Synthesis and Study of SrTiO3/TiO2 Hybrid Perovskite Nanotubes by Electrochemical Anodization
Abstract
1. Introduction
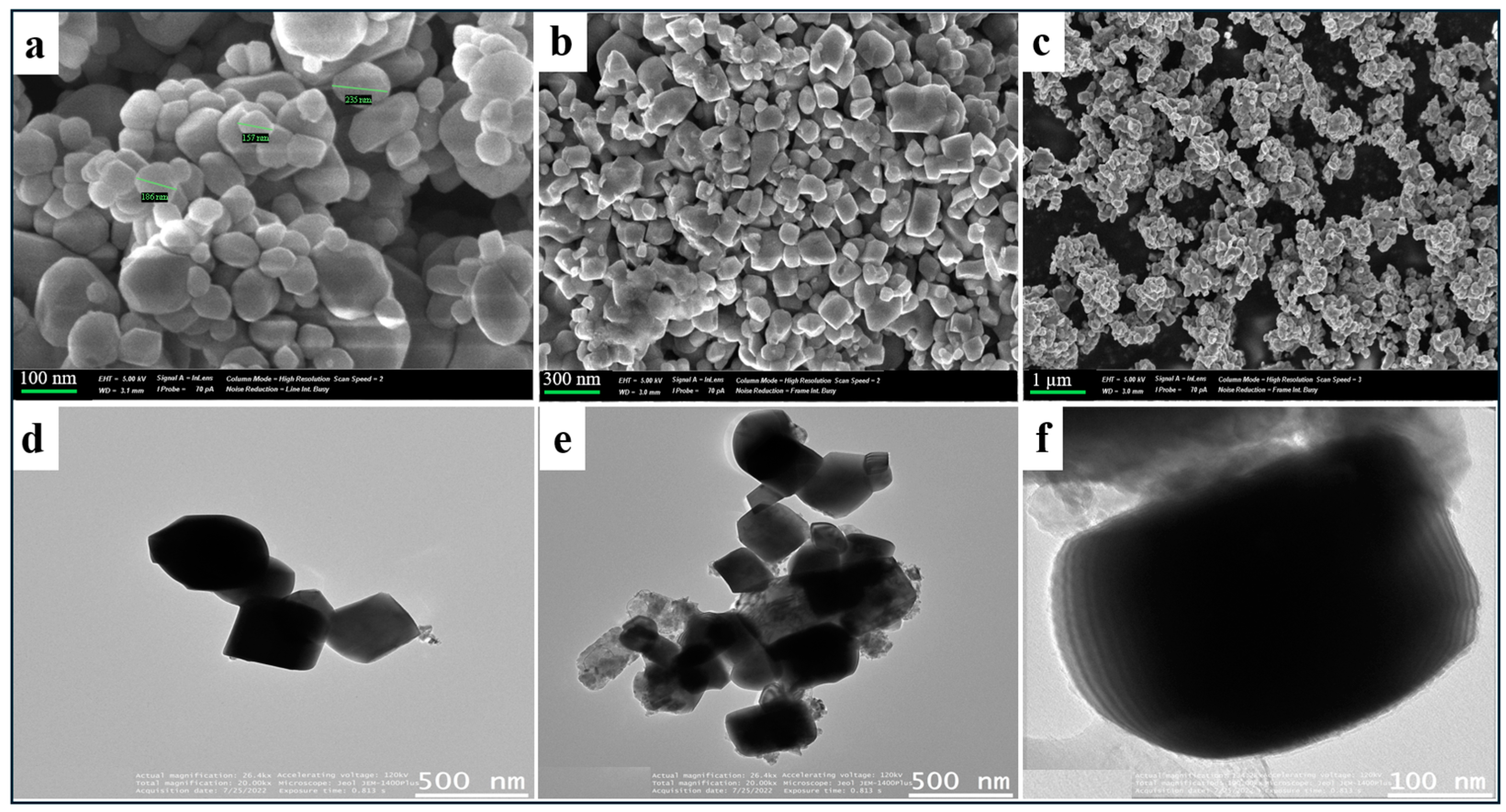
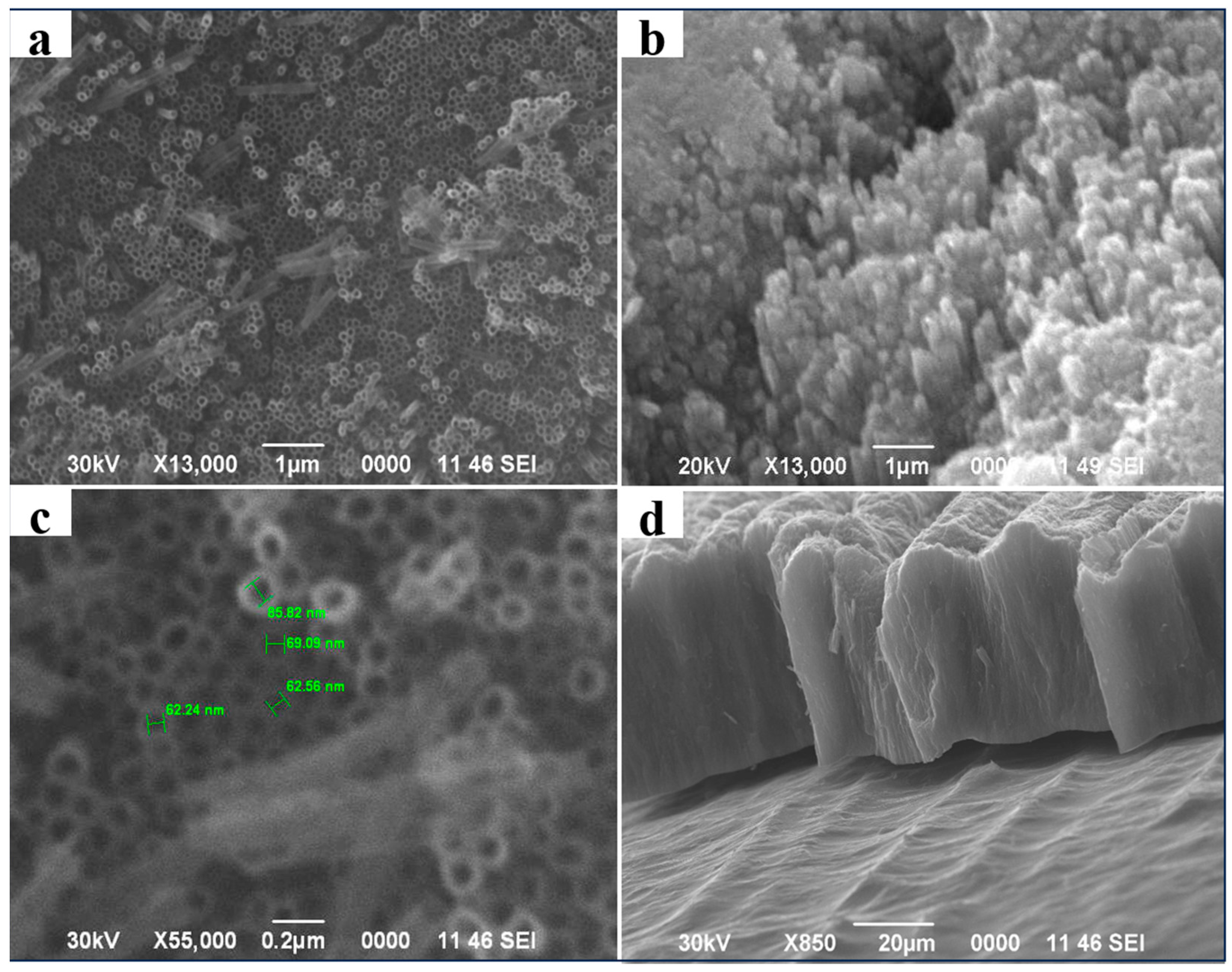
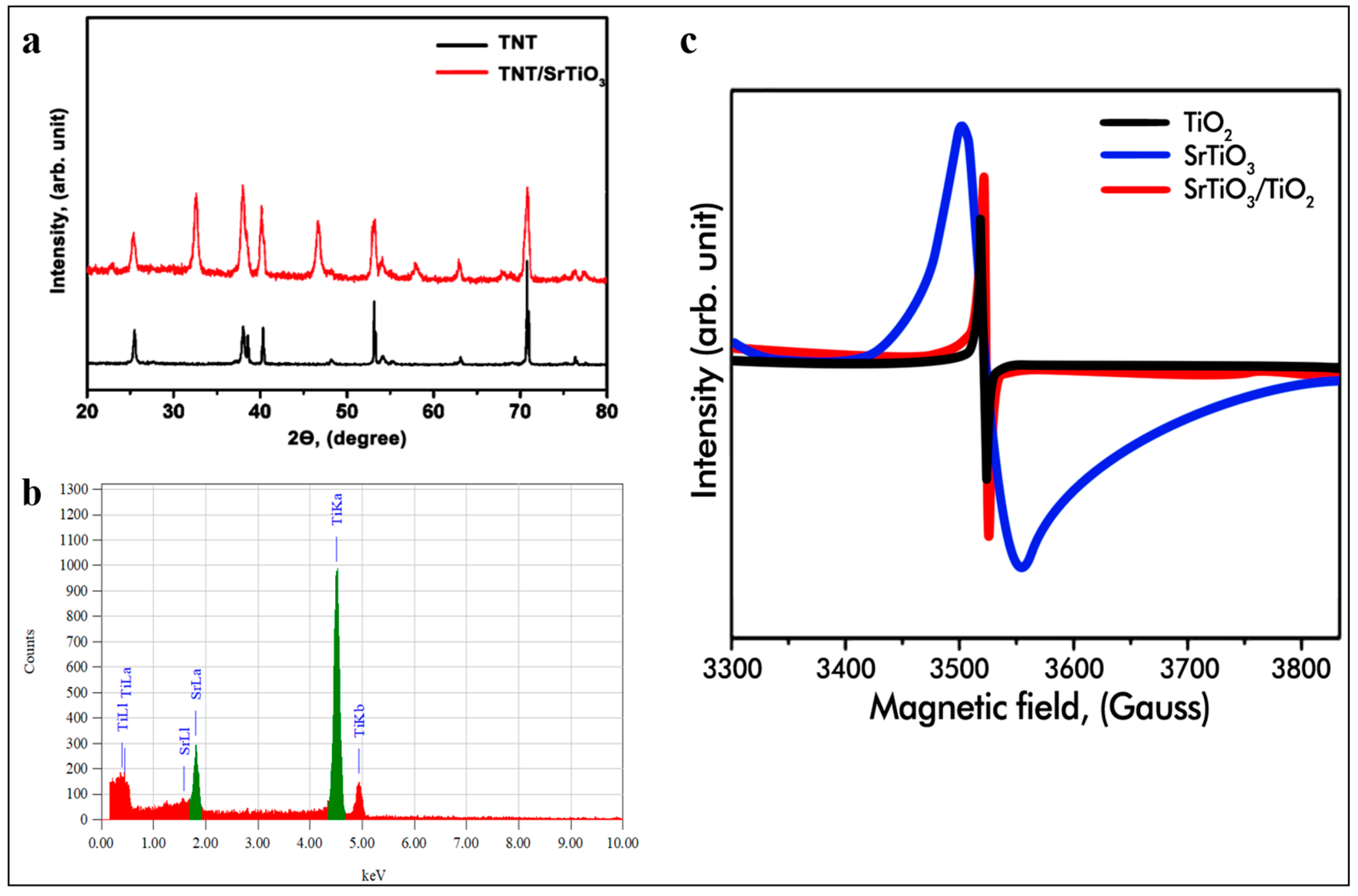
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Synthesis of SrTiO3
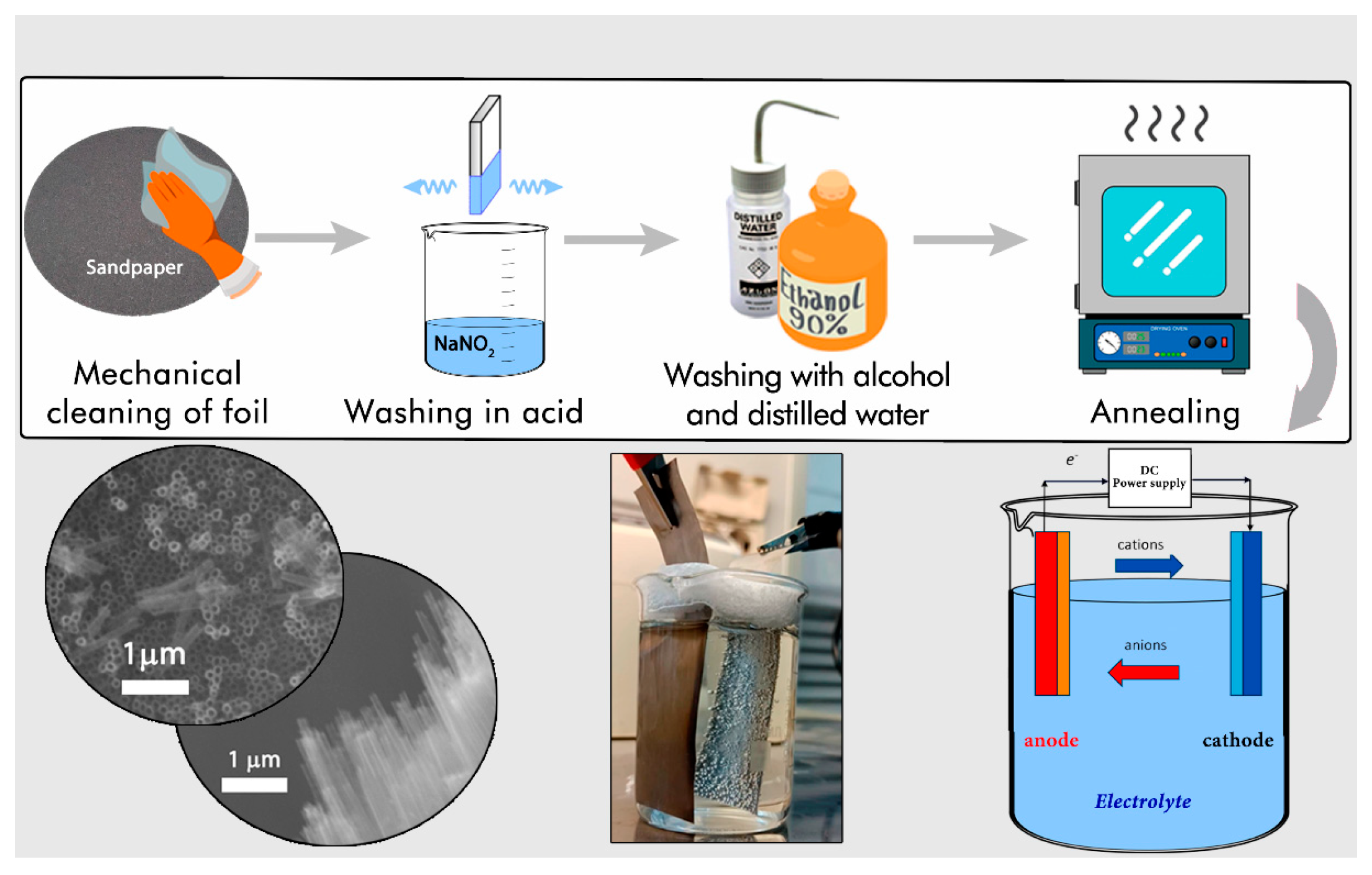
3.3. Nanotube Synthesis
3.4. Synthesis of TNT@SrTiO3
3.5. Material Characterization Techniques
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Z.; Wang, Q.; Xu, H.; Zhang, W.; Zhou, Q.; Zeng, H.; Yang, J.; Zhu, J.; Zhu, X. TiO2 Nanotube Arrays with a Volume Expansion Factor Greater than 2.0: Evidence against the Field-Assisted Ejection Theory. Electrochem. Commun. 2020, 114, 106717. [Google Scholar] [CrossRef]
- Prikhodko, N.; Yeleuov, M.; Abdisattar, A.; Askaruly, K.; Taurbekov, A.; Tolynbekov, A.; Rakhymzhan, N.; Daulbayev, C. Enhancing Supercapacitor Performance through Graphene Flame Synthesis on Nickel Current Collectors and Active Carbon Material from Plant Biomass. J. Energy Storage 2023, 73, 108853. [Google Scholar] [CrossRef]
- Askaruly, K.; Yeleuov, M.; Taurbekov, A.; Sarsembayeva, B.; Tolynbekov, A.; Zhylybayeva, N.; Azat, S.; Abdisattar, A.; Daulbayev, C. A Facile Synthesis of Graphite-Coated Amorphous SiO2 from Biosources as Anode Material for Libs. Mater. Today Commun. 2023, 34, 105136. [Google Scholar] [CrossRef]
- Yeleuov, M.; Daulbayev, C.; Taurbekov, A.; Abdisattar, A.; Ebrahim, R.; Kumekov, S.; Prikhodko, N.; Lesbayev, B.; Batyrzhan, K. Synthesis of Graphene-like Porous Carbon from Biomass for Electrochemical Energy Storage Applications. Diam. Relat. Mater. 2021, 119, 108560. [Google Scholar] [CrossRef]
- Galstyan, V.; Macak, J.M.; Djenizian, T. Anodic TiO2 Nanotubes: A Promising Material for Energy Conversion and Storage. Appl. Mater. Today 2022, 29, 101613. [Google Scholar] [CrossRef]
- Taurbekov, A.; Abdisattar, A.; Atamanov, M.; Yeleuov, M.; Daulbayev, C.; Askaruly, K.; Kaidar, B.; Mansurov, Z.; Castro-Gutierrez, J.; Celzard, A.; et al. Biomass Derived High Porous Carbon via CO2 Activation for Supercapacitor Electrodes. J. Compos. Sci. 2023, 7, 444. [Google Scholar] [CrossRef]
- Saddique, Z.; Imran, M.; Javaid, A.; Kanwal, F.; Latif, S.; dos Santos, J.C.S.; Kim, T.H.; Boczkaj, G. Bismuth-Based Nanomaterials-Assisted Photocatalytic Water Splitting for Sustainable Hydrogen Production. Int. J. Hydrogen Energy 2024, 52, 594–611. [Google Scholar] [CrossRef]
- Yergaziyeva, G.; Kuspanov, Z.; Mambetova, M.; Khudaibergenov, N.; Makayeva, N.; Daulbayev, C. Advancements in Catalytic, Photocatalytic, and Electrocatalytic CO2 Conversion Processes: Current Trends and Future Outlook. J. CO2 Util. 2024, 80, 102682. [Google Scholar] [CrossRef]
- Wang, S.-J.; Su, D.; Zhu, Y.-F.; Lu, C.-H.; Zhang, T. The State-of-the-Art Review on Rational Design for Cavitation Assisted Photocatalysis. Mater. Des. 2023, 234, 112377. [Google Scholar] [CrossRef]
- Kuspanov, Z.; Bakbolat, B.; Baimenov, A.; Issadykov, A.; Yeleuov, M.; Daulbayev, C. Photocatalysts for a Sustainable Future: Innovations in Large-Scale Environmental and Energy Applications. Sci. Total Environ. 2023, 885, 163914. [Google Scholar] [CrossRef]
- Megbenu, H.K.; Daulbayev, C.; Nursharip, A.; Tauanov, Z.; Poulopoulos, S.; Busquets, R.; Baimenov, A. Photocatalytic and Adsorption Performance of MXene@Ag/Cryogel Composites for Sulfamethoxazole and Mercury Removal from Water Matrices. Environ. Technol. Innov. 2023, 32, 103350. [Google Scholar] [CrossRef]
- Baimenov, A.; Montagnaro, F.; Inglezakis, V.J.; Balsamo, M. Experimental and Modeling Studies of Sr2+ and Cs+ Sorption on Cryogels and Comparison to Commercial Adsorbents. Ind. Eng. Chem. Res. 2022, 61, 8204–8219. [Google Scholar] [CrossRef]
- Lee, D.-E.; Kim, M.-K.; Danish, M.; Jo, W.-K. State-of-the-Art Review on Photocatalysis for Efficient Wastewater Treatment: Attractive Approach in Photocatalyst Design and Parameters Affecting the Photocatalytic Degradation. Catal. Commun. 2023, 183, 106764. [Google Scholar] [CrossRef]
- Indira, K.; Mudali, U.K.; Nishimura, T.; Rajendran, N. A Review on TiO2 Nanotubes: Influence of Anodization Parameters, Formation Mechanism, Properties, Corrosion Behavior, and Biomedical Applications. J. Bio- Tribo-Corros. 2015, 1, 28. [Google Scholar] [CrossRef]
- Kuspanov, Z.; Umirzakov, A.; Serik, A.; Baimenov, A.; Yeleuov, M.; Daulbayev, C. Multifunctional Strontium Titanate Perovskite-Based Composite Photocatalysts for Energy Conversion and Other Applications. Int. J. Hydrogen Energy 2023, 48, 38634–38654. [Google Scholar] [CrossRef]
- Bakbolat, B.; Daulbayev, C.; Sultanov, F.; Beissenov, R.; Umirzakov, A.; Mereke, A.; Bekbaev, A.; Chuprakov, I. Recent Developments of TiO2-Based Photocatalysis in the Hydrogen Evolution and Photodegradation: A Review. Nanomaterials 2020, 10, 1790. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Nagappagari, L.R.; Lee, J.; Lee, H.; Lee, W.; Lee, K. Electrochemical Detection of 2,4,6-Trinitrotoluene Reduction in Aqueous Solution by Using Highly Ordered 1D TiO2 Nanotube Arrays. Mater. Today Commun. 2020, 25, 101389. [Google Scholar] [CrossRef]
- An, X.; Hua, W.; Rui, L.; Liu, P.; Li, X. Application of a New Nano-TiO2 Composite Antibacterial Agent in Nursing Management of Operating Room: Based on Real-Time Information Push Assistant System. Prev. Med. 2023, 172, 107541. [Google Scholar] [CrossRef]
- Yin, H.; Liu, H.; Shen, W.Z. The Large Diameter and Fast Growth of Self-Organized TiO2 Nanotube Arrays Achieved via Electrochemical Anodization. Nanotechnology 2009, 21, 035601. [Google Scholar] [CrossRef]
- Ghani, T.; Mujahid, M.; Mehmood, M.; Zhang, G.; Naz, S. Highly Ordered Combined Structure of Anodic TiO2 Nanotubes and TiO2 Nanoparticles Prepared by a Novel Route for Dye-Sensitized Solar Cells. J. Saudi Chem. Soc. 2019, 23, 1231–1240. [Google Scholar] [CrossRef]
- Jandosov, J.; Alavijeh, M.; Sultakhan, S.; Baimenov, A.; Bernardo, M.; Sakipova, Z.; Azat, S.; Lyubchyk, S.; Zhylybayeva, N.; Naurzbayeva, G.; et al. Activated Carbon/Pectin Composite Enterosorbent for Human Protection from Intoxication with Xenobiotics Pb(II) and Sodium Diclofenac. Molecules 2022, 27, 2296. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, Y.; Tian, R.; Gao, Q.; Wang, J.; Liu, Y.; Yang, F. Anodic Growth of TiO2 Nanotube Arrays: Effects of Substrate Curvature and Residual Stress. Surf. Coat. Technol. 2023, 469, 129783. [Google Scholar] [CrossRef]
- Roy, P.K.; Bera, J. Formation of SrTiO3 from Sr-Oxalate and TiO2. Mater. Res. Bull. 2005, 40, 599–604. [Google Scholar] [CrossRef]
- Kudaibergen, A.D.; Kuspanov, Z.B.; Issadykov, A.N.; Beisenov, R.E.; Mansurov, Z.A.; Yeleuov, M.A.; Daulbayev, C.B. Synthesis, Structure, and Energetic Characteristics of Perovskite Photocatalyst SrTiO3: An Experimental and DFT Study. Eurasian Chem.-Technol. J. 2023, 25, 139–146. [Google Scholar] [CrossRef]
- Paulose, M.; Prakasam, H.E.; Varghese, O.K.; Peng, L.; Popat, K.C.; Mor, G.K.; Desai, T.A.; Grimes, C.A. TiO2 Nanotube Arrays of 1000 μm Length by Anodization of Titanium Foil: Phenol Red Diffusion. J. Phys. Chem. C 2007, 111, 14992–14997. [Google Scholar] [CrossRef]
- Moulis, F.; Krýsa, J. Photocatalytic Degradation of Several VOCs (n-Hexane, n-Butyl Acetate and Toluene) on TiO2 Layer in a Closed-Loop Reactor. Catal. Today 2013, 209, 153–158. [Google Scholar] [CrossRef]
- Hou, X.; Lund, P.D.; Li, Y. Controlling Anodization Time to Monitor Film Thickness, Phase Composition and Crystal Orientation during Anodic Growth of TiO2 Nanotubes. Electrochem. Commun. 2022, 134, 107168. [Google Scholar] [CrossRef]
- Sopha, H.; Samoril, T.; Palesch, E.; Hromadko, L.; Zazpe, R.; Skoda, D.; Urbanek, M.; Ng, S.; Prikryl, J.; Macak, J.M. Ideally Hexagonally Ordered TiO2 Nanotube Arrays. ChemistryOpen 2017, 6, 480–483. [Google Scholar] [CrossRef] [PubMed]
- Beketova, D.; Motola, M.; Sopha, H.; Michalicka, J.; Cicmancova, V.; Dvorak, F.; Hromadko, L.; Frumarova, B.; Stoica, M.; Macak, J.M. One-Step Decoration of TiO2 Nanotubes with Fe3O4 Nanoparticles: Synthesis and Photocatalytic and Magnetic Properties. ACS Appl. Nano Mater. 2020, 3, 1553–1563. [Google Scholar] [CrossRef]
- Tak, M.; Tomar, H.; Mote, R.G. Synthesis of Titanium Nanotubes (TNT) and Its Influence on Electrochemical Micromachining of Titanium. Procedia CIRP 2020, 95, 803–808. [Google Scholar] [CrossRef]
- Daulbayev, C.; Sultanov, F.; Korobeinyk, A.V.; Yeleuov, M.; Azat, S.; Bakbolat, B.; Umirzakov, A.; Mansurov, Z. Bio-Waste-Derived Few-Layered Graphene/SrTiO3/PAN as Efficient Photocatalytic System for Water Splitting. Appl. Surf. Sci. 2021, 549, 149176. [Google Scholar] [CrossRef]
- Sultanov, F.; Daulbayev, C.; Azat, S.; Kuterbekov, K.; Bekmyrza, K.; Bakbolat, B.; Bigaj, M.; Mansurov, Z. Influence of Metal Oxide Particles on Bandgap of 1D Photocatalysts Based on SrTiO3/PAN Fibers. Nanomaterials 2020, 10, 1734. [Google Scholar] [CrossRef] [PubMed]
- Sultanov, F.; Daulbayev, C.; Bakbolat, B.; Daulbayev, O.; Bigaj, M.; Mansurov, Z.; Kuterbekov, K.; Bekmyrza, K. Aligned Composite SrTiO3/PAN Fibers as 1D Photocatalyst Obtained by Electrospinning Method. Chem. Phys. Lett. 2019, 737, 136821. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bissenova, M.; Umirzakov, A.; Mit, K.; Mereke, A.; Yerubayev, Y.; Serik, A.; Kuspanov, Z. Synthesis and Study of SrTiO3/TiO2 Hybrid Perovskite Nanotubes by Electrochemical Anodization. Molecules 2024, 29, 1101. https://doi.org/10.3390/molecules29051101
Bissenova M, Umirzakov A, Mit K, Mereke A, Yerubayev Y, Serik A, Kuspanov Z. Synthesis and Study of SrTiO3/TiO2 Hybrid Perovskite Nanotubes by Electrochemical Anodization. Molecules. 2024; 29(5):1101. https://doi.org/10.3390/molecules29051101
Chicago/Turabian StyleBissenova, Madina, Arman Umirzakov, Konstantin Mit, Almaz Mereke, Yerlan Yerubayev, Aigerim Serik, and Zhengisbek Kuspanov. 2024. "Synthesis and Study of SrTiO3/TiO2 Hybrid Perovskite Nanotubes by Electrochemical Anodization" Molecules 29, no. 5: 1101. https://doi.org/10.3390/molecules29051101
APA StyleBissenova, M., Umirzakov, A., Mit, K., Mereke, A., Yerubayev, Y., Serik, A., & Kuspanov, Z. (2024). Synthesis and Study of SrTiO3/TiO2 Hybrid Perovskite Nanotubes by Electrochemical Anodization. Molecules, 29(5), 1101. https://doi.org/10.3390/molecules29051101





