Comparative Study of ZnO and ZnO-Ag Particle Synthesis via Flame and Spray Pyrolysis for the Degradation of Methylene Blue
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Precursor Solution Preparation
3.2.2. Material Fabrication
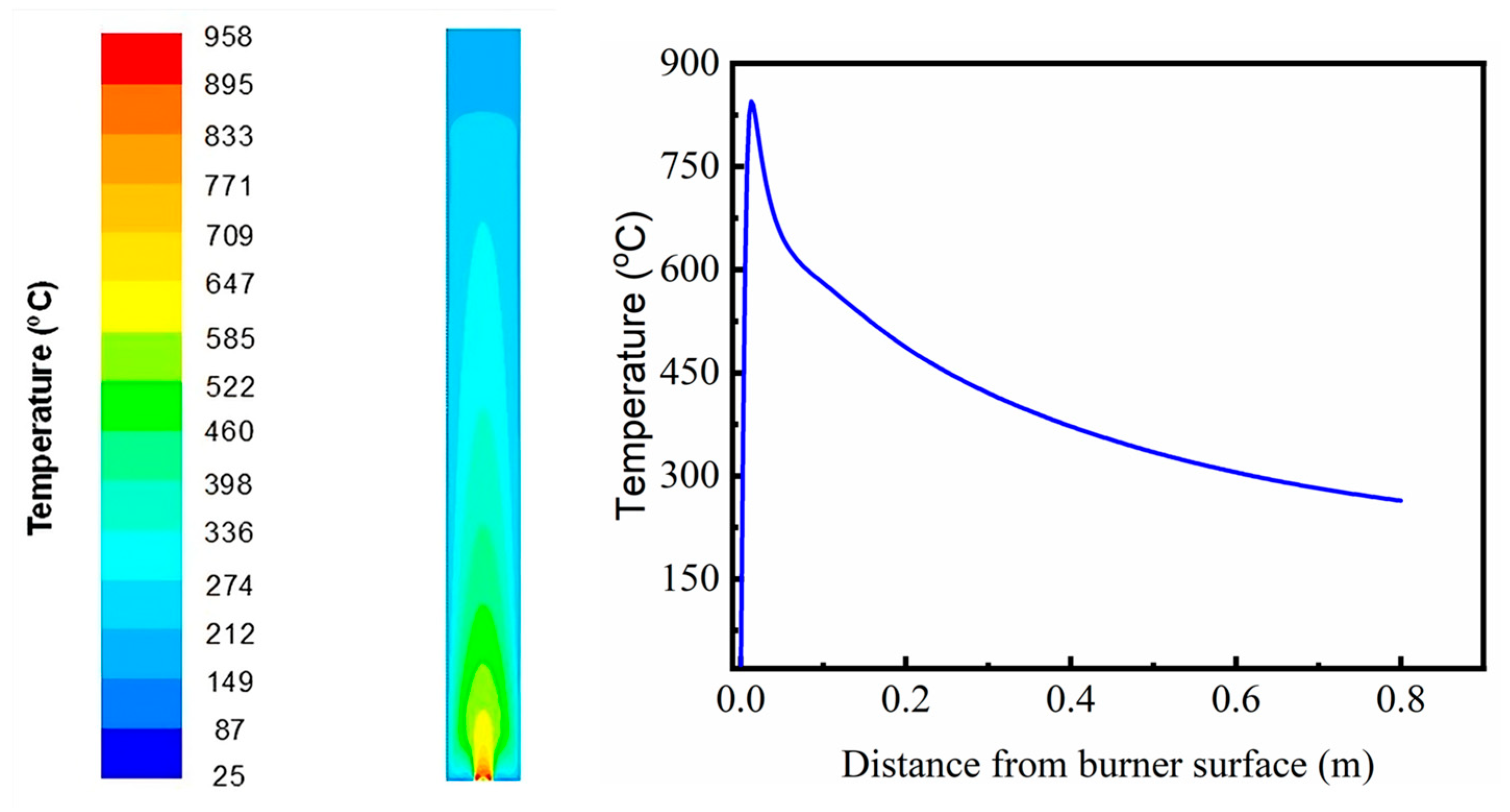
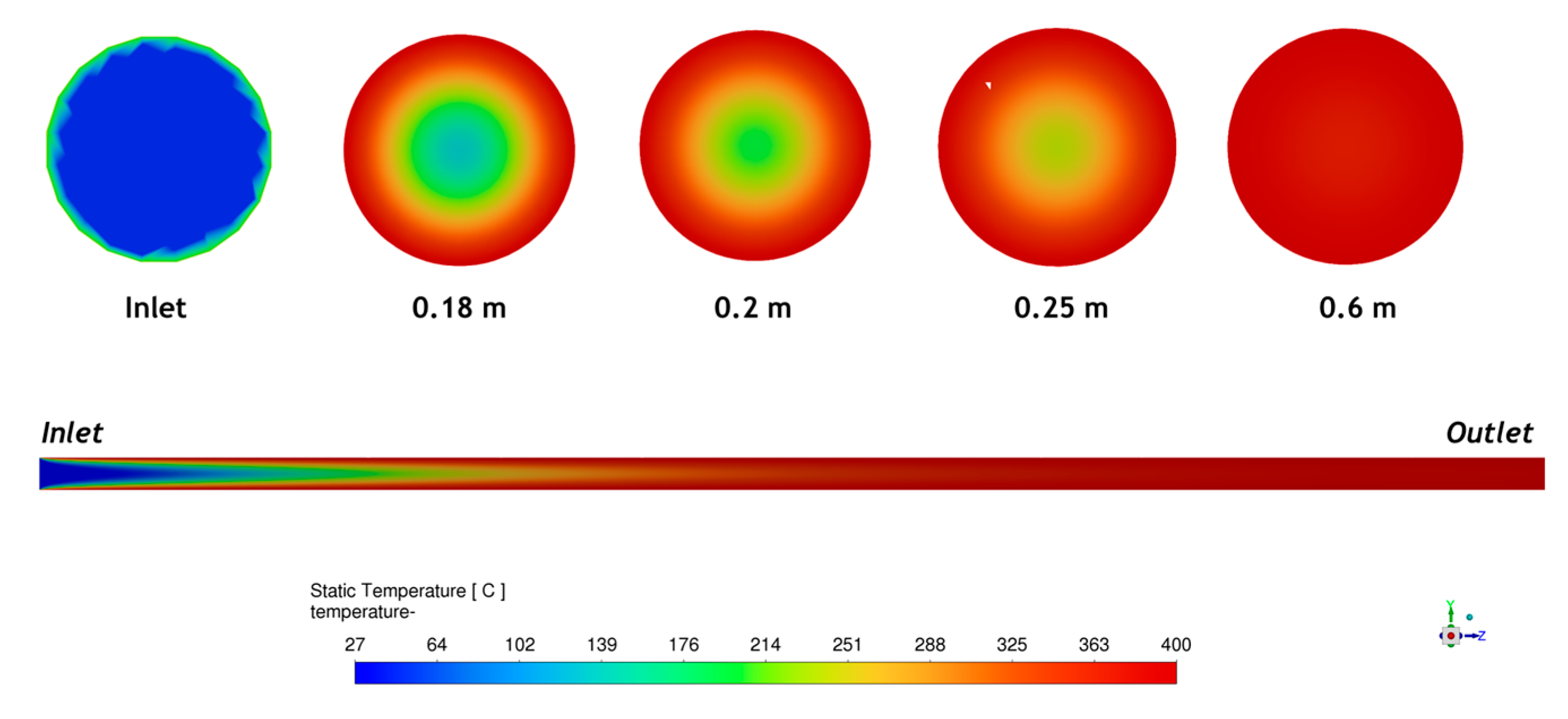
3.3. Numerical Simulations
3.4. Characterizations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haleem, A.; Shafiq, A.; Chen, S.-Q.; Nazar, M. A Comprehensive Review on Adsorption, Photocatalytic and Chemical Degradation of Dyes and Nitro-Compounds over Different Kinds of Porous and Composite Materials. Molecules 2023, 28, 1081. [Google Scholar] [CrossRef]
- Brocchetto, G.; Sciscenko, I.; Minella, M.; Craveri, L.; Bertozzi, E.; Malaguti, M.; Coha, M.; Tiraferri, A.; Vione, D. Combination of Membrane-Based Pre-Treatment Techniques and Heterogeneous Photocatalysis to Obtain High-Quality Effluents from Produced Water. Molecules 2025, 30, 2532. [Google Scholar] [CrossRef]
- Yang, X.; Yu, H.; Hong, L.; Huang, Z.; Zeng, Q.; Yao, X.; Qiu, Y. Enhanced Oxidation of Carbamazepine Using Mn(II)-Activated Peracetic Acid: A Novel Advanced Oxidation Process Involving the Significant Role of Ligand Effects. Molecules 2025, 30, 2690. [Google Scholar] [CrossRef]
- Chen, S.-F.; Chen, W.-J.; Song, H.; Liu, M.; Mishra, S.; Ghorab, M.A.; Chen, S.; Chang, C. Microorganism-Driven 2,4-D Biodegradation: Current Status and Emerging Opportunities. Molecules 2024, 29, 3869. [Google Scholar] [CrossRef]
- Kusdianto, K.; Jiang, D.; Kubo, M.; Shimada, M. Fabrication of TiO2—Ag nanocomposite thin films via one-step gas-phase deposition. Ceram. Int. 2017, 43, 5351–5355. [Google Scholar] [CrossRef]
- Lou, J.; Wang, L.; Huang, Y.; Xing, J.; Yang, X. Boosting Photocatalytic Performance of ZnO Nanowires via Building Heterojunction with Conjugated 2,4,6-Triaminopyrimidine-g-C3N4. Molecules 2024, 29, 3716. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Xiang, Y.; Yu, Y.; Zou, C. Bamboo-like MnO2/TiO2 Nanotube Arrays with Enhanced Photocatalytic Degradation. Coatings 2024, 14, 894. [Google Scholar] [CrossRef]
- La Greca, E.; Armeli Iapichino, M.T.; Herrera Beurnio, M.C.; Urbano Navarro, F.J.; Liotta, L.F.; Scirè, S.; Fiorenza, R. Influence of Ni Addition on Au/CeO2 Photocatalysts for Solar Photocatalytic H2 Production by Glycerol Photoreforming. Catalysts 2025, 15, 555. [Google Scholar] [CrossRef]
- Haq, S.; Sarfraz, A.; Menaa, F.; Shahzad, N.; Din, S.U.; Almukhlifi, H.A.; Alshareef, S.A.; Al Essa, E.M.; Shahzad, M.I. Green Synthesis of NiO-SnO2 Nanocomposite and Effect of Calcination Temperature on Its Physicochemical Properties: Impact on the Photocatalytic Degradation of Methyl Orange. Molecules 2022, 27, 8420. [Google Scholar] [CrossRef] [PubMed]
- Kusdianto, K.; Hudandini, M.; Kusuma, T.C.; Widiyastuti, W.; Madhania, S.; Machmudah, S.; Nurtono, T.; Puspitasari, D.; Winardi, S. Photocatalytic degradation of organic waste derived from textile dye by ZnO-Ag nanocomposite synthesized by spray pyrolysis. AIP Conf. Proc. 2020, 2219, 30001. [Google Scholar] [CrossRef]
- Samriti; Pujara, A.; Thakur, S.; Palai, T.; Gupta, R.; Swart, H.C.; Kuznetsov, A.; Prakash, J. Insight into the sunlight-driven photocatalytic activity of ZnO and ZnO/Ag hybrid nanostructures. Next Mater. 2025, 8, 100733. [Google Scholar] [CrossRef]
- Hudandini, M.; Puri, N.R.; Winardi, S.; Widiyastuti, W.; Shimada, M.; Kusdianto, K. Photocatalytic Activity of ZnO/Ag Nanoparticles Fabricated by a Spray Pyrolysis Method with Different O2:N2 Carrier Gas Ratios and Ag Contents. Catalysts 2022, 12, 1374. [Google Scholar] [CrossRef]
- Hakim, A.L.; Sari, I.E.; Nur Sabrina, B.J.; Nurkhalizah, Z.A.; Ajeng Zahabiya, E.K.; Hidayat, A.N.; Sulistiono, D.O.; Sopiah, R.N.; Kusumawati, Y.; Ediati, R. Impregnation of porous TiO2/ZnO into MCM-41 synthesized from coal fly ash for enhanced photocatalytic activity of TiO2/ZnO@MCM-41 composites in methylene blue removal. Case Stud. Chem. Environ. Eng. 2025, 11, 101152. [Google Scholar] [CrossRef]
- Rajendran, R.; Rojviroon, O.; Arumugam, P.; Ramasamy, G.; Paramasivam, S.; Rojviroon, T. Synergistic effects of activated Carbon-Supported TiO2/ZnO nanocomposites for photocatalytic dye degradation and antibacterial activity. J. Mol. Liq. 2025, 433, 127792. [Google Scholar] [CrossRef]
- Ahmad, I.; Shukrullah, S.; Naz, M.Y.; Ahmed, E.; Ahmad, M.; Akhtar, M.S.; Ullah, S.; Farooq, M.U.; Iqbal, S.; Assiri, M.A.; et al. Microwave-assisted one-pot hydrothermal synthesis of V and La co-doped ZnO/CNTs nanocomposite for boosted photocatalytic hydrogen production. Int. J. Hydrog. Energy 2022, 47, 15505–15515. [Google Scholar] [CrossRef]
- Yu, H.; Xu, H.; Hao, T.; Yuan, Y.; Zhang, B.; Wang, H.; Shao, G.; Fan, B.; Lu, H. Facile synthesis of ZnO/halloysite nanotube composite with greatly enhanced photocatalytic performance. Colloids Surf. A Physicochem. Eng. Asp. 2024, 688, 133633. [Google Scholar] [CrossRef]
- Nguyen, T.X.Q.; Chen, S.-S.; Pasawan, M.; Chang, H.-M. Enhanced photocatalytic activity of g-C3N4–n-p type flower-like ZnO/BiOBr heterojunction for hexavalent chromium and dye wastewater degradation. Environ. Technol. Innov. 2023, 31, 103154. [Google Scholar] [CrossRef]
- Chowdhury, S.; Lau, K.S.; Najm, A.S.; Naeem, H.S.; Holi, A.M.; Amin, N.; Laref, A.; Jamal, M.S.; Chia, C.H. Structural, morphological, and optical properties of Al-doped ZnO Material synthesized via sol-gel and spin coating: Insights into crystallinity and doping effects. Mater. Res. Bull. 2025, 190, 113516. [Google Scholar] [CrossRef]
- Deepa, G.; Balasundaram, O.N. Society based photovoltaic application of dye sensitized solar cell of Indium doped ZnO photoanode using cactus fruit via solvothermal method. Opt. Mater. 2024, 157, 116138. [Google Scholar] [CrossRef]
- Cherif, S.; Rezzaz-Yazid, H.; Hemidouche, S.; Farsi, A.; Mostefaoui, S.; Belmedani, M.; Djelal, H.; Sadaoui, Z. Comparative study on the photocatalytic efficiency of ZnO nanoparticles synthesized via chemical and eco-friendly coprecipitation methods. Ceram. Int. 2025, 51, 4737–4749. [Google Scholar] [CrossRef]
- Rout, D.R.; Jena, H.M. Enhanced Cr(VI) adsorption using ZnO decorated graphene composite: Batch and continuous studies. J. Taiwan Inst. Chem. Eng. 2022, 140, 104534. [Google Scholar] [CrossRef]
- Riwayati, I.; Winardi, S.; Madhania, S.; Shimada, M.; Kusdianto. Green synthesis of ZnO nanoparticles using Cosmos caudatus: Effects of calcination temperature and precursor type on photocatalytic and antimicrobial activities. Results Eng. 2024, 24, 103594. [Google Scholar] [CrossRef]
- Hosseinmardi, A.; Shojaee, N.; Keyanpour-Rad, M.; Ebadzadeh, T. A study on the photoluminescence properties of electrospray deposited amorphous and crystalline nanostructured ZnO thin films. Ceram. Int. 2012, 38, 1975–1980. [Google Scholar] [CrossRef]
- Merenda, A.; Gangadoo, S.; Johannessen, B.; Wilson, K.; Chapman, J.; Lee, A.F. Atomic layer deposition of antibacterial ZnO ultrathin films over SBA-15. Mater. Today Chem. 2025, 44, 102566. [Google Scholar] [CrossRef]
- Lei, P.-H.; Cheng, C.-H. Fabrication of Ag nanoparticle/ZnO thin films using dual-plasma-enhanced metal-organic chemical vapor deposition (DPEMOCVD) system incorporated with photoreduction method and its application. Mater. Sci. Semicond. Process. 2017, 57, 220–226. [Google Scholar] [CrossRef]
- Saravanavel, G.; Honnali, S.K.; Lourdes, K.S.; John, S.; Gunasekhar, K.R. Study on the thermoelectric properties of Al-ZnO thin-film stack fabricated by physical vapour deposition process for temperature sensing. Sens. Actuators A Phys. 2021, 332, 113097. [Google Scholar] [CrossRef]
- Kusdianto, K.; Widiyastuti, W.; Shimada, M.; Nurtono, T.; Machmudah, S.; Winardi, S. Photocatalytic Activity of ZnO-Ag Nanocomposites Prepared by a One-step Process using Flame Pyrolysis. Int. J. Technol. 2019, 10, 291–319. [Google Scholar] [CrossRef]
- Schulte, M.L.; Catharina Sender, V.; Baumgarten, L.; Beck, A.; Nilayam, A.R.L.; Saraçi, E.; Grunwaldt, J.-D. Tuning Flame Spray Pyrolysis for Variation of the Crystallite Size in Cu/ZnO/ZrO2 and its Influence on the Performance in CO2-to-Methanol Synthesis. Eur. J. Inorg. Chem. 2025, 28, e202400684. [Google Scholar] [CrossRef]
- Du, W.; Feng, K.; Li, C.; Li, S.; Abidin, Z.U.; Yin, H.; Chen, S. Controlled synthesis of zinc oxide nanoparticles through flame spray pyrolysis and evaluation of their anticancer effects against gastric cancer cell. Arab. J. Chem. 2023, 16, 105192. [Google Scholar] [CrossRef]
- Kathwate, L.H.; Kanwate, A.D.; Sarnikar, Y.P.; Rakhade, H.M.; Kore, A.; Barse, N.S.; Mendhe, A.C. Fabrication of Ni-doped ZnO thin films via spray pyrolysis method towards highly selective and sensitive acetone gas sensing. Inorg. Chem. Commun. 2025, 175, 114152. [Google Scholar] [CrossRef]
- Ruiz-Duarte, E.V.; Molina-Jiménez, J.P.; Avila, D.A.; Torres, C.O.; Horta-Piñeres, S.D. Rapid Synthesis of Highly Crystalline ZnO Nanostructures: Comparative Evaluation of Two Alternative Routes. Crystals 2025, 15, 640. [Google Scholar] [CrossRef]
- Modi, S.; Yadav, V.K.; Thakur, R.; Kumari, M.; Gnanamoorthy, G.; Patel, A.; Choudhary, N.; Fulekar, M.H. Comparative Analysis of Morphological and Structural Properties of Zinc Oxide Nanoparticles Synthesized by Sonochemical Method and Microbial Method. Plasmonics 2025, 20, 5027–5044. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Q.; Xu, M.; Yuan, H.; Chen, Y.; Zhang, J.; Luo, K.; Zhang, J.; You, B. Novel and efficient synthesis of Ag-ZnO nanoparticles for the sunlight-induced photocatalytic degradation. Appl. Surf. Sci. 2019, 476, 632–640. [Google Scholar] [CrossRef]
- Hussain, A.; Fiaz, S.; Almohammedi, A.; Waqar, A. Optimizing photocatalytic performance with Ag-doped ZnO nanoparticles: Synthesis and characterization. Heliyon 2024, 10, e35725. [Google Scholar] [CrossRef]
- Dehimi, M.; Touam, T.; Chelouche, A.; Boudjouan, F.; Djouadi, D.; Solard, J.; Fischer, A.; Boudrioua, A.; Doghmane, A. Effects of Low Ag Doping on Physical and Optical Waveguide Properties of Highly Oriented Sol-Gel ZnO Thin Films. Adv. Condens. Matter Phys. 2015, 2015, 740208. [Google Scholar] [CrossRef]
- Sengupta, A.; Gupta, A.K.; Mishra, I.M.; Suresh, S. One Dimensional Modeling of Jet Diffusion Flame. J. Appl. Res. Technol. 2019, 16, 320–333. [Google Scholar] [CrossRef][Green Version]
- Bhat, M.; Luo, S.; Zhang, J.; Zhang, C.; Zhou, B.; Deng, S. Multi-component precursor droplet evaporation in spray synthesis of cathode materials. Chem. Eng. J. 2024, 479, 147417. [Google Scholar] [CrossRef]
- Mäkelä, J.M.; Haapanen, J.; Harra, J.; Juuti, P.; Kujanpää, S. Liquid Flame Spray—A Hydrogen-Oxygen Flame Based Method for Nanoparticle Synthesis and Functional Nanocoatings. KONA Powder Part. J. 2017, 34, 141–154. [Google Scholar] [CrossRef]
- Van Werde, K.; Mondelaers, D.; Vanhoyland, G.; Nelis, D.; Van Bael, M.K.; Mullens, J.; Van Poucke, L.C.; Van Der Veken, B.; Desseyn, H.O. Thermal decomposition of the ammonium zinc acetate citrate precursor for aqueous chemical solution deposition of ZnO. J. Mater. Sci. 2002, 37, 81–88. [Google Scholar] [CrossRef]
- Duan, Y.; Li, J.; Yang, X.; Hu, L.; Wang, Z.; Liu, Y.; Wang, C. Kinetic analysis on the non-isothermal dehydration by integral master-plots method and TG–FTIR study of zinc acetate dihydrate. J. Anal. Appl. Pyrolysis 2008, 83, 1–6. [Google Scholar] [CrossRef]
- Ma, Y.; Zhai, C.; Dai, J.; Gu, F. Photocatalytic and photothermal catalytic CO2 reduction with H2O from regulatory mechanism to catalyst structure design: A review. Sol. Energy 2025, 296, 113558. [Google Scholar] [CrossRef]
- Saadi, H.; Atmani, E.H.; Fazouan, N. Enhanced photocatalytic degradation of methylene blue dye by ZnO nanoparticles: Synthesis, characterization, and efficiency assessment. Environ. Prog. Sustain. Energy 2025, 44, e14529. [Google Scholar] [CrossRef]
- Maddu, A.; Meliafatmah, R.; Rustami, E. Enhancing Photocatalytic Degradation of Methylene Blue Using ZnO/Carbon Dots Nanocomposite Derived From Coffee Grounds. Pol. J. Environ. Stud. 2021, 30, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. Review on the improvement of the photocatalytic and antibacterial activities of ZnO. J. Alloys Compd. 2017, 727, 792–820. [Google Scholar] [CrossRef]
- Uribe-López, M.C.; Hidalgo-López, M.C.; López-González, R.; Frías-Márquez, D.M.; Núñez-Nogueira, G.; Hernández-Castillo, D.; Alvarez-Lemus, M.A. Photocatalytic activity of ZnO nanoparticles and the role of the synthesis method on their physical and chemical properties. J. Photochem. Photobiol. A Chem. 2021, 404, 112866. [Google Scholar] [CrossRef]
- Widiyastuti, W.; Purwanto, A.; Wang, W.-N.; Iskandar, F.; Setyawan, H.; Okuyama, K. Nanoparticle formation through solid-fed flame synthesis: Experiment and modeling. AIChE J. 2009, 55, 885–895. [Google Scholar] [CrossRef]
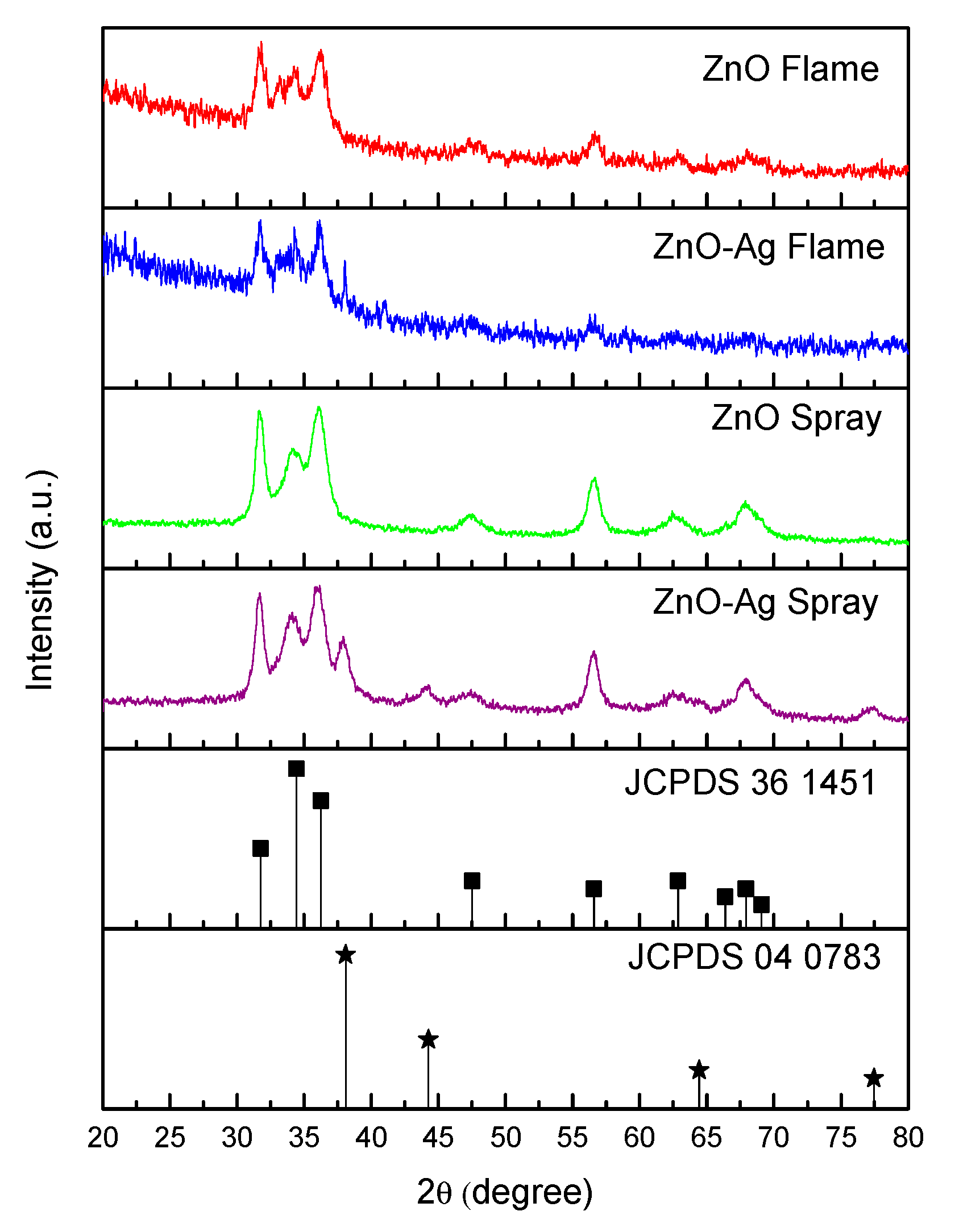
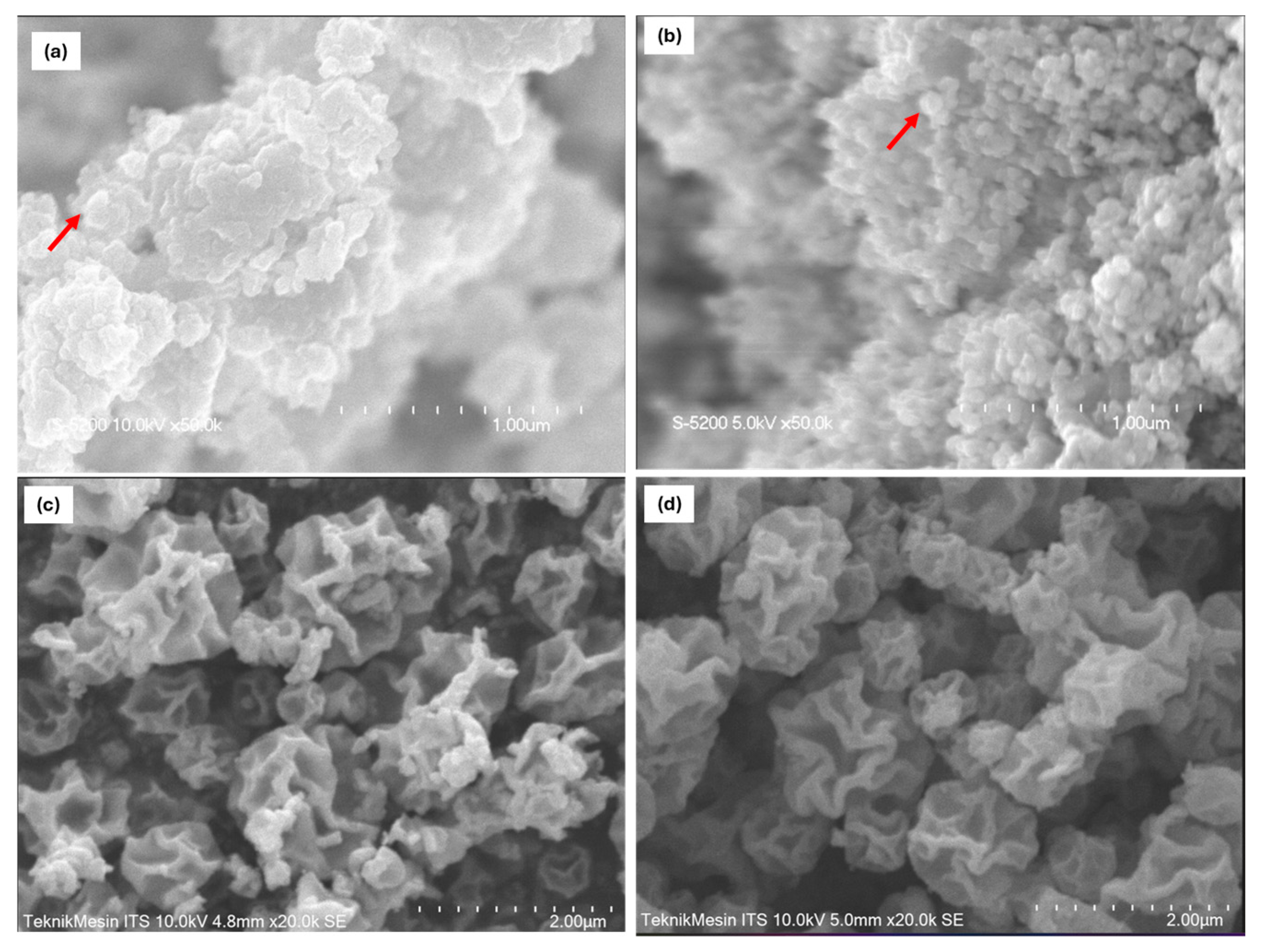
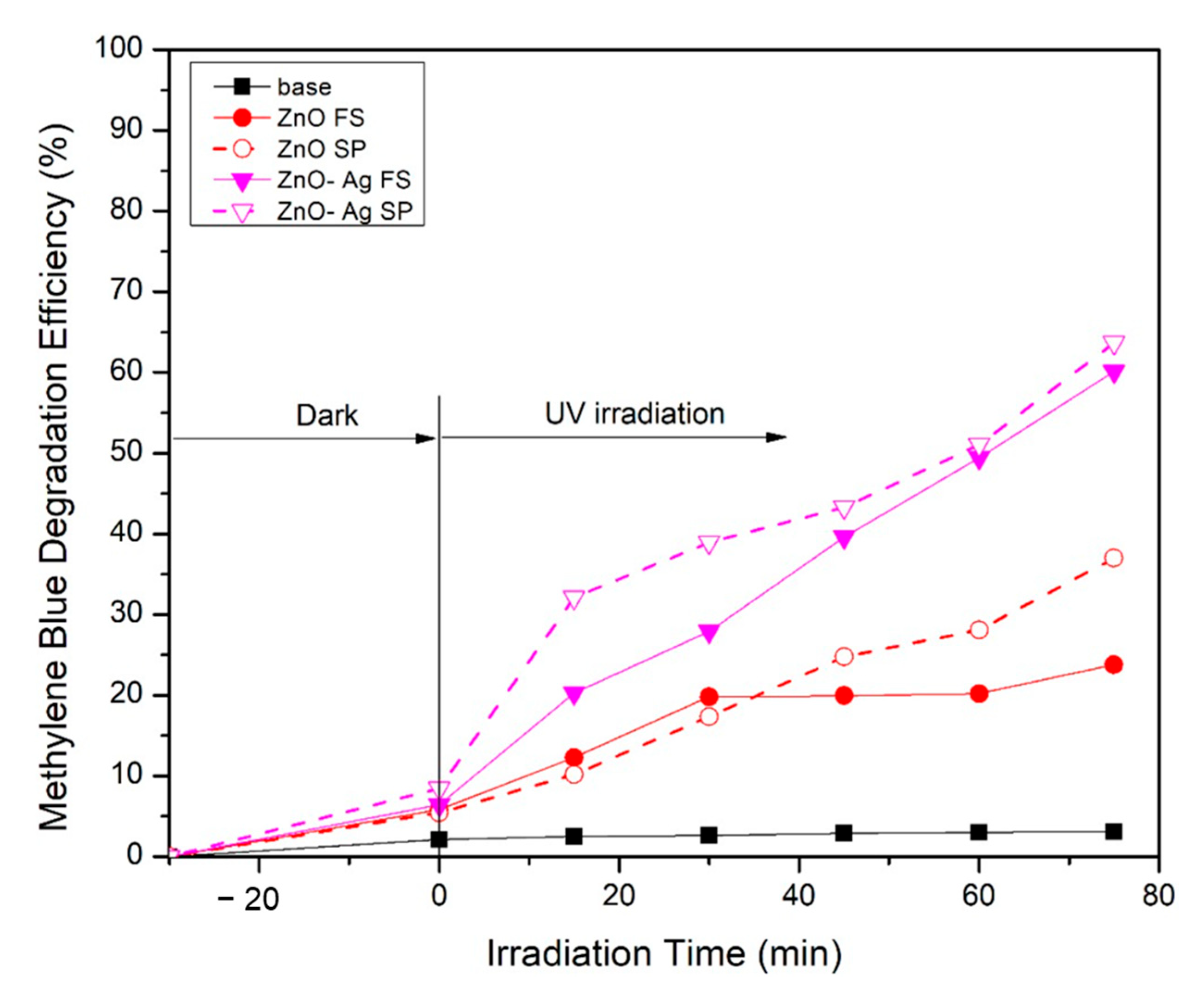
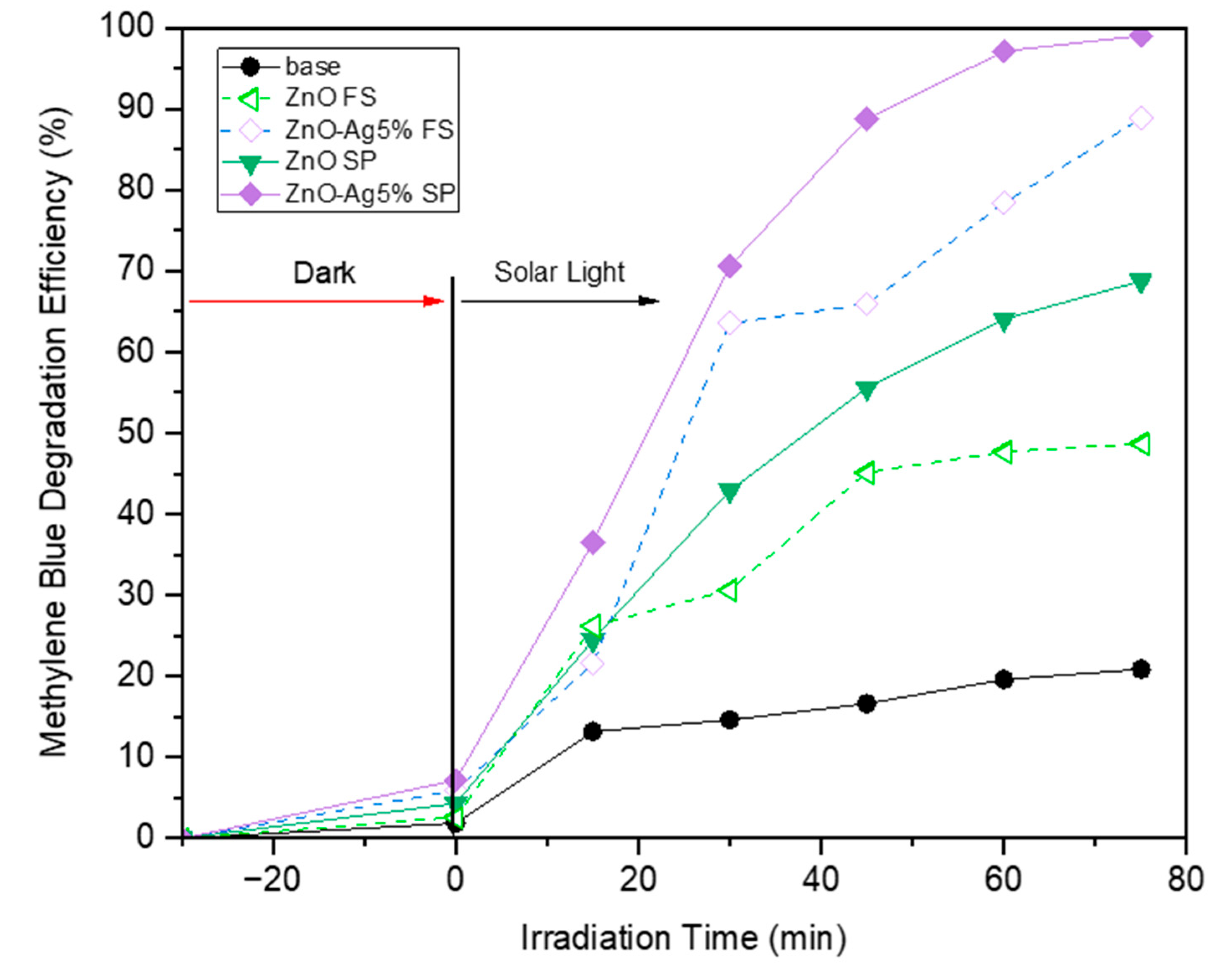
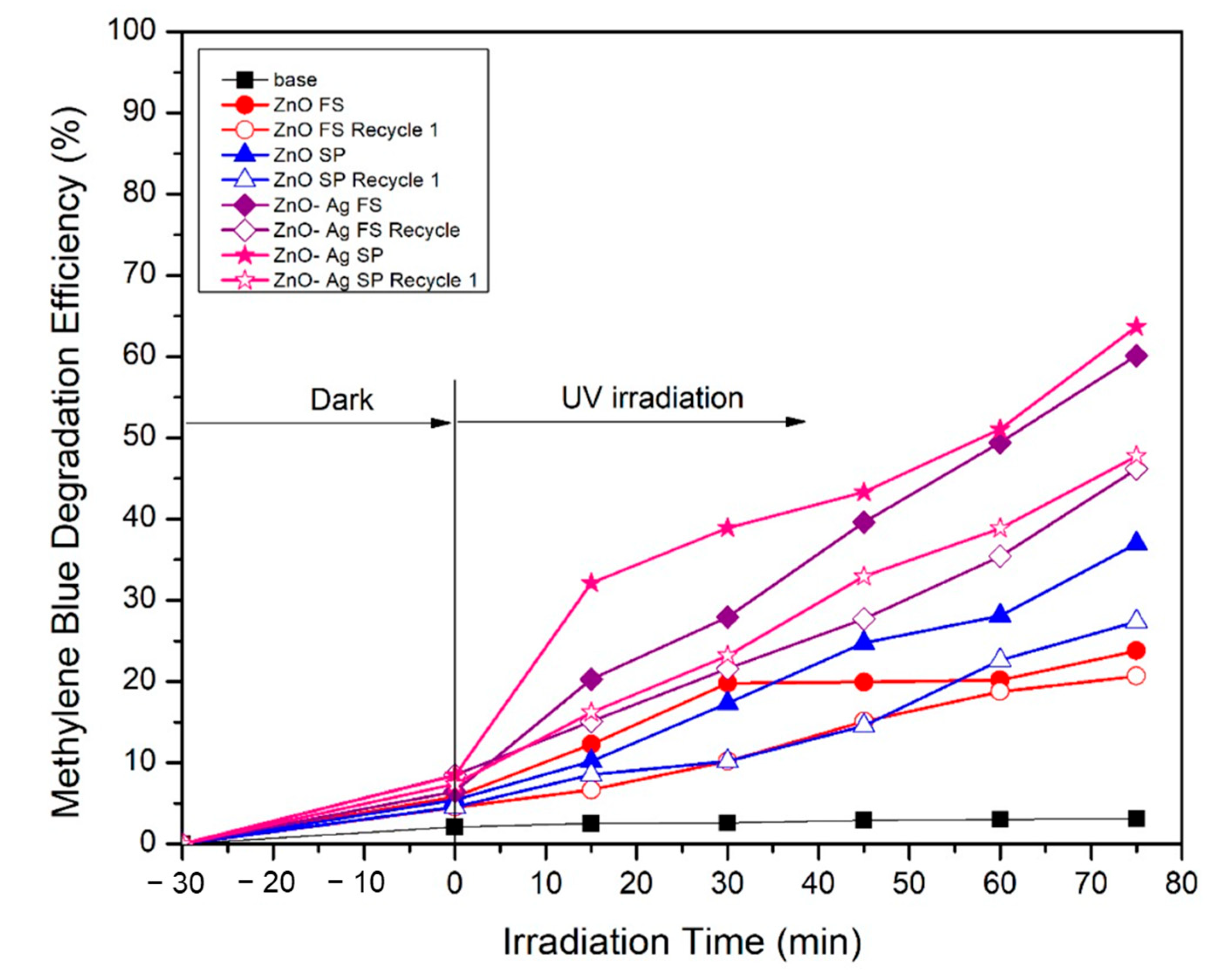
| Variable | Crystalline Phase a | Crystallite Size b [nm] | Average Diameter c [nm] | Specific Surface Area d [m2/g] | Pore Diameter d [nm] |
|---|---|---|---|---|---|
| ZnO Flame | H. Wurtzite | 3.3 | 40 | 237 | 4.5 |
| ZnO-Ag Flame | H. Wurtzite | 5.7 | 46 | 280 | 5.7 |
| ZnO Spray | H. Wurtzite | 6.5 | 1130 | 238 | 18.6 |
| ZnO-Ag Spray | H. Wurtzite | 5.8 | 1230 | 284 | 9.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kusdianto; Puri, N.R.; Setiawan, A.; Winardi, S.; Widiyastuti; Madhania, S.; Rozy, M.I.F.; Shimada, M. Comparative Study of ZnO and ZnO-Ag Particle Synthesis via Flame and Spray Pyrolysis for the Degradation of Methylene Blue. Molecules 2025, 30, 3364. https://doi.org/10.3390/molecules30163364
Kusdianto, Puri NR, Setiawan A, Winardi S, Widiyastuti, Madhania S, Rozy MIF, Shimada M. Comparative Study of ZnO and ZnO-Ag Particle Synthesis via Flame and Spray Pyrolysis for the Degradation of Methylene Blue. Molecules. 2025; 30(16):3364. https://doi.org/10.3390/molecules30163364
Chicago/Turabian StyleKusdianto, Nurdiana Ratna Puri, Adhi Setiawan, Sugeng Winardi, Widiyastuti, Suci Madhania, Mohammad Irwan Fatkhur Rozy, and Manabu Shimada. 2025. "Comparative Study of ZnO and ZnO-Ag Particle Synthesis via Flame and Spray Pyrolysis for the Degradation of Methylene Blue" Molecules 30, no. 16: 3364. https://doi.org/10.3390/molecules30163364
APA StyleKusdianto, Puri, N. R., Setiawan, A., Winardi, S., Widiyastuti, Madhania, S., Rozy, M. I. F., & Shimada, M. (2025). Comparative Study of ZnO and ZnO-Ag Particle Synthesis via Flame and Spray Pyrolysis for the Degradation of Methylene Blue. Molecules, 30(16), 3364. https://doi.org/10.3390/molecules30163364







