Designing a Small Molecule for PET Radiotracing: [18F]MC225 in Human Trials for Early Diagnosis in CNS Pathologies
Abstract
1. Introduction
2. Localization, Structure, and P-gp Activity
2.1. Localization
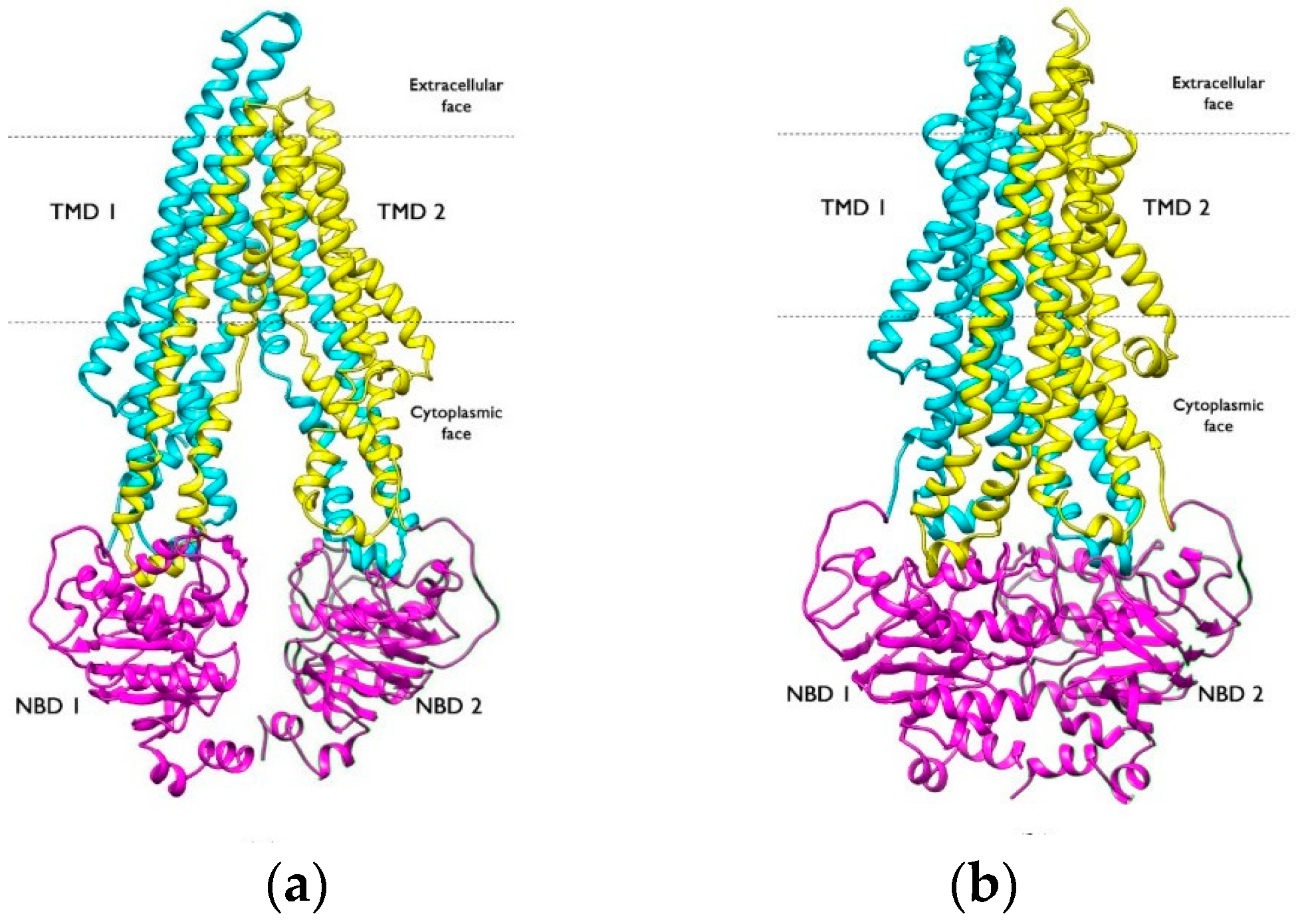
2.2. Structure
3. Role of P-gp in CNS Diseases
3.1. Role of P-gp in Alzheimer’s Disease
3.2. Role of P-gp in Parkinson’s Disease
3.3. Role of P-gp in Other Diseases
4. P-gp and Multidrug Resistance (MDR)
5. PET Imaging and the Importance of P-gp
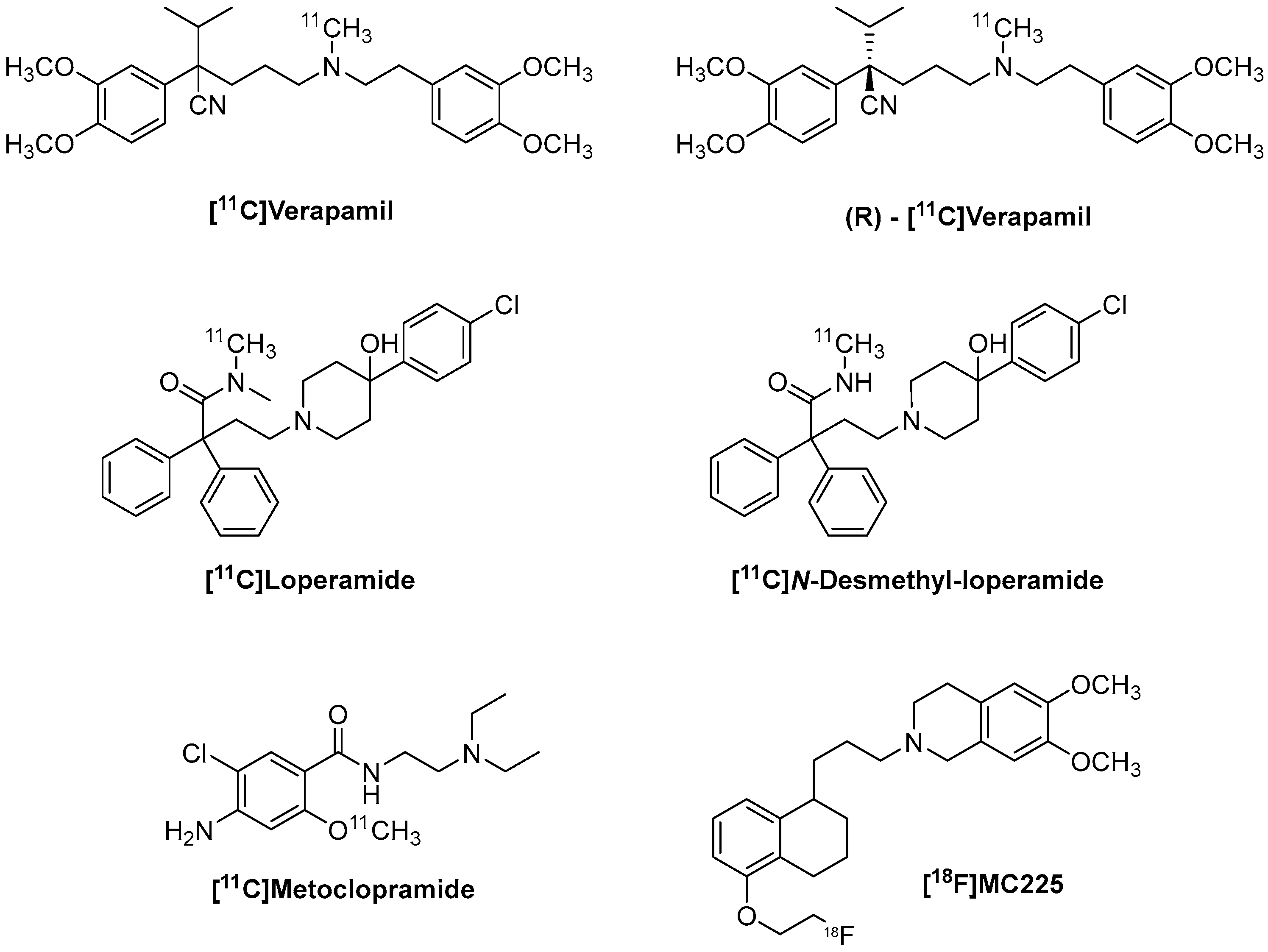
6. [11C]Verapamil and (R)-[11C]Verapamil
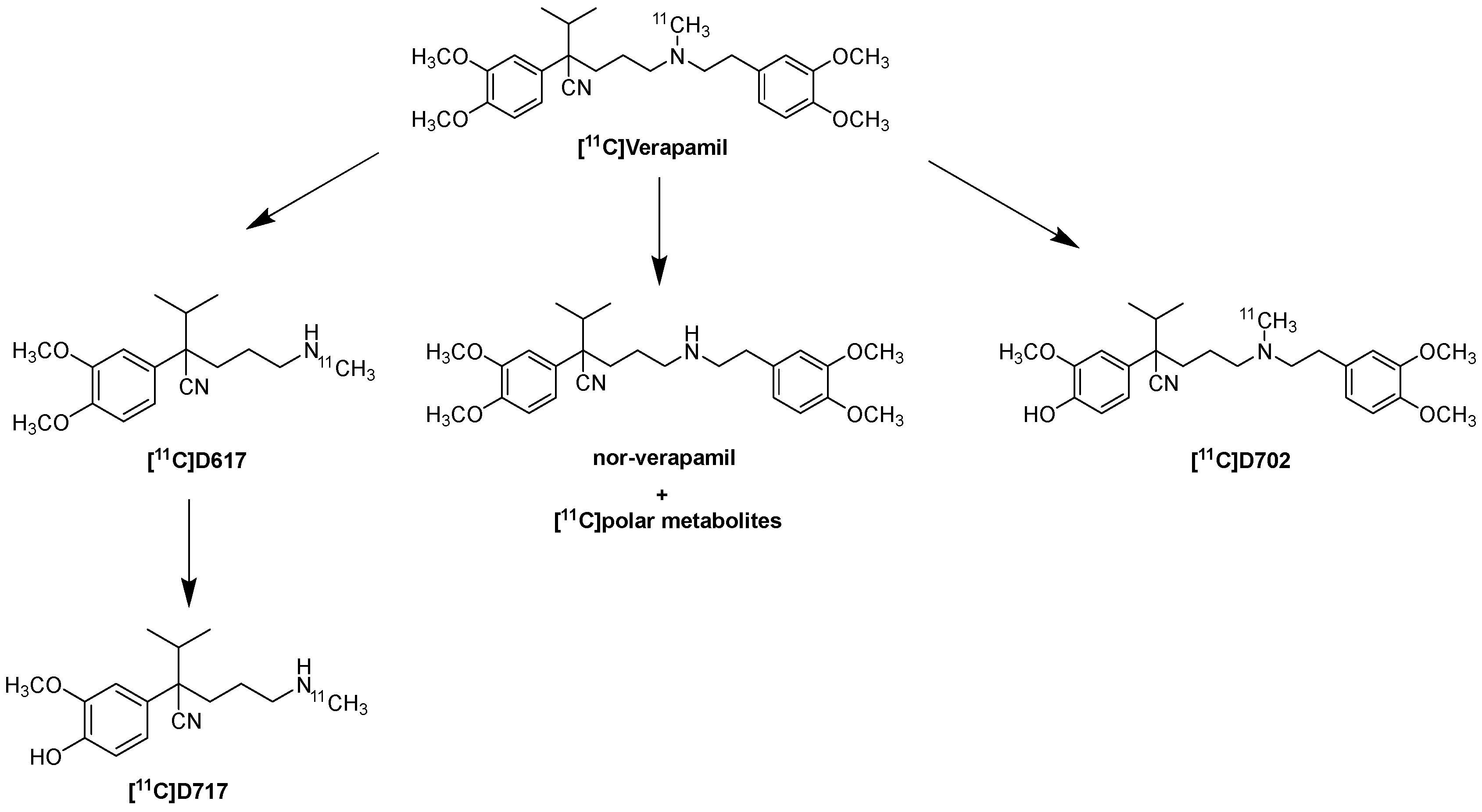
6.1. Metabolism and Kinetic Evaluation of [11C]Verapamil and (R)-[11C]Verapamil
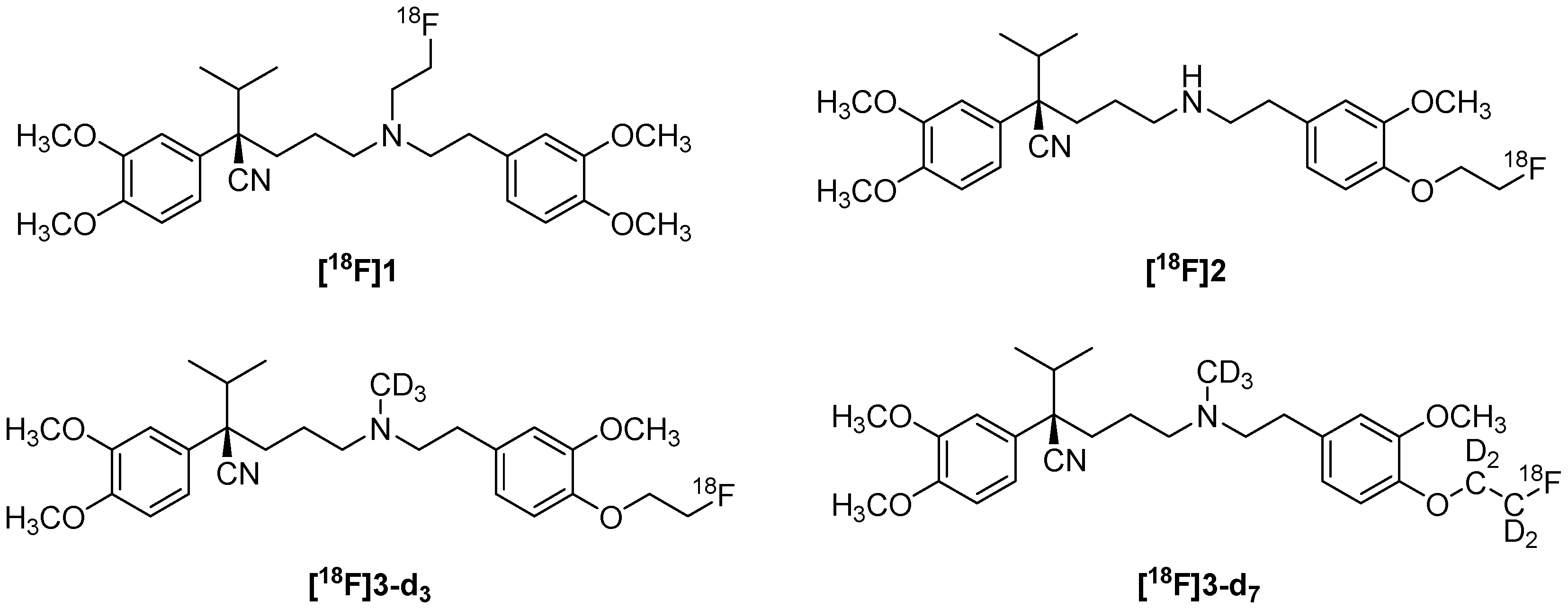
6.2. Fluorinated Verapamil Analogues
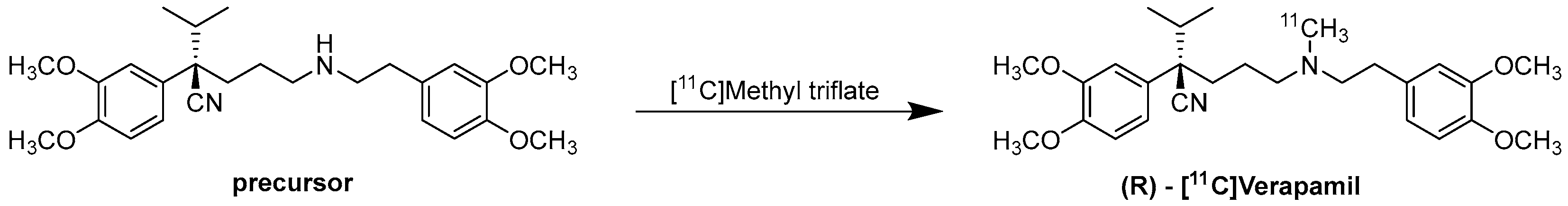
6.3. Radiochemistry of (R)-[11C]Verapamil
7. [11C]Loperamide and [11C]-N-Desmethyl-Loperamide
Radiochemistry of [11C]-N-Desmethyl-Loperamide
8. [11C]Metoclopramide
Radiochemistry of [11C]Metoclopramide
9. [18F]MC225
9.1. Radiochemistry of [18F]MC225
9.2. Clinical Trials in Progress
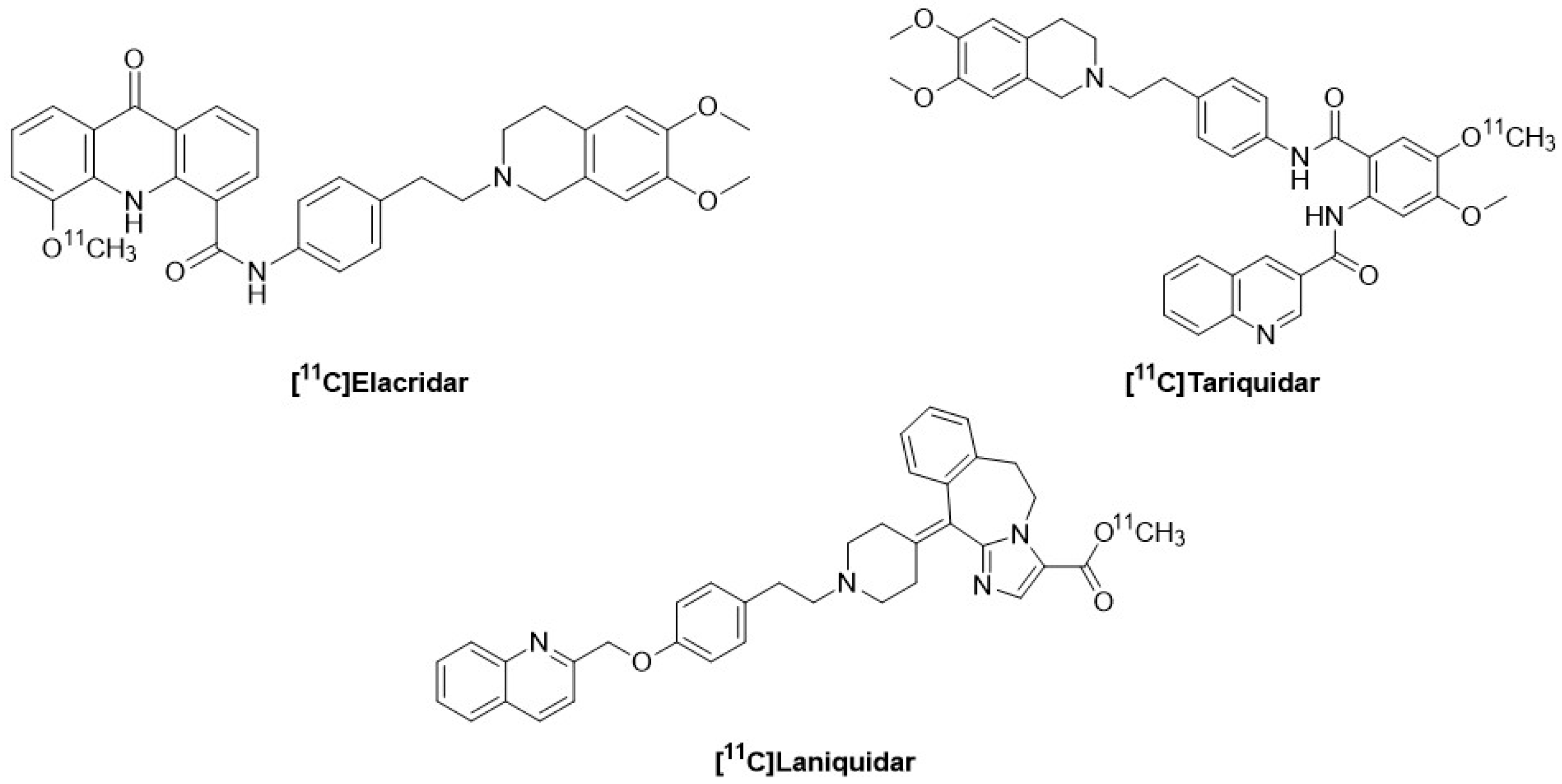
10. [11C]-Labeled P-gp Inhibitors: Tariquidar, Elacridar, Laniquidar
11. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xing, J.; Huang, S.; Heng, Y.; Mei, H.; Pan, X. Computational Insights into Allosteric Conformational Modulation of P-Glycoprotein by Substrate and Inhibitor Binding. Molecules 2020, 25, 6006. [Google Scholar] [CrossRef]
- Halder, J.; Pradhan, D.; Kar, B.; Ghosh, G.; Rath, G. Nanotherapeutics approaches to overcome P-glycoprotein-mediated multi-drug resistance in cancer. Nanomed. Nanotechnol. Biol. Med. 2022, 40, 102494. [Google Scholar] [CrossRef]
- Mairinger, S.; Wanek, T.; Kuntner, C.; Doenmez, Y.; Strommer, S.; Stanek, J.; Capparelli, E.; Chiba, P.; Muller, M.; Colabufo, N.A.; et al. Synthesis and preclinical evaluation of the radiolabeled P-glycoprotein inhibitor [11C]MC113. Nucl. Med. Biol. 2012, 39, 1219–1225. [Google Scholar] [CrossRef]
- Van Waarde, A.; Ramakrishnan, N.K.; Rybczynska, A.A.; Elsinga, P.H.; Berardi, F.; De Jong, J.R.; Kwizera, C.; Perrore, R.; Cantore, M.; Sijbesma, J.W.; et al. Synthesis and Preclinical Evaluation of Novel PET Probes for P-Glycoprotein Function and Expression. J. Med. Chem. 2009, 52, 4524–4532. [Google Scholar] [CrossRef]
- Silva, R.; Vilas-Boas, V.; Carmo, H.; Dinis-Oliveira, R.J.; Carvalho, F.; De Lourdes Bastos, M.; Fernando, R. Modulation of P-glycoprotein efflux pump: Induction and activation as a therapeutic strategy. Pharmacol. Ther. 2015, 149, 1–123. [Google Scholar] [CrossRef]
- Ohashi, R.; Watanabe, R.; Esaki, T.; Taniguchi, T.; Torimoto-Katori, N.; Watanabe, T.; Ogasawara, Y.; Takayashi, T. Development of Simplified in Vitro P-Glycoprotein Substrate Assay and in Silico Prediction Models To Evaluate Transport Potential of P-Glycoprotein. Mol. Pharm. 2019, 16, 1851–1863. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-T.-L.; Duong, V.-A.; Maeng, H.-J. Pharmaceutical Formulations with P-Glycoprotein Inhibitory Effect as Promising Approaches for Enhancing Oral Drug Absorption and Bioavailability. Pharmaceutics 2021, 13, 1103. [Google Scholar] [CrossRef] [PubMed]
- Husain, A.; Makadia, V.; Valicherla, G.R.; Riyazuddin, M.; Gayen, J.R. Approaches to minimize the effects of P-glycoprotein in drug transport: A review. Drug Dev. Res. 2022, 83, 825–841. [Google Scholar] [CrossRef]
- Staud, F.; Ceckova, M.; Micuda, S.; Pavek, P. Expression and Function of P-Glycoprotein in Normal Tissues: Effect on Pharmacokinetics. In Multi-Drug Resistance in Cancer; Zhou, J., Ed.; Humana Press: Totowa, NJ, USA, 2010; pp. 199–222. Available online: http://link.springer.com/10.1007/978-1-60761-416-6_10 (accessed on 15 April 2024).
- Milojkovic, M.; Milacic, N.; Radovic, J.; Ljubisavljevic, S. MDR1 gene polymorphisms and P-glycoprotein expression in respiratory diseases. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 2015, 159, 341–346. [Google Scholar] [CrossRef]
- Mairinger, S.; Hernández-Lozano, I.; Filip, T.; Sauberer, M.; Löbsch, M.; Stanek, J.; Wanek, T.; Sake, J.A.; Pekar, T.; Ehrhardt, C.; et al. Impact of P-gp and BCRP on pulmonary drug disposition assessed by PET imaging in rats. J. Control. Release 2022, 349, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Al-Jayyoussi, G.; Price, D.F.; Francombe, D.; Taylor, G.; Smith, M.W.; Morris, C.; Edwards, C.D.; Eddershaw, P.; Gumbleton, M. Selectivity in the impact of P-glycoprotein upon pulmonary absorption of airway-dosed substrates: A study in ex vivo lung models using chemical inhibition and genetic knockout. J. Pharm. Sci. 2013, 102, 3382–3394. [Google Scholar] [CrossRef]
- Kozlosky, D.; Barrett, E.; Aleksunes, L.M. Regulation of Placental Efflux Transporters during Pregnancy Complications. Drug Metab. Dispos. 2022, 50, 1364–1375. [Google Scholar] [CrossRef]
- Gil, S.; Saura, R.; Forestier, F.; Farinotti, R. P-glycoprotein expression of the human placenta during pregnancy. Placenta 2005, 26, 268–270. [Google Scholar] [CrossRef]
- Eyal, S.; Chung, F.S.; Muzi, M.; Link, J.M.; Mankoff, D.A.; Kaddoumi, A.; O’ Sullivan, F.; Hebert, M.F.; Unadkat, J.D. Simultaneous PET Imaging of P-Glycoprotein Inhibition in Multiple Tissues in the Pregnant Nonhuman Primate. J. Nucl. Med. 2009, 50, 798–806. [Google Scholar] [CrossRef]
- Liu, W.; Mossel, P.; Schwach, V.; Slart, R.H.J.A.; Luurtsema, G. Cardiac PET Imaging of ATP Binding Cassette (ABC) Transporters: Opportunities and Challenges. Pharmaceuticals 2023, 16, 1715. [Google Scholar] [CrossRef] [PubMed]
- Solbach, T.; Konig, J.; Fromm, M.; Zolk, O. ATP-Binding Cassette Transporters in the Heart. Trends Cardiovasc. Med. 2006, 16, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Jaitak, V. Natural products as multidrug resistance modulators in cancer. Eur. J. Med. Chem. 2019, 176, 268–291. [Google Scholar] [CrossRef]
- Mora Lagares, L.; Pérez-Castillo, Y.; Minovski, N.; Novič, M. Structure–Function Relationships in the Human P-Glycoprotein (ABCB1): Insights from Molecular Dynamics Simulations. Int. J. Mol. Sci. 2021, 23, 362. [Google Scholar] [CrossRef]
- Mora Lagares, L.; Novič, M. Recent Advances on P-Glycoprotein (ABCB1) Transporter Modelling with In Silico Methods. Int. J. Mol. Sci. 2022, 23, 14804. [Google Scholar] [CrossRef] [PubMed]
- Ahmed Juvale, I.I.; Abdul Hamid, A.A.; Abd Halim, K.B.; Che Has, A.T. P-glycoprotein: New insights into structure, physiological function, regulation and alterations in disease. Heliyon 2022, 8, e09777. [Google Scholar] [CrossRef]
- Aller, S.G.; Yu, J.; Ward, A.; Weng, Y.; Chittaboina, S.; Zhuo, R.; Harrell, P.M.; Trinh, Y.T.; Zhang, Q.; Urbatsch, I.L.; et al. Structure of P-Glycoprotein Reveals a Molecular Basis for Poly-Specific Drug Binding. Science 2009, 323, 1718–1722. [Google Scholar] [CrossRef]
- Mukhametov, A.; Raevsky, O.A. On the mechanism of substrate/non-substrate recognition by P-glycoprotein. J. Mol. Graph. Model. 2017, 71, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Mollazadeh, S.; Sahebkar, A.; Hadizadeh, F.; Behravan, J.; Arabzadeh, S. Structural and functional aspects of P-glycoprotein and its inhibitors. Life Sci. 2018, 214, 118–123. [Google Scholar] [CrossRef]
- Gil-Martins, E.; Barbosa, D.J.; Silva, V.; Remião, F.; Silva, R. Dysfunction of ABC transporters at the blood-brain barrier: Role in neurological disorders. Pharmacol. Ther. 2020, 213, 107554. [Google Scholar] [CrossRef]
- Qosa, H.; Miller, D.S.; Pasinelli, P.; Trotti, D. Regulation of ABC efflux transporters at blood-brain barrier in health and neurological disorders. Brain Res. 2015, 1628, 298–316. [Google Scholar] [CrossRef] [PubMed]
- Rapposelli, S.; Digiacomo, M.; Balsamo, A. P-gp Transporter and its Role in Neurodegenerative Diseases. CTMC 2009, 9, 209–217. [Google Scholar] [CrossRef]
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chételat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer disease. Nat. Rev. Dis. Primers 2021, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- De-Paula, V.J.; Radanovic, M.; Diniz, B.S.; Forlenza, O.V. Alzheimer’s Disease. In Protein Aggregation and Fibrillogenesis in Cerebral and Systemic Amyloid Disease; Harris, J.R., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 329–352. Available online: https://link.springer.com/10.1007/978-94-007-5416-4_14 (accessed on 23 April 2024).
- Soria Lopez, J.A.; González, H.M.; Léger, G.C. Alzheimer’s disease. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 231–255. Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780128047668000133 (accessed on 23 April 2024).
- Jeynes, B.; Provias, J. An investigation into the role of P-glycoprotein in Alzheimer’s disease lesion pathogenesis. Neurosci. Lett. 2011, 487, 389–393. [Google Scholar] [CrossRef]
- Bartels, A.L. Blood-Brain Barrier P-Glycoprotein Function in Neurodegenerative Disease. CPD 2011, 17, 2771–2777. [Google Scholar] [CrossRef]
- Lam, F.C.; Liu, R.; Lu, P.; Shapiro, A.B.; Renoir, J.; Sharom, F.J.; Reiner, P.B. β-Amyloid efflux mediated by p-glycoprotein. J. Neurochem. 2001, 76, 1121–1128. [Google Scholar] [CrossRef]
- Xing, Z.-K.; Du, L.-S.; Fang, X.; Liang, H.; Zhang, S.-N.; Shi, L.; Chun-Xiang, K.; Tian-Xiong, H.; Qing, Y. The relationship among amyloid-β deposition, sphingomyelin level, and the expression and function of P-glycoprotein in Alzheimer’s disease pathological process. Neural Regen. Res. 2023, 18, 1300. [Google Scholar] [PubMed]
- Jeynes, B.; Provias, J. P-Glycoprotein Altered Expression in Alzheimer’s Disease: Regional Anatomic Variability. J. Neurodegener. Dis. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Ding, Y.; Zhong, Y.; Baldeshwiler, A.; Abner, E.L.; Bauer, B.; Hartz, A.M.S. Protecting P-glycoprotein at the blood–brain barrier from degradation in an Alzheimer’s disease mouse model. Fluids Barriers CNS 2021, 18, 10. [Google Scholar] [CrossRef]
- Pan, Y.; Nicolazzo, J.A. Impact of aging, Alzheimer’s disease and Parkinson’s disease on the blood-brain barrier transport of therapeutics. Adv. Drug Deliv. Rev. 2018, 135, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Shin, J.-Y.; Lee, Y.-S.; Yun, S.P.; Maeng, H.-J.; Lee, Y. Brain Endothelial P-Glycoprotein Level Is Reduced in Parkinson’s Disease via a Vitamin D Receptor-Dependent Pathway. Int. J. Mol. Sci. 2020, 21, 8538. [Google Scholar] [CrossRef]
- Desai, B.S.; Monahan, A.J.; Carvey, P.M.; Hendey, B. Blood–Brain Barrier Pathology in Alzheimer’s and Parkinson’s Disease: Implications for Drug Therapy. Cell Transpl. 2007, 16, 285–299. [Google Scholar] [CrossRef]
- Pizarro-Galleguillos, B.M.; Kunert, L.; Brüggemann, N.; Prasuhn, J. Neuroinflammation and Mitochondrial Dysfunction in Parkinson’s Disease: Connecting Neuroimaging with Pathophysiology. Antioxidants 2023, 12, 1411. [Google Scholar] [CrossRef] [PubMed]
- Colabufo, N.A.; Berardi, F.; Cantore, M.; Contino, M.; Inglese, C.; Niso, M.; Perrone, R. Perspectives of P-Glycoprotein Modulating Agents in Oncology and Neurodegenerative Diseases: Pharmaceutical, Biological, and Diagnostic Potentials. J. Med. Chem. 2010, 53, 1883–1897. [Google Scholar] [CrossRef]
- Bartels, A.L.; Van Berckel, B.N.M.; Lubberink, M.; Luurtsema, G.; Lammertsma, A.A.; Leenders, K.L. Blood–brain barrier P-glycoprotein function is not impaired in early Parkinson’s disease. Park. Relat. Disord. 2008, 14, 505–508. [Google Scholar] [CrossRef]
- Hou, J.; Liu, Q.; Li, Y.; Sun, H.; Zhang, J. An In Vivo Microdialysis Study of FLZ Penetration through the Blood-Brain Barrier in Normal and 6-Hydroxydopamine Induced Parkinson’s Disease Model Rats. BioMed Res. Int. 2014, 2014, 1–11. [Google Scholar]
- Bartels, A.L.; Kortekaas, R.; Bart, J.; Willemsen, A.T.M.; De Klerk, O.L.; De Vries, J.J.; Joost, C.H.; Leenders, K.L. Blood–brain barrier P-glycoprotein function decreases in specific brain regions with aging: A possible role in progressive neurodegeneration. Neurobiol. Aging 2009, 30, 1818–1824. [Google Scholar] [CrossRef]
- Kao, Y.-H.; Chern, Y.; Yang, H.-T.; Chen, H.-M.; Lin, C.-J. Regulation of P-glycoprotein expression in brain capillaries in Huntington’s disease and its impact on brain availability of antipsychotic agents risperidone and paliperidone. J. Cereb. Blood Flow. Metab. 2016, 36, 1412–1423. [Google Scholar] [CrossRef]
- De Klerk, O.L.; Willemsen, A.T.M.; Bosker, F.J.; Bartels, A.L.; Hendrikse, N.H.; Den Boer, J.A.; Dierck, R.A. Regional increase in P-glycoprotein function in the blood-brain barrier of patients with chronic schizophrenia. Psychiatry Res. Neuroimaging 2010, 183, 151–156. [Google Scholar] [CrossRef]
- Toyohara, J. Importance P-Gp PET Imaging Pharmacology. CPD 2016, 22, 5830–5836. [Google Scholar] [CrossRef]
- De Klerk, O.L.; Willemsen, A.T.M.; Roosink, M.; Bartels, A.L.; Harry Hendrikse, N.; Bosker, F.J. Locally increased P-glycoprotein function in major depression: A PET study with [11C]verapamil as a probe for P-glycoprotein function in the blood–brain barrier. Int. J. Neuropsychopharm 2009, 12, 895. [Google Scholar] [CrossRef] [PubMed]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef] [PubMed]
- Wanek, T.; Kuntner, C.; Bankstahl, J.P.; Bankstahl, M.; Stanek, J.; Sauberer, M.; Mairinger, S.; Strommer, S.; Wacheck, V.; Loscher, W.; et al. A comparative small-animal PET evaluation of [11C]tariquidar, [11C]elacridar and (R)-[11C]verapamil for detection of P-glycoprotein-expressing murine breast cancer. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 149–159. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carocci, A.; Catalano, A.; Turi, F.; Lovece, A.; Cavalluzzi, M.M.; Bruno, C.; Colabufo, N.A.; Contino, M.; Perrone, M.G.; Franchini, C.; et al. Stereoselective Modulation of P-Glycoprotein by Chiral Small Molecules. ChemMedChem 2016, 11, 93–101. [Google Scholar] [CrossRef]
- Palmeira, A.; Sousa, E.; Helena Vasconcelos, M.; Pinto, M.; Fernandes, M.X. Structure and Ligand-based Design of P-glycoprotein Inhibitors: A Historical Perspective. Curr. Pharm. Des. 2012, 18, 4197–4214. [Google Scholar] [CrossRef]
- Pettersson, M.; Hou, X.; Kuhn, M.; Wager, T.T.; Kauffman, G.W.; Verhoest, P.R. Quantitative Assessment of the Impact of Fluorine Substitution on P-Glycoprotein (P-gp) Mediated Efflux, Permeability, Lipophilicity, and Metabolic Stability. J. Med. Chem. 2016, 59, 5284–5296. [Google Scholar] [CrossRef]
- Dong, J.; Qin, Z.; Zhang, W.-D.; Cheng, G.; Yehuda, A.G.; Ashby, C.R.; Chen, Z.-S.; Cheng, X.-D.; Qin, J.-J. Medicinal chemistry strategies to discover P-glycoprotein inhibitors: An update. Drug Resist. Updates 2020, 49, 100681. [Google Scholar] [CrossRef]
- Waarde, A.V. Introduction on PET: Description of Basics and Principles. In Trends on the Role of PET in Drug Development; World Scientific: London, UK, 2012; pp. 1–13. Available online: http://www.worldscientific.com/doi/abs/10.1142/9789814317740_0001 (accessed on 23 April 2024).
- Van Den Hoff, J. Principles of quantitative positron emission tomography. Amino Acids 2005, 29, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Haider, A.; Chen, J.; Xiao, Z.; Gobbi, L.; Honer, M.; Grether, U.; Arnold, S.E.; Josephson, L.; Liang, S.H. The Repertoire of Small-Molecule PET Probes for Neuroinflammation Imaging: Challenges and Opportunities beyond TSPO. J. Med. Chem. 2021, 64, 17656–17689. [Google Scholar] [CrossRef] [PubMed]
- Iking, J.; Staniszewska, M.; Kessler, L.; Klose, J.M.; Lückerath, K.; Fendler, W.P.; Herrmann, K.; Rischpler, C. Imaging Inflammation with Positron Emission Tomography. Biomedicines 2021, 9, 212. [Google Scholar] [CrossRef] [PubMed]
- West, C.M.L.; Jones, T.; Price, P. The potential of positron-emission tomography to study anticancer-drug resistance. Nat. Rev. Cancer 2004, 4, 457–469. [Google Scholar] [CrossRef]
- Nerella, S.G.; Singh, P.; Sanam, T.; Digwal, C.S. PET Molecular Imaging in Drug Development: The Imaging and Chemistry Perspective. Front. Med. 2022, 9, 812270. [Google Scholar] [CrossRef]
- Ehman, E.C.; Johnson, G.B.; Villanueva-Meyer, J.E.; Cha, S.; Leynes, A.P.; Larson, P.E.Z.; Hope, T.A. PET/MRI: Where might it replace PET/CT? Magn. Reson. Imaging 2017, 46, 1247–1262. [Google Scholar] [CrossRef]
- Syvänen, S.; Eriksson, J. Advances in PET Imaging of P-Glycoprotein Function at the Blood-Brain Barrier. ACS Chem. Neurosci. 2013, 4, 225–237. [Google Scholar] [CrossRef]
- Van Dongen, G.A.M.S.; Visser, G.W.M.; Lub-de Hooge, M.N.; De Vries, E.G.; Perk, L.R. Immuno-PET: A Navigator in Monoclonal Antibody Development and Applications. Oncologist 2007, 12, 1379–1389. [Google Scholar] [CrossRef]
- Serdons, K.; Verbruggen, A.; Bormans, G.M. Developing new molecular imaging probes for PET. Methods 2009, 48, 104–111. [Google Scholar] [CrossRef]
- Miller, P.W.; Long, N.J.; Vilar, R.; Gee, A.D. Synthesis of 11C, 18F, 15O, and 13N Radiolabels for Positron Emission Tomography. Angew. Chem. Int. Ed. 2008, 47, 8998–9033. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Conti, P.S. Radiopharmaceutical chemistry for positron emission tomography. Adv. Drug Deliv. Rev. 2010, 62, 1031–1051. [Google Scholar] [CrossRef]
- Elsinga, P.; Dierckx, R. Small Molecule PET-Radiopharmaceuticals. CPD 2014, 20, 2268–2274. [Google Scholar] [CrossRef]
- Vaalburg, W.; Hendrikse, N.; Elsinga, P.; Bart, J.; Vanwaarde, A. P-glycoprotein activity and biological response. Toxicol. Appl. Pharmacol. 2005, 207, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Elsinga, P.; Hendrikse, N.; Bart, J.; Vaalburg, W.; Waarde, A. PET Studies on P-Glycoprotein Function in the Blood-Brain Barrier: How it Affects Uptake and Binding of Drugs within the CNS. CPD 2004, 10, 1493–1503. [Google Scholar] [CrossRef]
- Hendrikse, N.H.; Schinkel, A.H.; De Vries, E.G.E.; Fluks, E.; Van Der Graaf, W.T.A.; Willemsen, A.T.M.; Vaalburg, W.; Franssen, E.J. Complete in vivo reversal of P-glycoprotein pump function in the blood-brain barrier visualized with positron emission tomography. Br. J. Pharmacol. 1998, 124, 1413–1418. [Google Scholar] [CrossRef]
- Unadkat, J.D.; Ke, B.; Eyal, S. The Impact of the BBB on the Pharmacokinetics of Drugs: Investigations by PET. In Trends on the Role of PET in Drug Development; World Scientific: London, UK, 2012; pp. 575–614. Available online: http://www.worldscientific.com/doi/abs/10.1142/9789814317740_0021 (accessed on 11 May 2024).
- Marie, S.; Tournier, N. Imagerie TEP pour l’étude des répercussions fonctionnelles de la P-glycoprotéine en neuropharmacocinétique. Therapies 2020, 75, 623–632. [Google Scholar] [CrossRef]
- Hanke, N.; Türk, D.; Selzer, D.; Wiebe, S.; Fernandez, É.; Stopfer, P.; Nock, V.; Lehr, T. A Mechanistic, Enantioselective, Physiologically Based Pharmacokinetic Model of Verapamil and Norverapamil, Built and Evaluated for Drug–Drug Interaction Studies. Pharmaceutics 2020, 12, 556. [Google Scholar] [CrossRef]
- Arakawa, R.; Ito, H.; Okumura, M.; Morimoto, T.; Seki, C.; Takahashi, H. No inhibitory effect on P-glycoprotein function at blood–brain barrier by clinical dose of clarithromycin: A human PET study with [11C]verapamil. Ann. Nucl. Med. 2010, 24, 83–87. [Google Scholar] [CrossRef]
- Toyohara, J.; Okamoto, M.; Aramaki, H.; Zaitsu, Y.; Shimizu, I.; Ishiwata, K. (R)-[11C]Emopamil as a novel tracer for imaging enhanced P-glycoprotein function. Nucl. Med. Biol. 2016, 43, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Abrahim, A.; Luurtsema, G.; Bauer, M.; Karch, R.; Lubberink, M.; Pataraia, E. Peripheral metabolism of (R)-[11C]verapamil in epilepsy patients. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 116–123. [Google Scholar] [CrossRef]
- Robinson, M.A.; Mehvar, R. Enantioselective Distribution of Verapamil and Norverapamil into Human and Rat Erythrocytes: The Role of Plasma Protein Binding. Biopharm. Drug Dispos. 1996, 17, 577–587. [Google Scholar] [CrossRef]
- Waldegger, S.; Niemeyer, G.; Mörike, K.; Wagner, C.A.; Suessbrich, H.; Busch, A.E.; Lang, F.; Eichelbaum, M. Effect of Verapamil Enantiomers and Metabolites on Cardiac K+ Channels Expressed in Xenopus Oocytes. Cell Physiol. Biochem. 1999, 9, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Luurtsema, G.; Molthoff, C.F.M.; Windhorst, A.D.; Smit, J.W.; Keizer, H.; Boellaard, R. (R)- and (S)-[11C]verapamil as PET-tracers for measuring P-glycoprotein function: In vitro and in vivo evaluation. Nucl. Med. Biol. 2003, 30, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Takashima, T.; Yokoyama, C.; Mizuma, H.; Yamanaka, H.; Wada, Y.; Onoe, K. Developmental Changes in P-Glycoprotein Function in the Blood–Brain Barrier of Nonhuman Primates: PET Study with R-11C-Verapamil and 11C-Oseltamivir. J. Nucl. Med. 2011, 52, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Verbeek, J.; Syvänen, S.; Schuit, R.C.; Eriksson, J.; De Lange, E.C.; Windhorst, A.D. Synthesis and preclinical evaluation of [11C]D617, a metabolite of (R)-[11C]verapamil. Nucl. Med. Biol. 2012, 39, 530–539. [Google Scholar] [CrossRef]
- Luurtsema, G.; Molthoff, C.F.M.; Schuit, R.C.; Windhorst, A.D.; Lammertsma, A.A.; Franssen, E.J.F. Evaluation of (R)-[11C]verapamil as PET tracer of P-glycoprotein function in the blood–brain barrier: Kinetics and metabolism in the rat. Nucl. Med. Biol. 2005, 32, 87–93. [Google Scholar] [CrossRef]
- Lubberink, M.; Luurtsema, G.; Van Berckel, B.N.; Boellaard, R.; Toornvliet, R.; Windhorst, A.D. Evaluation of Tracer Kinetic Models for Quantification of P-Glycoprotein Function using (R)-[11C]Verapamil and PET. J. Cereb. Blood Flow. Metab. 2007, 27, 424–433. [Google Scholar] [CrossRef]
- Ikoma, Y.; Takano, A.; Ito, H.; Kusuhara, H.; Sugiyama, Y.; Arakawa, R.; Fukumura, T.; Nakao, R.; Suzuki, K.; Suhara, T. Quantitative analysis of 11C-verapamil transfer at the human blood-brain barrier for evaluation of P-glycoprotein function. J. Nucl. Med. 2006, 47, 1531–1537. [Google Scholar] [PubMed]
- Lee, Y.-J.; Maeda, J.; Kusuhara, H.; Okauchi, T.; Inaji, M.; Nagai, Y. In Vivo Evaluation of P-glycoprotein Function at the Blood-Brain Barrier in Nonhuman Primates Using [11C]Verapamil. J. Pharmacol. Exp. Ther. 2006, 316, 647–653. [Google Scholar] [CrossRef]
- Wanek, T.; Mairinger, S.; Langer, O. Radioligands targeting P-glycoprotein and other drug efflux proteins at the blood–brain barrier. Label. Comp. Radiopharm. 2013, 56, 68–77. [Google Scholar] [CrossRef]
- Sasongko, L.; Link, J.; Muzi, M.; Mankoff, D.; Yang, X.; Collier, A.; Shoner, S.C.; Unadkat, J.U. Imaging P-glycoprotein transport activity at the human blood-brain barrier with positron emission tomography. Clin. Pharmacol. Ther. 2005, 77, 503–514. [Google Scholar] [CrossRef]
- Bart, J.; Willemsen, A.T.M.; Groen, H.J.M.; Van Der Graaf, W.T.A.; Wegman, T.D.; Vaalburg, W. Quantitative assessment of P-glycoprotein function in the rat blood–brain barrier by distribution volume of [11C]verapamil measured with PET. NeuroImage 2003, 20, 1775–1782. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.C.; Simpson, M.; Zeitlinger, M.; Bauer, M.; Karch, R.; Abrahim, A.; Feurstein, T.; Schütz, M.; Kletter, K.; Müller, M.; et al. A Combined Accelerator Mass Spectrometry-Positron Emission Tomography Human Microdose Study with 14C- and 11C-Labelled Verapamil. Clin. Pharmacokinet. 2011, 50, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Toornvliet, R.; Vanberckel, B.; Luurtsema, G.; Lubberink, M.; Geldof, A.; Bosch, T. Effect of age on functional P-glycoprotein in the blood-brain barrier measured by use of (R)-[11C]verapamil and positron emission tomography. Clin. Pharmacol. Ther. 2006, 79, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Takano, A.; Kusuhara, H.; Suhara, T.; Ieiri, I.; Morimoto, T.; Lee, Y.-J.; Maeda, J.; Ikoma, Y.; Ito, H.; Suzuki, K.; et al. Evaluation of in vivo P-glycoprotein function at the blood-brain barrier among MDR1 gene polymorphisms by using 11C-verapamil. J. Nucl. Med. 2006, 47, 1427–1433. [Google Scholar]
- Ghosh, K.K.; Padmanabhan, P.; Yang, C.-T.; Mishra, S.; Halldin, C.; Gulyás, B. Dealing with PET radiometabolites. EJNMMI Res. 2020, 10, 109. [Google Scholar] [CrossRef]
- Raaphorst, R.M.; Luurtsema, G.; Schuit, R.C.; Kooijman, E.J.M.; Elsinga, P.H.; Lammertsma, A.A.; Windhorst, A.D. Synthesis and Evaluation of New Fluorine-18 Labeled Verapamil Analogs To Investigate the Function of P-Glycoprotein in the Blood–Brain Barrier. ACS Chem. Neurosci. 2017, 8, 1925–1936. [Google Scholar] [CrossRef]
- Raaphorst, R.M.; Luurtsema, G.; Schokker, C.J.; Attia, K.A.; Schuit, R.C.; Elsinga, P.H.; Lammertsma, A.A.; Windhorstl, A.D. Improving metabolic stability of fluorine-18 labeled verapamil analogs. Nucl. Med. Biol. 2018, 64–65, 47–56. [Google Scholar] [CrossRef]
- Bankstahl, J.P.; Kuntner, C.; Abrahim, A.; Karch, R.; Stanek, J.; Wanek, T. Tariquidar-Induced P-Glycoprotein Inhibition at the Rat Blood–Brain Barrier Studied with (R)-11C-Verapamil and PET. J. Nucl. Med. 2008, 49, 1328–1335. [Google Scholar] [CrossRef][Green Version]
- Wagner, C.C.; Bauer, M.; Karch, R.; Feurstein, T.; Kopp, S.; Chiba, P. A Pilot Study to Assess the Efficacy of Tariquidar to Inhibit P-glycoprotein at the Human Blood–Brain Barrier with (R)-11C-Verapamil and PET. J. Nucl. Med. 2009, 50, 1954–1961. [Google Scholar] [CrossRef]
- Wandel, C.; Kim, R.; Wood, M.; Wood, A. Interaction of Morphine, Fentanyl, Sufentanil, Alfentanil, and Loperamide with the Efflux Drug Transporter P-glycoprotein. Anesthesiology 2002, 96, 913–920. [Google Scholar] [CrossRef]
- Wang, M.; Gao, M.; Zheng, Q.-H. A high-yield route to synthesize the P-glycoprotein radioligand [ C]N-desmethyl-loperamide and its parent radioligand [ C]loperamide. Bioorganic Med. Chem. Lett. 2013, 23, 5259–5263. [Google Scholar] [CrossRef]
- Kim, K.-A.; Chung, J.; Jung, D.-H.; Park, J.-Y. Identification of cytochrome P450 isoforms involved in the metabolism of loperamide in human liver microsomes. Eur. J. Clin. Pharmacol. 2004, 60, 575–581. [Google Scholar] [CrossRef]
- Seneca, N.; Zoghbi, S.S.; Shetty, H.U.; Tuan, E.; Kannan, P.; Taku, A. Effects of ketoconazole on the biodistribution and metabolism of [11C]loperamide and [11C]N-desmethyl-loperamide in wild-type and P-gp knockout mice. Nucl. Med. Biol. 2010, 37, 335–345. [Google Scholar] [CrossRef]
- Zoghbi, S.S.; Liow, J.-S.; Yasuno, F.; Hong, J.; Tuan, E.; Lazarova, N.; Gladding, R.L.; Pike, V.W.; Innis, R.B. 11C-Loperamide and Its N-Desmethyl Radiometabolite Are Avid Substrates for Brain Permeability-Glycoprotein Efflux. J. Nucl. Med. 2008, 49, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Seneca, N.; Zoghbi, S.S.; Liow, J.-S.; Kreisl, W.; Herscovitch, P.; Jenko, K. Human Brain Imaging and Radiation Dosimetry of 11C-N-Desmethyl-Loperamide, a PET Radiotracer to Measure the Function of P-Glycoprotein. J. Nucl. Med. 2009, 50, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Lazarova, N.; Zoghbi, S.S.; Hong, J.; Seneca, N.; Tuan, E.; Gladding, R.L. Synthesis and Evaluation of [N-methyl-11C]N-Desmethyl-loperamide as a New and Improved PET Radiotracer for Imaging P-gp Function. J. Med. Chem. 2008, 51, 6034–6043. [Google Scholar] [CrossRef] [PubMed]
- Kannan, P.; Brimacombe, K.R.; Kreisl, W.C.; Liow, J.-S.; Zoghbi, S.S.; Telu, S. Lysosomal trapping of a radiolabeled substrate of P-glycoprotein as a mechanism for signal amplification in PET. Proc. Natl. Acad. Sci. USA 2011, 108, 2593–2598. [Google Scholar] [CrossRef]
- Liow, J.-S.; Kreisl, W.; Zoghbi, S.S.; Lazarova, N.; Seneca, N.; Gladding, R.L.; Taku, A.; Herscovitch, P.; Pike, V.W.; Innis, R.B. P-Glycoprotein Function at the Blood–Brain Barrier Imaged Using 11C-N-Desmethyl -Loperamide in Monkeys. J. Nucl. Med. 2009, 50, 108–115. [Google Scholar] [CrossRef]
- Wanek, T.; Römermann, K.; Mairinger, S.; Stanek, J.; Sauberer, M.; Filip, T. Factors Governing P-Glycoprotein-Mediated Drug–Drug Interactions at the Blood–Brain Barrier Measured with Positron Emission Tomography. Mol. Pharm. 2015, 12, 3214–3225. [Google Scholar] [CrossRef]
- Kannan, P.; Brimacombe, K.R.; Zoghbi, S.S.; Liow, J.-S.; Morse, C.; Taku, A.K.; Pike, V.W.; Halldin, C.; Innis, R.B.; Gottesman, M.M.; et al. N-desmethyl -Loperamide Is Selective for P-Glycoprotein among Three ATP-Binding Cassette Transporters at the Blood-Brain Barrier. Drug Metab. Dispos. 2010, 38, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Moerman, L.; De Naeyer, D.; Boon, P.; De Vos, F. P-glycoprotein at the blood-brain barrier: Kinetic modeling of 11C-desmethylloperamide in mice using a 18F-FDG μPET scan to determine the input function. EJNMMI Res. 2011, 1, 12. [Google Scholar] [CrossRef] [PubMed]
- Farwell, M.D.; Chong, D.J.; Iida, Y.; Bae, S.A.; Easwaramoorthy, B.; Ichise, M. Imaging P-glycoprotein function in rats using [11C]-N-desmethyl-loperamide. Ann. Nucl. Med. 2013, 27, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Moerman, L.; Dumolyn, C.; Boon, P.; De Vos, F. The influence of mass of [11C]-laniquidar and [11C]-N-desmethyl-loperamide on P-glycoprotein blockage at the blood–brain barrier. Nucl. Med. Biol. 2012, 39, 121–125. [Google Scholar] [CrossRef]
- Kreisl, W.C.; Liow, J.-S.; Kimura, N.; Seneca, N.; Zoghbi, S.S.; Morse, C.L.; Herscovitch, P.; Pike, V.W.; Innis, R. P-Glycoprotein Function at the Blood–Brain Barrier in Humans Can Be Quantified with the Substrate Radiotracer 11C-N-Desmethyl -Loperamide. J. Nucl. Med. 2010, 51, 559–566. [Google Scholar] [CrossRef]
- Damont, A.; Goutal, S.; Auvity, S.; Valette, H.; Kuhnast, B.; Saba, W. Imaging the impact of cyclosporin A and dipyridamole on P-glycoprotein (ABCB1) function at the blood-brain barrier: A [11C]-N-desmethyl-loperamide PET study in nonhuman primates. Eur. J. Pharm. Sci. 2016, 91, 98–104. [Google Scholar] [CrossRef]
- Kreisl, W.C.; Bhatia, R.; Morse, C.L.; Woock, A.E.; Zoghbi, S.S.; Shetty, H.U.; Pike, V.W.; Innis, R.B. Increased Permeability-Glycoprotein Inhibition at the Human Blood–Brain Barrier Can Be Safely Achieved by Performing PET During Peak Plasma Concentrations of Tariquidar. J. Nucl. Med. 2015, 56, 82–87. [Google Scholar] [CrossRef]
- Moerman, L.; Wyffels, L.; Slaets, D.; Raedt, R.; Boon, P.; De Vos, F. Antiepileptic drugs modulate P-glycoproteins in the brain: A mice study with 11C-desmethylloperamide. Epilepsy Research 2011, 94, 18–25. [Google Scholar] [CrossRef]
- Pichler, V.; Ozenil, M.; Bamminger, K.; Vraka, C.; Hacker, M.; Langer, O. Pitfalls and solutions of the fully-automated radiosynthesis of [11C]metoclopramide. EJNMMI Radiopharm. Chem. 2019, 4, 31. [Google Scholar] [CrossRef]
- Livezey, M.R.; Briggs, E.D.; Bolles, A.K.; Nagy, L.D.; Fujiwara, R.; Furge, L.L. Metoclopramide is metabolized by CYP2D6 and is a reversible inhibitor, but not inactivator, of CYP2D6. Xenobiotica 2014, 44, 309–319. [Google Scholar] [CrossRef]
- Breuil, L.; Ziani, N.; Leterrier, S.; Hugon, G.; Caillé, F.; Bouilleret, V. Impact of Cytochrome Induction or Inhibition on the Plasma and Brain Kinetics of [11C]metoclopramide, a PET Probe for P-Glycoprotein Function at the Blood-Brain Barrier. Pharmaceutics 2022, 14, 2650. [Google Scholar] [CrossRef] [PubMed]
- Caillé, F.; Goutal, S.; Marie, S.; Auvity, S.; Cisternino, S.; Kuhnast, B.; Pottier, G.; Tournier, N. Positron Emission Tomography Imaging Reveals an Importance of Saturable Liver Uptake Transport for the Pharmacokinetics of Metoclopramide. Contrast Media Mol. Imaging 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- Pottier, G.; Marie, S.; Goutal, S.; Auvity, S.; Peyronneau, M.-A.; Stute, S. Imaging the Impact of the P-Glycoprotein (ABCB1) Function on the Brain Kinetics of Metoclopramide. J. Nucl. Med. 2016, 57, 309–314. [Google Scholar] [CrossRef]
- Auvity, S.; Tournier, N. Impact of Acute Alcohol Exposure on P-Glycoprotein Function at the Blood-Brain Barrier Assessed Using 11C-Metoclopramide PET Imaging. Clin. Pharma Ther. 2019, 105, 812–813. [Google Scholar] [CrossRef] [PubMed]
- Auvity, S.; Caillé, F.; Marie, S.; Wimberley, C.; Bauer, M.; Langer, O.; Buvat, I.; Goutal, S.; Tournier, N. P-Glycoprotein (ABCB1) Inhibits the Influx and Increases the Efflux of 11C-Metoclopramide Across the Blood–Brain Barrier: A PET Study on Nonhuman Primates. J. Nucl. Med. 2018, 59, 1609–1615. [Google Scholar] [CrossRef]
- Tournier, N.; Bauer, M.; Pichler, V.; Nics, L.; Klebermass, E.-M.; Bamminger, K. Impact of P-Glycoprotein Function on the Brain Kinetics of the Weak Substrate 11C-Metoclopramide Assessed with PET Imaging in Humans. J. Nucl. Med. 2019, 60, 985–991. [Google Scholar] [CrossRef]
- Zoufal, V.; Mairinger, S.; Brackhan, M.; Krohn, M.; Filip, T.; Sauberer, M. Imaging P-Glycoprotein Induction at the Blood–Brain Barrier of a β-Amyloidosis Mouse Model with 11 C-Metoclopramide PET. J. Nucl. Med. 2020, 61, 1050–1057. [Google Scholar] [CrossRef]
- Breuil, L.; El Biali, M.; Vodovar, D.; Marie, S.; Auvity, S.; Bauer, M. Parametric Imaging of P-Glycoprotein Function at the Blood–Brain Barrier Using kE,brain-maps Generated from [11C]Metoclopramide PET Data in Rats, Nonhuman Primates and Humans. Mol. Imaging Biol. 2023, 25, 1135–1141. [Google Scholar] [CrossRef]
- Bauer, M.; Barna, S.; Blaickner, M.; Prosenz, K.; Bamminger, K.; Pichler, V. Human Biodistribution and Radiation Dosimetry of the P-Glycoprotein Radiotracer [11C]Metoclopramide. Mol. Imaging Biol. 2021, 23, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Van Der Aart, J.; Hallett, W.A.; Rabiner, E.A.; Passchier, J.; Comley, R.A. Radiation dose estimates for carbon-11-labelled PET tracers. Nucl. Med. Biol. 2012, 39, 305–314. [Google Scholar] [CrossRef]
- Hernández-Lozano, I.; Mairinger, S.; Sauberer, M.; Stanek, J.; Filip, T.; Wanek, T. Influence of Cation Transporters (OCTs and MATEs) on the Renal and Hepatobiliary Disposition of [11C]Metoclopramide in Mice. Pharm. Res. 2021, 38, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Bamminger, K.; Pichler, V.; Weber, M.; Binder, S.; Maier-Salamon, A.; Tahir, A.; Jäger, W.; Haslacher, W.; Tournier, T.; et al. Impaired Clearance From the Brain Increases the Brain Exposure to Metoclopramide in Elderly Subjects. Clin. Pharma Ther. 2021, 109, 754–761. [Google Scholar] [CrossRef]
- Mairinger, S.; Hernández-Lozano, I.; Filip, T.; Löbsch, M.; Stanek, J.; Zeitlinger, M. Influence of P-glycoprotein on pulmonary disposition of the model substrate [11C]metoclopramide assessed by PET imaging in rats. Eur. J. Pharm. Sci. 2023, 183, 106404. [Google Scholar] [CrossRef]
- Goutal, S.; Novell, A.; Leterrier, S.; Breuil, L.; Selingue, E.; Gerstenmayer, M.; Marie, S.; Saubaméa, B.; Caillé, F.; Langer, O.; et al. Imaging the impact of blood-brain barrier disruption induced by focused ultrasound on P-glycoprotein function. J. Control. Release 2023, 361, 483–492. [Google Scholar] [CrossRef]
- El Biali, M.; Wölfl-Duchek, M.; Jackwerth, M.; Mairinger, S.; Weber, M.; Bamminger, K. St. John’s wort extract with a high hyperforin content does not induce P-glycoprotein activity at the human blood–brain barrier. Clin. Transl. Sci. 2024, 17, e13804. [Google Scholar] [CrossRef]
- Garcia-Varela, L.; Attia, K.; Sembrano, J.C.; Jacquet, O.; Antunes, I.F.; Kwizera, C. A new approach to produce [18F]MC225 via one-step synthesis, a PET radiotracer for measuring P-gp function. EJNMMI Radiopharm. Chem. 2021, 6, 24. [Google Scholar] [CrossRef]
- Savolainen, H.; Windhorst, A.D.; Elsinga, P.H.; Cantore, M.; Colabufo, N.A.; Willemsen, A.T. Evaluation of [ 18 F]MC225 as a PET radiotracer for measuring P-glycoprotein function at the blood–brain barrier in rats: Kinetics, metabolism, and selectivity. J. Cereb. Blood Flow. Metab. 2017, 37, 1286–1298. [Google Scholar] [CrossRef] [PubMed]
- Savolainen, H.; Cantore, M.; Colabufo, N.A.; Elsinga, P.H.; Windhorst, A.D.; Luurtsema, G. Synthesis and Preclinical Evaluation of Three Novel Fluorine-18 Labeled Radiopharmaceuticals for P-Glycoprotein PET Imaging at the Blood–Brain Barrier. Mol. Pharm. 2015, 12, 2265–2275. [Google Scholar] [CrossRef] [PubMed]
- García-Varela, L.; Arif, W.M.; Vállez García, D.; Kakiuchi, T.; Ohba, H.; Harada, N. Pharmacokinetic Modeling of [ 18 F]MC225 for Quantification of the P-Glycoprotein Function at the Blood–Brain Barrier in Non-Human Primates with PET. Mol. Pharm. 2020, 17, 3477–3486. [Google Scholar] [CrossRef]
- García-Varela, L.; Vállez García, D.; Aguiar, P.; Kakiuchi, T.; Ohba, H.; Harada, N. Head-to-head comparison of (R)-[11C]verapamil and [18F]MC225 in non-human primates, tracers for measuring P-glycoprotein function. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4307–4317. [Google Scholar] [CrossRef]
- García-Varela, L.; Rodríguez-Pérez, M.; Custodia, A.; Moraga-Amaro, R.; Colabufo, N.A.; Aguiar, P. In Vivo Induction of P-Glycoprotein Function can be Measured with [ 18 F]MC225 and PET. Mol. Pharm. 2021, 18, 3073–3085. [Google Scholar] [CrossRef]
- Garcia-Varela, L.; Mossel, P.; Aguiar, P.; Vazquez-Matias, D.A.; Van Waarde, A.; Willemsen, A.T.M. Dose-response assessment of cerebral P-glycoprotein inhibition in vivo with [18F]MC225 and PET. J. Control. Release 2022, 347, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Mossel, P.; Garcia Varela, L.; Arif, W.M.; Van Der Weijden, C.W.J.; Boersma, H.H.; Willemsen, A.T.M. Evaluation of P-glycoprotein function at the blood–brain barrier using [18F]MC225-PET. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4105–4106. [Google Scholar] [CrossRef]
- Muzi, M.; Mankoff, D.A.; Link, J.M.; Shoner, S.; Collier, A.C.; Sasongko, L.; Unadkat, J.D. Imaging of Cyclosporine Inhibition of P-Glycoprotein Activity Using 11C-Verapamil in the Brain: Studies of Healthy Humans. J. Nucl. Med. 2009, 50, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Toyohara, J.; Sakata, M.; Tago, T.; Colabufo, N.A.; Luurtsema, G. Automated synthesis, preclinical toxicity, and radiation dosimetry of [18F]MC225 for clinical use: A tracer for measuring P-glycoprotein function at the blood-brain barrier. EJNMMI Res. 2020, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Fusi, F.; Durante, M.; Gorelli, B.; Perrone, M.G.; Colabufo, N.A.; Saponara, S. MC225, a Novel Probe for P-glycoprotein PET Imaging at the Blood–brain Barrier: In Vitro Cardiovascular Safety Evaluation. J. Cardiovasc. Pharmacol. 2017, 70, 405–410. [Google Scholar] [CrossRef]
- Toyohara, J.; Sakata, M.; Ishibashi, K.; Mossel, P.; Imai, M.; Wagatsuma, K. First clinical assessment of [18F]MC225, a novel fluorine-18 labelled PET tracer for measuring functional P-glycoprotein at the blood–brain barrier. Ann. Nucl. Med. 2021, 35, 1240–1252. [Google Scholar] [CrossRef]
- Mossel, P.; Arif, W.M.; De Souza, G.S.; Varela, L.G.; Van Der Weijden, C.W.J.; Boersma, H.H. Quantification of P-glycoprotein function at the human blood-brain barrier using [18F]MC225 and PET. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 3917–3927. [Google Scholar] [CrossRef]
- Savolainen, H.; Meerlo, P.; Elsinga, P.H.; Windhorst, A.D.; Dierckx, R.A.J.O.; Colabufo, N.A.; Van Waarde, A.; Luurtsema, G. P-glycoprotein Function in the Rodent Brain Displays a Daily Rhythm, a Quantitative In Vivo PET Study. AAPS J. 2016, 18, 1524–1531. [Google Scholar] [CrossRef]
- García-Varela, L.; Vállez García, D.; Rodríguez-Pérez, M.; Van Waarde, A.; Sijbesma, J.W.A.; Schildt, A. Test–Retest Repeatability of [ 18 F]MC225-PET in Rodents: A Tracer for Imaging of P-gp Function. ACS Chem. Neurosci. 2020, 11, 648–658. [Google Scholar] [CrossRef]
- O’Brien, F.E.; Dinan, T.G.; Griffin, B.T.; Cryan, J.F. Interactions between antidepressants and P-glycoprotein at the blood–brain barrier: Clinical significance of in vitro and in vivo findings. Br. J. Pharmacol. 2012, 165, 289–312. [Google Scholar] [CrossRef] [PubMed]
- Kurdziel, K.A.; Kalen, J.D.; Hirsch, J.I.; Wilson, J.D.; Agarwal, R.; Barrett, D. Imaging multidrug resistance with 4-[18F]fluoropaclitaxel. Nucl. Med. Biol. 2007, 34, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Bauer, F.; Kuntner, C.; Bankstahl, J.P.; Wanek, T.; Bankstahl, M.; Stanek, J. Synthesis and in vivo evaluation of [11C]tariquidar, a positron emission tomography radiotracer based on a third-generation P-glycoprotein inhibitor. Bioorganic Med. Chem. 2010, 18, 5489–5497. [Google Scholar] [CrossRef]
- Dörner, B.; Kuntner, C.; Bankstahl, J.P.; Bankstahl, M.; Stanek, J.; Wanek, T. Synthesis and Small-Animal Positron Emission Tomography Evaluation of [11C]-Elacridar As a Radiotracer to Assess the Distribution of P-Glycoprotein at the Blood−Brain Barrier. J. Med. Chem. 2009, 52, 6073–6082. [Google Scholar] [CrossRef] [PubMed]
- Dörner, B.; Kuntner, C.; Bankstahl, J.P.; Wanek, T.; Bankstahl, M.; Stanek, J. Radiosynthesis and in vivo evaluation of 1-[18F]fluoroelacridar as a positron emission tomography tracer for P-glycoprotein and breast cancer resistance protein. Bioorganic Med. Chem. 2011, 19, 2190–2198. [Google Scholar] [CrossRef]
- Müllauer, J.; Karch, R.; Bankstahl, J.P.; Bankstahl, M.; Stanek, J.; Wanek, T. Assessment of cerebral P-glycoprotein expression and function with PET by combined [11C]inhibitor and [11C]substrate scans in rats. Nucl. Med. Biol. 2013, 40, 755–763. [Google Scholar] [CrossRef]
- Bauer, M.; Blaickner, M.; Philippe, C.; Wadsak, W.; Hacker, M.; Zeitlinger, M.; Langer, O. Whole-Body Distribution and Radiation Dosimetry of 11C-Elacridar and 11C-Tariquidar in Humans. J. Nucl. Med. 2016, 57, 1265–1268. [Google Scholar] [CrossRef]
- Hernández Lozano, I.; Bauer, M.; Wulkersdorfer, B.; Traxl, A.; Philippe, C.; Weber, M. Measurement of Hepatic ABCB1 and ABCG2 Transport Activity with [11C]Tariquidar and PET in Humans and Mice. Mol. Pharm. 2020, 17, 316–326. [Google Scholar] [CrossRef]
- Luurtsema, G.; Schuit, R.C.; Klok, R.P.; Verbeek, J.; Leysen, J.E.; Lammertsma, A.A. Evaluation of [11C]laniquidar as a tracer of P-glycoprotein: Radiosynthesis and biodistribution in rats. Nucl. Med. Biol. 2009, 36, 643–649. [Google Scholar] [CrossRef]
- Froklage, F.E.; Boellaard, R.; Bakker, E.; Hendrikse, N.H.; Reijneveld, J.C.; Schuit, R.C.; van Berckel, B.N.M.; Lammertsma, A.; Postnov, A. Quantification of 11C-Laniquidar Kinetics in the Brain. J. Nucl. Med. 2015, 56, 1730–1735. [Google Scholar] [CrossRef] [PubMed]
- Postnov, A.; Froklage, F.E.; Van Lingen, A.; Reijneveld, J.C.; Hendrikse, N.H.; Windhorst, A.D.; Schuit, R.C.; Eriksson, J.; Lammertsma, A.A.; Huisman, M.C. Radiation Dose of the P-Glycoprotein Tracer 11C-Laniquidar. J. Nucl. Med. 2013, 54, 2101–2103. [Google Scholar] [CrossRef] [PubMed]
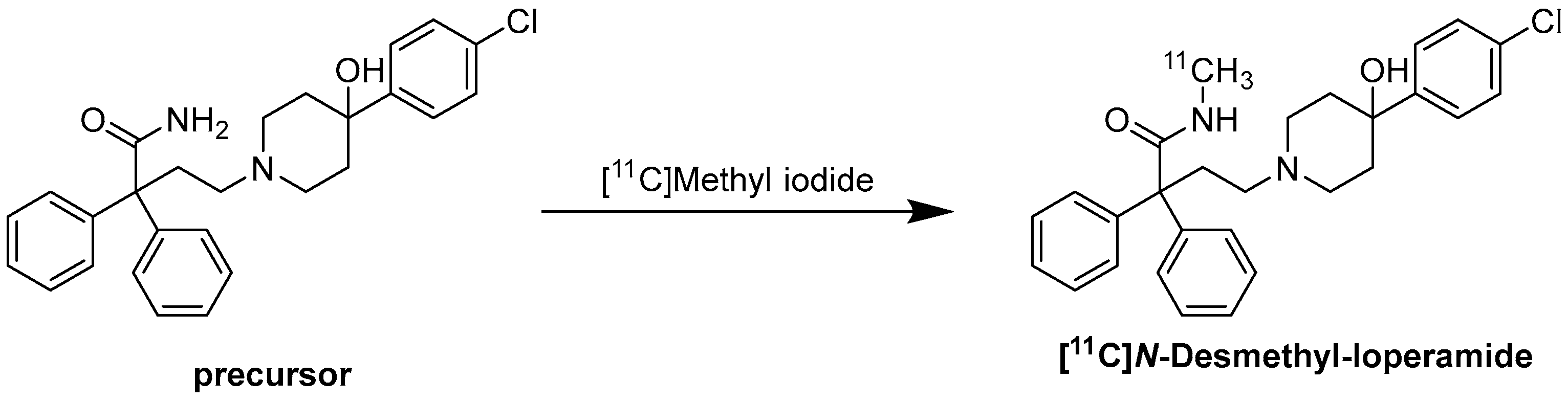
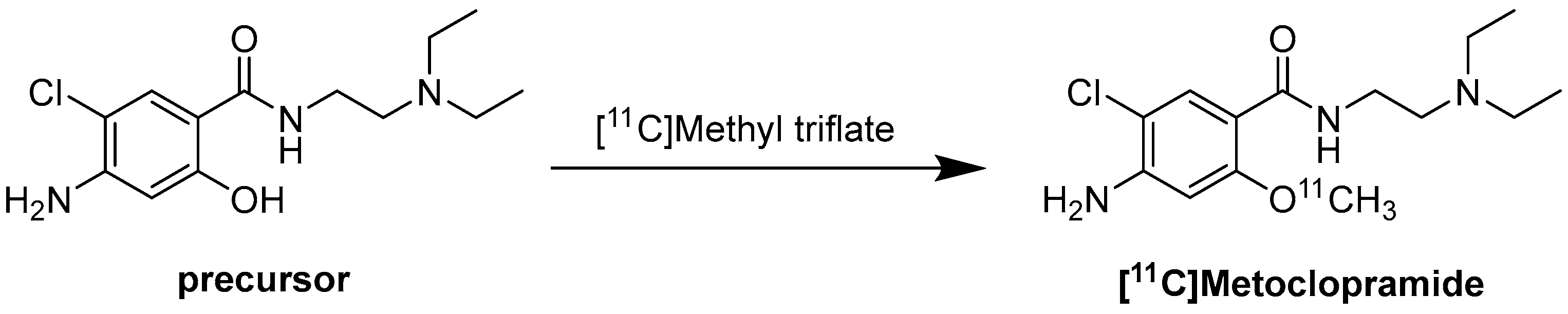

| Organ/District | Localization | Physiological/Pharmacological Role |
|---|---|---|
| Intestine | Epithelial cells of the colon and ileum (↑); jejunum, duodenum, and stomach (↓) | Absorption and bioavailability; interacts with CYP3A4; |
| Liver | Bile ducts (↑) | Elimination of endogenous and xenobiotic metabolites |
| Kidneys | Proximal tubules (↑) | Excretion of endogenous and xenobiotic metabolites |
| CNS | Endothelial cells of BBB (↑) | Protects the CNS by limiting xenobiotic passage |
| Respiratory system | Ciliated epithelial cells, collecting ducts, lateral surfaces of serous cells in bronchial glands (↑); absent in goblet cells | Modulates pulmonary absorption of P-gp substrates (e.g., COPD drugs) |
| Placenta | Microvillous surface of syncytiotrophoblasts (↑) | Efflux of hydrophobic/cationic drugs into maternal blood; fetal protection |
| Heart | Arterioles and cardiac capillaries (↑) | Reduces intracellular drug concentration, lowering bioavailability |
| Tracer | Function | Advantages | Disadvantages |
|---|---|---|---|
| (R)-[11C]Verapamil | Avid substrate | “Gold standard” for comparing other P-gp ligands; extensive literature background and established models. | Poor sensitivity to moderate reductions in P-gp; strong dependence on metabolic correction; 11C-labeled. |
| [11C]dLop | Avid substrate | Very high dynamic range after P-gp blockade; strong increase in uptake with Tariquidar or other inhibitors; good selectivity for P-gp. | Low sensitivity to moderate decreases in P-gp function; presence of potential lipophilic metabolites; 11C-labeled. |
| [11C]Metoclopramide | Weak substrate | Metabolism present but manageable; ideal for detecting moderate pathophysiological changes; improved sensitivity for clinically relevant changes; validated in patients (epilepsy) without administering inhibitors. | Being a weak substrate, P-gp can limit both entry and increase efflux; there is still a need to standardize simplified outcomes; 11C-labeled. |
| [18F]MC225 | Weak substrate | Longer half-life (easier logistics, multicenter possibilities, and longer scans); higher uptake than all previous tracers; sensitive to both down- and upregulation; reduced interaction with other proteins; good clinical translational potential (satisfactory initial evaluation in humans). | Studies are being carried out, and the radiotracer is being employed in Phase II clinical trials (in humans). Moreover, there are over 25 papers regarding this radiotracer. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastropasqua, F.; Luurtsema, G.; Filosa, C.; Colabufo, N.A. Designing a Small Molecule for PET Radiotracing: [18F]MC225 in Human Trials for Early Diagnosis in CNS Pathologies. Molecules 2025, 30, 3696. https://doi.org/10.3390/molecules30183696
Mastropasqua F, Luurtsema G, Filosa C, Colabufo NA. Designing a Small Molecule for PET Radiotracing: [18F]MC225 in Human Trials for Early Diagnosis in CNS Pathologies. Molecules. 2025; 30(18):3696. https://doi.org/10.3390/molecules30183696
Chicago/Turabian StyleMastropasqua, Francesco, Gert Luurtsema, Cristina Filosa, and Nicola Antonio Colabufo. 2025. "Designing a Small Molecule for PET Radiotracing: [18F]MC225 in Human Trials for Early Diagnosis in CNS Pathologies" Molecules 30, no. 18: 3696. https://doi.org/10.3390/molecules30183696
APA StyleMastropasqua, F., Luurtsema, G., Filosa, C., & Colabufo, N. A. (2025). Designing a Small Molecule for PET Radiotracing: [18F]MC225 in Human Trials for Early Diagnosis in CNS Pathologies. Molecules, 30(18), 3696. https://doi.org/10.3390/molecules30183696