Advancing Brain Health Naturally: β-Caryophyllene and Xanthohumol as Neuroprotective Agents
Abstract
1. Introduction
2. Results and Discussion
2.1. Studies Evaluating β-Caryophyllene Activity on NDDS
2.2. Studies Evaluating Xanthohumol Activity on NDDS
2.3. Drug-Delivery Systems for β-Caryophyllene
| Carrier Type | Polymer/Lipid | Impact on BCP Characteristics | Ref. |
|---|---|---|---|
| Nanostructured lipid carriers | Compritol 888ATO and linseed oil | ↑ Solubility, ↑ stability, and cumulative release | [70] |
| Lipid nanocarriers | Medium-chain triglyceride, coconut oil, cocoa butter, olive oil, soybean oil | ↑ Stability, ↑ bioaccessibility | [79] |
| Self-emulsifying drug-delivery system (VESIsorb®) | Medium-chain triglycerides, natural vegetable oils, PEG | ↑ Oral bioavailability, ↓ inter-individual variability, and fast absorption | [71] |
| Liposomes | Soybean phosphatidylcholine | ↑ Dispersibility, ↑ cellular uptake, | [72] |
| Phospholipids | Neuroprotection, BBB repair, ↓brain edema | [74] | |
| Liposomal powder (Rephyll®) | Phospholipids | ↑ Stability, sustained release, ↑ clinical efficacy | [73] |
| Hydrogel containing nanoemulsified BCP | Hydroxyethyl cellulose | ↑ Stability, controlled release, ↑anti-inflammatory activity | [75] |
| Microemulsion hydrogel | Isopropyl myristate, Phospholipon 90, Carbopol 940 | ↑ Skin permeation, ↑ anti-inflammatory activity | [76] |
| Microemulsion | Copaiba oil-resin | ↑ Antimicrobial and anti-inflammatory activity | [77] |
| Nanoemulsion | Medium chain triglycerides (capric and caprylic acids) | Dose reduction, ↑ anti-inflammatory effect | [78] |
| Lecithin, oleylamine | Direct brain delivery, ↑ anticonvulsant effect | [86] | |
| Cyclodextrin inclusion complexes | Methyl-β-cyclodextrin β-cyclodextrin | ↑ Solubility, ↑ stability, ↑ oral bioavailability | [81,82] |
| PEGylated nanoparticles | PEG 400, polyvinyl alcohol (PVA), poly-caprolactone (PCL), β-CD, and chitosan | Controlled release, ↑ stability, ↑ ability to permeate BBB | [89] |
| Nanoparticles | PEG, PLGA | ↑ Stability, prolonged circulation, ↑ oral bioavailability | [84] |
| Chitosan | ↑ Mucoadhesion and retention, ↓ toxicity | [85] | |
| Fibrous carriers | Natural fibers (hemp, coconut, rice) | ↑ mucosal absorption, controlled release, | US10933016B2 |
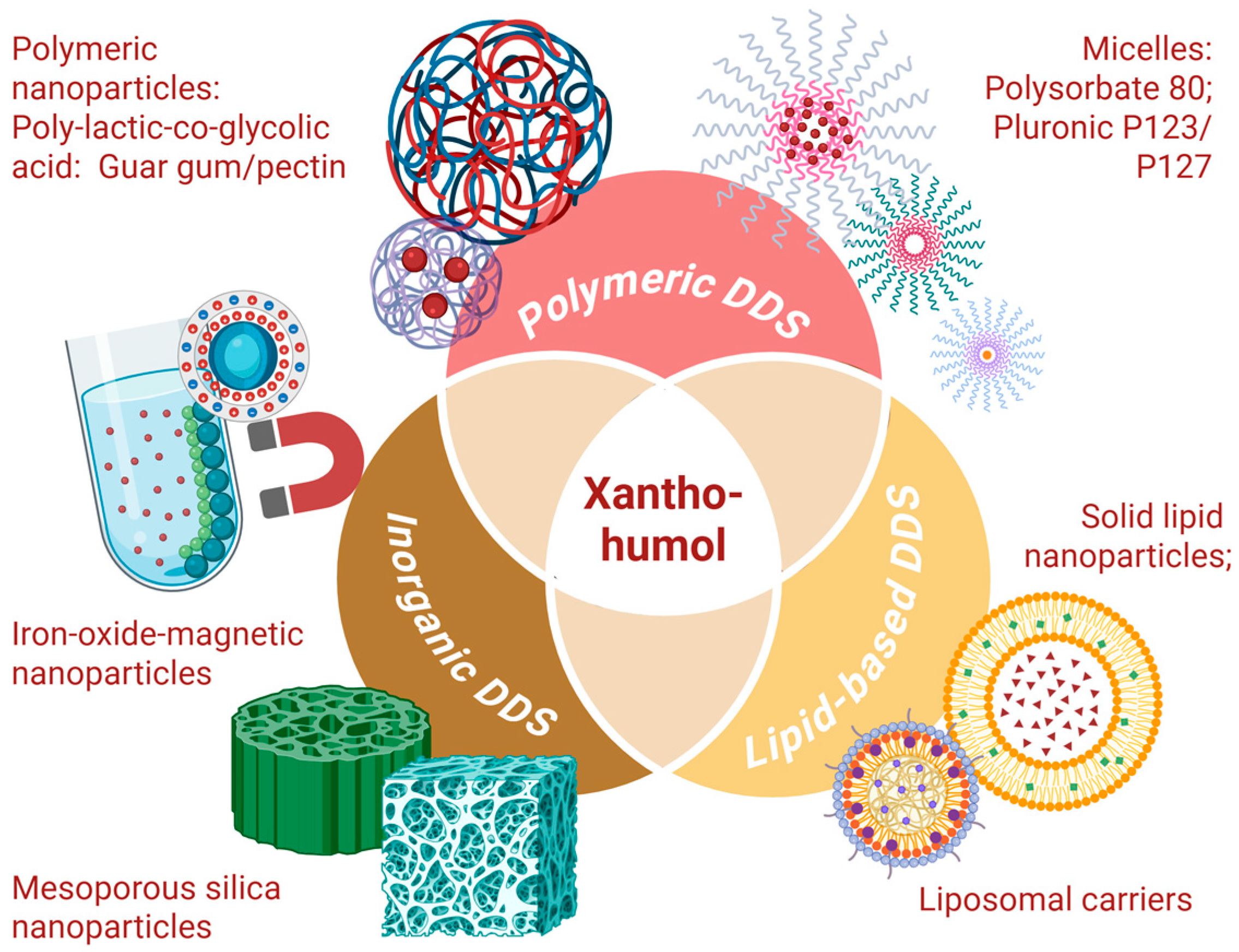
2.4. Drug-Delivery Systems for Xanthohumol
2.4.1. Polymeric DDS for Xanthohumol
2.4.2. Lipid-Based DDS for Xanthohumol
2.4.3. Inorganic Carriers for Xanthohumol
2.5. Clinical Trials and Future Perspectives
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| XAN | Xanthohumol |
| NDDs | Neurodegenerative diseases |
| AD | Alzheimer’s disease |
| ALS | amyotrophic lateral sclerosis |
| BCP | β-Caryophyllene |
| BBB | Blood–brain barrier |
| CB2 | Cannabinoid receptor 2 |
| HLB | Hydrophilic lipophilic balance |
| MβCD | Methyl-β-cyclodextrin |
| MS | Multiple sclerosis |
| NO | Nitric oxide |
| PLGA | Poly(lactic-co-glycolic) acid |
| PEG | Polyethylene glycol |
| PCL | Poly-caprolactone |
| PPARγ | Peroxisome proliferatoractivated receptor-γ |
| PVA | Polyvinyl alcohol |
| TC | Trans-caryophyllene |
| TNBC | Triple-negative breast cancer |
| CD | Cyclodextrin |
| DDS | Drug-delivery system |
| TNF-α | Tumor necrosis factor α |
| IL-6 | Interleukin 6 |
| DSC | Differential scanning calorimetry |
| PXRD | Powder x-ray diffraction |
| FDA | Food and Drug Administration |
References
- Lamptey, R.N.L.; Chaulagain, B.; Trivedi, R.; Gothwal, A.; Layek, B.; Singh, J. A Review of the Common Neurodegenerative Disorders: Current Therapeutic Approaches and the Potential Role of Nanotherapeutics. IJMS 2022, 23, 1851. [Google Scholar] [CrossRef]
- Kovacs, G.G. Concepts and Classification of Neurodegenerative Diseases. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 145, pp. 301–307. ISBN 978-0-12-802395-2. [Google Scholar]
- Relja, M. Pathophysiology and Classification of Neurodegenerative Diseases. EJIFCC 2004, 15, 97–99. [Google Scholar]
- Bates, G.P.; Dorsey, R.; Gusella, J.F.; Hayden, M.R.; Kay, C.; Leavitt, B.R.; Nance, M.; Ross, C.A.; Scahill, R.I.; Wetzel, R.; et al. Huntington Disease. Nat. Rev. Dis. Primers 2015, 1, 15005. [Google Scholar] [CrossRef] [PubMed]
- Holland, N.; Robbins, T.W.; Rowe, J.B. The Role of Noradrenaline in Cognition and Cognitive Disorders. Brain 2021, 144, 2243–2256. [Google Scholar] [CrossRef]
- Postuma, R.B.; Aarsland, D.; Barone, P.; Burn, D.J.; Hawkes, C.H.; Oertel, W.; Ziemssen, T. Identifying Prodromal Parkinson’s Disease: Pre-Motor Disorders in Parkinson’s Disease. Mov. Disord. 2012, 27, 617–626. [Google Scholar] [CrossRef]
- Kasanuki, K.; Josephs, K.A.; Ferman, T.J.; Murray, M.E.; Koga, S.; Konno, T.; Sakae, N.; Parks, A.; Uitti, R.J.; Van Gerpen, J.A.; et al. Diffuse Lewy Body Disease Manifesting as Corticobasal Syndrome: A Rare Form of Lewy Body Disease. Neurology 2018, 91, e268–e279. [Google Scholar] [CrossRef]
- Saranza, G.M.; Whitwell, J.L.; Kovacs, G.G.; Lang, A.E. Corticobasal Degeneration. In International Review of Neurobiology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 149, pp. 87–136. ISBN 978-0-12-817730-3. [Google Scholar]
- Rahul; Siddique, Y. Neurodegenerative Disorders and the Current State, Pathophysiology, andManagement of Parkinson’s Disease. CNSNDDT 2022, 21, 574–595. [Google Scholar] [CrossRef]
- Gupta, R.; Advani, D.; Yadav, D.; Ambasta, R.K.; Kumar, P. Dissecting the Relationship Between Neuropsychiatric and Neurodegenerative Disorders. Mol. Neurobiol. 2023, 60, 6476–6529. [Google Scholar] [CrossRef] [PubMed]
- Chopade, P.; Chopade, N.; Zhao, Z.; Mitragotri, S.; Liao, R.; Chandran Suja, V. Alzheimer’s and Parkinson’s Disease Therapies in the Clinic. Bioeng. Transl. Med. 2023, 8, e10367. [Google Scholar] [CrossRef] [PubMed]
- Bordoloi, S.; Pathak, K.; Devi, M.; Saikia, R.; Das, J.; Kashyap, V.H.; Das, D.; Ahmad, M.Z.; Abdel-Wahab, B.A. Some Promising Medicinal Plants Used in Alzheimer’s Disease: An Ethnopharmacological Perspective. Discov. Appl. Sci. 2024, 6, 215. [Google Scholar] [CrossRef]
- Tavan, M.; Hanachi, P.; de la Luz Cádiz-Gurrea, M.; Segura Carretero, A.; Mirjalili, M.H. Natural Phenolic Compounds with Neuroprotective Effects. Neurochem. Res. 2024, 49, 306–326. [Google Scholar] [CrossRef] [PubMed]
- Assis, L.C.; Straliotto, M.R.; Engel, D.; Hort, M.A.; Dutra, R.C.; Bem, A.F. de β-Caryophyllene Protects the C6 Glioma Cells against Glutamate-Induced Excitotoxicity through the Nrf2 Pathway. Neuroscience 2014, 279, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Santos, N.A.G.; Martins, N.M.; Sisti, F.M.; Fernandes, L.S.; Ferreira, R.S.; de Freitas, O.; Santos, A.C. The Cannabinoid Beta-Caryophyllene (BCP) Induces Neuritogenesis in PC12 Cells by a Cannabinoid-Receptor-Independent Mechanism. Chem.-Biol. Interact. 2017, 261, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zeng, Z.; Wang, B.; Guo, S. Trans-Caryophyllene Inhibits Amyloid β (Aβ) Oligomer-Induced Neuroinflammation in BV-2 Microglial Cells. Int. Immunopharmacol. 2017, 51, 91–98. [Google Scholar] [CrossRef]
- Wang, G.; Ma, W.; Du, J. β-Caryophyllene (BCP) Ameliorates MPP+ Induced Cytotoxicity. Biomed. Pharmacother. 2018, 103, 1086–1091. [Google Scholar] [CrossRef]
- Askari, V.R.; Shafiee-Nick, R. Promising Neuroprotective Effects of β-Caryophyllene against LPS-Induced Oligodendrocyte Toxicity: A Mechanistic Study. Biochem. Pharmacol. 2019, 159, 154–171. [Google Scholar] [CrossRef]
- Askari, V.R.; Shafiee-Nick, R. The Protective Effects of β-Caryophyllene on LPS-Induced Primary Microglia M1/M2 Imbalance: A Mechanistic Evaluation. Life Sci. 2019, 219, 40–73. [Google Scholar] [CrossRef]
- Gouthamchandra, K.; Venkataramana, S.H.; Sathish, A.; Amritharaj; Basavegowda, L.H.; Puttaswamy, N.; Kodimule, S.P. Anti-Inflammatory and Neuroprotective Activity of Viphyllin a Standardized Extract of β-Caryophyllene from Black Pepper (Piper nigrum L.) and Its Associated Mechanisms in Mouse Macrophage Cells and Human Neuroblastoma SH-SY5Y Cells. Fortune J. Health Sci. 2024, 7, 25–36. [Google Scholar]
- Zhang, Y.; Huang, Q.; Wang, S.; Liao, Z.; Jin, H.; Huang, S.; Hong, X.; Liu, Y.; Pang, J.; Shen, Q.; et al. The Food Additive β-Caryophyllene Exerts Its Neuroprotective Effects Through the JAK2-STAT3-BACE1 Pathway. Front. Aging Neurosci. 2022, 14, 814432. [Google Scholar] [CrossRef]
- Anbaraki, A.; Dindar, Z.; Mousavi-Jarrahi, Z.; Ghasemi, A.; Moeini, Z.; Evini, M.; Saboury, A.A.; Seyedarabi, A. The Novel Anti-Fibrillary Effects of Volatile Compounds α-Asarone and β-Caryophyllene on Tau Protein: Towards Promising Therapeutic Agents for Alzheimer’s Disease. Int. J. Biol. Macromol. 2024, 271, 132401. [Google Scholar] [CrossRef]
- Rathod, S.S.; Agrawal, Y.O. β-Caryophyllene (CB2 Agonist) Mitigates Rotenone-Induced Neurotoxicity and Apoptosis in SH-SY5Y Neuroblastoma Cells via Modulation of GSK-3β/NRF2/HO-1 Axis. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 1–21. [Google Scholar] [CrossRef]
- Fontes, L.B.A.; Dias, D.D.S.; Aarestrup, B.J.V.; Aarestrup, F.M.; Da Silva Filho, A.A.; do Amaral Corrêa, J.O. β-Caryophyllene Ameliorates the Development of Experimental Autoimmune Encephalomyelitis in C57BL/6 Mice. Biomed. Pharmacother. 2017, 91, 257–264. [Google Scholar] [CrossRef]
- Machado, K.D.C.; Paz, M.F.C.J.; Oliveira Santos, J.V.D.; da Silva, F.C.C.; Tchekalarova, J.D.; Salehi, B.; Islam, M.T.; Setzer, W.N.; Sharifi-Rad, J.; de Castro e Sousa, J.M.; et al. Anxiety Therapeutic Interventions of β-Caryophyllene: A Laboratory-Based Study. Nat. Product. Commun. 2020, 15, 1934578X20962229. [Google Scholar] [CrossRef]
- Cheng, Y.; Dong, Z.; Liu, S. β-Caryophyllene Ameliorates the Alzheimer-Like Phenotype in APP/PS1 Mice through CB2 Receptor Activation and the PPARγ Pathway. Pharmacology 2014, 94, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ojha, S.; Javed, H.; Azimullah, S.; Haque, M.E. β-Caryophyllene, a Phytocannabinoid Attenuates Oxidative Stress, Neuroinflammation, Glial Activation, and Salvages Dopaminergic Neurons in a Rat Model of Parkinson Disease. Mol. Cell Biochem. 2016, 418, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Viveros-Paredes, J.M.; González-Castañeda, R.E.; Gertsch, J.; Chaparro-Huerta, V.; López-Roa, R.I.; Vázquez-Valls, E.; Beas-Zarate, C.; Camins-Espuny, A.; Flores-Soto, M.E. Neuroprotective Effects of β-Caryophyllene against Dopaminergic Neuron Injury in a Murine Model of Parkinson’s Disease Induced by MPTP. Pharmaceuticals 2017, 10, 60. [Google Scholar] [CrossRef]
- Alberti, T.B.; Barbosa, W.L.R.; Vieira, J.L.F.; Raposo, N.R.B.; Dutra, R.C. (−)-β-Caryophyllene, a CB2 Receptor-Selective Phytocannabinoid, Suppresses Motor Paralysis and Neuroinflammation in a Murine Model of Multiple Sclerosis. Int. J. Mol. Sci. 2017, 18, 691. [Google Scholar] [CrossRef]
- Lou, J.; Teng, Z.; Zhang, L.; Yang, J.; Ma, L.; Wang, F.; Tian, X.; An, R.; Yang, M.; Zhang, Q.; et al. β-Caryophyllene/Hydroxypropyl-β-Cyclodextrin Inclusion Complex Improves Cognitive Deficits in Rats with Vascular Dementia through the Cannabinoid Receptor Type 2-Mediated Pathway. Front. Pharmacol. 2017, 8, 2. [Google Scholar] [CrossRef]
- Mesquita, H.L.; Fontes, L.B.A.; Cinsa, L.A.; Filho, A.A.D.S.; Nagato, A.C.; Aarestrup, B.J.V.; do Amaral Corrêa, J.O.; Aarestrup, F.M. β-Caryophyllene Causes Remyelination and Modifies Cytokines Expression in C57BL/6 Mice with Experimental Autoimmune Encephalomyelitis. J. App Pharm. Sci. 2019, 9, 27–33. [Google Scholar] [CrossRef][Green Version]
- Kanojia, U.; Chaturbhuj, S.G.; Sankhe, R.; Das, M.; Surubhotla, R.; Krishnadas, N.; Gourishetti, K.; Nayak, P.G.; Kishore, A. Beta-Caryophyllene, a CB2R Selective Agonist, Protects Against Cognitive Impairment Caused by Neuro-Inflammation and Not in Dementia Due to Ageing Induced by Mitochondrial Dysfunction. CNS Neurol. Disord. Drug Targets 2021, 20, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Flores-Soto, M.E.; Corona-Angeles, J.A.; Tejeda-Martinez, A.R.; Flores-Guzman, P.A.; Luna-Mujica, I.; Chaparro-Huerta, V.; Viveros-Paredes, J.M. β-Caryophyllene Exerts Protective Antioxidant Effects through the Activation of NQO1 in the MPTP Model of Parkinson’s Disease. Neurosci. Lett. 2021, 742, 135534. [Google Scholar] [CrossRef]
- Askari, V.R.; Baradaran Rahimi, V.; Shafiee-Nick, R. Low Doses of β-Caryophyllene Reduced Clinical and Paraclinical Parameters of an Autoimmune Animal Model of Multiple Sclerosis: Investigating the Role of CB2 Receptors in Inflammation by Lymphocytes and Microglial. Brain Sci. 2023, 13, 1092. [Google Scholar] [CrossRef] [PubMed]
- Brand-Rubalcava, P.A.; Tejeda-Martínez, A.R.; González-Reynoso, O.; Nápoles-Medina, A.Y.; Chaparro-Huerta, V.; Flores-Soto, M.E. β-Caryophyllene Decreases Neuroinflammation and Exerts Neuroprotection of Dopaminergic Neurons in a Model of Hemiparkinsonism through Inhibition of the NLRP3 Inflammasome. Park. Relat. Disord. 2023, 117, 105906. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Molina, A.R.; Tejeda-Martínez, A.R.; Viveros-Paredes, J.M.; Chaparro-Huerta, V.; Urmeneta-Ortíz, M.F.; Ramírez-Jirano, L.J.; Flores-Soto, M.E. Beta-Caryophyllene Inhibits the Permeability of the Blood–Brain Barrier in MPTP-Induced Parkinsonism. Neurología 2025, 40, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.J.; Gomez-Pinilla, F.; Ling, P.-R. Beta-Caryophyllene, An Anti-Inflammatory Natural Compound, Improves Cognition. J. Food Nutr. Sci. 2021, 3, 31–43. [Google Scholar]
- Stevens, J.F.; Page, J.E. Xanthohumol and Related Prenylflavonoids from Hops and Beer: To Your Good Health! Phytochemistry 2004, 65, 1317–1330. [Google Scholar] [CrossRef]
- Kundu, P.; Holden, S.; Paraiso, I.L.; Sudhakar, R.; McQuesten, C.; Choi, J.; Miranda, C.L.; Maier, C.S.; Bobe, G.; Stevens, J.F.; et al. ApoE Isoform-Dependent Effects of Xanthohumol on High Fat Diet-Induced Cognitive Impairments and Hippocampal Metabolic Pathways. Front. Pharmacol. 2022, 13, 954980. [Google Scholar] [CrossRef]
- Min, B.; Park, C.W.; Ahn, Y.; Hong, K.-B.; Cho, H.-J.; Lee, J.H.; Jo, K.; Suh, H.J. Effect of Hop Mixture Containing Xanthohumol on Sleep Enhancement in a Mouse Model and ROS Scavenging Effect in Oxidative Stress-Induced HT22 Cells. Food Sci. Technol. 2022, 42, e29922. [Google Scholar] [CrossRef]
- Urmann, C.; Bieler, L.; Priglinger, E.; Aigner, L.; Couillard-Despres, S.; Riepl, H.M. Neuroregenerative Potential of Prenyl- and Pyranochalcones: A Structure–Activity Study. J. Nat. Prod. 2021, 84, 2675–2682. [Google Scholar] [CrossRef]
- Zamzow, D.R.; Elias, V.; Legette, L.L.; Choi, J.; Stevens, J.F.; Magnusson, K.R. Xanthohumol Improved Cognitive Flexibility in Young Mice. Behav. Brain Res. 2014, 275, 1–10. [Google Scholar] [CrossRef]
- Legette, L.; Ma, L.; Reed, R.L.; Miranda, C.L.; Christensen, J.M.; Rodriguez-Proteau, R.; Stevens, J.F. Pharmacokinetics of Xanthohumol and Metabolites in Rats after Oral and Intravenous Administration. Mol. Nutr. Food Res. 2012, 56, 466–474. [Google Scholar] [CrossRef]
- Sasazawa, Y.; Kanagaki, S.; Tashiro, E.; Nogawa, T.; Muroi, M.; Kondoh, Y.; Osada, H.; Imoto, M. Xanthohumol Impairs Autophagosome Maturation through Direct Inhibition of Valosin-Containing Protein. ACS Chem. Biol. 2012, 7, 892–900. [Google Scholar] [CrossRef]
- Yao, J.; Zhang, B.; Ge, C.; Peng, S.; Fang, J. Xanthohumol, a Polyphenol Chalcone Present in Hops, Activating Nrf2 Enzymes To Confer Protection against Oxidative Damage in PC12 Cells. J. Agric. Food Chem. 2015, 63, 1521–1531. [Google Scholar] [CrossRef]
- Orhan, I.E.; Jedrejek, D.; Senol, F.S.; Salmas, R.E.; Durdagi, S.; Kowalska, I.; Pecio, L.; Oleszek, W. Molecular Modeling and in Vitro Approaches towards Cholinesterase Inhibitory Effect of Some Natural Xanthohumol, Naringenin, and Acyl Phloroglucinol Derivatives. Phytomedicine 2018, 42, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, J.; Chen, X.; Liu, P.; Wang, S.; Song, F.; Zhang, Z.; Zhu, F.; Huang, X.; Liu, J.; et al. The Prenylflavonoid Xanthohumol Reduces Alzheimer-Like Changes and Modulates Multiple Pathogenic Molecular Pathways in the Neuro2a/APPswe Cell Model of AD. Front. Pharmacol. 2018, 9, 199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wu, Q.; Yao, X.; Zhao, J.; Zhong, W.; Liu, Q.; Xiao, S. Xanthohumol Inhibits Tau Protein Aggregation and Protects Cells against Tau Aggregates. Food Funct. 2019, 10, 7865–7874. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.; Zhang, B.; Hou, Y.; Yao, J.; Xu, Q.; Xu, J.; Fang, J. Xanthohumol Analogues as Potent Nrf2 Activators against Oxidative Stress Mediated Damages of PC12 Cells. ACS Chem. Neurosci. 2019, 10, 2956–2966. [Google Scholar] [CrossRef]
- Donoso, F.; Ramírez, V.T.; Golubeva, A.V.; Moloney, G.M.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Naturally Derived Polyphenols Protect Against Corticosterone-Induced Changes in Primary Cortical Neurons. Int. J. Neuropsychopharmacol. 2019, 22, 765–777. [Google Scholar] [CrossRef]
- Alonso, P.; Albasanz, J.L.; Martín, M. Modulation of Adenosine Receptors by Hops and Xanthohumol in Cell Cultures. ACS Chem. Neurosci. 2021, 12, 2373–2384. [Google Scholar] [CrossRef]
- Molteni, L.; Bruzzone, C.; Ami, D.; De Luigi, A.; Colombo, L.; Moretti, L.; Natalello, A.; Palmioli, A.; Airoldi, C. Xanthohumol Destabilizes the Structure of Amyloid-β (Aβ) Oligomers and Promotes the Formation of High-Molecular-Weight Amorphous Aggregates. Int. J. Biol. Macromol. 2025, 319, 145720. [Google Scholar] [CrossRef]
- Tejero, A.; León-Navarro, D.A.; Martín, M. Effect of Xanthohumol, a Bioactive Natural Compound from Hops, on Adenosine Pathway in Rat C6 Glioma and Human SH-SY5Y Neuroblastoma Cell Lines. Nutrients 2024, 16, 1792. [Google Scholar] [CrossRef]
- Yen, T.-L.; Hsu, C.-K.; Lu, W.-J.; Hsieh, C.-Y.; Hsiao, G.; Chou, D.-S.; Wu, G.-J.; Sheu, J.-R. Neuroprotective Effects of Xanthohumol, a Prenylated Flavonoid from Hops (Humulus lupulus), in Ischemic Stroke of Rats. J. Agric. Food Chem. 2012, 60, 1937–1944. [Google Scholar] [CrossRef]
- Rancán, L.; Paredes, S.D.; García, I.; Muñoz, P.; García, C.; López De Hontanar, G.; De La Fuente, M.; Vara, E.; Tresguerres, J.A.F. Protective Effect of Xanthohumol against Age-Related Brain Damage. J. Nutr. Biochem. 2017, 49, 133–140. [Google Scholar] [CrossRef]
- Jiao, Y.; Cao, Y.; Lu, X.; Wang, J.; Saitgareeva, A.; Kong, X.; Song, C.; Li, J.; Tian, K.; Zhang, S.; et al. Xanthohumol Protects Neuron from Cerebral Ischemia Injury in Experimental Stroke. Mol. Biol. Rep. 2020, 47, 2417–2425. [Google Scholar] [CrossRef]
- Wang, C.C.; Ho, Y.H.; Hung, C.F.; Kuo, J.R.; Wang, S.J. Xanthohumol, an Active Constituent from Hope, Affords Protection against Kainic Acid-Induced Excitotoxicity in Rats. Neurochem. Int. 2020, 133, 104629. [Google Scholar] [CrossRef]
- Rahman, S.U.; Ali, T.; Hao, Q.; He, K.; Li, W.; Ullah, N.; Zhang, Z.; Jiang, Y.; Li, S. Xanthohumol Attenuates Lipopolysaccharide-Induced Depressive Like Behavior in Mice: Involvement of NF-κB/Nrf2 Signaling Pathways. Neurochem. Res. 2021, 46, 3135–3148. [Google Scholar] [CrossRef]
- Sun, X.-L.; Zhang, J.-B.; Guo, Y.-X.; Xia, T.-S.; Xu, L.-C.; Rahmand, K.; Wang, G.-P.; Li, X.-J.; Han, T.; Wang, N.-N.; et al. Xanthohumol Ameliorates Memory Impairment and Reduces the Deposition of β-Amyloid in APP/PS1 Mice via Regulating the mTOR/LC3II and Bax/Bcl-2 Signalling Pathways. J. Pharm. Pharmacol. 2021, 73, 1230–1239. [Google Scholar] [CrossRef]
- Liu, W.; He, K.; Wu, D.; Zhou, L.; Li, G.; Lin, Z.; Yang, X.; Liu, J.; Pui Man Hoi, M. Natural Dietary Compound Xanthohumol Regulates the Gut Microbiota and Its Metabolic Profile in a Mouse Model of Alzheimer’s Disease. Molecules 2022, 27, 1281. [Google Scholar] [CrossRef]
- Liu, Y.; Shao, J.; Qiao, R.; Li, J.; Li, C.; Cao, W. Xanthohumol Improves Cognitive Impairment by Regulating miRNA-532-3p/Mpped1 in Ovariectomized Mice. Psychopharmacology 2023, 240, 1169–1178. [Google Scholar] [CrossRef]
- Xiao-Lei, S.; Tian-Shuang, X.; Yi-Ping, J.; Na-Ni, W.; Ling-Chuan, X.; Ting, H.; Hai-Liang, X. Humulus lupulus L. Extract and Its Active Constituent Xanthohumol Attenuate Oxidative Stress and Nerve Injury Induced by Iron Overload via Activating AKT/GSK3β and Nrf2/NQO1 Pathways. J. Nat. Med. 2023, 77, 12–27. [Google Scholar] [CrossRef]
- Hu, F.-F.; Pan, S.-Y.; Chu, J.-Y.; Liu, J.-J.; Duan, T.-T.; Luo, Y.; Zhou, W.; Wang, Z.-M.; Liu, W.; Zeng, Y. Xanthohumol Protects Against Neuronal Excitotoxicity and Mitochondrial Dysfunction in APP/PS1 Mice: An Omics-Based Study. Nutrients 2024, 16, 3754. [Google Scholar] [CrossRef]
- Liu, W.; Chen, X.; Zhao, J.; Yang, C.; Huang, G.; Zhang, Z.; Liu, J. Protective Signature of Xanthohumol on Cognitive Function of APP/PS1 Mice: A Urine Metabolomics Approach by Age. Front. Pharmacol. 2024, 15, 1423060. [Google Scholar] [CrossRef]
- Liu, W.; Chen, X.; Yang, C.; Lin, Z.; Huang, X.; Zhang, Z.; Liu, J. Preventive Effects of Xanthohumol in APP/PS1 Mice Based on Multi-Omics Atlas. Brain Res. Bull. 2025, 224, 111316. [Google Scholar] [CrossRef]
- Khandale, N.; Birla, D.; Alam, M.S.; Bashir, B.; Vishwas, S.; Kumar, A.; Potale, Y.; Gupta, G.; Negi, P.; Alam, A.; et al. Quality by Design Endorsed Fabrication of Xanthohumol Loaded Solid Nanostructured Lipid Carrier Based Powder for Effective Treatment of Alzheimer’s Disease in Rats. J. Drug Deliv. Sci. Technol. 2025, 107, 106792. [Google Scholar] [CrossRef]
- Wang, X.; Ho, S.-L.; Poon, C.-Y.; Yan, T.; Li, H.-W.; Wong, M.S. Amyloid-β Aggregation Inhibitory and Neuroprotective Effects of Xanthohumol and Its Derivatives for Alzheimer’s Diseases. CAR 2019, 16, 836–842. [Google Scholar] [CrossRef]
- Bashir, B.; Singh, S.K.; Gulati, M.; Vishwas, S.; Dua, K. Xanthohumol Loaded Self-Nano Emulsifying Drug Delivery System: Harnessing Neuroprotective Effects in Alzheimer’s Disease Management. Alzheimer’s Dement. 2024, 20, e087955. [Google Scholar] [CrossRef]
- Alam, M.S.; Khandale, N.; Birla, D.; Bashir, B.; Vishwas, S.; Kulkarni, M.P.; Rajput, R.P.; Pandey, N.K.; Loebenberg, R.; Davies, N.M.; et al. Formulation and Optimization of Xanthohumol Loaded Solid Dispersion for Effective Treatment of Parkinson’s Disease in Rats: In Vitro and in Vivo Assessment. J. Drug Deliv. Sci. Technol. 2024, 102, 106385. [Google Scholar] [CrossRef]
- Ghazwani, M.; Hani, U.; Alqarni, M.H.; Alam, A. Beta Caryophyllene-Loaded Nanostructured Lipid Carriers for Topical Management of Skin Disorders: Statistical Optimization, In Vitro and Dermatokinetic Evaluation. Gels 2023, 9, 550. [Google Scholar] [CrossRef]
- Mödinger, Y.; Knaub, K.; Dharsono, T.; Wacker, R.; Meyrat, R.; Land, M.H.; Petraglia, A.L.; Schön, C. Enhanced Oral Bioavailability of β-Caryophyllene in Healthy Subjects Using the VESIsorb® Formulation Technology, a Novel Self-Emulsifying Drug Delivery System (SEDDS). Molecules 2022, 27, 2860. [Google Scholar] [CrossRef]
- Di Sotto, A.; Paolicelli, P.; Nardoni, M.; Abete, L.; Garzoli, S.; Di Giacomo, S.; Mazzanti, G.; Casadei, M.A.; Petralito, S. SPC Liposomes as Possible Delivery Systems for Improving Bioavailability of the Natural Sesquiterpene β-Caryophyllene: Lamellarity and Drug-Loading as Key Features for a Rational Drug Delivery Design. Pharmaceutics 2018, 10, 274. [Google Scholar] [CrossRef]
- Amalraj, A.; Jacob, J.; Varma, K.; Gopi, S. Preparation and Characterization of Liposomal β-Caryophyllene (Rephyll) by Nanofiber Weaving Technology and Its Effects on Delayed Onset Muscle Soreness (DOMS) in Humans: A Randomized, Double-Blinded, Crossover-Designed, and Placebo-Controlled Study. ACS Omega 2020, 5, 24045–24056. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Teng, Z.; Liu, D.; Wang, Y.; Lou, J.; Dong, Z. β-Caryophyllene Liposomes Attenuate Neurovascular Unit Damage After Subarachnoid Hemorrhage in Rats. Neurochem. Res. 2020, 45, 1758–1768. [Google Scholar] [CrossRef] [PubMed]
- Parisotto-Peterle, J.; Bidone, J.; Lucca, L.G.; Araújo, G.D.M.S.; Falkembach, M.C.; Da Silva Marques, M.; Horn, A.P.; Dos Santos, M.K.; Da Veiga, V.F.; Limberger, R.P.; et al. Healing Activity of Hydrogel Containing Nanoemulsified β-Caryophyllene. Eur. J. Pharm. Sci. 2020, 148, 105318. [Google Scholar] [CrossRef]
- Alharthi, S.; Ziora, Z.M.; Mustafa, G.; Chaubey, P.; El Kirdasy, A.F.; Alotaibi, G. β-Caryophyllene-Loaded Microemulsion-Based Topical Hydrogel: A Promising Carrier to Enhance the Analgesic and Anti-Inflammatory Outcomes. Gels 2023, 9, 634. [Google Scholar] [CrossRef]
- De Oliveira Neves, J.K.; Apolinário, A.C.; Alcantara Saraiva, K.L.; Da Silva, D.T.C.; De Freitas Araújo Reis, M.Y.; De Lima Damasceno, B.P.G.; Pessoa, A.; Moraes Galvão, M.A.; Soares, L.A.L.; Veiga Júnior, V.F.D.; et al. Microemulsions Containing Copaifera Multijuga Hayne Oil-Resin: Challenges to Achieve an Efficient System for β-Caryophyllene Delivery. Ind. Crops Prod. 2018, 111, 185–192. [Google Scholar] [CrossRef]
- Weimer, P.; Kirsten, C.N.; De Araújo Lock, G.; Nunes, K.A.A.; Rossi, R.C.; Koester, L.S. Co-Delivery of Beta-Caryophyllene and Indomethacin in the Oily Core of Nanoemulsions Potentiates the Anti-Inflammatory Effect in LPS-Stimulated Macrophage Model. Eur. J. Pharm. Biopharm. 2023, 191, 114–123. [Google Scholar] [CrossRef]
- Lee, M.H.; Lee, I.Y.; Chun, Y.G.; Kim, B.-K. Formulation and Characterization of β-Caryophellene-Loaded Lipid Nanocarriers with Different Carrier Lipids for Food Processing Applications. LWT 2021, 149, 111805. [Google Scholar] [CrossRef]
- Baranda, E.R.; Santos, J.S.; Toledo, A.L.M.M.; Barradas, T.N. Design and Characterization of Stable β-Caryophyllene-Loaded Nanoemulsions: A Rational HLB-Based Approach for Enhanced Volatility Control and Sustained Release. Beilstein Arch. 2025, 2025, 36. [Google Scholar]
- Santos, P.S.; Souza, L.K.M.; Araújo, T.S.L.; Medeiros, J.V.R.; Nunes, S.C.C.; Carvalho, R.A.; Pais, A.C.C.; Veiga, F.J.B.; Nunes, L.C.C.; Figueiras, A. Methyl-β-Cyclodextrin Inclusion Complex with β-Caryophyllene: Preparation, Characterization, and Improvement of Pharmacological Activities. ACS Omega 2017, 2, 9080–9094. [Google Scholar] [CrossRef]
- Liu, H.; Yang, G.; Tang, Y.; Cao, D.; Qi, T.; Qi, Y.; Fan, G. Physicochemical Characterization and Pharmacokinetics Evaluation of β-Caryophyllene/β-Cyclodextrin Inclusion Complex. Int. J. Pharm. 2013, 450, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Nurul Hidayati, E.S. pH-Sensitive Niosomal Nanoencapsulation of Beta- Caryophyllene and Its Novel Pathway in Triple Negative Breast Cancer. Biointerface Res. Appl. Chem. 2025, 15, 1–18. [Google Scholar] [CrossRef]
- El-Hammadi, M.M.; Small-Howard, A.L.; Fernández-Arévalo, M.; Martín-Banderas, L. Development of Enhanced Drug Delivery Vehicles for Three Cannabis-Based Terpenes Using Poly(Lactic-Co-Glycolic Acid) Based Nanoparticles. Ind. Crops Prod. 2021, 164, 113345. [Google Scholar] [CrossRef]
- Porto, D.S.; Fretes Argenta, D.; Ziech, C.C.; Balleste, M.P.; Dreyer, J.P.; Micke, G.A.; Campos, Â.M.; Caumo, K.S.; Caon, T. Amoebicidal Potential of β-Caryophyllene-Loaded Polymeric Nanoparticles. ACS Appl. Polym. Mater. 2024, 6, 14447–14457. [Google Scholar] [CrossRef]
- Nogueira, C.; Lemos-Senna, E.; Da Silva Vieira, E.; Sampaio, T.B.; Mallmann, M.P.; Oliveira, M.S.; Bernardi, L.S.; Oliveira, P.R. β-Caryophyllene Cationic Nanoemulsion for Intranasal Delivery and Treatment of Epilepsy: Development and in Vivo Evaluation of Anticonvulsant Activity. J. Nanopart Res. 2023, 25, 19. [Google Scholar] [CrossRef]
- Tang, Q.; Xu, F.; Wei, X.; Gu, J.; Qiao, P.; Zhu, X.; Yin, S.; Ouyang, D.; Dong, J.; Yao, J.; et al. Investigation of β-Caryophyllene as Terpene Penetration Enhancer: Role of Stratum Corneum Retention. Eur. J. Pharm. Sci. 2023, 183, 106401. [Google Scholar] [CrossRef] [PubMed]
- Yakimov, I.D.; Kolmogorov, I.M.; Le-Deygen, I.M. Beta-Caryophyllene Induces Significant Changes in the Lipid Bilayer at Room and Physiological Temperatures: ATR-FTIR Spectroscopy Studies. Biophysica 2023, 3, 501–512. [Google Scholar] [CrossRef]
- Alberti, T.B.; Coelho, D.S.; Maraschin, M. β-Caryophyllene Nanoparticles Design and Development: Controlled Drug Delivery of Cannabinoid CB2 Agonist as a Strategic Tool towards Neurodegeneration. Mater. Sci. Eng. C 2021, 121, 111824. [Google Scholar] [CrossRef]
- Długosz, A.; Błaszak, B.; Czarnecki, D.; Szulc, J. Mechanism of Action and Therapeutic Potential of Xanthohumol in Prevention of Selected Neurodegenerative Diseases. Molecules 2025, 30, 694. [Google Scholar] [CrossRef]
- Christaki, S.; Spanidi, E.; Panagiotidou, E.; Athanasopoulou, S.; Kyriakoudi, A.; Mourtzinos, I.; Gardikis, K. Cyclodextrins for the Delivery of Bioactive Compounds from Natural Sources: Medicinal, Food and Cosmetics Applications. Pharmaceuticals 2023, 16, 1274. [Google Scholar] [CrossRef] [PubMed]
- Kirchinger, M.; Bieler, L.; Tevini, J.; Vogl, M.; Haschke-Becher, E.; Felder, T.K.; Couillard-Després, S.; Riepl, H.; Urmann, C. Development and Characterization of the Neuroregenerative Xanthohumol C/Hydroxypropyl-β-Cyclodextrin Complex Suitable for Parenteral Administration. Planta Medica 2019, 85, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Oledzka, E. Xanthohumol—A Miracle Molecule with Biological Activities: A Review of Biodegradable Polymeric Carriers and Naturally Derived Compounds for Its Delivery. Int. J. Mol. Sci. 2024, 25, 3398. [Google Scholar] [CrossRef]
- Khayyal, M.T.; El-Hazek, R.M.; El-Sabbagh, W.A.; Frank, J.; Behnam, D.; Abdel-Tawab, M. Micellar Solubilization Enhances the Anti-Inflammatory Effect of Xanthohumol. Phytomedicine 2020, 71, 153233. [Google Scholar] [CrossRef]
- Ronka, S.; Kowalczyk, A.; Baczyńska, D.; Żołnierczyk, A.K. Pluronics-Based Drug Delivery Systems for Flavonoids Anticancer Treatment. Gels 2023, 9, 143. [Google Scholar] [CrossRef]
- Jain, R.A. The Manufacturing Techniques of Various Drug Loaded Biodegradable Poly(Lactide-Co-Glycolide) (PLGA) Devices. Biomaterials 2000, 21, 2475–2490. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, M.; Macedo, A.S.; Lima, S.A.C.; Reis, S.; Soares, R.; Fonte, P. Evaluation of the Antitumour and Antiproliferative Effect of Xanthohumol-Loaded PLGA Nanoparticles on Melanoma. Materials 2021, 14, 6421. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Thapa, R.; Hariani, H.N.; Volyanyuk, M.; Ogle, S.D.; Orloff, K.A.; Ankireddy, S.; Lai, K.; Žiniauskaitė, A.; Stubbs, E.B.; et al. Poly(Lactic-Co-Glycolic Acid) Nanoparticles Encapsulating the Prenylated Flavonoid, Xanthohumol, Protect Corneal Epithelial Cells from Dry Eye Disease-Associated Oxidative Stress. Pharmaceutics 2021, 13, 1362. [Google Scholar] [CrossRef]
- Hanmantrao, M.; Chaterjee, S.; Kumar, R.; Vishwas, S.; Harish, V.; Porwal, O.; Alrouji, M.; Alomeir, O.; Alhajlah, S.; Gulati, M.; et al. Development of Guar Gum-Pectin-Based Colon Targeted Solid Self-Nanoemulsifying Drug Delivery System of Xanthohumol. Pharmaceutics 2022, 14, 2384. [Google Scholar] [CrossRef] [PubMed]
- Harish, V.; Tewari, D.; Mohd, S.; Govindaiah, P.; Babu, M.R.; Kumar, R.; Gulati, M.; Gowthamarajan, K.; Madhunapantula, S.V.; Chellappan, D.K.; et al. Quality by Design Based Formulation of Xanthohumol Loaded Solid Lipid Nanoparticles with Improved Bioavailability and Anticancer Effect against PC-3 Cells. Pharmaceutics 2022, 14, 2403. [Google Scholar] [CrossRef]
- Buczek, A.; Rzepiela, K.; Stępniak, A.; Buczkowski, A.; Broda, M.A.; Pentak, D. Xanthohumol in Liposomal Form in the Presence of Cyclodextrins: Drug Delivery and Stability Analysis. Food Chem. 2025, 492, 145453. [Google Scholar] [CrossRef]
- Paul, W.; Sharma, C.P. Inorganic Nanoparticles for Targeted Drug Delivery. In Biointegration of Medical Implant Materials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 333–373. ISBN 978-0-08-102680-9. [Google Scholar]
- Matthews, T.; Wang, X.E.; Dang, T.; Pruner, J.; Li, Y. Preparation and Characterization of Xanthohumol-Incorporated Sub-5 Ultrafine Iron Oxide Nanoparticles: Investigation of Their Effects on Multiple Myeloma Cell Growth (Abstract ID: 190025). J. Pharmacol. Exp. Ther. 2025, 392, 103487. [Google Scholar] [CrossRef]
- Cauda, V.; Canavese, G. Mesoporous Materials for Drug Delivery and Theranostics. Pharmaceutics 2020, 12, 1108. [Google Scholar] [CrossRef] [PubMed]
- Krajnović, T.; Pantelić, N.Đ.; Wolf, K.; Eichhorn, T.; Maksimović-Ivanić, D.; Mijatović, S.; Wessjohann, L.A.; Kaluđerović, G.N. Anticancer Potential of Xanthohumol and Isoxanthohumol Loaded into SBA-15 Mesoporous Silica Particles against B16F10 Melanoma Cells. Materials 2022, 15, 5028. [Google Scholar] [CrossRef] [PubMed]
- He, X.-H.; Galaj, E.; Bi, G.-H.; He, Y.; Hempel, B.; Wang, Y.-L.; Gardner, E.L.; Xi, Z.-X. β-Caryophyllene, an FDA-Approved Food Additive, Inhibits Methamphetamine-Taking and Methamphetamine-Seeking Behaviors Possibly via CB2 and Non-CB2 Receptor Mechanisms. Front. Pharmacol. 2021, 12, 722476. [Google Scholar] [CrossRef] [PubMed]
- Clinicaltrials.Gov. Available online: https://clinicaltrials.gov/ (accessed on 8 January 2025).
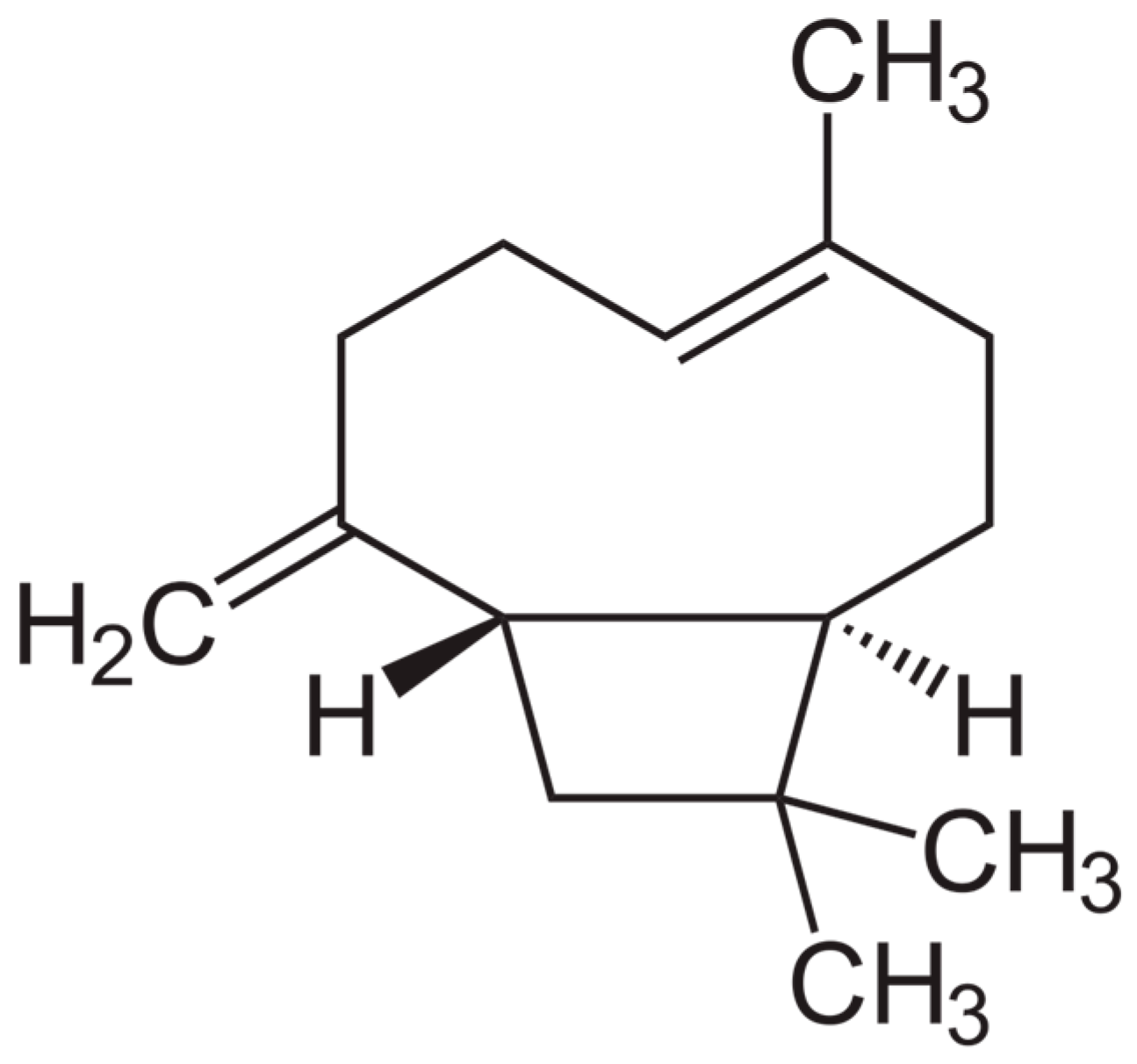

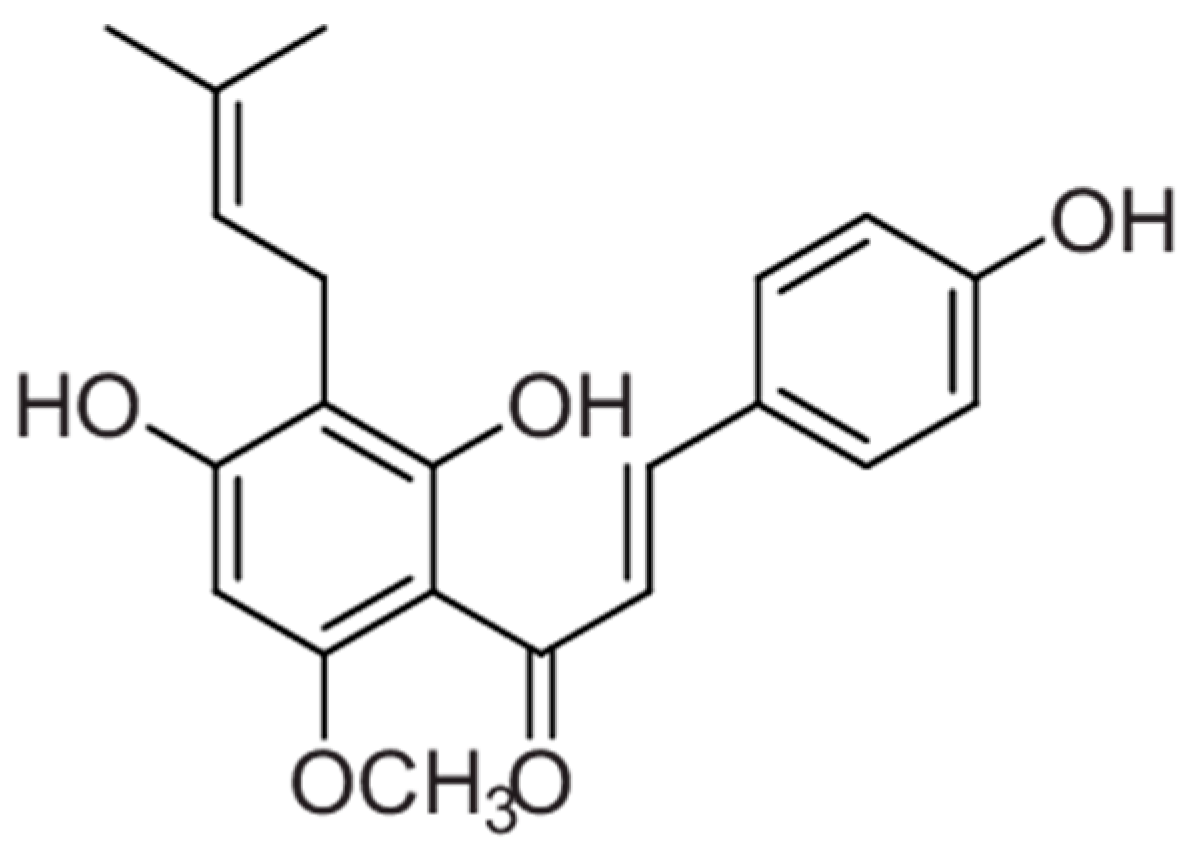
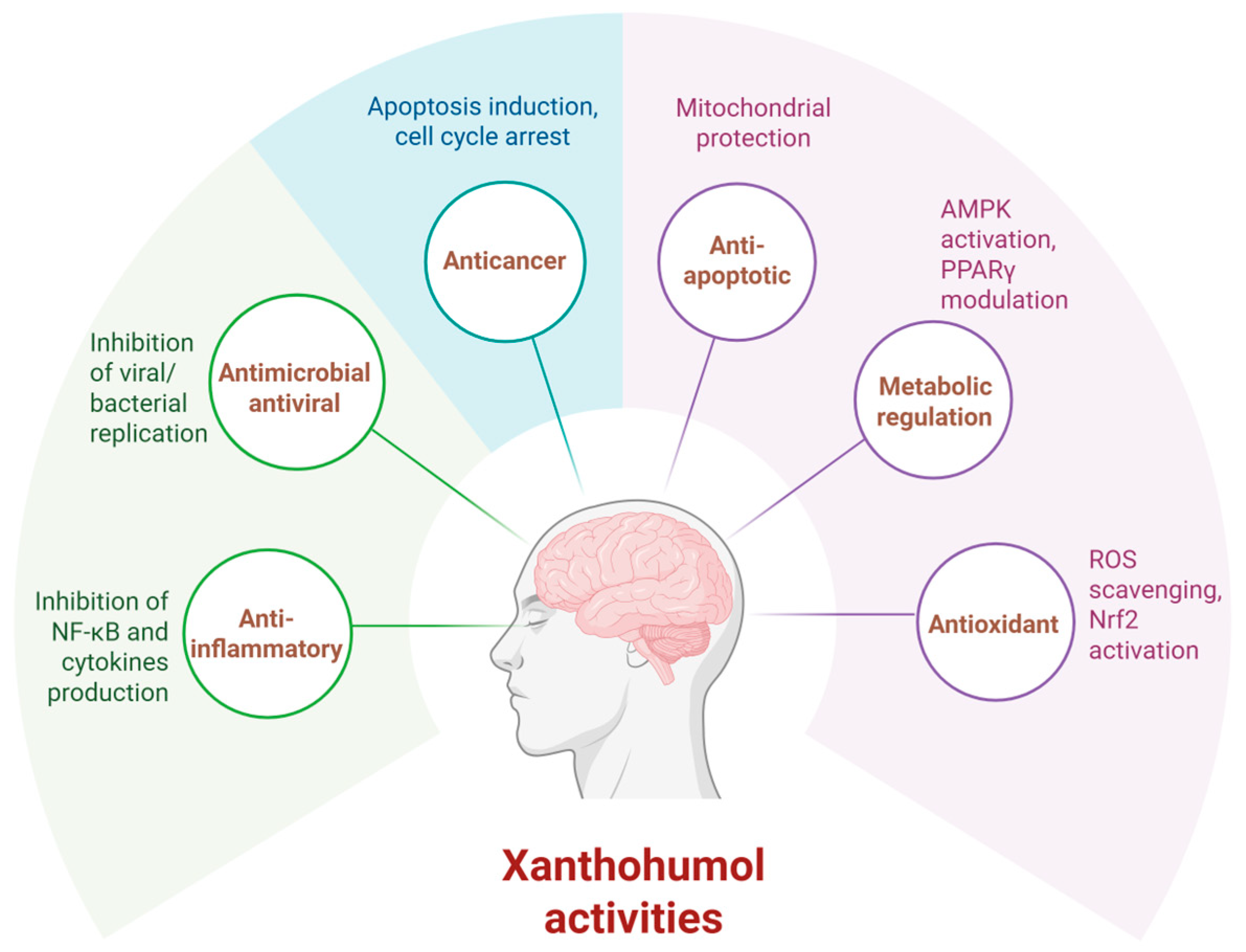
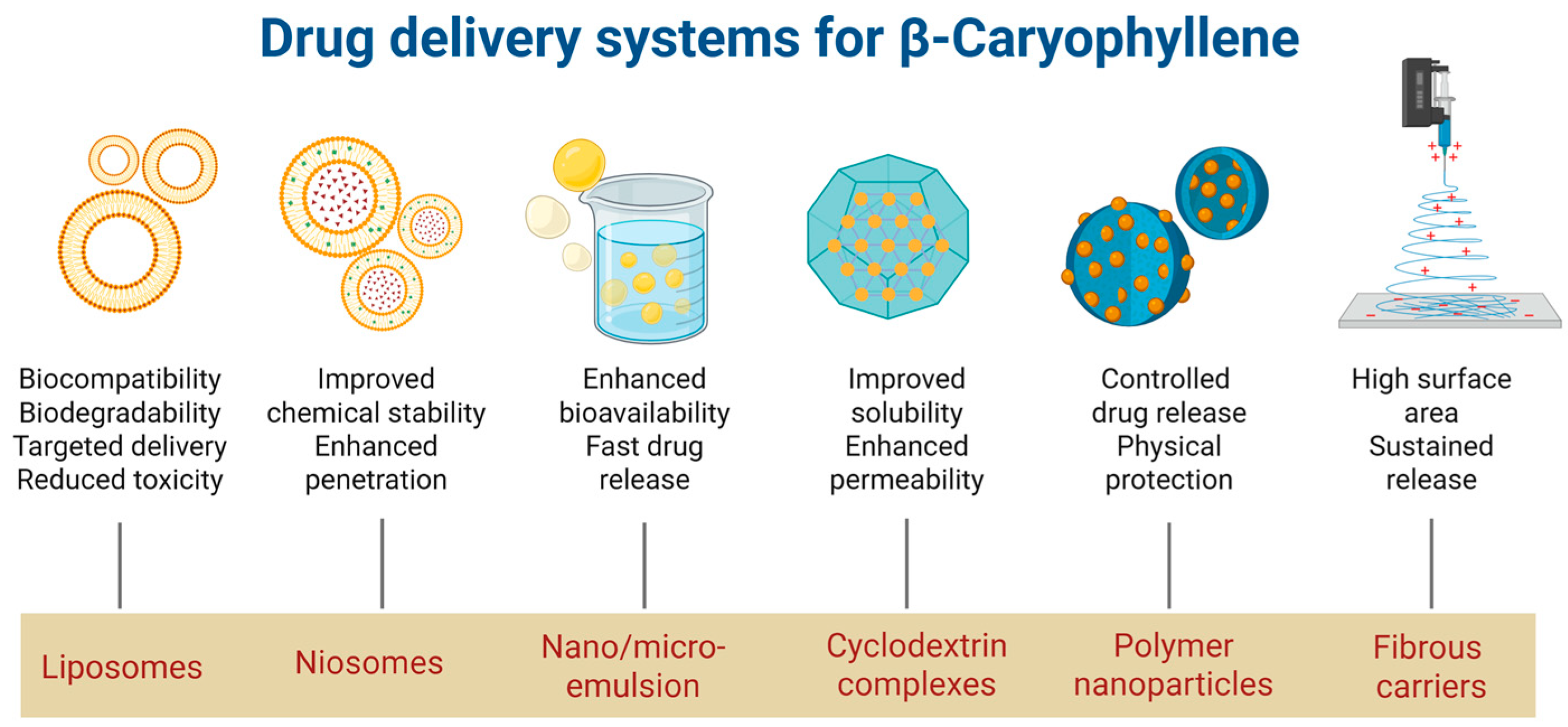
| Study Objectives | Study Design | Main Results | Mechanism of Action | Ref. |
|---|---|---|---|---|
| To evaluate the protective effects of BCP against glutamate-induced cytotoxicity in C6 glioma cells. | Cells: C6 rat glioma cells (ATCC CCL-107). Treatments: Glu-induced cytotoxicity: Cells exposed to 0.05–10 mM Glu for 24 h. BCP co-administration: Glu (1 mM) + BCP (0.5–1 µM) for 1 or 24 h. BCP pretreatment: BCP (0.5–3 µM) for 24 h before Glu (1 mM) exposure. | BCP inhibited ROS production and reestablished the mitochondrial membrane potential (CB2R/Nrf2 signaling pathway), which led to the prevention of C6 glioma cell line from Glu-induced cytotoxicity. | BCP activated Nrf2 and improved antioxidant defenses, partly via CB2 receptor activation. | [14] |
| To investigate whether BCP promotes neuritogenesis independently of CB2 receptor activation. | Cell lines: PC12 and SH-SY5Y. Treatment: PC12 cells treated with BCP (10–50 μM) ± NGF or K252a (TrkA inhibitor) for 72 h; SH-SY5Y cells treated with BCP (10 μM) ± retinoic acid for 72 h. | BCP stimulated the process of neuritogenesis and synaptogenesis in PC12 cells through a mechanism that did not involve CB2 receptors. | Activation of NGF-specific receptor trkA. Neuritogenesis and axonal protein upregulation, independent of CB2 or NGF expression. | [15] |
| To investigate whether trans-caryophyllene (TC) protects against Aβ1-42-induced neuroinflammation in microglial cells, relevant to AD. | BV-2 microglial cells were pretreated with TC (10, 25, 50 μM) and then stimulated with Aβ1-42. Cytotoxicity, NO/PGE2 production, iNOS/COX-2 expression, pro-inflammatory cytokines, TLR4 expression, and NF-κB signaling were assessed. | Pretreatment of BV2 microglial cells with BCP before Aβ stimulation resulted in inhibition of NO, prostaglandin E2 (PGE2) production, inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) expression, as well as secretion of proinflammatory cytokines. Activated overexpression of toll-like receptor 4 (TLR4), phosphorylation and degradation of IκBα, nuclear translocation of p65, and transcriptional activity of NF-κB, induced by Aβ1-42 were reduced. | - | [16] |
| To evaluate the neuroprotective effects of BCP against MPP+-induced neurotoxicity in SH-SY5Y cells. | SH-SY5Y cells were treated with MPP+ (50 μM) ± BCP (1 or 2.5 μM) for 24 h. | BCP enhanced cell viability, reduced the release of lactic dehydrogenase, and decreased the generation of reactive oxygen species (ROS). | BCP protects via CB2R activation, reducing oxidative stress, preventing apoptosis, and modulating HO-1/JNK pathways. | [17] |
| To investigate the protective effects of BCP against LPS-induced oligodendrocyte toxicity and the involvement of CB2, Nrf2, PPAR-γ, and SMase pathways. | OLN-93 oligodendrocyte cells treated with LPS; BCP tested at low (0.2–1 μM) and high (10–50 μM) concentrations; effects on ROS, NO, TNF-α, GSH, and signaling pathways were measured; SMase inhibitors (imipramine, fluoxetine) tested for synergy. | The protective effects of BCP are mediated through the CB2 receptor in different pathways at high and low concentrations. BCP prevented the increased production of nitric oxide (NO), ROS, and tumor necrosis factor (TNF-α). | BCP protects oligodendrocytes via CB2 activation, modulating Nrf2/HO-1 (low dose) and PPAR-γ (high dose), with additional benefit from SMase inhibition. | [18] |
| To evaluate the protective effects of BCP, a selective CB2 agonist, on LPS-induced microglial inflammation and M1/M2 imbalance and to identify signaling pathways involved (CB2, PPAR-γ, SMase). | Cells: Primary microglia isolated from adult C57BL/6 mice. Treatments: BCP dose-response: 0.2–25 µM or JWH-133 (1 µM) ± LPS (1 µg/mL) for 24 h. CB2 involvement: Pre-incubation with AM630 (1 µM) 30 min before BCP/JWH-133 + LPS. | A low concentration of BCP showed selective anti-inflammatory activity. BCP modulated microglia and could affect neuroinflammatory conditions and microglial cells. | - | [19] |
| To evaluate the anti-inflammatory and neuroprotective effects of Viphyllin, a standardized BCP extract, in macrophages and neuronal cells under oxidative stress. | Cells: RAW 264.7 (macrophages), SH-SY5Y (neuroblastoma). Viability: RAW 264.7: 5–50 μg/mL Viphyllin, 24 h; SH-SY5Y: 12–24 μg/mL Viphyllin ± H2O2 (500 μM). NO Production: RAW 264.7: 5–50 μg/mL Viphyllin + LPS (1 μg/mL), 24 h. | Regulated LPS-mediated inflammation in macrophages and exerted an antiapoptotic effect against neuronal damage induced by H2O2. | Anti-inflammatory effects via MAPK pathway inhibition and neuroprotection through anti-apoptotic modulation under oxidative stress. | [20] |
| To investigate whether BCP exerts neuroprotection in an AD cell model. | PC-12 cells overexpressing amyloid-β precursor protein. Groups: control, empty vector, APP overexpression, and BCP (5, 10, 20 μM). | BCP enhanced the viability of PC-12 cells while protecting cell morphology and counteracted the neurotoxic effects of amyloid-β. | BCP protects neurons by inhibiting the JAK2-STAT3-BACE1 signaling pathway, reducing Aβ-induced toxicity. | [21] |
| To evaluate whether α-asarone and BCP can inhibit tau fibrillation, disassemble tau fibrils, and protect neuronal cells against tau-induced toxicity. | Tau aggregation and fibrillation were assessed using SDS-PAGE, AFM, ThT/ANS fluorescence, and β-sheet content analysis; SH-SY5Y cells were exposed to 5 μM tau samples treated with α-asarone or BCP for 24 h. | BCP inhibited tau fibrillation and aggregation, leading to the formation of various structural and morphological intermediate species. BCP enhanced cell viability. | - | [22] |
| To investigate the neuroprotective effects of BCP against rotenone-induced neurotoxicity in SH-SY5Y cells. | In silico: molecular docking of BCP with GSK-3β, NRF2, HO-1. In vitro: SH-SY5Y cells pretreated with rotenone. BCP (100 µg/mL) tested for effects. | BCP increased cell viability, reduced ROS levels, and altered cellular pathways associated with inflammation, redox processes, and apoptosis. | BCP protects cells by modulating the GSK-3β/NRF2/HO-1 axis, reducing oxidative stress, inflammation, and apoptosis. | [23] |
| To evaluate the immunomodulatory and therapeutic effects of BCP in vitro and in vivo in a mouse model of multiple sclerosis. | Splenocytes from MS-induced C57BL/6 mice treated with BCP (4, 20, 40 μM) were assessed in vitro. In vivo, MS-mice received oral BCP (25 or 50 mg/kg/day). | In vitro and in vivo BCP inhibited the production of NO, H2O2, TNF- α, and Interferon-gamma (IFN-γ). BCP administered orally at a dose of 50 mg/kg/day showed a reduction in the number of inflammatory infiltrates and attenuated neurological damage in the CNS. | - | [24] |
| To evaluate the anxiolytic, antioxidant, and toxicity effects of BCP in vitro and in vivo. | Swiss albino mice were tested for anxiolytic activity using elevated plus-maze, rota-rod, light/dark, and hiding sphere models. BCP—10, 25, and 50 mg/kg, intraperitoneally. Antioxidant activity was assessed via DPPH, ABTS, and S. cerevisiae assays. | BCP exerted dose-dependent anxiolytic and antioxidant effects on experimental animals. It has not shown toxicity in A. salina. BCP showed protective and restorative activity in S. cerevisiae strains against the harmful effects of hydrogen peroxide. | - | [25] |
| To evaluate whether BCP prevents cognitive decline and neuroinflammation in an APP/PS1 Alzheimer’s disease model and to determine the roles of CB2 and PPARγ pathways. | APP/PS1 transgenic mice received oral BCP (BCP—16, 48, or 144 mg/kg, orally, 10 weeks). CB2 (AM630) and PPARγ (GW9662) antagonists were used to confirm pathway involvement. | BCP exhibited anti-inflammatory activity, related to the activation of CB2 and PPARγ receptors, which resulted in enhanced memory and cognitive function, a decrease in β-amyloid accumulation, and the suppression of gliosis, as well as the release of pro-inflammatory cytokines. | BCP exerts effects through CB2 receptor activation and subsequent engagement of the PPARγ pathway. | [26] |
| To evaluate the neuroprotective effect of BCP against rotenone-induced oxidative stress and neuroinflammation in a rat model of PD. | Male Wistar rats. BCP administered once daily for 4 weeks at 50 mg/kg body weight prior to rotenone challenge (2.5 mg/kg body weight). | BCP restored dopaminergic neurons, reduced the levels of proinflammatory cytokines and inflammatory mediators, restored antioxidant enzymes, and prevented lipid peroxidation and the depletion of glutathione. | - | [27] |
| To investigate the neuroprotective effects and mechanisms of BCP in an MPTP-induced murine model of PD. | PD induce mice model: BCP 10 mg/kg, 5 days (i.p. or oral). | BCP improved motor function, protected dopaminergic neurons, reduced glial activation, and lowered inflammatory cytokines via CB2R. | - | [28] |
| To investigate the therapeutic effects of BCP on experimental autoimmune encephalomyelitis, a murine model of multiple sclerosis, and to explore the underlying immunomodulatory mechanisms. | C57BL/6 mice were treated with BCP (25 or 50 mg/kg, twice a day, orally). | The immunomodulatory effect of BCP is associated with the inhibition of microglial cells, CD4+ T-lymphocytes, CD8+ T-lymphocytes, and protein expression of pro-inflammatory cytokines. BCP reduced axonal demyelination and modulated the Th1/Treg immune balance. | Neuroprotective and immunomodulatory effects are mediated via CB2 receptor activation. | [29] |
| To evaluate the effects of a β-caryophyllene–hydroxypropyl-β-cyclodextrin (HPβCD/BCP) inclusion complex on cognitive deficits in a vascular dementia rat model and to investigate underlying mechanisms. | VD rats were treated i.p. for 4 weeks with HPβCD/BCP (16–144 mg/kg), AM630 (3 mg/kg), or saline. | HPβCD/BCP reduced cognitive impairment and nerve fiber loss, enhanced cerebral blood flow, and inhibited neuronal cell death in rats. | BCP demonstrated its neuroprotective properties by stimulating the CB2 pathway. | [30] |
| To assess the effects of BCP on IL-17, transcription factors (T-bet, GATA-3), and remyelination in mice as a model of MS. | EAE was induced in C57BL/6 mice and treated with BCP (25 or 50 mg/kg/day) by gavage from day 10 post-induction. CNS tissue was analyzed after 9 days of treatment for cytokines, transcription factors, and remyelination. | BCP administered at a daily dose of 50 mg/kg resulted in reduced levels of IL-17 in the brain, medulla, and cerebellum; a reduction in T-bet was observed in both the medulla and cerebellum, while GATA-3 levels increased in the cerebellum. | Anti-inflammatory and neuroprotective effects via modulation of Th1/Th17 (T-bet/IL-17) and Th2 (GATA-3) responses. | [31] |
| To evaluate the neuroprotective effects of BCP against dementia induced by neuroinflammation and aging in animal models. | Male SD rats: AlCl3-induced dementia. Female SD rats: Doxorubicin-induced chemobrain model. D-galactose-induced mitochondrial dysfunction model. BCP given orally at 50 and 100 mg/kg. | At a dosage of 100 mg/kg, BCP had a protective activity against dementia caused by neuroinflammation but did not influence neuronal aging related to mitochondrial dysfunction. | - | [32] |
| To evaluate the neuroprotective effect of BCP against MPTP-induced Parkinsonism. | Male C57BL/6J mice; 4 groups: 1. saline control, 2. MPTP (5 mg/kg i.p. × 7 days), 3. BCP (10 mg/kg p.o. × 7 days), 4. MPTP + BCP (BCP from day 4 onward, 7 days). | BCP inhibited oxidative stress-induced cell death of dopaminergic neurons and simultaneously increased the expression and enzymatic activity of NQO1. | - | [33] |
| To evaluate the protective effects of low-dose BCP in an EAE mouse model of MS and explore its CB2-dependent immunomodulatory mechanisms. | Female C57BL/6 mice were immunized with MOG35–55 + CFA and pertussis toxin to induce EAE. Mice received low-dose BCP (2.5 or 5 mg/kg/day) ± CB2 antagonist (AM630). | Low doses of BCP influenced EAE, which is a chronic model for MS. The protective impacts of BCP are facilitated by the CB2 receptor. BCP modulated the adaptive (lymphocytes) and innate (microglia) immune systems from an inflammatory (Th1/Th17/M1) to an anti-inflammatory (Th2/Treg/M2) phase. | Protective effects were CB2 receptor–dependent. | [34] |
| To evaluate the neuroprotective effects of BCP on dopaminergic neurons. | Male C57BL/6 mice were randomly assigned to 4 groups: Sham, 6-OHDA, BCP (10 mg/kg, oral, 5 days), and 6-OHDA + BCP. BCP was administered orally either alone or after 6-OHDA. | Treatment with BCP improved motor dysfunction; exhibited a neuroprotective effect on dopaminergic neurons; and decreased expression levels of NLRP3, caspase-1, and malondialdehyde (MDA). | BCP protects dopaminergic neurons by inhibiting NLRP3 inflammasome activation and reducing oxidative stress. | [35] |
| To test whether CB2 receptor agonism via BCP can correct blood–brain barrier (BBB) permeability in the MPTP mouse model of PD. | MPTP-induced PD mouse model (male C57BL/6J); 4 groups n = 20/group, one of the groups—10 mg/kg, i.g. BCP. | BCP reduced the permeability of the BBB, likely by altering the expression of TJ proteins and decreasing oxidative stress levels. | BCP activation of CB2 reduces BBB disruption and oxidative stress, protecting dopaminergic neurons. | [36] |
| To determine the effect of BCP supplementation on cognitive function and quality of life in older adults with memory complaints. | Prospective, randomized 8-week trial; 52 participants (mean age 67 ± 5 years, classified obese by BMI); randomized to receive BCP 90 mg (n = 29) or 180 mg (n = 29). Cognitive performance (4 online brain games) and quality of life assessed at baseline, week 4, and week 8. | BCP is suggested to improve cognitive function in an elderly population. | – | [37] |
| Study Objectives | Study Design | Main Results | Mechanism of Action | Ref. |
|---|---|---|---|---|
| Investigate how XAN affects autophagy at the molecular level. | Human epidermoid carcinoma A431 cells and Hella cells. XAN treatment with 0, 10, 30 μM. | XAN inhibited autophagosome maturation. | XAN binds directly to the N domain of VCP. | [44] |
| Assess neuroprotective effects of XAN under oxidative stress. | PC12 cells were pretreated with XAN 0.1–0.5 µM. | XAN upregulated cytoprotective genes and protected cells from oxidative damage. | Activated Nrf2 pathway via α, β-unsaturated ketone, enhancing antioxidant defenses. | [45] |
| Investigate XAN and related hop-derived compounds for their inhibitory effects on AChE and BChE—key enzymes in AD. | In vitro ELISA assays to assess enzyme inhibition and molecular docking for binding interactions of XAN (30–70 µM). | XAN inhibits cholinesterases by binding to active site residues, supporting its potential as a lead compound for AD drugs. | Xanthohumol and 3-hydroxy-xanthohumol showed moderate AChE and BChE inhibition (IC50 ~30–70 µM). 8-prenylnaringenin also inhibited BChE. | [46] |
| Evaluate the therapeutic potential of XAN in AD. | N2a/APP and HEK293/tau cell lines. XAN treatment 52 μM. | XAN reduced Aβ accumulation, inhibited APP processing, and ameliorated tau hyperphosphorylation. | Modulation of PP2A, GSK3β, ER stress, oxidative stress, proteasome function, and cytoskeletal proteins. | [47] |
| Investigate the effects of XAN on tau protein aggregation related to AD. | Biochemical binding assays, tau aggregation/disaggregation studies, and cell-based toxicity/apoptosis assays. N2a cells were treated with XAN (0–100 μM). | Inhibits aggregation and disaggregates tau fibrils. Reduces tau-induced apoptosis, with low cytotoxicity. | XAN inhibits tau protein aggregation and disaggregate tau filaments. | [48] |
| Identify XAN analogues that activate Nrf2 and protect against oxidative stress in neurodegeneration. | PC12 cell model, H2O2/6-OHDA-induced injury, cytotoxicity and Nrf2 pathway analysis. XAN treatment 5 and 10 μM. | Two analogues with removed prenyl group showed low toxicity and rescued cells from oxidative injury. | Activation of Nrf2 pathway via nuclear translocation and protection abolished by Nrf2 knockdown. | [49] |
| Investigate neuroprotective effects of XAN and quercetin against corticosterone-induced cytotoxicity in cortical cells. | Primary cortical cultures from postnatal day 1 Sprague Dawley male rats were prepared. Quercetin (0.03–3 µM) and XAN (0.2–5 µM) were added for 24 h, then replaced with 200 µM corticosterone for 96 h. | Both polyphenols prevented corticosterone-induced loss of cell viability and restored Bdnf mRNA levels. | XAN neuroprotection is mediated via Nrf2 activation. | [50] |
| Assess hops and XAN effects on oxidative stress and adenosine receptors. | C6 and SH-SY5Y cells treated with 500 μL hops extracts, XAN 10–100 μM, and/or 50 μM H2O2 for 30 min or 24 h. | Hops reversed oxidative stress-induced cell death; XAN did not but modulated adenosine receptors. | Modulation of adenosine A1 and A2 receptors. | [51] |
| Investigate how XAN and related prenylflavonoids interact with Aβ1-42 oligomers to modulate amyloid aggregation. | Structural and molecular analysis of prenylflavonoids with Aβ1-42 and evaluation of anti-amyloidogenic properties at molecular level. XAN is tested with 0.5 μM, 0.15 mM and without defined concentrations. | XAN strongly inhibited Aβ1-42 aggregation at low concentrations, stabilized amorphous aggregates, and prevented β-sheet fibril formation. | XAN forms stable complexes with Aβ1-42 oligomers via conformational flexibility, redirecting aggregation toward less toxic forms. | [52] |
| Investigate the effect of XAN on the adenosinergic pathway, potentially involved in AD pathology. | Cell culture study using C6 (rat glioma) and SH-SY5Y (human neuroblastoma) cells treated with 10 µM XAN. | XAN may protect against AD by enhancing A1 receptor-mediated inhibition of excitotoxicity and modulating adenosine metabolism. | XAN increased A1 receptor levels. No effect on A2A receptors or adenylate cyclase activity. CD73 (5′-nucleotidase) significantly decreased in C6 cells. | [53] |
| Investigate the neuroprotective effects of XAN in rats with MCAO-induced cerebral ischemia. | Rats were treated with XAN (0.2–0.4 mg/kg, i.p.) 10 min before MCAO. | Reduced infarct size and improved neurobehavior. | Suppressed HIF-1α, TNF-α, iNOS, and caspase-3 expression. Inhibited platelet aggregation and hydroxyl radical formation. | [54] |
| Investigate the neuroprotective effects of XAN on age-related brain inflammation and apoptosis. | Male SAMP8 mice (aging model) treated with XAN (1 or 5 mg/kg/day) for 30 days and comparisons made with young/old SAMR1 controls. | Anti-inflammatory and anti-apoptotic effects, and preservation of synaptic markers suggest XAN protects against aging-induced neurodegeneration. | XAN reduced expression of pro-inflammatory (TNF-α, IL-1β) and pro-apoptotic (AIF, BAD, BAX) markers. A dose of 5 mg/kg restored synaptic proteins (BDNF, synapsin, and synaptophysin). | [55] |
| Investigate the neuroprotective effects of XAN in ischemic stroke. | Male Sprague Dawley rats were divided into sham, MCAO, and XAN (0.4 mg/kg, i.p.) groups (n = 12). MCAO and XAN groups underwent 60 min middle cerebral artery occlusion with 24 h reperfusion; sham rats had surgery without occlusion. | XAN reduced brain infarct size, improved neurological function, and reduced oxidative stress and neuronal apoptosis. | Inhibition of p38-MAPK phosphorylation and activation of Nrf2-mediated antioxidant response. | [56] |
| Assess the protective effects of XAN against glutamate-induced excitotoxicity. | Male Sprague Dawley rats were randomly assigned to 6 groups: control, XAN 10 or 50 mg/kg, kainic acid (KA) 15 mg/kg, and XAN + KA. XAN was given i.p. 30 min before KA. Seizures were monitored for 4 h. | XAN reduced KA-induced seizures, glutamate elevation, and neuronal death in the CA3 region of the hippocampus. | XAN upregulated mitochondrial fusion protein Mfn-2 and antiapoptotic Bcl-2, inhibited Apaf-1 and caspase-3 activation, preserving mitochondrial function and promoting neuron survival. | [57] |
| Evaluate the protective effects of XAN against LPS-induced depressive-like behaviors via neuroinflammation and oxidative stress pathways. | Mice were pretreated with XAN (10/20 mg/kg) before LPS (1 mg/kg) induction, behavior tests. | XAN reduced neuroinflammation. | XAN activates Nrf2/HO-1 antioxidant pathway and inhibits NF-κB signaling. | [58] |
| Assess whether XAN improves memory. | Male APP/PS1 and 10 C57BL/6J mice, divided into 5 groups: CON, APP/PS1, APP/PS1 + NAC (100 mg/kg), APP/PS1 + XAN-L (30 mg/kg), APP/PS1 + XAN-H (90 mg/kg) for 6 days/week for 2 months. | XAN improved memory performance, increased SOD, reduced IL-6 and IL-1β, decreased hippocampal Aβ deposition, and promoted autophagy and anti-apoptotic signals. It shows anti-inflammatory and antioxidant effects. | Activation of mTOR/LC3 (autophagy) and Bax/Bcl-2 (apoptosis inhibition) pathways. | [59] |
| Examine how XAN modulates gut microbiota and cognitive function in APP/PS1 mice as a potential AD treatment. | APP/PS1 and C57 WT mice; preventive (2-month-old) and therapeutic (6-month-old) studies; 4 groups per study: WT + corn oil, WT + XAN (5 mg/kg), APP/PS1 + corn oil, APP/PS1 + XAN (5 mg/kg) for 90 days. | XAN shows protective role in regulating gut microbiome composition and metabolism in an animal model of AD. | - | [60] |
| Investigate how XAN repairs cognitive impairment caused by estrogen deprivation. | Thirty C57BL/6J female mice divided in 5 groups: sham-operated, OVX + saline (vehicle), and OVX + xanthohumol at 10, 25, or 50 mg/kg. XAN was administered intraperitoneally every 2 days. | XAN improved learning and memory in OVX mice; miR-532-3p was downregulated, and Mpped1 expression was restored; overexpression of Mpped1 improved cognition. | XAN inhibits miR-532-3p, thereby restoring Mpped1 expression in the hippocampus to reverse cognitive impairment. | [61] |
| Evaluate the neuroprotective effects of HLE and XAN against iron-induced nerve damage. | Male C57BL/6 mice were randomly assigned to 8 groups: control, iron dextran plus vehicle, iron dextran + humic acid (0.1 mg/kg/day), iron dextran + N-acetylcysteine (100 mg/kg/day), iron dextran + low-dose hawthorn leaf extract (3 g/kg/day), iron dextran + high-dose hawthorn leaf extract (9 g/kg/day), iron dextran + low-dose XAN (30 mg/kg/day), and iron dextran + high-dose XAN (90 mg/kg/day). | HLE and XAN improved memory, reduced oxidative stress, and increased antioxidant enzyme activity. | Activation of AKT/GSK3β and Nrf2/NQO1/HO-1 signaling pathways. | [62] |
| Explore how XAN prevents memory loss in AD. | APP/PS1 mice: wild-type, Alzheimer’s disease model (AD), or AD + XAN, 0.5 mg/kg groups for 90 days. | Inhibited excitotoxicity, enhanced mitochondrial function, regulated systemic glutamate. | Reduced excitatory receptor expression, boosted ATP and mitophagy, lowered glutamate levels. | [63] |
| Explore the preventive and therapeutic mechanisms of XAN in AD using metabolite analysis. | APP/PS1 mice. Animals received XAN (5 mg/kg, gavage, every other day, 90 days), corn oil (vehicle), or memantine (5 mg/kg, positive control). | XAN improved cognition in older mice. | XAN influences cognition via endogenous metabolite regulation. | [64] |
| Identify therapeutic targets of different XAN doses in early Alzheimer’s using proteomics and microbiomics. | APP/PS1 mice Treated with 0.5 mg/kg and 5 mg/kg XAN. | 0.5 mg/kg XAN improved memory, neurogenesis and reduced inflammation. | XAN modulates neurodegeneration pathways and gut microbiota. Low dose may optimize brain–gut signaling and cognitive benefits. | [65] |
| Improve oral bioavailability and therapeutic efficacy of XAN for AD. | AD was induced by AlCl3 (100 mg/kg, p.o.) for 56 days in rats. Treatments: XAN (30 mg/kg), donepezil (1 mg/kg), and XAN NLCs (15, 30 mg/kg), with corresponding controls. Behavioral tests were performed on days 56, 70, and 84. | Enhancements in cognitive and motor performance, along with decreased levels of AChE, Aβ, oxidative stress, and neuroinflammation. | - | [66] |
| Study Overview | Phase | Status | Trial ID |
|---|---|---|---|
| Xanthohumol | |||
| Determine the pharmacokinetic profile of xanthohumol after oral intake. | Not applicable | Completed | NCT01367431 |
| Evaluate the capability of xanthohumol to prevent damage of DNA and reduce oxidative stress. | Phase 1 | Completed | NCT02432651 |
| Determine the effect of xanthohumol on metabolic syndrome progression. | Not applicable | Unknown | NCT03561116 |
| Determine the effect of xanthohumol and iso-alpha acids on the immune response of healthy participants (placebo-controlled crossover study). | Not applicable | Completed | NCT04847193 |
| Determine the biological effect of xanthohumol exposure and its metabolism by intestinal microorganisms (double-masked, placebo-controlled, randomized clinical trial). | Phase 1 | Ongoing | NCT03735420 |
| Evaluate the safety and tolerability of xanthohumol and its effect on adults with Crohn’s disease (double-masked, placebo-controlled, randomized clinical trial). | Phase 2 | Ongoing | NCT04590508 |
| Determine the effect of xanthohumol on clinical course, inflammatory response and outcome of patients with COVID-related acute respiratory failure. | Early phase 1 | Suspended | NCT05463393 |
| Evaluate the rate and extend of the plasma presence of xanthohumol and micelle-incorporated xanthohumol in healthy men and women. | Not applicable | Completed | NCT05524714 |
| Evaluate the capability of xanthohumol to reduce the inflammatory response in patients with septic shock (randomized, double-blind, placebo-controlled study). | Phase 2 | Ongoing | NCT06225258 |
| Evaluate the effect of xanthohumol on the severity of symptoms and duration of viral infections (placebo-controlled study. | Not applicable | Ongoing | NCT06286657 |
| Investigate how xanthohumol affects the resting energy expenditure and substrate oxidation in healthy women. | Not applicable | Completed | NCT05711212 |
| Evaluate the effect of iso-alpha acids and xanthohumol on the immune response in overweight patients. | Not applicable | Ongoing | NCT06745102 |
| β-caryophyllene | |||
| Determine the pharmacokinetics of β-caryophyllene and to evaluate its analgesic effect on thermal pain (randomized, placebo-controlled, double-blind study). | Phase 2 | Withdrawn | NCT04794205 |
| Evaluate the effectiveness of combined treatment of oxygen–ozone injection and topical patches containing cannabidiol and β-caryophyllene, compared to oxygen–ozone injection alone in patients with neck pain (randomized, controlled study). | Phase 3 | Ongoing | NCT06099171 |
| Evaluate the analgesic effect of 20% topical cream containing β-caryophyllene alone or in combination with 0.025% capsicum oleoresin, in patients with knee osteoarthritis-induced pain (randomized, double-blind, placebo-controlled crossover trial). | Phase 2 | Completed | NCT03152578 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, S.; Dzhakova, Z.; Todorova, V.; Boyuklieva, R.; Simeonov, P.; Katsarov, P. Advancing Brain Health Naturally: β-Caryophyllene and Xanthohumol as Neuroprotective Agents. Molecules 2025, 30, 3702. https://doi.org/10.3390/molecules30183702
Ivanova S, Dzhakova Z, Todorova V, Boyuklieva R, Simeonov P, Katsarov P. Advancing Brain Health Naturally: β-Caryophyllene and Xanthohumol as Neuroprotective Agents. Molecules. 2025; 30(18):3702. https://doi.org/10.3390/molecules30183702
Chicago/Turabian StyleIvanova, Stanislava, Zoya Dzhakova, Velislava Todorova, Radka Boyuklieva, Plamen Simeonov, and Plamen Katsarov. 2025. "Advancing Brain Health Naturally: β-Caryophyllene and Xanthohumol as Neuroprotective Agents" Molecules 30, no. 18: 3702. https://doi.org/10.3390/molecules30183702
APA StyleIvanova, S., Dzhakova, Z., Todorova, V., Boyuklieva, R., Simeonov, P., & Katsarov, P. (2025). Advancing Brain Health Naturally: β-Caryophyllene and Xanthohumol as Neuroprotective Agents. Molecules, 30(18), 3702. https://doi.org/10.3390/molecules30183702










