4. Materials and Methods
General methods. All reactions sensitive to air and/or moisture were carried out under an argon atmosphere. The reactions were performed with the use of commercial reagents (Aldrich (St. Louis, MO, USA), Fluka (Waltham, MA, USA), Acros Organics (Geel, Belgium)). Anhydrous solvents were purified and dried (where appropriate) according to standard procedures [
34]. Dichloromethane was distilled over P
2O
5 and then over CaH
2 and stored over 4 Å molecular sieves (MS 4 Å). Powdered MS 4 Å and 3 Å molecular sieves (MS 4 Å and MS 3 Å, respectively) (Fluka, Seelze, Germany) were activated before glycosylation reactions by heating at 220 °C in high vacuum (0.2 mbar) for 6 h. Column chromatography was performed on silica gel 60 (40–63 μm, Merck, Darmstadt, Germany) using a Büchi C-815 Flash chromatograph (Büchi Labotechnic AG, Flawil, Switzerland). Thin-layer chromatography was carried out on plates with silica gel 60 on aluminum foil (Merck). Spots of compounds were visualized under UV light (254 nm) and by heating the plates (at ca. 150 °C) after immersion in a 1:10 (
v/
v) mixture of 85% aqueous H
3PO
4 and 95% EtOH. Gel permeation chromatography was performed on a 400 × 20 mm column packed with Bio-Beads S-X3 (200–400 mesh, Bio-Rad, Hercules, CA, USA) or on a 450 × 30 mm column packed with Bio-Beads S-X1 (200–400 mesh). A procedure for “co-evaporation” with water (or toluene) involved (multiple) addition of water (or toluene) and evaporation of volatiles on a rotary evaporator. Amberlite MB-3 mixed-bed ion-exchange resin (Fluka) (1 mL) was washed with H
2O (10 mL), 50% EtOH (5 mL), EtOH (5 mL), 50% EtOH (5 mL), and H
2O (10 mL) before use.
1H,
13C,
19F, and
29Si NMR spectra were recorded on a Bruker AVANCE NEO 300 spectrometer (300.23, 75.50, 282.47, and 59.65 MHz for
1H,
13C,
19F, and
29Si, respectively) or on a Bruker AVANCE 600 spectrometer (Billerica, MA, USA, 600.13 and 150.92 MHz for
1H and
13C, respectively). The
1H NMR chemical shifts are referred to the residual signal of CHCl
3 (δ
H 7.27 ppm), CHD
2OD (δ
H 3.31 ppm) for solutions in CD
3OD, and HDO (δ
H 4.79 ppm) for solutions in D
2O. The
13C NMR shifts to the central line of the CDCl
3 signal (δ
C 77.00 ppm), CD
3OD signal (δ
C 49.00 ppm), or the signal of external 1,4-dioxane in D
2O (δ
C 67.40 ppm). The
19F chemical shifts are given relative to the signal of external CFCl
3 (δ
F 0.00 ppm). The
29Si chemical shifts are given relative to the signal of external Me
4Si (δ
Si 0.00 ppm). Assignments of the signals in the NMR spectra were performed using
1H–
1H and
1H–
13C 2D-spectroscopy (COSY, HSQC, HMBC) and DEPT-135 experiments. Position of silyl groups was determined from
1H–
29Si HMBC experiments. High-resolution mass spectra (HRMS, electrospray ionization (ESI)) were recorded in a positive ion mode on Bruker micrOTOF II or maXis mass spectrometers for 2 × 10
−5 M solutions in MeCN. Optical rotations were measured using a JASCO P-2000 automatic digital polarimeter (Hachioji, Japan).
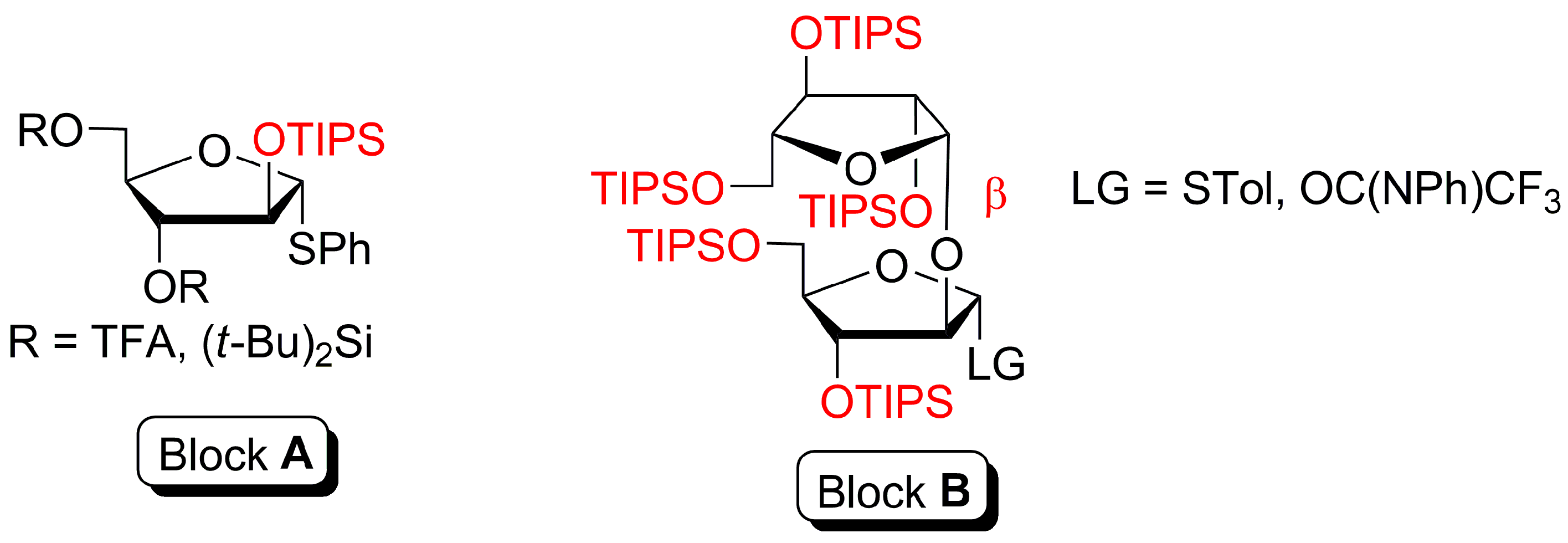
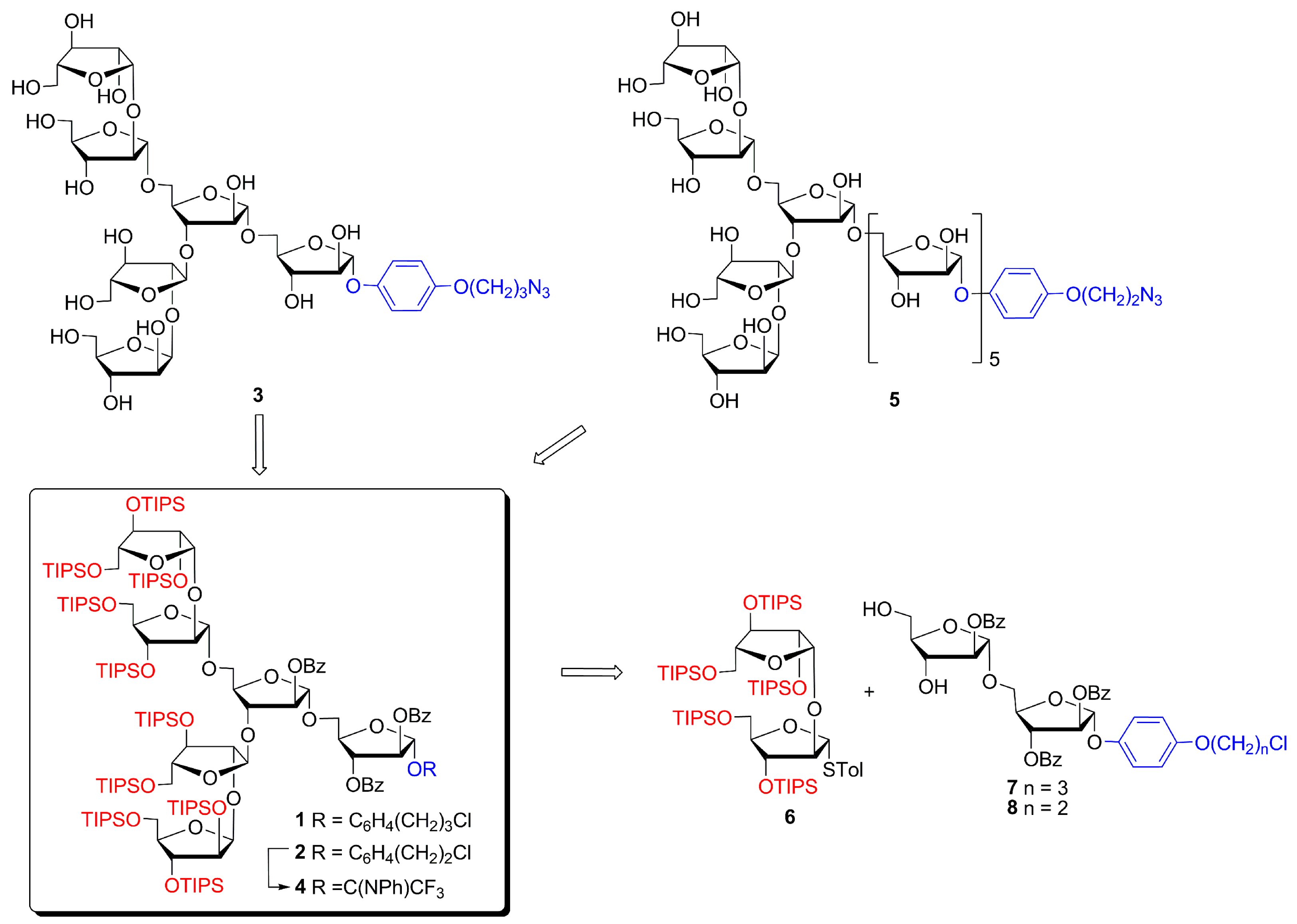
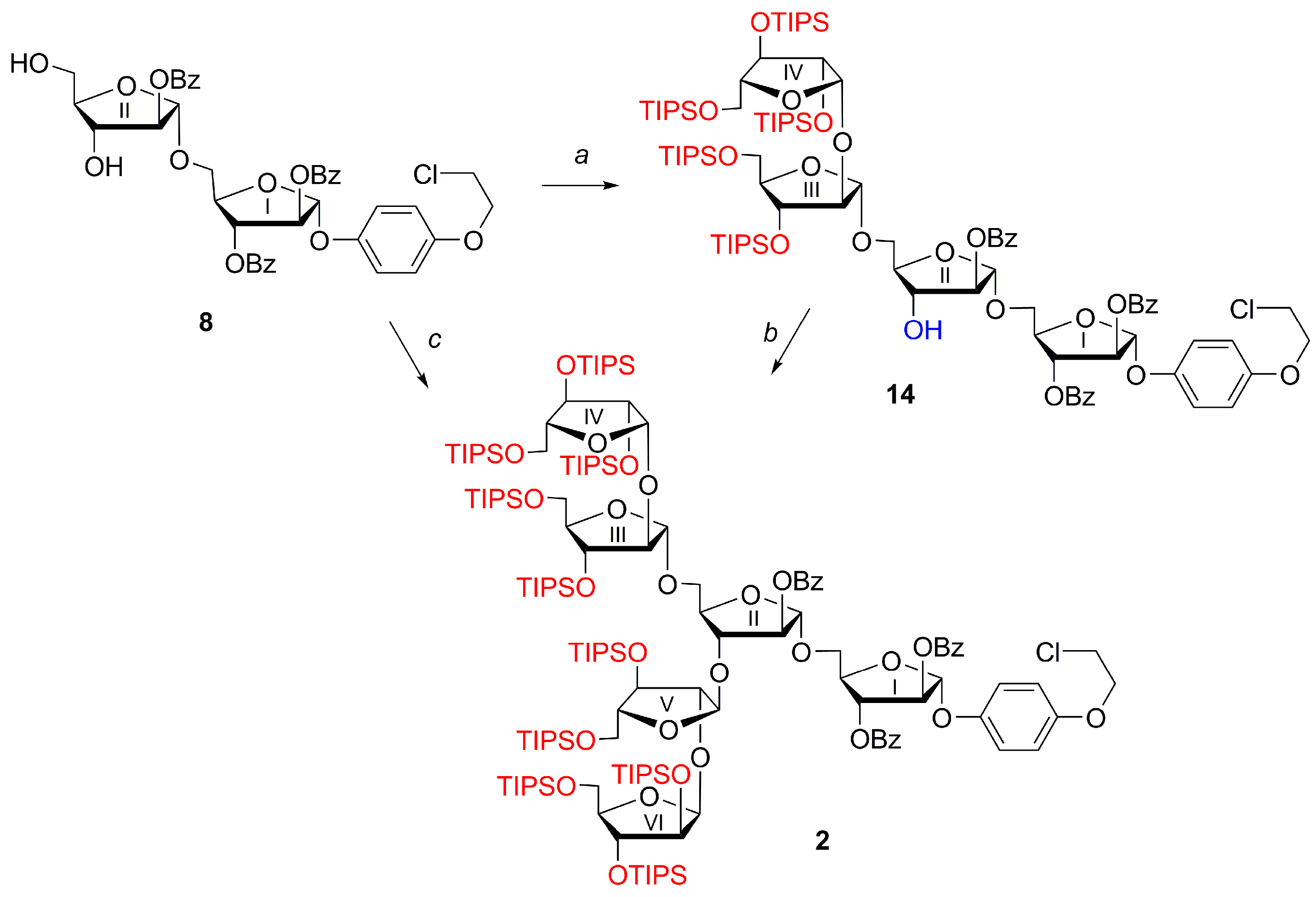
4-(2-Chloroethoxy)phenyl 2,3,5-tris-O-(triisopropylsilyl)-β-d-arabinofuranosyl-(1→2)-3,5-bis-O-(triisopropylsilyl)-α-d-arabinofuranosyl-(1→3)-[2,3,5-tris-O-(triisopropylsilyl)-β-d-arabinofuranosyl-(1→2)-3,5-bis-O-(triisopropylsilyl)-α-d-arabinofuranosyl-(1→5)]-2-O-benzoyl-α-d-arabinofuranosyl-(1→5)-2,3-di-O-benzoyl-α-d-arabinofuranoside (2)
(1) A mixture of disaccharide thioglycoside 6 (37 mg, 0.31 mmol) and tetrasaccharide alcohol 14 (19 mg, 0.25 mmol) was dried in vacuo for 2 h, then anhydrous CH2Cl2 (2 mL) was added under argon. Freshly activated powdered MS 4 Å (200 mg) (100 mg per 1 mL of solvent) was added under argon to the resulting solution. The suspension was stirred under argon at ~22 °C for 1 h, cooled to −60 °C, then NIS (7 mg, 0.31 mmol) and TfOH (1 μL) were added. Then, the temperature was allowed to rise slowly until −30 °C during 0.5 h and was kept at −30 °C for 10 min. Then the reaction was quenched by the addition of Py (50 μL), diluted with CH2Cl2 (15 mL), and filtered through a Celite pad. The solids were washed with CH2Cl2 (5 × 10 mL), and the filtrate was washed with a mixture of satd aq Na2S2O3 (50 mL) and satd aq NaHCO3 (50 mL). The aqueous layer was extracted with CH2Cl2 (2 × 5 mL). The combined organic extracts were filtered through a cotton wool plug, concentrated and dried in vacuo, then dissolved in toluene (2 mL) and subjected to chromatography on Bio-Beads S-X1 in toluene to give α-linked hexasaccharide 2 (52 mg, 90%); Rf = 0.50 (light petroleum–EtOAc 10:1); +31.0 (c 1.0 in CHCl3); 1H NMR (600 MHz, CDCl3): δ 0.95–1.15 (m, 210H, 10 × ((CH3)2CH)3Si), 3.63 (dd, 1H, J 9.5 Hz, J 4.5 Hz, H-5IVa or H-5VIa), 3.66 (dd, 1H, J 10.5 Hz, J 5.4 Hz, H-5Va), 3.71 (dd, 1H, J 9.5 Hz, J 4.5 Hz, H-5IVa or H-5VIa), 3.78 (t, 2H, J 6.0 Hz, CH2Cl), 3.73–3.99 (m, 11H, 8 × H-5, H-4IV, H-4VI, H-4V), 4.03–4.05 (m, 2H, H-2IV, H-2VI), 4.09 (d, 1H, J 1.3 Hz, H-2V), 4.11 (td, 1H, J 5.9 Hz, J 3.9 Hz, H-4III), 4.14 (d, 1H, J 1.1 Hz, H-2III), 4.15–4.19 (m, 1H, H-5Ib), 4.17 (t, 2H, J 6.0 Hz, CH2O), 4.22 (dd, 1H, J 7.0 Hz, J 2.1 Hz, H-3II), 4.30 (d, 1H, J 0.9 Hz, H-3VI), 4.29–4.32 (m, 1H, H-4II), 4.33 (d, 1H, J 0.8 Hz, H-3IV), 4.34 (d, 1H, J 4.0 Hz, H-3III), 4.45 (dd, 1H, J 3.7 Hz, J 1.4 Hz, H-3V), 4.60 (q, 1H, J 4.2 Hz, H-4I), 5.11 (s, 1H, H-1III), 5.17 (d, 1H, J 2.7 Hz, H-1VI), 5.23 (s, 1H, H-1V), 5.25 (d, 1H, J 2.8 Hz, H-1IV), 5.27 (s, 1H, H-1II), 5.41 (d, 1H, J 2.1 Hz, H-2II), 5.65 (dd, 1H, J 4.5 Hz, J 2.0 Hz, H-3I), 5.71 (d, 1H, J 1.6 Hz, H-2I), 5.80 (s, 1H, H-1I), 6.82–6.87 (m, 2H, OC6H4O (H-3, H-5)), 7.03–7.09 (m, 2H, OC6H4O (H-2, H-6)), 7.38–7.44 (m, 2H, 2II-O-PhCO (H-3, H-5)), 7.44–7.62 (m, 7H, PhCO), 8.01–8.05 (m, 2H, 2II-O-PhCO (H-2, H-6)), 8.10–8.13 (m, 2H, PhCO (H-2, H-6)), 8.13–8.16 (m, 2H, PhCO (H-2, H-6)); 13C NMR (151 MHz, CDCl3): δ 11.93 (((CH3)2CH)3Si), 11.96 (((CH3)2CH)3Si), 11.97 (((CH3)2CH)3Si), 12.01 (((CH3)2CH)3Si), 12.18 (((CH3)2CH)3Si), 12.23 (((CH3)2CH)3Si), 12.24 (2 × ((CH3)2CH)3Si), 12.3 (((CH3)2CH)3Si), 12.4 (((CH3)2CH)3Si), 17.9 (2 × ((CH3)2CH)3Si), 17.95 (2 × ((CH3)2CH)3Si), 17.96 (2 × ((CH3)2CH)3Si), 18.02 (2 × ((CH3)2CH)3Si), 18.03 (((CH3)2CH)3Si), 18.05 (((CH3)2CH)3Si), 18.08 (((CH3)2CH)3Si), 18.09 (2 × ((CH3)2CH)3Si), 18.11 (((CH3)2CH)3Si), 18.12 (4 × ((CH3)2CH)3Si), 18.17 (((CH3)2CH)3Si), 18.19 (((CH3)2CH)3Si), 41.9 (CH2Cl), 63.7 (C-5IV, C-5VI), 63.8 (C-5IV, C-5VI), 64.1 (C-5V), 64.7 (C-5III), 65.1 (C-5II), 65.3 (C-5I), 68.7 (CH2O), 77.5 (C-3I), 77.6 (C-3V), 78.1 (C-2IV or C-2VI), 78.2 (C-3VI), 78.3 (C-2IV or C-2VI), 78.48 (C-3III, C-3IV), 78.51 (C-3III, C-3IV), 81.0 (C-3II), 81.9 (C-2I), 82.2 (C-4II), 82.9 (C-4I), 84.0 (C-2II), 85.68 (C-4IV, C-4VI), 85.74 (C-4IV, C-4VI), 87.6 (C-4III), 88.2 (C-4V), 90.5 (C-2III), 91.3 (C-2V), 104.69 (C-1IV), 104.71 (C-1I), 105.7 (C-1VI), 106.0 (C-1II), 106.3 (C-1V), 106.8 (C-1III), 115.8 (OC6H4O (C-3, C-5)), 118.3 (OC6H4O (C-2, C-6)), 128.2 (2II-O-PhCO (C-3, C-5)), 128.4 (PhCO (C-3, C-5)), 128.6 (PhCO (C-3, C-5)), 129.1 (PhCO (C-1)), 129.5 (PhCO (C-1)), 129.81 (PhCO (C-1)), 129.84 (2II-O-PhCO (C-2, C-6)), 129.95 (PhCO (C-2, C-6)), 130.03 (PhCO (C-2, C-6)), 132.8 (2II-O-PhCO (C-4)), 133.2 (PhCO (C-4)), 133.4 (PhCO (C-4)), 151.0 (OC6H4O (C-1)), 153.5 (OC6H4O (C-4)), 165.1 (2II-O-PhCO), 165.5 (PhCO), 165.6 (PhCO); 29Si INEPT NMR (60 MHz, CDCl3): δ 12.36, 12.93, 13.26, 13.48, 13.51, 13.53, 13.56, 13.79, 13.83, 13.87; HRMS (ESI): m/z [M + 2NH4]2+ Calcd for C149H277ClN2O29Si102+ 2855.7294; Found: 2855.7251;
(2) A mixture of disaccharide thioglycoside 6 (127 mg, 0.11 mmol) and disaccharide 8 (22 mg, 0.03 mmol) was dried in vacuo for 2 h, then anhydrous CH2Cl2 (3 mL) was added under argon. Freshly activated powdered MS 4 Å (300 mg) (100 mg per 1 mL of solvent) was added under argon to the resulting solution. The suspension was stirred under argon at ~22 °C for 1 h, cooled to −60 °C, then NIS (7 mg, 0.31 mmol) and TfOH (1 μL) were added. Then, the temperature was allowed to rise slowly to −30 °C during 0.5 h and was kept at −30 °C for 10 min. Then the reaction was quenched by the addition of Py (50 μL), diluted with CH2Cl2 (15 mL), and filtered through a Celite pad. The solids were washed with CH2Cl2 (5 × 10 mL), and the filtrate was washed with a mixture of satd aq Na2S2O3 (50 mL) and satd aq NaHCO3 (50 mL). The aqueous layer was extracted with CH2Cl2 (2×5 mL). The combined organic extracts were filtered through a cotton wool plug, concentrated and dried in vacuo, then dissolved in toluene (2 mL) and subjected to chromatography on Bio-Beads S-X1 in toluene to give hexasaccharide 2 (80 mg, 97%).
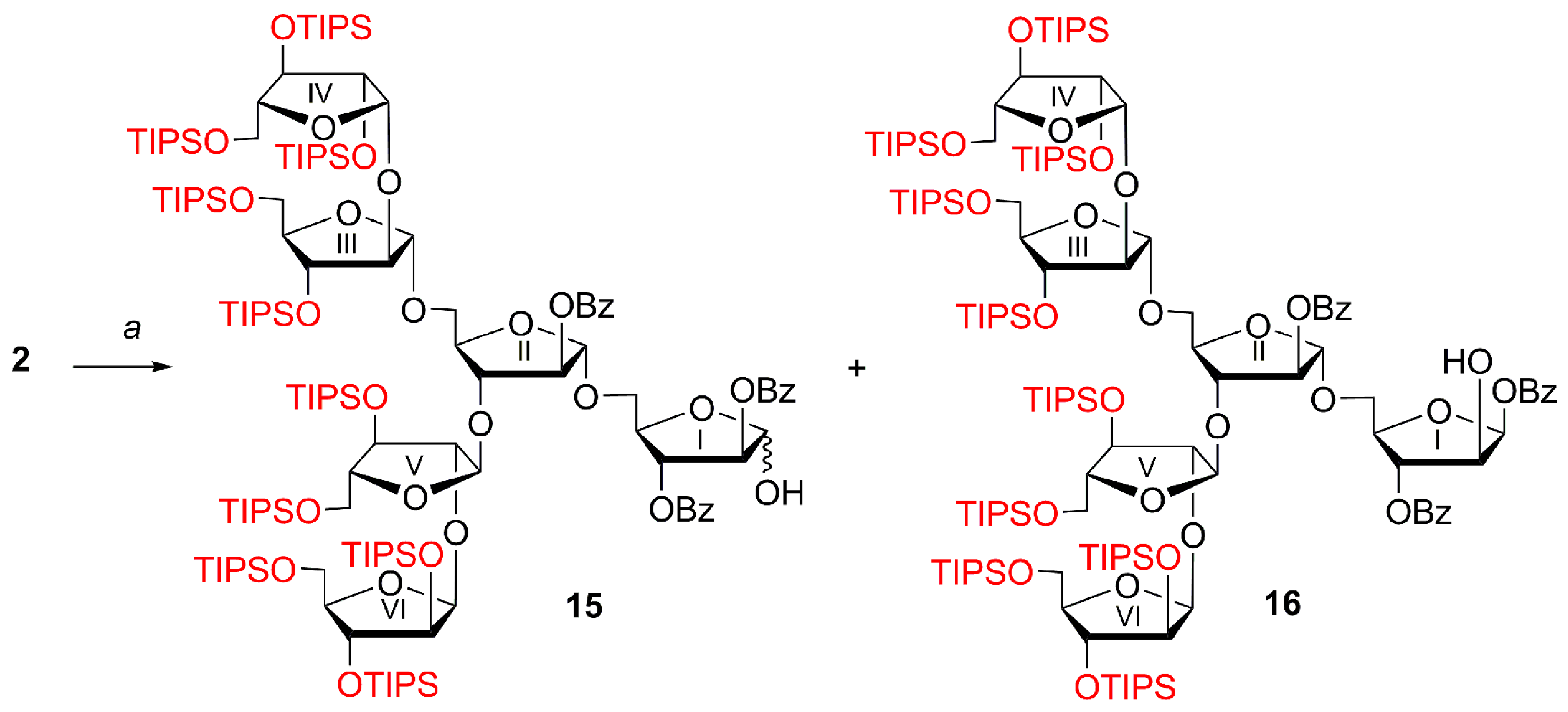
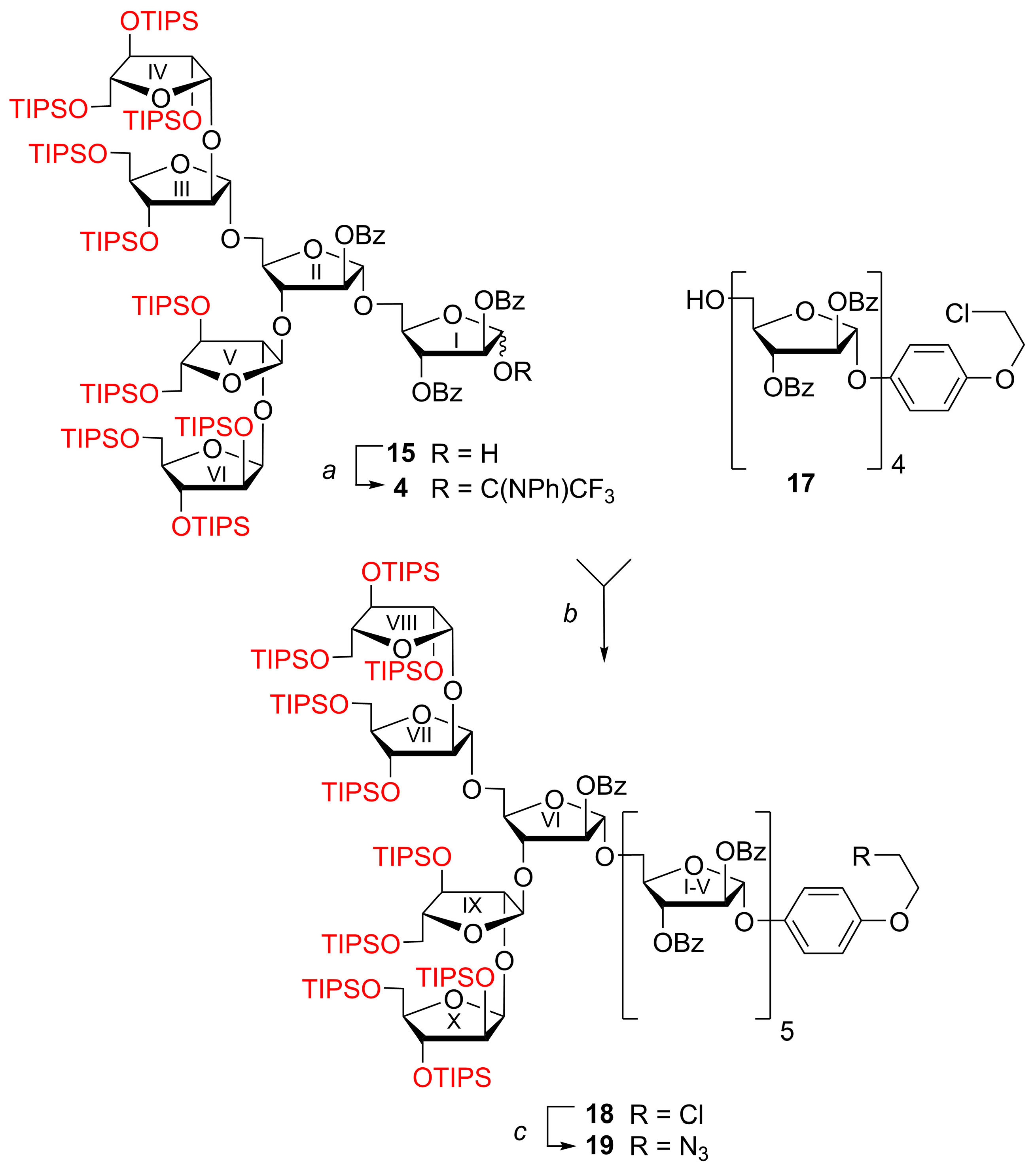
2,3,5-Tris-O-(triisopropylsilyl)-β-d-arabinofuranosyl-(1→2)-3,5-bis-O-(triisopropylsilyl)-α-d-arabinofuranosyl-(1→3)-[2,3,5-tris-O-(triisopropylsilyl)-β-d-arabinofuranosyl-(1→2)-3,5-bis-O-(triisopropylsilyl)-α-d-arabinofuranosyl-(1→5)]-2-O-benzoyl-α-d-arabinofuranosyl-(1→5)-2,3-di-O-benzoyl-d-arabinofuranosyl N-phenyltrifluoroacetimidate (4)
To a solution of hemiacetal of hexasaccharide
15 (31 mg, 0.012 mmol) in CH
2Cl
2 (1 mL), Cs
2CO
3 (30 mg, 0.093 mmol) and CF
3C(NPh)Cl [
35] (4 μL, 0.023 mmol) were added at 0 °C. The reaction mixture was stirred at 20 °C for 3h. The reaction mixture was diluted with CH
2Cl
2 (20 mL) and then filtered through the cotton wool plug. The filtrate concentrated under reduced pressure, and the residue was dried in vacuo and subjected to gel chromatography on Bio-Beads S-X1 in toluene to give imidate
4 as a mixture of anomers (α:β = ~1:1.3 according to NMR), (32 mg, 96%).
Rf = 0.76 (light petroleum–EtOAc, 8.5:1.5); selected signals:
1H NMR (300 MHz, CDCl
3): δ 0.78–1.35 (m, 483H), 3.61–4.01 (m, 38H), 4.01–4.19 (m, 16H), 4.19–4.39 (m, 13H), 4.43–4.48 (m, 2H), 4.51–4.59 (m, 1H), 4.61–4.71 (m, 1H), 5.08 (s, 1H), 5.10 (s, 1H), 5.16–5.28 (m, 11H), 5.36 (d, 1H,
J 1.9 Hz), 5.62 (d, 1H,
J 3.7 Hz), 5.73 (s, 1H), 5.84–5.97 (m, 2H), 6.51–6.57 (m, 2H, PhN (H-2, H-6)β), 6.79 (s, 1H, H-1), 6.85–6.91 (m, 2H, PhN (H-2, H-6)α), 6.98–7.10 (m, 2H, PhN (H-4)), 7.12–7.20 (m, 2H, PhN (H-3, H-5)), 7.22–7.31 (m, 5H, PhCO (H-3, H-5)), 7.36–7.63 (m, 25H, PhCO (H-3, H-5); PhCO (H-4), 7.97–8.17 (m, 16H, PhCO (H-2, H-6));
13C NMR (76 MHz, CDCl
3): δ 12.0 (((CH
3)
2CH)
3Si), 12.2 (((CH
3)
2CH)
3Si), 12.4 (((CH
3)
2CH)
3Si), 18.0 (((
CH
3)
2CH)
3Si), 18.1 (((
CH
3)
2CH)
3Si), 63.7, 63.8, 64.1, 64.6, 64.7, 67.8, 78.3, 78.4, 83.7, 85.7, 87.6, 88.2, 90.8, 91.2, 97.3 (C-1), 105.4 (C-1), 105.89 (C-1), 106.91 (C-1), 119.3 (PhN (C-2, C-6)), 119.7 (PhN (C-2, C-6)), 124.0, 128.2, 128.4, 128.49, 128.53, 128.6, 129.85 (Ph
CO (C-2, C-6)), 129.94 (Ph
CO (C-2, C-6)), 130.0 (Ph
CO (C-2, C-6)), 132.9 (
PhCO (C-4)), 133.4 (
PhCO (C-4)), 133.6 (
PhCO (C-4)), 165.1 (CO), 165.4 (CO);
29Si INEPT NMR (60 MHz, CDCl
3): δ 12.39, 12.45, 12.84, 12.94, 13.26, 13.38, 13.47, 13.50, 13.53, 13.55, 13.74, 13.79, 13.83, 13.88, 13.91;
19F NMR (282 MHz, CDCl
3): δ −65.76 (CF
3); HRMS (ESI):
m/
z [M + NH
4]
+ Calcd for C
149H
270F
3N
2O
28Si
10+ 2872.7404; found: 2872.7378.
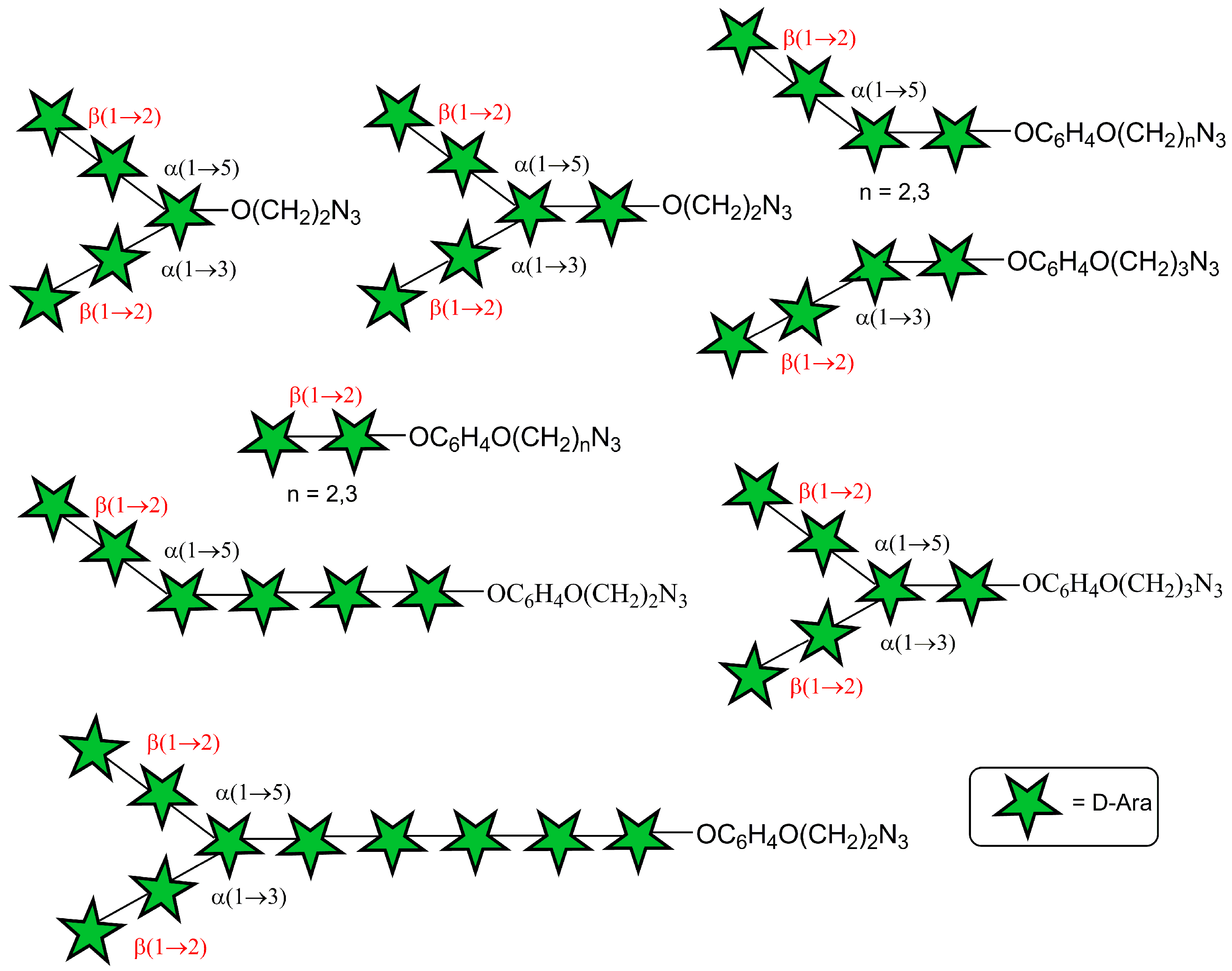
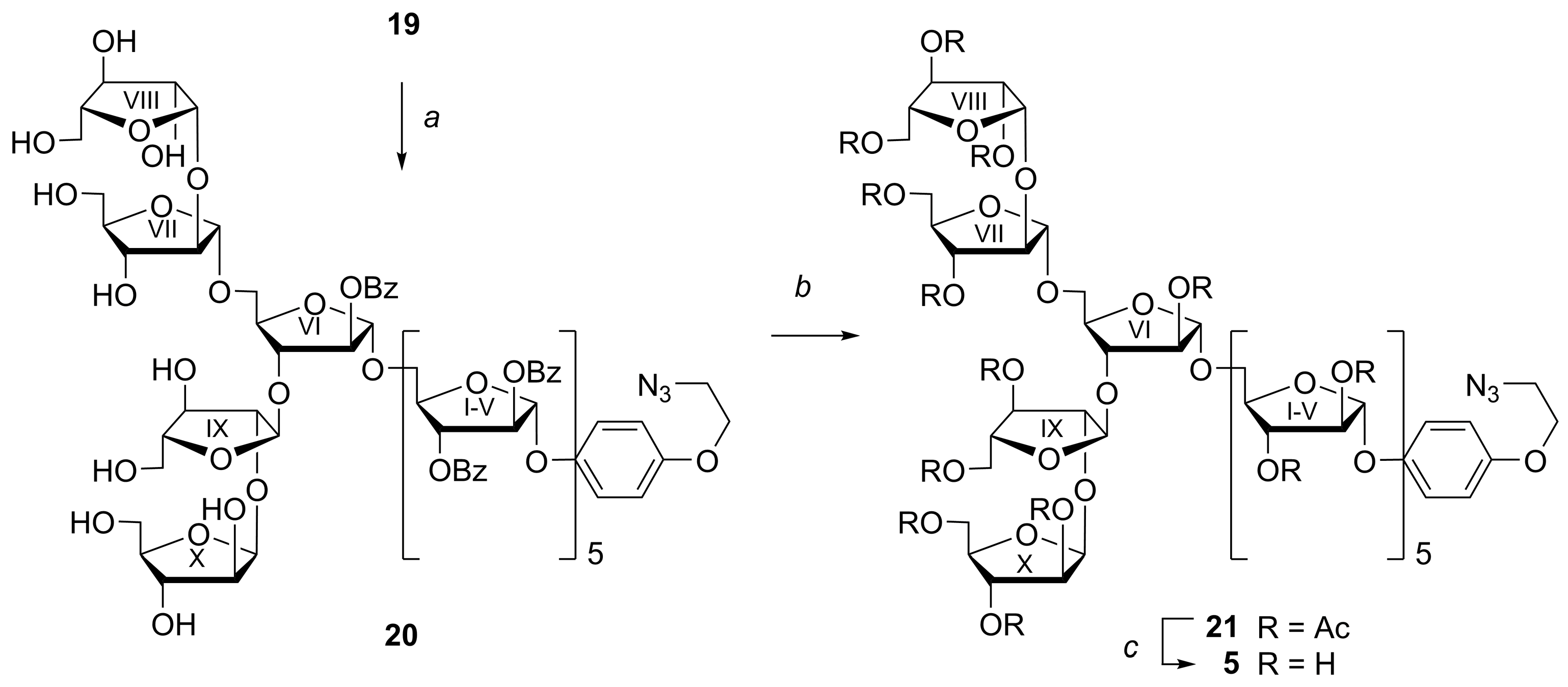
4-(2-Azidoethoxy)phenyl β-d-arabinofuranosyl-(1→2)-α-d-arabinofuranosyl-(1→3)-[β-d-arabinofuranosyl-(1→2)-α-d-arabinofuranosyl-(1→5)]-α-d-arabinofuranosyl-(1→5)-α-d-arabinofuranosyl-(1→5)-α-d-arabinofuranosyl-(1→5)-α-d-arabinofuranosyl-(1→5)-α-d-arabinofuranosyl-(1→5)-α-d-arabinofuranoside (5)
Acetylated decasaccharide 21 (1.5 mg, 0.0006 mmol) was dissolved in anhydrous CH2Cl2 (0.2 mL) and anhydrous MeOH (0.8 mL), followed by the addition of 1 M methanolic MeONa (20 μL). The reaction mixture was kept at ~20 °C for 24 h. Then, the reaction mixture was neutralized with Dowex 50W X8 (H+) ion-exchange resin (the resin was washed with MeOH before addition) and then filtered. The resin was washed with MeOH (3 × 20 mL). The combined filtrate was concentrated under reduced pressure. The residue was co-evaporated with toluene (2 × 2 mL), dried in vacuo, dissolved in water (1 mL), and applied on a small column (ID 5 mm) packed with Amberlite MB-3 mixed-bed ion-exchange resin (1 mL), which was eluted with H2O (15 mL). The eluate was lyophilized and additionally purified by reversed phase chromatography on a Sep-Pak C18 cartridge (particle size: 55–105 μm, pore size: 125 Å, sorbent substrate: silica, sorbent weight: 360 mg), gradient: 0→100% MeCN in H2O) to give deprotected decasaccharide 5 (0.8 mg, 85%). Rf = 0.50 (MeCN–H2O 7.5:2.5); +15.1 (c 0.2 in MeOH); 1H NMR (600 MHz, CD3OD): δ 3.54–3.58 (m, 2H, CH2N3), 3.61–3.88 (m, 21H, H-5I-Xa, H-4VIII, X, H-5I-V, VII-Xb), 3.89–3.95 (m, 5H, H-5VIb, H-3II-V), 3.99–4.06 (m, 12H, H-2II-V, VIII, X, H-3I, VI-X), 4.06–4.11 (m, 4H, H-4II-V), 4.12 (dd, 2H, J 5.4 Hz, J 4.4 Hz, CH2O), 4.13–4.15 (m, 3H, H-2VII, H-2IX, H-4I), 4.16 (dd, 1H, J 2.7 Hz, J 1.5 Hz, H-2VI), 4.17–4.21 (m, 1H, H-4VI), 4.21 (dd, 1H, J 4.0 Hz, J 1.8 Hz, H-2I), 4.94–4.98 (m, 5H, H-1II-VI), 5.02–5.06 (m, 2H, H-1VIII, H-1X), 5.09 (d, 1H, J 2.2 Hz, H-1VII), 5.17 (d, 1H, J 2.4 Hz, H-1IX), 5.42 (d, 1H, J 2.0 Hz, H-1I), 6.86–6.91 (m, 2H, OC6H4O (H-3, H-5)), 6.97–7.03 (m, 2H, OC6H4O (H-2, H-6)); 13C NMR (151 MHz, CD3OD): δ 51.4 (CH2N3), 62.45 (C-5VII, C-5IX), 62.53 (C-5VII, C-5IX), 64.38 (C-5VIII, C-5X), 64.41 (C-5VIII, C-5X), 67.9 (2C, C-5I-VI), 68.0 (C-5I-VI), 68.3 (3C, C-5I-VI), 69.0 (CH2O), 75.8 (C-3VIII, C-3X), 75.9 (C-3VIII, C-3X), 76.3 (C-3VII, C-3IX), 76.5 (C-3VII, C-3IX), 78.8 (3C, C-2VIII, C-2X, C-3I-V), 78.9 (C-2VIII, C-2X, C-3I-V), 79.2 (3C, C-2VIII, C-2X, C-3I-V), 81.6 (C-2VI), 83.03 (C-4VI), 83.3 (C-2II-V), 83.8 (C-2I), 83.95 (C-4I-V, C-4VII-X), 84.03 (C-4I-V, C-4VII-X), 84.2 (4C, C-4I-V, C-4VII-X), 84.3 (C-4I-V, C-4VII-X), 84.4 (2C, C-4I-V, C-4VII-X), 84.6 (C-3VI), 89.3 (C-2VII), 89.6 (C-2IX), 102.5 (C-1VIII, C-1X), 102.6 (C-1VIII, C-1X), 107.1 (C-1IX), 107.4 (C-1VII), 108.7 (C-1I), 109.58 (C-1II-VI), 109.63 (C-1II-VI), 109.70 (2C, C-1II-VI), 109.74 (C-1II-VI), 116.6 (OC6H4O (C-3, C-5)), 119.3 (OC6H4O (C-2, C-6)), 152.7 (OC6H4O (C-1)), 155.2 (OC6H4O (C-4)); HRMS (ESI): m/z [M + NH4]+ Calcd for C58H93N4O42+ 1517.5259; found: 1517.5244.
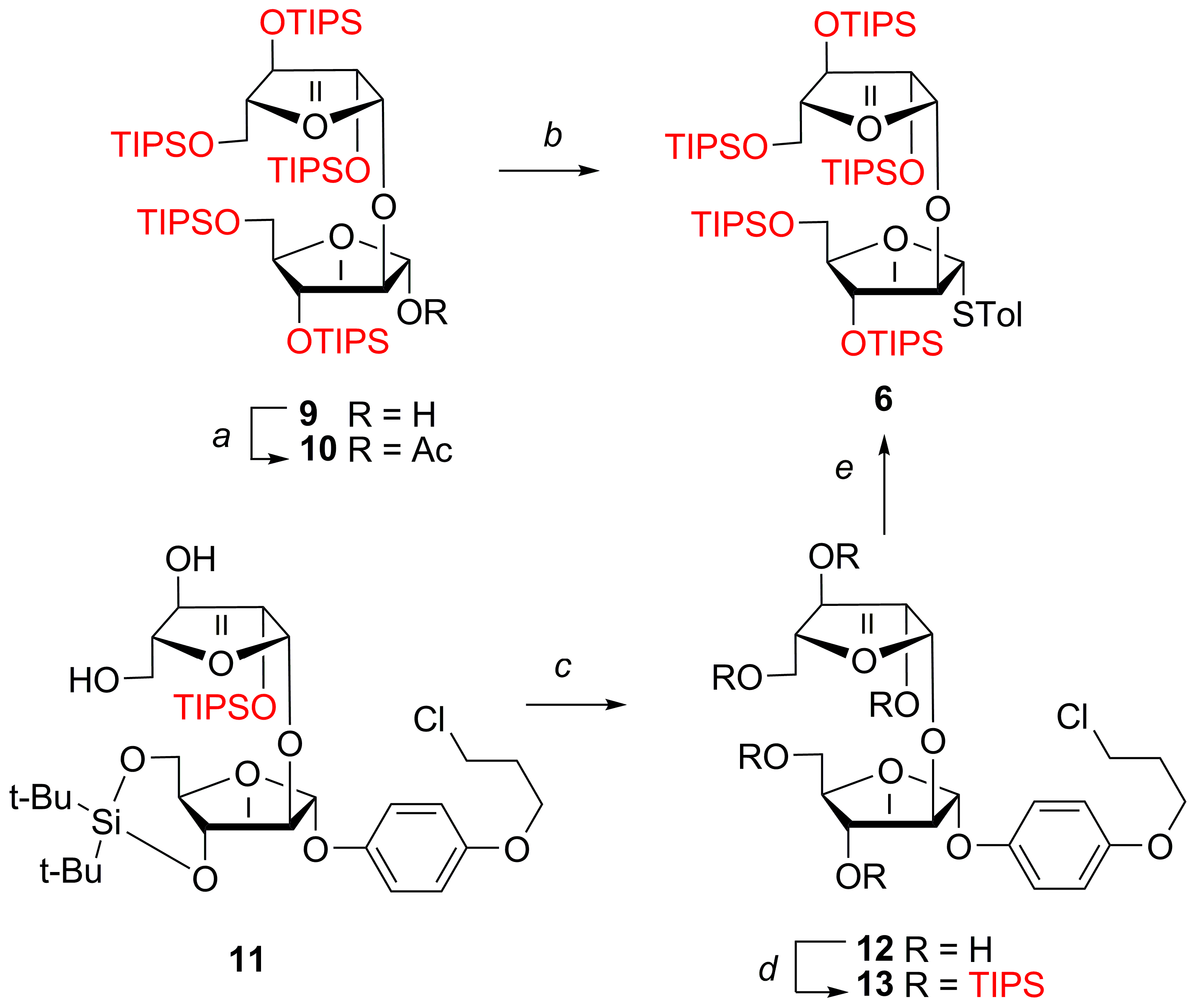
p-Tolyl 2,3,5-tris-O-(triisopropylsilyl)-β-d-arabinofuranosyl-(1→2)-1-thio-3,5-bis-O-(triisopropylsilyl)-α-d-arabinofuranoside (6)
(1) To a solution of acetate
10 (41 mg, 0.037 mmol) in ClCH
2CH
2Cl (1 mL),
p-TolSH (9 mg, 0.074 mmol) and BF
3·Et
2O (2 μL, 0.02 mmol) were added at 20 °C. The reaction mixture was stirred at 40 °C for 1 h. Then, the reaction mixture was diluted with CH
2Cl
2 (50 mL) and washed with H
2O (50 mL). The organic phase was filtered through a cotton wool plug, concentrated, and dried in vacuo. The residue was dissolved in 2,4,6-collidine (1 mL), then
i-Pr
3SiOTf (450 mL, mmol) was added. The reaction mixture was stirred at 70 °C for 1 h and then diluted with CH
2Cl
2 (50 mL), washed with 1 M KHSO
4 (50 mL), H
2O (50 mL), and NaHCO
3 (50 mL). Combined organic extracts were filtered through a cotton wool plug, concentrated, and dried in vacuo. The residue was purified by silica gel chromatography (gradient: 0%→15% CH
2Cl
2 in petroleum ether) to give known [
28] silylated disaccharide
6 (27 mg, 63%).
(2) To a solution of CPP-glycoside
13 (91 mg, 0.075 mmol) in ClCH
2CH
2Cl (2 mL),
p-TolSH (19 mg, 0.15 mmol) and BF
3·Et
2O (4 μL, 0.02 mmol) at −5 °C were added. Then, the temperature was raised to 0 °C and the reaction mixture was stirred at 0 °C for 2.5 h. Then, the reaction mixture was diluted with CH
2Cl
2 (50 mL) and washed with H
2O (50 mL). The organic extract was filtered through a cotton wool plug, concentrated, and dried in vacuo. The residue was purified by silica gel chromatography (gradient: 0%→15% CH
2Cl
2 in petroleum ether) to give known [
28] silylated disaccharide
6 (44 mg, 50%; 54% with respect to the reacted starting material).
2,3,5-Tris-O-(triisopropylsilyl)-β-d-arabinofuranosyl-(1→2)-1-O-acetyl-3,5-bis-O-(triisopropylsilyl)-α-d-arabinofuranose (10)
To a solution of known arabinofuranose
9 [
28] (48 mg, 0.045 mmol) in anhydrous Py (1 mL), Ac
2O (1 mL) was added at 0 °C (ice–water bath). The reaction mixture was stirred at 20 °C for 24 h. The reaction was quenched by the addition of MeOH (1 μL) at 0 °C (ice–water bath), then concentrated under reduced pressure, co-evaporated with toluene (5×5 mL), and dried in vacuo to give acetate
10 (42 mg, 84%);
Rf = 0.50 (light petroleum–CH
2Cl
2 1:1);
−1.7 (c 2.1 in CHCl
3);
1H NMR (300 MHz, CDCl
3): δ 0.99–1.20 (m, 105H, 5 × ((CH
3)
2CH)
3Si), 2.05 (s, 3H, CH
3CO), 3.68 (dd, 1H,
J 8.4 Hz,
J 3.6 Hz, H-5
IIa), 3.71–3.82 (m, 2H, H-5
Ia, H-5
Ib), 3.82–3.99 (m, 2H, H-4
II, H-5
IIb), 4.04 (dd, 1H,
J 2.7 Hz,
J 1.0 Hz, H-2
II), 4.21 (d, 1H,
J 0.8 Hz, H-2
I), 4.25 (td, 1H,
J 6.4 Hz,
J 2.4 Hz, H-4
I), 4.32 (d, 1H,
J 1.1 Hz, H-3
II), 4.56 (d, 1H,
J 2.5 Hz, H-3
I), 5.25 (d, 1H,
J 2.7 Hz, H-1
II), 6.16 (s, 1H, H-1
I);
13C NMR (76 MHz, CDCl
3): δ 12.0 (2 × ((CH
3)
2CH)
3Si), 12.2 (2 × ((CH
3)
2CH)
3Si), 12.3 ((CH
3)
2CH)
3Si), 17.9 (2 × ((
CH
3)
2CH)
3Si), 17.97 (2 × ((
CH
3)
2CH)
3Si), 18.01 ((
CH
3)
2CH)
3Si), 18.0 ((
CH
3)
2CH)
3Si), 18.07 ((
CH
3)
2CH)
3Si), 18.11 (3 × ((
CH
3)
2CH)
3Si), 21.2 (CH
3CO), 63.6 (C-5
II), 64.2 (C-5
I), 77.1 (C-3
I), 78.0 (C-2
II), 78.1 (C-3
II), 86.0 (C-4
II), 90.4 (C-2
I), 90.8 (C-4
I), 101.0 (C-1
I), 105.3 (C-1
II), 170.1 (CO);
29Si INEPT NMR (60 MHz, CDCl
3) δ 13.67 (5
I-
O-TIPS, 5
II-
O-TIPS), 13.80 (5
I-
O-TIPS, 5
II-
O-TIPS), 13.96 (3
II-
O-TIPS), 14.02 (3
I-
O-TIPS), 14.29 (2
II-
O-TIPS); HRMS (ESI):
m/z [M + NH
4]
+ Calcd for C
57H
124NO
10Si
5+ 1122.8066; Found: 1122.8063; [M + K]
+ Calcd for C
57H
120KO
10Si
5+: 1143.7359; found: 1143.7357.
4-(3-Chloropropoxy)phenyl 2,3,5-tris-O-(triisopropylsilyl)-β-d-arabinofuranosyl-(1→2)-3,5-bis-O-(triisopropylsilyl)-α-d-arabinofuranoside (13)
Known 4-(3-chloropropoxy)phenyl 3,5-
O-(di-
tert-butylsilylene)-2-
O-[2-
O-(triisopropylsilyl)-β-
d-arabinofuranosyl]-α-
d-arabinofuranoside
11 [
28] (147 mg, 0.19 mmol) was dissolved in THF (2 mL), then 1 M TBAF in THF (0.77 mL, 0.77 mmol) was added to the reaction mixture at 0 °C. The reaction mixture was kept at 0 °C for 3 h. After that, the reaction mixture was concentrated under reduced pressure, co-evaporated with toluene (2 × 2 mL), and dried in vacuo. The residue was purified by silica gel chromatography (gradient: 5%→10% MeOH in CH
2Cl
2) to give 4-(3-chloropropoxy)phenyl 2-
O-(β-
d-arabinofuranosyl)-α-
d-arabinofuranoside
12, containing 3 mol. % of 4-(3-fluoropropoxy)phenyl 2-
O-(β-
d-arabinofuranosyl)-α-
d-arabinofuranoside (72 mg, 81%) and 15 mol. % of
n-Bu
4N
+ salts. Selected signals for
12:
1H NMR (300 MHz, CD
3OD): δ 2.18 (p, 2H,
J 6.2 Hz, CH
2), 3.62–3.86 (m, 7H), 3.94–4.09 (m, 5H), 4.13–4.20 (m, 1H, H-3
I), 4.33–4.38 (m, 1H, H-2
I), 5.03 (d, 1H,
J 4.2 Hz, H-1
II), 5.56 (d, 1H,
J 2.5 Hz, H-1
I), 6.79–6.92 (m, 2H, OC
6H
4O), 6.93–7.05 (m, 2H, OC
6H
4O); selected signals for minor 4-(3-fluoropropoxy)phenyl 2-
O-(β-
d-arabinofuranosyl)-α-
d-arabinofuranoside:
1H NMR (300 MHz, CD
3OD): 4.60 (dt, 2H,
J 47.3 Hz,
J 5.9 Hz, CH
2F),
19F NMR (282 MHz, CD
3OD): δ −223.86 (tt,
2J
H–F 47.3 Hz,
3J
H–F 25.4 Hz, CH
2F). Then,
i-Pr
3SiOTf (0.42 mL, 1.57 mmol) was added to the solution of crude pentaol
12 (72 mg, 0.16 mmol) in 2,4,6-collidine (1.5 mL) at 20 °C. The reaction mixture was stirred at 80 °C for 20 h and then diluted with CH
2Cl
2 (30 mL), washed with 1 M KHSO
4 (3 × 30 mL), and satd aq NaHCO
3 (3 × 30 mL). Organic extracts were filtered, concentrated under reduced pressure, and purified by silica gel chromatography (gradient: petroleum ether–EtOAc, 0%→6%) to give silylated disaccharide
13, containing 3% 4-(3-fluoropropoxy)phenyl derivative (155 mg, 80%).
Rf = 0.80 (light petroleum–EtOAc 10:1);
+21.1 (c 0.25 in CHCl
3);
1H NMR (600 MHz, CDCl
3): δ 1.03–1.22 (m, 105H, ((CH
3)
2CH)
3Si), 2.15 (dt, 1H,
J 25.8 Hz,
J 6.0 Hz, C
H2CH
2F), 2.22 (p, 2H,
J 6.1 Hz, CH
2), 3.71 (dd, 1H,
J 9.3 Hz,
J 4.5 Hz, H-5
IIa), 3.75 (t, 2H,
J 6.4 Hz, CH
2Cl), 3.78 (dd, 1H,
J 10.7 Hz,
J 5.6 Hz, H-5
Ia), 3.87 (dd, 1H,
J 10.7 Hz,
J 5.3 Hz, H-5
Ib), 3.88 (dd, 1H,
J 10.4 Hz,
J 4.5 Hz, H-4
II), 3.94 (dd, 1H,
J 10.4 Hz,
J 9.3 Hz, H-5
IIb), 4.01 (d, 1H,
J 2.9 Hz, H-2
II), 4.07 (t, 2H,
J 5.8 Hz, CH
2O), 4.20 (td, 1H,
J 5.5 Hz,
J 4.4 Hz, H-4
I), 4.33 (d, 1H,
J 1.1 Hz, H-3
II), 4.41 (dd, 1H,
J 2.2 Hz,
J 1.0 Hz, H-2
I), 4.52 (dd, 1H,
J 4.5 Hz,
J 2.2 Hz, H-3
I), 4.65 (dt, 1H,
J 47.1 Hz,
J 5.8 Hz, CH
2F), 5.29 (d, 1H,
J 2.8 Hz, H-1
II), 5.53 (d, 1H,
J 0.8 Hz, H-1
I), 6.78–6.84 (m, 2H, OC
6H
4O (H-3, H-5)), 6.94–7.00 (m, 2H, OC
6H
4O (H-2, H-6));
13C NMR (151 MHz, CDCl
3): δ 11.98 (((CH
3)
2CH)
3Si), 12.03 (((CH
3)
2CH)
3Si), 12.30 (((CH
3)
2CH)
3Si), 12.32 (((CH
3)
2CH)
3Si), 12.4 (((CH
3)
2CH)
3Si), 17.96 (((
CH
3)
2CH)
3Si), 17.98 (((
CH
3)
2CH)
3Si), 18.08 (((
CH
3)
2CH)
3Si), 18.11 (((
CH
3)
2CH)
3Si), 18.14 (((
CH
3)
2CH)
3Si), 18.15 (((
CH
3)
2CH)
3Si), 32.4 (CH
2), 41.6 (CH
2Cl), 63.7 (C-5
II), 63.8 (C-5
I), 64.9 (CH
2O), 77.1 (C-3
I), 78.1 (C-2
II), 78.3 (C-3
II), 85.9 (C-4
II), 87.9 (C-4
I), 91.2 (C-2
I), 104.8 (C-1
II), 105.6 (C-1
I), 115.3 (OC
6H
4O (C-3, C-5)), 118.1 (OC
6H
4O (C-2, C-6)), 151.6 (OC
6H
4O (C-1)), 153.7 (OC
6H
4O (C-4));
29Si INEPT NMR (60 MHz, CDCl
3): δ 13.36 (3
I-
O-TIPS), 13.65 (5
I-
O-TIPS, 5
II-
O-TIPS), 13.70 (3
II-
O-TIPS), 14.05 (2
II-
O-TIPS); HRMS (ESI):
m/
z [M + NH
4]
+ Calcd for C
64H
131ClNO
10Si
5+ 1248.8302; found: 1248.8296.
4-(2-Chloroethoxy)phenyl 2,3,5-tris-O-(triisopropylsilyl)-β-d-arabinofuranosyl-(1→2)-3,5-bis-O-(triisopropylsilyl)-α-d-arabinofuranosyl-(1→5)-2-O-benzoyl-α-d-arabinofuranosyl-(1→5)-2,3-di-O-benzoyl-α-d-arabinofuranoside (14)
A mixture of disaccharide thioglycoside
6 (82 mg, 0.07 mmol) and disaccharide diol
8 [
28] (19 mg, 0.25 mmol) was dried in vacuo for 2 h, then anhydrous CH
2Cl
2 (2 mL) was added under argon. Freshly activated powdered MS 4 Å (200 mg) (100 mg per 1 mL of solvent) was added under argon to the resulting solution. The suspension was stirred under argon at ~22 °C for 1 h, cooled to −60 °C, then NIS (16 mg, 0.7 mmol) and, after 10 min, AgOTf (1 mg) was added. Then, the temperature was allowed to rise slowly to −20 °C and was kept at −20 °C for 1 h. Then, the reaction was quenched by the addition of satd aq NaHCO
3 (50 μL), diluted with CH
2Cl
2 (15 mL), and filtered through a Celite pad. The solids were washed with CH
2Cl
2 (5 × 10 mL), and the filtrate was washed with a mixture of satd aq Na
2S
2O
3 (50 mL) and satd aq NaHCO
3 (50 mL). The aqueous layer was extracted with CH
2Cl
2 (2 × 5 mL). The combined organic extracts were filtered through a cotton wool plug, concentrated and dried in vacuo, then dissolved in toluene (2 mL) and subjected to chromatography on Bio-Beads S-X1 in toluene. The fractions eluted just after the void volume were collected, concentrated under reduced pressure, and the residue was purified by silica gel chromatography (gradient: EtOAc in petroleum ether, 0%→30%) to give α-linked tetrasaccharide
14 (36 mg, 80%).
Rf = 0.20 (light petroleum–EtOAc 10:1);
1H NMR (600 MHz, CDCl
3): δ 0.89 –1.14 (m, 105H, 5 × ((CH
3)
2CH)
3Si), 3.43 (d, 1H,
J 4.1 Hz, HO-3
II), 3.66 (dd, 1H,
J 9.5 Hz,
J 4.6 Hz, H-5
IVa), 3.68 (dd, 1H,
J 11.1 Hz,
J 3.9 Hz, H-5
IIa), 3.73 (dd, 1H,
J 10.4 Hz,
J 6.4 Hz, H-5
IIIa), 3.77–3.80 (m, 1H, H-5
IIIb), 3.79 (t, 2H,
J 5.9 Hz, CH
2Cl), 3.82 (dd, 1H,
J 10.3 Hz,
J 4.6 Hz, H-4
IV), 3.90 (dd, 1H,
J 10.2 Hz,
J 9.4 Hz, H-5
IVb), 3.92 (dd, 1H,
J 11.1 Hz,
J 3.7 Hz, H-5
Ia), 3.98 (d, 1H,
J 3.0 Hz, H-2
IV), 4.00 (dd, 1H,
J 11.2 Hz,
J 3.5 Hz, H-5
IIb), 4.07 (td, 1H,
J 6.0 Hz,
J 3.6 Hz, H-4
III), 4.17 (d, 1H,
J 1.2 Hz, H-2
III), 4.19 (t, 2H,
J 6.0 Hz, CH
2O), 4.21 (dd, 1H,
J 11.0 Hz,
J 4.5 Hz, H-5
Ib), 4.29 (d, 1H,
J 1.1 Hz, H-3
IV), 4.31 (dt, 1H,
J 7.1 Hz,
J 3.5 Hz, H-3
II), 4.34–4.38 (m, 2H, H-4
II, H-3
III), 4.58–4.63 (m, 1H, H-4
I), 5.06 (s, 1H, H-1
III), 5.17 (dd, 1H,
J 3.7 Hz,
J 1.4 Hz, H-2
II), 5.20 (d, 1H,
J 2.6 Hz, H-1
IV), 5.37 (d, 1H,
J 1.4 Hz, H-1
II), 5.68 (dd, 1H,
J 5.0 Hz,
J 1.8 Hz, H-3
I), 5.75 (d, 1H,
J 1.7 Hz, H-2
I), 5.83 (s, 1H, H-1
I), 6.84–6.91 (m, 2H, OC
6H
4O (H-3, H-5)), 7.04–7.11 (m, 2H, OC
6H
4O (H-2, H-6)), 7.41–7.51 (m, 6H, 3 × PhCO (H-3, H-5)), 7.55–7.64 (m, 3H, 3 × PhCO (H-4)), 7.99–8.04 (m, 2H, 2
II-
O-PhCO (H-2, H-6)), 8.07–8.11 (m, 2H, 2
I-
O-PhCO (H-2, H-6)), 8.09–8.14 (m, 2H, 3
I-
O-PhCO (H-2, H-6));
13C NMR (151 MHz, CDCl
3): δ 11.9 (((CH
3)
2CH)
3Si), 12.0 (((CH
3)
2CH)
3Si), 12.1 (((CH
3)
2CH)
3Si), 12.2 (((CH
3)
2CH)
3Si), 12.3 (((CH
3)
2CH)
3Si), 17.9 (2 × ((
CH
3)
2CH)
3Si), 17.95 (3 × ((
CH
3)
2CH)
3Si), 17.97 (((
CH
3)
2CH)
3Si), 18.06 (((
CH
3)
2CH)
3Si), 18.09 (((
CH
3)
2CH)
3Si), 18.13 (((
CH
3)
2CH)
3Si), 18.14 (((
CH
3)
2CH)
3Si), 41.9 (CH
2Cl), 63.7 (C-5
IV), 64.4 (C-5
II), 64.6 (C-5
III), 66.0 (C-5
I), 68.7 (CH
2O), 76.1 (C-3
II), 77.3 (C-3
I), 77.9 (C-3
III), 78.0 (C-2
IV), 78.1 (C-3
IV), 82.1 (C-2
I, C-4
II), 82.5 (C-4
I), 85.8 (C-4
IV), 86.9 (C-2
II), 88.2 (C-4
III), 90.8 (C-2
III), 104.8 (C-1
I), 105.0 (C-1
IV), 105.4 (C-1
II), 106.3 (C-1
III), 115.8 (OC
6H
4O (C-3, C-5)), 118.3 (OC
6H
4O (C-2, C-6)), 128.4 (Ph
CO (C-3, C-5)), 128.5 (Ph
CO (C-3, C-5)), 128.6 (Ph
CO (C-3, C-5)), 128.9 (Ph
CO (C-1)), 129.1 (Ph
CO (C-1)), 129.3 (Ph
CO (C-1)), 129.87 (Ph
CO (C-2, C-6)), 129.92 (Ph
CO (C-2, C-6)), 129.94 (Ph
CO (C-2, C-6)), 133.4 (Ph
CO (C-4)), 133.5 (Ph
CO (C-4)), 133.6 (Ph
CO (C-4)), 150.7 (OC
6H
4O (C-1)), 153.7 (OC
6H
4O (C-4)), 165.5 (2
I-
O-Ph
CO), 165.7 (3
I-
O-Ph
CO), 166.8 (2
II-
O-Ph
CO);
29Si INEPT NMR (60 MHz, CDCl
3): δ 13.54, 13.59, 13.70, 13.80, 13.89; HRMS (ESI):
m/
z [M + NH
4]
+ Calcd for C
94H
193ClNO
21Si
5+ 1810.9777; found: 1810.9764; [M + K]
+ Calcd for C
94H
153ClKO
21Si
5+: 1831.9071; found: 1831.9063.
2,3,5-Tris-O-(triisopropylsilyl)-β-d-arabinofuranosyl-(1→2)-3,5-bis-O-(triisopropylsilyl)-α-d-arabinofuranosyl-(1→3)-[2,3,5-tris-O-(triisopropylsilyl)-β-d-arabinofuranosyl-(1→2)-3,5-bis-O-(triisopropylsilyl)-α-d-arabinofuranosyl-(1→5)]-2-O-benzoyl-α-d-arabinofuranosyl-(1→5)-2,3-di-O-benzoyl-d-arabinofuranose (15) and 2,3,5-tris-O-(triisopropylsilyl)-β-d-arabinofuranosyl-(1→2)-3,5-bis-O-(triisopropylsilyl)-α-d-arabinofuranosyl-(1→3)-[2,3,5-tris-O-(triisopropylsilyl)-β-d-arabinofuranosyl-(1→2)-3,5-bis-O-(triisopropylsilyl)-α-d-arabinofuranosyl-(1→5)]-2-O-benzoyl-α-d-arabinofuranosyl-(1→5)-1,3-di-O-benzoyl-β-d-arabinofuranose (16)
CEP glycoside 2 (44 mg, 0.016 mmol) was dissolved in CH2Cl2 (2 mL) and MeCN (1 mL), then H2O (0.1 mL) was added at 0 °C, followed by (NH4)2Ce(NO3)6 (52.6 mg, 0.096 mmol). The reaction mixture was stirred at 0 °C for 4 h. Then, Na2SO3 (110 mg) and H2O (1 mL) were added to the reaction mixture at 0 °C, and the reaction mixture was kept at 0 °C for 16 h. Then, the reaction mixture was diluted with CH2Cl2 (50 mL), washed with H2O (50 mL), concentrated under reduced pressure and purified by silica gel column chromatography (gradient: petroleum ether–EtOAc, 3%→8%) to give hemiacetal 15 (29 mg, 70%) as a mixture of anomers 15, and contaminated with the product 16 of migration of benzoyl group from O-2 to O-1. The ratio of 15α:15β:16 = 1:0.45:0.28 according to NMR; Rf = 0.50 (light petroleum–EtOAc, 8.5:1.5); selected signals: 1H NMR (300 MHz, CDCl3): δ 0.75–1.18 (m, 363H, ((CH3)2CH)3Si), 3.40 (s, 1H, HO-1Iα), 4.65 (ddd, 1H, J 6.3 Hz, J 5.2 Hz, J 3.3 Hz, H-4Iα), 5.05 (s, 1H, H-1III of 16), 5.10 (s, 1H, H-1IIIβ), 5.10 (s, 1H, H-1IIIα), 5.16 (s, 1H, H-1Vβ), 5.17–5.18 (m, 2H, H-1IIβ, H-1II or H-1V of 16), 5.31 (d, 1H, J 3.2 Hz, H-2IIβ), 5.35 (d, 1H, J 2.0 Hz, H-2IIα), 5.40–5.48 (m, 2H, H-3Iα, H-3I of 16), 5.51 (d, 1H, J 2.4 Hz, H-2Iα), 5.56 (dd, 1H, J 6.2 Hz, J 4.7 Hz, H-2Iβ), 5.63 (s, 1H, H-1Iα), 5.72 (dd, 1H, J 5.5 Hz, J 5.5 Hz, H-1Iβ), 5.86 (dd, 1H, J 6.2 Hz, J 5.0 Hz, H-3Iβ), 6.60 (d, 1H, J 4.5 Hz, H-1I of 16), 7.33–7.66 (m, 16H, PhCO (H-3, H-4, H-5)), 7.92–8.19 (m, 11H, PhCO (H-2, H-6)); 13C NMR (151 MHz, CDCl3): δ 11.97 (((CH3)2CH)3Si), 11.99 (((CH3)2CH)3Si), 12.02 (((CH3)2CH)3Si), 12.03 (((CH3)2CH)3Si), 12.2 (((CH3)2CH)3Si), 12.28 (((CH3)2CH)3Si), 12.31 (((CH3)2CH)3Si), 12.36 (((CH3)2CH)3Si), 12.39 (((CH3)2CH)3Si), 17.95 (((CH3)2CH)3Si), 17.97 (((CH3)2CH)3Si), 17.99 (((CH3)2CH)3Si), 18.01 (((CH3)2CH)3Si), 18.03 (((CH3)2CH)3Si), 18.05 (((CH3)2CH)3Si), 18.08 (((CH3)2CH)3Si), 18.09 (((CH3)2CH)3Si), 18.11 (((CH3)2CH)3Si), 18.13 (((CH3)2CH)3Si), 18.14 (((CH3)2CH)3Si), 18.17 (((CH3)2CH)3Si), 18.18 (((CH3)2CH)3Si), 18.20 (((CH3)2CH)3Si), 63.7 (C-5), 63.76 (2 × C-5), 63.82 (C-5), 64.16 (C-5), 64.18 (C-5), 64.5 (C-5), 64.7 (C-5), 65.3 (C-5II minor), 65.4 (C-5IIα), 66.0 (C-5IIβ), 66.6 (C-5Iα), 67.4 (C-5I of 16), 67.6 (C-5Iβ), 76.5 (C-3Iβ), 76.7 (C-2I of 16), 77.4, 77.5, 77.7, 78.08, 78.14, 78.19, 78.21, 78.23, 78.25, 78.28, 78.32, 78.37, 78.43, 78.47, 78.52, 79.7, 80.06 (C-3IIβ), 80.12, 80.6, 80.7 (C-3IIα), 81.0 (C-3I of 16), 81.1, 81.8 (C-4Iα), 82.1, 82.2, 82.6 (C-2Iα), 83.6 (C-2IIα), 83.7 (C-2II of 16), 84.1 (C-2IIβ), 85.7 (C-4IV, C-4VI), 85.7 (C-4IV, C-4VI), 85.6 (C-4IV, C-4VI), 85.7 (C-4IV, C-4VI), 85.8 (C-4IV, C-4VI), 87.5 (C-4III), 87.54 (C-4III), 87.59 (C-4III of 16), 87.8 (C-4V), 88.3 (C-4V), 88.4 (C-4V of 16), 90.5 (C-2III), 90.70 (C-2III), 90.74 (C-2III of 16), 91.2 (C-2V), 91.4 (C-2V of 16), 91.6 (C-2V), 95.4 (C-1Iβ), 97.0 (C-1I of 16), 100.9 (C-1Iα), 104.7 (C-1), 104.75 (C-1), 104.78 (C-1 minor), 105.2 (C-1), 105.5 (C-1), 105.48 (C-1 of 16), 105.93 (C-1), 105.94 (C-1 of 16), 105.98 (C-1), 106.04 (C-1), 106.2 (C-1), 106.4 (C-1 of 16), 106.69 (C-1III of 16), 106.73 (C-1IIIβ), 106.8 (C-1IIIα), 128.20 (PhCO (C-3, C-5) of 16), 128.23 (PhCO (C-3, C-5)), 128.27 (PhCO (C-3, C-5)), 128.30 (PhCO (C-3, C-5)), 128.37 (2 × PhCO (C-3, C-5)), 128.44 (PhCO (C-3, C-5) of 16), 128.5 (PhCO (C-3, C-5)), 129.3 (PhCO (C-1)), 129.4 (PhCO (C-1)), 129.48 (PhCO (C-1)), 129.49 (PhCO (C-1)), 129.54 (PhCO (C-1)), 129.8 (PhCO (C-1)), 129.85 (PhCO (C-2, C-6)), 129.86 (PhCO (C-2, C-6)), 129.92 (PhCO (C-2, C-6) of 16), 129.94 (PhCO (C-2, C-6)), 130.0 (2 × PhCO (C-2, C-6)), 130.1 (PhCO (C-2, C-6)), 132.9 (PhCO (C-4)), 133.07 (PhCO (C-4)), 133.12 (PhCO (C-4)), 133.2 (PhCO (C-4)), 133.27 (PhCO (C-4)), 133.29 (PhCO (C-4)), 133.5 (PhCO (C-4) of 16), 165.16 (PhCO of 16), 165.20 (PhCO), 165.58 (PhCO), 165.59 (PhCO), 165.7 (PhCO), 165.8 (2 × PhCO); HRMS (ESI): m/z [M + NH4]+ Calcd for C141H266NO28Si10+ 2701.7109; found: 2701.7093.
4-(2-Chloroethoxy)phenyl 2,3,5-tris-O-(triisopropylsilyl)-β-d-arabinofuranosyl-(1→2)-3,5-bis-O-(triisopropylsilyl)-α-d-arabinofuranosyl-(1→3)-[2,3,5-tris-O-(triisopropylsilyl)-β-d-arabinofuranosyl-(1→2)-3,5-bis-O-(triisopropylsilyl)-α-d-arabinofuranosyl-(1→5)]-2-O-benzoyl-α-d-arabinofuranosyl-(1→5)-2,3-di-O-benzoyl-α-d-arabinofuranosyl-(1→5)-2,3-di-O-benzoyl-α-d-arabinofuranosyl-(1→5)-2,3-di-O-benzoyl-α-d-arabinofuranosyl-(1→5)-2,3-di-O-benzoyl-α-d-arabinofuranosyl-(1→5)-2,3-di-O-benzoyl-α-d-arabinofuranoside (18)
A mixture of hexasaccharide imidate
5 (32 mg, 0.011 mmol) and known tetrasaccharide glycosyl acceptor
17 [
33] (21 mg, 0.013 mmol) was dried
in vacuo for 2 h, then anhydrous CH
2Cl
2 (2 mL) was added under argon. Freshly activated powdered MS 4 Å (200 g) (100 mg per 1 mL of solvent) was added under argon to the resulting solution. The suspension was stirred under argon at ~22 °C for 1 h, then cooled to −60 °C, followed by the addition of TfOH (1 μL). Then, the temperature was allowed to rise during 1 h to −40 °C and this temperature was kept for 1 h. After an additional 20 min, the reaction was quenched by the addition of Py (50 µL). The reaction mixture was diluted with CH
2Cl
2 (15 mL) and filtered through a Celite pad. The solids were washed with CH
2Cl
2 (5 × 10 mL), and the filtrate was washed with satd aq NaHCO
3 (20 mL). The aqueous layer was extracted with CH
2Cl
2 (2 × 5 mL). The combined organic layer was filtered through a cotton wool plug, concentrated under reduced pressure, the residue was dried in vacuo, then dissolved in toluene (2 mL) and subjected to chromatography on Bio-Beads S-X1 in toluene. The fractions eluted just after the void volume were collected and concentrated under reduced pressure to give decasaccharide
18 (34 mg, 72%; 51% over 3 steps starting from
2).
Rf = 0.28 (light petroleum–EtOAc 6:1);
+31.9 (c 1.13 in CHCl
3);
1H NMR (600 MHz, CDCl
3): δ 0.91–1.16 (m, 210H, 10 × ((CH
3)
2CH)
3Si), 3.63 (dd, 1H,
J 9.3 Hz,
J 4.4 Hz, H-5
VIIIa or H-5
Xa), 3.65 (dd, 1H,
J 10.7 Hz,
J 5.5 Hz, H-5
IXa), 3.78 (t, 2H,
J 5.9 Hz, CH
2Cl), 3.69–3.98 (m, 16H, H-5
Xa or H-5
VIIIa, H-5
I-VIIa, H-5
VI-Xb, H-4
VIII-X), 4.02–4.04 (m, 2H, H-2
VIII, H-2
X), 4.06 (d, 1H,
J 1.2 Hz, H-2
IX), 4.08–4.12 (m, 2H, H-4
VII, H-5
Vb), 4.13 (s, 1H, H-2
VII), 4.16 (t, 2H,
J 5.9 Hz, CH
2O), 4.14–4.24 (m, 5H, H-5
I-IV, H-3
VI), 4.26–4.28 (m, 1H, H-4
VI), 4.29 (s, 1H, H-3
X), 4.32 (s, 1H, H-3
VIII), 4.34 (d, 1H,
J 3.9 Hz, H-3
VII), 4.45 (d, 1H,
J 3.4 Hz, H-3
IX), 4.55–4.64 (m, 5H, H-4
I-V), 5.10 (s, 1H, H-1
VII), 5.15 (d, 1H,
J 2.6 Hz, H-1
X), 5.23 (s, 1H, H-1
IX), 5.24 (d, 1H,
J 2.8 Hz, H-1
VIII), 5.29 (s, 1H, H-1
VI), 5.37 (s, 2H, 2 × H-1), 5.38–5.41 (m, 3H, H-2
VI, 2 × H-1), 5.50 (d, 1H,
J 4.4 Hz, H-3
V), 5.58 (d, 1H,
J 1.1 Hz, H-2
V), 5.62–5.67 (m, 6H, H-3
II-IV, H-2
II-IV), 5.75–5.79 (m, 2H, H-3
I, H-2
I), 5.82 (s, 1H, H-1
I), 6.80–6.86 (m, 2H, OC
6H
4O (H-3, H-5)), 7.02–7.08 (m, 2H, OC
6H
4O (H-2, H-6)), 7.18–7.27 (m, 8H, 4 × PhCO (H-3, H-5)), 7.34–7.55 (m, 24H, 7 × PhCO (H-3, H-5), 10 × PhCO (H-4)), 7.55–7.61 (m, 1H, PhCO (H-4)), 7.84–7.92 (m, 8H, 4 × PhCO (H-2, H-6)), 7.97–8.06 (m, 10H, 5 × PhCO (H-2, H-6)), 8.06–8.11 (m, 2H, PhCO (H-2, H-6)), 8.09–8.14 (m, 2H, PhCO (H-2, H-6));
13C NMR (151 MHz, CDCl
3): δ 11.97 (((CH
3)
2CH)
3Si), 11.99 (((CH
3)
2CH)
3Si), 12.01 (((CH
3)
2CH)
3Si), 12.1 (((CH
3)
2CH)
3Si), 12.2 (((CH
3)
2CH)
3Si), 12.27 (2 × ((CH
3)
2CH)
3Si), 12.29 (2 × ((CH
3)
2CH)
3Si), 12.4 (((CH
3)
2CH)
3Si), 17.94 (((
CH
3)
2CH)
3Si), 17.96 (((
CH
3)
2CH)
3Si), 17.97 (((
CH
3)
2CH)
3Si), 18.02 (((
CH
3)
2CH)
3Si), 18.04 (((
CH
3)
2CH)
3Si), 18.06 (((
CH
3)
2CH)
3Si), 18.09 (((
CH
3)
2CH)
3Si), 18.13 (((
CH
3)
2CH)
3Si), 18.19 (((
CH
3)
2CH)
3Si), 18.20 (((
CH
3)
2CH)
3Si), 41.9 (CH
2Cl), 63.8 (C-5
VIII, C-5
X), 63.9 (C-5
VIII, C-5
X), 64.3 (C-5
IX), 64.9 (C-5
VII), 65.2 (C-5
VI), 65.3 (C-5
V), 65.8 (2C, C-5
I, C-5
II, C-5
III, C-5
IV), 65.86 (C-5
I, C-5
II, C-5
III, C-5
IV), 65.91 (C-5
I, C-5
II, C-5
III, C-5
IV), 68.8 (CH
2O), 77.2 (C-3
I), 77.2 (2C, C-3
II, C-3
III, C-3
IV, C-3
V), 77.3 (C-3
II, C-3
III, C-3
IV, C-3
V), 77.6 (C-3
II, C-3
III, C-3
IV, C-3
V), 77.7 (C-3
IX), 78.2 (C-2
VIII or C-2
X), 78.3 (C-3
X), 78.4(C-2
VIII or C-2
X), 78.5 (2C, C-3
VII, C-3
VIII), 81.0 (C-3
VI), 81.5 (2C, C-2
II, C-2
III, C-2
IV, C-2
V), 81.6 (2C, C-2
II, C-2
III, C-2
IV, C-2
V), 81.9 (C-2
I), 82.2 (3C, C-4
II, C-4
III, C-4
IV, C-4
V, C-4
VI), 82.26 (C-4
II, C-4
III, C-4
IV, C-4
V, C-4
VI), 82.30 (C-4
II, C-4
III, C-4
IV, C-4
V, C-4
VI), 82.8 (C-4
I), 84.0 (C-2
VI), 85.7 (C-4
VIII, C-4
X), 85.6 (C-4
VIII, C-4
X), 87.7 (C-4
VII), 88.6 (C-4
IX), 90.6 (C-2
VII), 91.4 (C-2
IX), 104.8 (C-1
VIII), 104.9 (C-1
I), 105.5 (C-1
X), 105.85 (C-1
VI), 105.89 (C-1), 106.0 (3C, C-1
II, C-1
III, C-1
IV), 106.2 (C-1
IX), 106.9 (C-1
VII), 115.9 (OC
6H
4O (C-3, C-5)), 118.3 (OC
6H
4O (C-2, C-6)), 128.1 (
PhCO (C-3, C-5)), 128.15 (
PhCO (C-3, C-5)), 128.17 (
PhCO (C-3, C-5)), 128.20 (
PhCO (C-3, C-5)), 128.3 (
PhCO (C-3, C-5)), 128.4 (
PhCO (C-3, C-5)), 128.6 (
PhCO (C-3, C-5)), 128.49 (3 ×
PhCO (C-3, C-5)), 128.54 (
PhCO (C-3, C-5)), 129.1–129.29 (
PhCO (C-1)), 129.29 (
PhCO (C-1)), 129.36 (
PhCO (C-1)), 129.41 (
PhCO (C-1)), 129.5 (
PhCO (C-1)), 129.78 (3 ×
PhCO (C-2, C-6)), 129.81 (2 ×
PhCO (C-2, C-6)), 129.83 (3 ×
PhCO (C-2, C-6)), 129.86 (
PhCO (C-2, C-6)), 129.92 (
PhCO (C-2, C-6)), 130.0 (
PhCO (C-2, C-6)), 132.75 (
PhCO (C-4)), 132.82 (
PhCO (C-4)), 132.9 (
PhCO (C-4)), 133.0 (
PhCO (C-4)), 133.1 (2 ×
PhCO (C-4)), 133.2 (
PhCO (C-4)), 133.3 (
PhCO (C-4)), 133.35 (
PhCO (C-4)), 133.44 (
PhCO (C-4)), 133.5 (
PhCO (C-4)), 150.8 (OC
6H
4O (C-1)), 153.7 (OC
6H
4O (C-4)), 165.0 (Ph
CO), 165.2 (Ph
CO), 165.2 (Ph
CO), 165.2 (Ph
CO), 165.2 (Ph
CO), 165.3 (Ph
CO), 165.37 (Ph
CO), 165.44 (Ph
CO), 165.5 (Ph
CO), 165.57 (Ph
CO), 165.64 (Ph
CO);
29Si INEPT NMR (60 MHz, CDCl
3): δ 12.30 (3
VII-
O-TIPS), 12.84 (3
IX-
O-TIPS), 13.21 (5-
O-TIPS), 13.44 (5-
O-TIPS), 13.49 (3
X-
O-TIPS, 5-
O-TIPS), 13.50 (3
X-
O-TIPS, 5-
O-TIPS), 13.54 (5-
O-TIPS), 13.76 (3
VIII-
O-TIPS), 13.82 (2
VIII-
O-TIPS, 2
X-
O-TIPS), 13.86 (2
VIII-
O-TIPS, 2
X-
O-TIPS); HRMS (ESI):
m/
z [M + 2NH
4]
2+ Calcd for C
225H
341ClN
2O
53Si
102+ 2117.0710; found: 2117.0702.
4-(2-Azidoethoxy)phenyl 2,3,5-tris-O-(triisopropylsilyl)-β-d-arabinofuranosyl-(1→2)-3,5-bis-O-(triisopropylsilyl)-α-d-arabinofuranosyl-(1→3)-[2,3,5-tris-O-(triisopropylsilyl)-β-d-arabinofuranosyl-(1→2)-3,5-bis-O-(triisopropylsilyl)-α-d-arabinofuranosyl-(1→5)]-2-O-benzoyl-α-d-arabinofuranosyl-(1→5)-2,3-di-O-benzoyl-α-d-arabinofuranosyl-(1→5)-2,3-di-O-benzoyl-α-d-arabinofuranosyl-(1→5)-2,3-di-O-benzoyl-α-d-arabinofuranosyl-(1→5)-2,3-di-O-benzoyl-α-d-arabinofuranosyl-(1→5)-2,3-di-O-benzoyl-α-d-arabinofuranoside (19)
A mixture of decasaccharide CEP glycoside 18 (30 mg, 0.007 mmol), NaN3 (3 mg, 0.042 mmol), and 18-crown-6 (2 mg, 0.006 mmol) in DMF (1 mL) was stirred at 80 °C for 16 h. The reaction mixture was concentrated under reduced pressure, co-evaporated with toluene (2×2 mL), and dried in vacuo. The residue was dissolved in EtOAc (50 mL), washed with water (50 mL), and the aqueous layer was extracted with EtOAc (5 mL). The combined organic extracts were filtered through a cotton wool plug, concentrated under reduced pressure, and purified by silica gel column chromatography (gradient: EtOAc in petroleum ether, 2%→20%) to give azide 19 (25 mg, 84%); +29.7 (c 2.55 in CHCl3); 1H NMR (600 MHz, CDCl3): δ 0.91–1.13 (m, 210H, 10 × ((CH3)2CH)3Si), 3.56 (t, 2H, J 5.0 Hz, CH2N3), 3.63 (dd, 1H, J 9.3 Hz, J 4.4 Hz, H-5VIIIa or H-5Xa), 3.66 (dd, 1H, J 10.5 Hz, J 5.5 Hz, H-5IXa), 3.69–3.98 (m, 16H, H-5Xa or H-5VIIIa, H-5I-VIIa, H-5VI-Xb, H-4VIII-X), 4.03 (d, 1H, J 2.8 Hz, H-2VIII), 4.04 (d, 1H, J 2.7 Hz, H-2X), 4.07 (d, 1H, J 1.4 Hz, H-2IX), 4.08 (t, 2H, J 5.1 Hz, CH2O), 4.07–4.13 (m, 2H, H-4VII, H-5Vb), 4.13 (d, 1H, J 1.2 Hz, H-2VII), 4.14–4.24 (m, 5H, H-5I-IVb, H-3VI), 4.27 (ddd, 1H, J 7.2 Hz, J 5.0 Hz, J 1.9 Hz, H-4VI), 4.29 (d, 1H, J 1.0 Hz, H-3X), 4.32 (d, 1H, J 0.9 Hz, H-3VIII), 4.34 (dt, 1H, J 4.0 Hz, J 0.8 Hz, H-3VII), 4.45 (dd, 1H, J 3.3 Hz, J 1.6 Hz, H-3IX), 4.54–4.64 (m, 5H, H-4I-V), 5.09 (s, 1H, H-1VII), 5.16 (d, 1H, J 2.7 Hz, H-1X), 5.23 (s, 1H, H-1IX), 5.24 (d, 1H, J 2.8 Hz, H-1VIII), 5.29 (s, 1H, H-1VI), 5.37 (s, 1H, H-1), 5.37 (s, 1H, H-1), 5.37–5.41 (m, 3H, H-2VI, 2 × H-1), 5.50 (d, 1H, J 4.4 Hz, H-3V), 5.58 (d, 1H, J 1.2 Hz, H-2V), 5.61–5.67 (m, 6H, H-3II-IV, H-2II-IV), 5.74–5.79 (m, 1H, H-3I), 5.77 (s, 1H, H-2I), 5.82 (s, 1H, H-1I), 6.80–6.86 (m, 2H, OC6H4O (H-3, H-5)), 7.02–7.08 (m, 2H, OC6H4O (H-2, H-6)), 7.19–7.27 (m, 8H, 4 × PhCO (H-3, H-5)), 7.34–7.55 (m, 24H, 7 × PhCO (H-3, H-5), 10 × PhCO (H-4)), 7.55–7.61 (m, 1H, PhCO (H-4)), 7.84–7.92 (m, 8H, 4 × PhCO (H-2, H-6)), 7.97–8.06 (m, 10H, 5 × PhCO (H-2, H-6)), 8.06–8.11 (m, 2H, PhCO (H-2, H-6)), 8.09–8.14 (m, 2H, PhCO (H-2, H-6)); 13C NMR (151 MHz, CDCl3): δ 11.97 (((CH3)2CH)3Si), 11.99 (((CH3)2CH)3Si), 12.01 (((CH3)2CH)3Si), 12.04 (((CH3)2CH)3Si), 12.2 (((CH3)2CH)3Si), 12.26 (2 × ((CH3)2CH)3Si), 12.28 (((CH3)2CH)3Si), 12.29 (((CH3)2CH)3Si), 12.4 (((CH3)2CH)3Si), 17.9 (((CH3)2CH)3Si), 17.96 (((CH3)2CH)3Si), 17.97 (((CH3)2CH)3Si), 18.02 (((CH3)2CH)3Si), 18.04 (((CH3)2CH)3Si), 18.06 (((CH3)2CH)3Si), 18.09 (((CH3)2CH)3Si), 18.13 (((CH3)2CH)3Si), 18.19 (((CH3)2CH)3Si), 18.20 (((CH3)2CH)3Si), 50.2 (CH2N3), 63.8 (C-5VIII, C-5X), 63.9 (C-5VIII, C-5X), 64.3 (C-5IX), 64.8 (C-5VII), 65.2 (C-5V, C-5VI), 65.3 (C-5V, C-5VI), 65.76 (2C, C-5I, C-5II, C-5III, C-5IV), 65.83 (C-5I, C-5II, C-5III, C-5IV), 65.9 (C-5I, C-5II, C-5III, C-5IV), 67.6 (CH2O), 77.10 (C-3I), 77.2 (2C, C-3II, C-3III, C-3IV, C-3V), 77.3 (C-3II, C-3III, C-3IV, C-3V), 77.6 (C-3II, C-3III, C-3IV, C-3V), 77.7 (C-3IX), 78.15 (C-2VIII or C-2X), 78.24 (C-3X), 78.4 (C-2VIII or C-2X), 78.51 (C-3VII, C-3VIII), 78.53 (C-3VII, C-3VIII), 81.0 (C-3VI), 81.5 (2C, C-2II, C-2III, C-2IV, C-2V), 81.59 (C-2II, C-2III, C-2IV, C-2V), 81.61 (C-2II, C-2III, C-2IV, C-2V), 81.9 (C-2I), 82.15 (C-4II, C-4III, C-4IV, C-4V, C-4VI), 82.17 (C-4II, C-4III, C-4IV, C-4V, C-4VI), 82.19 (C-4II, C-4III, C-4IV, C-4V, C-4VI), 82.26 (C-4II, C-4III, C-4IV, C-4V, C-4VI), 82.30 (C-4II, C-4III, C-4IV, C-4V, C-4VI), 82.8 (C-4I), 84.0 (C-2VI), 85.7 (C-4VIII, C-4X), 85.8 (C-4VIII, C-4X), 87.6 (C-4VII), 88.3 (C-4IX), 90.6 (C-2VII), 91.4 (C-2IX), 104.8 (C-1VIII), 104.9 (C-1I), 105.6 (C-1X), 105.8 (C-1VI), 105.87 (C-1II, C-1III, C-1IV, C-1V), 105.94 (C-1II, C-1III, C-1IV, C-1V), 105.97 (2C, C-1II, C-1III, C-1IV, C-1V), 106.2 (C-1IX), 106.9 (C-1VII), 115.6 (OC6H4O (C-3, C-5)), 118.3 (OC6H4O (C-2, C-6)), 128.10 (PhCO (C-3, C-5)), 128.14 (PhCO (C-3, C-5)), 128.16 (PhCO (C-3, C-5)), 128.20 (PhCO (C-3, C-5)), 128.3 (PhCO (C-3, C-5)), 128.4 (PhCO (C-3, C-5)), 128.46 (PhCO (C-3, C-5)), 128.49 (2 × PhCO (C-3, C-5)), 128.50 (PhCO (C-3, C-5)), 128.54 (PhCO (C-3, C-5)), 129.0 (PhCO (C-1)), 129.1 (PhCO (C-1)), 129.15 (PhCO (C-1)), 129.17 (3 × PhCO (C-1)), 129.22 (PhCO (C-1)), 129.28 (PhCO (C-1)), 129.34 (PhCO (C-1)), 129.4 (PhCO (C-1)), 129.76 (PhCO (C-2, C-6)), 129.78 (2 × PhCO (C-2, C-6)), 129.80 (PhCO (C-2, C-6)), 129.81 (PhCO (C-2, C-6)), 129.83 (3 × PhCO (C-2, C-6)), 129.86 (PhCO (C-2, C-6)), 129.92 (PhCO (C-2, C-6)), 130.0 (PhCO (C-2, C-6)), 132.7 (PhCO (C-4)), 132.8 (PhCO (C-4)), 132.9 (PhCO (C-4)), 133.0 (PhCO (C-4)), 133.1 (PhCO (C-4)), 133.2 (PhCO (C-4)), 133.2 (PhCO (C-4)), 133.3 (PhCO (C-4)), 133.4 (PhCO (C-4)), 133.44 (PhCO (C-4)), 133.49 (PhCO (C-4)), 150.7 (OC6H4O (C-1)), 153.6 (OC6H4O (C-4)), 165.01 (PhCO), 165.11 (PhCO), 165.14 (PhCO), 165.18 (PhCO), 165.23 (PhCO), 165.3 (PhCO), 165.37 (PhCO), 165.44 (PhCO), 165.5 (PhCO), 165.57 (PhCO), 165.64 (PhCO); 29Si INEPT NMR (60 MHz, CDCl3): δ 12.27, 12.83, 13.20, 13.44, 13.48, 13.50, 13.53, 13.76, 13.81, 13.85; HRMS (ESI): m/z [M + 2NH4]2+ Calcd for C225H341N5O53Si102+ 2120.5912; Found: 2120.5897.
4-(2-Azidoethoxy)phenyl 2,3,5-tri-O-acetyl-β-d-arabinofuranosyl-(1→2)-3,5-di-O-acetyl-α-d-arabinofuranosyl-(1→3)-[2,3,5-tri-O-acetyl-β-d-arabinofuranosyl-(1→2)-3,5-di-O-acetyl-α-d-arabinofuranosyl-(1→5)]-2-O-acetyl-α-d-arabinofuranosyl-(1→5)-2,3-di-O-acetyl-α-d-arabinofuranosyl-(1→5)-2,3-di-O-benzoyl-α-d-arabinofuranosyl-(1→5)-2,3-di-O-acetyl-α-d-arabinofuranosyl-(1→5)-2,3-di-O-acetyl-α-d-arabinofuranosyl-(1→5)-2,3-di-O-acetyl-α-d-arabinofuranoside (21)
Protected AEP decasaccharide 19 (25 mg, 0.059 mmol) was dissolved in THF (1 mL), then AcOH (4 μL, 0.059 mmol) and 1 M TBAF in THF (178 μL, 0.18 mmol) was added to the reaction mixture at 0 °C. The reaction mixture was stirred at 40 °C for 3 h. Then, the reaction mixture was concentrated under reduced pressure, co-evaporated with toluene (2 × 2 mL), dried in vacuo, and purified by silica gel column chromatography (gradient: MeOH in CH2Cl2, 2%→20%) to give benzoylated polyol of decasaccharide 20 (12 mg) isolated as a mixture with n-Bu4N+ salts according to MS and NMR data. Rf = 0.42 (CH2Cl2–MeOH 4:1); HRMS (ESI): m/z [M + Na]+ Calcd for C135H133N3NaO53 2666.7696; found: 2666.7635. Then, the obtained reaction mixture was dissolved in MeOH (1 mL), followed by the addition of 1 M methanolic MeONa (50 µL, 0.05 mmol). The reaction mixture was kept at ~20 °C for 16 h. Then, the reaction mixture was neutralized with Dowex 50W×8 (H+) ion-exchange resin (the resin was washed with MeOH before addition) and then filtered. The resin was washed with MeOH (50 mL). The filtrate was concentrated under reduced pressure, and the residue was dried in vacuo. The obtained crude product was dissolved in anhydrous Py (1 mL), then Ac2O (1 mL) was added at 0 °C (ice–water bath). The reaction mixture was stirred at 20 °C for 24 h. The reaction was quenched by the addition of MeOH (1 μL) at 0 °C (ice–water bath), then concentrated under reduced pressure, co-evaporated with toluene (5 × 5 mL), and dried in vacuo. The residue was purified by silica gel chromatography (gradient: 5%→70% acetone in petroleum ether) to give acetylated decasaccharide 21 (2 mg, 14% over 3 steps). Rf = 0.20 (light petroleum–acetone 1:1); +53.9 (c 0.15 in CHCl3); 1H NMR (600 MHz, CDCl3): δ 2.08 (s, 9H, 3 × CH3CO), 2.08 (s, 9H, 3 × CH3CO), 2.09 (s, 6H, 2 × CH3CO), 2.09 (s, 3H, CH3CO), 2.10 (s, 3H, CH3CO), 2.11 (s, 3H, CH3CO), 2.11 (s, 15H, 5 × CH3CO), 2.11 (s, 3H, CH3CO), 2.12 (s, 6H, 2 × CH3CO), 2.14 (s, 3H, CH3CO), 2.14 (s, 3H, CH3CO), 3.58 (t, 2H, J 5.0 Hz, CH2N3), 3.70–3.78 (m, 6H, H-5I-VIa), 3.86–3.90 (m, 1H, H-5VIb), 3.92–3.98 (m, 5H, H-5I-Vb), 4.11–4.13 (m, 3H, CH2O), 4.10–4.26 (m, 15H, H-4II-X, H-3VI, H-5VII-Xa, H-5VIIb or H-5IXb), 4.25 (d, 1H, J 2.0 Hz, H-2VII), 4.29 (d, 1H, J 1.7 Hz, H-2IX), 4.32 (dd, 1H, J 11.5 Hz, J 3.9 Hz, H-5VIIb or H-5IXb), 4.34–4.39 (m, 3H, H-4I, H-5VIIIb, H-5Xb), 4.95–5.01 (m, 4H, H-2VIII, H-2X, H-3VII, H-3IX), 5.02 (s, 1H, H-1VII), 5.04–5.07 (m, 3H, H-3V, H-2VI, H-1IX), 5.12–5.18 (m, 12H, H-1II-VI, H-2II-V, H-3II-IV), 5.29 (dd, 1H, J 5.3 Hz, J 1.9 Hz, H-3I), 5.32–5.37 (m, 3H, H-3VIII, H-3X, H-2I), 5.39 (d, 1H, J 4.7 Hz, H-1X), 5.44 (d, 1H, J 4.7 Hz, H-1VIII), 5.61 (s, 1H, H-1I), 6.84–6.88 (m, 2H, OC6H4O (H-3, H-5)), 6.98–7.03 (m, 2H, OC6H4O (H-2, H-6)); 13C NMR (151 MHz, CDCl3): δ 20.3 (CH3CO), 20.4 (CH3CO), 20.6 (CH3CO), 20.67 (CH3CO), 20.69 (CH3CO), 20.73 (CH3CO), 20.75 (CH3CO), 20.80 (CH3CO), 50.2 (CH2N3), 63.4 (C-5VII, C-5IX), 63.7 (C-5VII, C-5IX), 64.2 (C-5VI), 65.0 (C-5I-V, C-5VIII, C-5X), 65.1 (C-5I-V, C-5VIII, C-5X), 65.18 (C-5I-V, C-5VIII, C-5X), 65.24 (3C, C-5I-V, C-5VIII, C-5X), 65.7 (C-5I-V, C-5VIII, C-5X), 67.6 (CH2O), 75.5 (C-3VIII, C-3X), 75.7 (C-3VIII, C-3X), 76.4 (C-3I), 76.6 (C-3II-V, C-3VII, C-3IX, C-2VIII, C-2X), 76.7 (C-3II-V, C-3VII, C-3IX, C-2VIII, C-2X), 76.8 (C-3II-V, C-3VII, C-3IX, C-2VIII, C-2X), 76.9 (C-3II-V, C-3VII, C-3IX, C-2VIII, C-2X), 77.1 (C-3II-V, C-3VII, C-3IX, C-2VIII, C-2X), 77.4 (C-3II-V, C-3VII, C-3IX, C-2VIII, C-2X), 77.7 (C-3II-V, C-3VII, C-3IX, C-2VIII, C-2X), 78.9 (C-4VIII, C-4X), 78.6 (C-4VIII, C-4X), 80.3 (C-4II-VII, C-4IX, C-3VI, C-2II-V), 80.7 (C-4II-VII, C-4IX, C-3VI, C-2II-V), 80.8 (C-4II-VII, C-4IX, C-3VI, C-2II-V), 81.1 (C-4II-VII, C-4IX, C-3VI, C-2II-V), 81.2 (C-4II-VII, C-4IX, C-3VI, C-2II-V), 81.47 (C-4II-VII, C-4IX, C-3VI, C-2II-V), 81.51 (C-4II-VII, C-4IX, C-3VI, C-2II-V), 81.53 (C-4II-VII, C-4IX, C-3VI, C-2II-V), 81.68 (2C, C-4II-VII, C-4IX, C-3VI, C-2II-V), 81.73 (C-4II-VII, C-4IX, C-3VI, C-2II-V), 81.9 (C-4I), 82.0 (C-2I), 82.2 (C-4II-VII, C-4IX, C-3VI, C-2II-V), 83.4 (C-2VI), 83.9 (C-2IX), 84.2 (C-2VII), 99.3 (C-1X), 99.5 (C-1VIII), 104.8 (C-1I), 105.2 (C-1II-VII, C-1IX), 105.35 (3C, C-1II-VII, C-1IX), 105.39 (C-1II-VII, C-1IX), 105.43 (C-1II-VII, C-1IX), 105.7 (C-1II-VII, C-1IX), 115.6 (OC6H4O (C-3, C-5)), 118.3 (OC6H4O (C-2, C-6)), 150.4 (OC6H4O (C-1)), 153.8 (OC6H4O (C-4)), 169.7 (CO), 169.7 (CO), 169.9 (CO), 170.00 (CO), 170.02 (CO), 170.1 (CO), 170.2 (CO), 170.18 (CO), 170.20 (CO), 170.3 (CO), 170.46 (CO), 170.51 (CO), 170.6 (CO); HRMS (ESI): m/z [M + 2NH4]2+ Calcd for C100H139N5O632+ 1208.8908; found: 1208.8948.