Construction of Hollow TiO2/ZnS Heterojunction Photocatalysts for Highly Enhanced Photodegradation of Tetracycline Hydrochloride
Abstract
1. Introduction
2. Results and Discussion
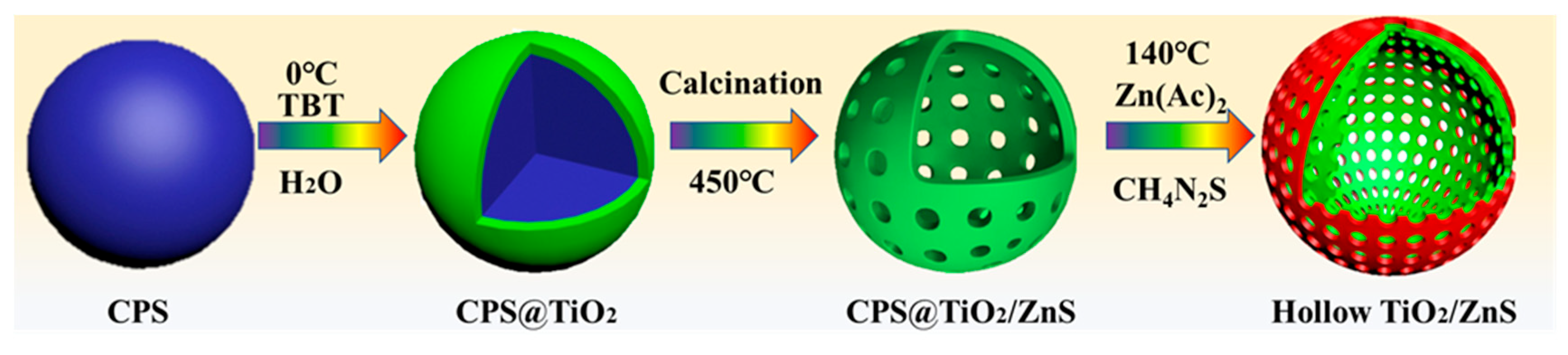
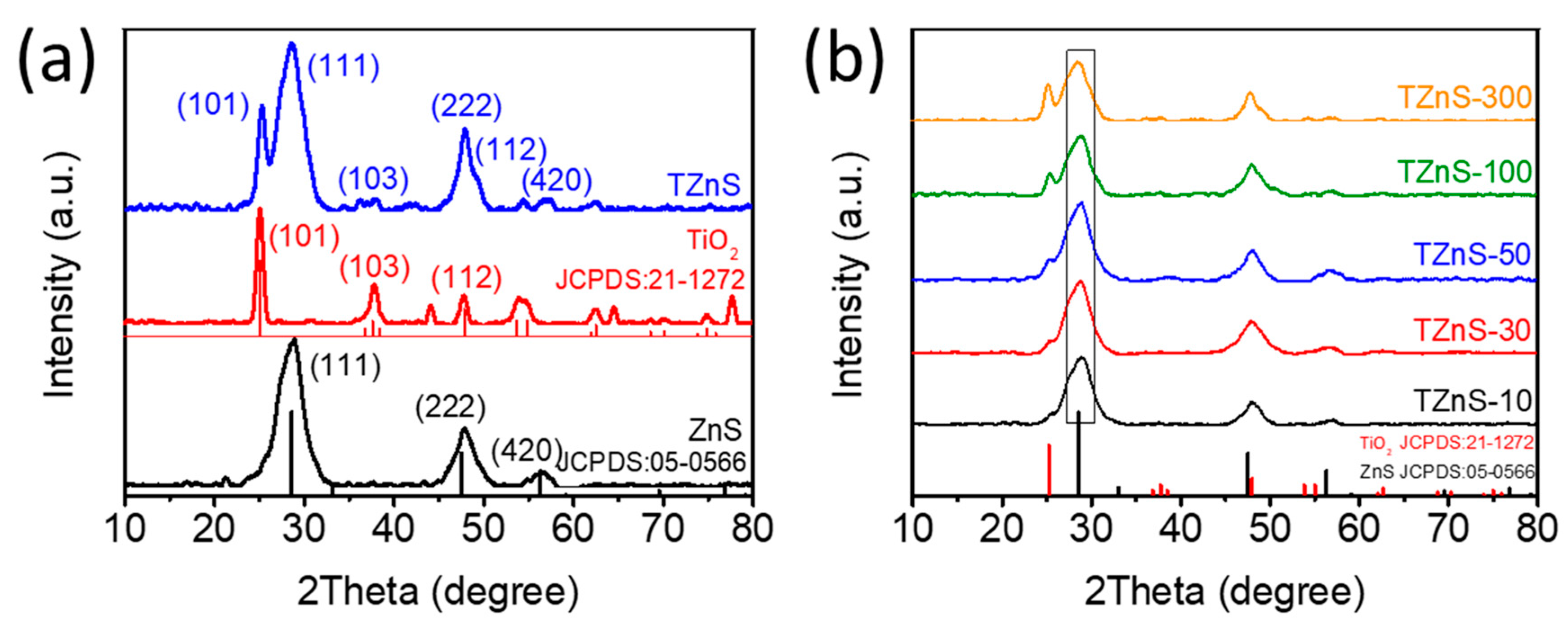
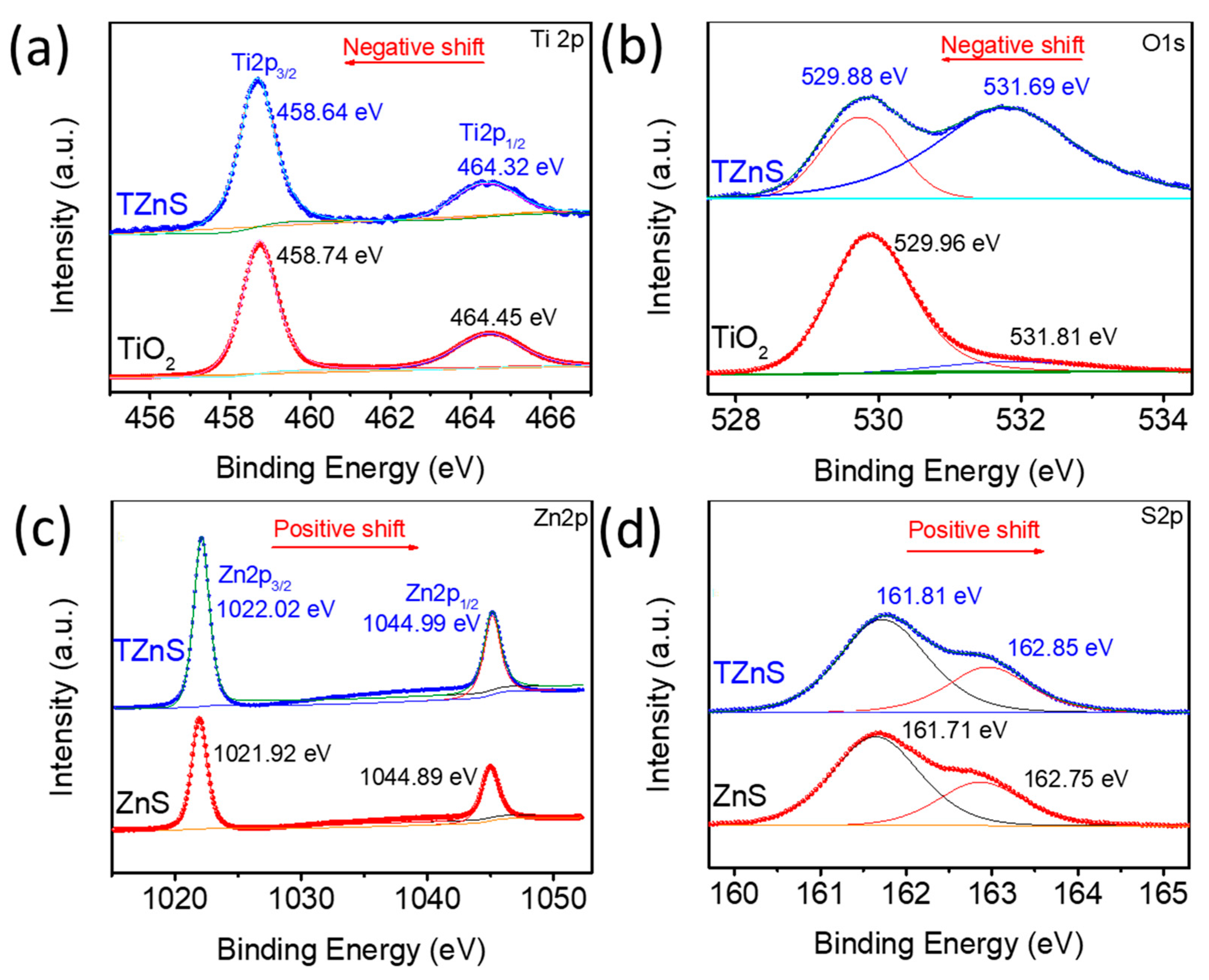
2.1. Structure Characterization
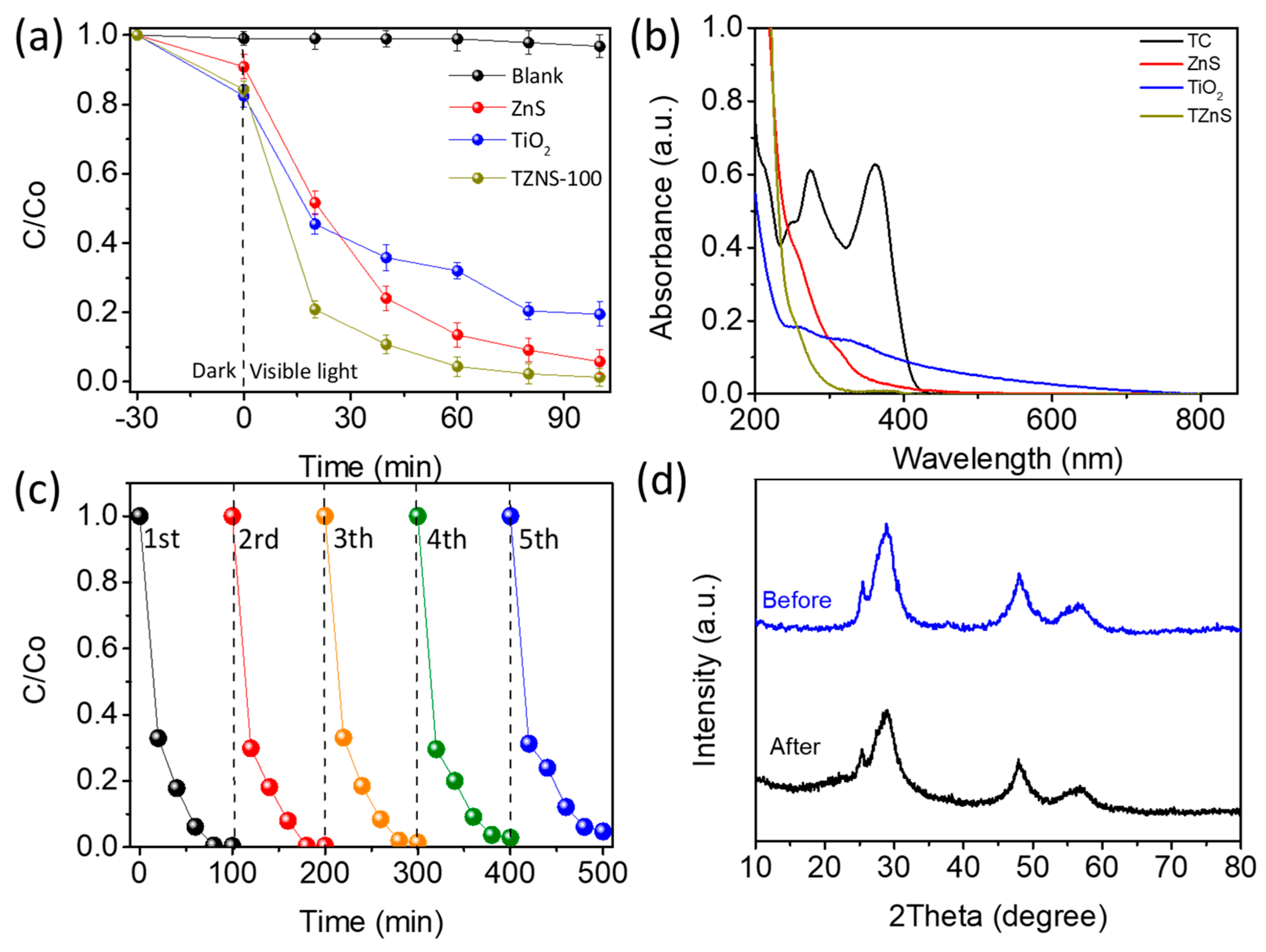
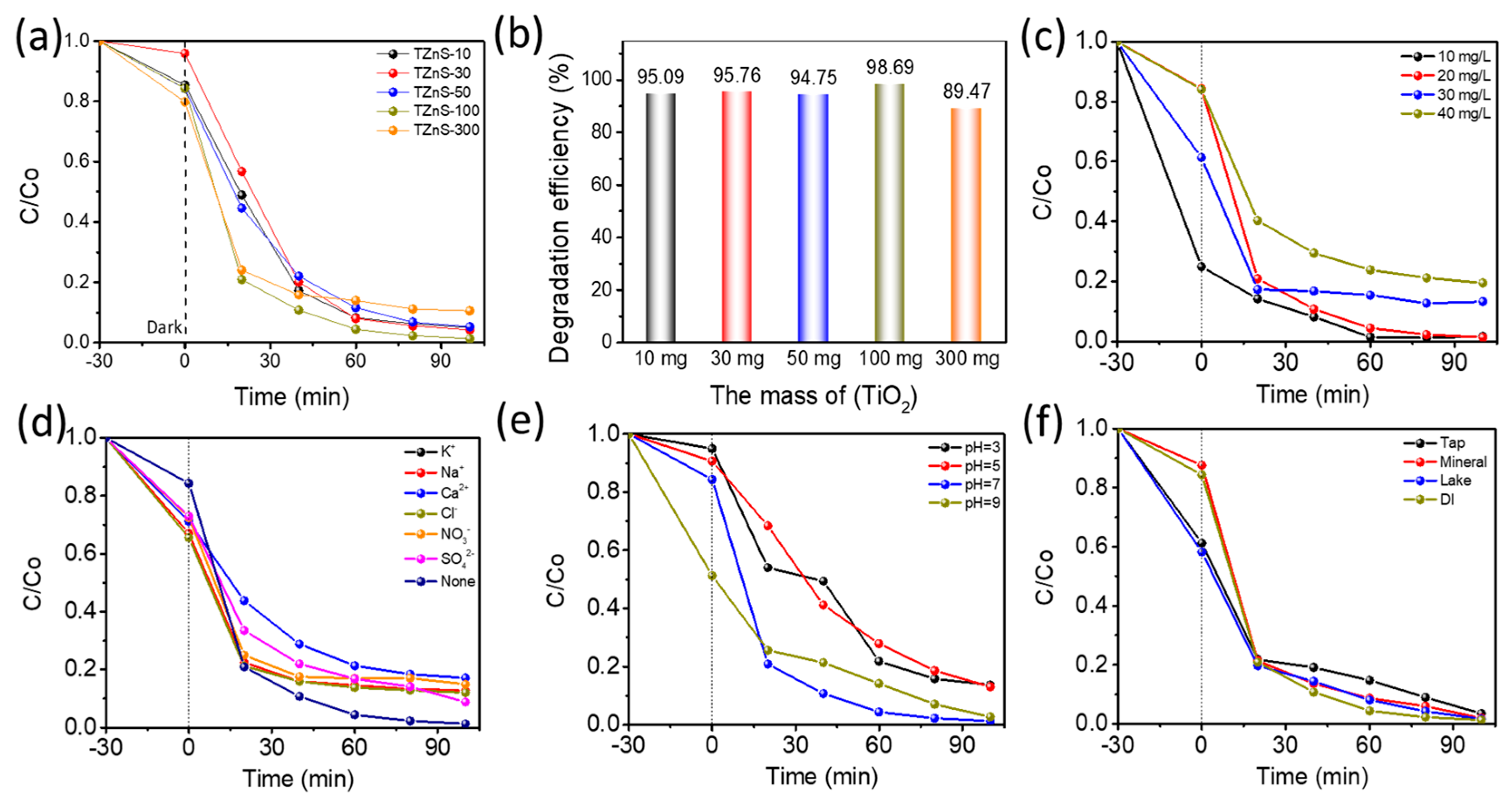
2.2. Photocatalyst Activity
2.2.1. Visible Light Degradation of TC
2.2.2. Effect of Different Reaction Conditions
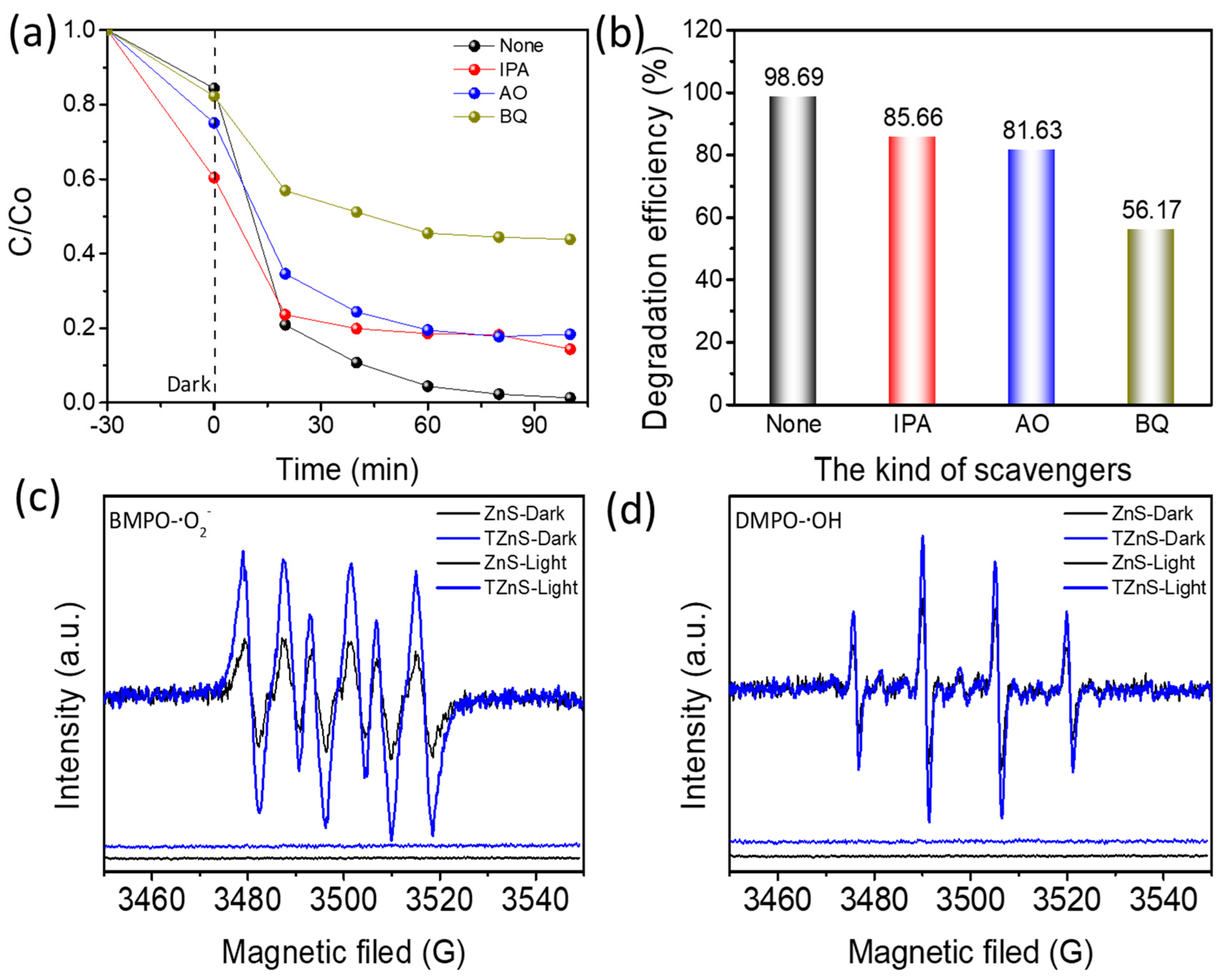
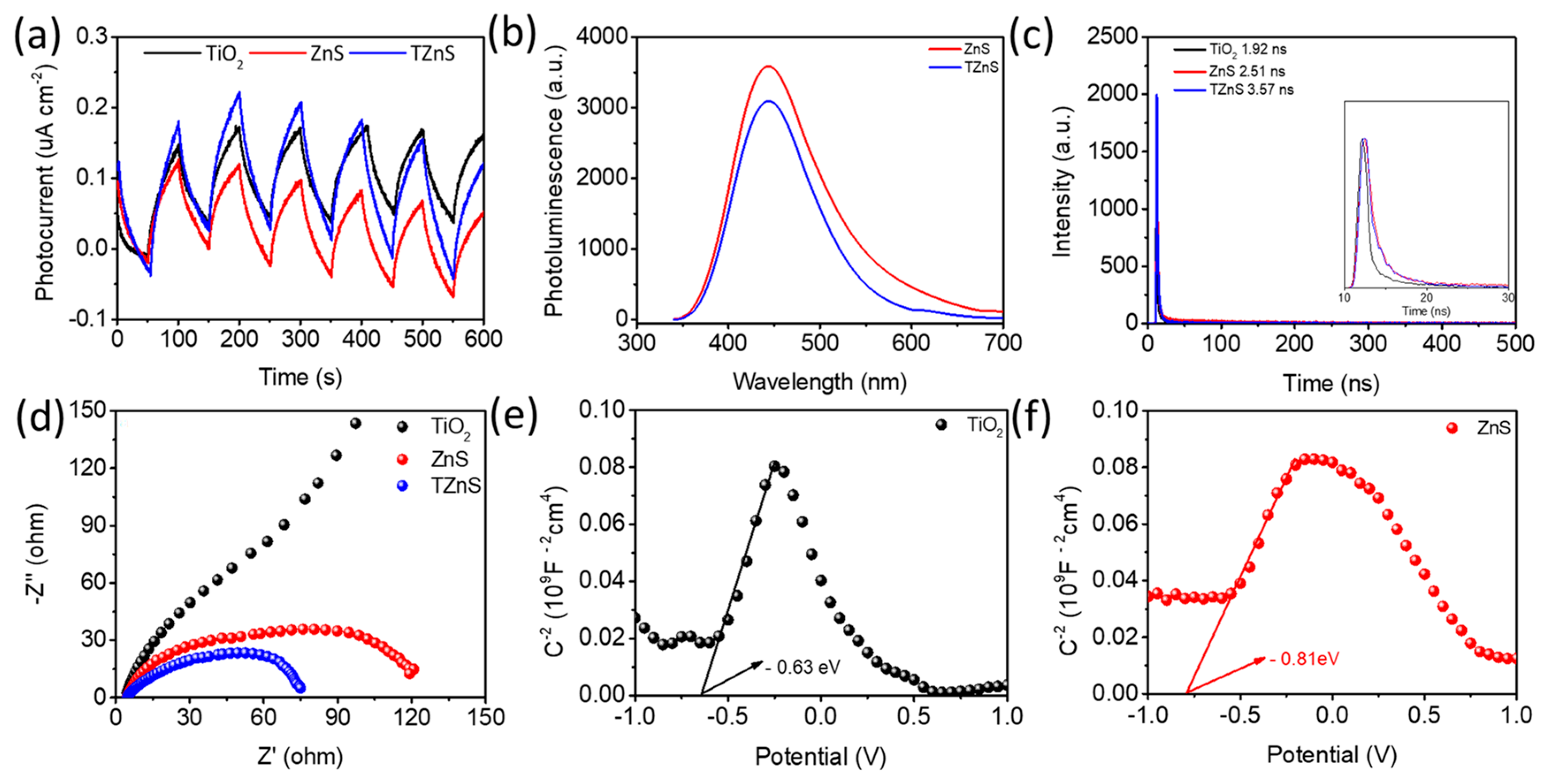
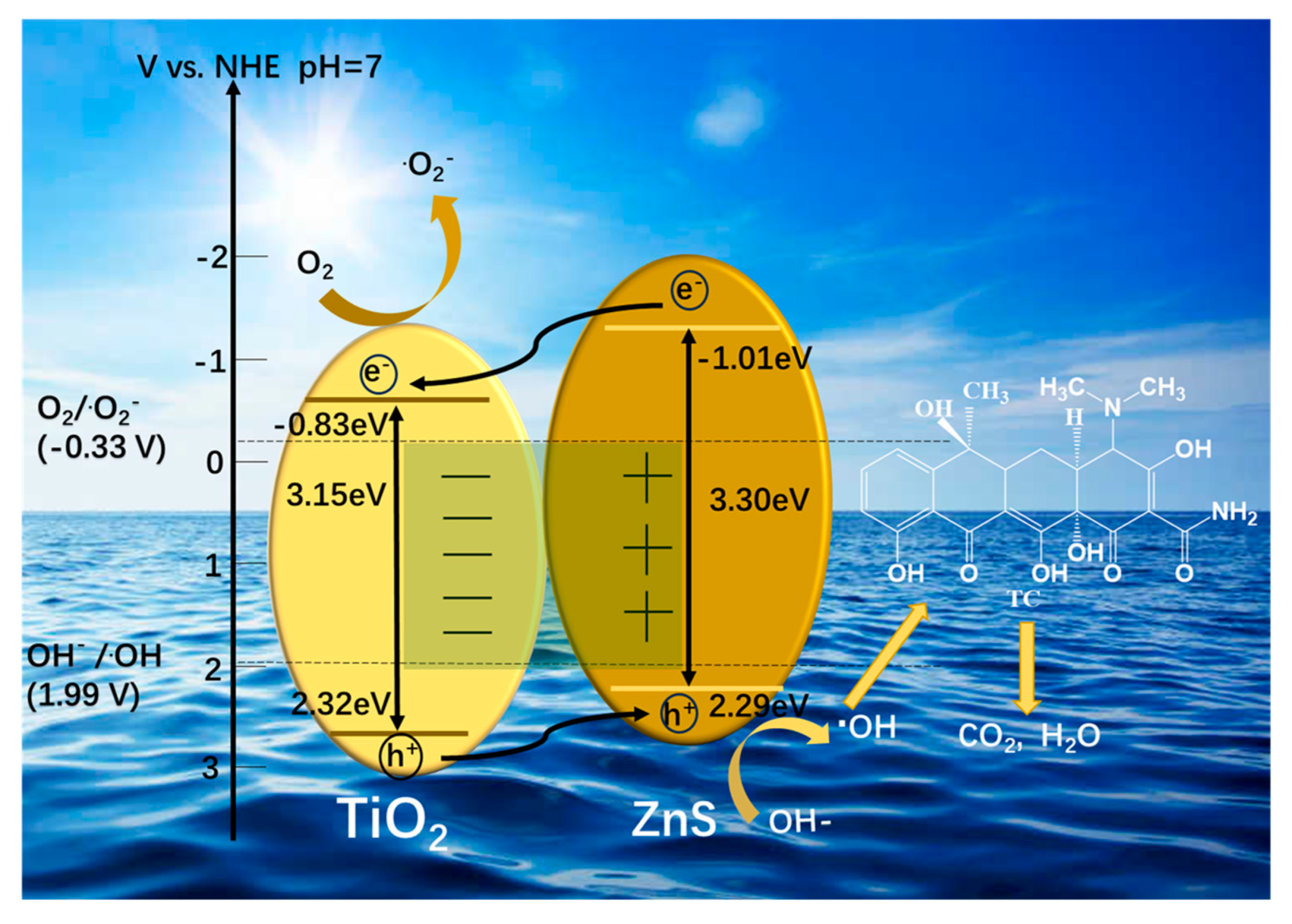
2.3. Possible Photocatalytic Mechanism
2.3.1. Active Species Determination
2.3.2. Charge Transfer Kinetics
2.3.3. Photocatalytic Mechanism
3. Experimental Section
3.1. Chemicals
3.2. Synthesis of TiO2/ZnS
3.3. Photocatalyst Characterization
3.4. Photocatalytic Activity Test
3.5. Electrochemical Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ding, D.; Wang, B.; Zhang, X.; Zhang, J.; Zhang, H.; Liu, X.; Gao, Z.; Yu, Z. The spread of antibiotic resistance to humans and potential protection strategies. Ecotoxicol. Environ. Saf. 2023, 254, 114734. [Google Scholar] [CrossRef]
- Wu, X.D.; Zhang, X.Y.; Liao, H.Z.; Guo, J.; Ma, Z.H.; Fu, Z.L. Microplastics and tetracycline affecting apoptosis, enzyme activities and metabolism processes in the Aurelia aurita polyps: Insights into combined pollutant effects. Front. Mar. Sci. 2025, 12, 1545131. [Google Scholar] [CrossRef]
- Zhou, P.F.; Han, X.Y.; Wu, L.X.; Qi, M.Y.; Shen, Y.B.; Nie, J.N.; Liu, X.N.; Wang, F.; Bian, L.; Liang, J.S. Facile construction of novel BiOBr/black-TiO2/tourmaline composites for the synergistic degradation of tetracycline in aqueous. Sep. Purif. Technol. 2025, 362, 131976. [Google Scholar] [CrossRef]
- Li, M.; Liu, Y.; Yang, S.Y.; Zhang, Y.; Wei, L.; Zhu, B.C. Efficient charge carrier transfer in TiO2/CulnS2 S-scheme heterojunction to boost photocatalytic degradation of tetracycline hydrochloride. J. Mater. Sci. Technol. 2025, 224, 245–256. [Google Scholar] [CrossRef]
- Balayeva, N.O.; Mamiyev, Z.; Dillert, R.; Zheng, N.; Bahnemann, D.W. Rh/TiO2-photocatalyzed acceptorless dehydrogenation of N-heterocycles upon visible-light illumination. ACS Catal. 2020, 10, 5542–5553. [Google Scholar] [CrossRef]
- Xu, H.Y.; Zhang, L.; Huang, K.Q.; Sun, D.C.; Tong, X.; Li, X.T.; Yang, Y.; Ji, X. Novel integrating sphere structure solar photocatalytic reactor design: Efficient conversion of nitrate wastewater into high-value ammonia. Renew. Energy 2025, 243, 122611. [Google Scholar] [CrossRef]
- Wang, W.B.; Liu, J.; Cui, M.X.; Li, X.H.; Niu, L.Y.; Wu, X.; Chen, Y.N.; Li, X.W.; Zhu, H.C.; Wang, D.; et al. Z-type ZnIn2S4 homojunction for high performance photocatalytic hydrogen evolution. Chem. Eng. J. 2025, 507, 160370. [Google Scholar] [CrossRef]
- Haghighi, P.; Haghighat, F. TiO2-based photocatalytic oxidation process for indoor air VOCs removal: A comprehensive review. Build. Environ. 2024, 249, 111108. [Google Scholar] [CrossRef]
- Ferreira, O.; Gil, M.; Carapeto, A.; Barreto, A.; Pereira, M.; Rodrigues, M.S.; Habib, L.; Gomes, L.C.; Teixeira-Santos, R.; Mergulhao, F.J.M.; et al. An engineered, biocide-grafted TiO2-based nanohybrid material with enhanced photocatalytic and antimicrobial activity. J. Clean. Prod. 2025, 494, 144937. [Google Scholar] [CrossRef]
- Zhao, Y.; Linghu, X.; Shu, Y.; Zhang, J.; Chen, Z.; Wu, Y.; Shan, D.; Wang, B. Classification and catalytic mechanisms of heterojunction photocatalysts and the application of titanium dioxide TiO2-based heterojunctions in environmental remediation. J. Environ. Chem. Eng. 2022, 10, 108077. [Google Scholar] [CrossRef]
- Mamiyev, Z.; Balayeva, N.O. Metal sulfide photocatalysts for hydrogen generation: A review of recent advances. Catalysts 2022, 12, 1316. [Google Scholar] [CrossRef]
- Lu, R.; Zhao, S.Z.; Yang, Y.; Wang, Y.; Huang, H.L.; Hu, Y.D.; Rodriguez, R.D.; Chen, J.J. Efficient adsorption and photocatalytic degradation of organic contaminants using TiO2/(Bi2O3/Bi2O2.33) nanotubes. Mater. Today Chem. 2023, 32, 101641. [Google Scholar] [CrossRef]
- Zhang, Y.; Bo, X.; Zhu, T.; Zhao, W.; Cui, Y.; Chang, J. Synthesis of TiO2-ZnO n-n heterojunction with excellent visible light-driven photodegradation of tetracycline. Nanomaterials 2024, 14, 1802. [Google Scholar] [CrossRef]
- Wang, Q.P.; Wang, G.H.; Wang, J.; Li, J.M.; Wang, K.; Zhou, S.; Su, Y.R. In situ hydrothermal synthesis of ZnS/TiO2 nanofibers S-scheme heterojunction for enhanced photocatalytic H2 evolution. Adv. Sustain. Syst. 2023, 7, 2200027. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.; Xing, K.; Xiao, Y.; Zhao, M.; Cun, Y. Construction of Z-scheme heterojunction LaCoO3 modified ZnO for photocatalytic degradation of tetracycline under visible light irradiation. Catal. Commun. 2024, 186, 106815. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, T.W.; Li, H.Q.; Sun, C.Z.; Hu, J.; Cao, S.S. Generalized synthetic strategies toward oxygen vacancy-enriched ZnO-ZnS hollow porous spheres with enhanced photocatalytic hydrogen evolution. Inorg. Chem. 2025, 64, 3563–3571. [Google Scholar] [CrossRef]
- Ma, F.; Xu, X.Y.; Huo, C.; Sun, C.Z.; Li, Q.; Yin, Z.L.; Cao, S.S. Dual heterogeneous structures promote electrochemical properties and photocatalytic hydrogen evolution for inverse opal ZnO/ZnS/Co3O4 Crystals. Inorg. Chem. 2024, 63, 8782–8790. [Google Scholar] [CrossRef]
- Wu, D.; He, Y.X.; Lin, C.; Li, B.; Ma, J.P.; Ruan, L.J.; Feng, Y.J.; Ban, C.G.; Ding, J.J.; Wang, X.X.; et al. Atmosphere-driven metal-support synergy in ZnO/Au catalysts for efficient piezo-catalytic hydrogen evolution. J. Mater. 2025, 11, 100959. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, H.Y.; Yang, P.; Zhang, X. SnO2 nanoparticles endowed Pt-TiO2 bowl nanoarraysenhanced redox ability towards efficient tetracycline hydrochlorideremoval and H2 generation. Sustain. Mater. Technol. 2025, 44, e01358. [Google Scholar] [CrossRef]
- Jiang, Y.C.; Zhu, K.R.; Zhang, S.Y.; Wang, D.Z. Hierarchically porous ZnS/C photocatalyst toward tetracycline hydrochloride degradation under visible light: Efficiency and mechanism. J. Water Process Eng. 2025, 70, 107074. [Google Scholar] [CrossRef]
- Chen, Q.Q.; Yang, X.B.; Fu, X.P.; Pan, J.J.; Zhou, J.M.; Zhou, G.W.; Cheng, K.J. Fabrication of a novel Ag2S-ZnS/ZIF-8 hollow heterojunction by in-situ formation of Ag2S and ZnS on ZIF-8 with enhanced tetracycline degradation performance. Chem. Eng. J. 2024, 499, 156242. [Google Scholar] [CrossRef]
- Chen, L.; Chen, X.; Xiao, J. Flower-like Ti3C2T-TiO2 modified with ZnS nanoparticles as adsorption-catalytic cathodic material for lithium-sulfur batteries. J. Energy Storage 2025, 118, 116241. [Google Scholar] [CrossRef]
- Li, M.; Liu, L.; Li, Y.; Li, Y. Tuning piezoelectric driven photocatalysis by TiO2/ZnS heterojunction for mineral processing wastewater degradation. J. Water Process Eng. 2024, 64, 105727. [Google Scholar] [CrossRef]
- Liu, D.D.; Liang, H.; Xu, T.; Bai, J.; Li, C.P. Construction of ternary hollow TiO2-ZnS@ZnO heterostructure with enhanced visible-light photoactivity. J. Mol. Struct. 2022, 1248, 131493. [Google Scholar] [CrossRef]
- Li, C.Y.; Wu, J.H.; Wang, X.Z.; Cai, Y.X.; Jia, R.R.; Wang, W.W.; Xia, S.S.; Li, L.; Chen, Z.; Jin, C.C. One-step preparation of Z-scheme g-C3N4 nanorods/TiO2 microsphere with improved photodegradation of antibiotic residue. Ceram. Int. 2024, 50, 42127–42134. [Google Scholar] [CrossRef]
- Malik, M.; Nazir, M.A.; Khan, H.M.; Shah, S.S.A.; Anum, A.; Rehman, A.U.; Hashem, A.; Avila-Quezada, G.D.; Abd Allah, E.F. Light harvesting S-scheme g-C3N4/TiO2/M photocatalysts for efficient removal of hazardous moxifloxacin. Diam. Relat. Mater. 2024, 150, 111673. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, P.; Fei, X.; Wu, X.; Huang, Z.; Zhong, W.; Gong, Q.; Zheng, Y.; Zhang, Q.; Xie, S.; et al. Unusual facet and co-catalyst effects in TiO2-based photocatalytic coupling of methane. Nat. Commun. 2024, 15, 4453. [Google Scholar] [CrossRef]
- Cui, Y.; Ru, G.; Zhang, T.; Yang, K.; Liu, S.; Qi, W.; Ye, Q.; Liu, X.; Zhou, F. Schottky interface engineering in Ti3C2Tx/ZnS organic hydrogels for high-performance multifunctional flexible absorbers. Adv. Funct. Mater. 2024, 35, 2417346. [Google Scholar] [CrossRef]
- Wang, H.; Qi, H.F.; Sun, X.; Jia, S.Y.; Li, X.Y.; Miao, T.J.; Xiong, L.Q.; Wang, S.H.; Zhang, X.L.; Liu, X.Y.; et al. High quantum efficiency of hydrogen production from methanol aqueous solution with PtCu-TiO2 photocatalysts. Nat. Mater. 2023, 22, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Liu, H.; Xie, Z.; Xie, L.; Liu, G.; Xu, Y.; Chen, J.; Lu, C.Z. Excellent charge separation of NCQDs/ZnS nanocomposites for the promotion of photocatalytic H2 evolution. ACS Appl Mater Interfaces 2024, 16, 16601–16611. [Google Scholar] [CrossRef]
- Wen, D.; Feng, J.; Deng, R.; Li, K.; Zhang, H. Zn/Pt dual-site single-atom driven difunctional superimposition-augmented sonosensitizer for sonodynamic therapy boosted ferroptosis of cancer. Nat. Commun. 2024, 15, 9359. [Google Scholar] [CrossRef]
- Han, C.; Zeng, Z.; Zhang, X.; Liang, Y.; Kundu, B.K.; Yuan, L.; Tan, C.-L.; Zhang, Y.; Xu, Y.-J. All-in-one: Plasmonic janus heterostructures for efficient cooperative photoredox catalysis. Angew. Chem. Int. Ed. 2024, 63, e202408527. [Google Scholar] [CrossRef]
- Ban, C.G.; Wang, Y.; Feng, Y.J.; Zhu, Z.H.; Duan, Y.Y.; Ma, J.P.; Zhang, X.; Liu, X.; Zhou, K.; Zou, H.J.; et al. Photochromic single atom Ag/TiO2 catalysts for selective CO2 reduction to CH4. Energy Environ. Sci. 2024, 17, 518–530. [Google Scholar] [CrossRef]
- Chen, J.; Jia, Y.; Zhao, H.; Wu, D.; Du, Y.; Ren, X.; Wei, Q. Ternary heterojunction ZnS-ZnIn2S4-In2S3 efficiently catalyzes triethylamine to enhance electrochemiluminescence imaging signals. Adv. Funct. Mater. 2024, 35, 2420714. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Xu, Y.; Liu, Q.; Bahri, M.; Zhang, L.; Browning, N.D.; Cowan, A.J.; Tang, J. Efficient hole abstraction for highly selective oxidative coupling of methane by Au-sputtered TiO2 photocatalysts. Nat. Energy 2023, 8, 1013–1022. [Google Scholar] [CrossRef]
- Sun, X.; Chen, X.; Fu, C.; Yu, Q.; Zheng, X.S.; Fang, F.; Liu, Y.; Zhu, J.; Zhang, W.; Huang, W. Molecular oxygen enhances H2O2 utilization for the photocatalytic conversion of methane to liquid-phase oxygenates. Nat. Commun. 2022, 13, 6677. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, C.; Ai, C.; Xiang, Y.; Mao, C.; Qiao, W.; Wu, J.; Kubi, J.A.; Liu, X.; Wu, S.; et al. Photocurrent-directed immunoregulation accelerates osseointegration through activating calcium influx in macrophages. Adv. Funct. Mater. 2024, 34, 2406095. [Google Scholar] [CrossRef]
- Huang, F.; Wang, F.; Liu, Y.; Guo, L. Cu-ZnS modulated multi-carbon coupling enables high selectivity photoreduction CO2 to CH3CH2COOH. Adv. Mater. 2025, 37, e2416708. [Google Scholar] [CrossRef]
- Mamiyev, Z.Q.; Balayeva, N.O. Optical and structural studies of ZnS nanoparticles synthesized via chemical in situ technique. Chem. Phys. Lett. 2016, 646, 69–74. [Google Scholar] [CrossRef]
- Lee, H.K.; Kim, T.; Jang, Y.A.; Jeong, Y.; Lee, S.W.; Park, C.H. Near-infrared emissive CuInS2/ZnS quantum dot-embedded polymer scaffolds for photon upconversion imaging. Adv. Mater. 2025, 37, e2502333. [Google Scholar] [CrossRef]
- Lin, J.; Liao, J.; Dagnaw, F.W.; Li, J.; Xie, L.; Zhou, M.; Liu, C.; Jian, J.; Tong, Q. Silicon-based heterojunction photoanodes modified with copper-bipyridine catalyst for enhanced solar-driven water splitting. Appl. Surf. Sci. 2024, 648, 159089. [Google Scholar] [CrossRef]
- Jin, Y.; Yu, W.; Chen, L.; Yuan, R.; Liu, J.; Fu, Y.; Chai, Y. Dual-sensitized heterojunction Ag2S/ZnS/NiS composites with entire visible-light region absorption for ultrasensitive photoelectrochemical detection of tobramycin. Biosens. Bioelectron. 2024, 260, 116459. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, Y.; Zhao, Y.; Liu, E.; Li, Z.; Miao, Z. Bioinspired mortise-and-tenon stacking supramolecular engineering for efficient carrier separation in S-scheme heterojunctions. Adv. Funct. Mater. 2025, 2507648. [Google Scholar] [CrossRef]
- Wang, J.; Li, S.; Yang, Q.; Liu, Z.; Tang, F.; Yang, X. Synthesis of In2O3/ZnS type II heterojunctions with superior photocatalytic activity: Mechanism and application in tetracycline hydrochloride degradation. Colloids Surf. A Physicochem. Eng. Asp. 2025, 723, 137306. [Google Scholar] [CrossRef]
- Yu, S.; Li, C.; Lin, Y.; Zhang, J.; Liu, Y.; Yu, F. Interfacial N-Ti bond modulated COFs-TiO2 type-II heterojunctions with directional charge transfer for efficient photocatalytic uranium reduction. Sep. Purif. Technol. 2024, 341, 126888. [Google Scholar] [CrossRef]
- Shabbir, A.; Sardar, S.; Mumtaz, A. Mechanistic investigations of emerging type-II, Z-scheme and S-scheme heterojunctions for photocatalytic applications-a review. J. Alloys Compd. 2024, 1003, 175683. [Google Scholar] [CrossRef]
- Xiao, Y.G.; Wu, J.J.; Li, H.Q.; Liu, X.; Li, Q.; Zhang, Y.; Cao, S.S. In-situ regulation of hollow TiO2 with tunable anatase/rutile heterophase homojunction for promoting excellent photocatalytic degradation of pesticides. Ceram. Int. 2023, 49, 38070–38080. [Google Scholar] [CrossRef]
- Ma, J.J.; Xiao, Y.G.; Chen, J.R.; Shen, Y.; Xiao, S.S.; Cao, S.S. Dual-pathway charge transfer mechanism of (anatase/rutile TiO2-Ag3PO4 )2-Ag3PO4 hollow photocatalyst promotes efficient degradation of pesticides. J. Colloid Interface Sci. 2025, 678, 334–344. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Su, A.; Ding, Y.; Wu, Y.; Tan, Y.; Chang, J. Construction of Hollow TiO2/ZnS Heterojunction Photocatalysts for Highly Enhanced Photodegradation of Tetracycline Hydrochloride. Molecules 2025, 30, 3644. https://doi.org/10.3390/molecules30173644
Zhang Y, Su A, Ding Y, Wu Y, Tan Y, Chang J. Construction of Hollow TiO2/ZnS Heterojunction Photocatalysts for Highly Enhanced Photodegradation of Tetracycline Hydrochloride. Molecules. 2025; 30(17):3644. https://doi.org/10.3390/molecules30173644
Chicago/Turabian StyleZhang, Ying, Anhui Su, Yuqin Ding, Yuhan Wu, Yapeng Tan, and Jianguo Chang. 2025. "Construction of Hollow TiO2/ZnS Heterojunction Photocatalysts for Highly Enhanced Photodegradation of Tetracycline Hydrochloride" Molecules 30, no. 17: 3644. https://doi.org/10.3390/molecules30173644
APA StyleZhang, Y., Su, A., Ding, Y., Wu, Y., Tan, Y., & Chang, J. (2025). Construction of Hollow TiO2/ZnS Heterojunction Photocatalysts for Highly Enhanced Photodegradation of Tetracycline Hydrochloride. Molecules, 30(17), 3644. https://doi.org/10.3390/molecules30173644





