Abstract
This study investigates photocatalytic cells based on cocatalyst-loaded SrTiO3:Al and nano-SiO2 as a porous binder, immobilized on frosted glass. Comprehensive analysis confirmed the successful incorporation of aluminum into SrTiO3, increasing oxygen vacancy concentration and enhancing charge transfer. The deposition of RhCr2O3 and CoOOH cocatalysts significantly improved photocatalytic activity, boosting hydrogen and oxygen evolution rates to 3.8401 and 1.6319 mmol g−1 h−1, respectively. The introduction of nano-SiO2 increased hardness (0.23–0.25 GPa) and Young’s modulus (5.27–5.40 GPa), reinforcing structural integrity. The development of efficient photocatalytic panels requires a multifaceted strategy that considers chemical, mechanical, and optical properties together with stability, durability, and energy efficiency. Future research should focus on optimizing these key parameters to enhance system performance for industrial applications.
1. Introduction
The development of photocatalysts represents one of the most promising directions in modern science [1,2]. Their applications span a wide range of areas, including water and air purification [3], the degradation of organic pollutants [4], and photocatalytic hydrogen generation from water under solar irradiation [5,6]. Despite the active advancement of photocatalysts, their large-scale implementation is hindered by several factors, including low photocatalytic activity, high charge carrier recombination rates, and insufficient mechanical stability [7,8,9]. A key research focus is the development of new photocatalytic materials with enhanced efficiency and stability [10,11,12]. Among these materials, SrTiO3 is of particular interest due to its high chemical and thermal stability, excellent charge carrier mobility, and reduced recombination probability [13,14,15,16]. Despite its favourable properties, pristine SrTiO3 typically exhibits limited photocatalytic activity due to poor visible light absorption and high recombination rates, which can be significantly improved by cocatalyst loading and doping strategies [17,18]. To enhance the efficiency of SrTiO3, various modification strategies are actively being explored, including Al doping and the decoration with Rh-, Cr-, and Co-based nanoparticles [19,20,21]. It has been demonstrated that incorporating aluminum nanoparticles into the SrTiO3 structure reduces the recombination rate of photogenerated charge carriers [22]. According to several studies [23,24,25], the modification of SrTiO3 with aluminum nanoparticles enhances charge separation efficiency, increases light absorption, and improves the photocatalytic performance of the material. In addition, silicon dioxide (nano-SiO2) has emerged as an effective porous binder that enhances mechanical strength and improves the immobilisation of photocatalyst layers on solid supports.
For the large-scale implementation of photocatalytic materials, a promising approach is their integration into structural panels [26,27], that operate under solar irradiation [28,29]. However, the successful industrial deployment of photocatalytic panels requires overcoming several key limitations related to the mechanical stability of materials, their environmental durability, the efficiency of photocatalyst deposition, and their integration into existing energy systems [30,31]. One of the critical challenges is enhancing the mechanical strength of photocatalytic panels, which requires a clearer understanding of mechanical stress—namely, the internal force per unit area arising from external loads, thermal expansion, or structural deformation. Such stress can lead to fatigue, microcracking, or delamination over time, especially under cyclic loading. Previous studies have reported that mechanical stress can significantly impair the structural integrity and long-term performance of photocatalytic materials [32,33]. In addition, external factors such as intense ultraviolet radiation, temperature fluctuations, and moisture exposure can accelerate these effects, further highlighting the need for in-depth research on mechanical stability. Another crucial aspect of improving the efficiency of such systems is optimizing the methods for depositing the photocatalyst onto the substrate. Enhanced immobilization should minimize the overlap of active sites and ensure their maximum accessibility. Moreover, specialized protective coatings with hydrophobic or chemically resistant properties can extend the lifespan of photocatalytic panels [34,35]. Thus, the development of photocatalytic panels requires further research to enhance their stability, optimize photocatalyst deposition techniques, improve mechanical strength, and adapt them to real-world operating conditions.
This study examines photocatalytic cells based on modified SrTiO3 (cocatalyst-loaded STO:Al) and nano-SiO2, which serves as a porous binder, deposited on the surface of frosted glass. Their chemical, mechanical, and optical properties, as well as durability and photoefficiency, are investigated. The influence of composition and structural features on the mechanical properties of the panels is analyzed, allowing for an assessment of their potential for further integration into technological and energy solutions.
2. Experimental Part
2.1. Materials
Titanium(IV) oxide, anatase (TiO2, particle sizes < 25 nm, 99.7%), strontium nitrate Sr(NO3)2 ≥ 98%), silicon dioxide (SiO2 < 99.99%), aluminium oxide (Al2O3, particle sizes < 50 nm, 99.8%), rhodium(III) chloride hydride (RhCl3·6H2O, Rh 38–40%), cobalt (II) nitrate hexahydrate (Co(NO3)3·6H2O, ≥98%), and methylene blue (C16H18ClN3S·H2O, dye content, ≥82%) were purchased from Sigma–Aldrich (St. Louis, MO, USA). Oxalic acid ((COOH)2·2H2O, >98%), strontium chloride hexahydrate (SrCl2·6H2O, 99.7%), potassium chromate (K2CrO4, 99.5%), were purchased from Laborpharma (Almaty, Kazakhstan). All the chemicals were used without any additional treatment.
2.2. Synthesis of SrTiO3 and SrTiO3:Al
SrTiO3 powder was synthesized using the methodology detailed in our previous works [36,37,38]. Briefly, TiO2 was added to an aqueous solution of Sr(NO3)2 in a molar ratio of 1:1. Under vigorous stirring, 0.4 M (COOH)2·2H2O was introduced into the solution to maintain the pH level at 6–7. The resulting precipitate was dried for 16 h and then calcined at 900 °C for 1 h. Aluminum doping of the obtained SrTiO3 powder was carried out using a flux treatment method. For this purpose, Al2O3 and SrCl2 were added to the SrTiO3 powder as fluxing agents in a molar ratio of 1:0.02:10. All components were mixed in an agate mortar and placed in a crucible for heating at 1150 °C for 10 h. The samples were then subjected to ultrasonic treatment, washed five times with distilled water, and dried for 20 h. The resulting sample is called STO:Al.
2.3. Photodeposition of Cocatalysts
The dual cocatalysts Rh/Cr2O3 and CoOOH were deposited on the STO:Al photocatalyst using the photodeposition method, as detailed in our previous works [24,39]. Briefly, 0.1 g of STO:Al was dispersed in distilled water, sonicated, and irradiated under a 10 W UV lamp. RhCl3⋅6H2O, K2CrO4, and Co(NO3)3 solutions were sequentially introduced, with irradiation after each step. The samples were then thoroughly washed and dried at 60 °C. The final cocatalyst concentrations were 0.1% Rh, 0.05% Cr, and 0.05% Co. For conciseness, the cocatalyst-photodeposited samples are referred to as Cocat/STO:Al.
2.4. Application of Photocatalyst Powders on the Substrate
The photocatalyst powders were immobilized onto frosted glass substrates with an area of 25 cm2. Before immobilization, the glass substrates were treated with a concentrated with 0.1 M H2SO4 solution and cleaned with an alcohol solution to improve wettability and ensure strong binding of the photocatalytic particles. The photocatalyst layers were formed using the drop-casting method. A mixture of 25 mg of photocatalyst powder, 25 mg of nano-SiO2, and 500 μL of distilled water was prepared to fabricate laboratory-scale photocatalytic cells with a working area of 25 cm2. The suspension was sonicated for 10 min to break up agglomerates, then applied dropwise (50 μL per drop) onto frosted glass substrates, ensuring uniform distribution, and dried at 70 °C. This process was repeated 10 times, followed by annealing in air at 350 °C for 1 h.
2.5. Characterization
Diffraction measurements were performed using the D8 ADVANCE universal diffractometer (Bruker, Germany) in the Bragg–Brentano θ-θ geometry, equipped with a copper anode tube (wavelength 1.5406 Å). The operating parameters were set to 40 kV and 40 mA, with a 2θ range of 5–80°, a step size of 0.02°, and a scanning speed of 0.5 s per step. The morphology and elemental composition of the samples were analyzed using a scanning electron microscope (Zeiss Crossbeam 540, Oberkochen, Germany) at an accelerating voltage of 5–20 kV, equipped with an energy-dispersive X-ray spectrometer (EDX) (INCA X-Sight, Oxford Instruments). Surface characterization of the synthesized photocatalytic panels was conducted using a Hitachi TM4000 scanning electron microscope, equipped with an elemental analysis attachment and secondary electron (SE) and backscattered electron (BSE) detectors. X-ray photoelectron spectroscopy (XPS) was carried out using a VG Microtech Multilab 3000 spectrometer (VG Microtech Ltd., London, UK) with Mg and Al X-ray sources to analyze the valence states and elemental composition of the samples. The C1s peak at 284.8 eV was used as a reference for binding energy (BE) calibration. Ultraviolet-visible diffuse reflectance spectra (UV-Vis DRS) were recorded on a Perkin Elmer Lambda 35 spectrophotometer in the 200–800 nm range. Mechanical properties were measured using nanoindentation with the “NanoScan-4D Compact” instrument based on the irreversible indentation method (ISO 14577). A Berkovich three-sided diamond pyramid was used as the indenter, with a load of 8 mN. The load application time was 5 s, with a holding time of 0.5 s at the specified load. The analysis of the load–displacement curves was performed using the Oliver–Pharr method [37,38], which enables the determination of coating hardness (H, GPa) and Young’s modulus (E, GPa).
2.6. Photocatalytic Measurements
The photocatalytic activity of immobilized photocatalysts was evaluated in a photochemical reactor (Shanghai Leewen Scientific Instrument Co., Ltd., Shanghai, China) under irradiation from a 10 W mercury lamp(The illumination intensity is adjusted to 12 W/m2). The photocatalytic cell (with an active area of 25 cm2 and a catalyst mass of 25 mg) was placed in a sealed quartz reactor containing 50 mL of distilled water. Before the experiment, the reaction system was purged with argon to create an inert atmosphere. The generated gaseous products were analyzed using a “CHROMOS 1000” gas chromatograph, equipped with three packed columns (3 mm in diameter) filled with NaX and PORAPAK Q sorbents, directly connected to the reactor.
3. Results and Discussion
3.1. Characterization of Samples
3.1.1. Microstructural and Morphological Properties
Figure 1a presents the X-ray diffraction (XRD) patterns of the synthesized SrTiO3-based samples. Diffraction peaks corresponding to the (110), (111), (200), (211), and (220) planes are observed at 32.40°, 40.50°, 46.50°, 58.50°, and 68.50°, respectively, indicating the cubic phase of SrTiO3 (JCPDS #35–0734). The high intensity and sharpness of the peaks suggest a high degree of crystallinity in the material. No phase reflections corresponding to the added elements (Al, Rh, Cr, Co) are detected, likely due to their low concentration and weak crystallinity. It is also noted that flux-assisted doping improves the crystallinity of SrTiO3. Electron microscopy techniques were employed to investigate the morphology of the synthesized samples. According to SEM and TEM data (Figure 1b,d), the STO particles exhibit a cubic shape with sizes ranging from 200 to 400 nm. After flux-assisted Al doping (Figure 1c,e), the STO:Al facets become smoother due to the selective adsorption of Cl− on {111}, which lowers surface energy and promotes the reduction of {100} facets [40,41,42]. This treatment also leads to the formation of more uniform particles with an average size of ~150 nm. Figure 1f illustrates the presence of RhCr2O3 and CoOOH-based cocatalysts (5–15 nm) on the surface of STO:Al. Studies indicate that pristine STO and STO:Al exhibit lower activity compared to the modified samples, as the absence of cocatalysts limits the formation of active sites [24,43,44]. Cocatalysts not only provide active sites for photocatalytic reactions but also enhance charge transfer through the formation of a Schottky junction, thereby reducing electron–hole recombination and improving photocatalytic efficiency [45,46].
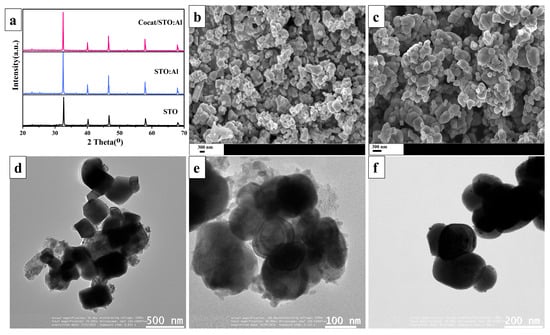
Figure 1.
(a) XRD patterns of obtained photocatalysts; SEM images of samples based on (b) STO and (c) STO:Al; TEM images of samples based on (d) STO, (e) STO:Al and (f) Cocat/STO:Al.
Figure S1 in the Supplementary Material presents SEM images of glass substrates before (a) and after (b) sandblasting treatment. The sandblasting process ensures sufficient adhesion for the effective fixation of the photocatalyst powder on the glass substrate surface. Figure S2 displays SEM images of the photocatalytic layer formed by the drop-casting method on a textured glass substrate. As shown in Figure S2d, the SrTiO3:Al-based powder layer is uniformly distributed due to the use of nano-SiO2 as a binder. This not only enhances adhesion to the substrate but also promotes the formation of a porous structure, optimizing the penetration of light, water, and the generated gases. The average thickness of the resulting coating is approximately 20 µm, which is optimal for efficient photocatalytic reactions (Figure S2d) [27].
3.1.2. Surface Chemistry and Optical Properties
XPS analysis was performed on STO:Al samples with deposited cocatalysts RhCr2O3 and CoOOH to study their chemical composition and surface states (Figure 2a–g). In the Ti 2p spectrum (Figure 2a), peaks at 458.9 and 464.5 eV are observed, corresponding to Ti4+ [19]. The HR-XPS O 1s spectrum (Figure 2b) shows two main signals: 530.3 eV, attributed to lattice oxygen (O2−), and 532.9 eV, corresponding to adsorbed oxygen (O_ads) [39]. It was established that Al doping increases the concentration of O_ads, which positively impacts the catalytic properties of the material compared to pristine STO [33]. The Sr 3d spectrum (Figure 2c) shows characteristic doublet peaks typical for Sr2+ in the perovskite structure. The Al 2p peak at 74.7 eV (Figure 2d) confirms the incorporation of Al into the STO lattice. It is known that Al3+ effectively suppresses undesirable Ti3+ defects and increases the concentration of oxygen vacancies, reducing recombination of photogenerated charges and stabilizing the defective structure [47,48].
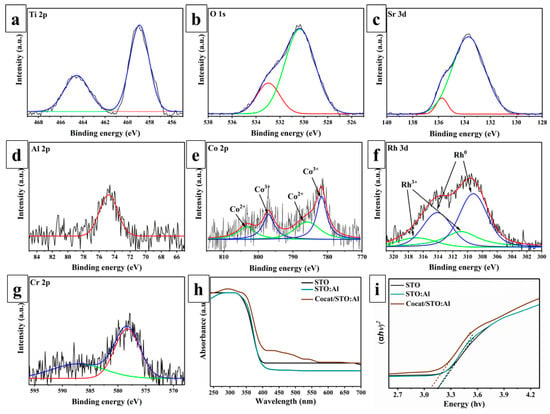
Figure 2.
High-resolution XPS spectrum of Cocat/STO:Al sample for (a) Ti 2p, (b) O 1s, (c) Sr 3d, (d) Al 2p, (e) Co 2p, (f) Rh 3d and (g) Cr 2p; (h) absorption spectra and (i) values of the bandgap of synthesized samples.
Furthermore, high-resolution XPS spectra confirmed the presence of cocatalysts on the surface of STO:Al. The Rh 3d spectrum (Figure 2f) exhibits peaks at 309.2/314.1 eV and 310.9/316.9 eV, corresponding to metallic Rh0 and oxidized Rh3+ species, respectively, with the amount of Rh3+ being relatively small [49]. The Rh 3d spectrum exhibited Rh3+ species along with traces of metallic Rh0 (Figure 2d). Metallic Rh acts as an electron sink by forming Schottky junctions with STO:Al, facilitating rapid electron capture, while Rh3+ contributes to interfacial redox cycling, accelerating the hydrogen evolution reaction (HER). Additionally, the presence of Cr3+ species from Cr2O3 (Figure 2e) was confirmed, which stabilizes Rh nanoparticles by forming a protective shell and enhances their durability under illumination. The Cr 2p spectrum (Figure 2g) displays peaks at 578.3 eV (Cr 2p3/2) and 587.1 eV (Cr 2p1/2), corresponding to Cr3+ [50]. The Co 2p spectrum (Figure 2e) contains components of both Co2+ and Co3+, with characteristic peaks at 803.4/785.9 eV and 797.0/781.7 eV, respectively. These species may originate not only from Co(III)OOH but also from oxide phases such as CoO. These observations indicate that the cocatalysts likely comprise a mixture of different oxidation states and chemical compositions, which serve as oxidative sites for hole accumulation and promote the oxygen evolution reaction (OER) through efficient utilization of photogenerated h+ [20].
The optical properties of STO, STO:Al, and Cocat/STO:Al samples were investigated by UV–vis spectroscopy. While STO and STO:Al primarily absorb light in the UV region (up to 400 nm), the Cocat/STO:Al composite shows extended absorption up to ~430 nm (Figure 2h). The Tauc plot analysis of the (αhν)2–hν dependence revealed a bandgap of 3.15 eV for STO and STO:Al, and 3.07 eV for Cocat/STO:Al (Figure 2i). Aluminum doping effectively suppresses Ti3+ defects but has no significant effect on the band gap width [21], whereas cocatalyst deposition enhances light absorption and charge separation efficiency, thereby reducing recombination losses.
3.2. Nanomechanical Testing: Nanoindentation
To evaluate the hardness and elastic modulus of the photocatalyst layers, nanoindentation tests were conducted. The load–displacement analysis allowed the determination of the mechanical properties of the coatings using the force law method according to Oliver and Pharr [51]. Young’s modulus, which characterizes the resistance to changes in interatomic distances, was calculated from the slope of the “loading–unloading” curve [52]. The tests were performed at 25 °C, and the values of hardness and Young’s modulus were determined as the average of five measurements, excluding the extreme values. The “loading–unloading” curves for measuring the mechanical properties typically exhibited a classical shape (Figure 3a), although, in some cases, steps were observed on the loading curve (red line). The unloading curves (black line), however, remained stable (Figure 3b). The presence of steps indicates localized areas of coating looseness, where the indenter penetrated to a depth of approximately 2 µm. Data from such areas were excluded from the analysis as artifacts. Figure 3 shows that the maximum indentation depth was approximately 425.99 nm, which is equivalent to about 2% of the average film thickness. All curves exhibited similar behavior in terms of plastic deformation, with the indentation imprint remaining stable, without signs of elastic relaxation.
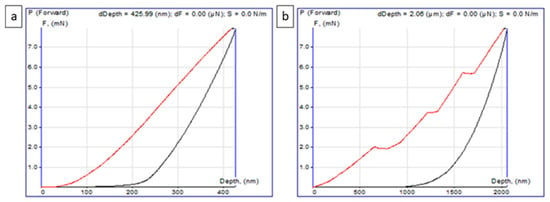
Figure 3.
Loading–unloading curves obtained during nanoindentation: (a) classical shape of the curve without discontinuities on the loading branch; (b) curve with characteristic step-like features on the loading branch (red line), while the unloading curve (black line) remains smooth.
Table 1 presents the results of the mechanical analysis of thin films. The STO:Al@nano-SiO2 coating demonstrates a hardness in the range of 0.18–0.20 GPa, which is attributed to its dense structure and the absence of cracks. A similar trend is observed for the Young’s modulus (3.77–4.89 GPa). The Cocat/STO:Al@nano-SiO2 sample shows a slight increase in hardness values (0.23–0.25 GPa) and Young’s modulus (5.27–5.40 GPa), indicating that the introduction of co-catalysts does not lead to significant morphological changes. For comparison, it has been reported in the literature [53] that the addition of ZnO nanoparticles to epoxy coatings increases their hardness and Young’s modulus by reducing the free volume in the matrix. Similarly, the introduction of nano-SiO2 in Cocat/STO:Al also contributes to the improvement of mechanical properties. In particular, the study [53] found that the maximum hardness (0.205 GPa) and Young’s modulus (4.95 GPa) were achieved with the addition of 5% SiO2 and 2% ZnO, while exceeding this concentration led to a decline in properties due to nanoparticle agglomeration. In our study, a similar trend is observed for Cocat/STO:Al, which demonstrates hardness values (0.23–0.25 GPa) and Young’s modulus (5.27–5.40 GPa). Thus, the obtained results confirm the patterns previously described in the literature and emphasize the effectiveness of modifying Cocat/STO:Al with nano-SiO2 to improve mechanical strength without significant morphological changes [54,55].

Table 1.
Hardness values and modulus of elasticity calculated for photocatalytic coatings.
3.3. Photocatalytic Performance
The photocatalytic activity of the samples immobilized on a glass substrate was investigated in the photocatalytic water splitting reaction for hydrogen (H2) and oxygen (O2) evolution. As shown in Figure 4, which presents the results of photocatalytic water decomposition using the STO:Al photocatalyst without (Figure 4a) and with (Figure 4b) photodeposited cocatalysts, the efficiency of the samples significantly depends on the presence of cocatalysts. The average H2 and O2 evolution rates for the STO:Al-based photocell were 0.0916 ± 0.0039 and 0.0389 ± 0.0013 mmol g−1 h−1, respectively, whereas the Cocat/STO:Al exhibited much higher values—3.8401 and 1.6319 mmol g−1 h−1. The relatively low efficiency of STO:Al compared to Cocat/STO:Al can be attributed to the accelerated charge transfer and separation of photogenerated charges, which reduces recombination, as well as the formation of active sites upon the deposition of cocatalysts via photodeposition. This significant improvement directly correlates with the XPS results, which confirmed the presence of Rh3+, Cr3+, and Co3+ species on the STO:Al surface. These oxidation states act as catalytically active centers, facilitating charge transfer processes: Rh and Cr species promote electron trapping and reduction reactions, while Co3+ sites acceler-ate hole utilization in oxidation reactions. Such cooperative effects suppress charge recombination and create efficient redox pathways, thereby enhancing overall photo-catalytic performance. However, in comparison to powder counterparts, the investigated samples show lower efficiency. Specifically, powder Cocat/STO:Al under similar conditions exhibited H2 and O2 evolution rates of 11.04 and 4.69 mmol g−1 h−1, respectively [24]. The decrease in activity after the photocatalyst was applied to the substrate is related to partial overlap of active particles. Nevertheless, the use of nano-SiO2 as a binder helps mitigate this effect by creating micropores that facilitate the contact of water and light with the active surface of the photocatalyst [27,28,56].
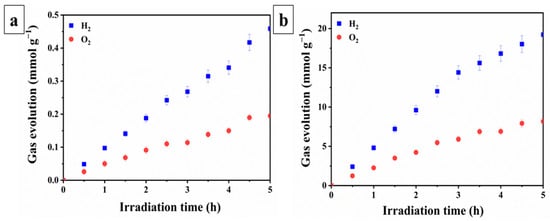
Figure 4.
Water splitting performance of photocatalytic sheets based on (a) STO:Al and (b) Cocat/STO:Al.
4. Conclusions and Future Prospectives
This study investigated photocatalytic cells based on a cocatalyst-loaded STO:Al photocatalyst and nano-SiO2 as a porous binder, immobilized on the surface of matte glass. Comprehensive analysis confirmed the successful incorporation of aluminum into SrTiO3, as confirmed by XPS analysis. This increased the concentration of oxygen vacancies and improved charge transfer. The deposition of co-catalysts (RhCr2O3 and CoOOH) significantly enhanced the photocatalytic activity by effectively separating charge carriers and creating active sites. Mechanical property analysis revealed that the addition of nano-SiO2 increased the hardness (0.23–0.25 GPa) and the Young’s modulus (5.27–5.40 GPa), confirming the suitability of its use for strengthening the structural integrity of photocatalytic films. Photocatalytic tests of the samples immobilized on glass substrates showed a significant increase in activity after the deposition of co-catalysts: the hydrogen and oxygen evolution rates increased to 3.8401 and 1.6319 mmol g−1 h−1, respectively. The development of efficient photocatalytic panels requires a comprehensive approach that includes the study of their chemical, mechanical, and optical properties, as well as the evaluation of stability, strength, and energy efficiency. Large-scale testing of such systems has already been conducted. A striking example is the work of Professor Kazunari Domen’s group, which demonstrated the safe operation of photocatalytic panel reactors with an effective area of 100 m2 [23]. These systems successfully operated for several months, demonstrating the possibility of large-scale water splitting, hydrogen and oxygen collection, and separation, which confirms the promising nature of the technology. Further research should focus on studying the influence of external factors and optimizing key aspects to improve the efficiency and stability of such systems.
For successful scaling of the technology to industrial applications, the following key aspects must be considered:
Mechanical Stability: Further investigation of the strength characteristics of photocatalytic panel cells during long-term operation, including fatigue resistance, thermal expansion, and resistance to mechanical stresses.
Environmental Impact: Studying the effects of prolonged UV exposure, temperature fluctuations, and humidity on the structural stability of photocatalytic films.
Optimization of Photocatalyst Application: Improving immobilization techniques to minimize overlap of active sites and enhance the catalyst’s accessibility for the reaction.
Development of Protective Coatings: The use of hydrophobic or chemically resistant materials to increase the lifespan of photocatalytic panels under real-world operating conditions.
Energy Efficiency and Integration: Exploring the potential for combining photocatalytic panels with other renewable energy sources (solar panels, thermoelectric modules) to increase the overall efficiency of the system.
Thus, the conducted research demonstrates significant progress in the development of photocatalytic materials. However, further work should focus on improving their stability, mechanical strength, and adaptability to real-world operating conditions, which is a crucial step toward their implementation in large-scale applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30183699/s1, Figure S1: SEM images of the glass substrate surface (a) before and (b) after sandblasting; Figure S2: SEM images of a photocatalytic layer formed by drip casting onto a textured glass substrate: (a,c) images at various magnifications, (b) energy dispersive X-ray spectroscopy (EDX) analysis showing the elemental composition, (d) layer thickness of a powder co-catalyst based on modified STO:Al.
Author Contributions
Conceptualization, methodology, writing—original draft preparation, writing—review & editing, A.B., A.D. and L.D.; formal analysis, resources, T.A. and T.Z.; project administration, investigation, funding acquisition E.M. and K.V.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research is funded by the Science Committee of the Ministry of Science and Higher Education and of the Republic of Kazakhstan (Grant No. BR21881930).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chakravorty, A.; Roy, S. A Review of Photocatalysis, Basic Principles, Processes, and Materials. Sustain. Chem. Environ. 2024, 8, 100155. [Google Scholar] [CrossRef]
- Ly, N.H.; Gnanasekaran, L.; Aminabhavi, T.M.; Vasseghian, Y.; Joo, S.W. Photogenerated Charge Carriers in Photocatalytic Materials for Solar Hydrogen Evolution. Curr. Opin. Chem. Eng. 2025, 47, 101087. [Google Scholar] [CrossRef]
- Unveiling the Photocatalytic Marvels: Recent Advances in Solar Heterojunctions for Environmental Remediation and Energy Harvesting. Available online: https://www.researchgate.net/publication/377549060_Unveiling_the_photocatalytic_marvels_Recent_advances_in_solar_heterojunctions_for_environmental_remediation_and_energy_harvesting (accessed on 27 August 2025).
- Roy, S.; Darabdhara, J.; Ahmaruzzaman, M. Sustainable Degradation of Pollutants, Generation of Electricity and Hydrogen Evolution via Photocatalytic Fuel Cells: An Inclusive Review. Environ. Res. 2023, 236, 116702. [Google Scholar] [CrossRef] [PubMed]
- Molaei, M.J. Recent Advances in Hydrogen Production through Photocatalytic Water Splitting: A Review. Fuel 2024, 365, 131159. [Google Scholar] [CrossRef]
- Serik, A.; Kuspanov, Z.; Daulbayev, C. Cost-Effective Strategies and Technologies for Green Hydrogen Production. Renew. Sustain. Energy Rev. 2026, 226, 116242. [Google Scholar] [CrossRef]
- Zakria, H.S.; Othman, M.H.D.; Kamaludin, R.; Kadir, S.H.S.A.; Kurniawan, T.A.; Jilani, A. Immobilization Techniques of a Photocatalyst into and onto a Polymer Membrane for Photocatalytic Activity. RSC Adv. 2021, 11, 6985–7014. [Google Scholar] [CrossRef]
- Wang, G.; Lv, S.; Shen, Y.; Li, W.; Lin, L.; Li, Z. Advancements in Heterojunction, Cocatalyst, Defect and Morphology Engineering of Semiconductor Oxide Photocatalysts. J. Materiomics 2024, 10, 315–338. [Google Scholar] [CrossRef]
- Nasir, J.A.; ur Rehman, Z.; Shah, S.N.A.; Khan, A.; Butler, I.S.; Catlow, C.R.A. Recent Developments and Perspectives in CdS-Based Photocatalysts for Water Splitting. J. Mater. Chem. A 2020, 8, 20752–20780. [Google Scholar] [CrossRef]
- Hassaan, M.A.; El-Nemr, M.A.; Elkatory, M.R.; Ragab, S.; Niculescu, V.-C.; El Nemr, A. Principles of Photocatalysts and Their Different Applications: A Review. Top. Curr. Chem. 2023, 381, 31. [Google Scholar] [CrossRef]
- Stabilization and Performance Enhancement Strategies for Halide Perovskite Photocatalysts-Chen-2023-Advanced Materials-Wiley Online Library. Available online: https://advanced.onlinelibrary.wiley.com/doi/abs/10.1002/adma.202203836 (accessed on 27 August 2025).
- Bhakar, U.; Agarwal, A.; Sanghi, S. Exploring SrTiO3 and (Ba,Sr)TiO3-Based Hydroelectric Cells with Defect-Driven Mechanisms for Green Power Generation. Ceram. Int. 2025, 51, 18672–18680. [Google Scholar] [CrossRef]
- González, L.; Cano-Valencia, M.J.; Vento-Lujano, E. Visible-Light Photocatalytic Performance of SrTiO3 Nanoparticles Modified with Cobalt. Opt. Mater. 2024, 157, 116231. [Google Scholar] [CrossRef]
- Baratov, A.; Kuspanov, Z.; Shaimerdenov, A.; Yergaziyeva, G.; Yerlanuly, Y.; Daulbayev, C. Enhancing Photogenerated Charge Separation in Perovskite Semiconductors via Dual Cocatalyst Engineering. J. Water Process Eng. 2025, 77, 108573. [Google Scholar] [CrossRef]
- Serik, A.; Kuspanov, Z.; Bissenova, M.; Idrissov, N.; Yeleuov, M.; Umirzakov, A.; Daulbayev, C. Effective Photocatalytic Degradation of Sulfamethoxazole Using PAN/SrTiO3 Nanofibers. J. Water Process Eng. 2024, 66, 106052. [Google Scholar] [CrossRef]
- Kuspanov, Z.; Umirzakov, A.; Serik, A.; Baimenov, A.; Yeleuov, M.; Daulbayev, C. Multifunctional Strontium Titanate Perovskite-Based Composite Photocatalysts for Energy Conversion and Other Applications. Int. J. Hydrogen Energy 2023, 48, 38634–38654. [Google Scholar] [CrossRef]
- Li, Y.; Li, R.; Zhai, Y.; Huang, Y.; Lee, S.; Cao, J. Improved Photocatalytic Activity of BaTiO3/La2Ti2O7 Heterojunction Composites via Piezoelectric-Enhanced Charge Transfer. Appl. Surf. Sci. 2021, 570, 151146. [Google Scholar] [CrossRef]
- Serik, A.; Idrissov, N.; Baratov, A.; Dikov, A.; Kislitsin, S.; Daulbayev, C.; Kuspanov, Z. Recent Progress in Photocatalytic Applications of Electrospun Nanofibers: A Review. Molecules 2024, 29, 4824. [Google Scholar] [CrossRef]
- Visible Light-Driven Photocatalysis of Al-Doped SrTiO3: Experimental and DFT Study. Available online: https://www.mdpi.com/1420-3049/29/22/5326 (accessed on 27 August 2025).
- Radu, I.; Borhan, A.; Gherca, D.; Dirtu, A.; Dirtu, D.; Popescu, D.; Husanu, M.; Pui, A. Cobalt Oxyhydroxide Co-Catalyst Loaded Onto Al:Srtio3 Surface to Boost Photocatalytic Performance. Mater. Chem. Phys. 2025, 332, 130274. [Google Scholar] [CrossRef]
- Zong, S.; Tian, L.; Guan, X.; Cheng, C.; Shi, J.; Guo, L. Photocatalytic Overall Water Splitting without Noble-Metal: Decorating CoP on Al-Doped SrTiO3. J. Colloid Interface Sci. 2022, 606, 491–499. [Google Scholar] [CrossRef]
- Abdelfattah, I.; El-Shamy, A.M. A Comparative Study for Optimizing Photocatalytic Activity of TiO2-Based Composites with ZrO2, ZnO, Ta2O5, SnO, Fe2O3, and CuO Additives. Sci. Rep. 2024, 14, 27175. [Google Scholar] [CrossRef]
- Radu, I.; Borhan, A.I.; Ghercă, D.; Popescu, D.G.; Borca, C.N.; Huthwelker, T.; Bulai, G.; Stoian, G.; Husanu, M.-A.; Pui, A. Enhancement of SrTiO3 Photocatalytic Efficiency by Al Doping: Answers from the Structure, Morphology and Electronic Properties Contributions. Ceram. Int. 2024, 50, 20664–20675. [Google Scholar] [CrossRef]
- Kuspanov, Z.; Serik, A.; Baratov, A.; Abdikarimova, U.; Idrissov, N.; Bissenova, M.; Daulbayev, C. Efficient Photocatalytic Hydrogen Evolution via Cocatalyst Loaded Al-Doped SrTiO3. Eurasian Chem.-Technol. J. 2024, 26, 133–140. [Google Scholar] [CrossRef]
- Murthy, D.H.K.; Nandal, V.; Furube, A.; Seki, K.; Katoh, R.; Lyu, H.; Hisatomi, T.; Domen, K.; Matsuzaki, H. Origin of Enhanced Overall Water Splitting Efficiency in Aluminum-Doped SrTiO3 Photocatalyst. Adv. Energy Mater. 2023, 13, 2302064. [Google Scholar] [CrossRef]
- Nishiyama, H.; Yamada, T.; Nakabayashi, M.; Maehara, Y.; Yamaguchi, M.; Kuromiya, Y.; Nagatsuma, Y.; Tokudome, H.; Akiyama, S.; Watanabe, T.; et al. Photocatalytic Solar Hydrogen Production from Water on a 100-M2 Scale. Nature 2021, 598, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, C.S.; Nalajala, N. A Scalable and Thin Film Approach for Solar Hydrogen Generation: A Review on Enhanced Photocatalytic Water Splitting. J. Mater. Chem. A 2021, 9, 1353–1371. [Google Scholar] [CrossRef]
- Xiong, A.; Ma, G.; Maeda, K.; Takata, T.; Hisatomi, T.; Setoyama, T.; Kubota, J.; Domen, K. Fabrication of Photocatalyst Panels and the Factors Determining Their Activity for Water Splitting. Catal. Sci. Technol. 2014, 4, 325–328. [Google Scholar] [CrossRef]
- Sun, T.; Benedictto, G.P.; Gonzalez, M.R.; Legnoverde, M.S.; Raymundo-Piñero, E.; Basaldella, E.I.; Ania, C. Photocatalytic Activity of NiZnAl Hydrotalcite-like Compound/Carbon Nitride Composites for the Degradation of Methylparaben. Catal. Today 2025, 459, 115432. [Google Scholar] [CrossRef]
- B., A.; Arasalike, J.; Rao, A.S.; Nagarkar, S.S.; Dutta, A.; Duttagupta, S.P.; Prabhu, S.S.; Pinto, R. Challenges in Photocatalytic Hydrogen Evolution: Importance of Photocatalysts and Photocatalytic Reactors. Int. J. Hydrogen Energy 2024, 81, 1442–1466. [Google Scholar] [CrossRef]
- Materials Advances in Photocatalytic Solar Hydrogen Production: Integrating Systems and Economics for a Sustainable Future-Gunawan-2024-Advanced Materials-Wiley Online Library. Available online: https://advanced.onlinelibrary.wiley.com/doi/full/10.1002/adma.202404618 (accessed on 27 August 2025).
- A Scalable Solar-Driven Photocatalytic System for Separated H2 and O2 Production from Water | Nature Communications. Available online: https://www.nature.com/articles/s41467-025-56314-x (accessed on 27 August 2025).
- Scaling up of Photocatalytic Systems for Large-Scale Hydrogen Generation|Applied Physics Reviews|AIP Publishing. Available online: https://pubs.aip.org/aip/apr/article-abstract/12/1/011303/3330351/Scaling-up-of-photocatalytic-systems-for-large?redirectedFrom=fulltext (accessed on 27 August 2025).
- Molar, J.; Herckes, P.; Fraser, M.P. Photocatalytic Abatement of Ambient NOx by TiO2 Coated Solar Panels. RSC Sustain. 2025, 3, 963–972. [Google Scholar] [CrossRef]
- SiO2/WO3/ZnO Based Self-Cleaning Coatings for Solar Cells | Journal of Sol-Gel Science and Technology. Available online: https://link.springer.com/article/10.1007/s10971-024-06351-7 (accessed on 27 August 2025).
- Kuspanov, Z.; Serik, A.; Tattibay, A.; Baratov, A.; Abdikarimova, U.; Bissenova, M.; Yeleyov, M.; Sakhiyev, S.; Daulbayev, C. Investigating and Correlating the Photocatalytic Activity of Synthesised Strontium Titanate Nanopowder with Calcination Temperature. Environ. Technol. Innov. 2024, 36, 103852. [Google Scholar] [CrossRef]
- Kudaibergen, A.D.; Kuspanov, Z.B.; Issadykov, A.N.; Beisenov, R.E.; Mansurov, Z.A.; Yeleuov, M.A.; Daulbayev, C.B. Synthesis, Structure, and Energetic Characteristics of Perovskite Photocatalyst SrTiO3: An Experimental and DFT Study. Eurasian Chem.-Technol. J. 2023, 25, 139–146. [Google Scholar] [CrossRef]
- Synthesis and Study of SrTiO3/TiO2 Hybrid Perovskite Nanotubes by Electrochemical Anodization. Available online: https://www.mdpi.com/1420-3049/29/5/1101 (accessed on 27 August 2025).
- Kuspanov, Z.; Serik, A.; Matsko, N.; Bissenova, M.; Issadykov, A.; Yeleuov, M.; Daulbayev, C. Efficient Photocatalytic Degradation of Methylene Blue via Synergistic Dual Co-Catalyst on SrTiO3@Al under Visible Light: Experimental and DFT Study. J. Taiwan Inst. Chem. Eng. 2024, 165, 105806. [Google Scholar] [CrossRef]
- Shen, Q.; Kang, W.; Ma, L.; Sun, Z.; Jin, B.; Li, H.; Miao, Y.; Jia, H.; Xue, J. Tuning the Anisotropic Facet of SrTiO3 to Promote Spatial Charge Separation for Enhancing Photocatalytic CO2 Reduction Properties. Chem. Eng. J. 2023, 478, 147338. [Google Scholar] [CrossRef]
- Crystal Facet Engineering on SrTiO3 Enhances Photocatalytic Overall Water Splitting | Journal of the American Chemical Society. Available online: https://pubs.acs.org/doi/10.1021/jacs.3c12062 (accessed on 27 August 2025).
- Hirayama, D.; Kawawaki, T.; Oguchi, S.; Ogano, M.; Kon, N.; Yasuda, T.; Higami, A.; Negishi, Y. Ultrafine Rhodium–Chromium Mixed-Oxide Cocatalyst with Facet-Selective Loading for Excellent Photocatalytic Water Splitting. J. Am. Chem. Soc. 2024, 146, 26808–26818. [Google Scholar] [CrossRef] [PubMed]
- Takata, T.; Jiang, J.; Sakata, Y.; Nakabayashi, M.; Shibata, N.; Nandal, V.; Seki, K.; Hisatomi, T.; Domen, K. Photocatalytic Water Splitting with a Quantum Efficiency of Almost Unity. Nature 2020, 581, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, X.; Zheng, S.; Lv, S.; Li, H.; Si, Z.; Wu, X.; Ran, R.; Weng, D.; Kang, F. Ni Single Atoms Anchored on Nitrogen-Doped Graphene as H2-Evolution Cocatalyst of SrTiO3(Al)/CoOx for Photocatalytic Overall Water Splitting. Carbon 2021, 183, 763–773. [Google Scholar] [CrossRef]
- Chen, G.; Li, R.; Huang, L. Advances in Photochemical Deposition for Controllable Synthesis of Heterogeneous Catalysts. Nanoscale 2023, 15, 13909–13931. [Google Scholar] [CrossRef]
- Wenderich, K.; Mul, G. Methods, Mechanism, and Applications of Photodeposition in Photocatalysis: A Review. Chem. Rev. 2016, 116, 14587–14619. [Google Scholar] [CrossRef]
- Zhao, Z.; Goncalves, R.V.; Barman, S.K.; Willard, E.J.; Byle, E.; Perry, R.; Wu, Z.; Huda, M.N.; Moulé, A.J.; Osterloh, F.E. Electronic Structure Basis for Enhanced Overall Water Splitting Photocatalysis with Aluminum Doped SrTiO3 in Natural Sunlight. Energy Environ. Sci. 2019, 12, 1385–1395. [Google Scholar] [CrossRef]
- Criteria for Efficient Photocatalytic Water Splitting Revealed by Studying Carrier Dynamics in a Model Al-doped SrTiO3 Photocatalyst-Li-2023-Angewandte Chemie International Edition-Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.202313537 (accessed on 27 August 2025).
- Effects of RhCrOx Cocatalyst Loaded on Different Metal Doped LaFeO3 Perovskites with Photocatalytic Hydrogen Performance under Visible Light Irradiation. Available online: https://www.mdpi.com/2073-4344/11/5/612 (accessed on 27 August 2025).
- Efficient Photocatalytic Water Splitting Using Al-Doped SrTiO3 Coloaded with Molybdenum Oxide and Rhodium–Chromium Oxide|ACS Catalysis. Available online: https://pubs.acs.org/doi/10.1021/acscatal.7b04264 (accessed on 27 August 2025).
- An Improved Technique for Determining Hardness and Elastic Modulus Using Load and Displacement Sensing Indentation Experiments | Journal of Materials Research. Available online: https://link.springer.com/article/10.1557/JMR.1992.1564 (accessed on 27 August 2025).
- Nanomechanical Properties of TiO2 Granular Thin Films|ACS Applied Materials & Interfaces. Available online: https://pubs.acs.org/doi/10.1021/am100455q (accessed on 27 August 2025).
- Effects of SiO2 and ZnO Nanoparticles on Epoxy Coatings and Its Performance Investigation Using Thermal and Nanoindentation Technique. Available online: https://www.mdpi.com/2073-4360/13/9/1490 (accessed on 27 August 2025).
- Zambrano-Mera, D.F.; Espinoza-González, R.; Villarroel, R.; Rosenkranz, A.; Carvajal, N.; Pintor-Monroy, M.I.; Montaño-Figueroa, A.G.; Arellano-Jiménez, M.J.; Quevedo-López, M.; Valenzuela, P.; et al. Optical and Mechanical Properties of Zr-Oxide Doped TiO2/SiO2 Anti-Reflective Coatings for PV Glass Covers. Sol. Energy Mater. Sol. Cells 2022, 243, 111784. [Google Scholar] [CrossRef]
- Jiang, J.; Dong, X.; Wang, H.; Wang, F.; Li, Y.; Lu, Z. Enhanced Mechanical and Photocatalytic Performance of Cement Mortar Reinforced by Nano-TiO2 Hydrosol-Coated Sand. Cem. Concr. Compos. 2023, 137, 104906. [Google Scholar] [CrossRef]
- Goto, Y.; Hisatomi, T.; Wang, Q.; Higashi, T.; Ishikiriyama, K.; Maeda, T.; Sakata, Y.; Okunaka, S.; Tokudome, H.; Katayama, M.; et al. A Particulate Photocatalyst Water-Splitting Panel for Large-Scale Solar Hydrogen Generation. Joule 2018, 2, 509–520. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).