Phytochemical Analysis and Chymotrypsin Inhibitory Potential of Galium sp. and Solidago sp. via Effect-Directed HPTLC Bioassay
Abstract
1. Introduction
2. Results
2.1. Extracts Yield
2.2. Total Phenolics Content (TPC), Total Flavonoids Content (TFC), and Antioxidant Activity
2.3. HPTLC-DPPH•
2.4. HPTLC-Chymotrypsin Inhibition
3. Discussion
4. Materials and Methods
4.1. Chamicals and Reagents
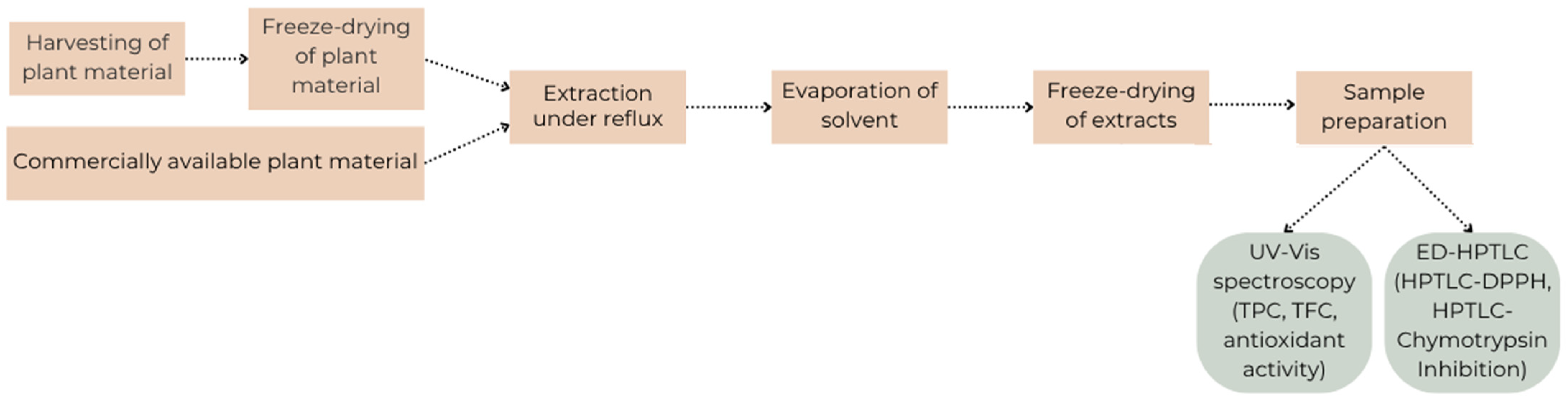
4.2. Procedure Diagram
4.3. Plant Material
4.4. Extraction and Preparation of Crude Extracts
4.5. UV-Vis Spectrophotometric Assays
4.5.1. Total Phenolic Content (TPC)
4.5.2. Total Flavonoids Content (TFC)
4.5.3. Antioxidant Activity—DPPH•
4.5.4. Antioxidant Activity—FRAP
4.5.5. Antioxidant Activity—ABTS+•
4.6. HPTLC
4.6.1. HPTLC-DPPH•
4.6.2. HPTLC-Chymotrypsin Inhibition
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ga C | Galium aparine L. commercial plant material |
| Ga L | Galium aparine L. lyophilized plant material self-collected from wild sites |
| Gv C | Galium verum L. commercial plant material |
| Gv L | Galium verum L. lyophilized plant material self-collected from wild sites |
| Sv C | Solidago virgaurea L. commercial plant material |
| Sv L | Solidago virgaurea L. lyophilized plant material self-collected from wild sites |
| Sc L | Solidago canadensis L. lyophilized plant material self-collected from wild sites |
References
- Wei, W.; Chen, Y.; Xie, D.; Zhou, Y. Molecular Insight into Chymotrypsin Inhibitor 2 Resisting Proteolytic Degradation. Phys. Chem. Chem. Phys. 2019, 21, 5049–5058. [Google Scholar] [CrossRef] [PubMed]
- Famutimi, O.G.; Adebiyi, V.G.; Akinmolu, B.G.; Dada, O.V.; Adewale, I.O. Trypsin, Chymotrypsin and Elastase in Health and Disease. Futur. J. Pharm. Sci. 2024, 10, 126. [Google Scholar] [CrossRef]
- Liu, K.; Woolman, M. Method for Determining Chymotrypsin Inhibitor Activity: Investigations into Sample Blank Measurements, Factors Involved, and Further Improvement. Sustain. Food Proteins 2025, 3, e70001. [Google Scholar] [CrossRef]
- Gunbatan, T.; Sucu, M.; Gokbulut, A.; Dilmac, E.; Gurbuz, I. Chymotrypsin and Trypsin Inhibitory Activity of Some Medicinal Plants Collected from Rize (Türkiye). Chem. Biodivers. 2024, 21, e202301879. [Google Scholar] [CrossRef]
- Liu, K. Chymotrypsin Inhibitor Assay: Expressing, Calculating, and Standardizing Inhibitory Activity in Absolute Amounts of Chymotrypsin Inhibited. Sustain. Food Proteins 2023, 1, 30–44. [Google Scholar] [CrossRef]
- Yakoby, N.; Raskin, I. A Simple Method to Determine Trypsin and Chymotrypsin Inhibitory Activity. J. Biochem. Biophys. Methods 2004, 59, 241–251. [Google Scholar] [CrossRef]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free Radicals, Antioxidants in Disease and Health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar] [CrossRef]
- Kulawik, A.; Cielecka-Piontek, J.; Zalewski, P. The Importance of Antioxidant Activity for the Health-Promoting Effect of Lycopene. Nutrients 2023, 15, 3812. [Google Scholar] [CrossRef]
- Chandimali, N.; Bak, S.G.; Park, E.H.; Lim, H.J.; Won, Y.S.; Kim, E.K.; Park, S.I.; Lee, S.J. Free Radicals and Their Impact on Health and Antioxidant Defenses: A Review. Cell Death Discov. 2025, 11, 19. [Google Scholar] [CrossRef]
- Ayoka, T.O.; Ezema, B.O.; Eze, C.N.; Nnadi, C.O. Antioxidants for the Prevention and Treatment of Non-Communicable Diseases. J. Explor. Res. Pharmacol. 2022, 7, 179–189. [Google Scholar] [CrossRef]
- Muscolo, A.; Mariateresa, O.; Giulio, T.; Mariateresa, R. Oxidative Stress: The Role of Antioxidant Phytochemicals in the Prevention and Treatment of Diseases. Int. J. Mol. Sci. 2024, 25, 3264. [Google Scholar] [CrossRef] [PubMed]
- Legerská, B.; Chmelová, D.; Ondrejovič, M.; Miertuš, S. The TLC-Bioautography as a Tool for Rapid Enzyme Inhibitors Detection—A Review. Crit. Rev. Anal. Chem. 2022, 52, 275–293. [Google Scholar] [CrossRef]
- Skrovankova, S.; Mlcek, J. Antioxidant Potential and Its Changes Caused by Various Factors in Lesser-Known Medicinal and Aromatic Plants. Horticulturae 2025, 11, 104. [Google Scholar] [CrossRef]
- Liu, K. Method Development and Optimization for Measuring Chymotrypsin and Chymotrypsin Inhibitor Activities. J. Food Sci. 2022, 87, 2018–2033. [Google Scholar] [CrossRef]
- Corni, G.; Brighenti, V.; Pellati, F.; Morlock, G.E. Effect-Directed Analysis of Bioactive Compounds in Cannabis Sativa L. by High-Performance Thin-Layer Chromatography. J. Chromatogr. A 2020, 1629, 461511. [Google Scholar] [CrossRef] [PubMed]
- Morlock, G.E. High-Performance Thin-Layer Chromatography Combined with Effect-Directed Assays and High-Resolution Mass Spectrometry as an Emerging Hyphenated Technology: A Tutorial Review. Anal. Chim. Acta 2021, 1180, 338644. [Google Scholar] [CrossRef] [PubMed]
- Móricz, Á.M.; Ott, P.G.; Yüce, I.; Darcsi, A.; Béni, S.; Morlock, G.E. Effect-Directed Analysis via Hyphenated High-Performance Thin-Layer Chromatography for Bioanalytical Profiling of Sunflower Leaves. J. Chromatogr. A 2018, 1533, 213–220. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Morton, D.W. Hyphenated TLC as a Tool in the Effect-Directed Discovery of Bioactive Natural Products. Appl. Sci. 2020, 10, 1123. [Google Scholar] [CrossRef]
- Morlock, G.E.; Lapin, T. Effect-Directed Analysis of Pimpinella Saxifraga L. root extract via HPTLC–UV/Vis/FLD–EDA–MS. J. Planar Chromatogr. Mod. TLC 2018, 31, 79–86. [Google Scholar] [CrossRef]
- Mocan, A.; Crişan, G.; Vlase, L.; Ivănescu, B.; Bădărău, A.S.; Arsene, A.L. Phytochemical Investigations on Four Galium Species (Rubiaceae) from Romania. Farmacia 2016, 64, 95–99. [Google Scholar]
- Vlase, L.; Mocan, A.; Hanganu, D.; Benedec, D.; Gheldiu, A.; Crişan, G. Comparative study of polyphenolic content, antioxidant and antimicrobial activity of four Galium species (Rubiaceae). Dig. J. Nanomater. Biostructures 2014, 9, 1085–1094. [Google Scholar]
- Shynkovenko, I.L.; Ilyina, T.V.; Kovalyova, A.M.; Goryacha, O.V.; Golembiovska, O.I.; Shemchuk, N.S.; Komissarenko, A.M. Phenolic compounds of the liquid extract from cleavers herb (Galium aparine L.). Vìsnik Farm. 2018, 3, 19–23. [Google Scholar] [CrossRef][Green Version]
- Matei, A.O.; Gatea, F.; Radu, G.L. Analysis of Phenolic Compounds in Some Medicinal Herbs by LC–MS. J. Chromatogr. Sci. 2015, 53, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Tămaş, M.; Stana, D.; Timiş, S. Comparative Phytochemical Research of Galium verum L. and G. mollugo L. Not. Bot. Horti Agrobot. Cluj-Napoca 2006, 34, 18–20. [Google Scholar]
- Anžlovar, S.; Koce, J.D. Antibacterial and Antifungal Activity of Aqueous and Organic Extracts from Indigenous and Invasive Species of Goldenrod (Solidago spp.) Grown in Slovenia. Phyton 2014, 54, 135–147. [Google Scholar]
- Wojnicz, D.; Tichaczek-Goska, D.; Gleńsk, M.; Hendrich, A.B. Is it Worth Combining Solidago virgaurea Extract and Antibiotics against Uropathogenic Escherichia coli rods? An In Vitro Model Study. Pharmaceutics 2021, 13, 573. [Google Scholar] [CrossRef]
- Judžentiene, A.; Budiene, J.; Labanauskas, L.; Stancelyte, D.; Nedveckyte, I. Allelopathic Activity of Canadian Goldenrod (Solidago canadensis L.) Extracts on Seed Germination and Growth of Lettuce (Lactuca sativa L.) and Garden Pepper Cress (Lepidium sativum L.). Plants 2023, 12, 1421. [Google Scholar] [CrossRef]
- Zekic, J.; Vovk, I.; Glavnik, V. Extraction and Analyses of Flavonoids and Phenolic Acids from Canadian Goldenrod and Giant Goldenrod. Forests 2021, 12, 40. [Google Scholar] [CrossRef]
- Poljuha, D.; Sladonja, B.; Božac, M.U.; Šola, I.; Damijanić, D.; Weber, T. The Invasive Alien Plant Solidago canadensis: Phytochemical Composition, Ecosystem Service Potential, and Application in Bioeconomy. Plants 2024, 13, 1745. [Google Scholar] [CrossRef]
- Senio, S.; Pereira, C.; Vaz, J.; Sokovic, M.; Barros, L.; Ferreira, I.C.F.R. Dehydration Process Influences the Phenolic Profile, Antioxidant and Antimicrobial Properties of Galium Aparine L. Ind. Crops Prod. 2018, 120, 97–103. [Google Scholar] [CrossRef]
- Öztürk, Ş.; Hazer, Y.; Kaşkatepe, B.; Ören, M. Determination of Total Phenol Contents, Antibacterial and Antioxidant Activity of Some Mosses Species. Karaelmas Fen. Ve Müh. Derg. 2022, 12, 86–92. [Google Scholar] [CrossRef]
- Chandra, S.; Khan, S.; Avula, B.; Lata, H.; Yang, M.H.; Elsohly, M.A.; Khan, I.A. Assessment of Total Phenolic and Flavonoid Content, Antioxidant Properties, and Yield of Aeroponically and Conventionally Grown Leafy Vegetables and Fruit Crops: A Comparative Study. Evid.-Based Complement. Altern. Med. 2014, 2014, 253875. [Google Scholar] [CrossRef] [PubMed]
- Sielicka, M.; Malecka, M.; Purlan, M. Comparison of the Antioxidant Capacity of Lipid-Soluble Compounds in Selected Cold-Pressed Oils Using Photochemiluminescence Assay (PCL) and DPPH Method. Eur. J. Lipid Sci. Technol. 2014, 116, 388–394. [Google Scholar] [CrossRef]
- Szôllôsi, R.; Szôllôsi Varga, I. Total Antioxidant Power in Some Species of Labiatae (Adaptation of FRAP Method). Acta Biol. Szeged. 2002, 46, 125–127. [Google Scholar]
- Lee, K.J.; Oh, Y.C.; Cho, W.K.; Ma, J.Y. Antioxidant and Anti-Inflammatory Activity Determination of One Hundred Kinds of Pure Chemical Compounds Using Offline and Online Screening HPLC Assay. Evid.-Based Complement. Altern. Med. 2015, 2015, 165457. [Google Scholar] [CrossRef]
- Islam, M.K.; Sostaric, T.; Lim, L.Y.; Hammer, K.; Locher, C. Antioxidant Hptlc-Dpph Fingerprinting of Honeys and Tracking of Antioxidant Constituents upon Thermal Exposure. Foods 2021, 10, 357. [Google Scholar] [CrossRef]
- Legerská, B.; Chmelová, D.; Ondrejovič, M. TLC-Bioautography as a Fast and Cheap Screening Method for the Detection of α-Chymotrypsin Inhibitors in Crude Plant Extracts. J. Biotechnol. 2020, 313, 11–17. [Google Scholar] [CrossRef]

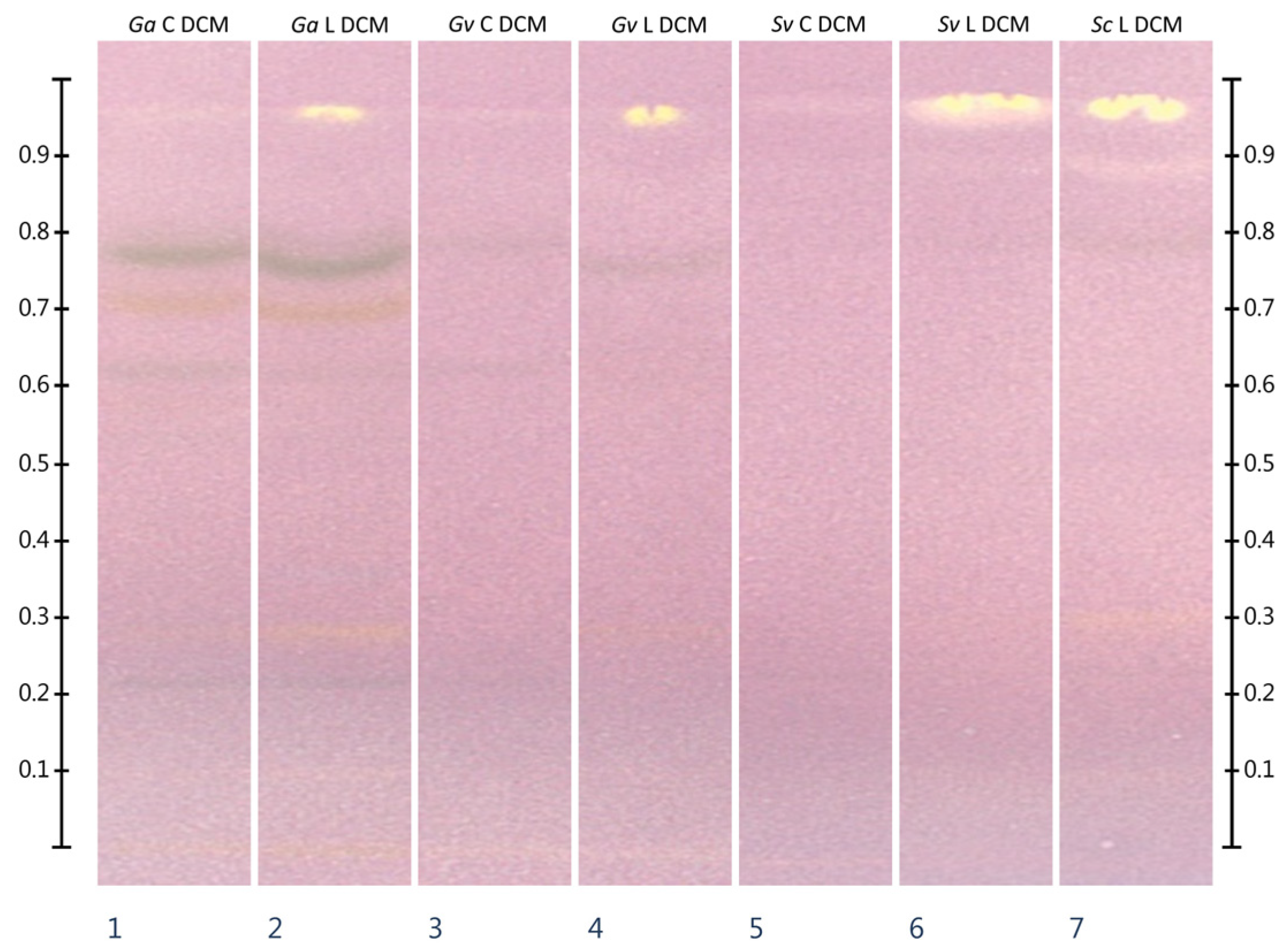
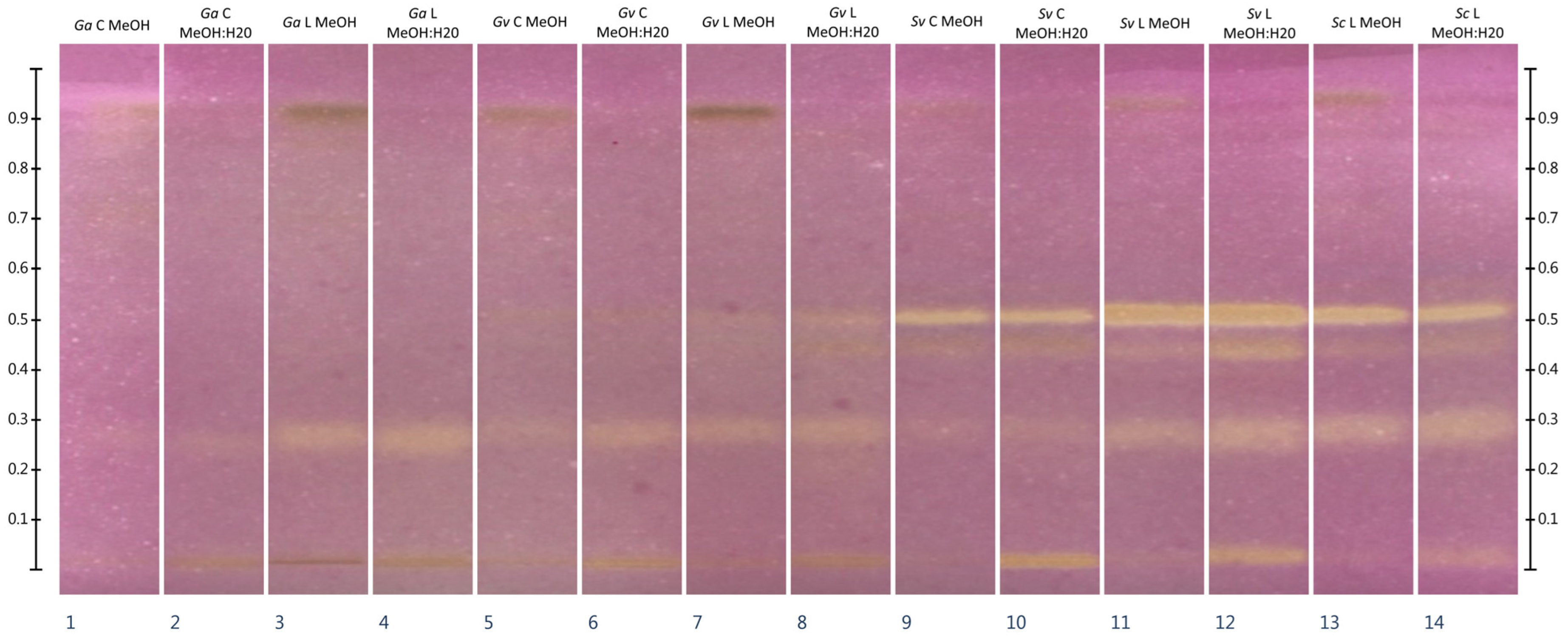

| Extract Type | Extract Yield from Plant Material [mg/g] | ||
|---|---|---|---|
| Methanol | Methanol:Water (70:30 v/v) | Dichloromethane | |
| G. aparine commercial | 116.64 | 162.12 | 10.86 |
| G. aparine lyophilized | 147.08 | 179.22 | 10.74 |
| G. verum commercial | 130.38 | 160.46 | 17.10 |
| G. verum lyophilized | 188.08 | 194.08 | 15.78 |
| S. virgaurea commercial | 117.78 | 218.88 | 17.12 |
| S. virgaurea lyophilized | 128.82 | 266.70 | 15.34 |
| S. canadensis lyophilized | 214.68 | 315.40 | 15.66 |
| Total Phenolics Content (TPC), Total Flavonoids Content (TFC) and Antioxidant Activity | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plant Extract | TPC [mg GAE/g Crude Extract] | TFC [mg RE/g Crude Extract] | DPPH• [TE/g Crude Extract] | FRAP [TE/g Crude Extract] | ABTS+• [TE/g Crude Extract] | |||||||
| MeOH | MeOH:H2O | MeOH | MeOH:H2O | MeOH | MeOH:H2O | DCM | MeOH | MeOH:H2O | MeOH | MeOH:H2O | DCM | |
| Ga C | 34.49 ± 1.12 | 37.18 ± 0.00 | 1.61 ± 0.09 | 0.16 ± 0.04 | 33.73 ± 3.57 | 50.76 ± 2.96 | 19.55 ± 1.51 | 17.48 ± 0.28 | 18.13 ± 0.51 | 32.71 ± 3.43 | 41.46 ± 1.62 | 18.68 ± 1.22 |
| Ga L | 56.73 ± 2.36 | 60.10 ± 2.70 | 0.74 ± 0.13 | 0.35 ± 0.04 | 78.36 ± 3.91 | 110.87 ± 4.44 | 27.07 ± 0.93 | 57.70 ± 0.61 | 67.31 ± 2.08 | 69.31 ± 4.05 | 109.69 ± 2.99 | 27.96 ± 0.37 |
| Gv C | 56.00 ± 1.12 | 84.62 ± 2.54 | 4.45 ± 0.33 | 1.67 ± 0.04 | 71.15 ± 4.96 | 122.37 ± 1.84 | 15.33 ± 0.35 | 49.15 ± 2.63 | 88.31 ± 2.19 | 75.79 ± 2.33 | 112.28 ± 7.56 | 11.39 ± 0.93 |
| Gv L | 76.04 ± 1.27 | 122.75 ± 0.00 | 6.32 ± 0.16 | 2.68 ± 0.15 | 66.55 ± 1.06 | 126.05 ± 1.66 | 20.17 ± 2.26 | 75.12 ± 0.56 | 144.41 ± 0.28 | 49.01 ± 2.94 | 118.76 ± 3.11 | 21.70 ± 0.28 |
| Sv C | 93.15 ± 5.50 | 129.11 ± 1.69 | 2.53 ± 0.49 | 1.47 ± 0.35 | 124.97 ± 3.91 | 177.42 ± 5.79 | 20.17 ± 1.70 | 107.52 ± 1.74 | 134.72 ± 4.44 | 149.85 ± 4.55 | 171.44 ± 8.23 | 12.47 ± 0.58 |
| Sv L | 184.89 ± 6.11 | 200.53 ± 4.48 | 4.34 ± 0.00 | 2.33 ± 0.12 | 163.47 ± 3.72 | 285.22 ± 4.22 | 17.17 ± 2.06 | 183.13 ± 8.00 | 294.84 ± 6.11 | 142.29 ± 1.98 | 249.39 ± 9.34 | 21.43 ± 1.10 |
| Sc L | 155.02 ± 1.47 | 157.95 ± 6.39 | 4.72 ± 0.19 | 3.93 ± 0.11 | 152.43 ± 1.62 | 218.98 ± 5.75 | 24.77 ± 3.14 | 169.40 ± 3.67 | 234.75 ± 3.10 | 125.56 ± 5.53 | 194.98 ± 8.91 | 25.96 ± 1.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rył, B.; Jasicka-Misiak, I. Phytochemical Analysis and Chymotrypsin Inhibitory Potential of Galium sp. and Solidago sp. via Effect-Directed HPTLC Bioassay. Molecules 2025, 30, 2746. https://doi.org/10.3390/molecules30132746
Rył B, Jasicka-Misiak I. Phytochemical Analysis and Chymotrypsin Inhibitory Potential of Galium sp. and Solidago sp. via Effect-Directed HPTLC Bioassay. Molecules. 2025; 30(13):2746. https://doi.org/10.3390/molecules30132746
Chicago/Turabian StyleRył, Bartosz, and Izabela Jasicka-Misiak. 2025. "Phytochemical Analysis and Chymotrypsin Inhibitory Potential of Galium sp. and Solidago sp. via Effect-Directed HPTLC Bioassay" Molecules 30, no. 13: 2746. https://doi.org/10.3390/molecules30132746
APA StyleRył, B., & Jasicka-Misiak, I. (2025). Phytochemical Analysis and Chymotrypsin Inhibitory Potential of Galium sp. and Solidago sp. via Effect-Directed HPTLC Bioassay. Molecules, 30(13), 2746. https://doi.org/10.3390/molecules30132746






