessed on 23 April 2025).
- Talapko, J.; Škrlec, I.; Alebić, T.; Jukić, M.; Včev, A. Malaria: The Past and the Present. Microorganisms 2019, 7, 179. [Google Scholar] [CrossRef]
- Agudelo Higuita, N.I.; Franco-Paredes, C.; Henao-Martínez, A.F.; Mendez Rojas, B.; Suarez, J.A.; Naranjo, L.; Alger, J. Migrants in Transit across Central America and the Potential Spread of Chloroquine Resistant Malaria—A Call for Action. Lancet Reg. Health—Americas 2023, 22, 100505. [Google Scholar] [CrossRef]
- Rogier, E.; Herman, C.; Huber, C.S.; Hamre, K.E.S.; Pierre, B.; Mace, K.E.; Présumé, J.; Mondélus, G.; Romilus, I.; Elismé, T.; et al. Nationwide Monitoring for Plasmodium falciparum Drug-Resistance Alleles to Chloroquine, Sulfadoxine, and Pyrimethamine, Haiti, 2016–2017. Emerg. Infect. Dis. 2020, 26, 902–909. [Google Scholar] [CrossRef]
- Sá, J.M.; Twu, O.; Hayton, K.; Reyes, S.; Fay, M.P.; Ringwald, P.; Wellems, T.E. Geographic Patterns of Plasmodium falciparum Drug Resistance Distinguished by Differential Responses to Amodiaquine and Chloroquine. Proc. Natl. Acad. Sci. USA 2009, 106, 18883–18889. [Google Scholar] [CrossRef]
- Shibeshi, M.A.; Kifle, Z.D.; Atnafie, S.A. Antimalarial Drug Resistance and Novel Targets for Antimalarial Drug Discovery. Infect. Drug Resist. 2020, 13, 4047–4060. [Google Scholar] [CrossRef]
- Malaria. Available online: https://www.who.int/news-room/fact-sheets/detail/malaria (accessed on 16 May 2024).
- Ariey, F.; Ménard, D. An Update on Artemisinin Resistance. Methods Mol. Biol. 2019, 2013, 141–149. [Google Scholar]
- White, N.J.; Chotivanich, K. Artemisinin-Resistant Malaria. Clin. Microbiol. Rev. 2024, 37, e00109-24. [Google Scholar] [CrossRef] [PubMed]
- Schreidah, C.; Giesbrecht, D.; Gashema, P.; Young, N.W.; Munyaneza, T.; Muvunyi, C.M.; Thwai, K.; Mazarati, J.-B.; Bailey, J.A.; Juliano, J.J.; et al. Expansion of Artemisinin Partial Resistance Mutations and Lack of Histidine Rich Protein-2 and -3 Deletions in Plasmodium falciparum Infections from Rukara, Rwanda. Malar. J. 2024, 23, 150. [Google Scholar] [CrossRef] [PubMed]
- Agaba, B.B.; Travis, J.; Smith, D.; Rugera, S.P.; Zalwango, M.G.; Opigo, J.; Katureebe, C.; Mpirirwe, R.; Bakary, D.; Antonio, M.; et al. Emerging Threat of Artemisinin Partial Resistance Markers (Pfk13 Mutations) in Plasmodium falciparum Parasite Populations in Multiple Geographical Locations in High Transmission Regions of Uganda. Malar. J. 2024, 23, 330. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, S.K.; Gabryszewski, S.J.; Small-Saunders, J.L.; Yeo, T.; Henrich, P.P.; Mok, S.; Fidock, D.A. Global Spread of Mutant PfCRT and Its Pleiotropic Impact on Plasmodium falciparum Multidrug Resistance and Fitness. mBio 2019, 10, 10–1128. [Google Scholar] [CrossRef]
- Dhingra, S.K.; Redhi, D.; Combrinck, J.M.; Yeo, T.; Okombo, J.; Henrich, P.P.; Cowell, A.N.; Gupta, P.; Stegman, M.L.; Hoke, J.M.; et al. A Variant PfCRT Isoform Can Contribute to Plasmodium falciparum Resistance to the First-Line Partner Drug Piperaquine. mBio 2017, 8, 10–1128. [Google Scholar] [CrossRef]
- de Azevedo Teotônio Cavalcanti, M.; Da Silva Menezes, K.J.; De Oliveira Viana, J.; de Oliveira Rios, É.; Corrêa de Farias, A.G.; Weber, K.C.; Nogueira, F.; dos Santos Nascimento, I.J.; de Moura, R.O. Current Trends to Design Antimalarial Drugs Targeting N -Myristoyltransferase. Future Microbiol. 2024, 19, 1601–1618. [Google Scholar] [CrossRef]
- Price, H.P.; Menon, M.R.; Panethymitaki, C.; Goulding, D.; McKean, P.G.; Smith, D.F. Myristoyl-CoA:Protein N-Myristoyltransferase, an Essential Enzyme and Potential Drug Target in Kinetoplastid Parasites. J. Biol. Chem. 2003, 278, 7206–7214. [Google Scholar] [CrossRef]
- Brannigan, J.A.; Smith, B.A.; Yu, Z.; Brzozowski, A.M.; Hodgkinson, M.R.; Maroof, A.; Price, H.P.; Meier, F.; Leatherbarrow, R.J.; Tate, E.W.; et al. N-Myristoyltransferase from Leishmania donovani: Structural and Functional Characterisation of a Potential Drug Target for Visceral Leishmaniasis. J. Mol. Biol. 2010, 396, 985–999. [Google Scholar] [CrossRef]
- Bayliss, T.; Robinson, D.A.; Smith, V.C.; Brand, S.; McElroy, S.P.; Torrie, L.S.; Mpamhanga, C.; Norval, S.; Stojanovski, L.; Brenk, R.; et al. Design and Synthesis of Brain Penetrant Trypanocidal N-Myristoyltransferase Inhibitors. J. Med. Chem. 2017, 60, 9790–9806. [Google Scholar] [CrossRef]
- Wright, M.H.; Heal, W.P.; Mann, D.J.; Tate, E.W. Protein Myristoylation in Health and Disease. J. Chem. Biol. 2010, 3, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Dian, C.; Pérez-Dorado, I.; Rivière, F.; Asensio, T.; Legrand, P.; Ritzefeld, M.; Shen, M.; Cota, E.; Meinnel, T.; Tate, E.W.; et al. High-Resolution Snapshots of Human N-Myristoyltransferase in Action Illuminate a Mechanism Promoting N-Terminal Lys and Gly Myristoylation. Nat. Commun. 2020, 11, 1132. [Google Scholar] [CrossRef] [PubMed]
- Pierre, M.; Traverso, J.A.; Boisson, B.; Domenichini, S.; Bouchez, D.; Giglione, C.; Meinnel, T. N -Myristoylation Regulates the SnRK1 Pathway in Arabidopsis. Plant Cell 2007, 19, 2804–2821. [Google Scholar] [CrossRef] [PubMed]
- Castrec, B.; Dian, C.; Ciccone, S.; Ebert, C.L.; Bienvenut, W.V.; Le Caer, J.-P.; Steyaert, J.-M.; Giglione, C.; Meinnel, T. Structural and Genomic Decoding of Human and Plant Myristoylomes Reveals a Definitive Recognition Pattern. Nat. Chem. Biol. 2018, 14, 671–679. [Google Scholar] [CrossRef]
- Das, U.; Kumar, S.; Dimmock, J.R.; Sharma, R.K. Inhibition of Protein N-Myristoylation: A Therapeutic Protocol in Developing Anticancer Agents. Curr. Cancer Drug Targets 2012, 12, 667–692. [Google Scholar] [CrossRef]
- Yang, S.H.; Shrivastav, A.; Kosinski, C.; Sharma, R.K.; Chen, M.-H.; Berthiaume, L.G.; Peters, L.L.; Chuang, P.-T.; Young, S.G.; Bergo, M.O. N-Myristoyltransferase 1 Is Essential in Early Mouse Development. J. Biol. Chem. 2005, 280, 18990–18995. [Google Scholar] [CrossRef]
- Sogabe, S.; Masubuchi, M.; Sakata, K.; Fukami, T.A.; Morikami, K.; Shiratori, Y.; Ebiike, H.; Kawasaki, K.; Aoki, Y.; Shimma, N.; et al. Crystal Structures of Candida Albicans N-Myristoyltransferase with Two Distinct Inhibitors. Chem. Biol. 2002, 9, 1119–1128. [Google Scholar] [CrossRef]
- Goncalves, V.; Brannigan, J.A.; Thinon, E.; Olaleye, T.O.; Serwa, R.; Lanzarone, S.; Wilkinson, A.J.; Tate, E.W.; Leatherbarrow, R.J. A Fluorescence-Based Assay for N-Myristoyltransferase Activity. Anal. Biochem. 2012, 421, 342–344. [Google Scholar] [CrossRef]
- Brannigan, J.A.; Wilkinson, A.J. Drug Discovery in Leishmaniasis Using Protein Lipidation as a Target. Biophys. Rev. 2021, 13, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, V.; Brannigan, J.A.; Whalley, D.; Ansell, K.H.; Saxty, B.; Holder, A.A.; Wilkinson, A.J.; Tate, E.W.; Leatherbarrow, R.J. Discovery of Plasmodium Vivax N -Myristoyltransferase Inhibitors: Screening, Synthesis, and Structural Characterization of Their Binding Mode. J. Med. Chem. 2012, 55, 3578–3582. [Google Scholar] [CrossRef] [PubMed]
- Frearson, J.A.; Brand, S.; McElroy, S.P.; Cleghorn, L.A.T.; Smid, O.; Stojanovski, L.; Price, H.P.; Guther, M.L.S.; Torrie, L.S.; Robinson, D.A.; et al. N-Myristoyltransferase Inhibitors as New Leads to Treat Sleeping Sickness. Nature 2010, 464, 728–732. [Google Scholar] [CrossRef]
- Brand, S.; Cleghorn, L.A.T.; McElroy, S.P.; Robinson, D.A.; Smith, V.C.; Hallyburton, I.; Harrison, J.R.; Norcross, N.R.; Spinks, D.; Bayliss, T.; et al. Discovery of a Novel Class of Orally Active Trypanocidal N -Myristoyltransferase Inhibitors. J. Med. Chem. 2012, 55, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.A.; Wyatt, P.G. Identification and Structure Solution of Fragment Hits against Kinetoplastid N -Myristoyltransferase. Acta Crystallogr. F Struct. Biol. Commun. 2015, 71, 586–593. [Google Scholar] [CrossRef]
- Harrison, J.R.; Brand, S.; Smith, V.; Robinson, D.A.; Thompson, S.; Smith, A.; Davies, K.; Mok, N.; Torrie, L.S.; Collie, I.; et al. A Molecular Hybridization Approach for the Design of Potent, Highly Selective, and Brain-Penetrant N-Myristoyltransferase Inhibitors. J. Med. Chem. 2018, 61, 8374–8389. [Google Scholar] [CrossRef]
- Goncalves, V.; Brannigan, J.A.; Laporte, A.; Bell, A.S.; Roberts, S.M.; Wilkinson, A.J.; Leatherbarrow, R.J.; Tate, E.W. Structure-Guided Optimization of Quinoline Inhibitors of Plasmodium N-Myristoyltransferase. Medchemcomm 2017, 8, 191–197. [Google Scholar] [CrossRef]
- Rackham, M.D.; Yu, Z.; Brannigan, J.A.; Heal, W.P.; Paape, D.; Barker, K.V.; Wilkinson, A.J.; Smith, D.F.; Leatherbarrow, R.J.; Tate, E.W. Discovery of High Affinity Inhibitors of Leishmania donovani N-Myristoyltransferase. Medchemcomm 2015, 6, 1761–1766. [Google Scholar] [CrossRef]
- Brannigan, J.A.; Roberts, S.M.; Bell, A.S.; Hutton, J.A.; Hodgkinson, M.R.; Tate, E.W.; Leatherbarrow, R.J.; Smith, D.F.; Wilkinson, A.J. Diverse Modes of Binding in Structures of Leishmania Major N -Myristoyltransferase with Selective Inhibitors. IUCrJ 2014, 1, 250–260. [Google Scholar] [CrossRef]
- Rackham, M.D.; Brannigan, J.A.; Rangachari, K.; Meister, S.; Wilkinson, A.J.; Holder, A.A.; Leatherbarrow, R.J.; Tate, E.W. Design and Synthesis of High Affinity Inhibitors of Plasmodium falciparum and Plasmodium Vivax N-Myristoyltransferases Directed by Ligand Efficiency Dependent Lipophilicity (LELP). J. Med. Chem. 2014, 57, 2773–2788. [Google Scholar] [CrossRef]
- Schlott, A.C.; Mayclin, S.; Reers, A.R.; Coburn-Flynn, O.; Bell, A.S.; Green, J.; Knuepfer, E.; Charter, D.; Bonnert, R.; Campo, B.; et al. Structure-Guided Identification of Resistance Breaking Antimalarial N-Myristoyltransferase Inhibitors. Cell Chem. Biol. 2019, 26, 991–1000.e7. [Google Scholar] [CrossRef]
- Yuan, M.; Song, Z.; Ying, M.; Zhu, H.; He, Q.; Yang, B.; Cao, J. N-Myristoylation: From Cell Biology to Translational Medicine. Acta Pharmacol. Sin. 2020, 41, 1005–1015. [Google Scholar] [CrossRef]
- dos Santos Nascimento, I.J.; de Aquino, T.M.; da Silva-Júnior, E.F. The New Era of Drug Discovery: The Power of Computer-Aided Drug Design (CADD). Lett. Drug Des. Discov. 2022, 19, 951–955. [Google Scholar] [CrossRef]
- dos Santos Nascimento, I.J.; de Aquino, T.M.; da Silva-Júnior, E.F. Repurposing FDA-Approved Drugs Targeting SARS-CoV2 3CL pro: A Study by Applying Virtual Screening, Molecular Dynamics, MM-PBSA Calculations and Covalent Docking. Lett. Drug Des. Discov. 2022, 19, 637–653. [Google Scholar] [CrossRef]
- Johri, S.; Kumar, B.K.; Dey, S.; Faheem; Balana-Fouce, R.; Gowri Chandra Sekhar, K.V.; Kunjiappan, S.; Murugesan, S. Inspection of In-House Designed Novel Thiochromone Amino-Acid Conjugate Derivatives as Lm-NMT Inhibitor—An in-Silico Analysis. J. Mol. Graph. Model. 2023, 119, 108397. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.F.; Junior, F.J.B.M.; da Silva, M.S.; Scotti, M.T.; Scotti, L. Computational and Investigative Study of Flavonoids Active against Trypanosoma cruzi and Leishmania Spp. Nat. Prod. Commun. 2015, 10, 1934578X1501000. [Google Scholar] [CrossRef]
- Olaleye, T.O.; Brannigan, J.A.; Roberts, S.M.; Leatherbarrow, R.J.; Wilkinson, A.J.; Tate, E.W. Peptidomimetic Inhibitors of N -Myristoyltransferase from Human Malaria and Leishmaniasis Parasites. Org. Biomol. Chem. 2014, 12, 8132–8137. [Google Scholar] [CrossRef]
- Bell, A.S.; Mills, J.E.; Williams, G.P.; Brannigan, J.A.; Wilkinson, A.J.; Parkinson, T.; Leatherbarrow, R.J.; Tate, E.W.; Holder, A.A.; Smith, D.F. Selective Inhibitors of Protozoan Protein N-Myristoyltransferases as Starting Points for Tropical Disease Medicinal Chemistry Programs. PLoS Negl. Trop. Dis. 2012, 6, e1625. [Google Scholar] [CrossRef]
- Hutton, J.A.; Goncalves, V.; Brannigan, J.A.; Paape, D.; Wright, M.H.; Waugh, T.M.; Roberts, S.M.; Bell, A.S.; Wilkinson, A.J.; Smith, D.F.; et al. Structure-Based Design of Potent and Selective Leishmania N-Myristoyltransferase Inhibitors. J. Med. Chem. 2014, 57, 8664–8670. [Google Scholar] [CrossRef]
- García-Sosa, A.T. Designing Ligands for Leishmania, Plasmodium, and Aspergillus N-Myristoyl Transferase with Specificity and Anti-Target-Safe Virtual Libraries. Curr. Comput. Aided Drug Des. 2018, 14, 131–141. [Google Scholar] [CrossRef]
- de Carvalho Gallo, J.C.; de Mattos Oliveira, L.; Araújo, J.S.C.; Santana, I.B.; dos Santos Junior, M.C. Virtual Screening to Identify Leishmania Braziliensis N-Myristoyltransferase Inhibitors: Pharmacophore Models, Docking, and Molecular Dynamics. J. Mol. Model. 2018, 24, 260. [Google Scholar] [CrossRef]
- Orabi, M.A.A.; Alshahrani, M.M.; Sayed, A.M.; Abouelela, M.E.; Shaaban, K.A.; Abdel-Sattar, E.S. Identification of Potential Leishmania N-Myristoyltransferase Inhibitors from Withania somnifera (L.) Dunal: A Molecular Docking and Molecular Dynamics Investigation. Metabolites 2023, 13, 93. [Google Scholar] [CrossRef]
- Novozhilova, N.M.; Bovin, N.V. Structure, Functions, and Biosynthesis of Glycoconjugates of Leishmania Spp. Cell Surface. Biochemistry 2010, 75, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Corpas-Lopez, V.; Moniz, S.; Thomas, M.; Wall, R.J.; Torrie, L.S.; Zander-Dinse, D.; Tinti, M.; Brand, S.; Stojanovski, L.; Manthri, S.; et al. Pharmacological Validation of N -Myristoyltransferase as a Drug Target in Leishmania donovani. ACS Infect. Dis. 2019, 5, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Hernández, D.; Vijayan, K.; Zigweid, R.; Fenwick, M.K.; Sankaran, B.; Roobsoong, W.; Sattabongkot, J.; Glennon, E.K.K.; Myler, P.J.; Sunnerhagen, P.; et al. Identification of Potent and Selective N-Myristoyltransferase Inhibitors of Plasmodium Vivax Liver Stage Hypnozoites and Schizonts. Nat. Commun. 2023, 14, 5408. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Brannigan, J.A.; Rangachari, K.; Heal, W.P.; Wilkinson, A.J.; Holder, A.A.; Leatherbarrow, R.J.; Tate, E.W. Discovery of Pyridyl-Based Inhibitors of Plasmodium falciparum N-Myristoyltransferase. Medchemcomm 2015, 6, 1767–1772. [Google Scholar] [CrossRef]
- Nicolau, M.S.P.; Resende, M.A.; Serafim, P.; Lima, G.Y.P.; Ueira-Vieira, C.; Nicolau-Junior, N.; Yoneyama, K.A.G. Identification of Potential Inhibitors for N-Myristoyltransferase (NMT) Protein of Plasmodium Vivax. J. Biomol. Struct. Dyn. 2023, 41, 7019–7031. [Google Scholar] [CrossRef]
- Wager, T.T.; Chandrasekaran, R.Y.; Hou, X.; Troutman, M.D.; Verhoest, P.R.; Villalobos, A.; Will, Y. Defining Desirable Central Nervous System Drug Space through the Alignment of Molecular Properties, in Vitro ADME, and Safety Attributes. ACS Chem. Neurosci. 2010, 1, 420–434. [Google Scholar] [CrossRef]
- Rackham, M.D.; Brannigan, J.A.; Moss, D.K.; Yu, Z.; Wilkinson, A.J.; Holder, A.A.; Tate, E.W.; Leatherbarrow, R.J. Discovery of Novel and Ligand-Efficient Inhibitors of Plasmodium falciparum and Plasmodium Vivax N -Myristoyltransferase. J. Med. Chem. 2013, 56, 371–375. [Google Scholar] [CrossRef]
- Garcia, M.L.; Oliveira, A.A.; Bueno, R.V.; Nogueira, V.H.R.; Souza, G.E.; Guido, R.V.C. QSAR Studies on Benzothiophene Derivatives as Plasmodium falciparum N-myristoyltransferase Inhibitors: Molecular Insights into Affinity and Selectivity. Drug Dev. Res. 2022, 83, 264–284. [Google Scholar] [CrossRef]
- Jameel, E.; Madhav, H.; Agrawal, P.; Raza, M.K.; Ahmedi, S.; Rahman, A.; Shahid, N.; Shaheen, K.; Gajra, C.H.; Khan, A.; et al. Identification of New Oxospiro Chromane Quinoline-Carboxylate Antimalarials That Arrest Parasite Growth at Ring Stage. J. Biomol. Struct. Dyn. 2023, 41, 15485–15506. [Google Scholar] [CrossRef] [PubMed]
- Santos-Garcia, L.; de Mecenas Filho, M.; Musilek, K.; Kuca, K.; Ramalho, T.; da Cunha, E. QSAR Study of N-Myristoyltransferase Inhibitors of Antimalarial Agents. Molecules 2018, 23, 2348. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sindhu, J.; Kumar, P. In-Silico Identification of Fingerprint of Pyrazolyl Sulfonamide Responsible for Inhibition of N-Myristoyltransferase Using Monte Carlo Method with Index of Ideality of Correlation. J. Biomol. Struct. Dyn. 2021, 39, 5014–5025. [Google Scholar] [CrossRef] [PubMed]
- Sheng, C.; Ji, H.; Miao, Z.; Che, X.; Yao, J.; Wang, W.; Dong, G.; Guo, W.; Lü, J.; Zhang, W. Homology Modeling and Molecular Dynamics Simulation of N-Myristoyltransferase from Protozoan Parasites: Active Site Characterization and Insights into Rational Inhibitor Design. J. Comput. Aided Mol. Des. 2009, 23, 375–389. [Google Scholar] [CrossRef]
- Masand, V.H.; El-Sayed, N.N.E.; Bambole, M.U.; Patil, V.R.; Thakur, S.D. Multiple Quantitative Structure-Activity Relationships (QSARs) Analysis for Orally Active Trypanocidal N-Myristoyltransferase Inhibitors. J. Mol. Struct. 2019, 1175, 481–487. [Google Scholar] [CrossRef]
- Scotti, L.; Ishiki, H.; Junior, F.J.B.M.; Ribeiro, F.F.; Yarla, N.S.; Sobral da Silva, M.; Filho, J.M.B.; Scotti, M.T. Computational and Metabolic Studies on a Set of N-Myristoyltransferase Inhibitors Against Trypanosoma brucei. Int. J. Quant. Struct.-Prop. Relatsh. 2018, 3, 80–94. [Google Scholar] [CrossRef]
- Spinks, D.; Smith, V.; Thompson, S.; Robinson, D.A.; Luksch, T.; Smith, A.; Torrie, L.S.; McElroy, S.; Stojanovski, L.; Norval, S.; et al. Development of Small-Molecule Trypanosoma brucei N-Myristoyltransferase Inhibitors: Discovery and Optimisation of a Novel Binding Mode. ChemMedChem 2015, 10, 1821–1836. [Google Scholar] [CrossRef]
- Sangha, R.; Jamal, R.; Spratlin, J.; Kuruvilla, J.; Sehn, L.H.; Beauchamp, E.; Weickert, M.; Berthiaume, L.G.; Mackey, J.R. A First-in-Human Phase I Trial of Daily Oral Zelenirstat, a N-Myristoyltransferase Inhibitor, in Patients with Advanced Solid Tumors and Relapsed/Refractory B-Cell Lymphomas. Investig. New Drugs 2024, 42, 386–393. [Google Scholar] [CrossRef]
- Galvin, B.D.; Li, Z.; Villemaine, E.; Poole, C.B.; Chapman, M.S.; Pollastri, M.P.; Wyatt, P.G.; Carlow, C.K.S. A Target Repurposing Approach Identifies N-Myristoyltransferase as a New Candidate Drug Target in Filarial Nematodes. PLoS Negl. Trop. Dis. 2014, 8, e3145. [Google Scholar] [CrossRef]
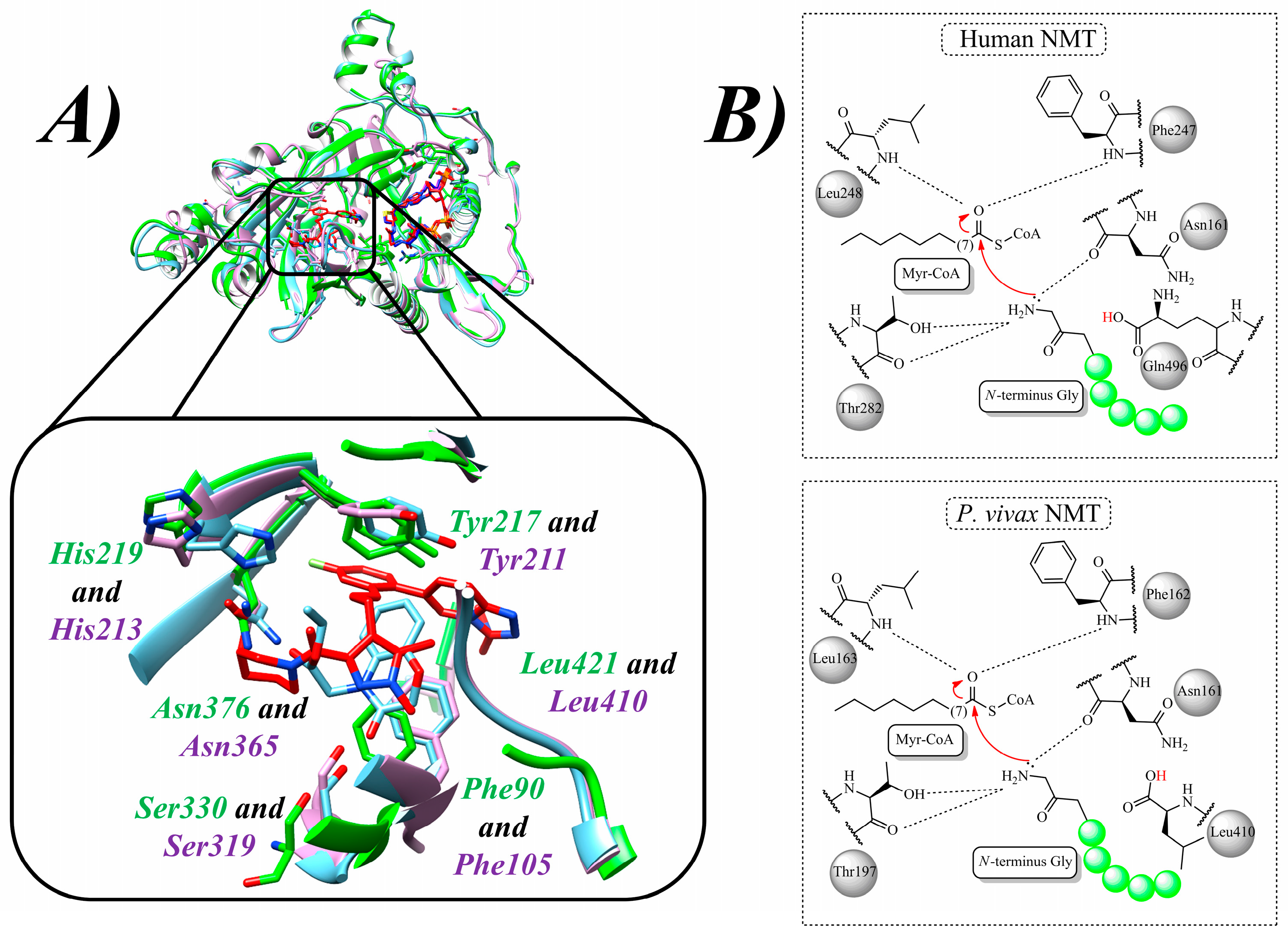
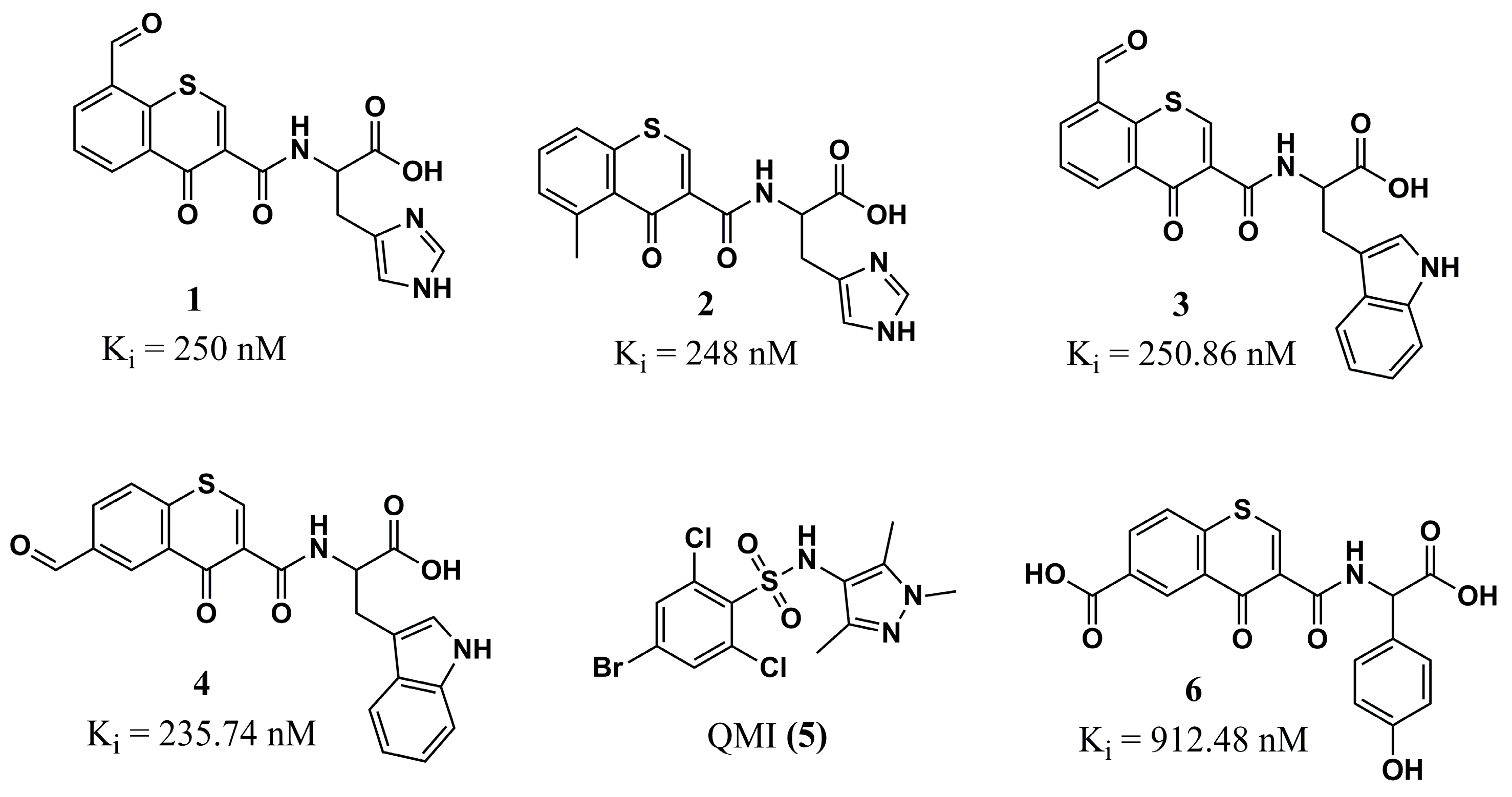
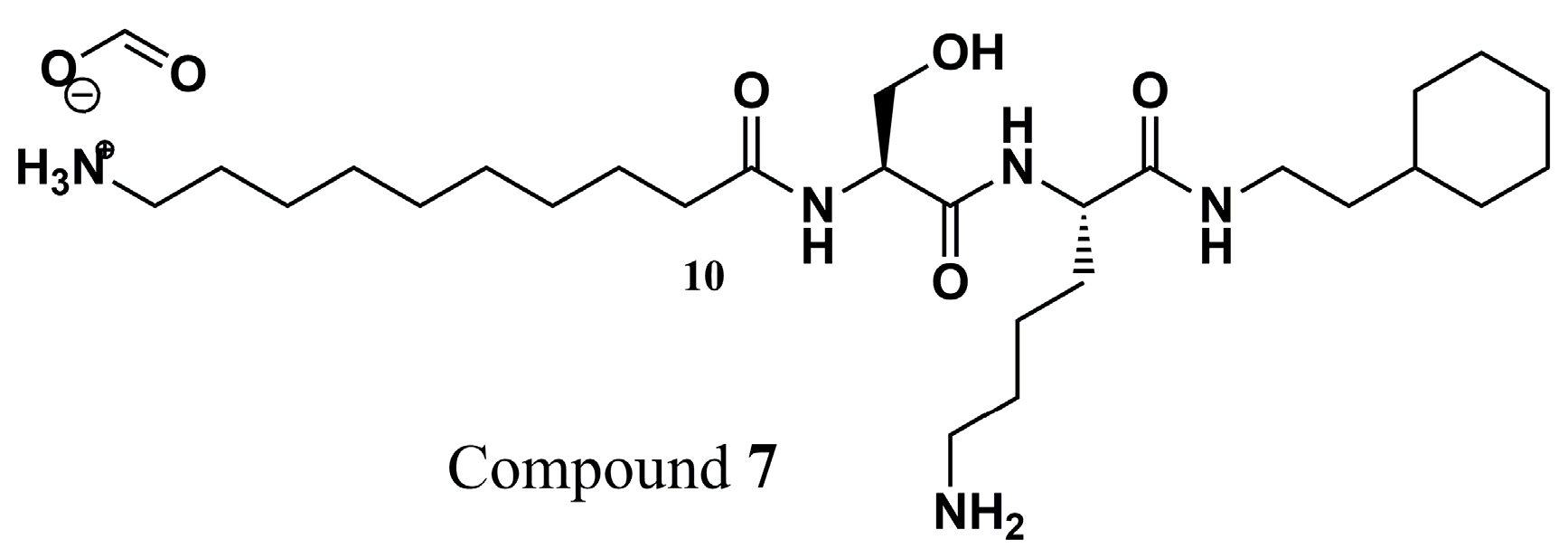



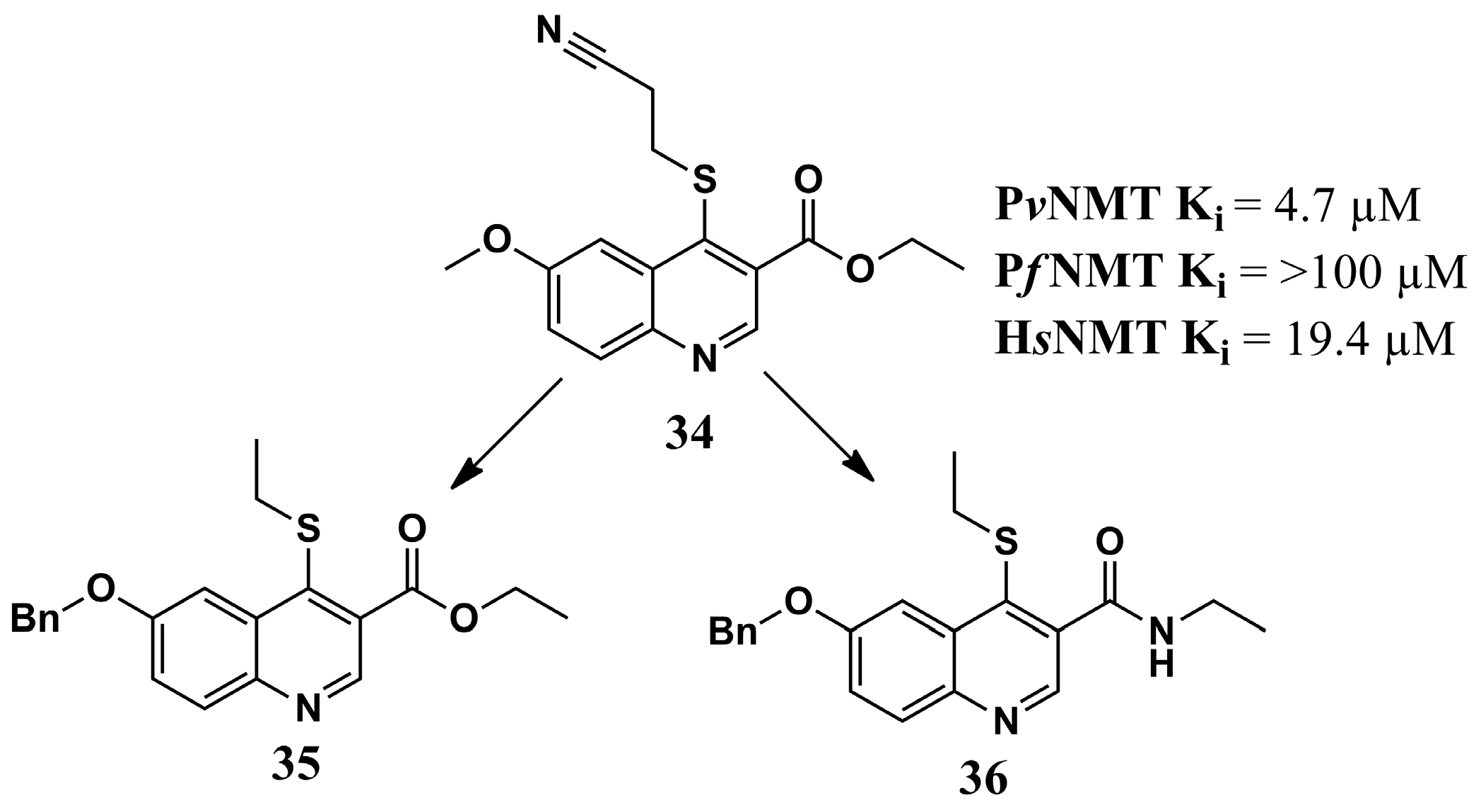
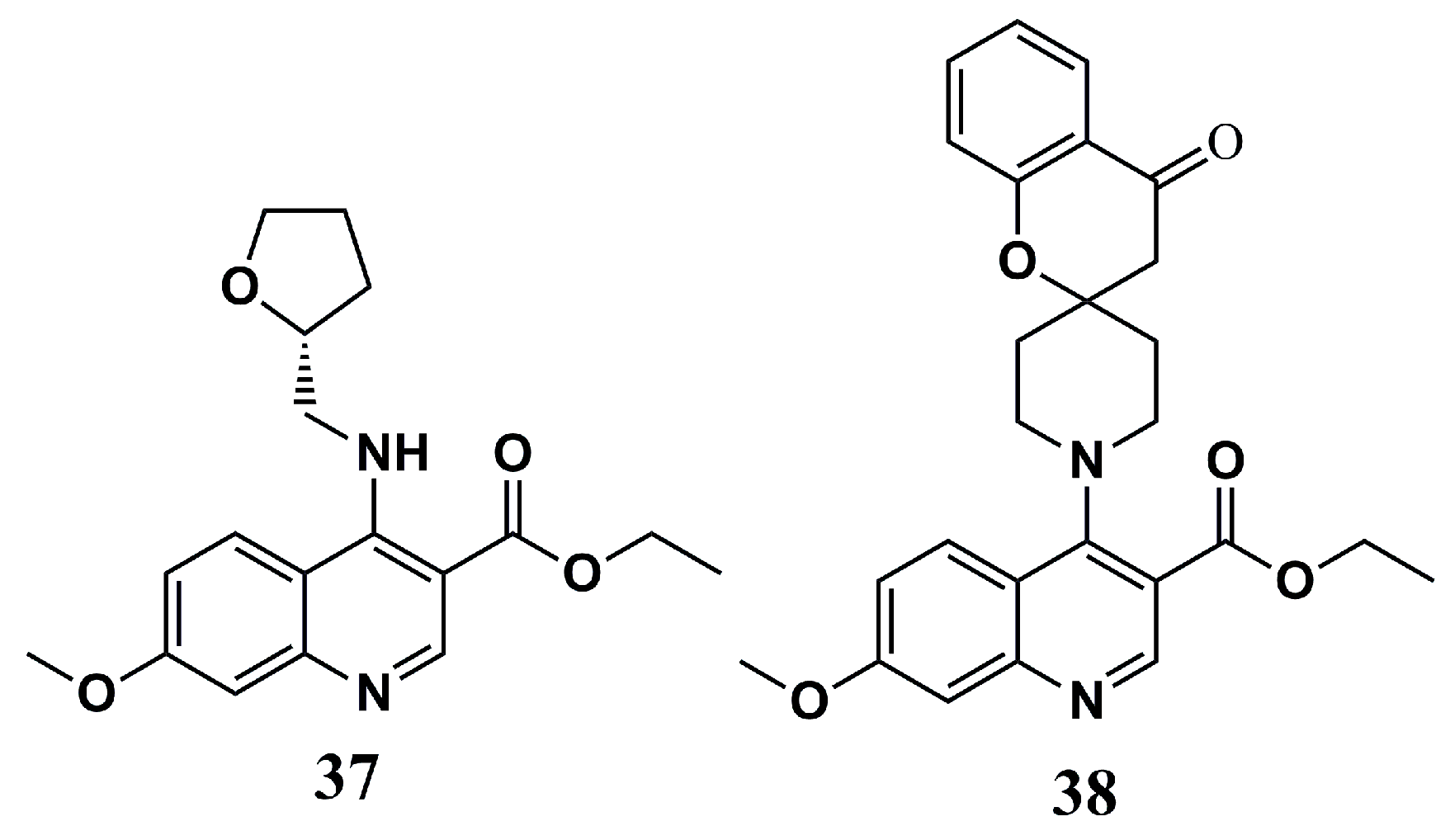
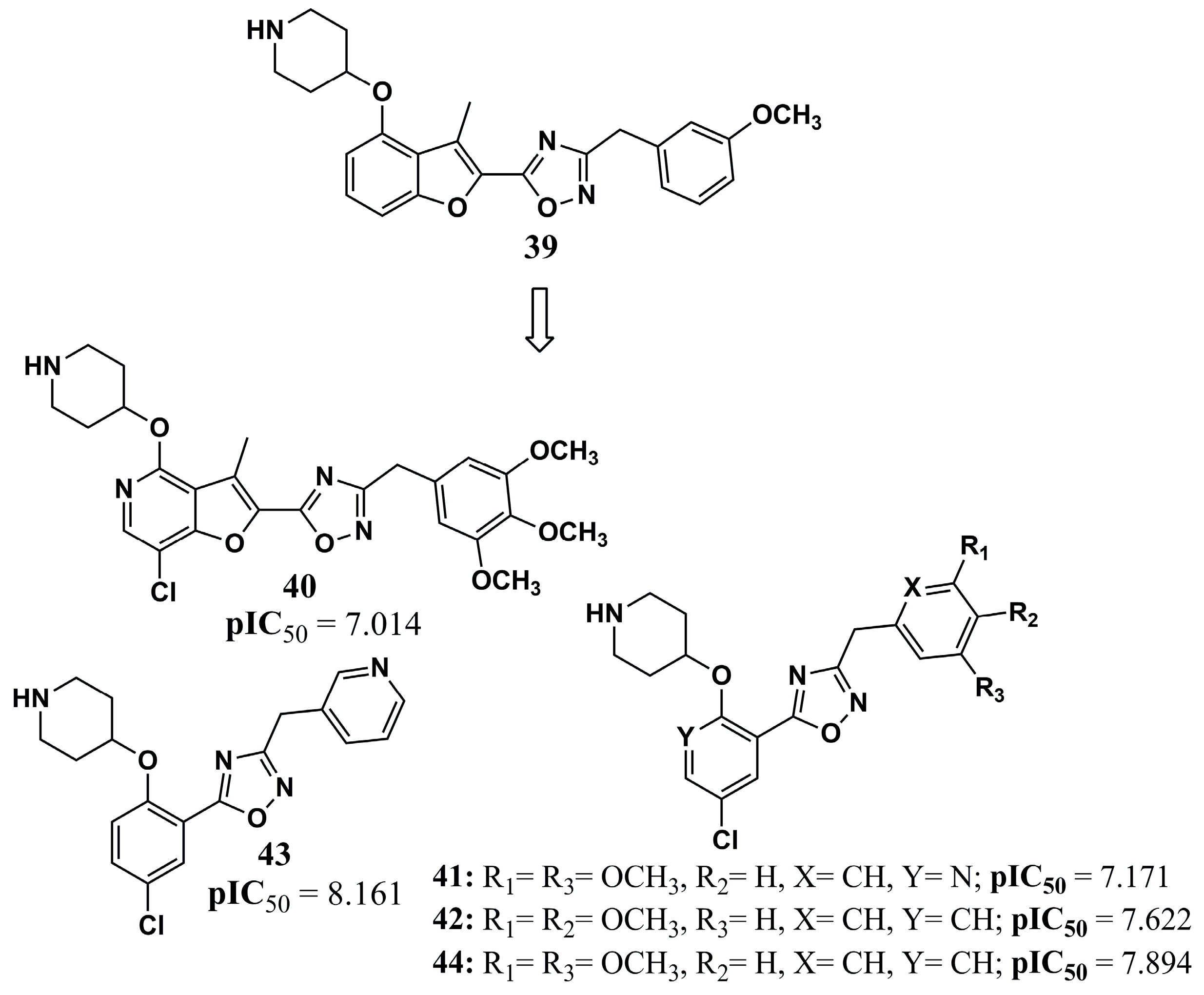

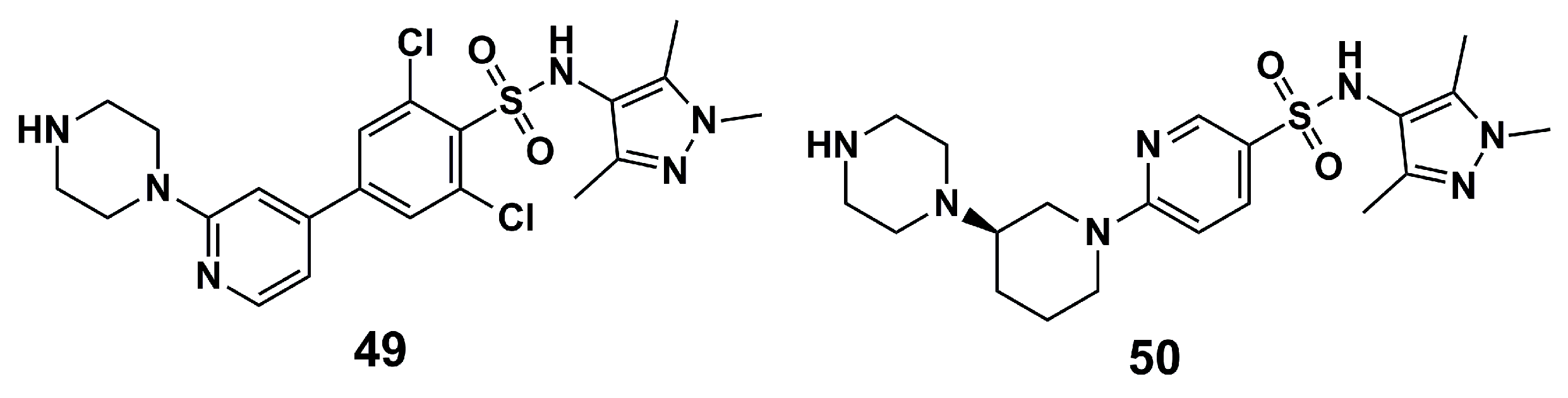
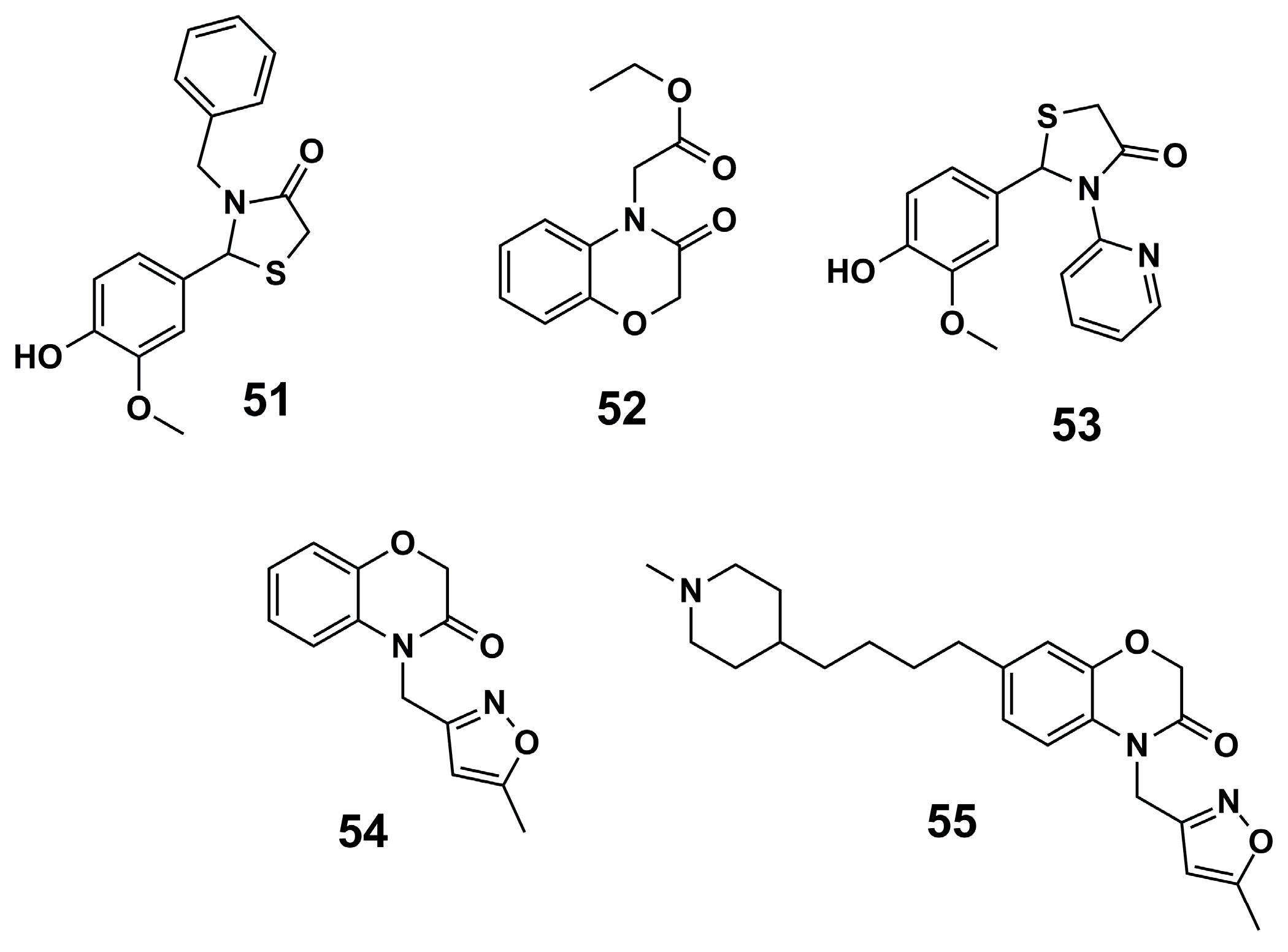
| N° | Proposed Disease | Activity Value | Ref. | N° | Proposed Disease | Activity Value | Ref. |
|---|---|---|---|---|---|---|---|
| (1) | Leishmaniasis | LmNMT: Ki = 250 nM | [86] | (29) | Malaria | - | [98] |
| (2) | Leishmaniasis | LmNMT: Ki = 248 nM | [86] | (30) | Malaria | - | [98] |
| (3) | Leishmaniasis | LmNMT: Ki = 250.86 nM | [86] | (31) | Malaria | - | [98] |
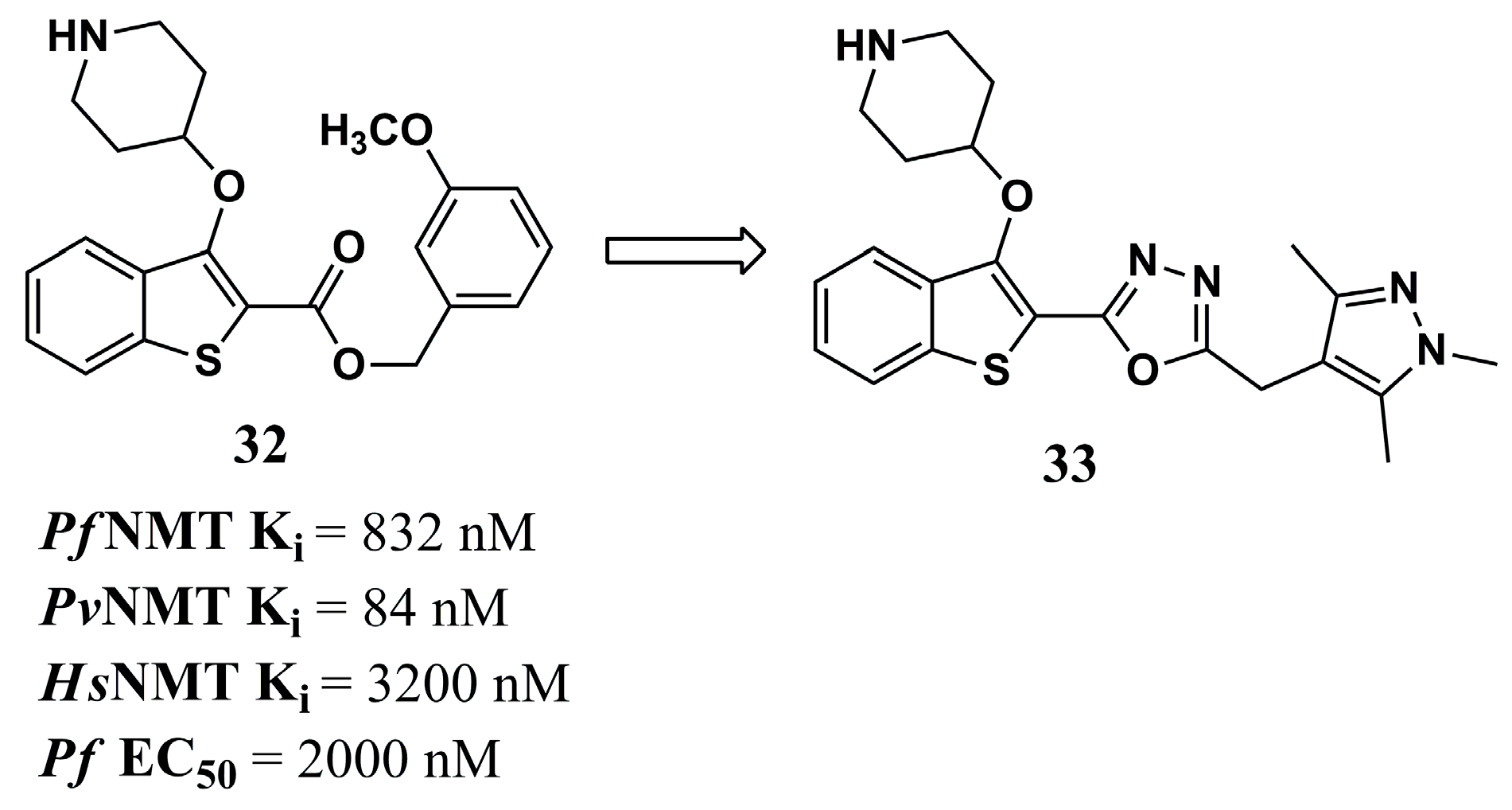
| (4) | Leishmaniasis | LmNMT: Ki = 235.74 nM | [86] | (32) | Malaria | PfNMT: Ki = 832 nM PvNMT: Ki = 84 nM | [81] |
| (5) | Leishmaniasis | - | [86] | (33) | Malaria | PfNMT: Ki = 8 nM PvNMT: Ki = 2 nM P. falciparum: EC50 = 302 nM | [81] |
| (6) | Leishmaniasis | LmNMT: Ki = 912.48 nM | [86] | (34) | Malaria | PvNMT: Ki = 4.7 µM PfNMT: Ki = >100 µM | [78] |
| (7) | Leishmaniasis | LdNMT: IC50 = 0.024 ± 0.003 µM HsNMT: IC50 = 0.06 ± 0.003 µM | [88] | (35) | Malaria | PvNMT: Ki = 0.44 µM PfNMT: Ki = 0.67 µM | [78] |
| (8) | Leishmaniasis | LdNMT: IC50 = 0.482 µM | [80] | (36) | Malaria | PvNMT: Ki = 0.34 µM PfNMT: Ki = 0.96 µM | [78] |
| (9) | Leishmaniasis | LmNMT: IC50 = 0.318 µM | [80] | (37) | Malaria | PvNMT: Ki = 0.34 µM PfNMT: Ki = 0.96 µM Pf3D7: IC50 = 3.96 µM PfINDO: IC50 = 6.38 µM | [78] and [102] |
| (10) | Leishmaniasis | LdNMT: IC50 = 0.077 µM | [80] | (38) | Malaria | Pf3D7: IC50 = 6.71 µM PfINDO: IC50 = 2.8 µM | [102] |
| (11) | Leishmaniasis | LdNMT: IC50 = 0.158 µM | [80] | (39) | Malaria | PfNMT: pIC50 = 7.301 | [103] |
| (12) | Leishmaniasis | LdNMT: Ki = 100 nM L. donovani: EC50 > 50 µM (amastigotes) | [90] | (40) | Malaria | PfNMT: pIC50 = 7.014 | [103] |
| (13) | Leishmaniasis | LdNMT: Ki = 1.6 nM L. donovani: EC50 = 10–30 µM (amastigotes) | [90] | (41) | Malaria | PfNMT: pIC50 = 7.171 | [103] |
| (14) | Leishmaniasis | Docking score: −13.93 kcal/mol Binding energy: −102.46 kcal/mol | [91] | (42) | Malaria | PfNMT: pIC50 = 7.622 | [103] |
| (15) | Leishmaniasis | Docking score: −14.75 kcal/mol Binding energy: −83.01 kcal/mol | [91] | (43) | Malaria | PfNMT: pIC50 = 8.161 | [103] |
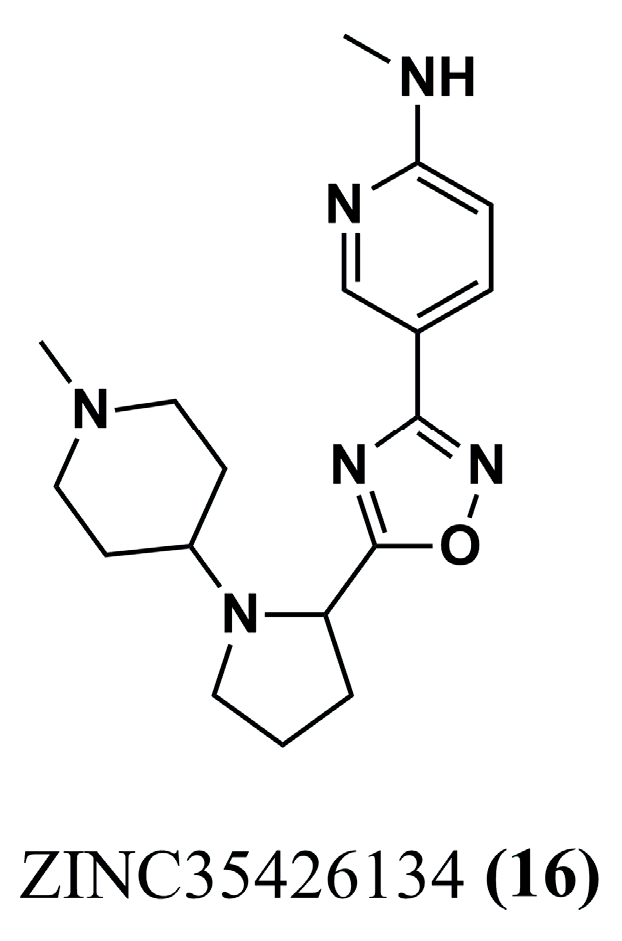
| (16) | Leishmaniasis | GRID score: −63.87 kcal/mol | [92] | (44) | Malaria | PfNMT: pIC50 = 7.894 | [103] |
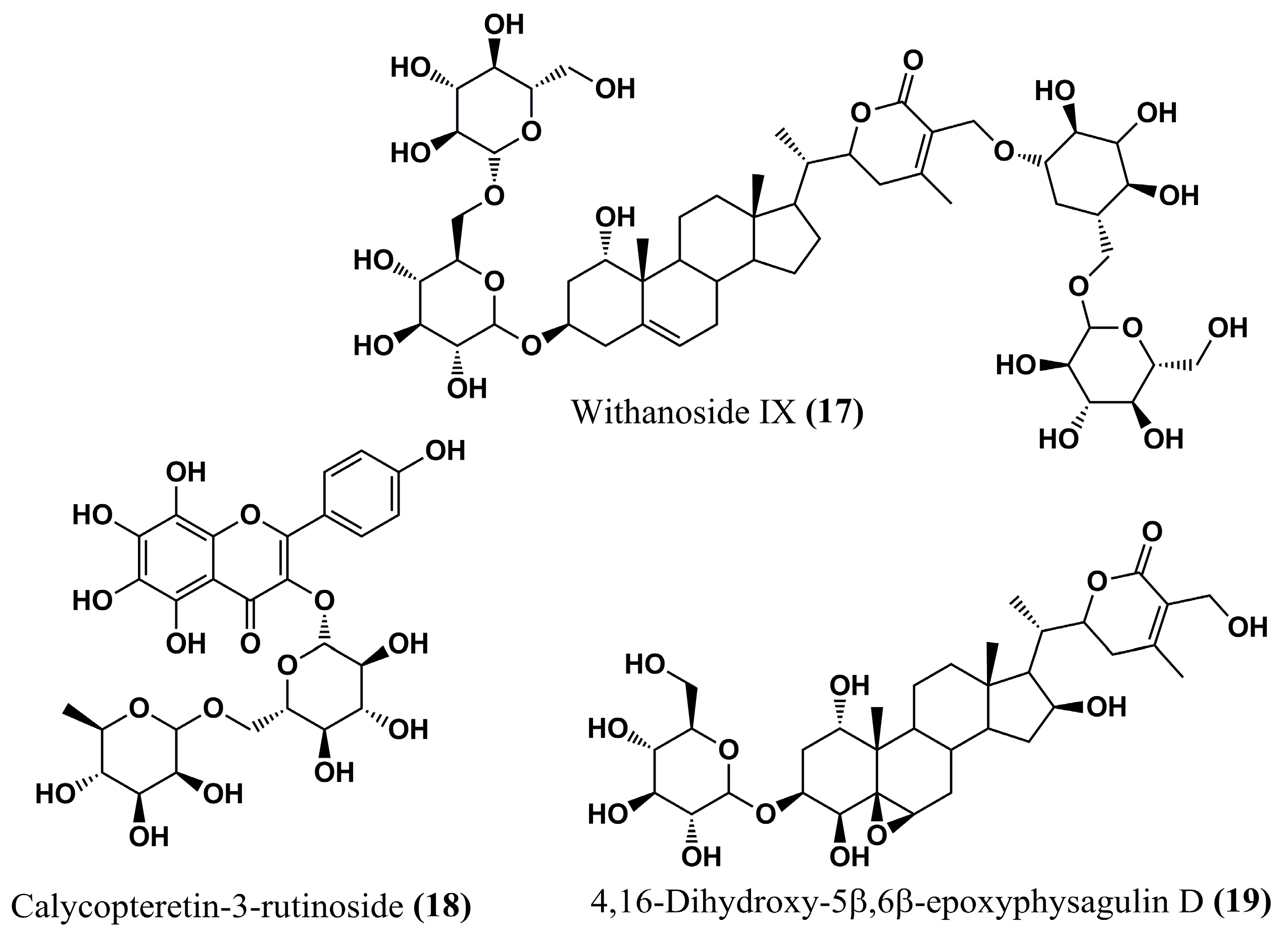
| (17) | Leishmaniasis | Binding affinity (docking): −22.2 kcal/mol | [93] | (45) | HAT | TbNMT: QSAR Analysis | [104] |
| (18) | Leishmaniasis | Binding affinity (docking): −23.3 kcal/mol | [93] | (46) | HAT | TbNMT: QSAR Analysis | [104] |
| (19) | Leishmaniasis | Binding affinity (docking): −24.0 kcal/mol | [93] | (47) | HAT | TbNMT: QSAR Analysis | [104] |
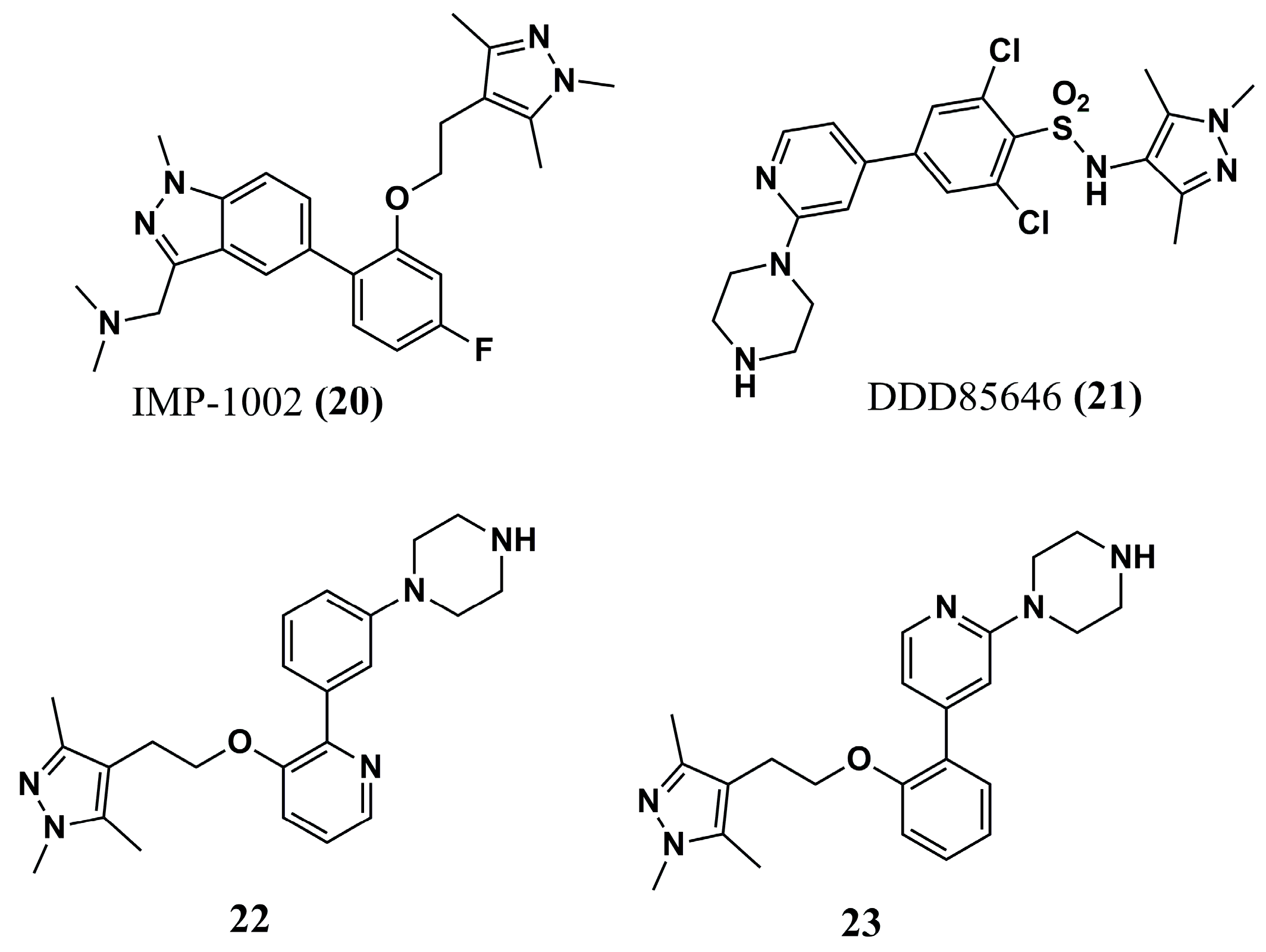
| (20) | Malaria | - | [96] | (48) | HAT Filariasis | TbNMT: QSAR Analysis CeNMT: IC50 = 10 nM BmNMT: IC50 = 10 nM | [104] and [110] |
| (21) | Malaria | - | [96] | (49) | HAT | TbNMT (predicted): pIC50 = 8.70 | [107] |
| (22) | Malaria | PvNMT: IC50 = 80.15 nM | [96] | (50) | HAT | TbNMT (predicted): pIC50 = 7.52 | [107] |
| (23) | Malaria | PvNMT: IC50 = 9.48 nM P. vivax: EC50 = 2.3–4.6 µM (schizonts) EC50 = 1.7 µM (hypnozoites) | [96] | (51) | HAT | TbNMT: IC50 = 22 µM T. brucei: EC50 > 50 µM | [108] |
| (24) | Malaria | PfNMT: Ki = 1.4 µM | [97] | (52) | HAT | TbNMT: IC50 = 12 µM T. brucei: EC50 > 50 µM | [108] |
| (25) | Malaria | PfNMT: Ki = 1.6 µM | [97] | (53) | HAT | T. brucei: IC50 = 0.27 µM EC50 = 6.3 µM | [108] |
| (26) | Malaria | PfNMT: Ki = 0.95 µM | [97] | (54) | HAT | TbNMT: IC50 = 2.9 µM | [108] |
| (27) | Malaria | PvNMT: Ki = 0.027 µM | [97] | (55) | HAT | TbNMT: IC50 < 0.002 µM T. brucei: EC50 = 0.007 µM | [108] |
| (28) | Malaria | PfNMT: Ki = 0.0017 µM | [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, F.d.F.G.R.; Moura, W.C.d.S.; Romário-Silva, D.; Araújo, R.S.A.d.; Morais, I.; Cortes, S.; Nogueira, F.; Moura, R.O.d.; Nascimento, I.J.d.S. N-Myristoyltransferase Inhibition in Parasitic Pathogens: Insights from Computer-Aided Drug Design. Molecules 2025, 30, 3703. https://doi.org/10.3390/molecules30183703
Campos FdFGR, Moura WCdS, Romário-Silva D, Araújo RSAd, Morais I, Cortes S, Nogueira F, Moura ROd, Nascimento IJdS. N-Myristoyltransferase Inhibition in Parasitic Pathogens: Insights from Computer-Aided Drug Design. Molecules. 2025; 30(18):3703. https://doi.org/10.3390/molecules30183703
Chicago/Turabian StyleCampos, Fernanda de França Genuíno Ramos, Willian Charles da Silva Moura, Diego Romário-Silva, Rodrigo Santos Aquino de Araújo, Inês Morais, Sofia Cortes, Fátima Nogueira, Ricardo Olimpio de Moura, and Igor José dos Santos Nascimento. 2025. "N-Myristoyltransferase Inhibition in Parasitic Pathogens: Insights from Computer-Aided Drug Design" Molecules 30, no. 18: 3703. https://doi.org/10.3390/molecules30183703
APA StyleCampos, F. d. F. G. R., Moura, W. C. d. S., Romário-Silva, D., Araújo, R. S. A. d., Morais, I., Cortes, S., Nogueira, F., Moura, R. O. d., & Nascimento, I. J. d. S. (2025). N-Myristoyltransferase Inhibition in Parasitic Pathogens: Insights from Computer-Aided Drug Design. Molecules, 30(18), 3703. https://doi.org/10.3390/molecules30183703










