Utility of the Redox Cycle of Nitrofurantoin for the Development of a New Chemiluminescence Method for Its Analysis in Milk Samples
Abstract
1. Introduction
2. Results and Discussion
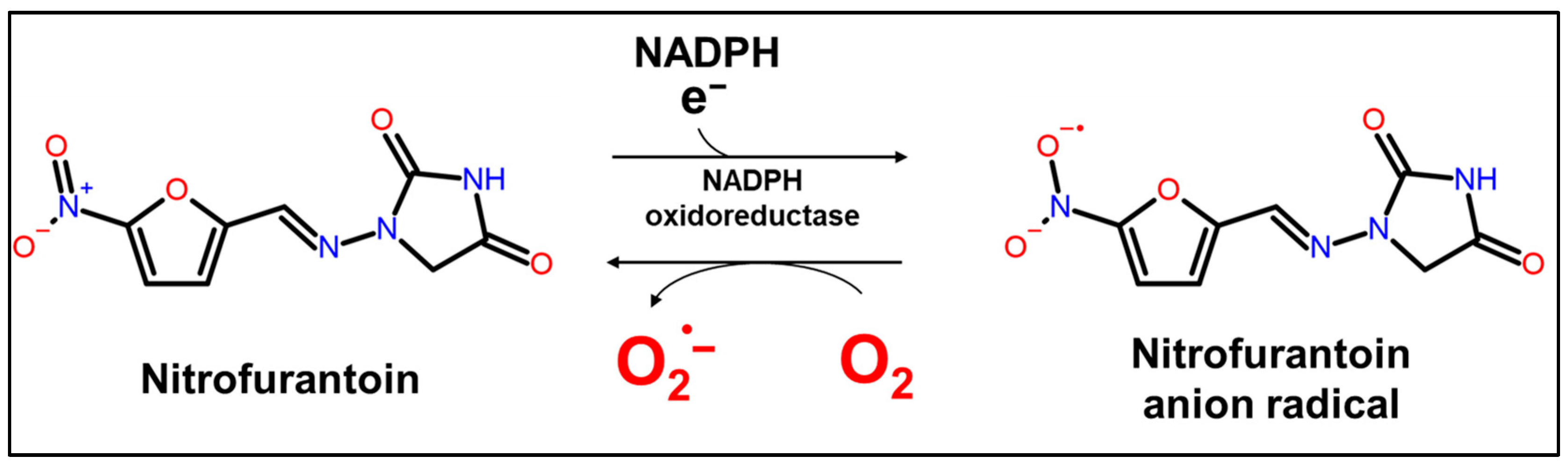
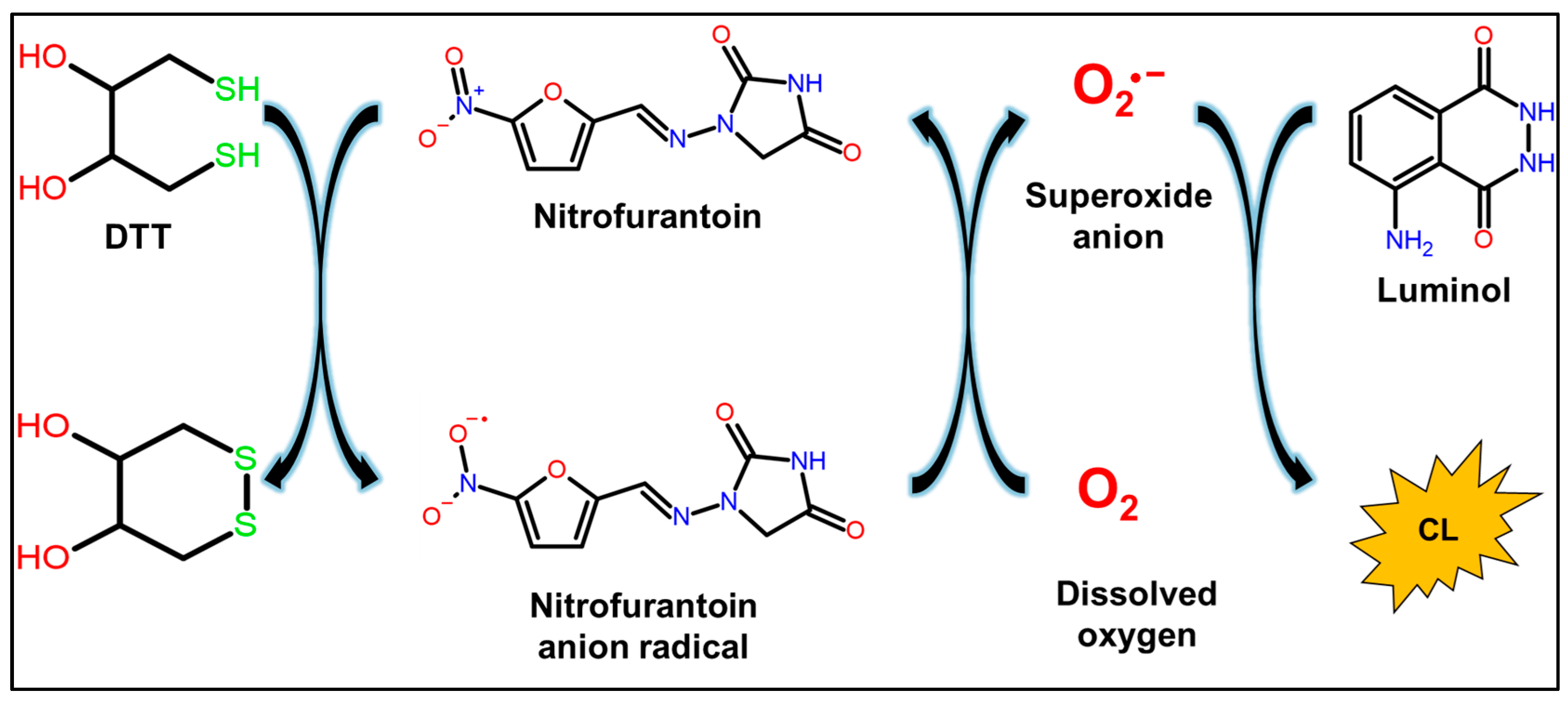
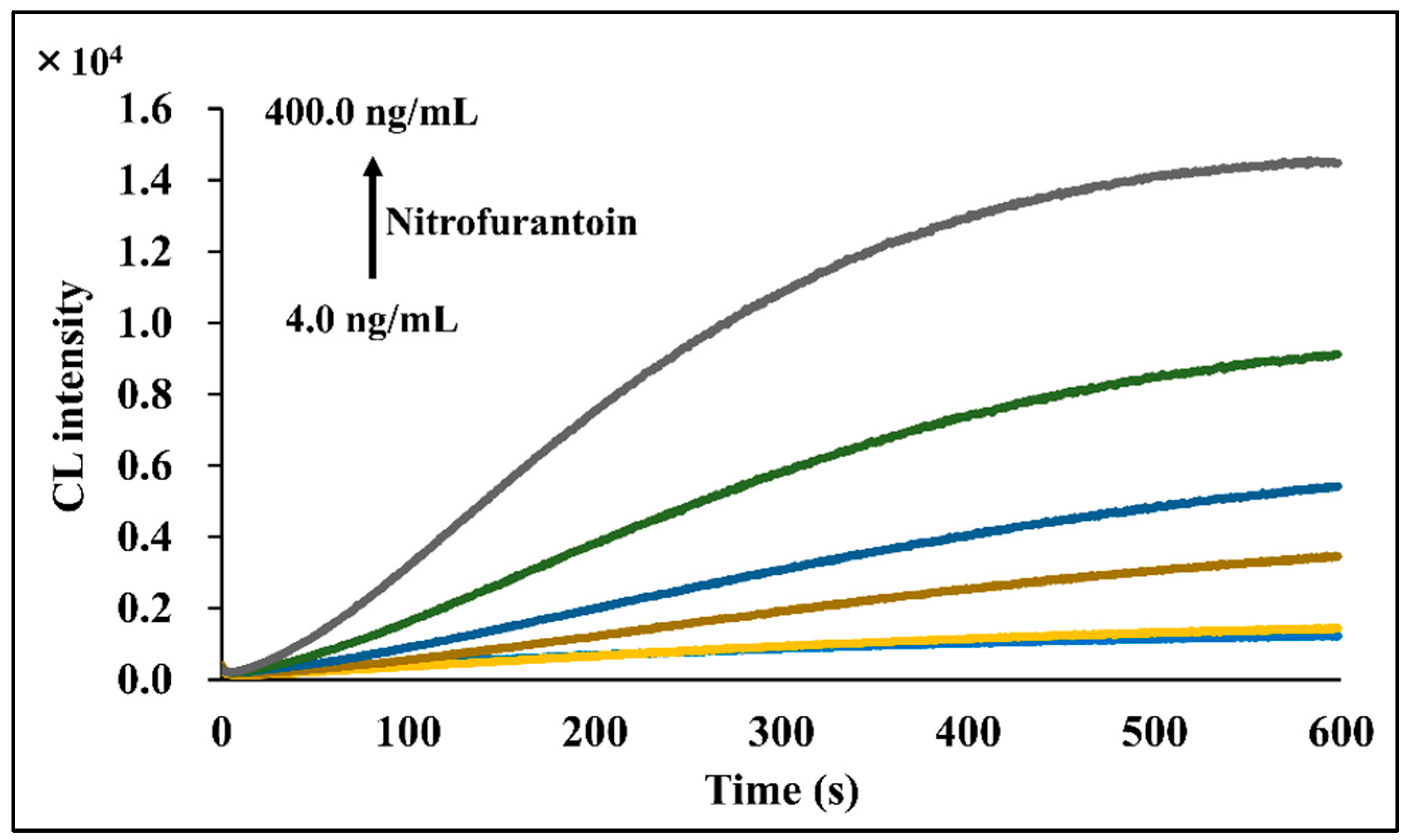
2.1. CL Profile of Luminol/DTT/Nitrofurantoin System
2.2. Optimization of CL Reaction Conditions
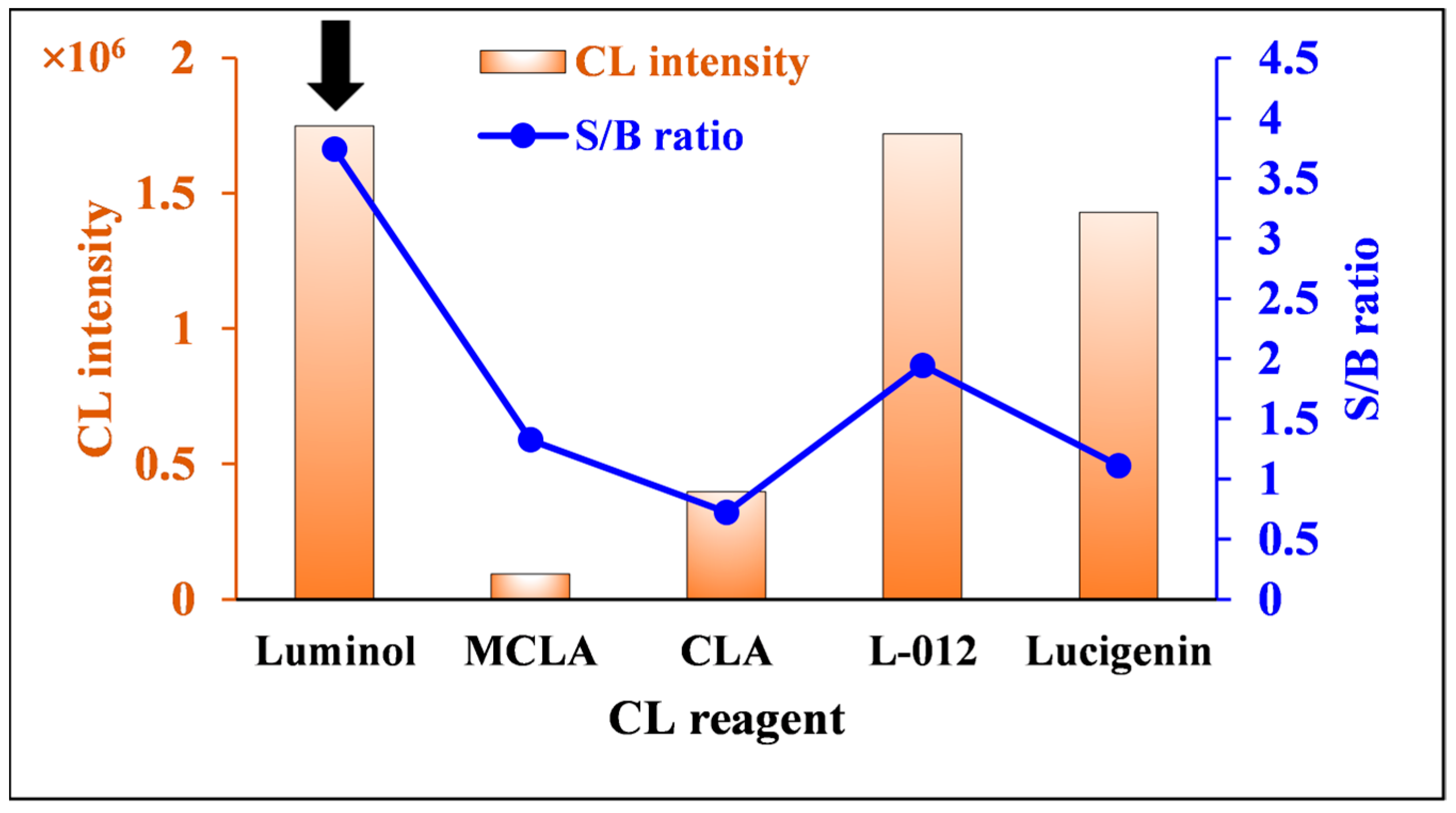
2.2.1. Selection of CL Reagent
2.2.2. Effect of Luminol Concentration
2.2.3. Effect of the Type of Reducing Agent
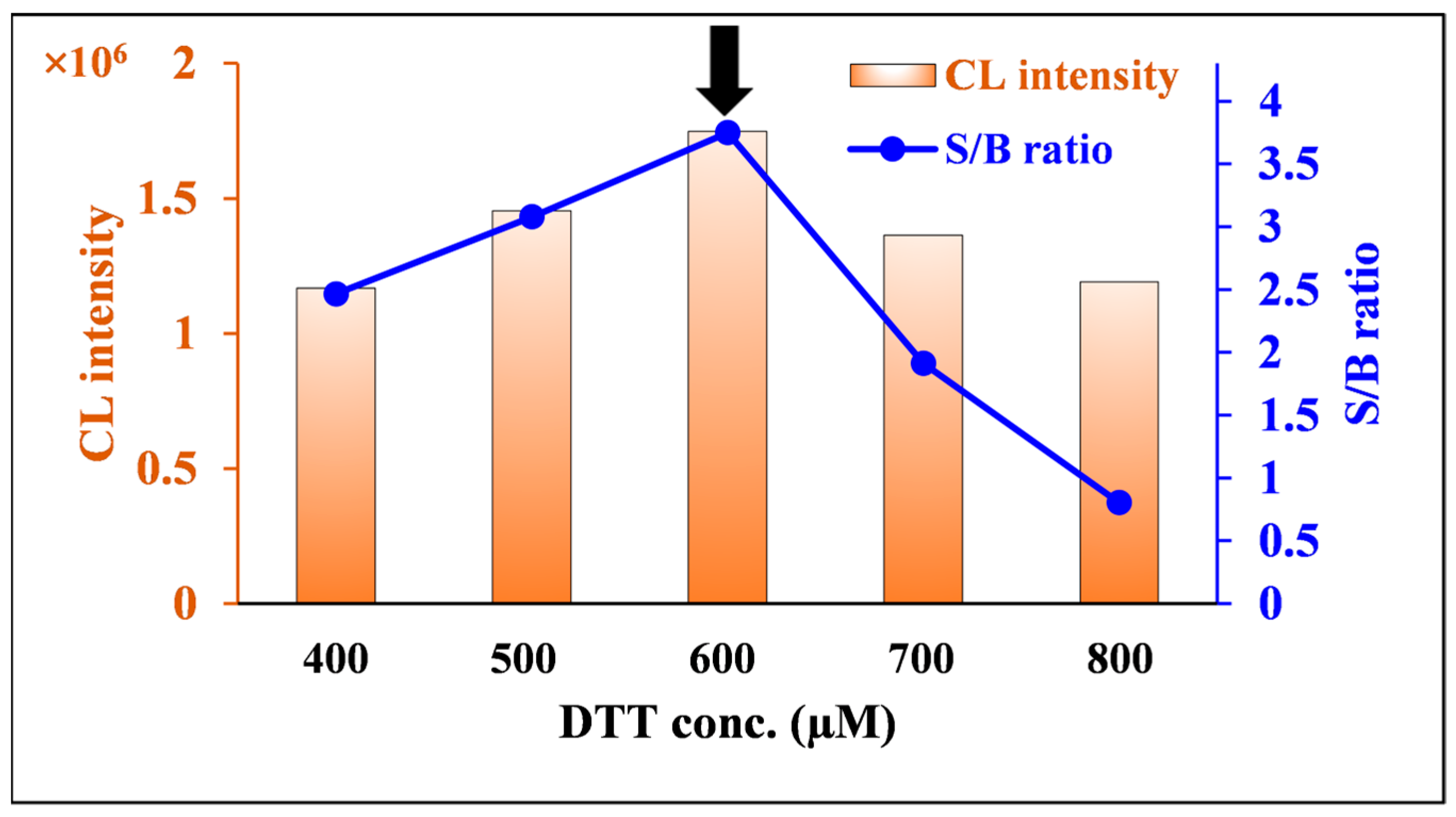
2.2.4. Effect of DTT Concentration
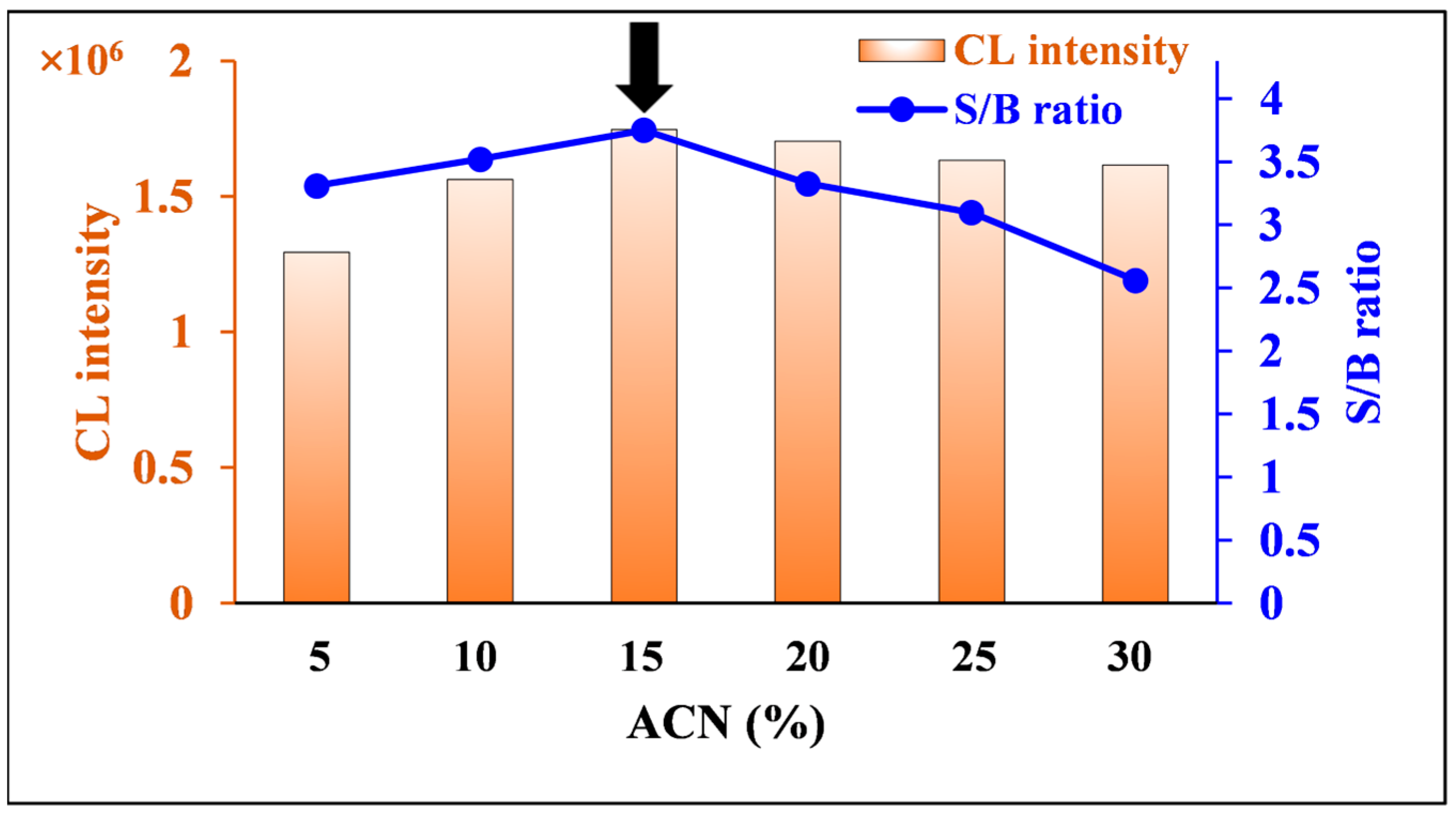
2.2.5. Effect of ACN Content in Nitrofurantoin Solvent
2.2.6. Effect of NaOH Concentration
2.3. Identification of ROS Produced by CL of Nitrofurantoin
2.4. Method Validation
2.5. Application of the Developed Method for the Determination of Nitrofurantoin in Milk Samples
2.6. Evaluation of the Method’s Practicability
3. Materials and Methods
3.1. Materials and Reagents
3.2. Equipment and Software
3.3. Procedure for Optimization of CL Conditions
3.4. Procedure for Identification of ROS Responsible for the CL of Nitrofurantoin
3.5. Procedure for Method Validation
3.6. Determination of Nitrofurantoin in Milk Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CL | Chemiluminescence |
| ROS | Reactive Oxygen Species |
| ICH | International Council for Harmonization |
| FDA | Food and Drug Administration |
| HPLC | High-Performance Liquid Chromatography |
| UHPLC | Ultra-High-Performance Liquid Chromatography |
| UV | Ultraviolet |
| QE HF HRMS | High-Field Quadrupole-Orbitrap High Resolution Mass Spectrometry |
| SERS | Surface-Enhanced Raman Spectroscopy |
| MIP | Molecularly Imprinted Polymer |
| DTT | Dithiothreitol |
| ACN | Acetonitrile |
| MCLA | 2-methyl-6-(4-methoxyphenyl)-3,7-dihydroimidazo [1,2-a]pyrazin-3-one |
| CLA | 2-methyl-6-phenyl-3,7-dihydroimidazo [1,2-a]pyrazin-3-one |
| S/B | Signal to Blank Ration |
| SOD | Superoxide dismutase |
| RCI | Relative CL Intensity |
| LOD | Limit of Detection |
| LOQ | Limit of Quantitation |
| S.D. | Standard Deviation |
| RSD | Relative Standard Deviation |
| BAGI | Blue Applicability Grade Index |
References
- Cvetkovski, A.; Ferretti, V. Crystal Structure and Packing Analysis of Nitrofurantoin N,N-Dimethylformamide Solvate. Crystallogr. Rep. 2016, 61, 611–615. [Google Scholar] [CrossRef][Green Version]
- Huttner, A.; Verhaegh, E.M.; Harbarth, S.; Muller, A.E.; Theuretzbacher, U.; Mouton, J.W. Nitrofurantoin Revisited: A Systematic Review and Meta-Analysis of Controlled Trials. J. Antimicrob. Chemother. 2015, 70, 2456–2464. [Google Scholar] [CrossRef] [PubMed]
- Guichard, P.; Laurentie, M.; Hurtaud-Pessel, D.; Verdon, E. Confirmation of Five Nitrofuran Metabolites Including Nifursol Metabolite in Meat and Aquaculture Products by Liquid Chromatography-Tandem Mass Spectrometry: Validation According to European Union Decision 2002/657/EC. Food Chem. 2021, 342, 128389. [Google Scholar] [CrossRef] [PubMed]
- Antunes, P.; Mourão, J.; Campos, J.; Peixe, L. Salmonellosis: The Role of Poultry Meat. Clin. Microbiol. Infect. 2016, 22, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Suriyamoorthy, P.; Rawson, A. Nitrofuran Residues in Animal Sourced Food: Sample Extraction and Identification Methods—A Review. Food Chem. Adv. 2023, 3, 100396. [Google Scholar] [CrossRef]
- Bessone, F.; Ferrari, A.; Hernandez, N.; Mendizabal, M.; Ridruejo, E.; Zerega, A.; Tanno, F.; Reggiardo, M.V.; Vorobioff, J.; Tanno, H.; et al. Nitrofurantoin-Induced Liver Injury: Long-Term Follow-up in Two Prospective DILI Registries. Arch. Toxicol. 2023, 97, 593–602. [Google Scholar] [CrossRef]
- Pacholak, A.; Żur-Pińska, J.; Piński, A.; Nguyen, Q.A.; Ligaj, M.; Luczak, M.; Nghiem, L.D.; Kaczorek, E. Potential Negative Effect of Long-Term Exposure to Nitrofurans on Bacteria Isolated from Wastewater. Sci. Total Environ. 2023, 872, 162199. [Google Scholar] [CrossRef]
- Ahmed, F.; Kokulnathan, T.; Umar, A.; Akbar, S.; Kumar, S.; Shaalan, N.M.; Arshi, N.; Alam, M.G.; Aljaafari, A.; Alshoaibi, A. Zinc Oxide/Phosphorus-Doped Carbon Nitride Composite as Potential Scaffold for Electrochemical Detection of Nitrofurantoin. Biosensors 2022, 12, 856. [Google Scholar] [CrossRef]
- Kijima, A.; Ishii, Y.; Takasu, S.; Matsushita, K.; Kuroda, K.; Hibi, D.; Suzuki, Y.; Nohmi, T.; Umemura, T. Chemical Structure-Related Mechanisms Underlying in Vivo Genotoxicity Induced by Nitrofurantoin and Its Constituent Moieties in Gpt Delta Rats. Toxicology 2015, 331, 125–135. [Google Scholar] [CrossRef]
- Damayanti, S.; Gunawan, U.; Ibrahim, S. Development of Molecular Imprinted Polymer Solid Phase Extraction (MISPE) for Separation Nitrofurantoin Residue in Chicken Eggs. Asian J. Pharm. Clin. Res. 2017, 10, 108. [Google Scholar] [CrossRef]
- Ibrahim, E.; Sallam, S.; Hadad, G. Simultaneous Determination of Nitrofurantoin and Phenazopyridine in Human Urine Samples by HPLC-UV. Rec. Pharm. Biomed. Sci. 2023, 7, 10–20. [Google Scholar] [CrossRef]
- Wijma, R.A.; Hoogtanders, K.E.J.; Croes, S.; Mouton, J.W.; Brüggemann, R.J.M. Development and Validation of a Fast and Sensitive UHPLC-DAD Assay for the Quantification of Nitrofurantoin in Plasma and Urine. J. Pharm. Biomed. Anal. 2019, 174, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Suo, D.; Song, Z.; Xiao, Z.; Wang, S.; Shang, W.; Du, Q.; Fan, X.; Wang, P. Enrichment and Determination of Nine Nitrofurans in Aquaculture Water and Aquatic Feed by Using Metal–Organic Framework NDO-Zn. Microchem. J. 2023, 190, 108639. [Google Scholar] [CrossRef]
- Karajgi, S.R.; Sunayana, M.; Potdar, S.S.; Kotnal, R.B. Novel First Order Derivative UV- Spectrophotometric Peak Detect Method for the Determination of Nitrofurantoin. Int. J. ChemTech Res. 2018, 11, 239–246. [Google Scholar] [CrossRef]
- Hadi, H.; Mouayed, M. Spectrophotometric Determination of Nitrofurantoin Drug in Its Pharmaceutical Formulations Using MBTH as a Coupling Reagent. Iraqi J. Pharm. Sci. 2017, 25, 7–14. [Google Scholar] [CrossRef]
- Al-Hashimi, B.R.; Omer, K.M.; Rahman, H.S.; Othman, H.H. Inner Filter Effect as a Sensitive Sensing Platform for Detection of Nitrofurantoin Using Luminescent Drug-Based Carbon Nanodots. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 244, 118835. [Google Scholar] [CrossRef]
- El Sharkasy, M.E.; Tolba, M.M.; Belal, F.; Walash, M.I.; Aboshabana, R. Turn-off Fluorescence of S,N-Doped Carbon Dots for Determination of Two Nitro-Containing Drugs in Dosage Forms and Human Plasma. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 289, 122246. [Google Scholar] [CrossRef]
- Liu, J.; Yang, B.; Wang, Y.; Zhang, F.; Liu, X.; Niu, S.; Yuan, Y.; Bi, S. Sensitive Detection of Furazolidone and Nitrofurantoin by Fluorescent Nanoprobe Coated with 3-Mercaptopropionic Acid on CdS Quantum Dots. Microchem. J. 2024, 197, 109773. [Google Scholar] [CrossRef]
- Yue, X.; Zhou, Z.; Li, M.; Jie, M.; Xu, B.; Bai, Y. Inner-Filter Effect Induced Fluorescent Sensor Based on Fusiform Al-MOF Nanosheets for Sensitive and Visual Detection of Nitrofuran in Milk. Food Chem. 2022, 367, 130763. [Google Scholar] [CrossRef]
- Cheng, Z.; Liu, X.; Zhao, B.; Liu, X.; Yang, X.; Zhang, X.; Feng, X. A Smartphone-Integrated Test Paper Sensing Platform for Visual and Intelligent Detection of Nitrofurantoin in Honey Samples. Food Chem. 2024, 445, 138783. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, Z.; Yue, Z.; Gao, J.; Wu, S.; Zhang, Z.; Li, G. Rapid Determination of Trace Nitrofurantoin in Cosmetics by Surface Enhanced Raman Spectroscopy Using Nanoarrayed Hydroxyl Polystyrene-based Substrate. J. Raman Spectrosc. 2019, 50, 1094–1102. [Google Scholar] [CrossRef]
- Zhang, H.; Lai, H.; Wu, X.; Li, G.; Hu, Y. CoFe2O4@HNTs/AuNPs Substrate for Rapid Magnetic Solid-Phase Extraction and Efficient SERS Detection of Complex Samples All-in-One. Anal. Chem. 2020, 92, 4607–4613. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, H.; Jiang, L. A Highly Reproducible SERS Sensor Based on an Au Nanoparticles/Graphene Oxide Hybrid Nanocomposite for Label-Free Quantitative Detection of Antibiotics. Analyst 2021, 146, 5740–5746. [Google Scholar] [CrossRef] [PubMed]
- Kokulnathan, T.; Wang, T.-J. Synthesis and Characterization of 3D Flower-like Nickel Oxide Entrapped on Boron Doped Carbon Nitride Nanocomposite: An Efficient Catalyst for the Electrochemical Detection of Nitrofurantoin. Compos. Part B Eng. 2019, 174, 106914. [Google Scholar] [CrossRef]
- Mariyappan, V.; Keerthi, M.; Chen, S.-M.; Jeyapragasam, T. Nanostructured Perovskite Type Gadolinium Orthoferrite Decorated RGO Nanocomposite for the Detection of Nitrofurantoin in Human Urine and River Water Samples. J. Colloid Interface Sci. 2021, 600, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhe, T.; Li, R.; Bai, F.; Jia, P.; Xu, Z.; Wang, X.; Bu, T.; Wu, H.; Wang, L. ZIF-Derived Co Nanoparticles Embedded into N-Doped Carbon Nanotube Composites for Highly Efficient Electrochemical Detection of Nitrofurantoin in Food. Food Chem. 2023, 418, 135948. [Google Scholar] [CrossRef]
- Babulal, S.M.; Koventhan, C.; Chen, S.M.; Hung, W. Construction of Sphere like Samarium Vanadate Nanoparticles Anchored Graphene Nanosheets for Enhanced Electrochemical Detection of Nitrofurantoin in Biological Fluids. Compos. Part B Eng. 2022, 237, 109847. [Google Scholar] [CrossRef]
- Amin, A.; Prasad, G.V.; Vinothkumar, V.; Jang, S.J.; Oh, D.E.; Kim, T.H. Reduced Graphene Oxide/β-Cyclodextrin Nanocomposite for the Electrochemical Detection of Nitrofurantoin. Chemosensors 2025, 13, 247. [Google Scholar] [CrossRef]
- Tzani, M.A.; Gioftsidou, D.K.; Kallitsakis, M.G.; Pliatsios, N.V.; Kalogiouri, N.P.; Angaridis, P.A.; Lykakis, I.N.; Terzidis, M.A. Direct and Indirect Chemiluminescence: Reactions, Mechanisms and Challenges. Molecules 2021, 26, 7664. [Google Scholar] [CrossRef]
- Taokaenchan, N.; Tangkuaram, T.; Pookmanee, P.; Phaisansuthichol, S.; Kuimalee, S.; Satienperakul, S. Enhanced Electrogenerated Chemiluminescence of Tris(2,2′-Bipyridyl)Ruthenium(II) System by l-Cysteine-Capped CdTe Quantum Dots and Its Application for the Determination of Nitrofuran Antibiotics. Biosens. Bioelectron. 2015, 66, 231–237. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Liu, J.-X.; Jiang, Z.-Q.; Wang, J.-P. Molecularly Imprinted Polymer Based Chemiluminescence Method for Detection of Nitrofurans. Aust. J. Chem. 2019, 72, 375. [Google Scholar] [CrossRef]
- Deepa, S.; Venkatesan, R.; Jayalakshmi, S.; Priya, M.; Kim, S.-C. Recent Advances in Catalyst-Enhanced Luminol Chemiluminescence System and Its Environmental and Chemical Applications. J. Environ. Chem. Eng. 2023, 11, 109853. [Google Scholar] [CrossRef]
- Satienperakul, S.; Tatana, C.; Taokaenchan, N.; Sirikeaw, W.; Liawruangrath, S. Determination of Selected Nitrofurans Using High Performance Liquid Chromatography with Post-Column Chemiluminescence Detection. Maejo Int. J. Sci. Technol. 2017, 11, 249–263. [Google Scholar]
- El-Maghrabey, M.; Kishikawa, N.; Kuroda, N. Novel Isotope-Coded Derivatization Method for Aldehydes Using 14 N/ 15 N-Ammonium Acetate and 9,10-Phenanthrenequinone. Anal. Chem. 2018, 90, 13867–13875. [Google Scholar] [CrossRef]
- El-Maghrabey, M.; Kishikawa, N.; Kuroda, N. 9,10-Phenanthrenequinone as a Mass-Tagging Reagent for Ultra-Sensitive Liquid Chromatography-Tandem Mass Spectrometry Assay of Aliphatic Aldehydes in Human Serum. J. Chromatogr. A 2016, 1462, 80–89. [Google Scholar] [CrossRef]
- El-Maghrabey, M.; Kishikawa, N.; Harada, S.; Ohyama, K.; Kuroda, N. Quinone-Based Antibody Labeling Reagent for Enzyme-Free Chemiluminescent Immunoassays. Application to Avidin and Biotinylated Anti-Rabbit IgG Labeling. Biosens. Bioelectron. 2020, 160, 112215. [Google Scholar] [CrossRef]
- El-Maghrabey, M.; Kishikawa, N.; Kamimura, S.; Ohyama, K.; Kuroda, N. Design of a Dual Functionalized Chemiluminescence Ultrasensitive Probe for Quinones Based on Their Redox Cycle. Application to the Determination of Doxorubicin in Lyophilized Powder and Human Serum. Sens. Actuators B Chem. 2021, 329, 129226. [Google Scholar] [CrossRef]
- Fukuda, M.; El-Maghrabey, M.H.; Kishikawa, N.; Ikemoto, K.; Kuroda, N. Ultrasensitive Determination of Pyrroloquinoline Quinone in Human Plasma by HPLC with Chemiluminescence Detection Using the Redox Cycle of Quinone. J. Pharm. Biomed. Anal. 2017, 145, 814–820. [Google Scholar] [CrossRef]
- Kim, S.Y.; Park, C.; Jang, H.-J.; Kim, B.; Bae, H.-W.; Chung, I.-Y.; Kim, E.S.; Cho, Y.-H. Antibacterial Strategies Inspired by the Oxidative Stress and Response Networks. J. Microbiol. 2019, 57, 203–212. [Google Scholar] [CrossRef]
- Giussani, A.; Farahani, P.; Martínez-Muñoz, D.; Lundberg, M.; Lindh, R.; Roca-Sanjuán, D. Molecular Basis of the Chemiluminescence Mechanism of Luminol. Chem—A Eur. J. 2019, 25, 5202–5213. [Google Scholar] [CrossRef]
- Carlsson, C.; Fégeant, B.; Svensson, E.; Wiklund, L.; Jonsson, M. On the Selectivity of Radical Scavengers Used to Probe Hydroxyl Radical Formation in Heterogeneous Systems. J. Phys. Chem. C 2022, 126, 12435–12440. [Google Scholar] [CrossRef]
- International Council for Harmonisation. Validation of Analytical Procedures Q2(R2); International Council for Harmonisation: Geneva, Switzerland, 2022. [Google Scholar]
- Manousi, N.; Wojnowski, W.; Płotka-Wasylka, J.; Samanidou, V. Blue Applicability Grade Index (BAGI) and Software: A New Tool for the Evaluation of Method Practicality. Green Chem. 2023, 25, 7598–7604. [Google Scholar] [CrossRef]
- Liu, M.; Zhe, T.; Zhu, L.; Li, F.; Ma, K.; Xuan, C.; Feng, Q.; Ouyang, S.; Wang, L. Interfacial Engineering of VS2/Ti3C2Tx MXene Heterojunctions for Portable Electrochemical Sensing of Nitrofurantoin Residues in Food and Water Samples. Food Chem. 2025, 486, 144624. [Google Scholar] [CrossRef]



| Scavenger | ROS | Concentration | RCI * |
|---|---|---|---|
| No scavenger | – | – | 100 |
| SOD | O2·− | 1 U/mL | 0.61 |
| 10 U/mL | 0.25 | ||
| Mannitol | •OH | 10 μM | 56.4 |
| 100 μM | 47.9 | ||
| Methanol | •OH | 1% | 71.1 |
| 10% | 52.8 | ||
| NaN3 | 1O2 | 10 μM | 63.6 |
| 100 μM | 53.7 |
| Concentration Added (ng/mL) | Recovery (%) | Intra-Day Precision (RSD, %) | Inter-Day Precision (RSD, %) |
|---|---|---|---|
| 8.0 | 102.5 | 3.3 | 8.6 |
| 40.0 | 98.0 | 1.4 | 2.4 |
| 80.0 | 99.5 | 5.5 | 6.2 |
| 160.0 | 99.8 | 5.5 | 6.0 |
| 400.0 | 101.3 | 1.2 | 1.8 |
| Method | Range (ng/mL) | LOD (ng/mL) | Ref. |
|---|---|---|---|
| HPLC-UV | 10,000–100,000 | 1119 | [10] |
| HPLC-UV | 200–20,000 | 10 | [11] |
| UHPLC-UV | 50–1250 | 27 | [12] |
| UHPLC-QE HF HRMS | 1–100 | 0.3 | [13] |
| UV-visible spectrophotometry | 5000–25,000 | – | [14] |
| UV-visible spectrophotometry | 500–30,000 | 163 | [15] |
| Fluorescence spectroscopy | 11,900–21,420 | 333 | [16] |
| Fluorescence spectroscopy | 500–8000 | 140 | [17] |
| Fluorescence spectroscopy | 570–28,580 | 10 | [18] |
| Optical detection | 200–19,040 | 200 | [19] |
| Optical detection | 21.4–38,080 | 21.4 | [20] |
| SERS | 500–10,000 | 50 | [21] |
| SERS | 50–1000 | 14 | [22] |
| SERS | 5–500 | 5 | [23] |
| Voltammetry | 12–54,740 | 2.4 | [24] |
| Voltammetry | 3.6–59,262 | 3.6 | [25] |
| Voltammetry | 12–130,900 | 4.4 | [26] |
| Voltammetry | 8.3–160,007 | 2 | [27] |
| Voltammetry | 119–28,560 | 11.4 | [28] |
| CL | 4.0–400.0 | 1.15 | This method |
| Proposed Method | Reported Method [17] | |||
|---|---|---|---|---|
| Concentration Added (ng/mL) | Recovery (%) | Precision (RSD, %) | Concentration Added (ng/mL) | Recovery (%) |
| 0 | Not detected | – | 0 | Not detected |
| 40.0 | 103.1 | 2.3 | 500.0 | 96.4 |
| 80.0 | 99.5 | 3.5 | 1000.0 | 102.3 |
| 400.0 | 97.5 | 4.4 | 1500.0 | 95.7 |
| Mean recovery ± S.D. | 100.03 ± 2.83 | 98.13 ± 3.62 | ||
| t-test (2.776) * | 0.71 | |||
| F-test (19.00) * | 1.63 | |||
| Attribute | Result | Color | Pictogram |
|---|---|---|---|
| Quantitative | Moderate blue | |
| Single element | While |  |
| Simple instruments | Moderate blue | |
| 1 | White | |
| Simple, low-cost sample preparation required | Moderate blue | |
| 5–10 | Moderate blue | |
| Common commercially available reagents | Dark blue | |
| Not needed | Dark blue | |
| Manual treatment and analysis | White | |
| Less than 10 mL of the food sample | Dark blue | |
| Overall score | 67.5 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Maghrabey, M.; Abdel-Hakim, A.; Tagaya, S.; Kuroda, N.; Kishikawa, N. Utility of the Redox Cycle of Nitrofurantoin for the Development of a New Chemiluminescence Method for Its Analysis in Milk Samples. Molecules 2025, 30, 3698. https://doi.org/10.3390/molecules30183698
El-Maghrabey M, Abdel-Hakim A, Tagaya S, Kuroda N, Kishikawa N. Utility of the Redox Cycle of Nitrofurantoin for the Development of a New Chemiluminescence Method for Its Analysis in Milk Samples. Molecules. 2025; 30(18):3698. https://doi.org/10.3390/molecules30183698
Chicago/Turabian StyleEl-Maghrabey, Mahmoud, Ali Abdel-Hakim, Shiho Tagaya, Naotaka Kuroda, and Naoya Kishikawa. 2025. "Utility of the Redox Cycle of Nitrofurantoin for the Development of a New Chemiluminescence Method for Its Analysis in Milk Samples" Molecules 30, no. 18: 3698. https://doi.org/10.3390/molecules30183698
APA StyleEl-Maghrabey, M., Abdel-Hakim, A., Tagaya, S., Kuroda, N., & Kishikawa, N. (2025). Utility of the Redox Cycle of Nitrofurantoin for the Development of a New Chemiluminescence Method for Its Analysis in Milk Samples. Molecules, 30(18), 3698. https://doi.org/10.3390/molecules30183698








