Isolating and Determining the Structures of Colored Products from the Reactions of Cannabinoids with Fast Blue RR
Abstract
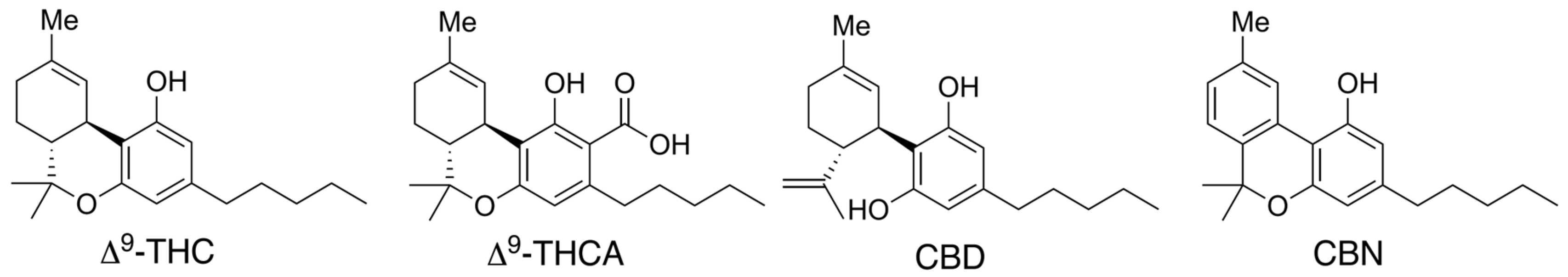
1. Introduction
2. Results
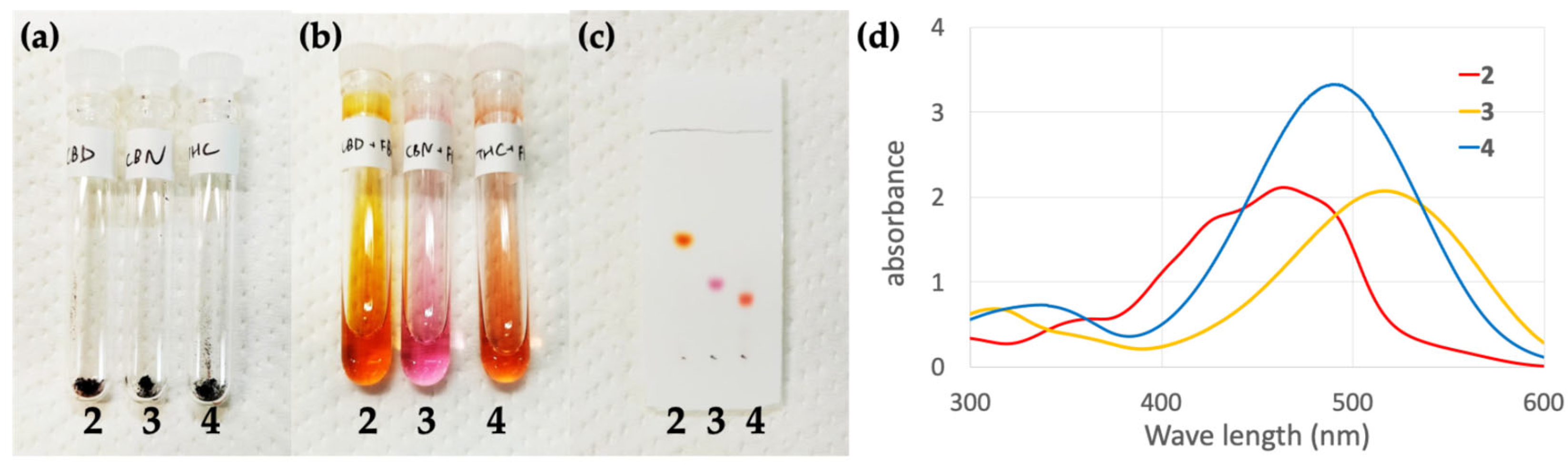
2.1. Colored Product 2 from the Reaction of CBD with Fast Blue RR
2.2. Colored Product 3 from the Reaction of CBN with Fast Blue RR
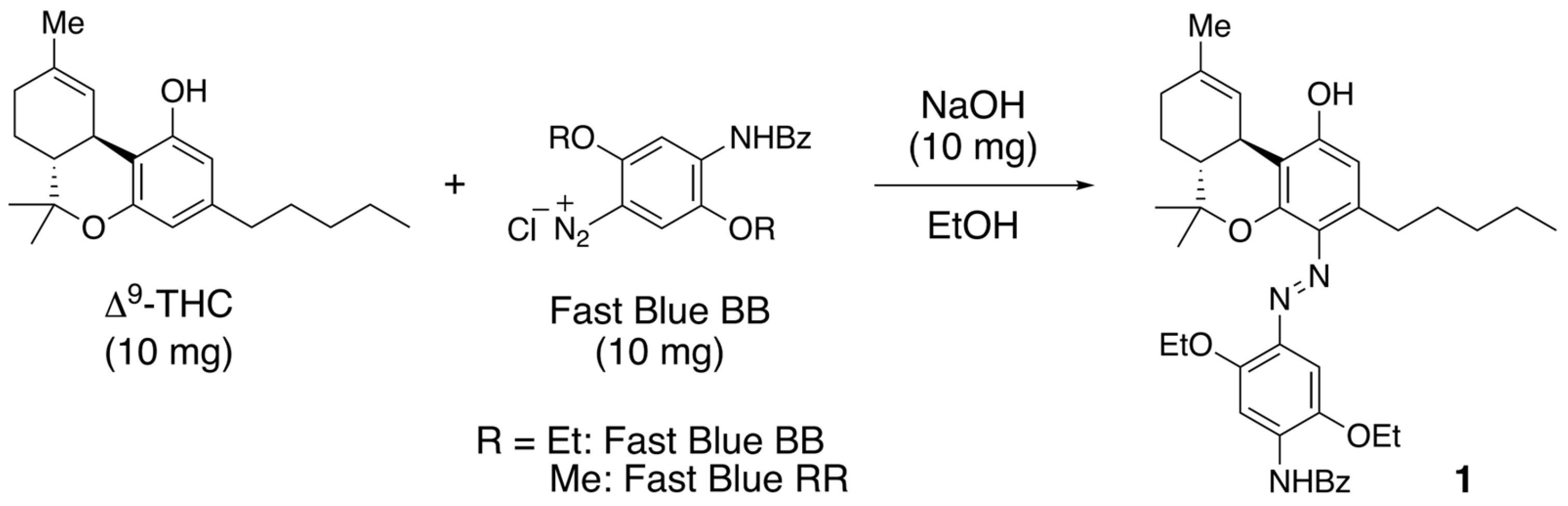
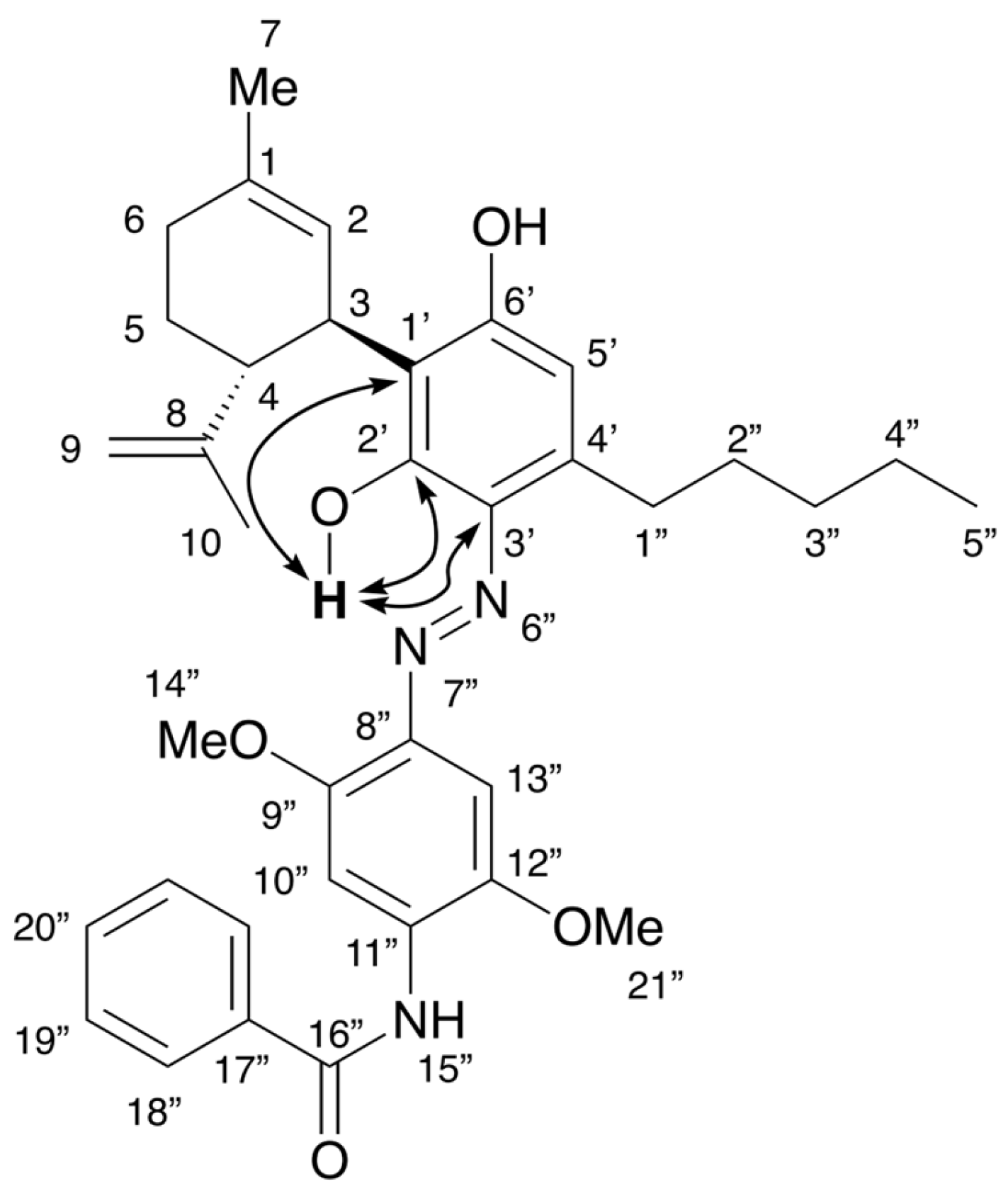
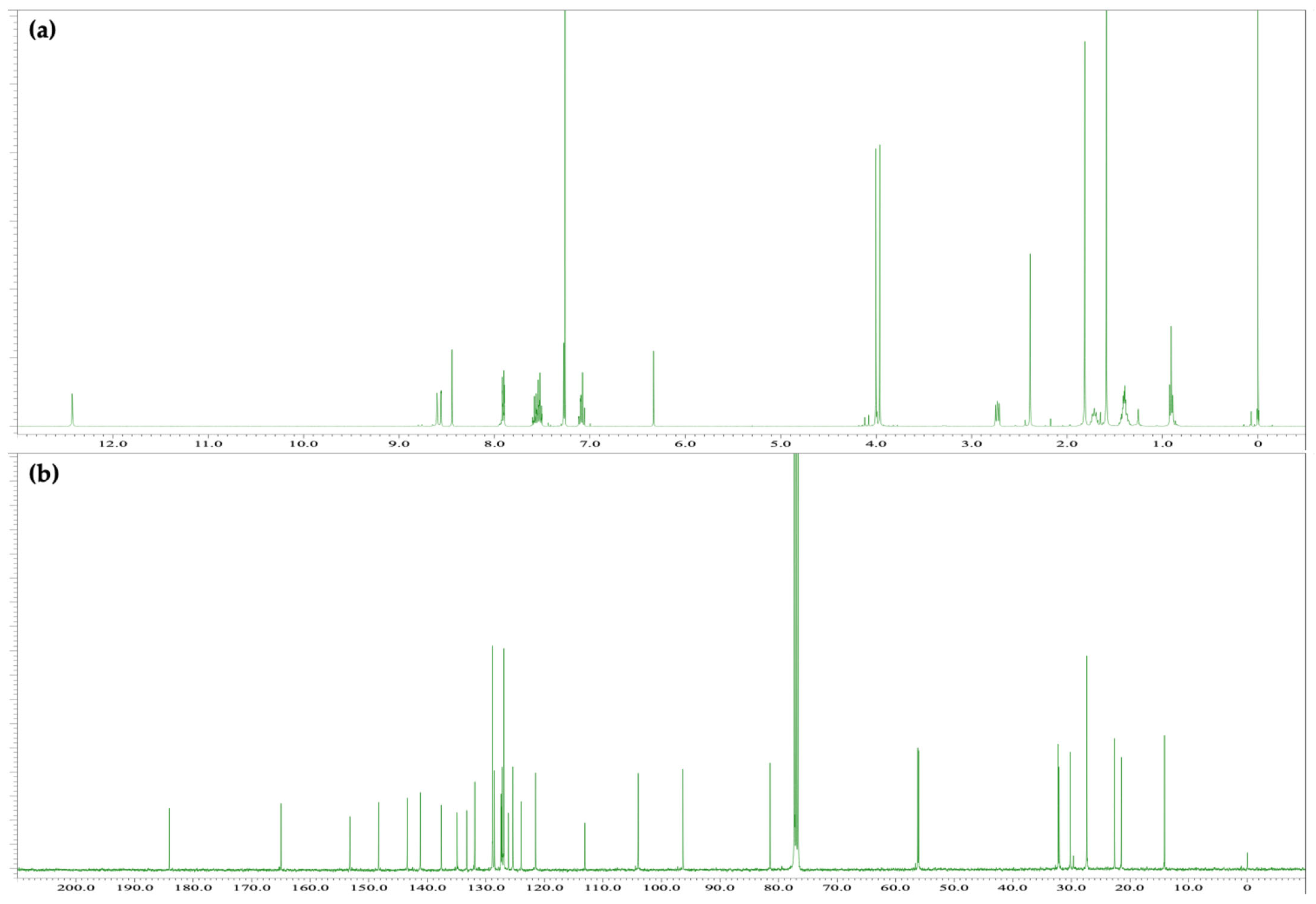
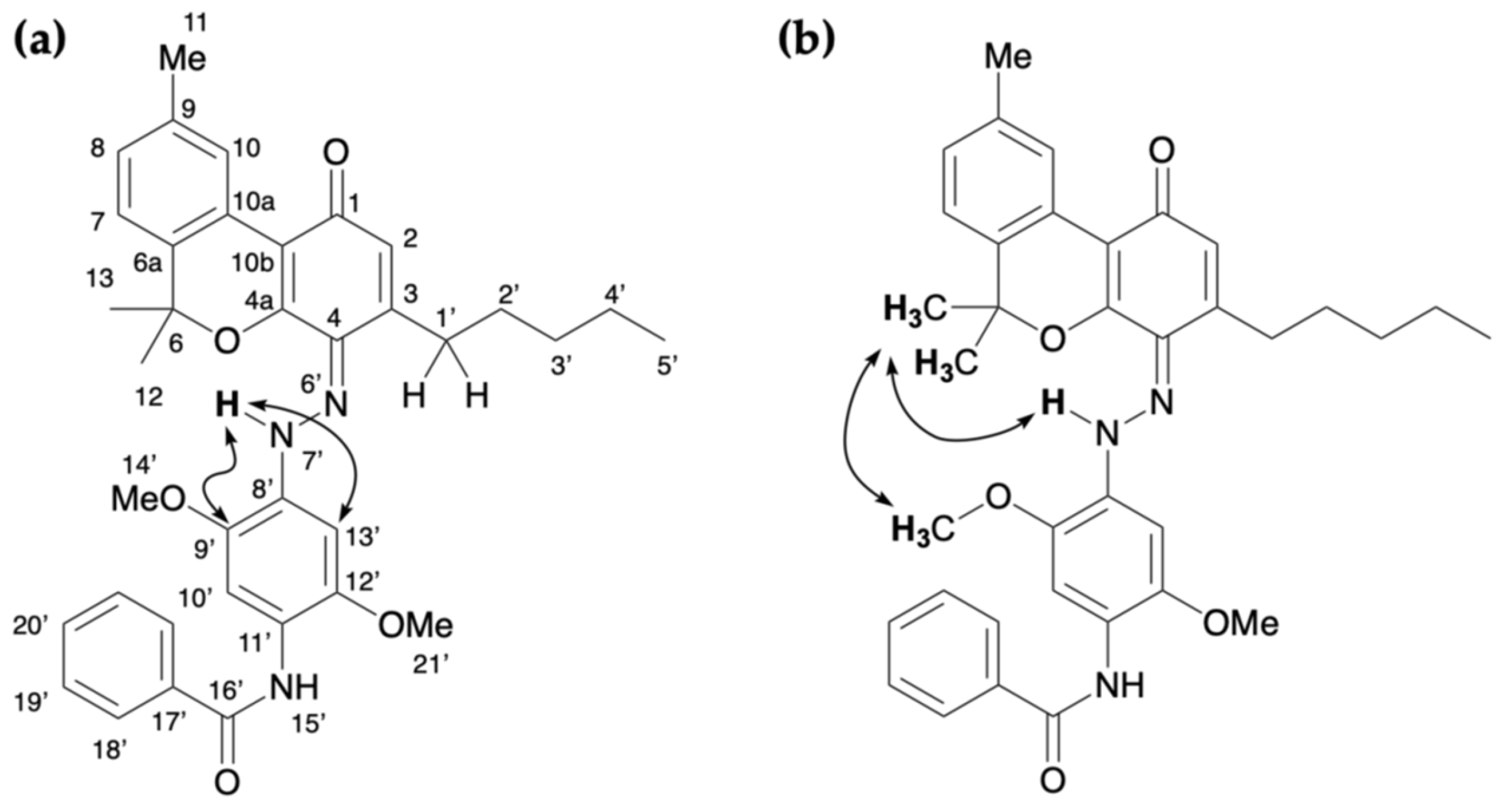
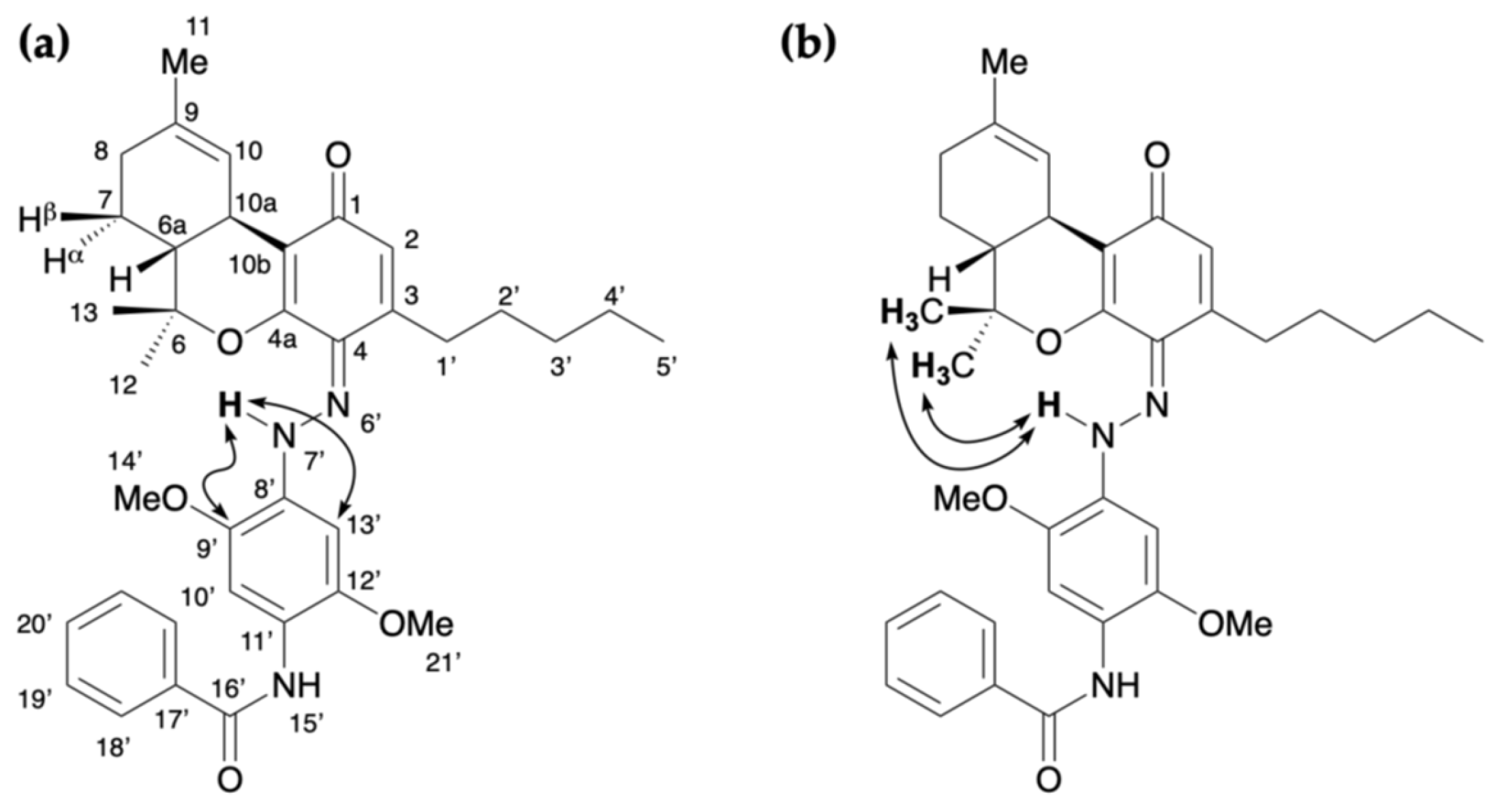
2.3. Colored Product 4 from the Reaction of Δ9-THC with Fast Blue RR
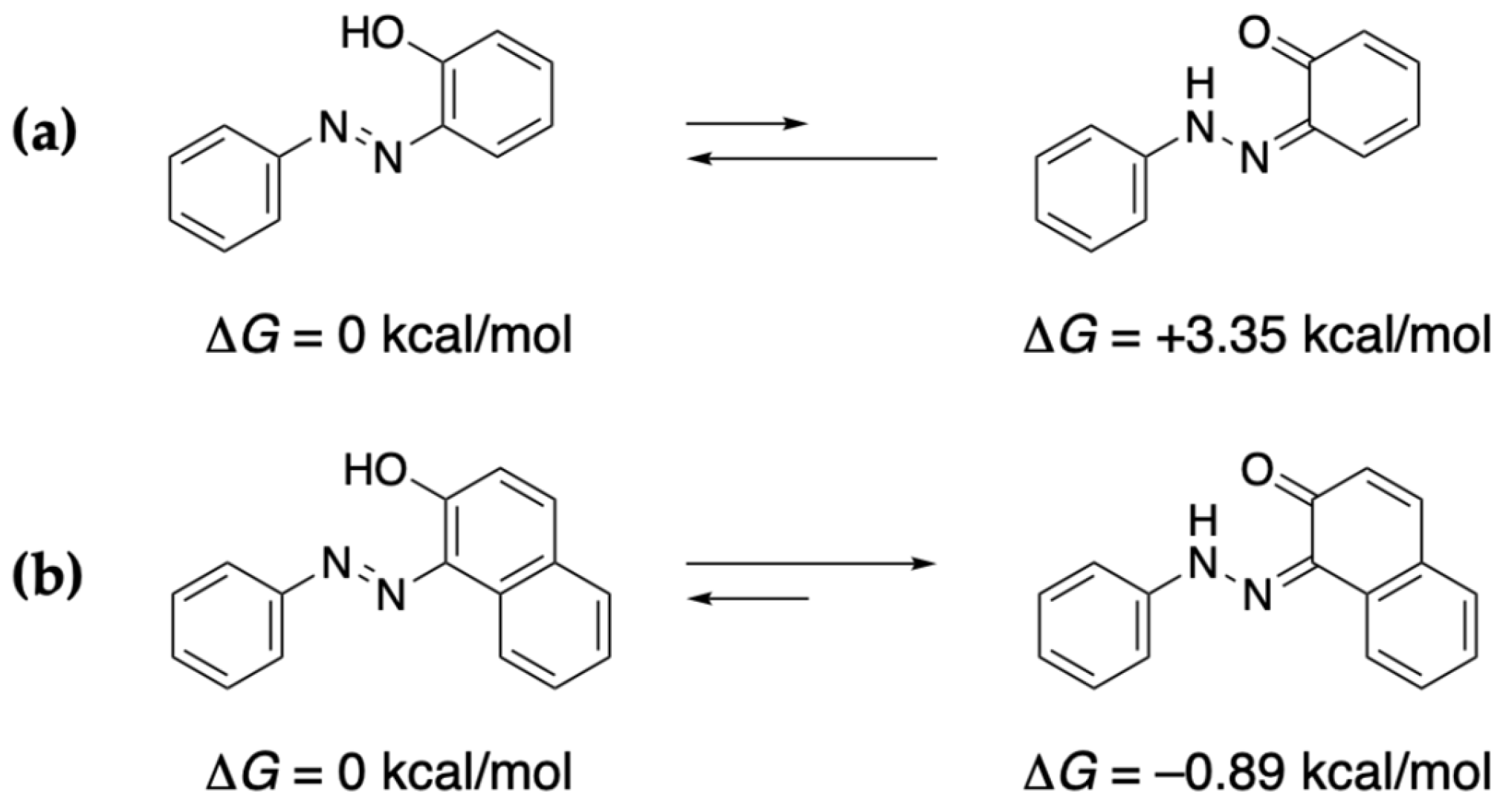
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. General Synthesis and Analysis Information
4.3. General Methods for Identifying Colored Products
4.4. Spectral Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hanus, L.O. Pharmacological and therapeutic secrets of plant and brain (endo)cannabinoids. Med. Res. Rev. 2009, 29, 213–271. [Google Scholar] [CrossRef]
- Leghissa, A.; Hildenbrand, Z.L.; Schug, K.A. A review of methods for the chemical characterization of cannabis natural products. J. Sep. Sci. 2018, 41, 398–415. [Google Scholar] [CrossRef]
- Ministry of Health, Labour and Welfare. Available online: https://www.mhlw.go.jp/content/11120000/001313370.pdf (accessed on 9 August 2025).
- Ministry of Health, Labour and Welfare. Available online: https://www.mhlw.go.jp/content/11126000/001279247.pdf (accessed on 15 August 2025).
- Tahir, M.N.; Shahbazi, F.; Rondeau-Gagné, S.; Trant, J.F. The biosynthesis of the cannabinoids. J. Cannabis Res. 2021, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Leweke, F.M.; Piomelli, D.; Pahlisch, F.; Muhl, D.; Gerth, C.W.; Hoyer, C.; Klosterkötter, J.; Hellmich, M.; Koethe, D. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl. Psychiatry 2012, 2, e94. [Google Scholar] [CrossRef] [PubMed]
- Tzadok, M.; Uliel-Siboni, S.; Linder, I.; Kramer, U.; Epstein, O.; Menascu, S.; Nissenkorn, A.; Yosef, O.B.; Hyman, E.; Granot, D.; et al. CBD-enriched medical cannabis for intractable pediatric epilepsy. The current Israeli experience. Seizure 2016, 35, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Strickland, J.C.; Jackson, H.; Schlienz, N.J.; Salpekar, J.A.; Martin, E.L.; Munson, J.; Bonn-Miller, M.O.; Vandrey, R. Cross-sectional and longitudinal evaluation of cannabidiol (CBD) product use and health among people with epilepsy. Epilepsy Behav. 2021, 122, 108205. [Google Scholar] [CrossRef]
- Khouchlaa, A.; Khouri, S.; Hajib, A.; Zeouk, I.; Amalich, S.; Msairi, S.; El Menyiy, N.; Rais, C.; Lahyaoui, M.; Khalid, A.; et al. Health benefits, pharmacological properties, and metabolism of cannabinol: A comprehensive review. Ind. Crops Prod. 2024, 213, 118359. [Google Scholar] [CrossRef]
- Watanabe, K.; Yamaori, S.; Yamamoto, I. A Study on the Culture and Sciences of the Cannabis and Marihuana XXIV. Bull. Hokuriku Univ. 2013, 37, 27–35. [Google Scholar]
- Balfour, A. Fourth Report of the Wellcome Tropical Research Laboratories at the Gordon Memorial College, Khartoum; Baillière, Tindall & Cox: London, UK, 1911. [Google Scholar]
- Adams, R.; Hunt, M.; Clark, J.H. Structure of cannabidiol, a product isolated from the marihuana extract of Minnesota wild hemp. I. J. Am. Chem. Soc. 1940, 62, 196–200. [Google Scholar] [CrossRef]
- Mechoulam, R.; Gaoni, Y. Recent advances in the chemistry of hashish. Fortschr. Chem. Org. Naturst. 1967, 25, 175–213. [Google Scholar] [CrossRef]
- Mechoulam, R.; Ben-Zvi, Z. Hashish–XIII: On the nature of the beam test. Tetrahedron 1968, 24, 5615–5624. [Google Scholar] [CrossRef] [PubMed]
- Ghamrawy, M.A. The detection of Cannabis indica. A new test. J. Egypt. Med. Assoc. 1937, 20, 193–208. [Google Scholar]
- Kovar, K.A.; Keilwagen, S.A. Zur Kenntnis der Ghamrawy-Reaktion auf Haschisch und Marihuana. Arch. Pharm. 1984, 317, 724–732. [Google Scholar] [CrossRef]
- Duquenois, P.; Moustapha, H.N. Identification and assay of Cannabis indica. J. Egypt. Med. Assoc. 1938, 21, 224–227. [Google Scholar]
- Kovar, K.A.; Keck, M.A. The Duquenois reaction for hashish and marihuana. Arch. Pharm. 1988, 321, 249–252. [Google Scholar] [CrossRef]
- Korte, F.; Sieper, H. Zur chemischen Klassifizierung von Pflanzen XXIV. Untersuchung von Haschisch-Inhaltsstoffen durch Dünnschichtchromatographie. J. Chromatogr. 1964, 13, 90–98. [Google Scholar] [CrossRef]
- França, H.S.; Acosta, A.; Jamal, A.; Romao, W.; Mulloor, J.; Almirall, J.R. Experimental and ab initio investigation of the products of reaction from Δ9-tetrahydrocannabinol (Δ9-THC) and the fast blue BB spot reagent in presumptive drug tests for cannabinoids. Forensic Chem. 2020, 17, 100212. [Google Scholar] [CrossRef]
- Mano-Sousa, B.J.; Maia, G.A.S.; Lima, P.L.; Campos, V.A.; Negri, G.; Chequer, F.M.D.; Duarte-Almeida, J.M. Color determination method and evaluation of methods for the detection of cannabinoids by thin-layer chromatography (TLC). J. Forensic Sci. 2021, 66, 854–865. [Google Scholar] [CrossRef]
- Tanaka, R.; Mizutani, S.; Kawamura, M.; Fuchino, H.; Kawahara, N.; Kikura–Hanajiri, R. Determination of 11 Cannabinoids in Cannabis sativa L. by Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry (LC-Q-TOF-MS). Yakugaku Zasshi 2023, 143, 411–418. [Google Scholar] [CrossRef]
- ElSohly, M.A.; Slade, D. Chemical constituents of marijuana: The complex mixture of natural cannabinoids. Life Sci. 2005, 78, 539–548. [Google Scholar] [CrossRef]
- Özen, A.S.; Doruker, P.; Aviyente, V. Effect of cooperative hydrogen bonding in azo–hydrazone tautomerism of azo dyes. J. Phys. Chem. A 2007, 111, 13506–13514. [Google Scholar] [CrossRef]
- Karci, F.; Ertan, N. Visible absorption spectra of some novel heteroarylazo disperse dyes derived from 2-hydroxy-1,4-nephthoquinone. Color. Technol. 2005, 121, 153–157. [Google Scholar] [CrossRef]
- Webster, G.R.B.; Sarna, L.P.; Mechoulam, R. Conversion of CBD to Δ8-THC and Δ9-THC. U.S. Patent 7399872B2, 15 July 2008. [Google Scholar]
- Choi, Y.H.; Hazekamp, A.; Peltenburg-Looman, A.M.G.; Frédérich, M.; Erkelens, C.; Lefeber, A.W.M.; Verpoorte, R. NMR assignments of the major cannabinoids and cannabiflavonoids isolated flowers of Cannabis sativa. Phytochem. Anal. 2004, 15, 345–354. [Google Scholar] [CrossRef]


| 1H | 13C | 1H | 13C | ||
|---|---|---|---|---|---|
| 1 | 140.0 | 1″ | 3.04−2.97 (m, 1H) 2.91−2.84 (m, 1H) | 32 | |
| 2 | 5.58 (s, 1H) | 124.3 | 2″ | 1.71−1.66 (m, 2H) | 31.6 |
| 3 | 4.22–4.16 (m, 1H) | 34.6 | 3″ | 1.41−1.34 (m, 2H) | 32 |
| 4 | 2.48–2.40 (m, 1H) | 46.7 | 4″ | 1.41−1.34 (m, 2H) | 22.6 |
| 5 | 1.85–1.80 (m, 2H) | 28.0 | 5″ | 0.88 (t, 3H) | 14.1 |
| 6 | 2.30–2.20 (m, 1H) 2.13–2.08 (m, 1H) | 30.3 | 8″ | 133.1 | |
| 7 | 1.80 (s, 3H) | 23.7 | 9″ | 149.4 | |
| 8 | 147.5 | 10″ | 8.52 (s, 1H) | 104.1 | |
| 9 | 4.53 (s, 1H) 4.46 (s, 1H) | 111.2 | 11″ | 129.6 | |
| 10 | 1.75 (s, 3H) | 18.9 | 12″ | 142.9 | |
| 1′ | 115.2 | 13″ | 7.47 (s, 1H) | 97.5 | |
| 2′ | 158.5 | 14″ | 4.04 (s, 3H) | 56.6 | |
| 2′-OH | 15.71 (s, 1H) | 15″-NH | 8.74 (s, 1H) | ||
| 3′ | 131.0 | 16″ | 165.2 | ||
| 4′ | 145.1 | 17″ | 134.5 | ||
| 5′ | 6.31 (s, 1H) | 110.4 | 18″ | 7.93−7.91 (m, 2H) | 127.0 |
| 6′ | 160.2 | 19″ | 7.61−7.51 (m, 2H) | 128.9 | |
| 6′-OH | 6.53 (brs, 1H) | 20″ | 7.61−7.51 (m, 1H) | 132.0 | |
| 21″ | 3.96 (s, 3H) | 56.3 |
| 1H | 13C | 1H | 13C | ||
|---|---|---|---|---|---|
| 1 | 184.0 | 3′ | 1.44−1.33 (m, 2H) | 32 | |
| 2 | 6.33 (s, 1H) | 125.4 | 4′ | 1.44−1.33 (m, 2H) | 22.6 |
| 3 | 127 | 5′ | 0.91 (t, 3H) | 14.1 | |
| 4 | 148.3 | 7′-NH | 12.43 (s, 1H) | ||
| 4a | 153.2 | 8′ | 127 | ||
| 6 | 81.5 | 9′ | 141.2 | ||
| 6a | 133.2 | 10′ | 8.44 (s, 1H) | 104.0 | |
| 7 | 7.07 (d, 1H) | 121.5 | 11′ | 124.0 | |
| 8 | 7.15 (dd, 1H) | 128.5 | 12′ | 143.4 | |
| 9 | 137.6 | 13′ | 7.28 (s, 1H) | 96.3 | |
| 10 | 8.56 (d, 1H) | 127 | 14′ | 4.00 (s, 3H) | 56 |
| 10a | 126.1 | 15′-NH | 8.60 (s, 1H) | ||
| 10b | 113.1 | 16′ | 165.0 | ||
| 11 | 2.39 (s, 3H) | 21.5 | 17′ | 134.9 | |
| 12, 13 | 1.82 (s, 6H) | 27.4 | 18′ | 7.92−7.90 (m, 2H) | 126.9 |
| 1′ | 2.75–2.71 (m, 2H) | 32 | 19′ | 7.60−7.50 (m, 2H) | 128.8 |
| 2′ | 1.75–1.68 (m, 2H) | 30.2 | 20′ | 7.60−7.50 (m, 1H) | 131.9 |
| 21′ | 3.96 (s, 3H) | 56 |
| 1H | 13C | 1H | 13C | ||
|---|---|---|---|---|---|
| 1 | 185.2 | 3′ | 1.42−1.33 (m, 2H) | 32 | |
| 2 | 6.22 (s, 1H) | 124.6 | 4′ | 1.42−1.33 (m, 2H) | 22.6 |
| 3 | 127 | 5′ | 0.89 (t, 3H) | 14.1 | |
| 4 | 148.6 | 7′-NH | 12.21 (s, 1H) | ||
| 4a | 154.3 | 8′ | 127 | ||
| 6 | 82.1 | 9′ | 140.9 | ||
| 6a | 1.76−1.63 (m, 1H) | 45.1 | 10′ | 8.41 (s, 1H) | 104.0 |
| 7 | 1.50−1.42 (m, 1H, 7a) 1.94−1.87 (m, 1H, 7b) | 24.6 | 11′ | 123 | |
| 8 | 2.21−2.14 (m, 2H) | 31.2 | 12′ | 143.4 | |
| 9 | 133.5 | 13′ | 7.24 (s, 1H) | 96.3 | |
| 10 | 6.24 (t, 1H) | 123 | 14′ | 3.96 (s, 3H) | 56 |
| 10a | 3.16−3.09 (m, 1H) | 32.8 | 15′-NH | 8.58 (s, 1H) | |
| 10b | 115.4 | 16′ | 165.0 | ||
| 11 | 1.67 (s, 3H) | 23.2 | 17′ | 135.0 | |
| 12 | 1.25 (s, 3H) | 20.0 | 18′ | 7.92−7.88 (m, 2H) | 126.9 |
| 13 | 1.65 (s, 3H) | 27.1 | 19′ | 7.60−7.49 (m, 2H) | 128.8 |
| 1′ | 2.77−2.59 (m, 2H) | 32 | 20′ | 7.60−7.49 (m, 1H) | 131.8 |
| 2′ | 1.76−1.63 (m, 2H) | 30.3 | 21′ | 3.95 (s, 3H) | 56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, K.; Nishiguchi, H.; Arai, R.; Hamajima, R.; Abe, H.; Ishida, A.; Tokeshi, M.; Higashi, K.; Saitoh, A.; Takahashi, H. Isolating and Determining the Structures of Colored Products from the Reactions of Cannabinoids with Fast Blue RR. Molecules 2025, 30, 3462. https://doi.org/10.3390/molecules30173462
Nakamura K, Nishiguchi H, Arai R, Hamajima R, Abe H, Ishida A, Tokeshi M, Higashi K, Saitoh A, Takahashi H. Isolating and Determining the Structures of Colored Products from the Reactions of Cannabinoids with Fast Blue RR. Molecules. 2025; 30(17):3462. https://doi.org/10.3390/molecules30173462
Chicago/Turabian StyleNakamura, Kayo, Hikari Nishiguchi, Ryosuke Arai, Riho Hamajima, Hiroko Abe, Akihiko Ishida, Manabu Tokeshi, Kyohei Higashi, Akiyoshi Saitoh, and Hideyo Takahashi. 2025. "Isolating and Determining the Structures of Colored Products from the Reactions of Cannabinoids with Fast Blue RR" Molecules 30, no. 17: 3462. https://doi.org/10.3390/molecules30173462
APA StyleNakamura, K., Nishiguchi, H., Arai, R., Hamajima, R., Abe, H., Ishida, A., Tokeshi, M., Higashi, K., Saitoh, A., & Takahashi, H. (2025). Isolating and Determining the Structures of Colored Products from the Reactions of Cannabinoids with Fast Blue RR. Molecules, 30(17), 3462. https://doi.org/10.3390/molecules30173462







