Photodegradation of Turmeric Oleoresin Under Fluorescent Light and White LED: Impacts on the Chemical Stability, Bioactivity, and Photosensitizing Property of Curcuminoids
Abstract
1. Introduction
2. Results and Discussion
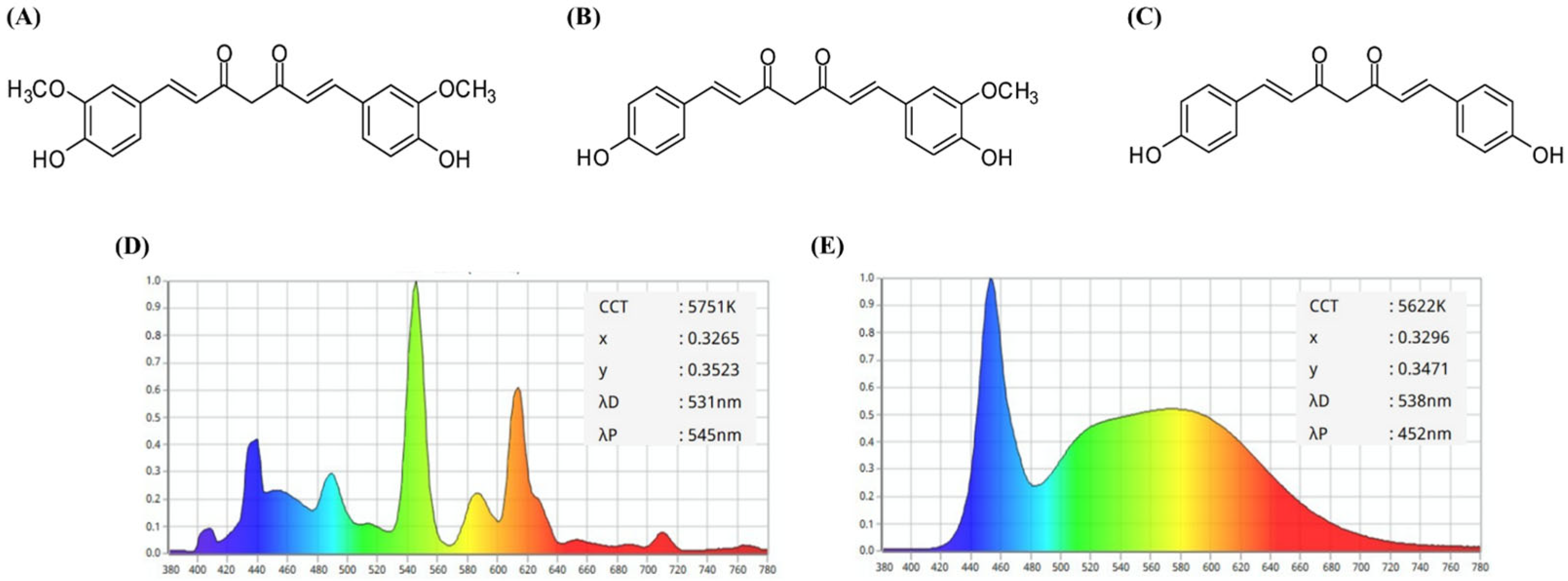
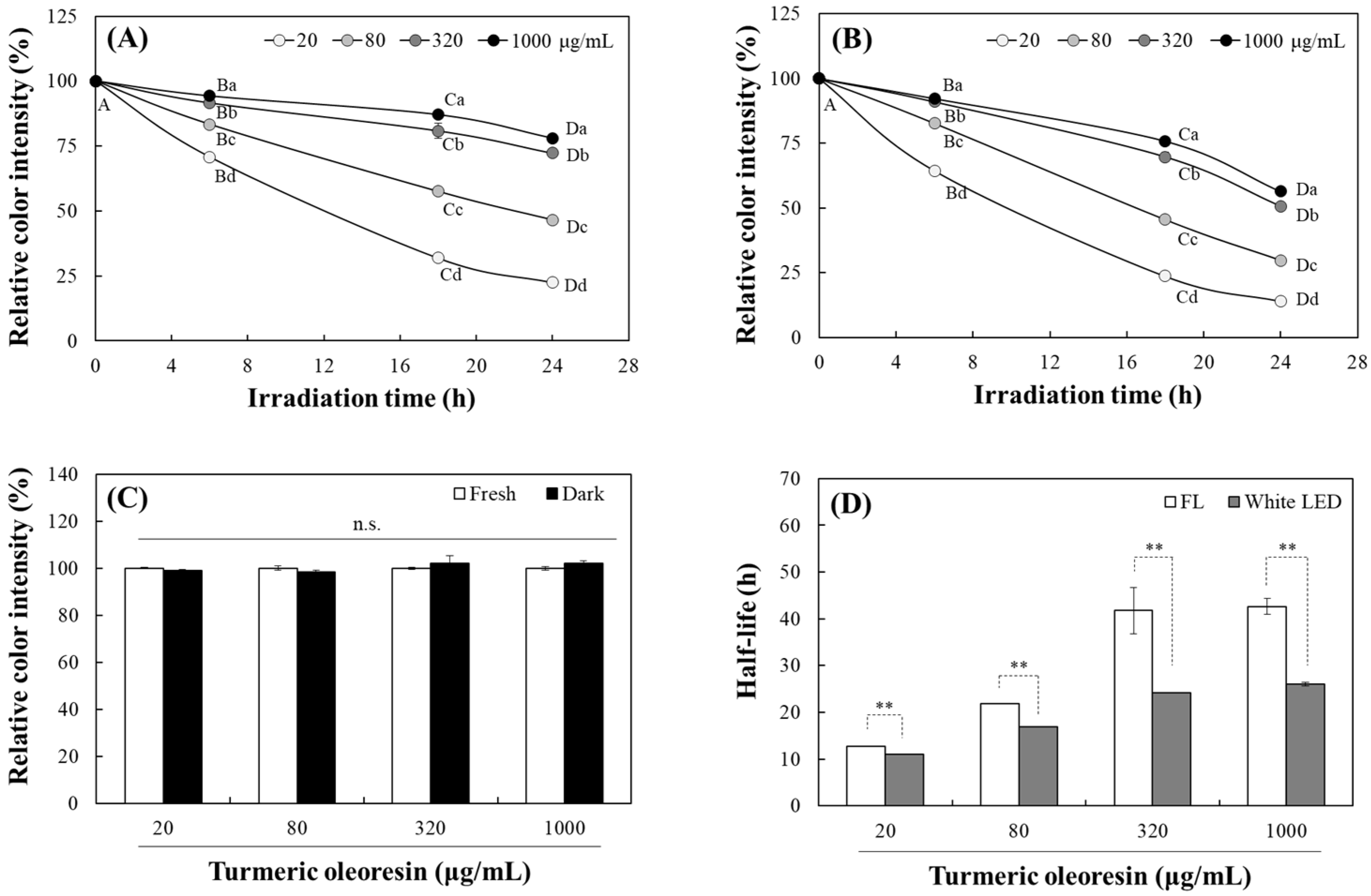
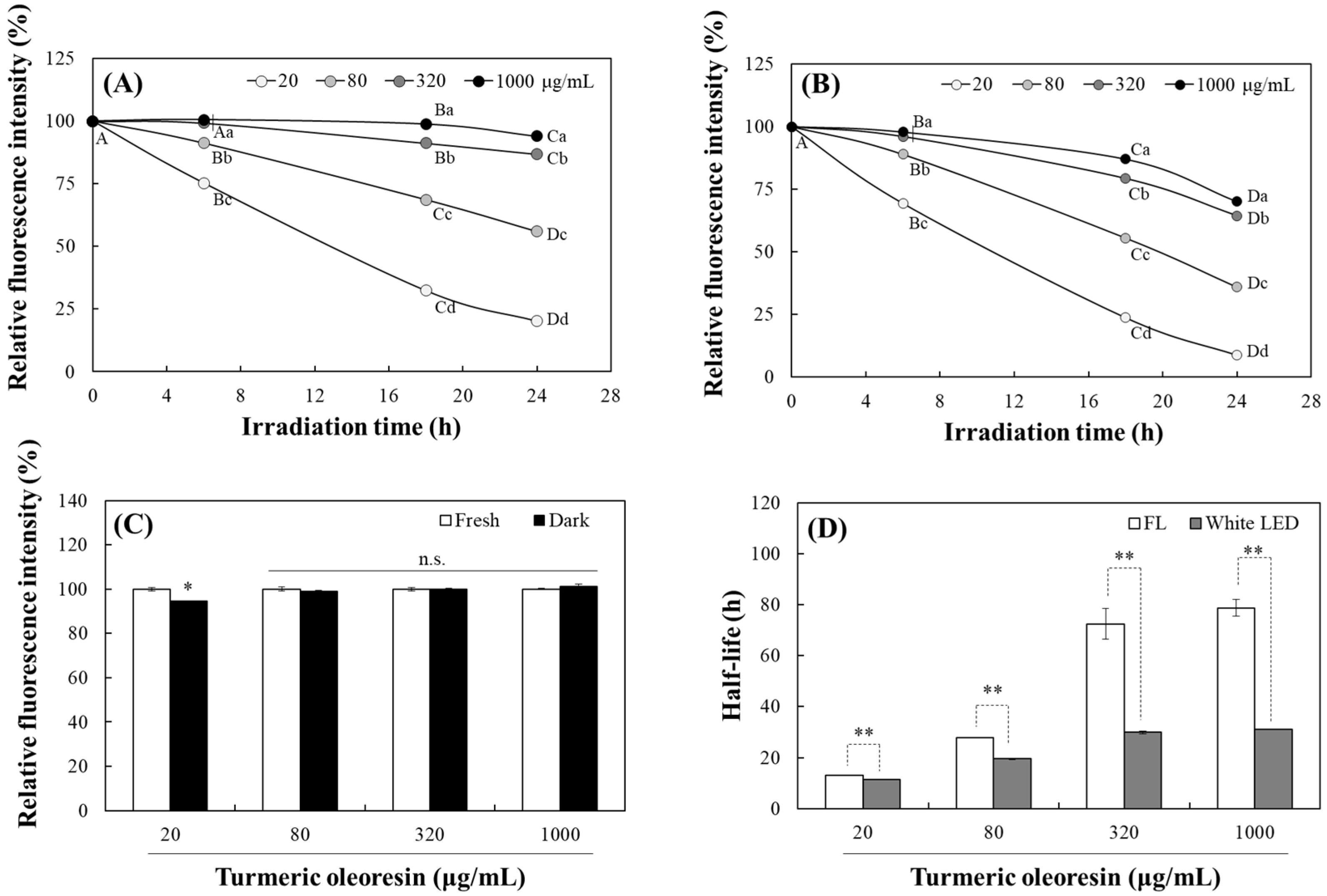
2.1. Changes in the Photostability of TO Under Light Irradiation
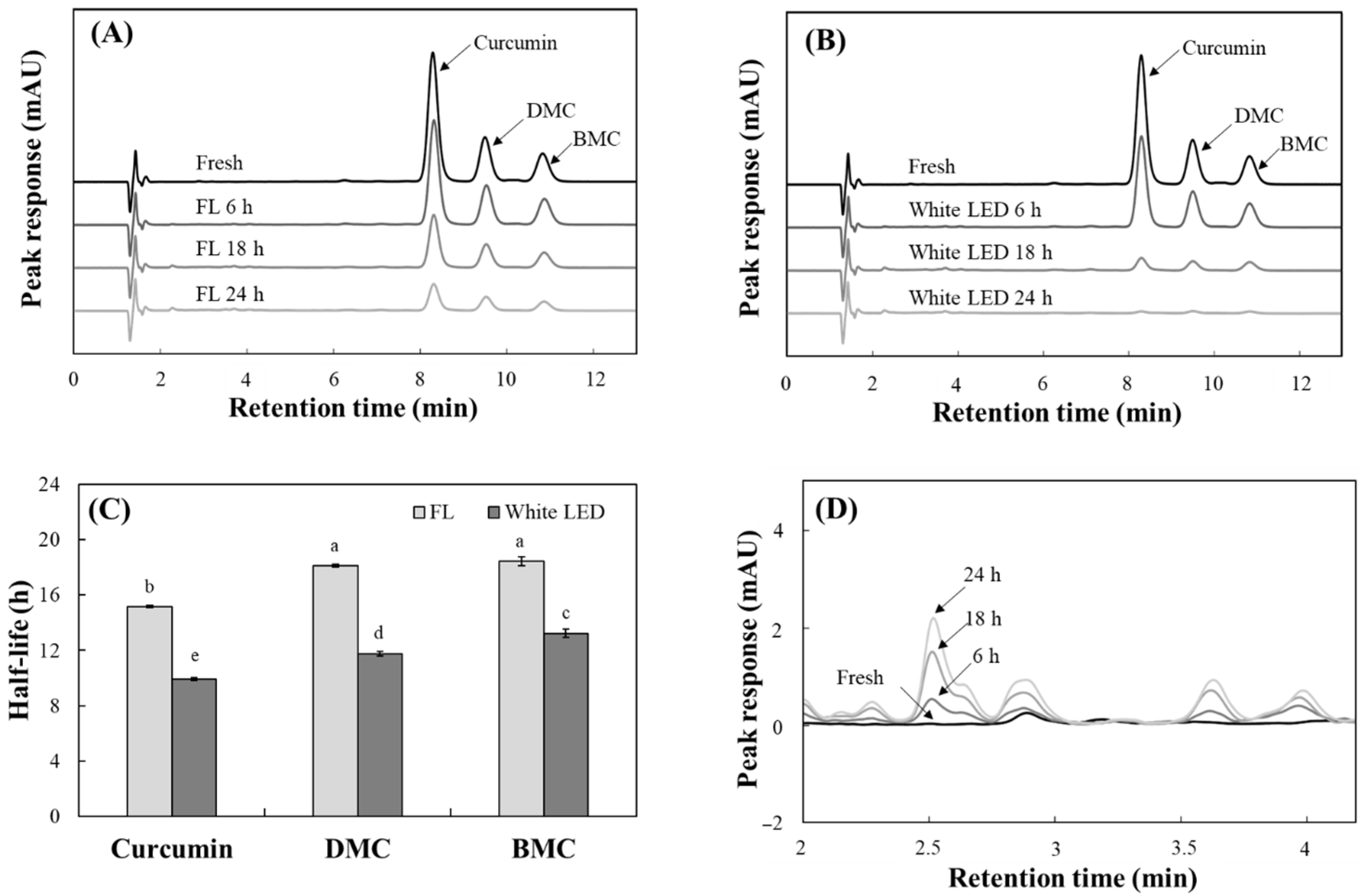
2.2. Analysis of the Effects on Individual Curcuminoid Levels During Light Irradiation
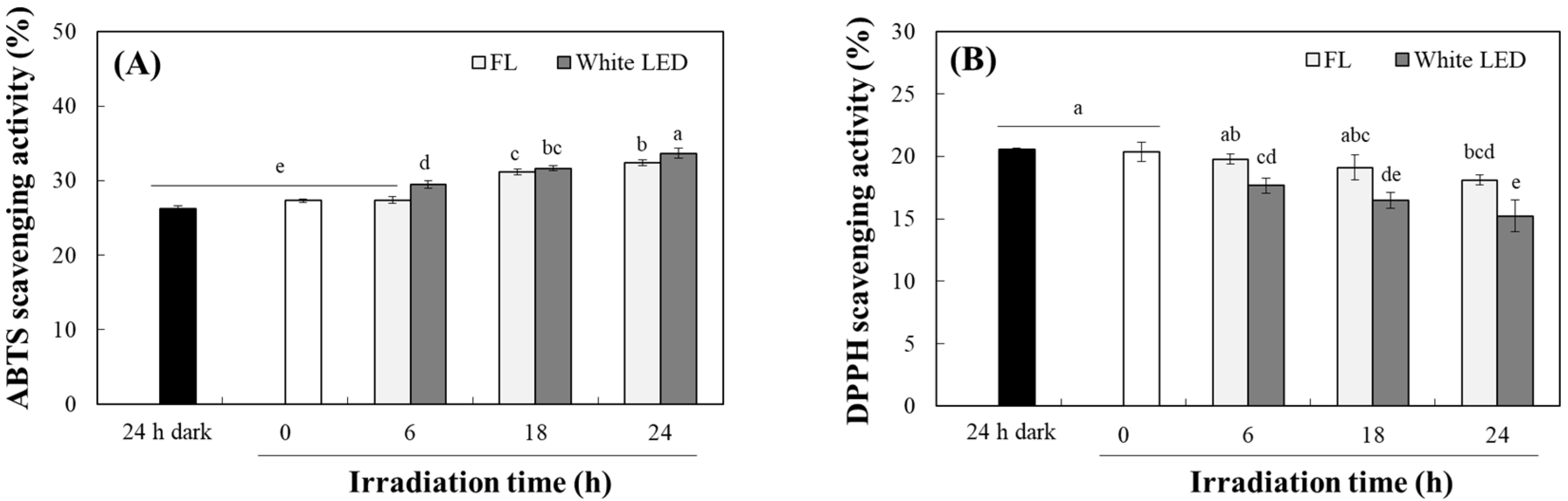
2.3. Changes in Antioxidant Activity of TO by Photodegradation
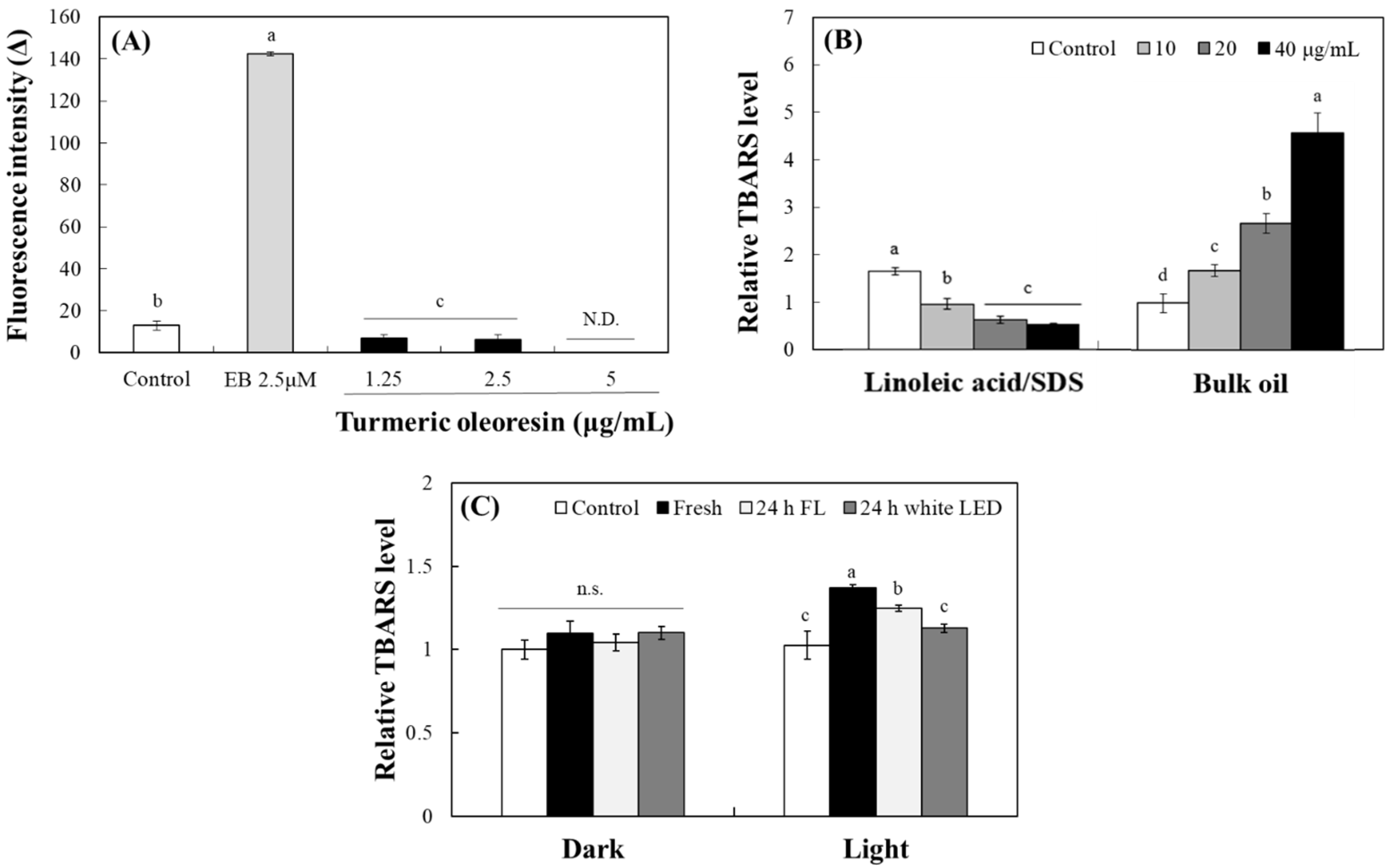
2.4. Photosensitizing Property of TO
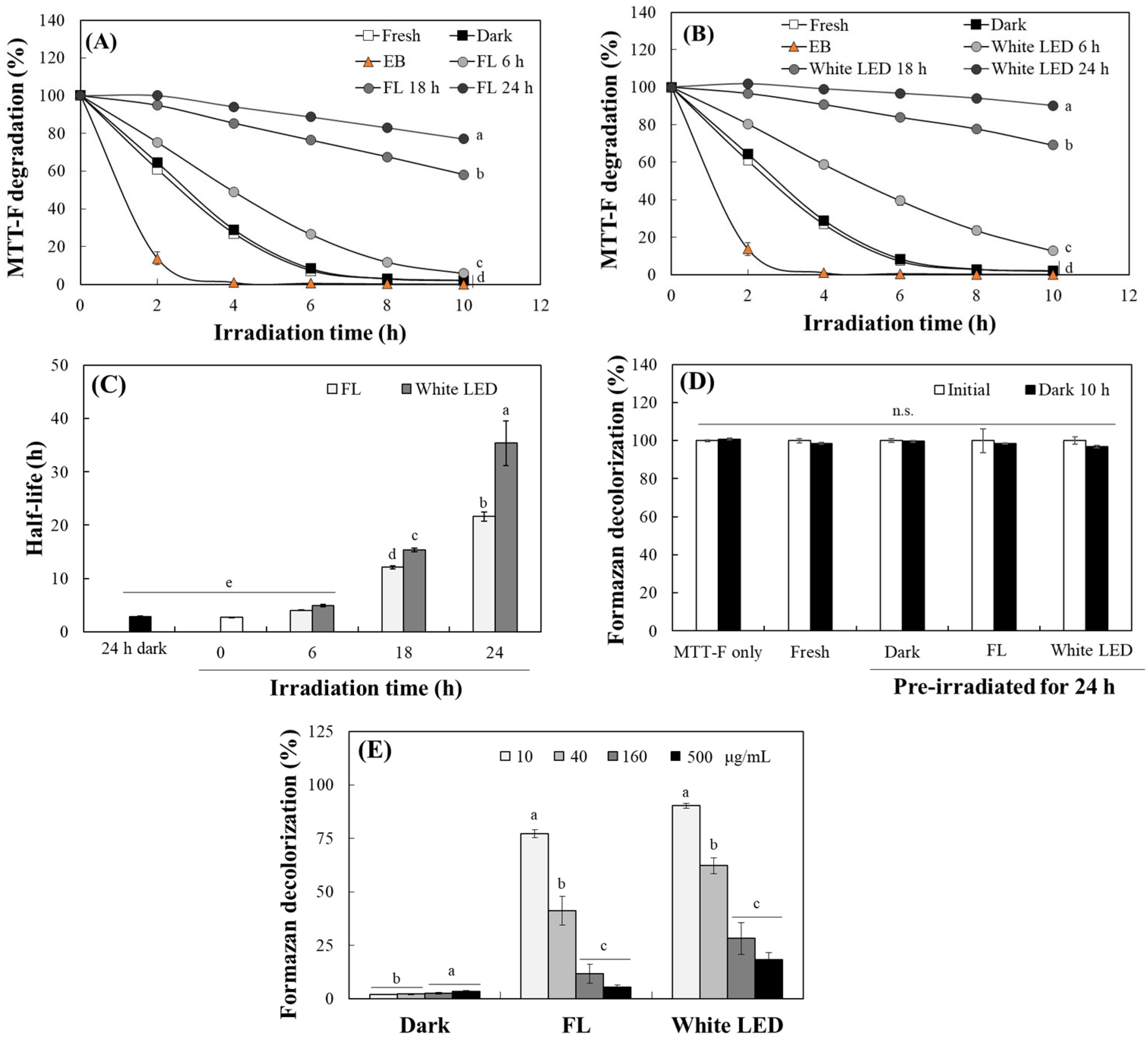
2.5. Measurement of the Photosensitivity of TO Using an MTT Formazan Probe
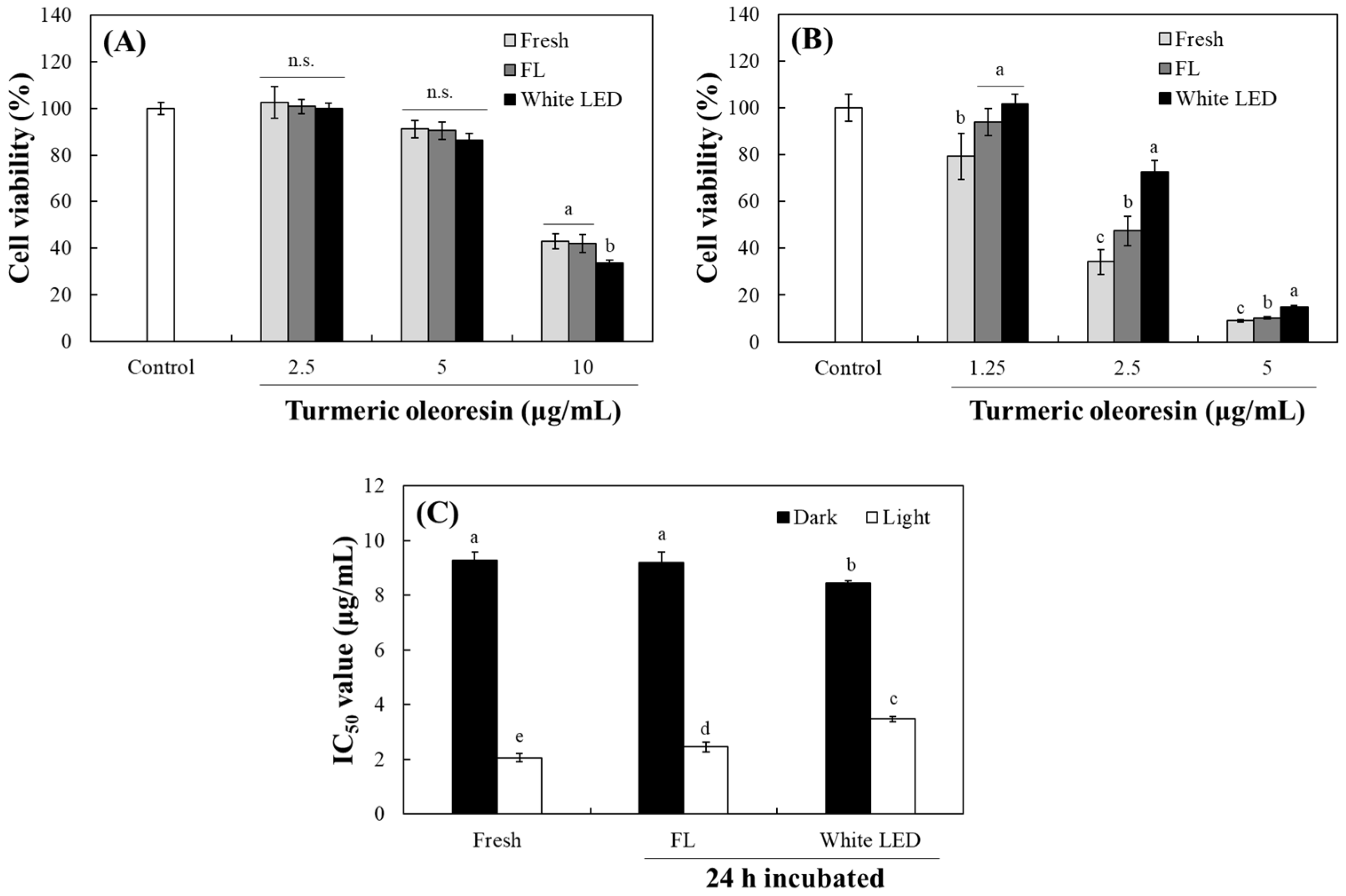
2.6. Changes in Cytotoxic Activity by Photodegradation of TO
3. Materials and Methods
3.1. Chemicals and Cell Lines
3.2. Light Sources and Irradiation
3.3. Analysis of TO Photostability Based on Changes in Color and Fluorescence
3.4. Analysis of Individual Curcuminoid Levels Using HPLC
3.5. Analysis of Antioxidant Activity
3.6. Effects of TO on ROS and Lipid Peroxidation
3.7. Measurement of Photosensitizing Activities of TO Using MTT Formazan Probe
3.8. Determination of Cytotoxic Effects
3.9. Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jayaprakasha, G.K.; Rao, L.J.M.; Sakariah, K.K. Chemistry and biological activities of Curcuma longa. Trends Food Sci. Technol. 2005, 16, 153–160. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Jagan Mohan Rao, L.; Sakariah, K.K. Antioxidant activities of curcumin, demethoxycurcumin and bisdemethoxycurcumin. Food Chem. 2006, 98, 720–724. [Google Scholar] [CrossRef]
- Llano, S.; Gómez, S.; Londoño, J.; Restrepo, Á. Antioxidant activity of curcuminoids: A theoretical and experimental study. Phys. Chem. Chem. Phys. 2019, 21, 3697–3706. [Google Scholar] [CrossRef]
- Priyadarsini, K.I.; Maity, D.K.; Naik, G.H.; Kumar, M.S.; Unnikrishnan, M.K.; Satav, J.G.; Mohan, H. Role of phenolic O-H and methylene hydrogen on the free radical reactions and antioxidant activity of curcumin. Free Radic. Biol. Med. 2003, 35, 475–484. [Google Scholar] [CrossRef]
- Giordano, A.; Tommonaro, G. Curcumin and Cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef]
- Gautam, S.C.; Gao, X.; Dulchavsky, S. Immunomodulation by curcumin. Adv. Exp. Med. Biol. 2007, 595, 321–341. [Google Scholar]
- Sandur, S.K.; Pandey, M.K.; Sung, B.; Ahn, K.S.; Murakami, A.; Sethi, G.; Limtrakul, P.; Badmaev, V.; Aggarwal, B.B. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis 2007, 28, 1765–1773. [Google Scholar] [CrossRef]
- Joshi, P.; Jain, S.; Sharma, V. Turmeric (Curcuma longa) a natural source of edible yellow colour. Int. J. Food Sci. Technol. 2009, 44, 2402–2406. [Google Scholar] [CrossRef]
- Govindarajan, V.S. Turmeric—Chemistry, technology, and quality. Crit. Rev. Food Sci. Nutr. 1980, 12, 199–301. [Google Scholar] [CrossRef] [PubMed]
- Vollono, L.; Falconi, M.; Gaziano, R.; Iacovelli, F.; Dika, E.; Terracciano, C.; Bianchi, L.; Campione, E. Potential of curcumin in skin disorders. Nutrients 2019, 11, 2169. [Google Scholar] [CrossRef] [PubMed]
- Tønnesen, H.H.; Karlsen, J. Studies on curcumin and curcuminoids. VI. Kinetics of curcumin degradation in aqueous solution. Z. Lebensm. Unters. Forsch. 1985, 180, 402–404. [Google Scholar] [CrossRef]
- Song, E.; Kang, S.; Hong, J. Changes in chemical properties, antioxidant activities, and cytotoxicity of turmeric pigments by thermal process. Korean J. Food Sci. Technol. 2018, 50, 21–27. [Google Scholar]
- Jankun, J.; Wyganowska-Świątkowska, M.; Dettlaff, K.; Jelińska, A.; Surdacka, A.; Wątróbska-Świetlikowska, D.; Skrzypczak-Jankun, E. Determining whether curcumin degradation/condensation is actually bioactivation (Review). Int. J. Mol. Med. 2016, 37, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-J.; Pan, M.-H.; Cheng, A.-L.; Lin, L.-I.; Ho, Y.-S.; Hsieh, C.-Y.; Lin, J.-K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Tønnesen, H.H.; Karlsen, J.; van Henegouwen, G.B. Studies on curcumin and curcuminoids. VIII. Photochemical stability of curcumin. Z. Lebensm. Unters. Forsch. 1986, 183, 116–122. [Google Scholar] [CrossRef]
- Jung, Y.N.; Hong, J. Changes in chemical properties and bioactivities of turmeric pigments by photo-degradation. AIMS Agric. Food 2021, 6, 754–767. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. Photophysics, photochemistry and photobiology of curcumin: Studies from organic solutions, bio-mimetics and living cells. J. Photochem. Photobiol. C 2009, 10, 81–95. [Google Scholar] [CrossRef]
- Naksuriya, O.; van Steenbergen, M.J.; Torano, J.S.; Okonogi, S.; Hennink, W.E. A kinetic degradation study of curcumin in its free form and loaded in polymeric micelles. AAPS J. 2016, 18, 777–787. [Google Scholar] [CrossRef]
- Yan, S.; Liao, X.; Xiao, Q.; Huang, Q.; Huang, X. Photostabilities and anti-tumor effects of curcumin and curcumin-loaded polydopamine nanoparticles. RSC Adv. 2024, 14, 13694–13702. [Google Scholar] [CrossRef]
- Pimputkar, S.; Speck, J.S.; DenBaars, S.P.; Nakamura, S. Prospects for LED lighting. Nat. Photonics 2009, 3, 180–182. [Google Scholar] [CrossRef]
- He, P.; Shi, Y.; Meng, T.; Yuan, T.; Li, Y.; Li, X.; Zhang, Y.; Fan, L.; Yang, S. Recent advances in white light-emitting diodes of carbon quantum dots. Nanoscale 2020, 12, 4826–4832. [Google Scholar] [CrossRef] [PubMed]
- Dalsgaard, T.K.; Sørensen, J.; Bakman, M.; Vognsen, L.; Nebel, C.; Albrechtsen, R.; Nielsen, J.H. Light-induced protein and lipid oxidation in cheese: Dependence on fat content and packaging conditions. Dairy Sci. Technol. 2010, 90, 565–577. [Google Scholar] [CrossRef]
- Appendino, G.; Allegrini, P.; de Combarieu, E.; Novicelli, F.; Ramaschi, G.; Sardone, N. Shedding light on curcumin stability. Fitoterapia 2022, 56, 105084. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Hong, J. Induction of lipid peroxidation and melanoma cell death by turmeric oleoresin through its photosensitizing properties. Korean J. Food Sci. Technol. 2022, 54, 59–65. [Google Scholar]
- Krieger-Liszkay, A. Singlet oxygen production in photosynthesis. J. Exp. Bot. 2005, 56, 337–346. [Google Scholar] [CrossRef]
- Li, D.; Xiao, Y.; Zhang, Z.; Liu, C. Light-induced oxidation and isomerization of all-trans-β-cryptoxanthin in a model system. J. Photochem. Photobiol. B Biol. 2015, 142, 51–58. [Google Scholar] [CrossRef]
- Salem, M.; Rohani, S.; Gillies, E.R. Curcumin, a promising anti-cancer therapeutic: A review of its chemical properties, bioactivity and approaches to cancer cell delivery. RSC Adv. 2014, 4, 10815–10829. [Google Scholar] [CrossRef]
- Barik, A.; Priyadarsini, K.I.; Mohan, H. Photophysical studies on binding of curcumin to bovine serum albumin. Photochem. Photobiol. 2003, 77, 597–603. [Google Scholar] [CrossRef]
- Shen, L.; Ji, H. The Pharmacology of curcumin: Is it the degradation products? Trends Mol. Med. 2012, 18, 138–144. [Google Scholar] [CrossRef]
- Schneider, C.; Gordon, O.N.; Edwards, R.L.; Luis, P.B. Degradation of curcumin: From mechanism to biological implications. J. Agric. Food Chem. 2015, 63, 7606–7614. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.A.; Pratap-Singh, A.; Kitts, D.D. Effect of pulsed light on curcumin chemical stability and antioxidant capacity. PLoS ONE 2023, 18, e0291000. [Google Scholar] [CrossRef]
- Peram, M.R.; Jalalpure, S.S.; Palkar, M.B.; Diwan, P.V. Stability studies of pure and mixture form of curcuminoids by reverse phase-HPLC method under various experimental stress conditions. Food Sci. Biotechnol. 2017, 26, 591–602. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Olszowy, M. The importance of solvent type in estimating antioxidant properties of phenolic compounds by ABTS assay. Eur. Food Res. Technol. 2013, 236, 1099–1105. [Google Scholar] [CrossRef]
- Locatelli, M.; Gindro, R.; Travaglia, F.; Coïsson, J.-D.; Rinaldi, M.; Arlorio, M. Study of the DPPH-scavenging activity: Development of a free software for the correct interpretation of data. Food Chem. 2009, 114, 889–897. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Farha, A.K.; Kim, G.; Gul, K.; Gan, R.Y.; Corke, H. Antimicrobial and anticancer applications and related mechanisms of curcumin-mediated photodynamic treatments. Trends Food Sci. Technol. 2020, 97, 341–354. [Google Scholar] [CrossRef]
- Xie, L.; Ji, X.; Zhang, Q.; Wei, Y. Curcumin combined with photodynamic therapy: Promising therapies for the treatment of cancer. Biomed. Pharmacother. 2022, 146, 112567. [Google Scholar] [CrossRef]
- Kang, S.; Oh, Y.J.; Kim, M.-R.; Jung, Y.N.; Song, E.; Lee, H.; Hong, J. Development of a convenient and quantitative method for evaluating photosensitizing activity using thiazolyl blue formazan dye. Molecules 2024, 29, 2471. [Google Scholar] [CrossRef]
- Gray, J.I. Measurement of lipid oxidation: A review. J. Am. Oil Chem. Soc. 1978, 55, 539–546. [Google Scholar] [CrossRef]
- Szlasa, W.; Supplitt, S.; Drąg-Zalesińska, M.; Przystupski, D.; Kotowski, K.; Szewczyk, A.; Kasperkiewicz, P.; Saczko, J.; Kulbacka, J. Effects of curcumin based PDT on the viability and the organization of actin in melanotic (A375) and amelanotic melanoma (C32)—In vitro studies. Biomed. Pharmacother. 2020, 132, 110883. [Google Scholar] [CrossRef]
- Niu, T.; Tian, Y.; Mei, Z.; Zhang, J.; Zhang, B.; Feng, Y.; Zhang, L. Inhibition of autophagy enhances curcumin united light irradiation-induced oxidative stress and tumor growth suppression in human melanoma cells. Sci. Rep. 2016, 6, 31383. [Google Scholar] [CrossRef][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Oh, J.; Hong, J. Photodegradation of Turmeric Oleoresin Under Fluorescent Light and White LED: Impacts on the Chemical Stability, Bioactivity, and Photosensitizing Property of Curcuminoids. Molecules 2025, 30, 3187. https://doi.org/10.3390/molecules30153187
Kim H, Oh J, Hong J. Photodegradation of Turmeric Oleoresin Under Fluorescent Light and White LED: Impacts on the Chemical Stability, Bioactivity, and Photosensitizing Property of Curcuminoids. Molecules. 2025; 30(15):3187. https://doi.org/10.3390/molecules30153187
Chicago/Turabian StyleKim, Heejeong, Juyeon Oh, and Jungil Hong. 2025. "Photodegradation of Turmeric Oleoresin Under Fluorescent Light and White LED: Impacts on the Chemical Stability, Bioactivity, and Photosensitizing Property of Curcuminoids" Molecules 30, no. 15: 3187. https://doi.org/10.3390/molecules30153187
APA StyleKim, H., Oh, J., & Hong, J. (2025). Photodegradation of Turmeric Oleoresin Under Fluorescent Light and White LED: Impacts on the Chemical Stability, Bioactivity, and Photosensitizing Property of Curcuminoids. Molecules, 30(15), 3187. https://doi.org/10.3390/molecules30153187






