Fluorimetric Determination of Eosin Y in Water Samples and Drinks Using Deep Eutectic Solvent-Based Liquid-Phase Microextraction
Abstract
1. Introduction
2. Results and Discussion
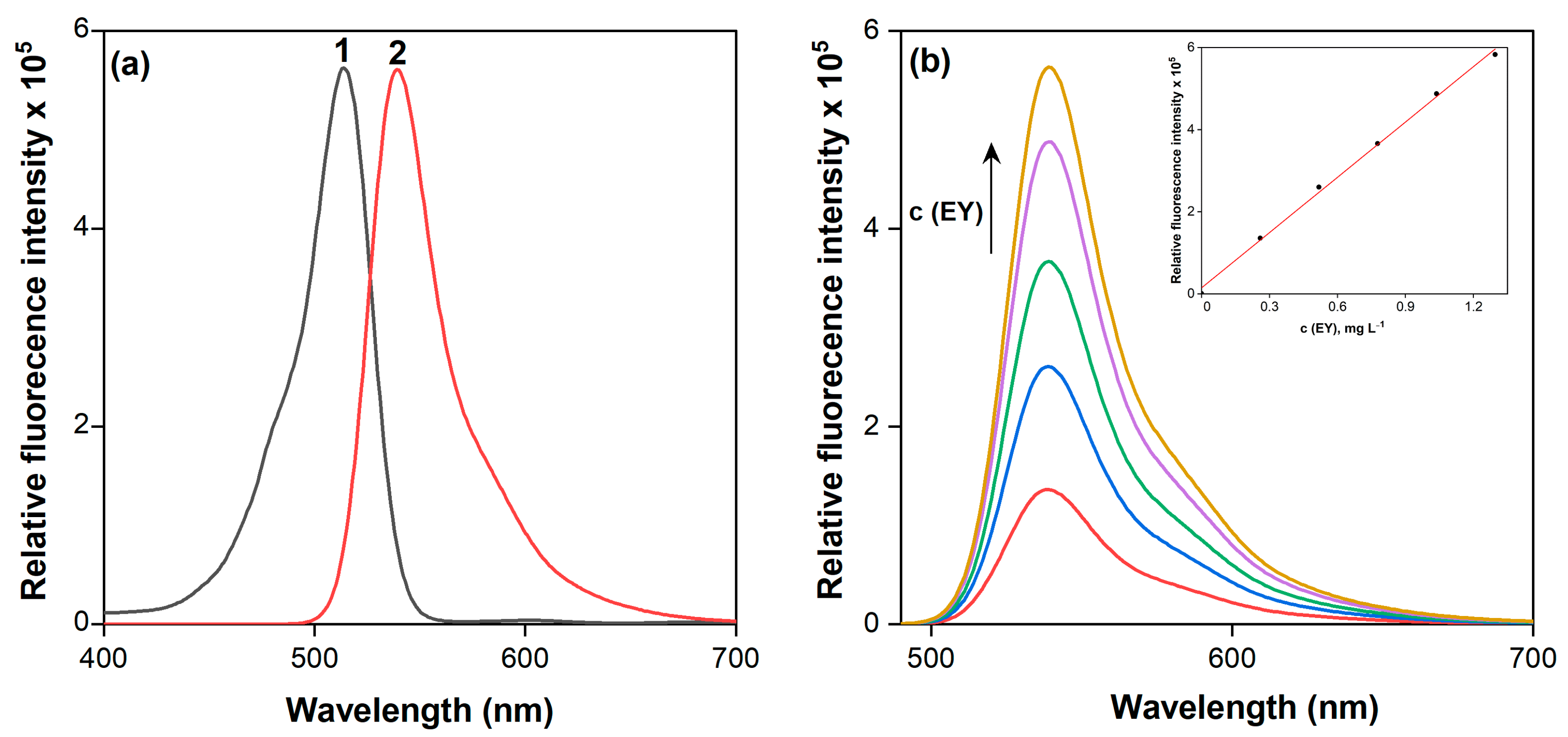
2.1. Protolitic and Fluorescence Properties of EY
2.2. DFT-Based Theoretical Calculations
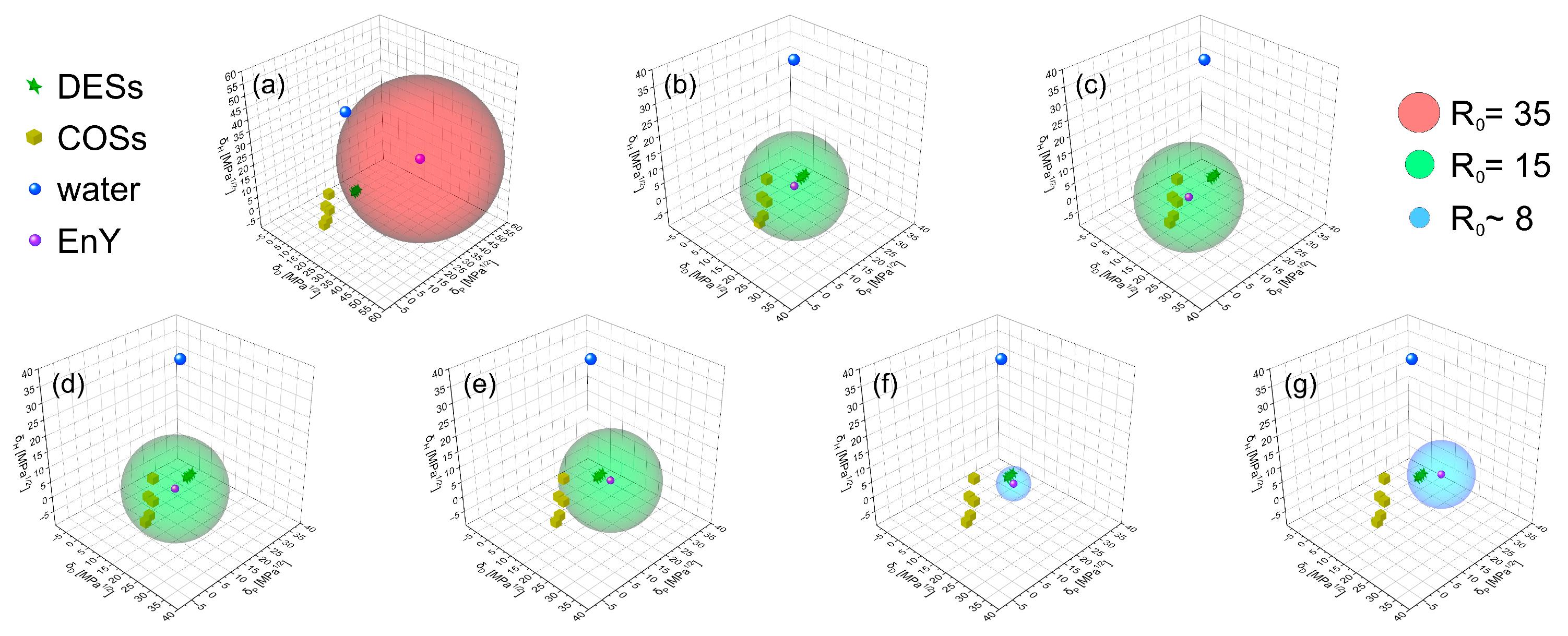
2.3. Hansen Solubility Parameters
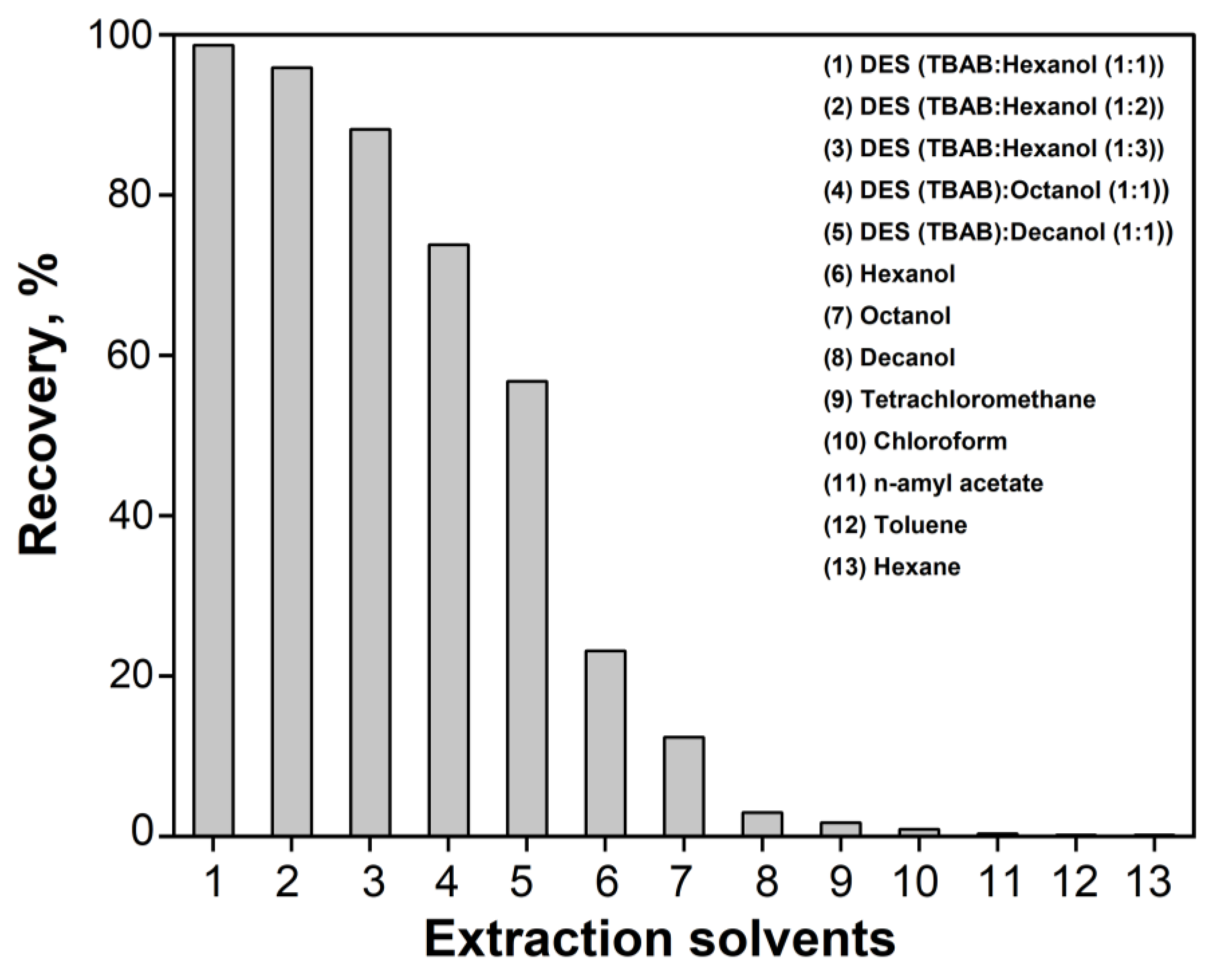
2.4. Optimization of EY Microextraction Using DES
2.4.1. Effect of Acidity
2.4.2. Effect of Vortex Mixing and Centrifugation
2.4.3. Stability of the Extracts over Time
2.5. Interference Study
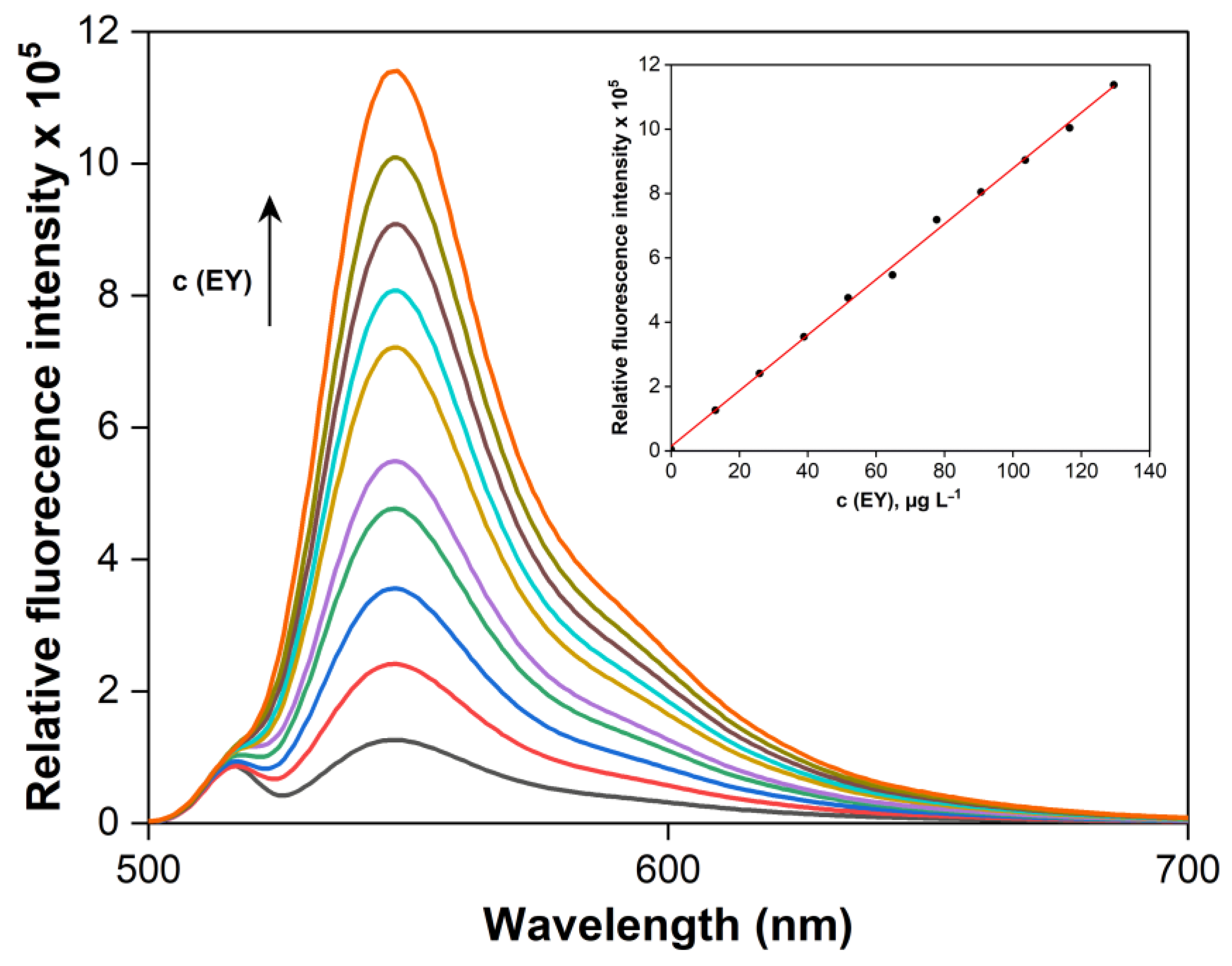
2.6. Validation Characteristics of the LPME-FLD Method
2.7. Application to Real Sample Analysis
3. Materials and Methods
3.1. Materials
Applied Instruments
3.2. Methods
3.2.1. Preparation of DESs
3.2.2. Sample Preparation
3.2.3. Procedure for the Determination of EY with LPME-FLD
3.2.4. Theoretical Methods and Software
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Ferreira, S.C.R.; Oliveira, M.C.; Pais, A.A.C.C.; Seixas De Melo, J.S. Shades of red: A chemical exploration of pigments and dyes in 19th century postage stamps by a multi-analytical mehodology. Talanta 2025, 285, 127409. [Google Scholar] [CrossRef]
- Kar, S.; Kumar, H.; Sangamesh, N.C.; Mishra, S.; Bajoria, A.A.; Saha, M. Safe food coloring agent as an alternative to Eosin stain. Cureus 2024, 16, e69817. [Google Scholar] [CrossRef] [PubMed]
- Dhasarathan, R.; Kavitha, B.; Aswathaman, H.; Senthilkumar, N.; Rani, S. Electrochemical sensors of food colorant—Eosin Y dye on carbon nanomaterials modified glassy carbon electrode. Mater. Today Proc. 2022, 48, 556–560. [Google Scholar] [CrossRef]
- Staneva, D.; Yordanova, S.; Vasileva-Tonkova, E.; Stoyanov, S.; Grabchev, I. Photophysical and antibacterial activity of light-activated quaternary eosin Y. Open Chem. 2019, 17, 1244–1251. [Google Scholar] [CrossRef]
- Gao, D.; Tian, Y.; Liang, F.; Jin, D.; Chen, Y.; Zhang, H.; Yu, A. Investigation on the pH-dependent binding of Eosin Y and bovine serum albumin by spectral methods. J. Lumin. 2007, 127, 515–522. [Google Scholar] [CrossRef]
- Sobika, M.; Karunanithi, S.; Dash, S. Studies on anionic dye Eosin Y in mixed anionic micellar medium. Results Chem. 2024, 7, 101220. [Google Scholar] [CrossRef]
- Al-Behadili, W.K.H.; Jawad, Y.M.; Whaab, W.S.A. Effect of solvent on intensity of absorption and fluorescence of Eosin Y dye and spectral properties of Eosin Y dye. J. Med. Chem. Sci. 2023, 6, 322–334. [Google Scholar] [CrossRef]
- Abbas, F.S.; Alaboodi, A.S.; Abojassim, A.A. Studying the effect of the solvent on the spectral properties of the Eosin-y Dye that used as medical laser medium. Cardiometry 2023, 25, 1463–1467. [Google Scholar] [CrossRef]
- de Freitas, C.F.; Estevao, B.M.; Pellosi, D.S.; Scarminio, I.S.; Caetano, W.; Hioka, N.; Batistela, V.R. Chemical equilibria of Eosin Y and its synthetic ester derivatives in non-ionic and ionic micellar environments. J. Mol. Liq. 2020, 327, 114794. [Google Scholar] [CrossRef]
- Vodolazkaya, N.A.; Kleshchevnikova, Y.A.; Mchedlov-Petrossyan, N.O. Differentiating impact of the AOT-stabilized droplets of water-in-octane microemulsions as examined using halogenated fluoresceins as molecular probes. J. Mol. Liq. 2013, 187, 381–388. [Google Scholar] [CrossRef]
- Erdinc, N.; Goktürk, S. Spectrophotometric and conductometric studies on the interaction of anionic dye Eosin-Y with cationic micelles. Anal. Chem. Lett. 2014, 4, 146–157. [Google Scholar] [CrossRef]
- Wang, J.; Chen, L.; Li, Y.; Shen, W.; Manley-Harris, M. A novel determination method for Ag(I) in environmental samples based on reduction of absorbance and fluorescence quenching of Eosin Y. Microchem. J. 2024, 196, 109588. [Google Scholar] [CrossRef]
- Saeed, M.A.; Mostafa, N.S.; Mahmoud, A.M.; Elsayed, M.A. Application of Eosin Y as a probe for spectroscopic determination of Aripiprazole in human plasma. Luminescence 2025, 40, e70131. [Google Scholar] [CrossRef]
- Abdel-Lateef, M.A.; Almahri, A.; Derayea, S.M.; Samir, E. Xanthene based resonance Rayleigh scattering and spectrofluorimetric probes for the determination of cyclobenzaprine: Application to content uniformity test. Rev. Anal. Chem. 2020, 9, 222–230. [Google Scholar] [CrossRef]
- Salim, M.M.; Marie, A.A.; Kamal, A.H.; Hammad, S.F.; Elkhoudary, M.M. Using of eosin Y as a facile fluorescence probe in alogliptin estimation: Application to tablet dosage forms and content uniformity testing. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 285, 121919. [Google Scholar] [CrossRef] [PubMed]
- Mahnashi, M.H.; Mahmoud, A.M.; El-Wekil, M.M.; Shahin, R.Y. An innovative enzyme-free ratiometric determination of uric acid based on polyethyleneimine modified graphene quantum dots pretreated with periodate combined with eosin Y. Microchem. J. 2023, 193, 109062. [Google Scholar] [CrossRef]
- Nandhini, N.T.; Rajeshkumar, S.; Mythili, S. The possible mechanism of eco-friendly synthesized nanoparticles on hazardous dyes degradation. Biocatal. Agric. Biotechnol. 2019, 19, 101138. [Google Scholar] [CrossRef]
- Pierau, L.; Andaloussi, S.A.; Chiappone, A.; Lajnef, S.; Peyrot, F.; Malval, J.-P.; Jockusch, S.; Versace, D.-L. Eosin Y derivatives for visible light-mediated free-radical polymerization: Application in 3D-photoprinting and bacterial photodynamic inactiva-tion. Eur. Polym. J. 2024, 214, 113143. [Google Scholar] [CrossRef]
- Nagar, B.; Dhar, B.B. Visible light-mediated thiolation of substituted 1,4-naphthoquinones using Eosin Y as a photoredox catalyst. J. Org. Chem. 2022, 87, 3195–3201. [Google Scholar] [CrossRef]
- Derayea, S.M.; Nagy, D.M. Application of a xanthene dye, eosin Y, as spectroscopic probe in chemical and pharmaceutical analysis; a review. Rev. Anal. Chem. 2018, 37, 20170020. [Google Scholar] [CrossRef]
- Alghamdi, A.F.; Kooli, F. A sensitive procedure for the rapid electrochemical determination of Eosin-Y dye using voltammetric techniques onto a mercury electrode surface. J. Mater. Environ. Sci. 2013, 4, 762–769. [Google Scholar]
- Brumley, W.C.; Farley, J.W. Determining eosin as a groundwater migration tracer by capillary electrophoresis/laser-induced fluorescence using a multiwavelength laser. Electrophoresis 2003, 24, 2335–2339. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, V.A.; Stokes, D.L.; Vo-Dinh, T. Vibrational spectral analysis of Eosin Y and Erythrosin B—Intensity studies for quantitative detection of the dyes. J. Raman Spectrosc. 1994, 25, 415–422. [Google Scholar] [CrossRef]
- Kaur, A.; Gupta, U. H-Point standard addition method for simultaneous determination of Eosin and Erytrosine. E-J. Chem. 2011, 8, 1979–1985. [Google Scholar] [CrossRef]
- Van Liedekerke, B.M.; Nelis, H.J.; Wittekind, D.H.; De Leenheer, A.P. Stability study of Azure B, Eosin Y and commercial Romanowsky Giemsa stock solutions using high performance liquid chromatography. Histochem. J. 1991, 23, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, F.; Degano, I.; Colombini, M.P. Development of a method based on high-performance liquid chromatography coupled with diode array, fluorescence, and mass spectrometric detectors for the analysis of eosin at trace levels. Sep. Sci. Plus 2020, 3, 207–215. [Google Scholar] [CrossRef]
- Kakalejčíková, S.; Bazel’, Y.; Thi, V.A.L.; Fizer, M. An innovative vortex-assisted liquid-liquid microextraction approach using deep eutectic solvent: Application for the spectrofluorometric determination of Rhodamine B in water, food and cosmetic samples. Molecules 2024, 29, 3397. [Google Scholar] [CrossRef]
- Kakalejčíková, S.; Harenčár, D.; Bazel’, Y.; Fizer, M. Fluorescence-based detection of Picric acid using vortex-assisted liquid–liquid microextraction: An innovative analytical approach. Processes 2025, 13, 1051. [Google Scholar] [CrossRef]
- Fizer, O.; Fizer, M.; Sidey, V.; Studenyak, Y. Predicting the end point potential break values: A case of potentiometric titration of lipophilic anions with cetylpyridinium chloride. Microchem. J. 2021, 160, 105758. [Google Scholar] [CrossRef]
- Fizer, O.; Fizer, M.; Sidey, V.; Studenyak, Y.; Mariychuk, R. Benchmark of different charges for prediction of the partitioning coefficient through the hydrophilic/lipophilic index. J. Mol. Model. 2018, 24, 141. [Google Scholar] [CrossRef]
- Das, T.; Mehta, C.H.; Nayak, U.Y. Multiple approaches for achieving drug solubility: An in silico perspective. Drug Discov. Today 2020, 25, 1206–1212. [Google Scholar] [CrossRef]
- Atiq, O.; Ricci, E.; Giacinti Baschetti, M.; Grazia De Angelis, M. Modelling solubility in semi-crystalline polymers: A critical comparative review. Fluid Phase Equilib. 2022, 556, 113412. [Google Scholar] [CrossRef]
- Klamt, A. The COSMO and COSMO-RS solvation models. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018, 8, e1338. [Google Scholar] [CrossRef]
- Khan, A.S.; Ibrahim, T.H.; Rashid, Z.; Khamis, M.I.; Nancarrow, P.; Jabbar, N.A. COSMO-RS based screening of ionic liquids for extraction of phenolic compounds from aqueous media. J. Mol. Liq. 2021, 328, 115387. [Google Scholar] [CrossRef]
- Wojeicchowski, J.P.; Ferreira, A.M.; Abranches, D.O.; Mafra, M.R.; Coutinho, J.A.P. Using COSMO-RS in the design of deep eutectic solvents for the extraction of antioxidants from Rosemary. ACS Sustain. Chem. Eng. 2020, 8, 12132–12141. [Google Scholar] [CrossRef]
- Darwish, A.S.; Lemaoui, T.; Al Yammahi, J.; Taher, H.; Benguerba, Y.; Banat, F.; Al Nashef, I.M. Molecular insights into potential hydrophobic deep eutectic solvents for furfural extraction guided by COSMO-RS and machine learning. J. Mol. Liq. 2023, 379, 121631. [Google Scholar] [CrossRef]
- Roslan, R.; Kurnia, K.A.; Hasanudin, N.; Hilmy, N.I.M.F.; Ruslan, M.S.H. Screen and design of deep eutectic solvents (DESs) for the extraction of delphinidin-3-sambubioside from Hibiscus sabdariffa via COSMO-RS. AIP Conf. Proc. 2024, 3041, 050003. [Google Scholar] [CrossRef]
- Lazović, M.; Cvijetić, I.; Jankov, M.; Milojković-Opsenica, D.; Trifković, J.; Ristivojević, P. COSMO-RS in prescreening of Natural Eutectic Solvents for phenolic extraction from Teucrium chamaedrys. J. Mol. Liq. 2023, 387, 122649. [Google Scholar] [CrossRef]
- Hansen, C.M. Solubility Parameters—An Introduction in Hansen Solubility Parameters. In A User’s Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 1–26. [Google Scholar] [CrossRef]
- Otárola-Sepúlveda, J.; Cea-Klapp, E.; Aravena, P.; Ormazábal-Latorre, S.; Canales, R.I.; Garrido, J.M.; Valerio, O. Assessment of Hansen solubility parameters in deep eutectic solvents for solubility predictions. J. Mol. Liq. 2023, 388, 122669. [Google Scholar] [CrossRef]
- Fernandes, C.C.; Paiva, A.; Haghbakhsh, R.; Duarte, A.R.C. Application of Hansen solubility parameters in the eutectic mixtures: Difference between empirical and semi-empirical models. Sci. Rep. 2025, 15, 3862. [Google Scholar] [CrossRef]
- Fernandes, C.C.; Paiva, A.; Haghbakhsh, R.; Duarte, A.R.C. Understanding the solubility behaviour of Ibuprofen and Xylitol in natural deep eutectic systems through Hansen solubility parameters and physicochemical properties. J. Mol. Liq. 2025, 428, 127544. [Google Scholar] [CrossRef]
- Moreira Novaes, F.J.; de Faria, D.C.; Zamboni Ferraz, F.; de Aquino Neto, F.R. Hansen solubility parameters applied to the extraction of phytochemicals. Plants 2023, 12, 3008. [Google Scholar] [CrossRef] [PubMed]
- Batistela, V.R.; Pellosi, D.S.; de Souza, F.D.; da Costa, W.F.; de Oliveira Santin, S.M.; de Souza, V.R.; Caetano, W.; de Oliveira, H.P.M.; Scarminio, I.S.; Hioka, N. pKa determinations of xanthene derivates in aqueous solutions by multivariate analysis applied to UV–Vis spectrophotometric data. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 79, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Levillain, P.; Fompeydie, D. Determination of equilibrium constants by derivative spectrophotometry. Application to the pKas of eosin. Anal. Chem. 1985, 57, 2561–2563. [Google Scholar] [CrossRef]
- Mchedlov-Petrossyan, N.O.; Kukhtik, V.I.; Egorova, S.I. Protolytic equilibria of Fluorescein halo derivatives in aqueous-organic systems. Russ. J. Gen. Chem. 2006, 76, 1607–1617. [Google Scholar] [CrossRef]
- Tamburello-Luca, A.A.; Hébert, P.; Antoine, R.; Brevet, P.F.; Girault, H.H. Optical surface second harmonic generation study of the two Acid/Base equilibria of Eosin B at the Air/Water interface. Langmuir 1997, 13, 4428–4434. [Google Scholar] [CrossRef]
- Vodolazkaya, N.A.; Gurina, Y.A.; Salamanova, N.V.; Mchedlov-Petrossyan, N.O. Spectroscopic study of acid–base ionization and tautomerism of fluorescein dyes in direct microemulsions at high bulk ionic strength. J. Mol. Liq. 2009, 145, 188–196. [Google Scholar] [CrossRef]
- Amat-Guerri, F.; López-González, M.M.C.; Sastre, R.; Martinez-Utrilla, R. Spectrophotometric determination of ionization and isomerization constants of Rose Bengal, eosin Y and some derivatives. Dye. Pigm. 1990, 13, 219–232. [Google Scholar] [CrossRef]
- Slyusareva, E.A.; Gerasimova, M.A. pH-dependence of the absorption and fluorescent properties of fluorone dyes in aqueous solutions. Russ. Phys. J. 2014, 56, 1370–1377. [Google Scholar] [CrossRef]
- Tóth, J.; Bazel, Y.; Balogh, I. A fully automated system with an optical immersion probe (OIP) for high-precision spectropho-tometric measurements. Talanta 2021, 226, 122185. [Google Scholar] [CrossRef]
- Kathiravan, V.A.; Jhonsi, M.A.; Renganathan, R. A Study on the fluorescence quenching of Eosin by certain organic dyes. Z. Phys. Chem. 2008, 222, 1013–1020. [Google Scholar] [CrossRef]
- Bedair, A.; Mansour, F. Homogeneous Liquid–Liquid Microextraction. In Microextraction Techniques: Fundamentals, Applications and Recent Developments; Springer: Cham, Switzerland, 2024; pp. 315–355. [Google Scholar] [CrossRef]
- Yamini, Y.; Rezazadeh, M.; Seidi, S. Liquid-phase microextraction—The different principles and configurations. Trends Anal. Chem. 2019, 112, 264–272. [Google Scholar] [CrossRef]
- Kakalejčíková, S.; Bazeľ, Y. A combination of vortex-assisted liquid–liquid microextraction with fluorescence detection: An innovative approach for a green and highly sensitive determination of sodium dodecyl sulfate in water samples and pharmaceutical. Microchem. J. 2024, 199, 110226. [Google Scholar] [CrossRef]
- Kunz, W.; Häckl, K. The hype with ionic liquids as solvents. Chem. Phys. Lett. 2016, 661, 6–12. [Google Scholar] [CrossRef]
- Bazel, Y.; Rečlo, M.; Chubirka, Y. Switchable hydrophilicity solvents in analytical chemistry. Five years of achievements. Microchem. J. 2020, 157, 105115. [Google Scholar] [CrossRef]
- Alshana, U.; Hassan, M.; Al-Nidawi, M.; Yilmaz, E.; Soylak, M. Switchable-hydrophilicity solvent liquid-liquid microextraction, Trends Anal. Chem. 2020, 131, 116025. [Google Scholar] [CrossRef]
- Andruch, V.; Kalyniukova, A.; Płotka-Wasylka, J.; Jatkowska, N.; Snigur, D.; Zaruba, S.; Płatkiewicz, J.; Zgoła-Grześkowiak, A.; Werner, J. Application of deep eutectic solvents in analytical sample pretreatment (update 2017–2022). Part A: Liquid phase microextraction. Microchem. J. 2023, 189, 108509. [Google Scholar] [CrossRef]
- Santana-Mayor, Á.; Rodríguez-Ramos, R.; Herrera-Herrera, A.V.; Socas-Rodríguez, B.; Rodríguez-Delgado, M.Á. Deep eutectic solvents. The new generation of green solvents in analytical chemistry. Trends Anal. Chem. 2021, 134, 116108. [Google Scholar] [CrossRef]
- Ramezani, A.M.; Ahmadi, R.; Yamini, Y. Homogeneous liquid-liquid microextraction based on deep eutectic solvents. Trends Anal. Chem. 2022, 149, 116566. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their application. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Alshana, U.; Soylak, M. Deep eutectic solvents in microextraction. In Analytical Sample Preparation with Nano- and Other High-Performance Materials; Elsevier: Amsterdam, The Netherlands, 2021; pp. 471–512. [Google Scholar] [CrossRef]
- De los Ríos, M.D.; Belmonte, R.M. Extending Microsoft excel and Hansen solubility parameters relationship to double Hansen’s sphere calculation. SN Appl. Sci. 2022, 4, 185. [Google Scholar] [CrossRef]
- Mchedlov-Petrossyan, N.O.; Kleshchevnikova, V.N. Influence of the cetyltrimethylammonium chloride micellar pseudophase on the protolytic equilibria of oxyxanthene dyes at high bulk phase ionic strength. J. Chem. Soc. Faraday Trans. 1994, 90, 629–640. [Google Scholar] [CrossRef]
- Vilková, M.; Płotka-Wasylka, J.; Andruch, V. The role of water in deep eutectic solvent-base extraction. J. Mol. Liq. 2020, 304, 112747. [Google Scholar] [CrossRef]
- Shishov, A.; Chromá, R.; Vakh, C.; Kuchár, J.; Simon, A.; Andruch, V.; Bulatov, A. In situ decomposition of deep eutectic solvent as a novel approach in liquid-liquid microextraction. Anal. Chim. Acta 2019, 1065, 49–55. [Google Scholar] [CrossRef]
- Shishov, A.; Makoś-Chełstowska, P.; Bulatov, A.; Andruch, V. Deep eutectic solvents or eutectic mixtures? Characterization of tetrabutylammonium bromide and nonanoic acid mixtures. J. Phys. Chem. B 2022, 126, 3889–3896. [Google Scholar] [CrossRef]
- Namlı, H.; Bişgin, A.T. Simultaneous deep eutectic solvent based microextraction for monitoring Brilliant blue and rhodamine B in foodstuff and industrial samples. J. Food Compos. Anal. 2024, 127, 105980. [Google Scholar] [CrossRef]
- Soylak, M.; Uzcan, F.; Goktas, O. Ultrasound-assisted quasi-hydrophobic deep eutectic solvent-based determination of trace Rhodamine B in water and food samples: A simple and green approach. J. Food Compos. Anal. 2023, 120, 105287. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE—Analytical GREEnness Metric approach and software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef]
- Manousi, N.; Wojnowski, W.; Płotka-Wasylka, J.; Samanidou, V. Blue applicability grade index (BAGI) and software: A new tool for the evaluation of method practicality. Green Chem. 2023, 25, 7598–7604. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Garcia-Ratés, M.; Neese, F. Efficient implementation of the analytical second derivatives of Hartree-Fock and hybrid DFT energies within the framework of the conductor-like polarizable continuum model. J. Comput. Chem. 2019, 40, 1816–1828. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.; Nevolianis, T.; Garcia-Ratés, M.; Riplinger, C.; Leonhard, K.; Smirnova, I. Predicting solvation free energies for neutral molecules in any solvent with openCOSMO-RS. Fluid Phase Equilib. 2025, 589, 114250. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system-Version 5.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- Stewart, J.J.P. MOPAC: A semiempirical molecular orbital program. J. Comput. Aided Mol. Des. 1990, 4, 1–103. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods VI: More modifications to the NDDO approximations and re-optimization of parameters. J. Mol. Model. 2013, 19, 1–32. [Google Scholar] [CrossRef]
- Klamt, A.; Schüürmann, G. COSMO: A New approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc. Perkin Trans. 1993, 2, 799–805. [Google Scholar] [CrossRef]
- Lu, T.; Chen, Q. Independent gradient model based on Hirshfeld partition (IGMH): A new method for visual study of interactions in chemical systems. J. Comput. Chem. 2022, 43, 539–555. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sanchez, P.; Contreras-Garcia, J.; Cohen, A.J.; Yang, W. Revealing noncovalent interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef]
- Lu, T. A comprehensive electron wavefunction analysis toolbox for chemists, Multiwfn. J. Chem. Phys. 2024, 161, 082503. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD—Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Chemaxon. Available online: https://chemaxon.com/blog/publication/predicted-pka-and-solubility-values-0 (accessed on 20 June 2025).
- Chemicalize. Available online: https://chemicalize.com (accessed on 20 June 2025).

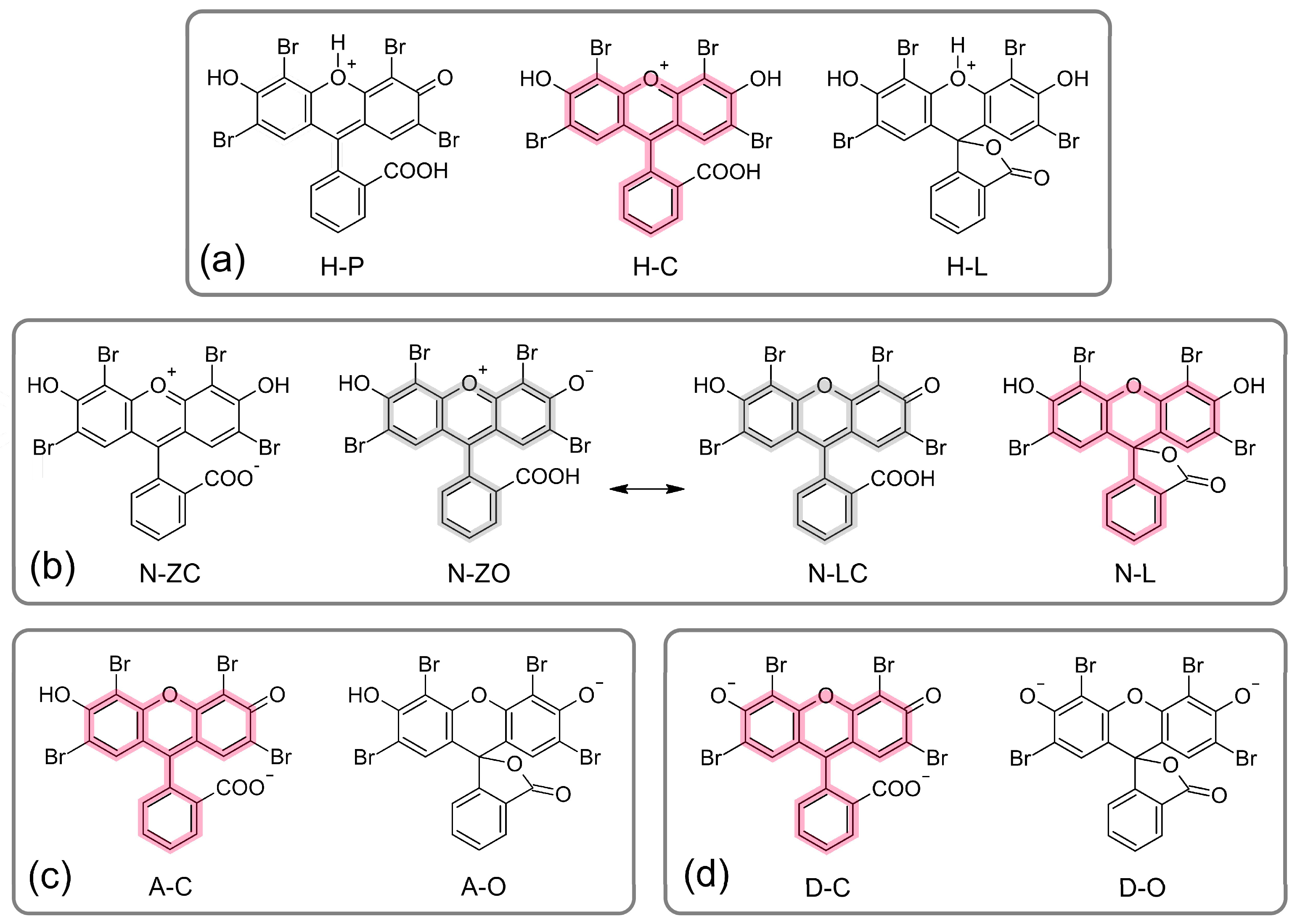



| H-C | H-P | H-L | N-ZC | N-ZO/N-LC | N-L | A-C | A-O | D-C | D-O | |
|---|---|---|---|---|---|---|---|---|---|---|
| water | ||||||||||
| ΔG, kcal/mol | 0.0 | 50.5 | 46.3 | 12.8 | 0.9 | 0.0 | 0.0 | 1.7 | 0.0 | 8.8 |
| p, % | 100 | 0.0 | 0.0 | 0.0 | 18.1 | 81.9 | 94.4 | 5.6 | 100 | 0.0 |
| octanol | ||||||||||
| ΔG, kcal/mol | 0.0 | 51.9 | 47.6 | 15.0 | 1.6 | 0.0 | 0.0 | 7.4 | 0.0 | 8.7 |
| p, % | 100 | 0.0 | 0.0 | 0.0 | 6.7 | 93.3 | 100 | 0.0 | 100 | 0.0 |
| EY Added, µg L−1 | Intra-Day | Inter-Day | ||||
|---|---|---|---|---|---|---|
| EY determined, µg L−1 | RSD, % | Recovery, % | EY determined, µg L−1 | RSD, % | Recovery, % | |
| 38.9 | 38.5 ± 1.3 | 2.7 | 99.0 | 41.3 ± 0.7 | 1.3 | 106.2 |
| 77.7 | 82.5 ± 1.3 | 1.3 | 106.2 | 81.0 ± 1.1 | 1.1 | 104.2 |
| EY Concentration, µg L−1 | ||||
|---|---|---|---|---|
| Samples | Added | Found | RSD, % | Recovery, % |
| Tap water | - | ≤LOQ | - | - |
| 38.9 | 38.1 ± 0.7 | 1.4 | 97.9 | |
| 77.7 | 74.3 ± 1.1 | 1.2 | 95.6 | |
| Energy drink | - | ≤LOQ | - | - |
| 38.9 | 40.6 ± 0.7 | 1.5 | 104.4 | |
| 77.7 | 78.3 ± 1.3 | 1.3 | 100.8 | |
| Ice Tea | - | ≤LOQ | - | - |
| 38.9 | 38.8 ± 0.8 | 1.7 | 99.7 | |
| 77.7 | 76.5 ± 0.8 | 0.8 | 98.5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakalejčíková, S.; Bazeľ, Y.; Drábiková, M.; Fizer, M. Fluorimetric Determination of Eosin Y in Water Samples and Drinks Using Deep Eutectic Solvent-Based Liquid-Phase Microextraction. Molecules 2025, 30, 3334. https://doi.org/10.3390/molecules30163334
Kakalejčíková S, Bazeľ Y, Drábiková M, Fizer M. Fluorimetric Determination of Eosin Y in Water Samples and Drinks Using Deep Eutectic Solvent-Based Liquid-Phase Microextraction. Molecules. 2025; 30(16):3334. https://doi.org/10.3390/molecules30163334
Chicago/Turabian StyleKakalejčíková, Sofia, Yaroslav Bazeľ, Mária Drábiková, and Maksym Fizer. 2025. "Fluorimetric Determination of Eosin Y in Water Samples and Drinks Using Deep Eutectic Solvent-Based Liquid-Phase Microextraction" Molecules 30, no. 16: 3334. https://doi.org/10.3390/molecules30163334
APA StyleKakalejčíková, S., Bazeľ, Y., Drábiková, M., & Fizer, M. (2025). Fluorimetric Determination of Eosin Y in Water Samples and Drinks Using Deep Eutectic Solvent-Based Liquid-Phase Microextraction. Molecules, 30(16), 3334. https://doi.org/10.3390/molecules30163334







