Boosting Photoelectrochemical Water Splitting via InPOx-Coated TiO2 Nanowire Photoanodes
Abstract
1. Introduction
2. Results and Discussion
2.1. Structure and Composition Characterization
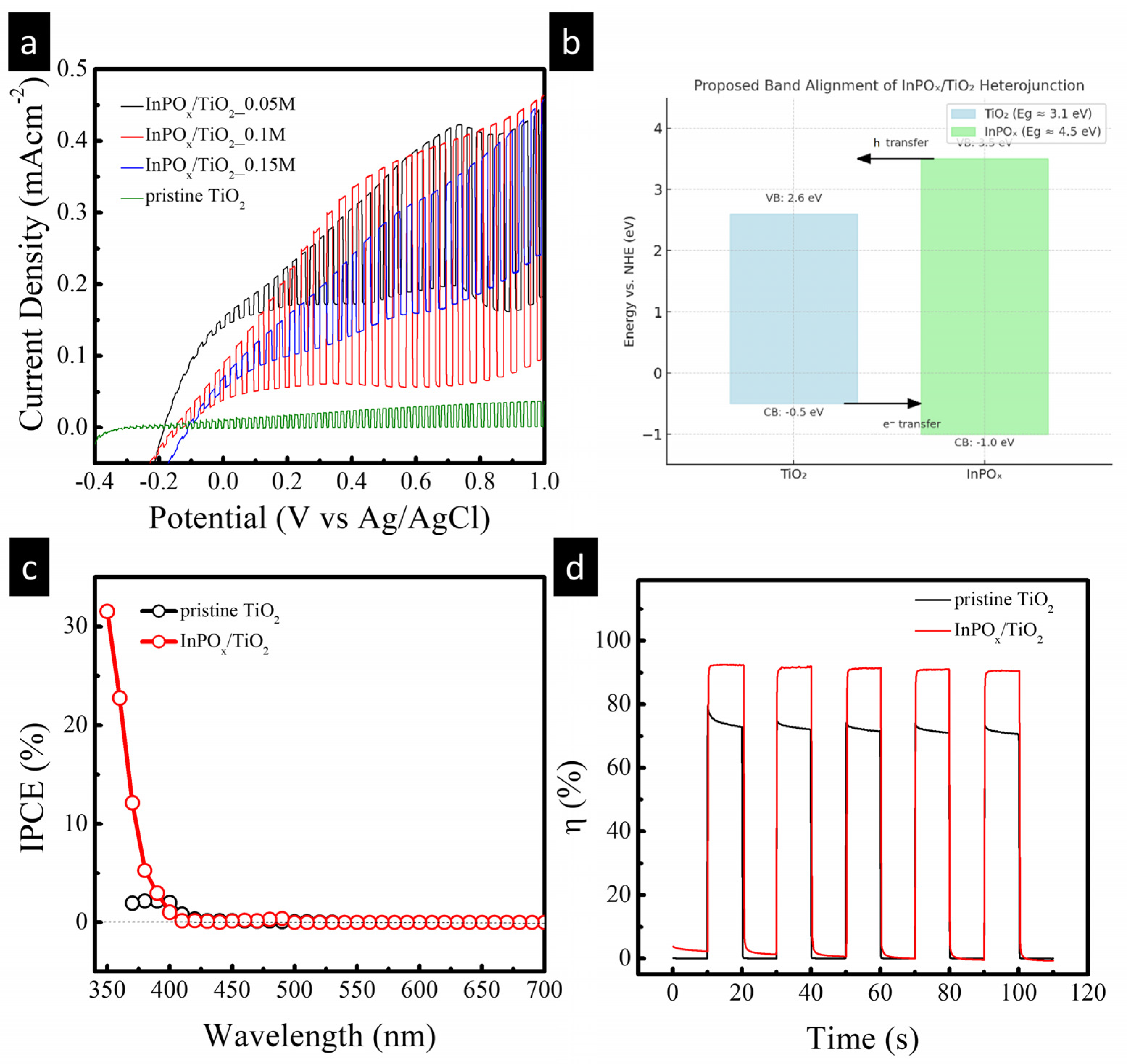
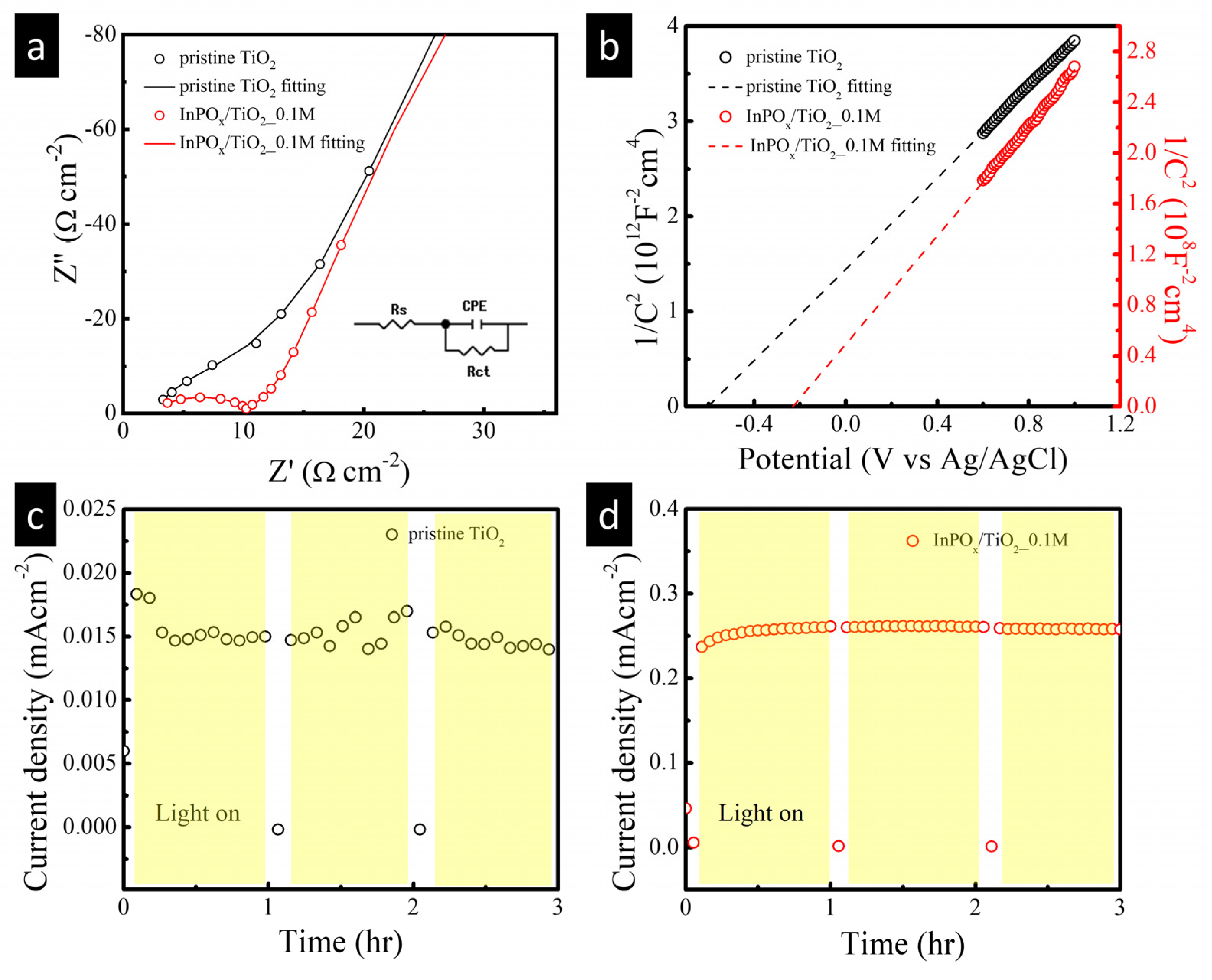
2.2. PEC Performance of InPOx/TiO2 NW Photoanode
3. Experimental Section
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.C.; Jui, H.Y.; Chen, Y.W.; Hsu, Y.K. Record High Photocurrent Density of In:Ag2S/ZnO Heterojunction Enabled by Controllable Doping for Efficient Solar Hydrogen Production. J. Alloys Compd. 2025, 1010, 177325. [Google Scholar] [CrossRef]
- Chen, Y.C.; Huang, Y.W.; Hsu, Y.K. Fine–Tuning Interfacial Band Edge Energetics at Sb2S3/TiO2 Heterojunction via Phase Control and Defect Engineering for Enhanced Solar Water Splitting Performance. J. Alloys Compd. 2025, 1022, 179841. [Google Scholar] [CrossRef]
- Kim, T.W.; Choi, K.S. Nanoporous BiVO4 Photoanodes with Dual-Layer Oxygen Evolution Catalysts for Solar Water Splitting. Science 2016, 343, 990–994. [Google Scholar] [CrossRef]
- Chen, Y.C.; Kuo, C.L.; Hsu, Y.K. Facile Preparation of Zn-doped Hematite Thin Film as Photocathode for Solar Hydrogen Generation. J. Alloys Compd. 2018, 768, 810–816. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Z.; Chen, D.; Guo, Z.; Ruan, M. Oxygen vacancies engineering in TiO2 homojunction/ZnFe-LDH for enhanced photoelectrochemical water oxidation. Chem. Engin. J. 2020, 395, 125101. [Google Scholar] [CrossRef]
- Shen, S.; Chen, J.; Wang, M.; Sheng, X.; Chen, X.; Feng, X.; Mao, S.S. Titanium dioxide nanostructures for photoelectrochemical applications. Prog. Mater. Sci. 2018, 98, 299–385. [Google Scholar] [CrossRef]
- Prabhakar, R.R.; Moehl, T.; Siol, S.; Suh, J.; Tilley, S.D. Sb2S3/TiO2 Heterojunction Photocathodes: Band Alignment and Water Splitting Properties. Chem. Mater. 2020, 32, 7247–7253. [Google Scholar] [CrossRef]
- Eidsvåg, H.; Bentouba, S.; Vajeeston, P.; Yohi, S.; Velauthapillai, D. TiO2 as a photocatalyst for water splitting—An experimental and theoretical review. Molecules 2021, 26, 1687. [Google Scholar] [CrossRef] [PubMed]
- Nisar, M.; Khan, N.; Qadir, M.I.; Shah, Z. Recent Advances in TiO2-Based Photocatalysts for Efficient Water Splitting to Hydrogen. Nanomaterials 2025, 15, 984. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, S.; Wu, Y.; Tian, Y.; Fu, H.; Zhan, S. P-doped In2S3 nanosheets coupled with InPOx overlayer: Charge-transfer pathways and highly enhanced photoelectrochemical water splitting. J. Catal. 2019, 375, 389–398. [Google Scholar] [CrossRef]
- Wager, J.F.; Wilmsen, C.W.; Kazmerski, L.L. Estimation of the band gap of InPO4. Appl. Phys. Lett. 1983, 42, 589–591. [Google Scholar] [CrossRef]
- Taudul, B.; Tielens, F.; Calatayud, M. On the Origin of Raman Activity in Anatase TiO2 (Nano)Materials: An Ab Initio Investigation of Surface and Size Effects. Nanomaterials 2023, 13, 1856. [Google Scholar] [CrossRef]
- Xue, X.; Chen, H.; Xiong, Y.; Chen, R.; Jiang, M.; Fu, G.; Xi, Z.; Zhang, X.L.; Ma, J.; Fang, W.; et al. Near-Infrared-Responsive Photo-Driven Nitrogen Fixation Enabled by Oxygen Vacancies and Sulfur Doping in Black TiO2−xSy Nanoplatelets. ACS Appl. Mater. Interfaces 2021, 13, 4975–4983. [Google Scholar] [CrossRef] [PubMed]
- Virieux, H.; Troedec, M.L.; Cros-Gagneux, A.; Ojo, W.S.; Delpech, F.; Nayral, C.; Martinez, H.; Chaudret, B. InP/ZnS Nanocrystals: Coupling NMR and XPS for Fine Surface and Interface Description. J. Am. Chem. Soc. 2012, 134, 19701–19708. [Google Scholar] [CrossRef] [PubMed]
- Eddy, D.R.; Permana, M.D.; Sakti, L.K.; Sheha, G.A.N.; Solihudin; Hidayat, S.; Takei, T.; Kumada, N.; Rahayu, I. Heterophase Polymorph of TiO2 (Anatase, Rutile, Brookite, TiO2 (B)) for Efficient Photocatalyst: Fabrication and Activity. Nanomaterials 2023, 13, 704. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Fan, W.; Lu, H.; Ge, Y.; Bai, H.; Shi, W. Fabrication of Au@CdS/RGO/TiO2 heterostructure for photoelectrochemical hydrogen production. New J. Chem. 2016, 40, 2287–2295. [Google Scholar] [CrossRef]
- Wang, Y.; Bai, W.; Wang, H.; Jiang, Y.; Han, S.; Sun, H.; Li, Y.; Jiang, G.; Zhao, Z.; Huan, Q. Promoted photoelectrocatalytic hydrogen evolution of a type II structure via an Al2O3 recombination barrier layer deposited using atomic layer deposition. Dalton Trans. 2017, 46, 10734–10741. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, H.; Huang, T.; Zhang, X.; Shi, Z.; Zhou, L.; Lan, Y.; Tang, G. High-Performance TiO2/ZnO Photoanodes for CdS Quantum Dot-Sensitized Solar Cells. J. Elec Materi 2019, 48, 7320–7327. [Google Scholar] [CrossRef]
- Łęcki, T.; Zarębska, K.; Sobczak, K.; Skompska, M. Photocatalytic degradation of 4-chlorophenol with the use of FTO/TiO2/SrTiO3 composite prepared by microwave-assisted hydrothermal method. Appl. Surf. Sci. 2019, 470, 991–1002. [Google Scholar] [CrossRef]
- Ni, S.; Guo, F.; Wang, D.; Jiao, S.; Wang, J.; Zhang, Y.; Wang, B.; Zhao, L. Optimal Sr-Doped Free TiO2@SrTiO3 Heterostructured Nanowire Arrays for High-Efficiency Self-Powered Photoelectrochemical UV Photodetector Applications. Crystals 2019, 9, 134. [Google Scholar] [CrossRef]
- Rambabu, Y.; Jaiswal, M.; Roy, S.C. Graphene Oxide Modified TiO2 Micro Whiskers and Their Photo Electrochemical Performance. J. Nanosci. Nanotechnol. 2016, 16, 4835–4839. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Zhou, L.; Lin, W.F.; Wu, Y. S-scheme NaxCoO2/g-C3N4 heterojunction photo-electrocatalysts for water splitting. Mater. Chem. Phys. 2024, 326, 129853. [Google Scholar] [CrossRef]
- Gao, T.; Liu, X.; Feng, Q.; Wu, X.; Wang, J.; Wang, G. Microwave assisted rapid synthesis of TiO2 NFs@COF S-scheme heterojunction photocatalyst for highly efficient photocatalytic hydrogen evolution. J. Colloid Interf. Sci. 2025, 698, 139075. [Google Scholar] [CrossRef] [PubMed]
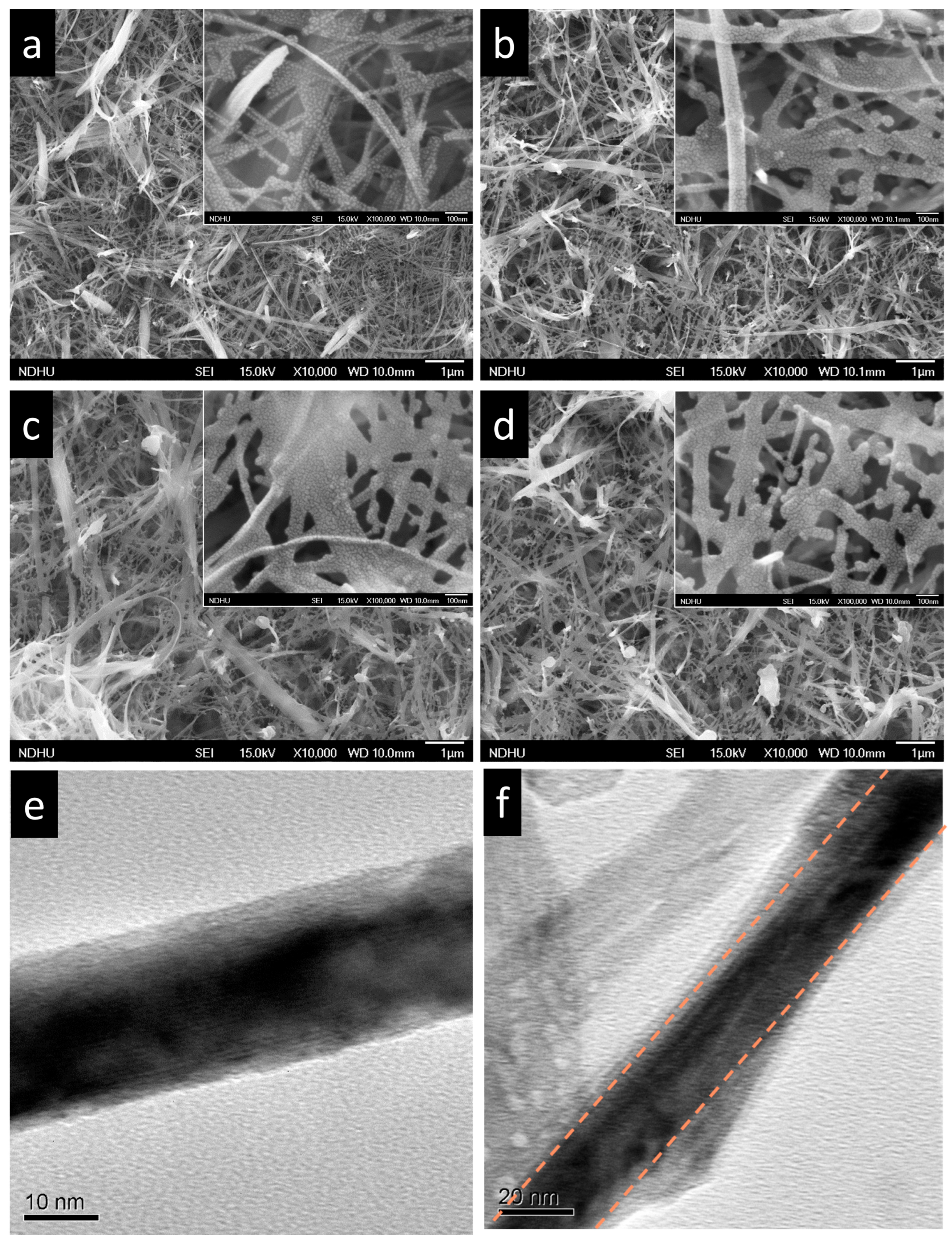
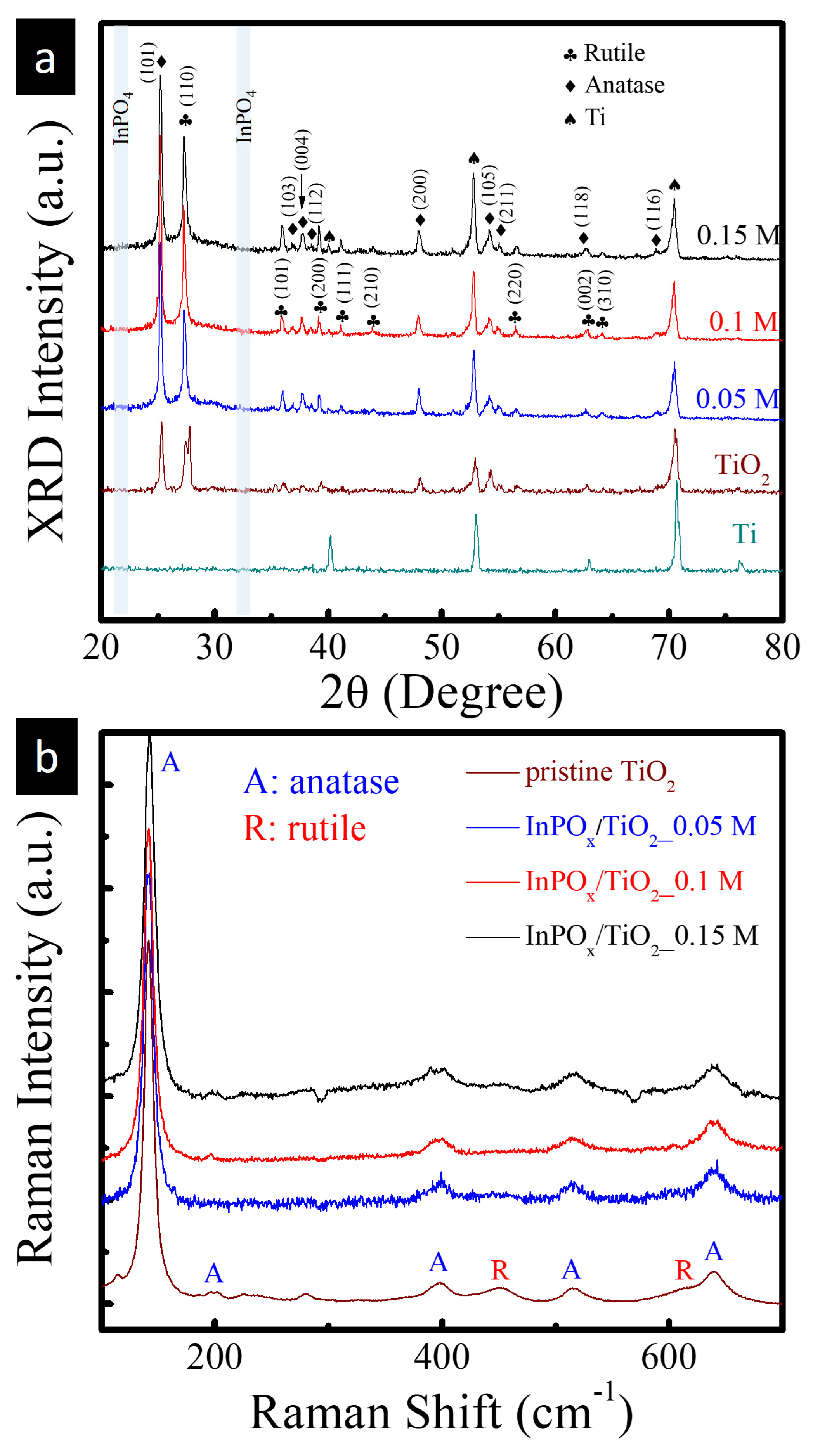
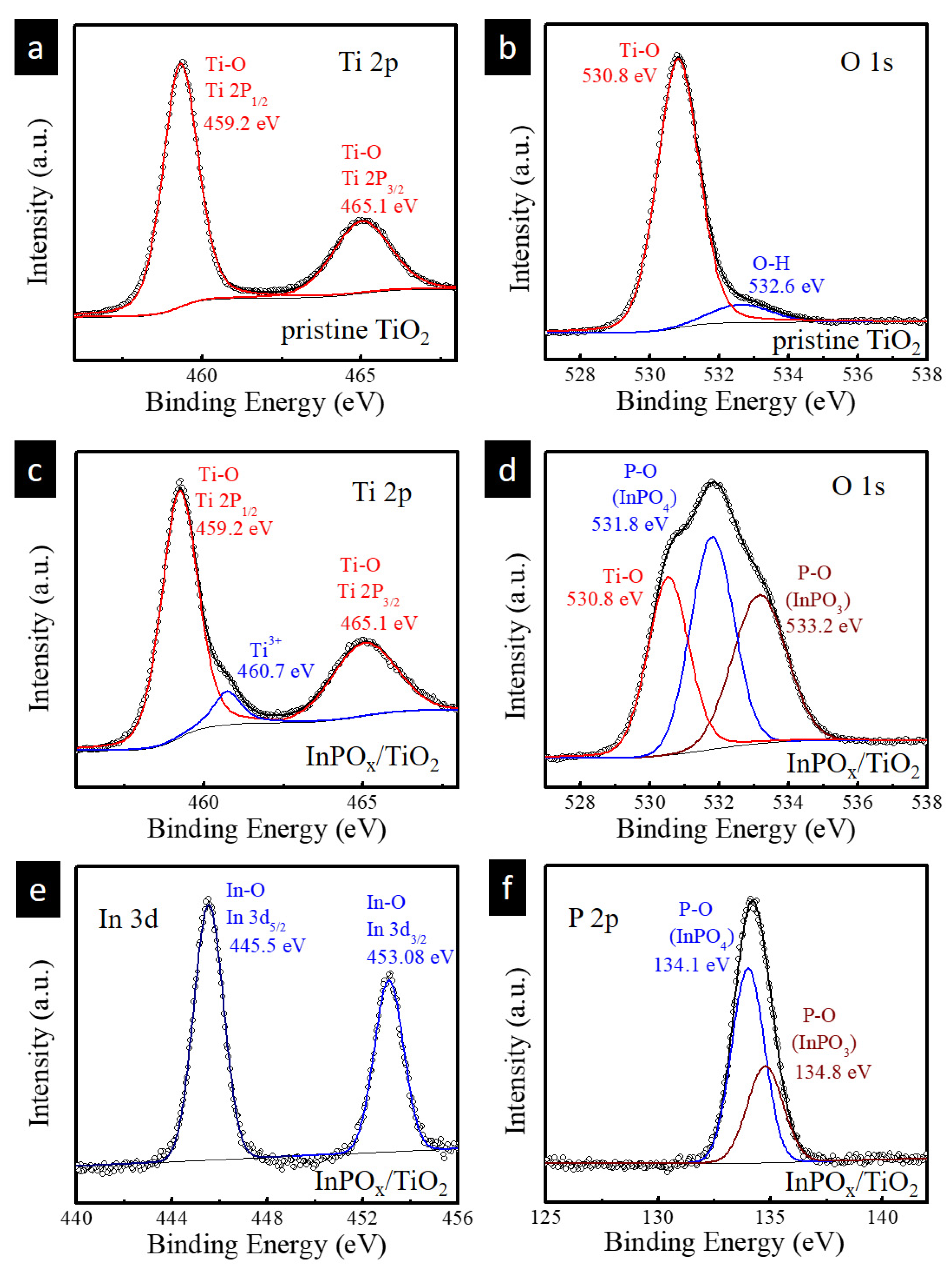
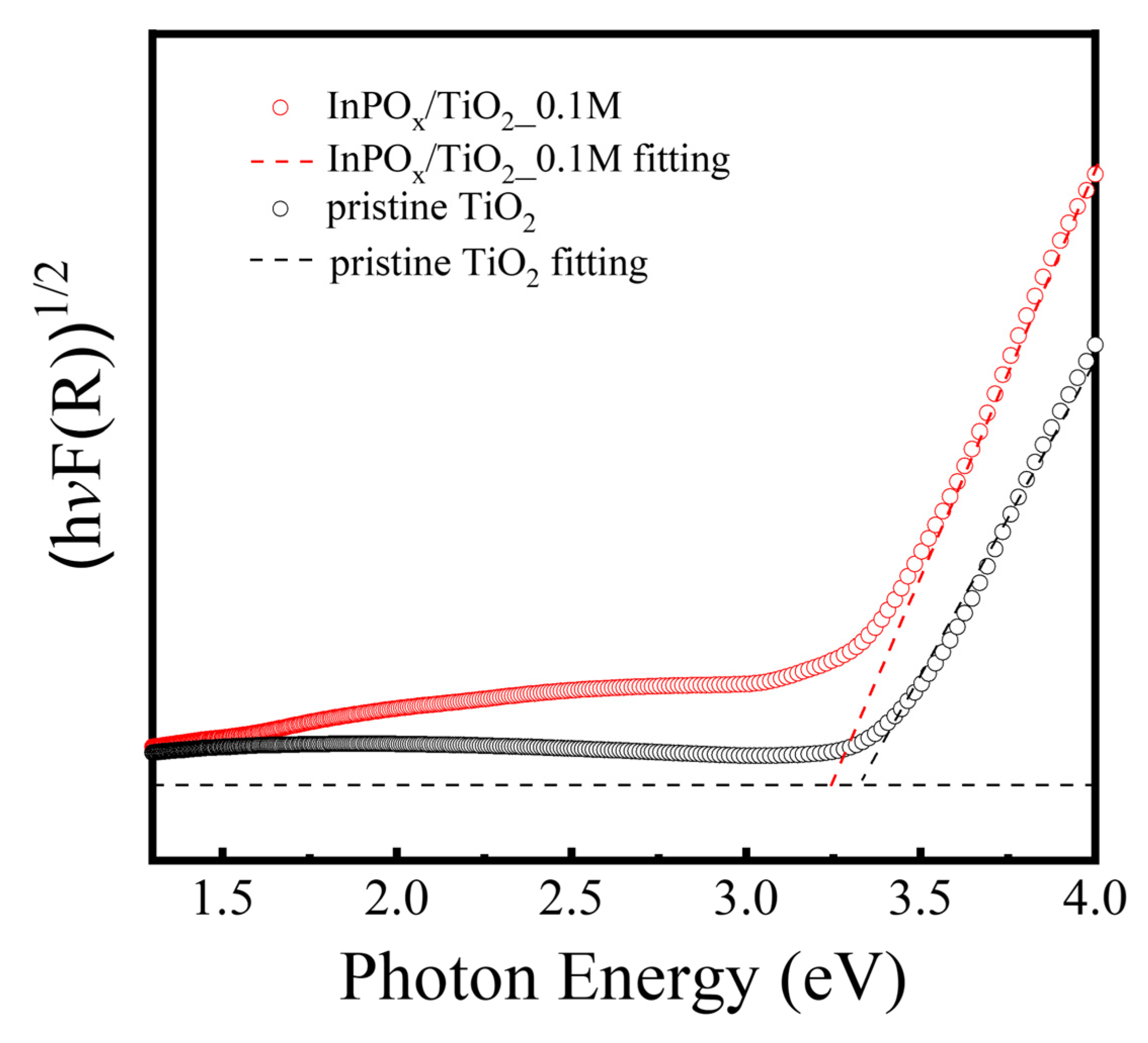
| Material Structure | Nanocomposite Photocurrent Density (mA/cm2) | BareTiO2 Photocurrent Density (mA/cm2) | Current Density Gain Ratio | Ref. |
|---|---|---|---|---|
| Au@CdS/RGO/TiO2 | 0.14 | 0.06 | 133% | [16] |
| Al2O3/TiO2 | ~0.2 | ~0.02 | 900% | [17] |
| ZnO/TiO2 | 2.37 | 0.68 | 248% | [18] |
| SrTiO3/TiO2 | ~0.025 | ~0.012 | 108% | [19] |
| SrTiO3/TiO2 | 3.48 | 1.5 | 132% | [20] |
| RGO/TiO2 | 2.45 | 1.92 | 27% | [21] |
| InPOx/TiO2 | 0.36 | 0.035 | 928% | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-C.; Lin, H.-Y.; Hsu, Y.-K. Boosting Photoelectrochemical Water Splitting via InPOx-Coated TiO2 Nanowire Photoanodes. Molecules 2025, 30, 3482. https://doi.org/10.3390/molecules30173482
Chen Y-C, Lin H-Y, Hsu Y-K. Boosting Photoelectrochemical Water Splitting via InPOx-Coated TiO2 Nanowire Photoanodes. Molecules. 2025; 30(17):3482. https://doi.org/10.3390/molecules30173482
Chicago/Turabian StyleChen, Ying-Chu, Heng-Yi Lin, and Yu-Kuei Hsu. 2025. "Boosting Photoelectrochemical Water Splitting via InPOx-Coated TiO2 Nanowire Photoanodes" Molecules 30, no. 17: 3482. https://doi.org/10.3390/molecules30173482
APA StyleChen, Y.-C., Lin, H.-Y., & Hsu, Y.-K. (2025). Boosting Photoelectrochemical Water Splitting via InPOx-Coated TiO2 Nanowire Photoanodes. Molecules, 30(17), 3482. https://doi.org/10.3390/molecules30173482





