Unlocking the Biochemical Potential of Diadema setosum Tests: A Pathway Toward Circular Marine Bioeconomy
Abstract
1. Introduction
2. Results and Discussion
2.1. Proximate Composition
2.2. Amino Acid Composition
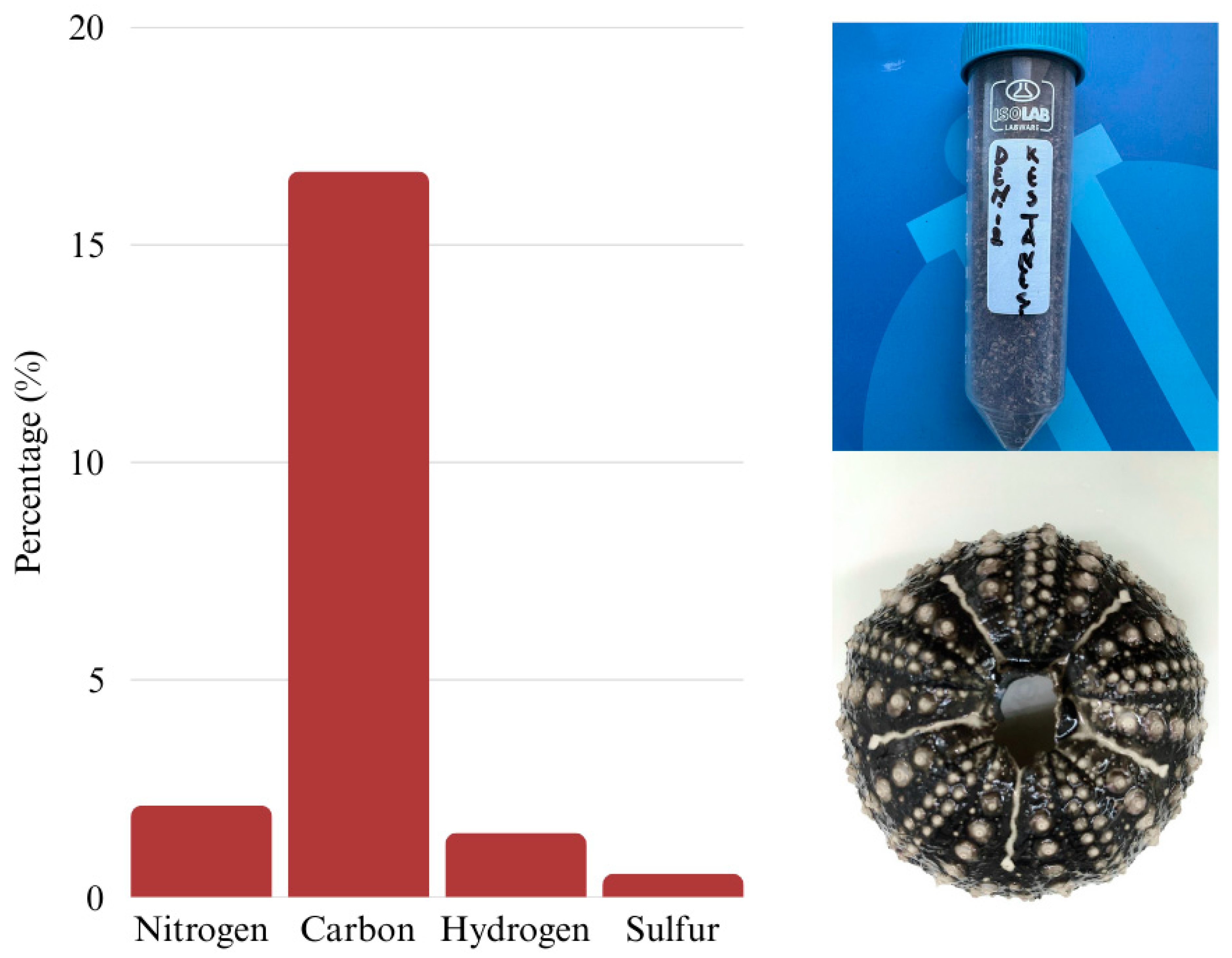
2.3. Elemental Composition
3. Materials and Methods
3.1. Materials
3.2. Proximate Analysis
3.3. Amino Acid Analysis
3.4. Elemental Characterization
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muthiga, N.A.; McClanahan, T.R. Chapter 23—Diadema. In Developments in Aquaculture and Fisheries Science; Lawrence, J.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 43, pp. 397–418. [Google Scholar]
- Lessios, H.A.; Kessing, B.D.; Pearse, J.S. Population Structure and Specialition In Tropic Seas: Global Phylogeography In Sea Urchin Diadema. Evolution 2001, 55, 955–975. [Google Scholar] [CrossRef]
- James, D.B.; Pearse, J.S. Echinoderms from the Gulf of Suez and Northern Red Sea. J. Mar. Biol. Assoc. India 1971, 1, 78–125. [Google Scholar]
- Yokes, M.B.; Galil, B. The first record of the needle-spined urchin Diadema setosum (Leske, 1778) (Echinodermata: Echinoidea: Diadematidae) from the Mediterranean Sea. Aquat. Invasions Issue 2006, 1, 188–190. [Google Scholar] [CrossRef]
- Turan, C.; Erguden, D.; Uygur, N. On the occurrence of Diadema setosum (Leske, 1778) in Antakya Bay, Eastern Mediterranean Sea. J. Black Sea / Mediterr. Environ. 2011, 17, 4–8. [Google Scholar]
- Vafidis, D.; Antoniadou, C.; Voulgaris, K.; Varkoulis, A.; Apostologamvrou, C. Abundance and population characteristics of the invasive sea urchin Diadema setosum (Leske, 1778) in the south Aegean Sea (eastern Mediterranean). J. Biol. Res. 2021, 28, 11. [Google Scholar] [CrossRef] [PubMed]
- Katsanevakis, S.; Acar, Ü.; Ammar, I.; Balci, B.A.; Bekas, P. New Mediterranean Biodiversity Records. Mediterr Mar. Sci. 2014, 15, 675–695. [Google Scholar] [CrossRef]
- Artüz, M.L.; Artüz, O.B. First and Northernmost Record of Diadema setosum (Leske, 1778) (Echinodermata: Echinoidea: Diadematidae) in the Sea of Marmara. Thalass. : Int. J. Mar. Sci. 2019, 35. [Google Scholar] [CrossRef]
- Ebert, T.A. Estimating mortality from growth parameters and a size distribution when recruitment is periodic. Limnol. Ocean-ogr. 1981, 26, 764–769. [Google Scholar] [CrossRef]
- TUİK. Export data of Diadema Setosum. Available online: https://www.tuik.gov.tr/ (accessed on 8 December 2024).
- Salma, W.; Wahyuni, S.; Yusuf, I.; Haya, L.O.M.Y.; Irawan, Y.; As’ad Armyn, S. Immune Nutrient Content of Sea Urchin (Diadema setosum) Gonads. Int. J. Nutr. Food Sci. 2016, 5, 330–336. [Google Scholar] [CrossRef][Green Version]
- Ahmed, H.O.; Mahdy, A.; Nasser, S.A.M.; El-Wakeil, K.F.A.; Obuid-Allah, A.H.; Hassan, M.M. Biochemical composition of some Echinodermata (Holothuroidea, Echinoidea) from the Red Sea, Egypt. Braz. J. Biol. 2022, 82, e246309. [Google Scholar] [CrossRef] [PubMed]
- Amarowicz, R.; Synowiecki, J.; Shahidi, F. Chemical composition of shells from red (Strongylocentrotus franciscanus) and green (Strongylocentrotus droebachiensis) sea urchin. Food Chem. 2012, 133, 822–826. [Google Scholar] [CrossRef]
- Garau, G.; Castaldi, P.; Deiana, S.; Campus, P.; Mazza, A.; Deiana, P.; Pais, A. Assessment of the use potential of edible sea urchins (Paracentrotus lividus) processing waste within the agricultural system: Influence on soil chemical and biological properties and bean (Phaseolus vulgaris) and wheat (Triticum vulgare) growth in an amended acidic soil. J. Environ. Manag. 2012, 109, 12–18. [Google Scholar] [CrossRef]
- Drozdov, A.; Sharmankina, V.; Zemnukhova, L.; Polyakova, N. Chemical composition of spines and tests of sea urchins. Biol. Bull. 2016, 43, 521–531. [Google Scholar] [CrossRef]
- Swift, D.M.; Sikes, C.S.; Wheeler, A.P. Analysis and function of organic matrix from sea urchin tests. J. Exp. Zool. 1986, 240, 65–73. [Google Scholar] [CrossRef]
- Kanold, J.M.; Guichard, N.; Immel, F.; Plasseraud, L.; Corneillat, M.; Alcaraz, G.; Brümmer, F.; Marin, F. Spine and test skeletal matrices of the Mediterranean sea urchin Arbacia lixula—A comparative characterization of their sugar signature. FEBS J. 2015, 282, 1891–1905. [Google Scholar] [CrossRef] [PubMed]
- Matveeva, V.; Shulgina, L.; Prikhodko, Y.; Shulgin, Y.; Madej, K.; Piekoszewski, W. Nutritional Value of Sea Urchin Roe (Strongylocentrotidae)—Study of Composition and Storage Conditions. Separations 2021, 8, 174. [Google Scholar] [CrossRef]
- Clark, M.S. Molecular mechanisms of biomineralization in marine invertebrates. J. Exp. Biol. 2020, 223, jeb206961. [Google Scholar] [CrossRef]
- Guo, C.L.; Fu, D.X.; He, H.; Jin, Q.Z. Metal elements in gonad and shell of sea urchin anthocidaris crassispina. Trop. Ocean. 2000, 19, 82–85. [Google Scholar]
- Weber, J.N. The incorporation of magnesium into the skeletal calcites of echinoderms. Am. J. Sci. 1969, 267, 537–566. [Google Scholar] [CrossRef]
- Shapkin, N.P.; Khalchenko, I.G.; Panasenko, A.E.; Drozdov, A.L. Sea urchin skeleton: Structure, composition, and application as a template for biomimetic materials. AIP Conf. Proc. 2017, 1851, 02006. [Google Scholar] [CrossRef]
- Lavigne, M.; Hill, T.; Sanford, E.; Gaylord, B.; Russell, A.; Lenz, E.; Hosfelt, J.; Young, M. The elemental composition of purple sea urchin (Strongylocentrotus purpuratus) calcite and potential effects of pCO2 during early life stages. Biogeosciences 2013, 10, 3465–3477. [Google Scholar] [CrossRef]
- Iglikowska, A.; Borszcz, T.; Drewnik, A.; Grabowska, M.; Humphreys-Williams, E.; Kędra, M.; Krzemińska, M.; Piwoni-Piórewicz, A.; Kukliński, P. Mg and Sr in Arctic echinoderm calcite: Nature or nurture? J. Mar. Syst. 2018, 180, 279–288. [Google Scholar] [CrossRef]
- Ugurlu, E. A biomonitoring study of Diadema setosum: Metal bioaccumulation and current status in Iskenderun Bay, eastern Mediterranean. Oceanol. Hydrobiol. Stud. 2023, 52, 484–492. [Google Scholar] [CrossRef]
- Yesudas, A.; Vidyalakshmi, D.; Sivan, G.; Shameem, K.; Akhil Prakash, E.; Priyaja, P. Comparative analysis of temporal variation of heavy metal accumulation by two sea urchin species from a harbour region, including pre and post COVID 19 lock down period. Sci. Total Environ. 2023, 877, 162879. [Google Scholar] [CrossRef]
- Al-Najjar, T.; Al Tawaha, M.; Wahsha, M.; Hilal, A.A. Heavy metals in the sea urchin Diadema setosum from the gulf of Aqaba. Fresenius Environ. Bull. 2018, 27, 4149–4155. [Google Scholar]
- Temara, A.; Aboutboul, P.; Warnau, M.; Jangoux, M.; Dubois, P. Kinetics of lead uptake by the skeleton of the asteroid Asterias rubens (Echinodermata). In Echinoderm Research 1995, Proceedings of the 4th Echinoderm Colloquium, London, United Kingdom, 10-13 April 1995; Balkema: Rotterdam, The Netherlands, 1995; pp. 79–82. [Google Scholar]
- Beeby, A. Toxic Metal Uptake and Essential Metal Regulation in Terrestrial Invertebrates: A Review. In Metal Ecotoxicology Concepts and Applications; Lewis Publishers: Chelsea, MI, USA, 1991; pp. 65–89. [Google Scholar]
- Temara, A.; Skei, J.M.; Gillan, D.; Warnau, M.; Jangoux, M.; Dubois, P. Validation of the Asteroid Asterias rubens (Echinodermata) as a Bioindicator of Spatial and Temporal Trends of Pb, Cd, and Zn Contamination in the Field. Mar. Environ. Res. 1998, 45, 341–356. [Google Scholar] [CrossRef]
- Carlin, D.J.; Naujokas, M.F.; Bradham, K.D.; Cowden, J.; Heacock, M.; Henry, H.F.; Lee, J.S.; Thomas, D.J.; Thompson, C.; Tokar, E.J.; et al. Arsenic and Environmental Health: State of the Science and Future Research Opportunities. Environ. Health Perspect. 2016, 124, 890–899. [Google Scholar] [CrossRef]
- World Health Organization (WHO); Food and Agriculture Organization of the United Nations (FAO). Safety Evaluation of Certain Contaminants in Food. In WHO Food Additives Series, No. 74; FAO JECFA Monographs 19 Bis; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- European Commission (EC). Commission Regulation (EU) 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006; Official Journal of the European Union: Brussels, Belgium, 2023. [Google Scholar]
- Turkish Food Codex (TFC). Turkish Food Codex Contaminants Regulation (Communiqué No: 2023/49); Ministry of Agriculture and Forestry: Ankara, Turkey, 2023. [Google Scholar]
- EFSA. Update of the risk assessment of inorganic arsenic in food. EFSA J. 2024, 22, e8488. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Analytical Chemists; AOAC International: Arlington, VA, USA, 1990; pp. 20–34. [Google Scholar]
- AOAC. Protein (crude) determination in animal feed: Copper catalyst kjeldahl method 984.13. In Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 1990. [Google Scholar]
- AOAC. Official method 920.153. Ash content. In Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2002. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Lee, Y.; Hwang, K.T. Changes in physicochemical properties of mulberry fruits (Morus alba L.) during ripening. Sci. Hortic. 2017, 217, 189–196. [Google Scholar] [CrossRef]
- Chan, P.T.; Matanjun, P. Chemical composition and physicochemical properties of tropical red seaweed, Gracilaria changii. Food Chem. 2017, 221, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Heiri, O.; Lotter, A.F.; Lemcke, G. Loss on ignition as a method for estimating organic and carbonate content in sediments: Reproducibility and comparability of results. J. Paleolimnol. 2001, 25, 101–110. [Google Scholar] [CrossRef]

| Component | Content (%) |
|---|---|
| Moisture (%) | 2.56 |
| Protein (%) | 8.03 |
| Lipid (%) | 0.72 |
| Ash (%) | 84.85 |
| Nitrogen (%) | 1.32 |
| Carbohydrate (%) | 3.84 |
| Amino Acid | Total Amino Acids (mg/g) | Free Amino Acids (mg/100 g) |
|---|---|---|
| Aspartic acid | 93.71 ± 0.04 | 0.71 ± 0.06 |
| Glutamic acid | 92.45 ± 0.10 | 5.12 ± 0.05 |
| Histidine | 15.10 ± 0.08 | 0.60 ± 0.04 |
| Arginine | 82.92 ± 0.06 | 4.65 ± 0.07 |
| Glycine | 118.30 ± 0.02 | 16.43 ± 0.02 |
| Alanine | 55.74 ± 0.02 | 5.31 ± 0.04 |
| Proline | 27.40 ± 0.02 | 0.70 ± 0.04 |
| Methionine | 10.62 ± 0.09 | 0.93 ± 0.05 |
| Tryptophan | 1.11 ± 0.06 | 0.90 ± 0.08 |
| Phenylalanine | 31.87 ± 0.07 | 1.81 ± 0.03 |
| Lysine | 78.30 ± 0.01 | 4.62 ± 0.06 |
| Leucine | 58.23 ± 0.10 | 4.45 ± 0.06 |
| Isoleucine | 32.50 ± 0.08 | 2.92 ± 0.01 |
| Serine | 32.68 ± 0.03 | 1.91 ± 0.06 |
| Valine | 45.11 ± 0.03 | 5.20 ± 0.03 |
| Threonine | 38.20 ± 0.03 | 3.27 ± 0.02 |
| Tyrosine | 27.82 ± 0.04 | 2.82 ± 0.10 |
| Component | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | TiO2 | MnO | Cl | P2O5 | Ig Loss |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Content (%) | 0.30 | 0.06 | 0.11 | 43.19 | 2.17 | 0.31 | 1.57 | 0.02 | 0.02 | 1.43 | 0.24 | 51.07 |
| Element | Sc | Ni | Cu | Zn | Ga | As | Rb | Sr | Zr | Mo |
| Content (mg/kg) | 22.60 | 30.00 | 9.70 | 38.30 | 4.60 | 5.60 | 19.80 | 2729.00 | 124.40 | 2.70 |
| Element | Cd | Ba | Nd | Sm | Yb | Hf | Pb | Th | U | Sn |
| Content (mg/kg) | 0.20 | 90.80 | 135.50 | 5.80 | 0.20 | 4.90 | 63.50 | 29.80 | 9.80 | N.D. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilgin Fıçıcılar, B.; Korkmaz, K. Unlocking the Biochemical Potential of Diadema setosum Tests: A Pathway Toward Circular Marine Bioeconomy. Molecules 2025, 30, 3700. https://doi.org/10.3390/molecules30183700
Bilgin Fıçıcılar B, Korkmaz K. Unlocking the Biochemical Potential of Diadema setosum Tests: A Pathway Toward Circular Marine Bioeconomy. Molecules. 2025; 30(18):3700. https://doi.org/10.3390/molecules30183700
Chicago/Turabian StyleBilgin Fıçıcılar, Bilge, and Koray Korkmaz. 2025. "Unlocking the Biochemical Potential of Diadema setosum Tests: A Pathway Toward Circular Marine Bioeconomy" Molecules 30, no. 18: 3700. https://doi.org/10.3390/molecules30183700
APA StyleBilgin Fıçıcılar, B., & Korkmaz, K. (2025). Unlocking the Biochemical Potential of Diadema setosum Tests: A Pathway Toward Circular Marine Bioeconomy. Molecules, 30(18), 3700. https://doi.org/10.3390/molecules30183700








