Chemical Exploration of Polysaccharides, Fatty Acids, and Antioxidants as Functional Ingredients from Colombian Macroalgae Acanthophora spicifera, Sargassum ramifolium, and Sargassum fluitans
Abstract
1. Introduction
2. Results and Discussion
2.1. Fatty Acids
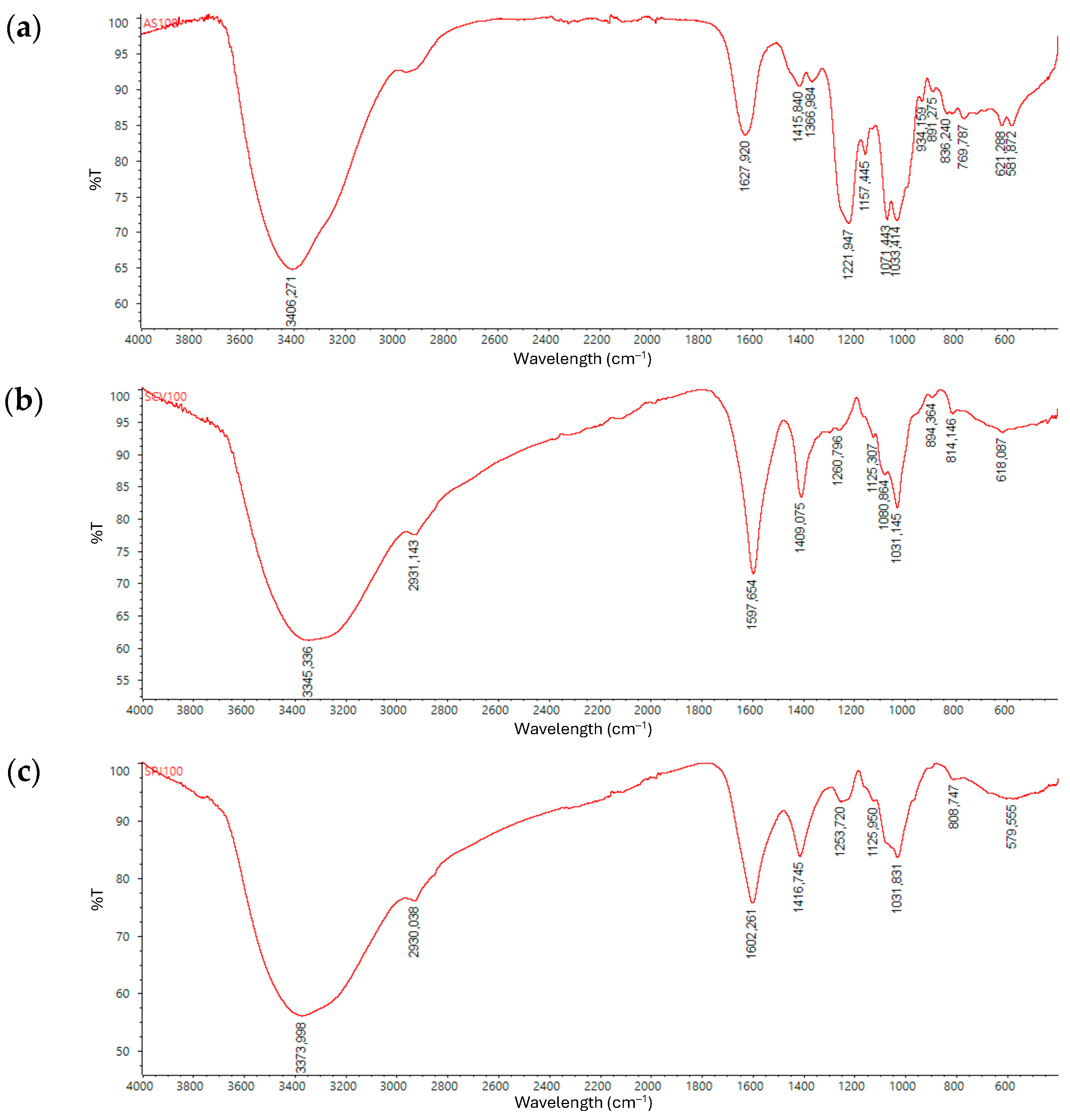
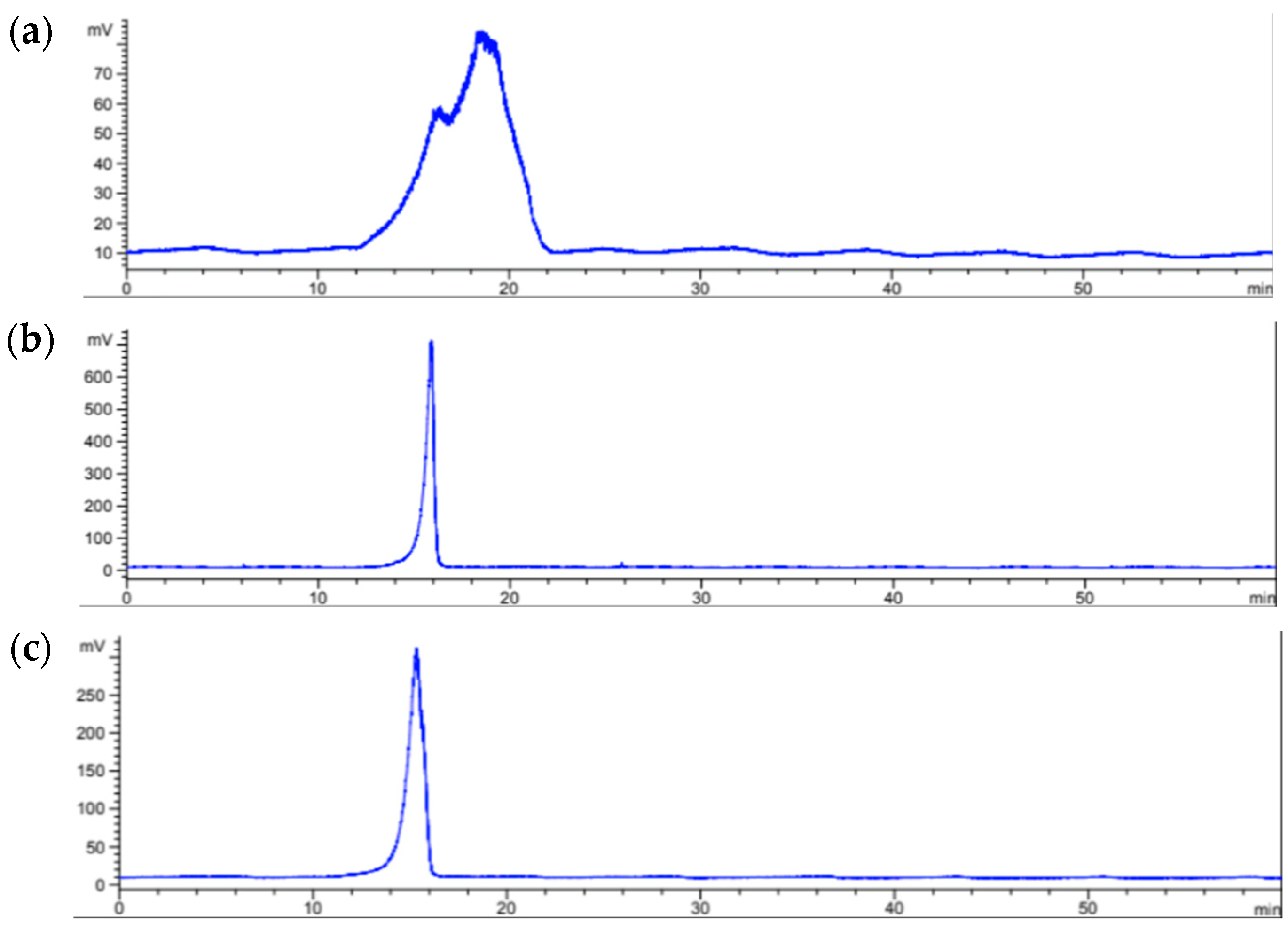
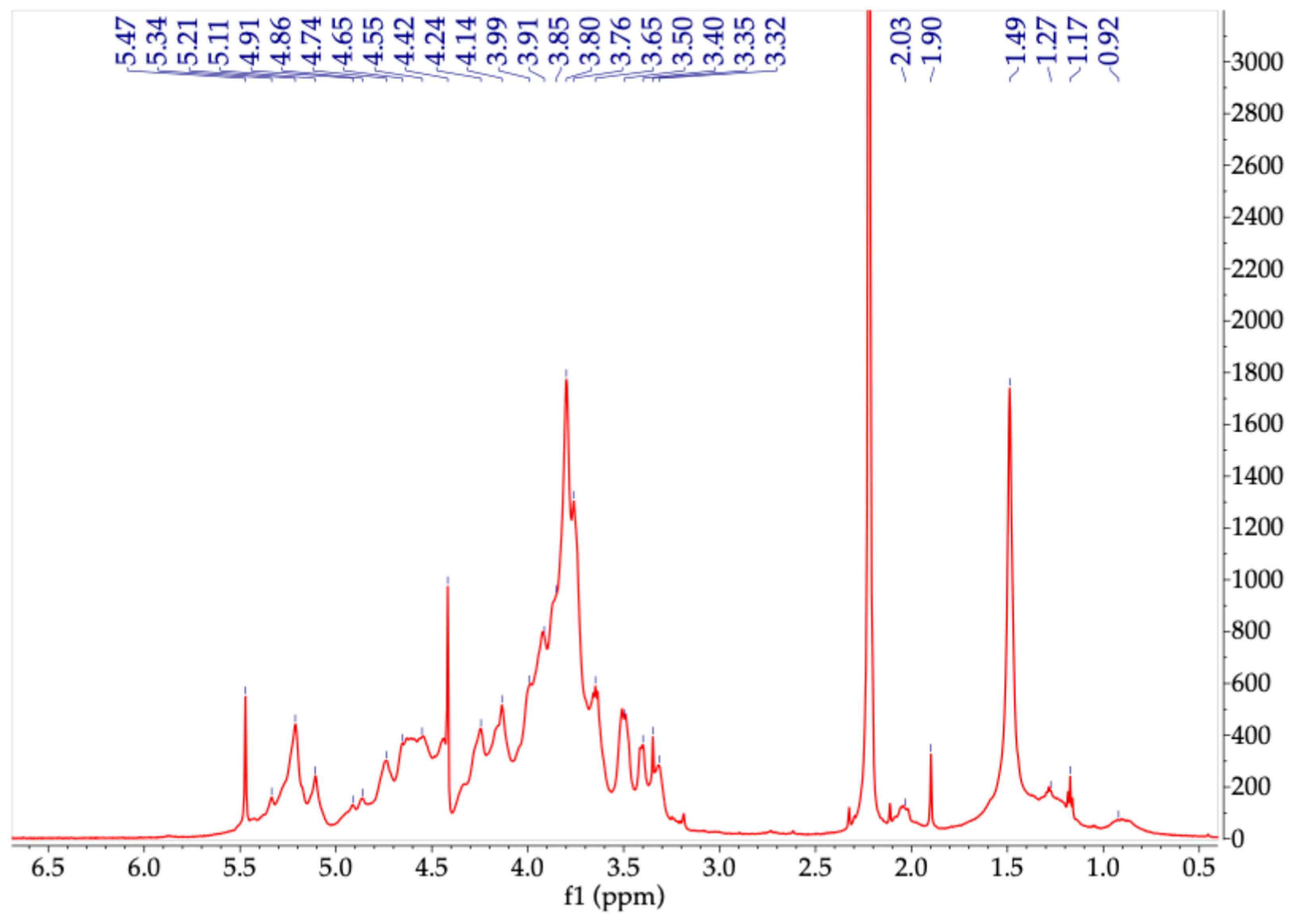
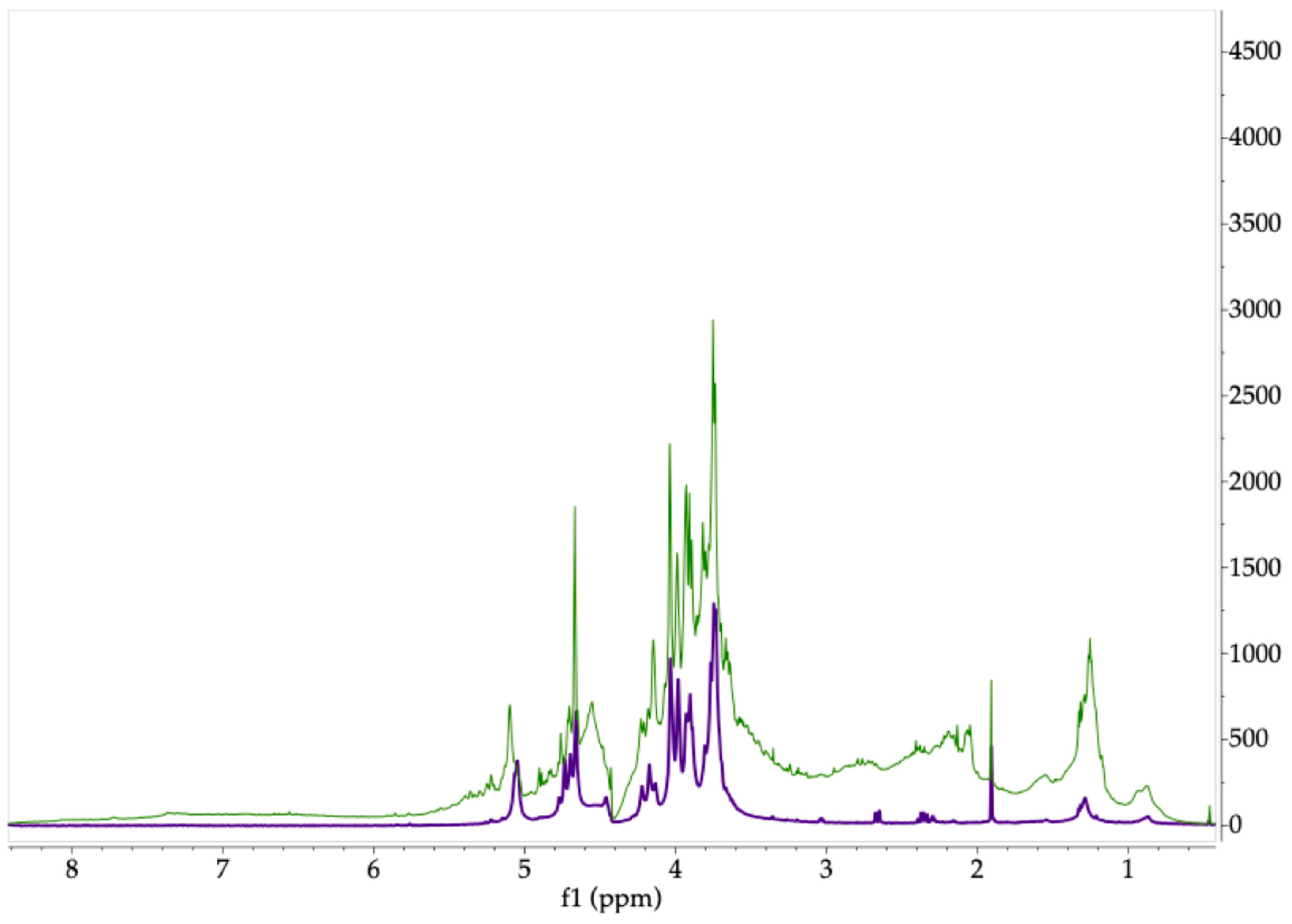
2.2. Polysaccharides
2.3. Polyphenols and Antioxidant Activity
Total Phenolic Compounds and Antioxidant Potential
3. Materials and Methods
3.1. Sample Collection
3.2. Reagents
3.3. Fatty Acid Analysis
3.4. Carbohydrate Analysis
3.4.1. Aqueous Extract
3.4.2. FT-IR Analysis
3.4.3. Size Exclusion Chromatographic Analysis
3.4.4. 1H-NMR Analysis
3.5. Total Phenolic Compounds and Antioxidant Potential Assays
3.5.1. Total Phenolic Compounds (TPC)
3.5.2. Antioxidant Assays
ABTS Assay
DPPH Assay
FRAP Assay
ORAC Assay
3.6. Statistical Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TPC | Total phenolic compounds |
| ORAC | Oxygen Radical Absorbance Capacity |
| FRAP | Ferric-reducing antioxidant power |
| TEAC | Trolox equivalent antioxidant capacity |
| ABTS | 2,2-azino-bis-3-ethylbenzothiazoline-6-sulphonic acid |
| GAE | Gallic acid equivalent |
| NMR | Nuclear magnetic resonance |
| HPLC | High performance liquid chromatography |
| FT-IR | Fourier-transform infrared spectroscopy |
| FAME | Fatty acid methyl ester |
References
- Biris-Dorhoi, E.-S.; Michiu, D.; Pop, C.R.; Rotar, A.M.; Tofana, M.; Pop, O.L.; Socaci, S.A.; Farcas, A.C. Macroalgae—A Sustainable Source of Chemical Compounds with Biological Activities. Nutrients 2020, 12, 3085. [Google Scholar] [CrossRef]
- Arias, A.; Feijoo, G.; Moreira, M.T. Macroalgae Biorefineries as a Sustainable Resource in the Extraction of Value-Added Compounds. Algal Res. 2023, 69, 102954. [Google Scholar] [CrossRef]
- Hentati, F.; Tounsi, L.; Djomdi, D.; Pierre, G.; Delattre, C.; Ursu, A.V.; Fendri, I.; Abdelkafi, S.; Michaud, P. Bioactive Polysaccharides from Seaweeds. Molecules 2020, 25, 3152. [Google Scholar] [CrossRef]
- Pal, A.; Kamthania, M.C.; Kumar, A. Bioactive Compounds and Properties of Seaweeds—A Review. Open Access Libr. J. 2014, 1, 1–17. [Google Scholar] [CrossRef]
- Lomartire, S.; Gonçalves, A.M.M. Algal Phycocolloids: Bioactivities and Pharmaceutical Applications. Mar. Drugs 2023, 21, 384. [Google Scholar] [CrossRef]
- Bi, D.; Yang, X.; Yao, L.; Hu, Z.; Li, H.; Xu, X.; Lu, J. Potential Food and Nutraceutical Applications of Alginate: A Review. Mar. Drugs 2022, 20, 564. [Google Scholar] [CrossRef]
- Pournaki, S.K.; Aleman, R.S.; Hasani-Azhdari, M.; Marcia, J.; Yadav, A.; Moncada, M. Current Review: Alginate in the Food Applications. J 2024, 7, 281–301. [Google Scholar] [CrossRef]
- Abka-khajouei, R.; Tounsi, L.; Shahabi, N.; Patel, A.K.; Abdelkafi, S.; Michaud, P. Structures, Properties and Applications of Alginates. Mar. Drugs 2022, 20, 364. [Google Scholar] [CrossRef] [PubMed]
- Tomić, S.L.; Babić Radić, M.M.; Vuković, J.S.; Filipović, V.V.; Nikodinovic-Runic, J.; Vukomanović, M. Alginate-Based Hydrogels and Scaffolds for Biomedical Applications. Mar. Drugs 2023, 21, 177. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Quito, E.-M.; Ruiz-Caro, R.; Veiga, M.-D. Carrageenan: Drug Delivery Systems and Other Biomedical Applications. Mar. Drugs 2020, 18, 583. [Google Scholar] [CrossRef] [PubMed]
- Kokkuvayil Ramadas, B.; Rhim, J.-W.; Roy, S. Recent Progress of Carrageenan-Based Composite Films in Active and Intelligent Food Packaging Applications. Polymers 2024, 16, 1001. [Google Scholar] [CrossRef]
- Hilliou, L. Structure–Elastic Properties Relationships in Gelling Carrageenans. Polymers 2021, 13, 4120. [Google Scholar] [CrossRef]
- Sohrabipour, J.; Rabiei, R.; Pirian, K. Fatty Acids Composition of Marine Macroalgae (Review). J. Phycol. Res. 2019, 3, 348–374. [Google Scholar] [CrossRef]
- Pereira, H.; Barreira, L.; Figueiredo, F.; Custódio, L.; Vizetto-Duarte, C.; Polo, C.; Rešek, E.; Engelen, A.; Varela, J. Polyunsaturated Fatty Acids of Marine Macroalgae: Potential for Nutritional and Pharmaceutical Applications. Mar. Drugs 2012, 10, 1920–1935. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xing, L.; Meng, W.; Zhang, X.; Li, J.; Dong, P. Molecular Weight Distribution and Structure Analysis of Phlorotannins in Sanhai Kelp (Saccharina Japonica) and Evaluation of Their Antioxidant Activities. Food Chem. 2025, 469, 142569. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Zhang, W.; Smid, S.D. Phlorotannins: A Review on Biosynthesis, Chemistry and Bioactivity. Food Biosci. 2021, 39, 100832. [Google Scholar] [CrossRef]
- Aina, O.; Bakare, O.O.; Daniel, A.I.; Gokul, A.; Beukes, D.R.; Fadaka, A.O.; Keyster, M.; Klein, A. Seaweed-Derived Phenolic Compounds in Growth Promotion and Stress Alleviation in Plants. Life 2022, 12, 1548. [Google Scholar] [CrossRef]
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Gonçalves, A.M.M.; da Silva, G.J.; Pereira, L. Seaweed Phenolics: From Extraction to Applications. Mar. Drugs 2020, 18, 384. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Pereira, A.G.; Lourenço-Lopes, C.; Garcia-Oliveira, P.; Cassani, L.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. Main Bioactive Phenolic Compounds in Marine Algae and Their Mechanisms of Action Supporting Potential Health Benefits. Food Chem. 2021, 341, 128262. [Google Scholar] [CrossRef]
- Eom, S.-H.; Kim, Y.-M.; Kim, S.-K. Antimicrobial Effect of Phlorotannins from Marine Brown Algae. Food Chem. Toxicol. 2012, 50, 3251–3255. [Google Scholar] [CrossRef]
- Liu, M.; Hansen, P.E.; Lin, X. Bromophenols in Marine Algae and Their Bioactivities. Mar. Drugs 2011, 9, 1273–1292. [Google Scholar] [CrossRef]
- Thomas, N.V.; Kim, S.-K. Potential Pharmacological Applications of Polyphenolic Derivatives from Marine Brown Algae. Environ. Toxicol. Pharmacol. 2011, 32, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Campos, N.H. (Ed.) Gavio Brigitte Historia de La Ficología En Colombia. In Colombia Bioazul: Dos Mares un País; Territorios por Explorar; Universidad Nacional de Colombia. (Sede Caribe): Saint Andrew, Colombia; Instituto de Estudios en Ciencias del Mar (CECIMAR): Santa Marta, Colombia, 2023; pp. 239–253. ISBN 978-958-505-136-2. [Google Scholar]
- Quintana-Manotas, H.L.; Hernández-Contreras, D.A.; Gavio, B.; Quintana-Manotas, H.L.; Hernández-Contreras, D.A.; Gavio, B. Updated List and New Records of Macroalgae from the Gulf of Morrosquillo, Colombian Caribbean. Acta Botánica Mex. 2024, 131, 1–23. [Google Scholar] [CrossRef]
- Kilar, J.A.; McLachlan, J. Ecological Studies of the Alga, Acanthophora Spicifera (Vahl) Børg. (Ceramiales: Rhodophyta): Vegetative Fragmentation. J. Exp. Mar. Biol. Ecol. 1986, 104, 1–21. [Google Scholar] [CrossRef]
- Pereira Júnior, L.C.; Nascimento, F.G.; Oliveira, S.R.B.D.; Lima, G.C.; Chagas, F.D.S.; Sombra, V.G.; Feitosa, J.P.A.; Soriano, E.M.; Souza, M.H.L.P.; Zocolo, G.J.; et al. Protective Effect against Gastric Mucosa Injury of a Sulfated Agaran from Acanthophora spicifera. Carbohydr. Polym. 2021, 261, 117829. [Google Scholar] [CrossRef]
- Guillén, P.O.; Motti, P.; Mangelinckx, S.; De Clerck, O.; Bossier, P.; Van Den Hende, S. Valorization of the Chemical Diversity of the Tropical Red Seaweeds Acanthophora and Kappaphycus and Their Applications in Aquaculture: A Review. Front. Mar. Sci. 2022, 9, 957290. [Google Scholar] [CrossRef]
- Fagundo-Mollineda, A.; Robledo, D.; Vásquez-Elizondo, R.M.; Freile-Pelegrín, Y. Antioxidant Activities in Holopelagic Sargassum Species from the Mexican Caribbean: Temporal Changes and Intra-Thallus Variation. Algal Res. 2023, 76, 103289. [Google Scholar] [CrossRef]
- Debue, M.; Guinaldo, T.; Jouanno, J.; Chami, M.; Barbier, S.; Berline, L.; Chevalier, C.; Daniel, P.; Daniel, W.; Descloitres, J.; et al. Understanding the Sargassum Phenomenon in the Tropical Atlantic Ocean: From Satellite Monitoring to Stranding Forecast. Mar. Pollut. Bull. 2025, 216, 117923. [Google Scholar] [CrossRef]
- Davis, D.; Simister, R.; Campbell, S.; Marston, M.; Bose, S.; McQueen-Mason, S.J.; Gomez, L.D.; Gallimore, W.A.; Tonon, T. Biomass Composition of the Golden Tide Pelagic Seaweeds Sargassum fluitans and S. natans (Morphotypes I and VIII) to Inform Valorisation Pathways. Sci. Total Environ. 2021, 762, 143134. [Google Scholar] [CrossRef]
- Ortega-Flores, P.A.; Serviere-Zaragoza, E.; De Anda-Montañez, J.A.; Freile-Pelegrín, Y.; Robledo, D.; Méndez-Rodríguez, L.C. Trace Elements in Pelagic Sargassum Species in the Mexican Caribbean: Identification of Key Variables Affecting Arsenic Accumulation in S. fluitans. Sci. Total Environ. 2022, 806, 150657. [Google Scholar] [CrossRef]
- Chale-Dzul, J.; Pérez-Cabeza de Vaca, R.; Quintal-Novelo, C.; Olivera-Castillo, L.; Moo-Puc, R. Hepatoprotective Effect of a Fucoidan Extract from Sargassum Fluitans Borgesen against CCl4-Induced Toxicity in Rats. Int. J. Biol. Macromol. 2020, 145, 500–509. [Google Scholar] [CrossRef]
- Emu, S.A.; Dulal, M.A.; Kali, T.D.; Chadni, M.S.; Rasul, M.G.; Mondal, M.N.; Ahsan, M.E.; Khan, M.; Shah, A.K.M.A. Effects of Extracting Solvents on Phytochemical, Antioxidant, and Antibacterial Activity of Some Seaweeds from the Bay of Bengal Offshore Island. Food Humanit. 2023, 1, 1157–1166. [Google Scholar] [CrossRef]
- Robledo, D.; Vázquez-Delfín, E.; Freile-Pelegrín, Y.; Vásquez-Elizondo, R.M.; Qui-Minet, Z.N.; Salazar-Garibay, A. Challenges and Opportunities in Relation to Sargassum Events Along the Caribbean Sea. Front. Mar. Sci. 2021, 8, 699664. [Google Scholar] [CrossRef]
- Pech-Puch, D.; Pérez-Povedano, M.; Lenis-Rojas, O.A.; Rodríguez, J.; Jiménez, C. Marine Natural Products from the Yucatan Peninsula. Mar. Drugs 2020, 18, 59. [Google Scholar] [CrossRef]
- Zubia, M.; Robledo, D.; Freile-Pelegrin, Y. Antioxidant Activities in Tropical Marine Macroalgae from the Yucatan Peninsula, Mexico. J. Appl. Phycol. 2007, 19, 449–458. [Google Scholar] [CrossRef]
- D’Armas, H.; Jaramillo-Jaramillo, C.; D’Armas, M.; Ordaz-González, G.J. Fatty acid profile, total phenolic content and antioxidant capacity of seaweeds in Salinas Bay, Ecuador. Rev. De Biol. Mar. Y Oceanogr. 2023, 58, 128–136. [Google Scholar] [CrossRef]
- Wazir, H.; Chay, S.Y.; Zarei, M.; Hussin, F.S.; Mustapha, N.A.; Wan Ibadullah, W.Z.; Saari, N. Effects of Storage Time and Temperature on Lipid Oxidation and Protein Co-Oxidation of Low-Moisture Shredded Meat Products. Antioxidants 2019, 8, 486. [Google Scholar] [CrossRef]
- Dhakal, S.; Pandey, D.; van der Heide, M.E.; Nørgaard, J.V.; Vrhovsek, U.; Khanal, P. Effect of Different Drying Methods on the Nutritional Composition and Phenolic Compounds of the Brown Macroalga, Fucus Vesiculosus (Fucales, Phaeophyceae). J. Appl. Phycol. 2024, 36, 3649–3663. [Google Scholar] [CrossRef]
- Matos, J.; Cardoso, C.; Serralheiro, M.L.; Bandarra, N.M.; Afonso, C. Seaweed Bioactives Potential as Nutraceuticals and Functional Ingredients: A Review. J. Food Compos. Anal. 2024, 133, 106453. [Google Scholar] [CrossRef]
- Calderon, D.; Juarez, H.; Osnaya, N.; Ortiz, M.; Trujillo, F.; Valenzuela, A.; Labra, N.; Santamaria, D.; Barragan, G. Oleic Acid Reduces Oxidative Stress in Rat Brain Induced by Some Anticancer Drugs. Chem.-Biol. Interact. 2024, 398, 111086. [Google Scholar] [CrossRef]
- Burns, J.L.; Nakamura, M.T.; Ma, D.W.L. Differentiating the Biological Effects of Linoleic Acid from Arachidonic Acid in Health and Disease. Prostaglandins Leukot. Essent. Fat. Acids 2018, 135, 1–4. [Google Scholar] [CrossRef]
- Quitral, V.; Morales, C.; Sepúlveda, M.; Schwartz, M. Propiedades Nutritivas y Saludables de Algas Marinas y Su Potencialidad Como Ingrediente Funcional. Rev. Chil. De Nutr. 2012, 39, 196–202. [Google Scholar] [CrossRef]
- Vandanjon, L.; Burlot, A.-S.; Zamanileha, E.F.; Douzenel, P.; Ravelonandro, P.H.; Bourgougnon, N.; Bedoux, G. The Use of FTIR Spectroscopy as a Tool for the Seasonal Variation Analysis and for the Quality Control of Polysaccharides from Seaweeds. Mar. Drugs 2023, 21, 482. [Google Scholar] [CrossRef] [PubMed]
- Tipson, R.S. Infrared Spectroscopy of Carbohydrates: A Review of the Literature; National Bureau of standards: Washington, DC, USA, 1968. [Google Scholar]
- Wiercigroch, E.; Szafraniec, E.; Czamara, K.; Pacia, M.Z.; Majzner, K.; Kochan, K.; Kaczor, A.; Baranska, M.; Malek, K. Raman and Infrared Spectroscopy of Carbohydrates: A Review. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 185, 317–335. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Xu, L.; Wang, B.; Ye, T.; Li, Y.; Wu, H. Optimization of Preparation Conditions, Molecular Structure Analysis and Antitumor Activity of Sulfated Sodium Alginate Oligosaccharides. Eur. Polym. J. 2023, 201, 112571. [Google Scholar] [CrossRef]
- Leal, D.; Matsuhiro, B.; Rossi, M.; Caruso, F. FT-IR Spectra of Alginic Acid Block Fractions in Three Species of Brown Seaweeds. Carbohydr. Res. 2008, 343, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Tojeira, A.; Vaz, D.C.; Mendes, A.; Bártolo, P. Preparation and Characterization of Films Based on Alginate and Aloe Vera. Int. J. Polym. Anal. Charact. 2011, 16, 449–464. [Google Scholar] [CrossRef]
- Marcin, N.; Thesnor, V.; Duvauchelle, V.; Ponce-Mora, A.; Gimeno-Mallench, L.; Narayanin-Richenapin, S.; Brelle, L.; Bejarano, E.; Yacou, C.; Sylvestre, M.; et al. Characterization of Alginates of Sargassum from the Archipelago of Guadeloupe. Separations 2024, 11, 226. [Google Scholar] [CrossRef]
- Ganesan, A.R.; Shanmugam, M.; Palaniappan, S.; Rajauria, G. Development of Edible Film from Acanthophora Spicifera: Structural, Rheological and Functional Properties. Food Biosci. 2018, 23, 121–128. [Google Scholar] [CrossRef]
- Schnoller, V.C.G.; Hernández-Carmona, G.; Hernández-Garibay, E.; López-Vivas, J.M.; Muñoz-Ochoa, M. Chemical Characterization of the Soluble Polysaccharides of the Red Alga Acanthophora Spicifera from La Paz Bay, Baja California Sur, México. Cienc. Mar. 2020, 46, 165–176. [Google Scholar] [CrossRef]
- Temjensangba, I.; Konark, T.; Reshmitha, R.; Krishnap, A. Evaluation of the Proximate Composition and Antioxidant Capacity of Some Seaweeds from the Konkan Coast of India. Algal Res. 2024, 83, 103730. [Google Scholar] [CrossRef]
- Singh, J.; Vishavnath; Sharma, V.; Singh, B. Development of Agar-Alginate Marine Polysaccharides-Based Hydrogels for Agricultural Applications to Reduce Environmental Hazards. Int. J. Biol. Macromol. 2025, 295, 139659. [Google Scholar] [CrossRef]
- Premarathna, A.D.; Ahmed, T.A.E.; Sooaar, A.; Rjabovs, V.; Critchley, A.T.; Hincke, M.T.; Tuvikene, R. Extraction and Functional Characterization of Fucoidans and Alginates from Ecklonia Maxima: A Focus on Skin, Immune, and Intestinal Health. Food Hydrocoll. 2025, 159, 110668. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological Activities and Pharmaceutical Applications of Polysaccharide from Natural Resources: A Review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Wu, D.; Li, M.; Dong, W. Polysaccharides, as Biological Macromolecule-Based Scaffolding Biomaterials in Cornea Tissue Engineering: A Review. Tissue Cell 2022, 76, 101782. [Google Scholar] [CrossRef]
- Williams, D.F. Chapter 36—Hydrogels in Regenerative Medicine. In Principles of Regenerative Medicine, 3rd ed.; Atala, A., Lanza, R., Mikos, A.G., Nerem, R., Eds.; Academic Press: Boston, UK, 2019; pp. 627–650. ISBN 978-0-12-809880-6. [Google Scholar]
- Raza, Z.A.; Munim, S.A.; Ayub, A. Recent Developments in Polysaccharide-Based Electrospun Nanofibers for Environmental Applications. Carbohydr. Res. 2021, 510, 108443. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.; Zhu, F. Ultrasound Modified Polysaccharides: A Review of Structure, Physicochemical Properties, Biological Activities and Food Applications. Trends Food Sci. Technol. 2021, 107, 491–508. [Google Scholar] [CrossRef]
- Ramadan, N.E.; Youssef, F.R.; Alshishtawy, A.A.K.; Elshikh, F.M.; Newir, O.; Abdelazeem, S.H.; Ma’ruf, N.K.; Shouman, H.; Ali, S.S.; El-Sheekh, M.M. Marine Algal Polysaccharides for Drug Delivery Applications: A Review. Int. J. Biol. Macromol. 2025, 295, 139551. [Google Scholar] [CrossRef]
- Rodríguez Sánchez, R.A.; Matulewicz, M.C.; Ciancia, M. NMR Spectroscopy for Structural Elucidation of Sulfated Polysaccharides from Red Seaweeds. Int. J. Biol. Macromol. 2022, 199, 386–400. [Google Scholar] [CrossRef]
- Knutsen, S.H.; Myslabodski, D.E.; Larsen, B.; Usov, A.I. A Modified System of Nomenclature for Red Algal Galactans. Bot. Mar. 1994, 37, 163–170. [Google Scholar] [CrossRef]
- Anand, J.; Sathuvan, M.; Babu, G.V.; Sakthivel, M.; Palani, P.; Nagaraj, S. Bioactive Potential and Composition Analysis of Sulfated Polysaccharide from Acanthophora spicifera (Vahl) Borgeson. Int. J. Biol. Macromol. 2018, 111, 1238–1244. [Google Scholar] [CrossRef]
- Duarte, M.E.R.; Cauduro, J.P.; Noseda, D.G.; Noseda, M.D.; Gonçalves, A.G.; Pujol, C.A.; Damonte, E.B.; Cerezo, A.S. The Structure of the Agaran Sulfate from Acanthophora spicifera (Rhodomelaceae, Ceramiales) and Its Antiviral Activity. Relation between Structure and Antiviral Activity in Agarans. Carbohydr. Res. 2004, 339, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Flores-Contreras, E.A.; Araújo, R.G.; Rodríguez-Aguayo, A.A.; Guzmán-Román, M.; García-Venegas, J.C.; Nájera-Martínez, E.F.; Sosa-Hernández, J.E.; Iqbal, H.M.N.; Melchor-Martínez, E.M.; Parra-Saldivar, R. Polysaccharides from the Sargassum and Brown Algae Genus: Extraction, Purification, and Their Potential Therapeutic Applications. Plants 2023, 12, 2445. [Google Scholar] [CrossRef]
- Muthu, S.; Altemimi, A.B.; Lakshmikanthan, M.; Krishnan, K.; ALKaisy, Q.H.; Awlqadr, F.H.; Hesarinejad, M.A. Phycocolloids from Sargassum Microcystum: Immunomodulatory and Antioxidant Activities of Alginic Acid and Fucoidan. Food Hydrocoll. Health 2025, 7, 100209. [Google Scholar] [CrossRef]
- Faidi, A.; Becheikh, M.E.H.; Lassoued, M.A.; stumbé, J.F.; Safta, F.; Sfar, S. Isolation of Sodium Alginate-like Polysaccharide from Padina Pavonica: Optimization, Characterization and Antioxidant Properties. J. Mol. Struct. 2025, 1321, 139737. [Google Scholar] [CrossRef]
- El-Sheekh, M.; Kassem, W.M.A.; Alwaleed, E.A.; Saber, H. Optimization and Characterization of Brown Seaweed Alginate for Antioxidant, Anticancer, Antimicrobial, and Antiviral Properties. Int. J. Biol. Macromol. 2024, 278, 134715. [Google Scholar] [CrossRef]
- Roy, D.; Sobuj, M.K.A.; Islam, M.S.; Haque, M.M.; Islam, M.A.; Islam, M.M.; Ali, M.Z.; Rafiquzzaman, S.M. Compositional, Structural, and Functional Characterization of Fucoidan Extracted from Sargassum polycystum Collected from Saint Martin’s Island, Bangladesh. Algal Res. 2024, 80, 103542. [Google Scholar] [CrossRef]
- Mabate, B.; Daub, C.D.; Malgas, S.; Pletschke, B.I. Characterisation of Sargassum elegans Fucoidans Extracted Using Different Technologies: Linking Their Structure to α-Glucosidase Inhibition. Algal Res. 2025, 85, 103885. [Google Scholar] [CrossRef]
- Urrea-Victoria, V.; Furlan, C.M.; dos Santos, D.Y.A.C.; Chow, F. Antioxidant Potential of Two Brazilian Seaweeds in Response to Temperature: Pyropia spiralis (Red Alga) and Sargassum stenophyllum (Brown Alga). J. Exp. Mar. Biol. Ecol. 2022, 549, 151706. [Google Scholar] [CrossRef]
- Santos, J.M.; Jesus, B.C.; Ribeiro, H.; Martins, A.; Marto, J.; Fitas, M.; Pinto, P.; Alves, C.; Silva, J.; Pedrosa, R.; et al. Extraction of Macroalgae Phenolic Compounds for Cosmetic Application Using Eutectic Solvents. Algal Res. 2024, 79, 103438. [Google Scholar] [CrossRef]
- Pradas-Baena, I.; Moreno-Rojas, J.M.; Luque de Castro, M.D. Chapter 1—Effect of Processing on Active Compounds in Fresh-Cut Vegetables. In Processing and Impact on Active Components in Food; Preedy, V., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 3–10. ISBN 978-0-12-404699-3. [Google Scholar]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical Evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu Assays to Assess the Antioxidant Capacity of Lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef]
- Siddik, M.A.B.; Francis, P.; Rohani, M.F.; Azam, M.S.; Mock, T.S.; Francis, D.S. Seaweed and Seaweed-Based Functional Metabolites as Potential Modulators of Growth, Immune and Antioxidant Responses, and Gut Microbiota in Fish. Antioxidants 2023, 12, 2066. [Google Scholar] [CrossRef]
- Oluwagunwa, O.A.; Alashi, A.M.; Riedl, K.; Aluko, R.E. Effect of Chlorophyll Content on the Multifunctional Properties of Telfairia Occidentalis Aqueous Leaf Polyphenolic Concentrate. S. Afr. J. Bot. 2024, 174, 85–95. [Google Scholar] [CrossRef]
- Tamayo-Rincón, V.M.; Colorado-Ríos, J.; Alvarez-Bustamante, D.J.; Urrea-Victoria, V.; Márquez-Fernández, D.M.; Salamanca, C.H.; Dall’Acqua, S.; Castellanos-Hernandez, L.; Martínez-Martínez, A. Sustainable Extraction of Prospective Cosmetic Ingredients from Colombian Marine Macroalgae Using Natural Deep Eutectic Solvents. Mar. Drugs 2025, 23, 239. [Google Scholar] [CrossRef]
- Valdez N, A.; Choez, I.; Van Der Hende, S.; Ruìz, O.; Manzano, P. Condiciones óptimas de extracción de compuestos antioxidante del alga roja Acanthophora spicifera. Rev. Bionatura 2023, 8, 1–8. [Google Scholar] [CrossRef]
- Cuong, D.X.; Boi, V.N.; Van, T.T.T.; Hau, L.N. Effect of Storage Time on Phlorotannin Content and Antioxidant Activity of Six Sargassum Species from Nhatrang Bay, Vietnam. J. Appl. Phycol. 2016, 28, 567–572. [Google Scholar] [CrossRef]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for Hydrophilic and Lipophilic Antioxidant Capacity (Oxygen Radical Absorbance Capacity (ORACFL)) of Plasma and Other Biological and Food Samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef]
- Vuppalapati, L.; Velayudam, R.; Nazeer Ahamed, K.F.H.; Cherukuri, S.; Kesavan, B.R. The Protective Effect of Dietary Flavonoid Fraction from Acanthophora Spicifera on Streptozotocin Induced Oxidative Stress in Diabetic Rats. Food Sci. Hum. Wellness 2016, 5, 57–64. [Google Scholar] [CrossRef]
- Sivakumar, S.; Sadaiyandi, V.; Swaminathan, S.; Ramalingam, R. Biocompatibility, Anti-Hemolytic, and Antibacterial Assessments of Electrospun PCL/Collagen Composite Nanofibers Loaded with Acanthophora spicifera Extracts Mediated Copper Oxide Nanoparticles. Biocatal. Agric. Biotechnol. 2024, 55, 102983. [Google Scholar] [CrossRef]
- Sali, V.K.; Malarvizhi, R.; Manikandamathavan, V.M.; Vasanthi, H.R. Isolation and Evaluation of Phytoconstituents from Red Alga Acanthophora spicifera as Potential Apoptotic Agents towards A549 and HeLa Cancer Cells Lines. Algal Res. 2018, 32, 172–181. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, X. A Critical Review of the Abilities, Determinants, and Possible Molecular Mechanisms of Seaweed Polysaccharides Antioxidants. Int. J. Mol. Sci. 2020, 21, 7774. [Google Scholar] [CrossRef]
- Santos, A.K.F. de S.; da Fonseca, D.V.; Salgado, P.R.R.; Muniz, V.M.; de Arruda Torres, P.; Lira, N.S.; da Silva Dias, C.; de Morais Pordeus, L.C.; Barbosa-Filho, J.M.; de Almeida, R.N. Antinociceptive Activity of Sargassum polyceratium and the Isolation of Its Chemical Components. Rev. Bras. Farmacogn. 2015, 25, 683–689. [Google Scholar] [CrossRef]
- Lahrsen, E.; Liewert, I.; Alban, S. Gradual Degradation of Fucoidan from Fucus Vesiculosus and Its Effect on Structure, Antioxidant and Antiproliferative Activities. Carbohydr. Polym. 2018, 192, 208–216. [Google Scholar] [CrossRef]
- Ruiz-Medina, M.A.; Sansón, M.; González-Rodríguez, Á.M. Changes in Antioxidant Activity of Fresh Marine Macroalgae from the Canary Islands during Air-Drying Process. Algal Res. 2022, 66, 102798. [Google Scholar] [CrossRef]
- Esim, N.; Dawar, P.; Arslan, N.P.; Orak, T.; Doymus, M.; Azad, F.; Ortucu, S.; Albayrak, S.; Taskin, M. Natural Metabolites with Antioxidant Activity from Micro-and Macro-Algae. Food Biosci. 2024, 62, 105089. [Google Scholar] [CrossRef]
- DeVries, J.W.; Kjos, L.; Groff, L.; Bob, M.; Kristi, C.; Patel, H.; Payne, M.; Leichtweis, H.; Shay, M.; Newcomer, L. Studies in Improvement of Official Method 996.06. J. AOAC Int. 1999, 82, 1146–1155. [Google Scholar] [CrossRef]
- House, S.D. Determination of Total, Saturated, and Monounsaturated Fats In Foodstuffs by Hydrolytic Extraction and Gas Chromatographic Quantitation: Collaborative Study. J. AOAC Int. 1997, 80, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Kupina, S.; Fields, C.; Roman, M.C.; Brunelle, S.L. Determination of Total Phenolic Content Using the Folin-C Assay: Single-Laboratory Validation, First Action 2017.13. J. AOAC Int. 2018, 101, 1466–1472. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Cano, A.; Acosta, M. The Hydrophilic and Lipophilic Contribution to Total Antioxidant Activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Plank, D.W.; Szpylka, J.; Sapirstein, H.; Woollard, D.; Zapf, C.M.; Lee, V.; Chen, C.-Y.O.; Liu, R.H.; Tsao, R.; Düsterloh, A.; et al. Determination of Antioxidant Activity in Foods and Beverages by Reaction with 2,2′-Diphenyl-1-Picrylhydrazyl (DPPH): Collaborative Study First Action 2012.04. J. AOAC Int. 2012, 95, 1562–1569. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- AOAC 2012.23; Total Antioxidant Activity Oxygen Radical Absorbance Capacity (ORAC) Using Fluorescein as the Fluorescence Probe. AOAC Official Methods of Analysis, Online, Ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2019.

| Fatty Acid | Acanthophora spicifera | Sargassum ramifolium | Sargassum fluitans |
|---|---|---|---|
| 14:0 | 0.05 | 0.04 | 0.02 |
| 15:0 | 0.01 | ND 1 | ND 1 |
| 16:0 | 0.33 | 0.31 | ND 1 |
| 16:1ω7 | 0.02 | 0.04 | 0.01 |
| 18:0 | ND 1 | 0.01 | ND 1 |
| 18:1ω9 | 0.10 | 0.13 | 0.04 |
| 18:2ω6 | 0.02 | 0.03 | 0.02 |
| 18:3ω3 | ND 1 | 0.01 | ND 1 |
| 20:4ω6 | ND 1 | 0.03 | 0.01 |
| 22:0 | ND 1 | 0.01 | ND 1 |
| Sample | Rt | Log MW | Mp (kDa) * |
|---|---|---|---|
| A. spicifera | 16.30–18.60 | 5.204 | 92–160 |
| S. ramifolium | 15.90 | 5.246 | 176 |
| S. fluitans | 15.70 | 5.267 | 185 |
| Alga | TPC | DPPH | ORAC | FRAP | ABTS |
|---|---|---|---|---|---|
| A. Spicifera | 366.04 ± 6.76 | 39.17 ± 1.73 | 541.09 ± 21.89 | 49.19 ± 0.98 | 283.42 ± 5.67 |
| S. ramifolium | 936.67 ± 18.25 | 102.84 ± 3.34 | 3362.67 ± 99.85 | 113.24 ± 6.18 | 1830.69 ± 88.62 |
| S. fluitans | 449.43 ± 6.06 | 57.69 ± 2.42 | 1898.04 ± 65.70 | 128.74 ± 2.55 | 1237.45 ± 18.08 |
| Statistical Measure | Test | ||||
|---|---|---|---|---|---|
| TPC | FRAP | ABTS | DPPH | ORAC | |
| F-value | 482.57 | 351.23 | 668.87 | 482.57 | 1214.40 |
| p-Value * | 2.36 × 10−7 | 6.07 × 10−7 | 8.90 × 10−8 | 2.36 × 10−7 | 1.50 × 10−8 |
| Groups | Statistical Measure * | Test | ||||
|---|---|---|---|---|---|---|
| TPC | FRAP | ABTS | DPPH | ORAC | ||
| A. spicifera –S. fluitans | Mean difference | 83.37 | 79.56 | 954.08 | 18.52 | 1357.4 |
| p-Value | 0.0003 | 0.0 | 0.0 | 0.0003 | 0.0 | |
| A. spicifera –S. ramifolium | Mean difference | 570.71 | 64.15 | 1548.64 | 63.68 | 2822.26 |
| p-Value | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| A. spicifera –S. fluitans | Mean difference | 487.34 | −15.41 | 594.56 | 45.16 | 1464.86 |
| p-Value | 0.0 | 0.0069 | 0.0 | 0.0 | 0.0 | |
| Species | Group | Order | Family | Collection Date | Collection Site | Voucher Number |
|---|---|---|---|---|---|---|
| A. spicifera | Red macroalgae | Ceramiale | Rhodomelacea | 28 February 2021 | Santa Marta | JIW00005017 |
| S. ramifolium | Brown macroalgae | Fucales | Sargassaceae | 1 March 2021 | Guajira | JIW00004940 |
| S. fluitans | Fucales | Sargassaceae | 1 February 2021 | San Andrés | JIW00004945 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colorado-Ríos, J.; Restrepo-Espinosa, D.C.; Restrepo-Moná, Y.; Monsalve, J.D.; Márquez-Fernández, D.M.; Castellanos, L.; Martínez-Martínez, A. Chemical Exploration of Polysaccharides, Fatty Acids, and Antioxidants as Functional Ingredients from Colombian Macroalgae Acanthophora spicifera, Sargassum ramifolium, and Sargassum fluitans. Molecules 2025, 30, 3333. https://doi.org/10.3390/molecules30163333
Colorado-Ríos J, Restrepo-Espinosa DC, Restrepo-Moná Y, Monsalve JD, Márquez-Fernández DM, Castellanos L, Martínez-Martínez A. Chemical Exploration of Polysaccharides, Fatty Acids, and Antioxidants as Functional Ingredients from Colombian Macroalgae Acanthophora spicifera, Sargassum ramifolium, and Sargassum fluitans. Molecules. 2025; 30(16):3333. https://doi.org/10.3390/molecules30163333
Chicago/Turabian StyleColorado-Ríos, Jhonny, Diana C. Restrepo-Espinosa, Yuli Restrepo-Moná, Juan David Monsalve, Diana M. Márquez-Fernández, Leonardo Castellanos, and Alejandro Martínez-Martínez. 2025. "Chemical Exploration of Polysaccharides, Fatty Acids, and Antioxidants as Functional Ingredients from Colombian Macroalgae Acanthophora spicifera, Sargassum ramifolium, and Sargassum fluitans" Molecules 30, no. 16: 3333. https://doi.org/10.3390/molecules30163333
APA StyleColorado-Ríos, J., Restrepo-Espinosa, D. C., Restrepo-Moná, Y., Monsalve, J. D., Márquez-Fernández, D. M., Castellanos, L., & Martínez-Martínez, A. (2025). Chemical Exploration of Polysaccharides, Fatty Acids, and Antioxidants as Functional Ingredients from Colombian Macroalgae Acanthophora spicifera, Sargassum ramifolium, and Sargassum fluitans. Molecules, 30(16), 3333. https://doi.org/10.3390/molecules30163333





