Design, Production and Quality Assessment of Antioxidant-Enriched Olive Paste Dips Using Agro-Food By-Products
Abstract
1. Introduction
2. Results and Discussion
2.1. Product Characterization
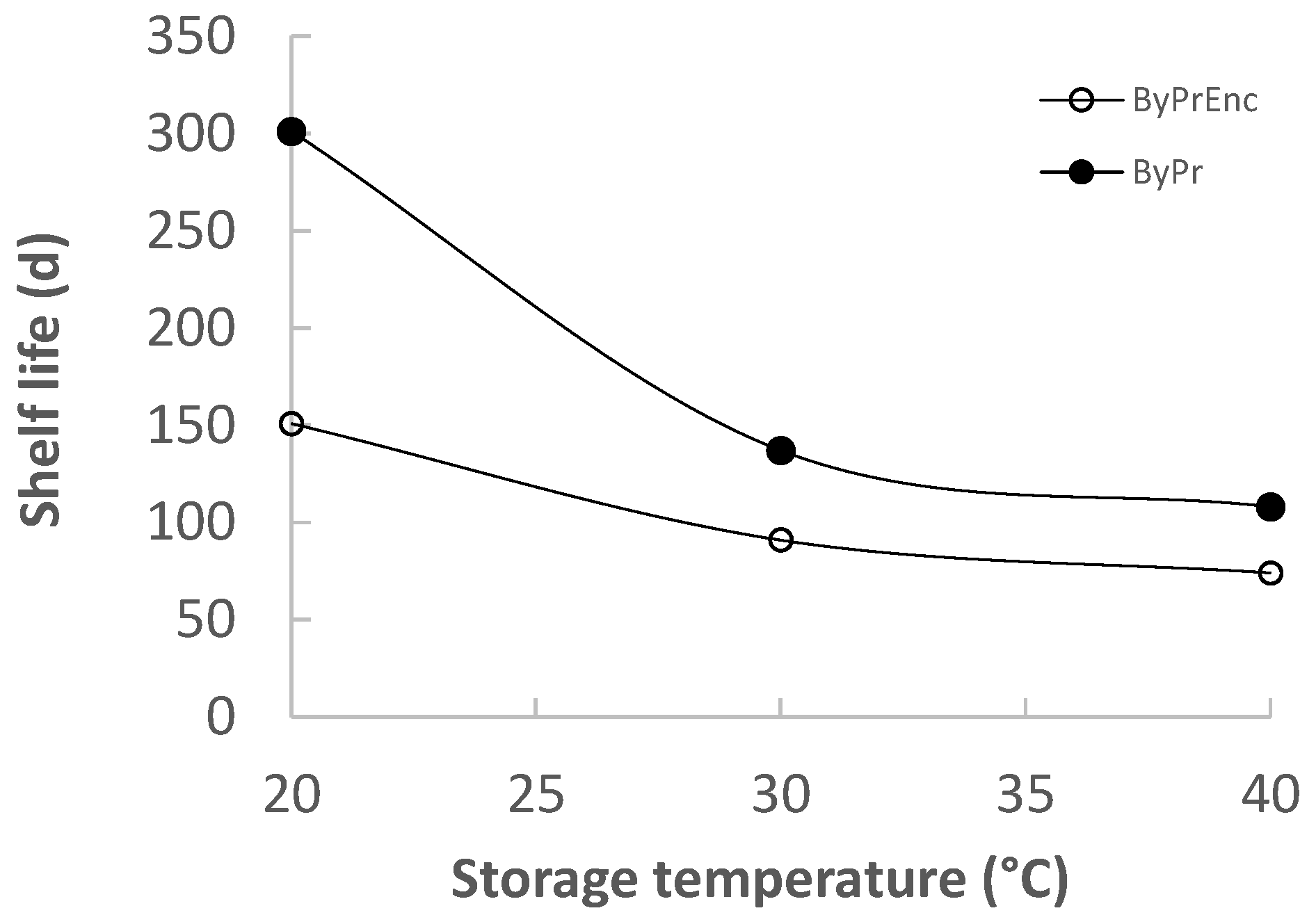
2.2. Shelf Life Determination
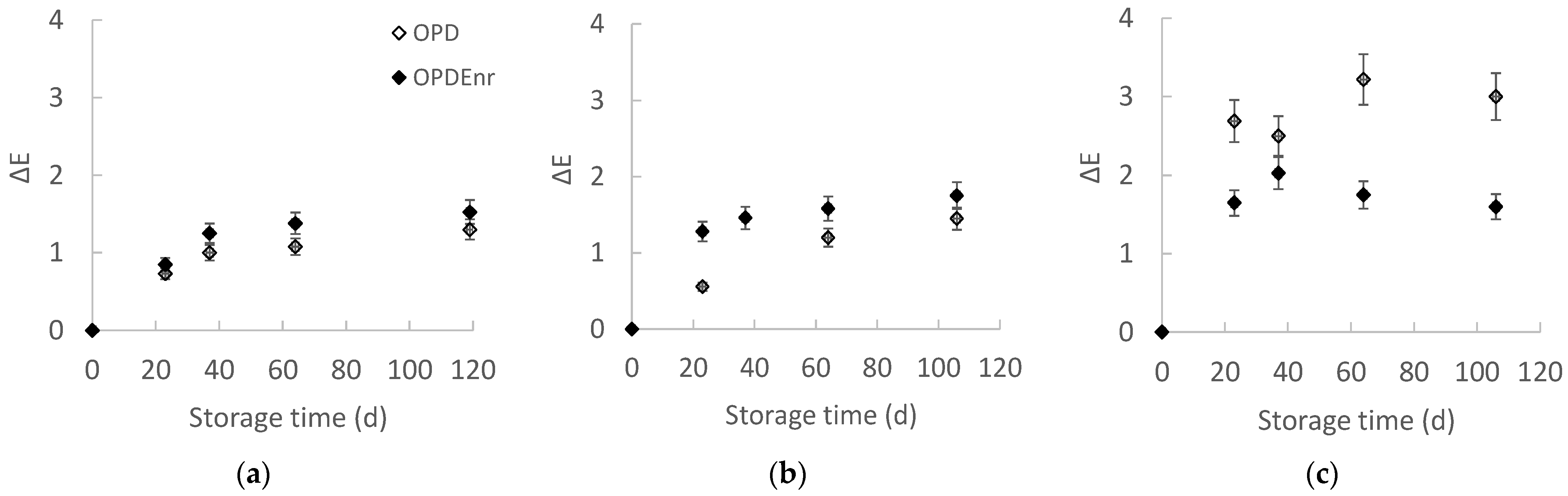
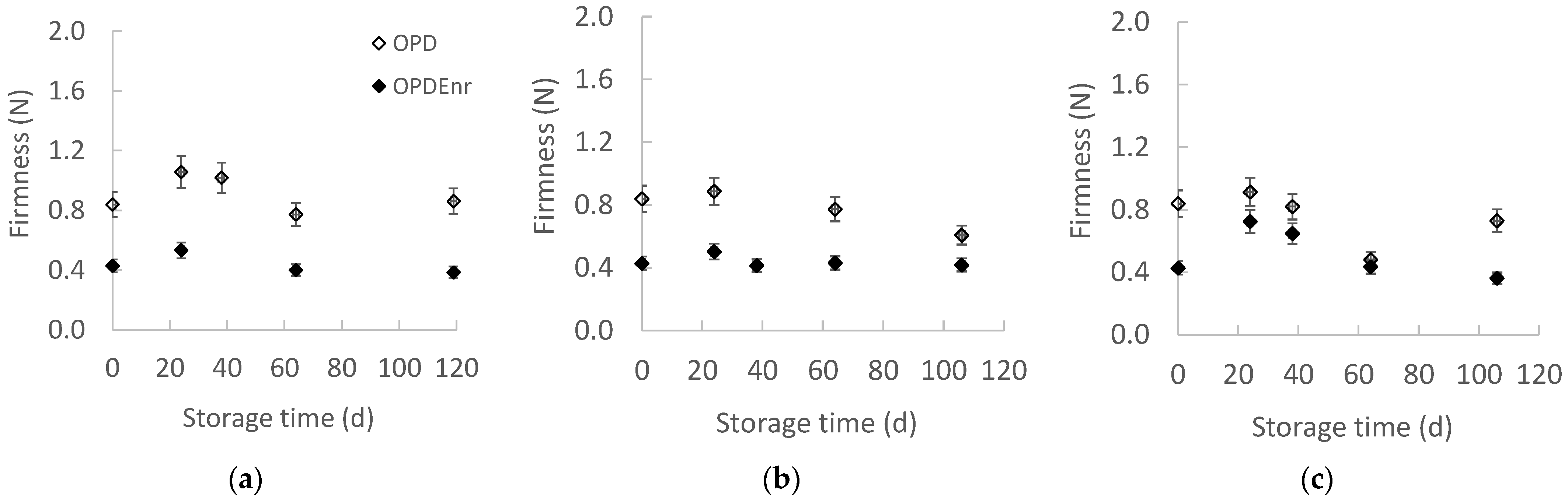
2.2.1. Evolution of Color and Texture of Olive Paste Dip Products During Storage
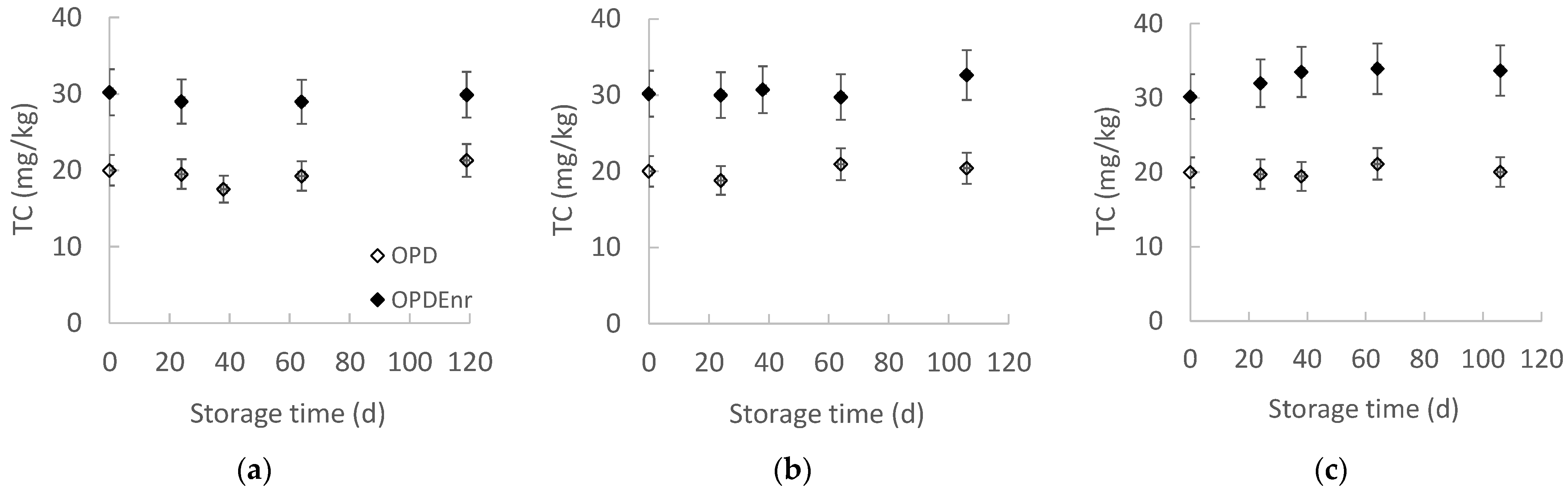
2.2.2. Evolution of Total Carotenoids of Olive Paste Dip Products During Storage
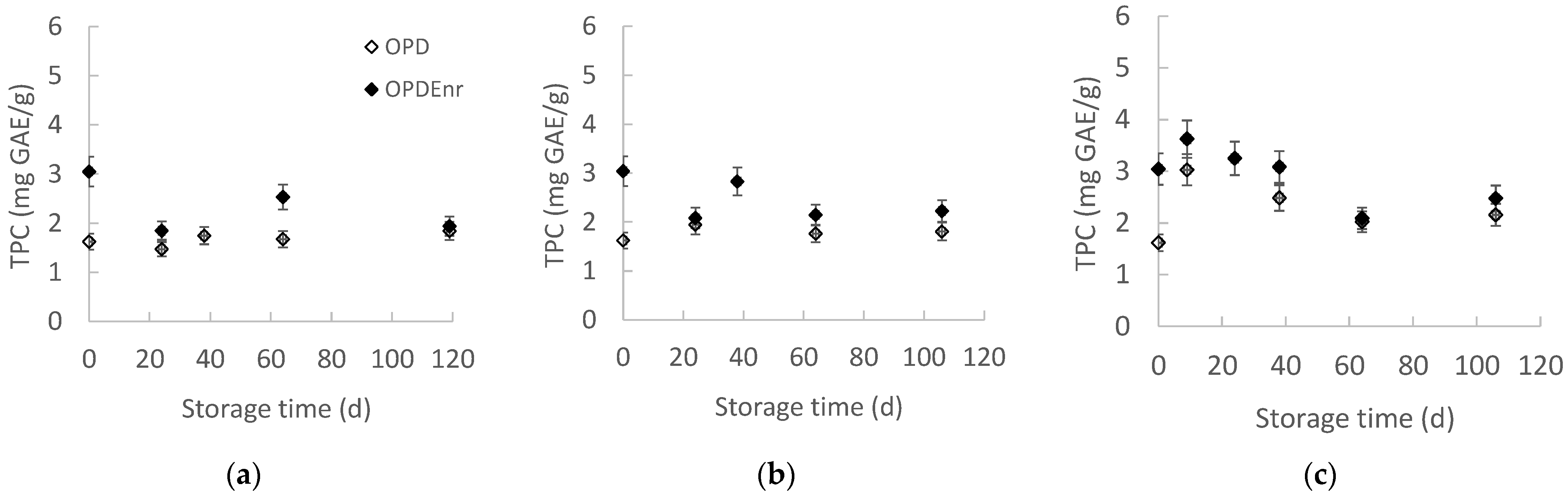
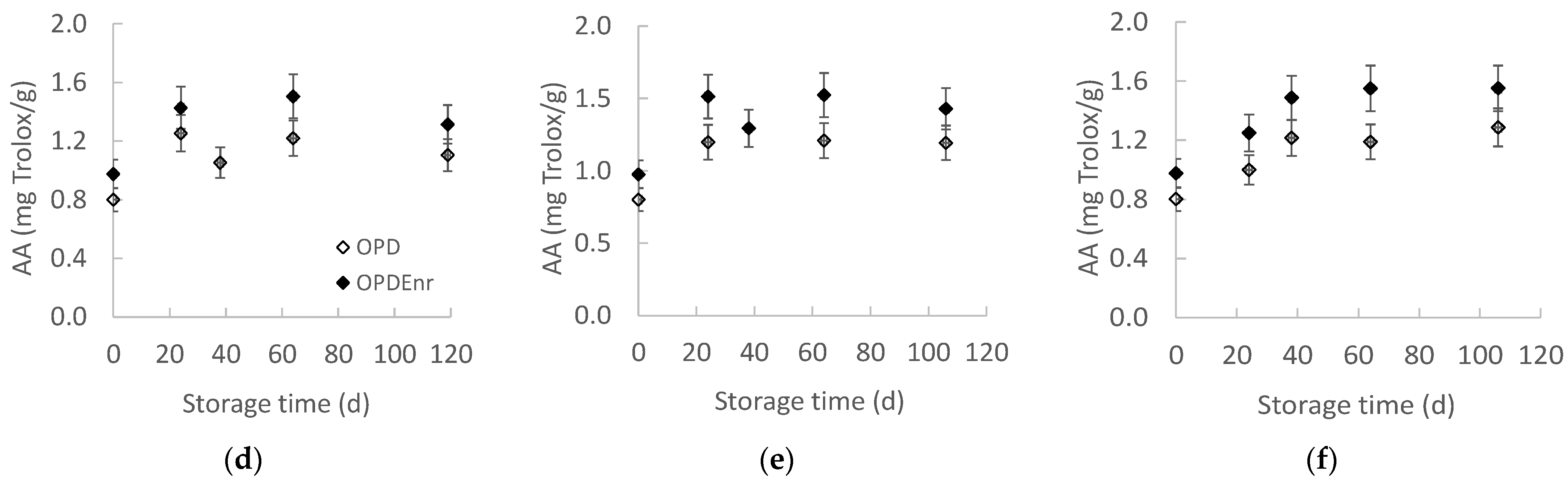
2.2.3. Evolution of Total Phenolic Content (TPC) and Antioxidant Activity (AA) of Olive Paste Dip Products During Storage
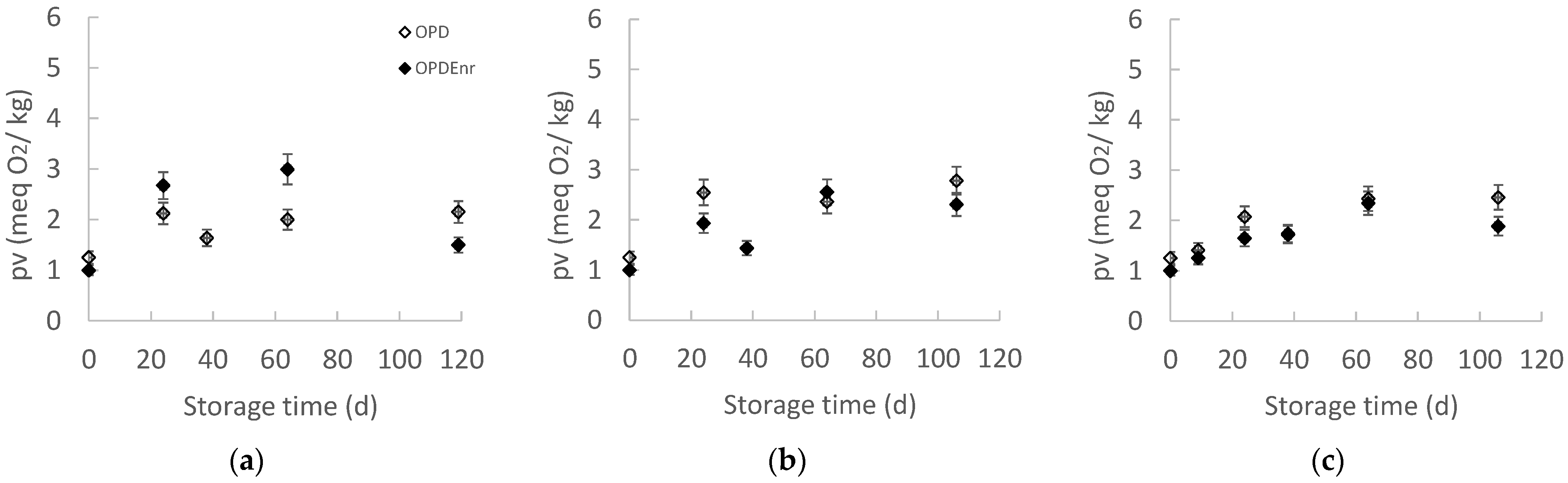
2.2.4. Evolution of Lipid Oxidation of Olive Paste Dip Products During Storage
2.2.5. Evolution of Microbial Load During Storage
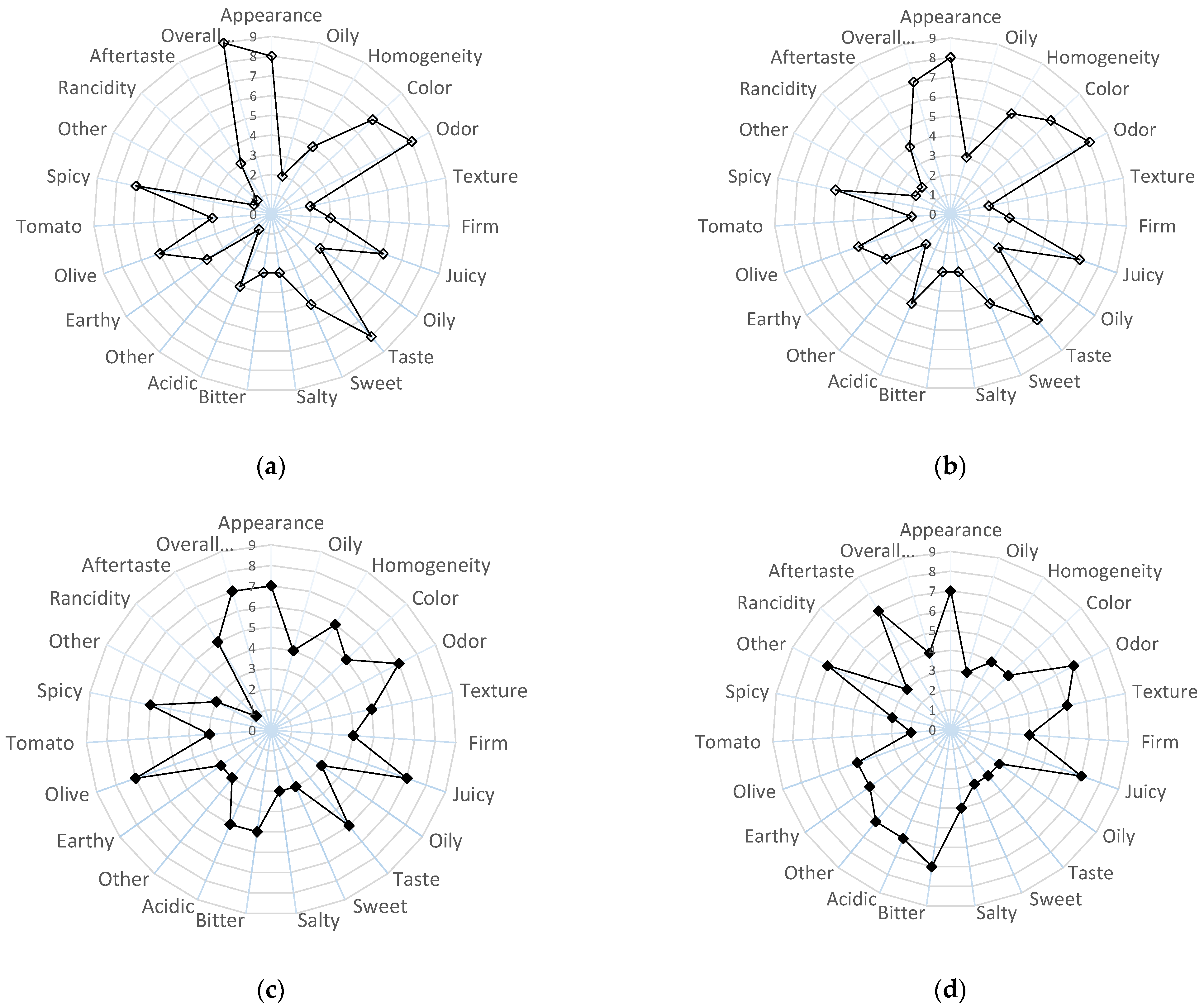
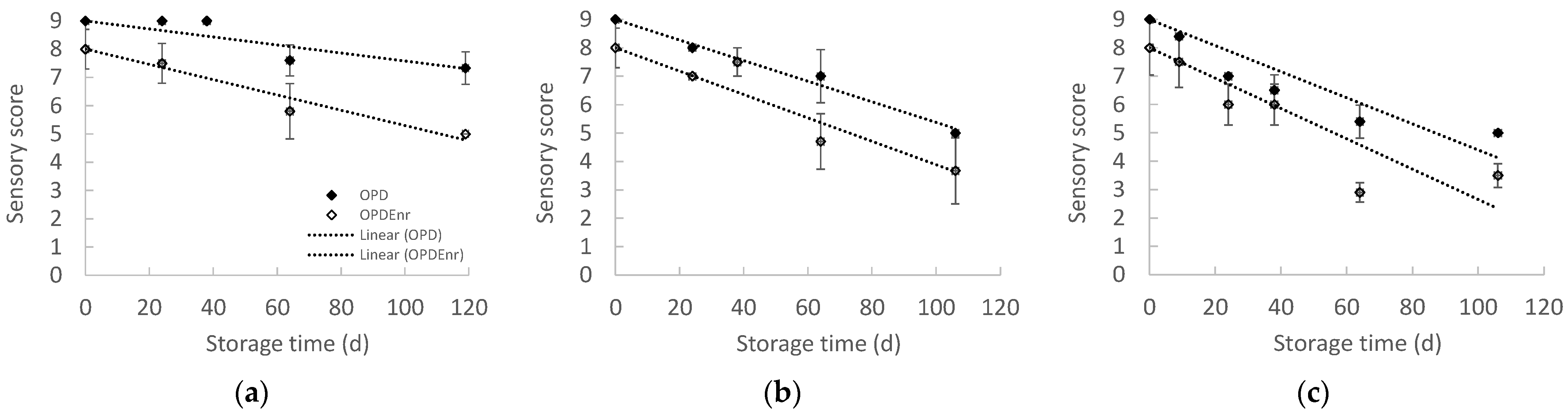
2.2.6. Evolution of Sensory Properties of Olive Paste Dip Products During Storage
3. Materials and Methods
3.1. Materials
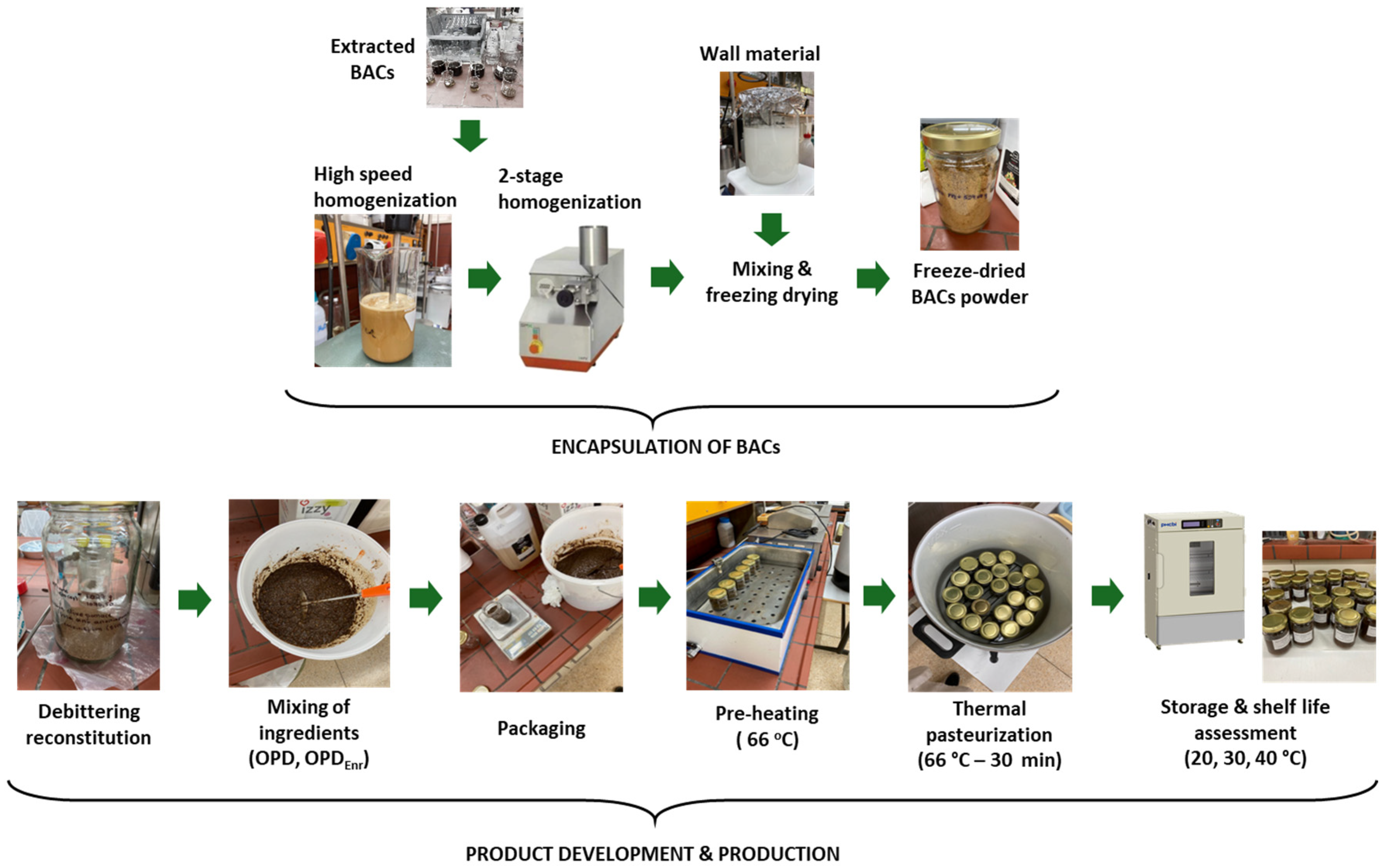
3.2. The Incorporation of BACs into the Product
3.3. Design and Production of Innovative Olive Paste Products
3.4. Determination of Physicochemical Parameters
3.5. Other Nutritional and Quality Parameters
3.5.1. Total Phenolic Content (TPC) and Antioxidant Activity (AA) Determination
3.5.2. Total Carotenoid Determination
3.5.3. Objective Color and Texture Determination
3.5.4. Lipid Oxidation: Peroxide Value (PV) Determination
3.5.5. Determination of Microbial Quality
3.5.6. Determination of Sensory Properties
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allison, B.J.; Simmons, C.W. Valorization of tomato pomace by sequential lycopene extraction and anaerobic digestion. Biomass Bioenergy 2017, 105, 331–341. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Sanchez-Zapata, E.; Sayas-Barberá, E.; Sendra, E.; Pérez-Álvarez, J.A.; Fernández-López, J. Tomato and tomato byproducts. human health benefits of lycopene and its application to meat products: A Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1032–1049. [Google Scholar] [CrossRef] [PubMed]
- Liadakis, G.; Katsouli, M.; Chanioti, S.; Giannou, V.; Tzia, C. Chapter One: Identification, quantification, and characterization of tomato processing by-products. In Tomato Processing by-Products. Sustainable Applications; Academic: Cambridge, MA, USA, 2022; pp. 1–32. [Google Scholar] [CrossRef]
- European Environment Agency. Diverting Waste from Landfill: Effectiveness of Waste Management Policies in the European Union; EEA Report: Copenhagen, Denmark, 2009; Volume 7, pp. 1–68. [Google Scholar] [CrossRef]
- Abbattista, R.; Ventura, G.; Calvano, C.D.; Cataldi, T.R.I.; Losito, I. Bioactive compounds in waste by-products from olive oil production: Applications and structural characterization by mass spectrometry techniques. Foods 2021, 10, 1236. [Google Scholar] [CrossRef]
- European Commission. Agriculture and Rural Development. Olive Oil in the EU. Available online: https://agriculture.ec.europa.eu/farming/crop-productions-and-plant-based-products/olive-oil_en (accessed on 27 February 2020).
- Skaltsounis, A.L.; Argyropoulou, A.; Aligiannis, N.; Xynos, N. 11—Recovery of high added value compounds from olive tree products and olive processing byproducts. In Olive and Olive Oil Bioactive Constituents, 1st ed.; Boskou, D., Ed.; Academic Press: Cambridge, MA, USA; AOCS Press: Urbana, IL, USA, 2015; pp. 333–356. [Google Scholar]
- Chanioti, S.; Tzia, C. Optimization of ultrasound-assisted extraction of oil from olive pomace using response surface technology: Oil recovery, unsaponifiable matter, total phenol content and antioxidant activity. LWT 2017, 79, 178–189. [Google Scholar] [CrossRef]
- Tsevdou, M.; Ntzimani, A.; Katsouli, M.; Dimopoulos, G.; Tsimogiannis, D.; Taoukis, P. Comparative study of microwave, pulsed electric fields, and high pressure processing on the extraction of antioxidants from olive pomace. Molecules 2024, 29, 2303. [Google Scholar] [CrossRef]
- Trombino, S.; Cassano, R.; Procopio, D.; Di Giola, M.L.; Barone, E. Valorization of tomato waste as a source of carotenoids. Molecules 2021, 26, 5062. [Google Scholar] [CrossRef]
- Strati, I.F.; Oreopoulou, V. Recovery of carotenoids from tomato processing by-products—A review. Food Res. Int. 2014, 65, 311–321. [Google Scholar] [CrossRef]
- Difonzo, G.; Troilo, M.; Squeo, G.; Pasqualone, A.; Caponio, F. Functional compounds from olive pomace to obtain high-added value foods—A review. J. Sci. Food Agric. 2021, 1, 15–26. [Google Scholar] [CrossRef]
- Katsouli, M.; Thanou, I.V.; Raftopoulou, E.; Ntzimani, A.; Taoukis, P.; Giannakourou, M.C. Bioaccessibility and stability Studies on encapsulated phenolics and carotenoids from olive and tomato pomace: Development of a functional fruit beverage. Appl. Sci. 2024, 14, 10495. [Google Scholar] [CrossRef]
- Araújo, M.; Pimentel, F.B.; Alves, R.C.; Oliveira, M.B.P. Phenolic compounds from olive mill wastes: Health effects, analytical approach and application as food antioxidants. Trends Food Sci. Technol. 2015, 45, 200–211. [Google Scholar] [CrossRef]
- Zbakh, H.; El Abbassi, A. Potential use of olive mill wastewater in the preparation of functional beverages: A review. J. Funct. Foods 2012, 4, 53–65. [Google Scholar] [CrossRef]
- Andreou, V.; Chanioti, S.; Stergiou, P.; Katsaros, G. Valorization of the olive oil production residue: Healthy ingredient for developing high value-added spread. Sustainability 2021, 13, 13984. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Koubaa, M.; Moubarik, A.; Lopes, R.P.; Saraiva, J.A.; Boussetta, N.; Grimi, N.; Barba, F.J. Emerging oppor-tunities for the effective valorization of wastes and by-products generated during olive oil production process: Non-conventional methods for the recovery of high-added value compounds. Trends Food Sci. Technol. 2015, 45, 296–310. [Google Scholar] [CrossRef]
- Meng, Q.; Long, P.; Zhou, J.; Ho, C.T.; Zou, X.; Chen, B.; Zhang, L. Improved Absorption of β-carotene by encapsulation in an oil-in-water nanoemulsion containing tea polyphenols in the aqueous phase. FoodRes. Int. 2019, 116, 731–736. [Google Scholar] [CrossRef]
- Ezhilarasi, P.N.; Karthik, P.; Chhanwal, N.; Chinnaswamy, A. Nanoencapsulation techniques for food bioactive components: A review. Food Bioproc.Technol. 2013, 6, 628–647. [Google Scholar] [CrossRef]
- Pérez-Andrés, J.M.; Charoux, C.M.; Cullen, P.J.; Tiwari, B.K. Chemical modifications of lipids and proteins by nonthermal food processing technologies. J. Agric. Food Chem. 2018, 66, 5041–5054. [Google Scholar] [CrossRef] [PubMed]
- Benshitrit, R.C.; Levi, C.S.; Tal, S.L.; Shimoni, E.; Lesmes, U. Development of oral food-grade delivery systems: Current knowledge and future challenges. Food Funct. 2012, 3, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Han, Y.; Jian, L.; Liao, W.; Zhang, Y.; Gao, Y. Fabrication, characterization, physicochemical stability of zein-chitosan nanocomplex for co-encapsulating curcumin and resveratrol. Carbohydr. Polym. 2020, 236, 116090. [Google Scholar] [CrossRef]
- Han, Y.; Pei, Y.; Wang, J.; Xiao, Z.; Miao, Y.; Wang, Z.; Zhang, F.; Hou, W.; Yi, Y.; Chen, S. Research progress on the nano-delivery systems of food-derived bioactive components. Food Biosci. 2024, 62, 105189. [Google Scholar] [CrossRef]
- Shin, G.H.; Kim, J.T.; Park, H.J. Recent developments in nanoformulations of lipophilic functional foods. Trends Food Sci. Technol. 2015, 46, 144–157. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, H.; Chen, H.; Lin, J.; Wang, Q. Food-grade nanoemulsions: Preparation, stability and application in encapsulation of bioactive compounds. Molecules 2019, 24, 4242. [Google Scholar] [CrossRef]
- Aboalnaja, K.O.; Yaghmoor, S.; Kumosani, T.A.; Mc Clements, D.J. Utilization of nanoemulsions to enhance bioactivity of pharmaceuticals, supplements, and nutraceuticals: Nanoemulsion delivery systems and nanoemulsion excipient systems. Expert. Opin. Drug Deliv. 2016, 13, 1327–1336. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols-a review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- da Silva Soares, B.; de Carvalho, C.W.P.; Garcia-Rojas, E.E. Microencapsulation of sacha inchi oil by complex coacervates using ovalbumin-tannic acid and pectin as wall materials. Food Bioproc. Technol. 2021, 14, 817–830. [Google Scholar] [CrossRef]
- Favaro-Trindade, C.S.; Patel, B.; Silva, M.P.; Comuniana, T.A.; Federici, E.; Jones, O.G.; Campanella, O.H. Microencapsulation as a tool to producing an extruded functional food. LWT 2020, 128, 1094. [Google Scholar] [CrossRef]
- Göktepe, S.; Ocak, B.; Özdestan, Ö. Physico-chemical, sensory, and antioxidant characteristics of olive paste enriched with microencapsulated thyme essential oil. Food Bioproc. Technol. 2021, 14, 2032–2045. [Google Scholar] [CrossRef]
- Lanza, B.; Di Serio, M.G.; Giansante, L.; Di Loreto, G.; Russi, F.; Di Giacinto, L. Effects of pasteurisation and storage on quality characteristics of table olives preserved in olive oil. Int. J. Food Sci. Technol. 2013, 48, 2630–2637. [Google Scholar] [CrossRef]
- Cosmai, L.; Campanella, D.; Summo, C.; Paradiso, V.M.; Pasqualone, A.; De Angelis, M.; Caponio, F. Combined effects of a natural Allium spp. extract and modified atmo spheres packaging on shelf life extension of olive-based paste. Int. J. Food Sci. Technol. 2017, 52, 1164–1175. [Google Scholar] [CrossRef]
- Cosmai, L.; Campanella, D.; De Angelis, M.; Summo, C.; Paradiso, V.M.; Pasqualone, A.; Caponio, F. Use of starter cultures for table olives fermentation as possibility to improve the quality of thermally stabilized olive-based paste. LWT 2018, 90, 381–388. [Google Scholar] [CrossRef]
- Nanis, I.; Hatzikamari, M.; Katharopoulos, E.; Boukouvala, E.; Ekateriniadou, E.; Litopoulou-Tzanetaki, E.; Gerasopoulos, D. Microbiological and physicochemical changes during fermentation of solid residue of olive mill wastewaters: Exploitation towards the production of an olive paste–type product. LWT 2020, 117, 108671. [Google Scholar] [CrossRef]
- Schwartz, M.; Quitral, V.; Daccarett, C.; Callejas, J. Development of spreadable olive paste from the Sevillana variety. Grasas Y Aceites 2009, 60, 451–457. [Google Scholar] [CrossRef]
- Choi, M.H.; Kim, G.H.; Lee, H.S. Effects of ascorbic acid retention on juice color and pigment stability in blood orange (Citrus sinensis) juice during refrigerated storage. Food Res. Int. 2002, 35, 753–759. [Google Scholar] [CrossRef]
- Stoll, L.; Costa, T.M.H.; Jablonski, A.; Flôres, S.H.; Rios, A.O. Microencapsulation of anthocyanins with different wall materials and its application in active biodegradable films. Food Bioproc. Technol. 2016, 9, 172–181. [Google Scholar] [CrossRef]
- Jafari, S.M.; Ghanbari, V.; Dehnad, D.; Ganje, M. Improving the storage stability of tomato paste by the addition of encapsu lated olive leaf phenolics and experimental growth modeling of A. flavus. Int. J. Food Microbiol. 2021, 338, 109018. [Google Scholar] [CrossRef]
- Talhaoui, N.; Taamalli, A.; Gómez-Caravaca, A.M.; Fernández Gutiérrez, A.; Segura-Carretero, A. Phenolic compounds in olive leaves: Analytical determination, biotic and abiotic influence, and health benefits. Food Res. Int. 2015, 77, 92–108. [Google Scholar] [CrossRef]
- Karagozlu, M.; Ocak, B.; Özdestan-Ocak, Ö. Effect of tannic acid concentration on the physicochemical, thermal, and anti oxidant properties of gelatin/gum Arabic–walled microcapsules containing Origanum onites L. essential oil. Food Bioproc. Technol. 2021, 15, 795–806. [Google Scholar] [CrossRef]
- Mahungu, S.M.; Diaz-Mercado, S.; Li, J.; Schwenk, M.; Singletary, K.; Faller, J. Stability of isoflavones during extrusion processing of corn/soy mixture. J. Agric. Food Chem. 1999, 47, 279–284. [Google Scholar] [CrossRef]
- Erskine, E.; Özkan, G.; Lu, B.; Capanoglu, E. Effects of fermentation process on the antioxidant capacity of fruit byproducts. ACS Omega 2023, 8, 4543–4553. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.I.F.S.; Jongen, W.M.F.; van Boekel, M.A.J.S. A review of Maillard reaction in food and implications to kinetic modelling. Trends Food Sci. Technol. 2000, 11, 364–373. [Google Scholar] [CrossRef]
- Bolchini, S.; Larcher, R.; Morozova, K.; Scampicchio, M.; Nardin, T. Screening of Antioxidant Maillard Reaction Products Using HPLC-HRMS and Study of Reaction Conditions for Their Production as Food Preservatives. Molecules 2024, 29, 4820. [Google Scholar] [CrossRef]
- Cam, M.; Icyer, N.C.; Erdogan, F. Pomegranate peel phenolics: Microencapsulation, storage stability and potential ingredient for functional food development. LWT 2014, 55, 117–123. [Google Scholar] [CrossRef]
- Lanza, B.; Amoruso, F. Sensory analysis of natural table olives: Relationship between appearance of defect and gustatory-kinaesthetic sensation changes. LWT 2016, 68, 365–372. [Google Scholar] [CrossRef]
- Gotoh, N.; Wada, S. The importance of peroxide value in assessing food quality and food safety. J. Amer Oil Chem. Soc. 2006, 83, 473–474. [Google Scholar] [CrossRef]
- Pleasance, E.A.; Kerr, W.L.; Pegg, R.B.; Swanson, R.B.; Cheely, A.N.; Huang, G.; Parrish, D.R.; Kerrihard, A.L. Effects of storage conditions on consumer and chemical assessments of raw ‘nonpareil’almonds over a two-year period. J. Food Sci. 2018, 83, 822–830. [Google Scholar] [CrossRef]
- Karaaslan, M.; Şengün, F.; Cansu, Ü.; Başyiğit, B.; Sağlam, H.; Karaaslan, A. Gum arabic/maltodextrin microencapsulation confers peroxidation stability and antimicrobial ability to pepper seed oil. Food Chem. 2021, 337, 127748. [Google Scholar] [CrossRef] [PubMed]
- Lanza, B.; Di Serio, M.G.; Iannucci, E.; Russi, F.; Marfisi, P. Nutritional, textural and sensorial characterisation of Italian table olives (Olea europaea L. cv. ‘Intosso d’Abruzzo’). Int. J. Food Sci. Technol. 2009, 45, 67–74. [Google Scholar] [CrossRef]
- European Food Safety Authority. Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food on a request from the Commission on the safety in use of lycopene as a food colour. EFSA J. 2008, 674, 1–66. [Google Scholar]
- Ramirez, E.; García-García, P.; de Castro, A.; Romero, C.; Brenes, M. Debittering of black dry-salted olives. Eur. J. Lipid Sci. Technol. 2013, 115, 1319–1324. [Google Scholar] [CrossRef]
- Tamer, C.E.; Incedayı, B.; Yıldız, B.; Çopur, Ö.U. The Use of vacuum impregnation for debittering green olives. Food Bioprocess Technol. 2013, 6, 3604–3612. [Google Scholar] [CrossRef]
- AOAC. Loss on Drying (Moisture) in Nuts and Nut Products. In Official Methods of Analysis of AOAC International, 22nd ed.; Latimer, G.W., Jr., Ed.; AOAC 925.40; Oxford University Press: Oxford, UK, 1995. [Google Scholar] [CrossRef]
- AOAC. Fat (Crude) in Nuts and Nut Products: Gravimetric methods. In Official Methods of Analysis AOAC International, 17th ed.; Horwitz, W., Ed.; AOAC 948.22; Oxford University Press: Oxford, UK, 2000. [Google Scholar] [CrossRef]
- AOAC. Fat (Crude) or Ether Extract in Animal Feed. In Official Methods of Analysis AOAC International, 22nd ed.; Latimer, G.W., Jr., Ed.; AOAC 920.39; Oxford University Press: Oxford, UK, 2023. [Google Scholar] [CrossRef]
- AOAC. Fat (Total, Saturated, and Unsaturated) in Foods: Hydrolytic Extraction Gas Chromatographic Method. In Official Methods of Analysis of AOAC International, 22nd ed.; Latimer, G.W., Jr., Ed.; AOAC 996.06; Oxford University Press: Oxford, UK, 2023. [Google Scholar] [CrossRef]
- ISO 1871; Food and Feed Products-General Guidelines for the Determination of Nitrogen by the Kjeldahl Method, 2nd ed. The International Organization for Standardization: Geneva, Switzerland, 2009. Available online: https://www.iso.org/obp/ui/#iso:std:iso:1871:ed-2:v1:en (accessed on 12 March 2025).
- AOAC. 991.43 Total, Soluble, and insoluble dietary fiber in foods: Enzymatic-gravimetric method, MES-TRIS Buffer. In Official Methods of Analysis of AOAC International, 22nd ed.; Latimer, G.W., Jr., Ed.; Oxford University Press: Oxford, UK, 2023. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Method 613: 2,3,7,8-Tetrachlorodibenzo-p-Dioxin by Capillary Column GC/MS. In Compendium of Methods for the Determination of Toxic Organic Compounds in Ambient Air; US EPA: Cincinnati, OH, USA, 1982. [Google Scholar]
- United States Environmental Protection Agency. Method 200.8: Determination of Trace Elements in Waters and Wastes by Inductively Coupled Plasma–Mass Spectrometry, Revision 5.4; US EPA: Cincinnati, OH, USA, 1994. [Google Scholar]
- Katsouli, M.; Giannou, V.; Tzia, C. Enhancement of physicochemical and encapsulation stability of O1/W/O2 multiple na noemulsions loaded with coenzyme Q10 or conjugated linoleic acid by incorporating polyphenolic extract. Food Funct. 2020, 11, 8878–8892. [Google Scholar] [CrossRef]
- Bancuta, O.R.; Chilian, A.; Bancuta, I.; Ion, R.M.; Setnescu, R.; Setnescu, T.; Gheboianu, A. Improvement of spectrophotometric method for determination of phenolic compounds by statistical investigations. Rom. Journ. Phys. 2016, 61, 1255–1264. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- AOCS Cd 8-53; Peroxide Value-Acetic Acid-Chloroform Method. AOCS: Champaign, IL, USA, 2003.
- ISO 13300-1:2006; Sensory Analysis—General Guidance for the Staff of a Sensory Evaluation Laboratory—Part 1: Staff Responsibilities. International Organization for Standardization: Geneva, Switzerland, 2022.
- ISO 13300-2:2006; Sensory Analysis—General Guidance for the Staff of a Sensory Evaluation Laboratory—Part 2: Recruitment and Training of Panel Leaders. International Organization for Standardization: Geneva, Switzerland, 2022.
- ISO 8589:2007; Sensory Analysis—General Guidance for the Design of Test Rooms. International Organization for Standardization: Geneva, Switzerland, 2017.
| Physicochemical/Quality Property | Product | |
|---|---|---|
| OPD | OPDEnr | |
| Fibers, total dietary (g/10 0 g) | 10.0 ± 0.8 | |
| Nitrogen content (g/100 g) | 0.49 ± 0.04 | |
| Proteins (g/100 g) | 3.1 ± 0.3 | |
| Lipids (g/100 g) | 15.4 ± 0.5 | |
| Saturated | 2.73 ± 0.21 | |
| Monounsaturated | 10.6 ± 0.83 | |
| Polyunsaturated | 2.01 ± 0.18 | |
| Carbohydrates (g/100 g) | 13.1 | |
| Sugars, total (g/100 g) | 1.26 ± 0.12 | |
| Sodium (mg Na/100 g) | 410 ± 50 | |
| Salt content (g/100 g) | 1.03 ± 0.13 | |
| Total energy (Kcal/100 g) | 223 | |
| pH | 3.984 ± 0.007 a | 4.059 ± 0.008 a |
| Ash content (g/100 g) | 1.40 ± 0.1 a | 1.50 ± 0.11 a |
| Moisture content (g/100 g) | 54.2 ± 0.06 a | 55.2 ± 0.4 a |
| Water activity (aw) | 0.898 ± 0.090 a | 0.908 ± 0.091 a |
| Titratable acidity (ΤA%) | 3.980 ± 0.018 a | 4.845 ± 0.193 b |
| Total phenolic content (TPC) (mg GAE/g) | 1.62 ± 0.08 a | 3.05 ± 0.10 b |
| Antioxidant capacity (AA) (mg Trolox/g) | 0.801 ± 0.075 a | 0.976 ± 0.032 b |
| Total carotenoids (TC) (mg/kg) | 20.0 ± 2.00 a | 30.2 ± 1.06 b |
| Color (CIE, L parameter) | 16.6 ± 2.01 a | 19.8 ± 0.21 b |
| Color (CIE, a parameter) | 7.47 ± 0.63 a | 8.64 ± 0.11 b |
| Color (CIE, b parameter) | 13.9 ± 1.73 a | 18.0 ± 0.01 b |
| Texture (firmness) (n) | 0.839 ± 0.093 a | 0.427 ± 0.040 b |
| Score of overall sensory quality | 9.0 ± 0.0 a | 8.0 ± 0.2 b |
| TMVC (logCFU/g) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Storage Time (d) | 20 °C | 30 °C | 40 °C | |||||
| OPD | OPDEnr | OPD | OPDEnr | OPD | OPDEnr | |||
| 0 | 2.76 ± 0.14 aA | 3.17 ± 0.27 aA | 0 | 2.76 ± 0.14 aA | 3.17 ± 0.27 aA | 0 | 2.76 ± 0.14 aA | 3.17 ± 0.27 aA |
| 24 | 3.41 ± 0.59 aA | 3.30 ± 0.15 aA | 24 | 3.16 ± 0.04 aA | 3.00 ± 0.45 aA | 24 | 2.93 ± 0.22 aA | <2.00 |
| 64 | 3.56 ± 0.23 aA | 3.30 ± 0.23 aA | 64 | 3.24 ± 0.34 aA | 3.05 ± 0.13 aA | 64 | 2.89 ± 0.80 aA | <2.00 |
| 119 | 3.12 ± 0.28 aA | 3.43 ± 0.59 aA | 106 | 3.18 ± 025 aA | 3.12 ± 0.34 aA | 106 | 2.30 ± 0.11 aA | <2.00 |
| Product | OPD | OPDEnr | ||
|---|---|---|---|---|
| T (°C) | ks (d−1) | R2 | ks (d−1) | R2 |
| 20 | 0.0166 ± 0.0047 aA | 0.896 | 0.0264 ± 0.0041 bA | 0.976 |
| 30 | 0.0363 ± 0.0033 aB | 0.984 | 0.0439 ± 0.0086 aB | 0.895 |
| 40 | 0.0461 ± 0.0072 aB | 0.871 | 0.0535 ± 0.0115 aB | 0.806 |
| Ea (kJ/mol) kref(d−1) (Tref = 25°C) | 39.2 ± 11.2 a 0.0236 ± 0.0028 a | 0.924 | 27.2 ± 6.33 a 0.0333 ± 0.0035 b | 0.948 |
| Ingredient | Product (g/100 g) | |
|---|---|---|
| OPD | OPDEnr | |
| Dry, debittered olive pomace | 16.7 | 16.7 |
| Pepper | 15.0 | 15.0 |
| Olive rings | 11.1 | 11.1 |
| Glycerol | 10.0 | 10.0 |
| Pomace oil | 10.0 | 7.5 |
| BACS | _ | 10.0 |
| Maltodextrin | 7.5 | _ |
| Vinegar | 7.0 | 7.0 |
| Tomato | 3.0 | 3.0 |
| Caper | 3.0 | 3.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dermesonlouoglou, E.; Limnaios, A.; Bouskou, I.; Ntzimani, A.; Tsevdou, M.; Taoukis, P. Design, Production and Quality Assessment of Antioxidant-Enriched Olive Paste Dips Using Agro-Food By-Products. Molecules 2025, 30, 3459. https://doi.org/10.3390/molecules30173459
Dermesonlouoglou E, Limnaios A, Bouskou I, Ntzimani A, Tsevdou M, Taoukis P. Design, Production and Quality Assessment of Antioxidant-Enriched Olive Paste Dips Using Agro-Food By-Products. Molecules. 2025; 30(17):3459. https://doi.org/10.3390/molecules30173459
Chicago/Turabian StyleDermesonlouoglou, Efimia, Athanasios Limnaios, Ioanna Bouskou, Athina Ntzimani, Maria Tsevdou, and Petros Taoukis. 2025. "Design, Production and Quality Assessment of Antioxidant-Enriched Olive Paste Dips Using Agro-Food By-Products" Molecules 30, no. 17: 3459. https://doi.org/10.3390/molecules30173459
APA StyleDermesonlouoglou, E., Limnaios, A., Bouskou, I., Ntzimani, A., Tsevdou, M., & Taoukis, P. (2025). Design, Production and Quality Assessment of Antioxidant-Enriched Olive Paste Dips Using Agro-Food By-Products. Molecules, 30(17), 3459. https://doi.org/10.3390/molecules30173459









