Abstract
A rational synthesis of the branched decaarabinofuranoside with 4-(2-azidoethoxy)phenyl aglycone (a Janus aglycone) related to the non-reducing terminal fragments of the arabinogalactan and lipoarabinomannan from Mycobacterium tuberculosis was proposed. Since the most challenging step is the formation of a 1,2-cis glycosidic linkage, we have significantly simplified access to a library of oligoarabinofuranosides derived from Mycobacterium tuberculosis polysaccharides using a silylated Ara-β-(1→2)-Ara disaccharide as the glycosyl donor. The application of a Janus aglycone also allowed us to reduce the number of reaction steps in glycoside synthesis. The obtained arabinans can be useful to further prepare conjugates as antigens for creating tuberculosis screening assays.
1. Introduction
The main causative agent of tuberculosis is Mycobacterium tuberculosis [1,2], and mycobacterial infections have received significant attention due to the increasing number of cases worldwide [3]. The components of the mycobacterial cell wall include arabinogalactan (AG) and lipoarabinomannan (LAM), which have a common motif consisting of α-(1→5), α-(1→3), and β-(1→2) glycosidic bonds. Due to the critical roles of AG and LAM in infectivity and pathogenicity of Mycobacterium tuberculosis, their structural fragments are currently attracting unprecedented attention for the study of pathways of biosynthesis of these glycopolymers [4], the development of diagnostics [5,6] and vaccines [7,8,9], as well as new effective antimicrobial drugs. It was found that neoglycoconjugates derived from synthetic fragments of LAM with bovine serum albumin (BSA), as well as neoglycoconjugates based on the non-reducing terminal branched hexasaccharide fragment (Ara6) with mycobacterial recombinant proteins, are important for the diagnosis of tuberculosis [6,10].
It should be noted that, despite many papers describing the preparation of oligoarabinofuranosides [11,12,13,14,15,16,17,18,19,20,21,22,23,24], the synthesis of the fragments of the mycobacterial cell wall remains a significant challenge. This is especially true for the preparation of oligoarabinofuranosides containing a large number of monosaccharide units (≥10), and the number of such studies remains limited.
It is known that 1,2-trans-α-arabinofuranosides can be easily synthesized using glycosyl donors with a participating acyl group at O-2. In contrast, the preparation of 1,2-cis-β-arabinofuranosides is more challenging, and 2-O-benzyl-containing glycosyl donors are often used for this purpose [13,16,18,20,21,22,24,25,26]. It should be noted that the removal of O-benzyl protective groups is incompatible with the azido group in the aglycone, which makes the synthesis less straightforward and requires additional steps [13].
We have recently showcased the benefits of glycosyl donors with O-2 protected by a triisopropylsilyl (TIPS) nonparticipating group (Figure 1, Block A, R = (t-Bu)2Si<) in the synthesis of the terminal oligoarabinoside fragments of M. tuberculosis such as penta- and hexaarabinofuranoside with α-(1→3)-, α-(1→5)- and β-(1→2)-linkages, containing 2-azidoethyl aglycone (Figure 2). Block A with a different type of remote protection was used by us in the synthesis of diarabinofuranosides Ara-β-(1→2)-Ara with 4-(2-azidoethoxy)phenyl (AEP) and 4-(3-azidopropoxy)phenyl (APP) aglycones. Moreover, the linear α-(1→5)-, β-(1→2)-linked tetrasaccharide AEP glycoside, as well as branched α-(1→3)-, α-(1→5)-, β-(1→2)-linked tetrasaccharide as an APP glycoside, were synthesized from Block A, (R = (t-Bu)2Si<) (Figure 2).
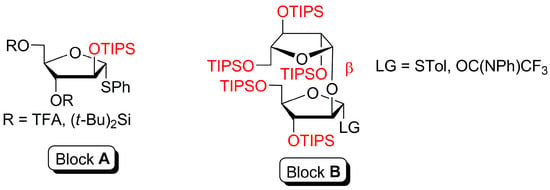
Figure 1.
Glycosyl donors with O-2 protected by triisopropylsilyl (TIPS) nonparticipating group for stereoselective 1,2-cis-glycosylation (Block A) [27]. Diarabinofuranoside Ara-β-(1→2)-Ara glycosyl donors for stereoselective 1,2-trans-glycosylation in the absence of participating group in glycosyl donor (Block B, R = STol [28], R = OC(NPh)CF3 [29]).
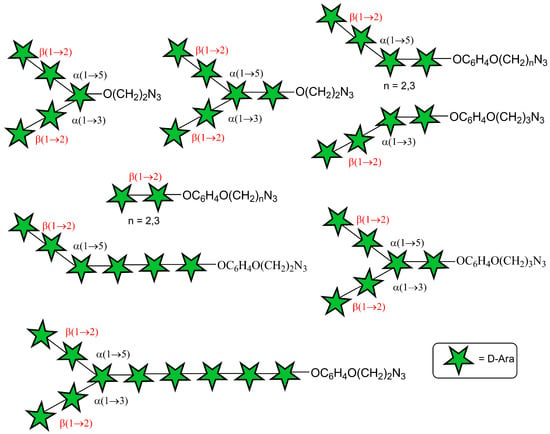
Figure 2.
The use of benzyl-free approach in the synthesis of a library of terminal linear and branched oligoarabinofuranosides related to the fragments of M. tuberculosis LAM with a 2-azidoethyl, 4-(2-azidoethoxy)phenyl (AEP), and 4-(3-azidopropoxy)phenyl (APP) aglycone (current work: synthesis of decaarabinofuranoside with AEP aglycone).
On the other hand, oligoarabinofuranosides with β-(1→2)-linked residues can be assembled using an Ara-β-(1→2)-Ara diarabinofuranoside glycosyl donor (Figure 1, Block B). In this case, the main difficulty is caused by the necessity to create a 1,2-trans-glycosidic linkage in the absence of a 2-O-acyl participating group. We have already demonstrated the successful stereoselective introduction of the silylated Ara-β-(1→2)-Ara disaccharide moiety (Figure 1, Block B), leading to more complex arabinans, including the formation of tetra [28] and branched hexaarabinofuranoside [30] 3 bearing APP aglycone and linear hexaarabinofuranoside with AEP aglycone [29] (Figure 2, Scheme 1).
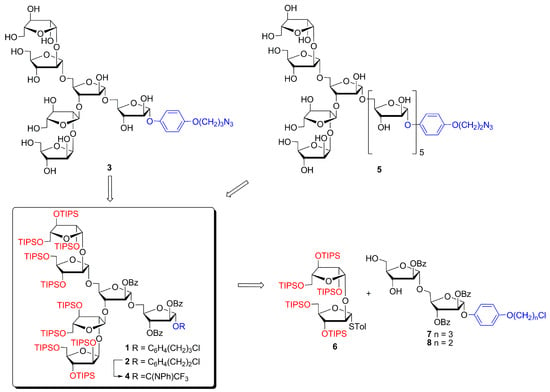
Scheme 1.
Hexaarabinofuranosides 1, 2 with 4-(ω-chloroalkoxy)phenyl aglycone in retrosynthetic analysis of deprotected hexa- (3) [30] and decaarabinofuranoside (5) fragments of M. tuberculosis LAM.
4-(ω-Chloroalkoxy)phenyl aglycones, due to their dual function, may be called Janus aglycones in analogy to the known nanoparticles [31]. 4-(ω-Chloroalkoxy)phenyl aglycones can be converted to 4-(ω-azidoalkoxy)phenyl arabinofuranosides [27]. The azido group in aglycone can be used for further preparation of corresponding glycosides with an amino group in aglycone [10] or it can be modified by using click-chemistry methods [32]. Moreover, both 4-(ω-chloroalkoxy)phenyl and 4-(ω-azidoalkoxy)phenyl glycosides are precursors of various glycosyl donors useful for the synthesis of more complex oligoarabinofuranosides [33].
It should be noted that the synthesis of oligosaccharides demanded selective multi-step protection and deprotection of numerous hydroxy groups and control of regio- and stereoselectivity. In this regard, developing strategies aimed at reducing the number of reaction steps still remains an important task. Since the most problematic glycosylation step is the creation of a 1,2-cis-glycosidic linkage, we significantly simplified the access to the library of oligoarabinofuranosides using Ara-β-(1→2)-Ara disaccharide as a glycosyl donor. Moreover, the application of 4-(ω-chloroalkoxy)phenyl aglycones also allows us to significantly reduce the number of reaction steps in glycoside synthesis.
Earlier, we synthesized branched hexaarabinofuranoside 1 with α-(1→3)-, α-(1→5)-, β-(1→2)-linkages and successfully converted it into deprotected AEP glycoside 3 for further conjugation [30] (Figure 1, Scheme 1). However, the synthesis of hexaarabinofuranoside 1 by glycosylation of benzoylated diol 7 with diarabinofuranoside glycosyl donor 6 under NIS/Et3SiOTf promotion was complicated by the formation of Et3Si-substituted products of monoglycosylation of the glycosyl acceptor at both the primary and the secondary position.
In the current work, we aimed to test an alternative promotion system in the synthesis of branched hexaarabinofuranoside 2 containing the homologous 4-(2-chloroethoxy)phenyl (CEP) aglycone. Additionally, we focused on converting hexaarabinofuranoside 2 to a glycosyl donor (2→4) with subsequent preparation of deprotected decaarabinofuranoside 5 bearing AEP aglycone (Scheme 1).
We hope that the application of our rational strategy based on the use of silylated Ara-β-(1→2)-Ara disaccharide 6 (Block B) will allow us easy access to the library of oligoarabinofuranosides. These oligosaccharides can then be used to create conjugates that act as antigens for tuberculosis screening assays.
2. Results
2.1. Synthesis of Branched α-(1→5)-, α-(1→3)-, β-(1→2)-Linked Hexaarabinofuranoside 2 with CEP Aglycone
Alternative Synthesis of the Known Silylated Ara-β-(1→2)-Ara p-Tolyl Thioglycoside 6 [28]
For the synthesis of hexaarabinofuranoside 2 with a 4-(2-chloroethoxy)phenyl (CEP) aglycone, similar to the preparation of hexaarabinofuranoside [30] 1 with a homologous 4-(3-chloropropoxy)phenyl (CPP) aglycone, we planned to use silylated Ara-β-(1→2)-Ara p-tolyl thioglycoside 6 with five TIPS groups [28]. In this study, we aim to investigate the conversion of silylated disaccharides 10 and 13, which contain acetyl and CPP groups at the anomeric position, to Ara-β-(1→2)-Ara p-tolyl thioglycoside 6 (Scheme 2). For this purpose, the previously obtained hemiacetal 9 [28] with five TIPS groups was acetylated with Ac2O/pyridine (Py), resulting in the single α-anomer of acetate 10. Next, thiolysis of 10 was carried out with p-TolSH/BF3·Et2O at 40 °C for 40 min, followed by silylation of the resulting desilylation products. After silica gel chromatography, disaccharide 6 was isolated in 63% yield.
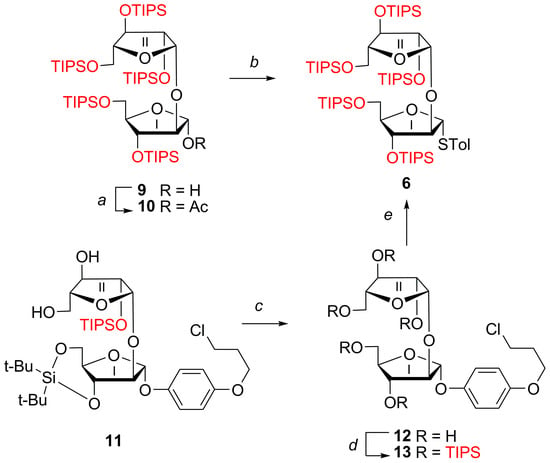
Scheme 2.
Synthesis of glycosyl donor Ara-β-(1→2)-Ara 6. Reagents and conditions: a. Ac2O, Py, 20 °C, 1 h (84%). b. (1) p-TolSH, BF3·Et2O, ClCH2CH2Cl, 40 °C (40 min); (2) TIPSOTf, 2,4,6-collidine, 70 °C, 1.5 h (63%). c. (1) Bu4NF, THF, 0 °C, 3 h; (2) silica gel chromatography (81%). d. TIPSOTf, 2,4,6-collidine, 70 °C, 12 h (80%). e. 13, p-TolSH, BF3·Et2O, −5 °C → 0 °C, 2.5 h (50%).
Alternatively, known disaccharide 11 with CPP aglycone was desilylated with TBAF in THF. The α-linked CPP-disaccharide 12, isolated in 81% yield, contained 3% of substitution product with 4-(3-fluoropropoxy)phenyl aglycone. Moreover, silica gel chromatography failed to remove Bu4N+ (15% according to NMR). The resulting crude pentaol 12 was silylated by TIPSOTf in 2,4,6-collidine to give α-linked CPP-disaccharide 13, also containing the product of substitution of a chlorine atom in the CPP aglycone with fluorine (3%). At the next stage, thiolysis of 13 with TolSH/BF3·Et2O was performed at −5 °C→0 °C for 2.5 h to give the target α-linked thioglycoside 6 isolated in a 50% yield. To the best of our knowledge, the products of the cleavage of the inter-saccharide glycosidic bond were not observed during thiolysis of 10 and 13. In contrast, the use of the acetylated disaccharide Ara-β-(1→2)-Ara with a 4-(ω-chloroalkoxy)phenyl aglycone [28] resulted in significant amounts of products arising from cleavage of this bond.
2.2. Synthesis of Branched Hexaarabinofuranoside 2 with the Use of Silylated Ara-β-(1→2)-Ara p-Tolyl Thioglycoside 6
At the next step, we performed the glycosylation of the diol of disaccharide with CEP aglycone 8 [28] with the Ara-β-(1→2)-Ara glycosyl donor 6 under AgOTf/NIS promotion. However, instead of the desired hexaarabinofuranoside 2, the formation of α-(1→5)-linked tetraarabinofuranoside 14 was observed.
The anomeric signals of tetraarabinofuranoside 14 were found at δH 5.06 (s, 1H, H-1III), 5.20 (d, 1H, J 2.6 Hz, H-1IV), 5.37 (s, 1H, H-1II), and 5.83 (s, 1H, H-1I) in the 1H NMR spectrum, which correlated with signals at δC 104.8 (C-1I), 105.0 (C-1IV), 105.4 (C-1II), and 106.3 (C-1III) in the 13C NMR spectrum. An additional confirmation of the structure of tetraarabinofuranoside 14 and the formation of a new glycosidic linkage at the primary position followed from the fact that the 1H–13C HMBC spectrum showed a correlation between the signals of H-1II at δH 5.37 ppm (s, 1H) and C-5I at δC 66.0 ppm, a correlation between the signals of H-1III at δH 5.06 ppm (s, 1H) and C-5II at δC 64.4 ppm, and a correlation between the signals of HIV at δH 5.20 (d, 1H, J 2.6 Hz) and C-2III at δC 90.8 ppm.
Then, we introduced the second disaccharide Ara-β-(1→2)-Ara fragment at C-3II of disaccharide 8 under TfOH/NIS promotion to form the key hexaarabinofuranoside 2 in 90% yield.
Moreover, we successfully obtained hexaarabinofuranoside 2 in a single step by bis-glycosylation of the diol 8 with p-tolyl thioglycoside Ara-β-(1→2)-Ara 6 under TfOH/NIS promotion in very high yield (97%) (Scheme 3).
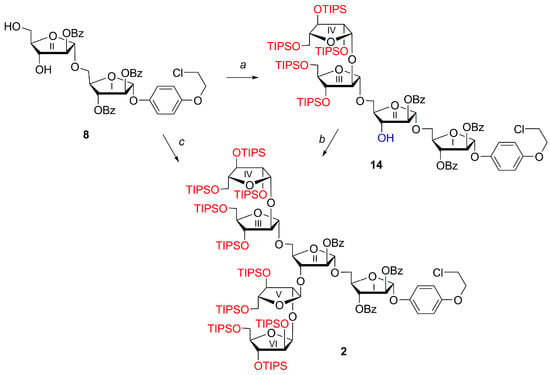
Scheme 3.
Synthesis of hexaarabinofuranoside 2. Reagents and conditions: a. 6, AgOTf, NIS, MS 4 Å, CH2Cl2, −60 °C → −20 °C, 1 h (80%). b. 6, TfOH, NIS, MS 4 Å, CH2Cl2, −60 °C → −30 °C, 0.5 h (90%). c. 6, TfOH, NIS, MS 4 Å, CH2Cl2, −30 °C, 0.5 h (97%).
Comparison of the 1H and 13C NMR spectra for hexaarabinofuranosides with CPP and CEP aglycones (1 and 2, respectively), as expected, revealed many common features.
The signals of ten TIPS groups in the 29Si NMR spectrum for hexaarabinofuranosides 1, 2 were observed in a similar range from δSi 12.3 to 13.9, and the signals of the corresponding carbon atoms of the isopropyl groups ((CH3)2CH)3Si) in the 13C NMR spectrum were observed in the range from δC 11.9 to 18.2. The signals of the isopropyl carbon atoms correlated with the 1H NMR proton signals observed at δH 0.86–1.15 (m, 210H, 10 × ((CH3)2CH)3Si).
The signals of the α-anomeric protons of the monosaccharide residues of hexaarabinofuranosides 2, 1 are found at δH 5.10 (s, 1H, H-1III), 5.23 (s, 1H, H-1V), 5.27 (s, 1H, H-1II), 5.79 (s, 1H, H-1I), and 5.11 (s, 1H, H-1III), 5.23 (s, 1H, H-1V), 5.27 (s, 1H, H-1II), 5.80 (s, 1H, H-1I), respectively. While the following signals for the two β-anomeric protons of the monosaccharide residues of hexaarabinofuranosides 2, 1 are present at δH 5.17 (d, 1H, J 2.5 Hz, H-1VI), 5.25 (d, 1H, J 2.8 Hz, H-1IV), and 5.17 (d, 1H, J 2.7 Hz, H-1VI), 5.25 (d, 1H, J 2.8 Hz, H-1IV), respectively.
The signals of the anomeric carbon atoms for all monosaccharide residues for hexaarabinofuranosides 2, 1 in the 13C NMR spectra appeared at δC 104.7 (C-1IV), 104.8 (C-1I), 105.4 (C-1VI), 106.0 (C-1II), 106.3 (C-1V), 106.9 (C-1III), and 104.69 (C-1IV), 104.71 (C-1I), 105.4 (C-1VI), 105.0 (C-1II), 106.3 (C-1V), 106.8 (C-1III), respectively. It is noteworthy that the low-field positions of the signals of C-1IV and C-1VI are untypical for β-arabinofuranosides, presumably due to the presence of bulky TIPS groups.
Moreover, similar correlations, confirming the formation of the glycosidic bonds between the corresponding monosaccharide residues in hexaarabinofuranosides 2, 1 were observed in a 1H–13C HMBC spectrum: the correlation of the H-1II proton signal at δH 5.27 (s, 1H) with the C-5I carbon atom signal at δC 65.3; the correlation of the H-1III proton signal at δH 5.1 (s, 1H) with the C-5II carbon atom signal at δC 65.14; the correlation of the H-1IV proton signal at δH 5.25 (d, 1H, J 2.8 Hz) with the C-2III carbon atom signal at δC 90.5; and the correlation of the H-1VI proton signal at δH 5.17 (d, 1H, J 2.5 Hz) with the C-2V carbon atom signal at δC 91.3.
The H-2III signal at δH 4.14 (d, 1H, J 0.8 Hz) correlates with the C-1IV signal at δC 104.7; the H-2III signal at 4.14 (d, 1H, J 1.1 Hz, H-2III) correlates with the C-1IV signal at δC 104.7 for hexaarabinofuranosides 2 and 1, respectively. Correlation of the H-2V signal at 4.08 (d, 1H, J 1.2 Hz) with the C-1VI signal at δC 105.4 and correlation of the H-2V signal at 4.09 (d, 1H, J 1.3 Hz, H-2V) with the C-1VI signal at δC 105.4 were also found for hexaarabinofuranosides 2 and 1, respectively.
2.3. Synthesis of Branched α-(1→5)-, α-(1→3)-, β-(1→2)-Linked Decaarabinofuranoside 5 with 4-(2-Azidoethoxy)phenyl Aglycone
2.3.1. Conversion of the Branched α-(1→5)-, α-(1→3)-, β-(1→2)-Linked Hexaarabinofuranoside 2 to Glycosyl Donor 4
In the obtained hexaarabinofuranoside 2, CEP aglycone was cleaved under oxidative conditions ((NH4)2Ce(NO3)6 in aqueous MeCN—CH2Cl2). After purification by silica gel column chromatography, hemiacetal 15 (70%) as a mixture of anomers (α:β = 1:0.45 according to NMR data), contaminated with the product 16 of migration of the benzoyl group from O-2 to O-1, was obtained (Scheme 4). The ratio of the compounds in the mixture (15α:15β:16 = 1:0.45:0.28 according to NMR data) was determined by integration of the signals of corresponding anomeric protons of the residues I in the 1H NMR spectrum: 5.62 (s, 1H, H-1Iα) for 15α, 5.72 (t, 1H, J 5.1 Hz, H-1Iβ) for 15β, and 6.60 (d, 1H, J 4.5 Hz, H-1I) for 16. The formation of the β-linked product 16 of the migration of the benzoyl group followed from the low-field position of the anomeric proton δH 6.60 (d, 1H, J 4.5 Hz, H-1I) in the 1H NMR spectrum, which correlated with the signal of anomeric carbon atom of monosaccharide residue δC 97.04 (C-1I) in the 13C NMR spectrum.
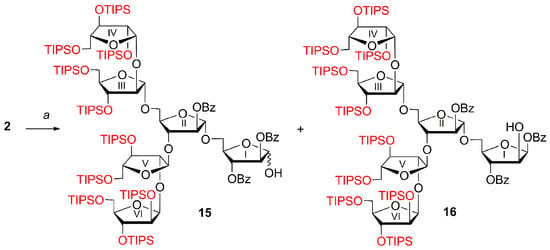
Scheme 4.
The formation of hemiacetal 15 of hexaarabinofuranose contaminated with the product 16 of benzoyl group migration from O-2 to O-1. Reagents and conditions: a. (NH4)2Ce(NO3)6, MeCN—CH2Cl2—H2O (2:1:0.1), 0 °C, 4 h (70%, 15α:15β:16 = 1:0.45:0.28 according to NMR data).
The mixture of hemiacetal 15 and β-linked benzoate 16 was treated with CF3C(NPh)Cl in the presence of Cs2CO3 (Scheme 5), then subjected to gel chromatography on Bio-Beads S-X1 in toluene. N-Phenyltrifluoroacetimidate 4 was obtained as a mixture of anomers (α:β ~ 1:1.3 according to NMR data) in high yield (96%). It should be noted that the characteristic signal of β-linked benzoate 16 in the low-field position δH 6.60 (d, 1H, J 4.5 Hz, H-1I minor) was absent in the 1H NMR spectrum. On the contrary, characteristic signals related to the N-phenyltrifluoroacetimidoyl group were present. The ratio of α and β isomers of N-phenyltrifluoroacetimidate 4 was determined by integrating the following signals in the 1H NMR spectrum: 6.51–6.57 (m, 2H, PhN (H-2, H-6)β) and 6.85–6.91 (m, 2H, PhN (H-2, H-6)α). Probably, a reverse migration of the benzoyl group from O-1 to O-2 for benzoate 16 took place under the basic conditions used for the imidate formation.
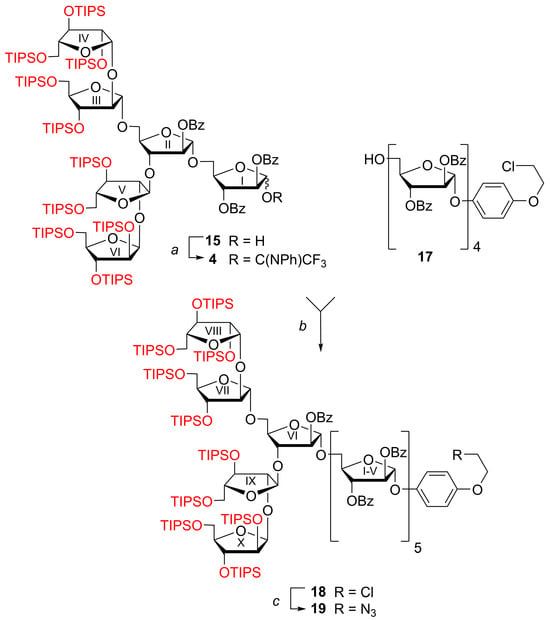
Scheme 5.
Synthesis of protected decaarabinofuranoside 19. Reagents and conditions: a. (1) CF3C(NPh)Cl, Cs2CO3, CH2Cl2, 20 °C, 3 h, (2) gel chromatography on Bio-Beads S-X1 in toluene, 4 (95 %). b. (1) TfOH, MS 4 Å, CH2Cl2, −40 °C, 1 h; (2) gel chromatography on Bio-Beads S-X1 in toluene 18 (72%; 51% over 3 steps starting from 2). c. NaN3, 18-crown-6, DMF, 80 °C, 16 h, 19 (84%). TIPS = ((CH3)2CH)3Si.
2.3.2. Synthesis of the Protected Decaarabinofuranoside 18 with CEP Aglycone
The glycosylating ability of the obtained N-phenyltrifluoroacetimidate of hexaarabinofuranoside 4 was tested in the synthesis of branched α-(1→5)-, α-(1→3)-, β-(1→2)-linked decaarabinofuranoside 18 with cleavable CEP aglycone. To this end, benzoylated tetraarabinofuranoside α-(1→5)-linked glycosyl acceptor 17 [33] was glycosylated by N-phenyltrifluoroacetimidate 4 promoted by TfOH (Scheme 5). After gel chromatography on Bio-Beads S-X1 in toluene, decaarabinofuranoside 18 was isolated in 72% yield (51% over 3 steps starting from 2). It should be noted that the use of glycosyl donor 4 generated from 2 and having a 2-O-acyl participating group ensures the creation of 1,2-trans-(α) glycosidic linkage. The signals of ten TIPS groups were observed for decaarabinofuranoside in the 29Si NMR spectrum. It is important to note that no desilylation products were found despite the fact that TfOH was used as the promotor.
The anomeric configuration of decaarabinofuranoside 18 was confirmed by NMR spectroscopy. For α-linked Ara residues, the following signals of the anomeric protons were observed: δH 5.10 (s, 1H, H-1VII), 5.23 (s, 1H, H-1IX), 5.29 (s, 1H, H-1VI), 5.37 (s, 2H, 2 × H-1), 5.38–5.41 (m, 3H, H-2VI, 2 × H-1), 5.82 (s, 1H, H-1I). The following signals for two β-anomeric protons of the monosaccharide residues of decaarabinofuranoside 18 are present: δH 5.15 (d, 1H, J 2.6 Hz, H-1X) and δH 5.24 (d, 1H, J 2.8 Hz, H-1VIII). The signals of the anomeric carbon atoms for all monosaccharide residues for decaarabinofuranoside 18 as in case of branched hexaarabinofuranosides 1 [30], 2 and α-(1→5)-, β-(1→2)-linked tetraarabinofuranoside [28] resonated in a low-field region at δC 104.8 (C-1VIII), 104.9 (C-1I), 105.5 (C-1X), 105.85 (C-1VI), 105.89 (C-1), 106.0 (3C, C-1II, C-1III, C-1IV), 106.2 (C-1IX), and 106.9 (C-1VII) in the 13C NMR spectra. Moreover, correlations confirming the presence of glycosidic bonds between the corresponding monosaccharide residues of decaarabinofuranoside 18 were found in its 1H–13C HMBC spectrum: the H-1VI signal at δH 5.29 (s, 1H) correlates with the C-5V signal at δC 65.3; the H-1VII signal at δH 5.10 (s, 1H) correlates with the C-5VI signal at δC 64.8; the H-1VIII signal at δH 5.24 (d, 1H, J 2.8 Hz, H-1VIII) correlates with the C-2VII signal at δC 90.6; the H-1IX signal at δH 5.23 (s, 1H, H-1IX) correlates with the C-3VI signal at δC 81.0 (C-3VI); and the H-1X signal at δH 5.15 (d, 1H, J 2.6 Hz, H-1X) correlates with the C-2IX signal at δC 91.4 (C-2IX).
2.3.3. Synthesis of the Branched α-(1→5)-, α-(1→3)-, β-(1→2)-Linked Decaarabinofuranoside 5 with AEP Aglycone
The chlorine atom in the aglycone of the resulting decaarabinofuranoside 18 was replaced with an azido group (NaN3, DMF, 18-crown-6) to give the corresponding AEP glycoside 19 in 84% yield (Scheme 5). Then all TIPS groups in azide 19 were removed by treatment with TBAF in THF in the presence of AcOH at 40 °C to give partially protected decaarabinofuranoside 20 isolated in a mixture with n-Bu4N+ salts after silica gel chromatography, which was treated with MeONa in MeOH with subsequent acetylation (Scheme 6). After purification by silica gel chromatography, acetylated decasaccharide 21 (14% over 3 steps) was obtained. The overall low deprotection yield can be attributed to the difficulty of the removal of the numerous (ten) triisopropylsilyl groups, followed by purification from by-products.
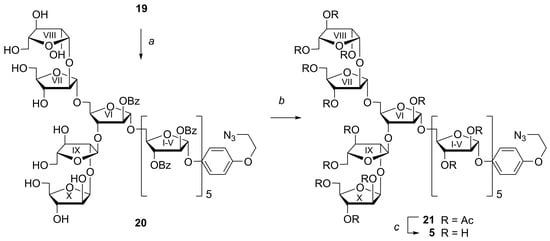
Scheme 6.
Synthesis of protected decaarabinofuranoside 5 with AEP aglycone. a. n-Bu4NF, AcOH, THF, 40 °C, 3 h. b. (1) MeONa, MeOH, 20 °C, 16 h; (2) Ac2O, pyridine, 0 °C, 20 h (14% over 3 steps including step a). c. MeONa, MeOH, 20 °C, 24 h (85%).
The anomeric configuration of acetylated decasaccharide 21 was verified by NMR spectroscopy. As expected, the signals of the two β-anomeric protons were found at δH 5.39 (d, 1H, J 4.7 Hz, H-1X) and δH 5.44 (d, 1H, J 4.7 Hz, H-1VIII), which correlated with the signals of the anomeric carbon atoms in the characteristic regions at δC 99.29 (C-1X) and 99.50 (C-1VIII), respectively. The obtained decasaccharide 21 was deacetylated by treatment with MeONa in MeOH to give the target deprotected decasaccharide 5 in 85% yield.
The presence of two β-anomeric AraVIII and AraX residues of the target deprotected decasaccharide 5 was confirmed by the signals of the C-1VIII and C-1X carbon atoms at δC 102.5, 102.6 (C-1VIII, C-1X). In the HSQC spectrum, the C-1VIII and C-1X carbon atom signals correlated with the proton signals at δH 5.02–5.06 (m, 2H, H-1VIII, H-1X). The signals of the eight α-linked arabinofuranose moieties were observed in the characteristic low-field regions at δC 107.1 (C-1IX), 107.4 (C-1VII), 108.7 (C-1I), 109.58, 109.63, 109.70 (2C), and 109.74 (C-1II-VI). The upfield position of C-2VIII and C-2X at δC 79.19 additionally confirms the β-configuration of the residues VIII and X. On the contrary, the low-field position of C-2VII, C-2IX at δC 89.3 (C-2VII), 89.6 (C-2IX), and C-3VI at δC 84.6, respectively, indicated that those residues are glycosylated.
3. Discussion
We suggested an alternative synthesis of diarabinofuranoside 6 and found that during the thiolysis of silylated disaccharides Ara-β-(1→2)-Ara (10 and 13) containing acetyl or CPP groups at the anomeric position, no products of cleavage of the inter-saccharide glycosidic bond were observed.
It is important to note that the previously proposed synthesis of 6 included the reaction of acetylated Ara-β-(1→2)-Ara bearing 4-(ω-chloroalkoxy)phenyl aglycone with TolSH/BF3·Et2O, which required a higher temperature and a longer time (20 °C for 16 h, then 60 °C for 2 h). Moreover, thiolysis of acetylated Ara-β-(1→2)-Ara was complicated by the formation of monosaccharides [28]. We can conclude that the protective groups in Ara-β-(1→2)-Ara have a significant effect on the course of the thiolysis. Thus, the presence of electron-donating TIPS groups is favorable for the reaction.
We tested various promotor systems, such as NIS/TfOH, NIS/TESOTf [30], and NIS/AgOTf to activate silylated Ara-β-(1→2)-Ara p-tolyl thioglycoside 6 in the synthesis of branched hexasaccharide 2. No glycosylation of the secondary 3′-OH of diarabinofuranoside glycosyl acceptor 14 was observed under mild activation conditions (NIS/AgOTf), and only α-(1→5)-linked tetraarabinofuranoside 14 was obtained. On the contrary, the use of a stronger activation system (NIS/TfOH) made possible stereospecific glycosylation of both primary (5′-OH) and secondary (3′-OH) hydroxy groups and afforded the desired hexaarabinofuranoside 2 in exceptionally high yield. We can conclude that the use of (NIS/TfOH) is more preferable for bis-glycosylation.
It should be noted that, like in the case of hexaarabinofuranoside 1 with the CPP aglycone, an exclusively α-configuration of the glycosidic linkage was observed in hexaarabinofuranoside 2 bearing the CEP aglycone. On the contrary, as we showed earlier, in the case of the use of Ara-β-(1→2)-Ara disaccharide glycosyl donor, which contains only O-benzoyl substituents, the absence of stereocontrol (α:β = 1:2) was observed [28].
TfOH also effectively facilitated the reaction between N-phenyltrifluoroacetimidate of hexaarabinofuranoside 4 and α-(1→5)-linked benzoylated tetrasaccharide glycosyl acceptor 17, resulting in the formation of decaarabinofuranoside 18. As we mentioned above, the nature of the protective group in the glycosyl donor is very essential in the outcome of glycosylation. Recently, we observed an unusual oligomerization of arabinofuranosides promoted by TfOH during the glycosylation of the primary hydroxyl group of the same α-(1→5)-linked tetraarabinofuranoside 17, which bears a 4-(2-chloroethoxy)phenyl aglycone by benzoylated N-phenyltrifluoroacetimidate [33]. To explain these unusual results, we suggested that silylated disaccharide Ara-β-(1→2)-Ara glycosyl donor 6 has superior reactivity compared to “disarmed” benzoylated glycosyl donors. Due to the higher reactivity of the former, side reactions are suppressed, and we did not observe the formation of the oligomerization products.
As the most challenging step in glycosylation is creating a 1,2-cis glycosidic linkage, we have significantly simplified access to a library of oligoarabinofuranosides from Mycobacterium tuberculosis using silylated Ara-β-(1→2)-Ara disaccharide. In addition, the use of a 4-(2-chloroethoxy)phenyl aglycone allowed us to reduce the number of reaction steps in glycosidic synthesis.
4. Materials and Methods
General methods. All reactions sensitive to air and/or moisture were carried out under an argon atmosphere. The reactions were performed with the use of commercial reagents (Aldrich (St. Louis, MO, USA), Fluka (Waltham, MA, USA), Acros Organics (Geel, Belgium)). Anhydrous solvents were purified and dried (where appropriate) according to standard procedures [34]. Dichloromethane was distilled over P2O5 and then over CaH2 and stored over 4 Å molecular sieves (MS 4 Å). Powdered MS 4 Å and 3 Å molecular sieves (MS 4 Å and MS 3 Å, respectively) (Fluka, Seelze, Germany) were activated before glycosylation reactions by heating at 220 °C in high vacuum (0.2 mbar) for 6 h. Column chromatography was performed on silica gel 60 (40–63 μm, Merck, Darmstadt, Germany) using a Büchi C-815 Flash chromatograph (Büchi Labotechnic AG, Flawil, Switzerland). Thin-layer chromatography was carried out on plates with silica gel 60 on aluminum foil (Merck). Spots of compounds were visualized under UV light (254 nm) and by heating the plates (at ca. 150 °C) after immersion in a 1:10 (v/v) mixture of 85% aqueous H3PO4 and 95% EtOH. Gel permeation chromatography was performed on a 400 × 20 mm column packed with Bio-Beads S-X3 (200–400 mesh, Bio-Rad, Hercules, CA, USA) or on a 450 × 30 mm column packed with Bio-Beads S-X1 (200–400 mesh). A procedure for “co-evaporation” with water (or toluene) involved (multiple) addition of water (or toluene) and evaporation of volatiles on a rotary evaporator. Amberlite MB-3 mixed-bed ion-exchange resin (Fluka) (1 mL) was washed with H2O (10 mL), 50% EtOH (5 mL), EtOH (5 mL), 50% EtOH (5 mL), and H2O (10 mL) before use. 1H, 13C, 19F, and 29Si NMR spectra were recorded on a Bruker AVANCE NEO 300 spectrometer (300.23, 75.50, 282.47, and 59.65 MHz for 1H, 13C, 19F, and 29Si, respectively) or on a Bruker AVANCE 600 spectrometer (Billerica, MA, USA, 600.13 and 150.92 MHz for 1H and 13C, respectively). The 1H NMR chemical shifts are referred to the residual signal of CHCl3 (δH 7.27 ppm), CHD2OD (δH 3.31 ppm) for solutions in CD3OD, and HDO (δH 4.79 ppm) for solutions in D2O. The 13C NMR shifts to the central line of the CDCl3 signal (δC 77.00 ppm), CD3OD signal (δC 49.00 ppm), or the signal of external 1,4-dioxane in D2O (δC 67.40 ppm). The 19F chemical shifts are given relative to the signal of external CFCl3 (δF 0.00 ppm). The 29Si chemical shifts are given relative to the signal of external Me4Si (δSi 0.00 ppm). Assignments of the signals in the NMR spectra were performed using 1H–1H and 1H–13C 2D-spectroscopy (COSY, HSQC, HMBC) and DEPT-135 experiments. Position of silyl groups was determined from 1H–29Si HMBC experiments. High-resolution mass spectra (HRMS, electrospray ionization (ESI)) were recorded in a positive ion mode on Bruker micrOTOF II or maXis mass spectrometers for 2 × 10−5 M solutions in MeCN. Optical rotations were measured using a JASCO P-2000 automatic digital polarimeter (Hachioji, Japan).
4-(2-Chloroethoxy)phenyl 2,3,5-tris-O-(triisopropylsilyl)-β-d-arabinofuranosyl-(1→2)-3,5-bis-O-(triisopropylsilyl)-α-d-arabinofuranosyl-(1→3)-[2,3,5-tris-O-(triisopropylsilyl)-β-d-arabinofuranosyl-(1→2)-3,5-bis-O-(triisopropylsilyl)-α-d-arabinofuranosyl-(1→5)]-2-O-benzoyl-α-d-arabinofuranosyl-(1→5)-2,3-di-O-benzoyl-α-d-arabinofuranoside (2)
(1) A mixture of disaccharide thioglycoside 6 (37 mg, 0.31 mmol) and tetrasaccharide alcohol 14 (19 mg, 0.25 mmol) was dried in vacuo for 2 h, then anhydrous CH2Cl2 (2 mL) was added under argon. Freshly activated powdered MS 4 Å (200 mg) (100 mg per 1 mL of solvent) was added under argon to the resulting solution. The suspension was stirred under argon at ~22 °C for 1 h, cooled to −60 °C, then NIS (7 mg, 0.31 mmol) and TfOH (1 μL) were added. Then, the temperature was allowed to rise slowly until −30 °C during 0.5 h and was kept at −30 °C for 10 min. Then the reaction was quenched by the addition of Py (50 μL), diluted with CH2Cl2 (15 mL), and filtered through a Celite pad. The solids were washed with CH2Cl2 (5 × 10 mL), and the filtrate was washed with a mixture of satd aq Na2S2O3 (50 mL) and satd aq NaHCO3 (50 mL). The aqueous layer was extracted with CH2Cl2 (2 × 5 mL). The combined organic extracts were filtered through a cotton wool plug, concentrated and dried in vacuo, then dissolved in toluene (2 mL) and subjected to chromatography on Bio-Beads S-X1 in toluene to give α-linked hexasaccharide 2 (52 mg, 90%); Rf = 0.50 (light petroleum–EtOAc 10:1); +31.0 (c 1.0 in CHCl3); 1H NMR (600 MHz, CDCl3): δ 0.95–1.15 (m, 210H, 10 × ((CH3)2CH)3Si), 3.63 (dd, 1H, J 9.5 Hz, J 4.5 Hz, H-5IVa or H-5VIa), 3.66 (dd, 1H, J 10.5 Hz, J 5.4 Hz, H-5Va), 3.71 (dd, 1H, J 9.5 Hz, J 4.5 Hz, H-5IVa or H-5VIa), 3.78 (t, 2H, J 6.0 Hz, CH2Cl), 3.73–3.99 (m, 11H, 8 × H-5, H-4IV, H-4VI, H-4V), 4.03–4.05 (m, 2H, H-2IV, H-2VI), 4.09 (d, 1H, J 1.3 Hz, H-2V), 4.11 (td, 1H, J 5.9 Hz, J 3.9 Hz, H-4III), 4.14 (d, 1H, J 1.1 Hz, H-2III), 4.15–4.19 (m, 1H, H-5Ib), 4.17 (t, 2H, J 6.0 Hz, CH2O), 4.22 (dd, 1H, J 7.0 Hz, J 2.1 Hz, H-3II), 4.30 (d, 1H, J 0.9 Hz, H-3VI), 4.29–4.32 (m, 1H, H-4II), 4.33 (d, 1H, J 0.8 Hz, H-3IV), 4.34 (d, 1H, J 4.0 Hz, H-3III), 4.45 (dd, 1H, J 3.7 Hz, J 1.4 Hz, H-3V), 4.60 (q, 1H, J 4.2 Hz, H-4I), 5.11 (s, 1H, H-1III), 5.17 (d, 1H, J 2.7 Hz, H-1VI), 5.23 (s, 1H, H-1V), 5.25 (d, 1H, J 2.8 Hz, H-1IV), 5.27 (s, 1H, H-1II), 5.41 (d, 1H, J 2.1 Hz, H-2II), 5.65 (dd, 1H, J 4.5 Hz, J 2.0 Hz, H-3I), 5.71 (d, 1H, J 1.6 Hz, H-2I), 5.80 (s, 1H, H-1I), 6.82–6.87 (m, 2H, OC6H4O (H-3, H-5)), 7.03–7.09 (m, 2H, OC6H4O (H-2, H-6)), 7.38–7.44 (m, 2H, 2II-O-PhCO (H-3, H-5)), 7.44–7.62 (m, 7H, PhCO), 8.01–8.05 (m, 2H, 2II-O-PhCO (H-2, H-6)), 8.10–8.13 (m, 2H, PhCO (H-2, H-6)), 8.13–8.16 (m, 2H, PhCO (H-2, H-6)); 13C NMR (151 MHz, CDCl3): δ 11.93 (((CH3)2CH)3Si), 11.96 (((CH3)2CH)3Si), 11.97 (((CH3)2CH)3Si), 12.01 (((CH3)2CH)3Si), 12.18 (((CH3)2CH)3Si), 12.23 (((CH3)2CH)3Si), 12.24 (2 × ((CH3)2CH)3Si), 12.3 (((CH3)2CH)3Si), 12.4 (((CH3)2CH)3Si), 17.9 (2 × ((CH3)2CH)3Si), 17.95 (2 × ((CH3)2CH)3Si), 17.96 (2 × ((CH3)2CH)3Si), 18.02 (2 × ((CH3)2CH)3Si), 18.03 (((CH3)2CH)3Si), 18.05 (((CH3)2CH)3Si), 18.08 (((CH3)2CH)3Si), 18.09 (2 × ((CH3)2CH)3Si), 18.11 (((CH3)2CH)3Si), 18.12 (4 × ((CH3)2CH)3Si), 18.17 (((CH3)2CH)3Si), 18.19 (((CH3)2CH)3Si), 41.9 (CH2Cl), 63.7 (C-5IV, C-5VI), 63.8 (C-5IV, C-5VI), 64.1 (C-5V), 64.7 (C-5III), 65.1 (C-5II), 65.3 (C-5I), 68.7 (CH2O), 77.5 (C-3I), 77.6 (C-3V), 78.1 (C-2IV or C-2VI), 78.2 (C-3VI), 78.3 (C-2IV or C-2VI), 78.48 (C-3III, C-3IV), 78.51 (C-3III, C-3IV), 81.0 (C-3II), 81.9 (C-2I), 82.2 (C-4II), 82.9 (C-4I), 84.0 (C-2II), 85.68 (C-4IV, C-4VI), 85.74 (C-4IV, C-4VI), 87.6 (C-4III), 88.2 (C-4V), 90.5 (C-2III), 91.3 (C-2V), 104.69 (C-1IV), 104.71 (C-1I), 105.7 (C-1VI), 106.0 (C-1II), 106.3 (C-1V), 106.8 (C-1III), 115.8 (OC6H4O (C-3, C-5)), 118.3 (OC6H4O (C-2, C-6)), 128.2 (2II-O-PhCO (C-3, C-5)), 128.4 (PhCO (C-3, C-5)), 128.6 (PhCO (C-3, C-5)), 129.1 (PhCO (C-1)), 129.5 (PhCO (C-1)), 129.81 (PhCO (C-1)), 129.84 (2II-O-PhCO (C-2, C-6)), 129.95 (PhCO (C-2, C-6)), 130.03 (PhCO (C-2, C-6)), 132.8 (2II-O-PhCO (C-4)), 133.2 (PhCO (C-4)), 133.4 (PhCO (C-4)), 151.0 (OC6H4O (C-1)), 153.5 (OC6H4O (C-4)), 165.1 (2II-O-PhCO), 165.5 (PhCO), 165.6 (PhCO); 29Si INEPT NMR (60 MHz, CDCl3): δ 12.36, 12.93, 13.26, 13.48, 13.51, 13.53, 13.56, 13.79, 13.83, 13.87; HRMS (ESI): m/z [M + 2NH4]2+ Calcd for C149H277ClN2O29Si102+ 2855.7294; Found: 2855.7251;
(2) A mixture of disaccharide thioglycoside 6 (127 mg, 0.11 mmol) and disaccharide 8 (22 mg, 0.03 mmol) was dried in vacuo for 2 h, then anhydrous CH2Cl2 (3 mL) was added under argon. Freshly activated powdered MS 4 Å (300 mg) (100 mg per 1 mL of solvent) was added under argon to the resulting solution. The suspension was stirred under argon at ~22 °C for 1 h, cooled to −60 °C, then NIS (7 mg, 0.31 mmol) and TfOH (1 μL) were added. Then, the temperature was allowed to rise slowly to −30 °C during 0.5 h and was kept at −30 °C for 10 min. Then the reaction was quenched by the addition of Py (50 μL), diluted with CH2Cl2 (15 mL), and filtered through a Celite pad. The solids were washed with CH2Cl2 (5 × 10 mL), and the filtrate was washed with a mixture of satd aq Na2S2O3 (50 mL) and satd aq NaHCO3 (50 mL). The aqueous layer was extracted with CH2Cl2 (2×5 mL). The combined organic extracts were filtered through a cotton wool plug, concentrated and dried in vacuo, then dissolved in toluene (2 mL) and subjected to chromatography on Bio-Beads S-X1 in toluene to give hexasaccharide 2 (80 mg, 97%).
2,3,5-Tris-O-(triisopropylsilyl)-β-d-arabinofuranosyl-(1→2)-3,5-bis-O-(triisopropylsilyl)-α-d-arabinofuranosyl-(1→3)-[2,3,5-tris-O-(triisopropylsilyl)-β-d-arabinofuranosyl-(1→2)-3,5-bis-O-(triisopropylsilyl)-α-d-arabinofuranosyl-(1→5)]-2-O-benzoyl-α-d-arabinofuranosyl-(1→5)-2,3-di-O-benzoyl-d-arabinofuranosyl N-phenyltrifluoroacetimidate (4)
To a solution of hemiacetal of hexasaccharide 15 (31 mg, 0.012 mmol) in CH2Cl2 (1 mL), Cs2CO3 (30 mg, 0.093 mmol) and CF3C(NPh)Cl [35] (4 μL, 0.023 mmol) were added at 0 °C. The reaction mixture was stirred at 20 °C for 3h. The reaction mixture was diluted with CH2Cl2 (20 mL) and then filtered through the cotton wool plug. The filtrate concentrated under reduced pressure, and the residue was dried in vacuo and subjected to gel chromatography on Bio-Beads S-X1 in toluene to give imidate 4 as a mixture of anomers (α:β = ~1:1.3 according to NMR), (32 mg, 96%). Rf = 0.76 (light petroleum–EtOAc, 8.5:1.5); selected signals: 1H NMR (300 MHz, CDCl3): δ 0.78–1.35 (m, 483H), 3.61–4.01 (m, 38H), 4.01–4.19 (m, 16H), 4.19–4.39 (m, 13H), 4.43–4.48 (m, 2H), 4.51–4.59 (m, 1H), 4.61–4.71 (m, 1H), 5.08 (s, 1H), 5.10 (s, 1H), 5.16–5.28 (m, 11H), 5.36 (d, 1H, J 1.9 Hz), 5.62 (d, 1H, J 3.7 Hz), 5.73 (s, 1H), 5.84–5.97 (m, 2H), 6.51–6.57 (m, 2H, PhN (H-2, H-6)β), 6.79 (s, 1H, H-1), 6.85–6.91 (m, 2H, PhN (H-2, H-6)α), 6.98–7.10 (m, 2H, PhN (H-4)), 7.12–7.20 (m, 2H, PhN (H-3, H-5)), 7.22–7.31 (m, 5H, PhCO (H-3, H-5)), 7.36–7.63 (m, 25H, PhCO (H-3, H-5); PhCO (H-4), 7.97–8.17 (m, 16H, PhCO (H-2, H-6)); 13C NMR (76 MHz, CDCl3): δ 12.0 (((CH3)2CH)3Si), 12.2 (((CH3)2CH)3Si), 12.4 (((CH3)2CH)3Si), 18.0 (((CH3)2CH)3Si), 18.1 (((CH3)2CH)3Si), 63.7, 63.8, 64.1, 64.6, 64.7, 67.8, 78.3, 78.4, 83.7, 85.7, 87.6, 88.2, 90.8, 91.2, 97.3 (C-1), 105.4 (C-1), 105.89 (C-1), 106.91 (C-1), 119.3 (PhN (C-2, C-6)), 119.7 (PhN (C-2, C-6)), 124.0, 128.2, 128.4, 128.49, 128.53, 128.6, 129.85 (PhCO (C-2, C-6)), 129.94 (PhCO (C-2, C-6)), 130.0 (PhCO (C-2, C-6)), 132.9 (PhCO (C-4)), 133.4 (PhCO (C-4)), 133.6 (PhCO (C-4)), 165.1 (CO), 165.4 (CO); 29Si INEPT NMR (60 MHz, CDCl3): δ 12.39, 12.45, 12.84, 12.94, 13.26, 13.38, 13.47, 13.50, 13.53, 13.55, 13.74, 13.79, 13.83, 13.88, 13.91; 19F NMR (282 MHz, CDCl3): δ −65.76 (CF3); HRMS (ESI): m/z [M + NH4]+ Calcd for C149H270F3N2O28Si10+ 2872.7404; found: 2872.7378.
4-(2-Azidoethoxy)phenyl β-d-arabinofuranosyl-(1→2)-α-d-arabinofuranosyl-(1→3)-[β-d-arabinofuranosyl-(1→2)-α-d-arabinofuranosyl-(1→5)]-α-d-arabinofuranosyl-(1→5)-α-d-arabinofuranosyl-(1→5)-α-d-arabinofuranosyl-(1→5)-α-d-arabinofuranosyl-(1→5)-α-d-arabinofuranosyl-(1→5)-α-d-arabinofuranoside (5)
Acetylated decasaccharide 21 (1.5 mg, 0.0006 mmol) was dissolved in anhydrous CH2Cl2 (0.2 mL) and anhydrous MeOH (0.8 mL), followed by the addition of 1 M methanolic MeONa (20 μL). The reaction mixture was kept at ~20 °C for 24 h. Then, the reaction mixture was neutralized with Dowex 50W X8 (H+) ion-exchange resin (the resin was washed with MeOH before addition) and then filtered. The resin was washed with MeOH (3 × 20 mL). The combined filtrate was concentrated under reduced pressure. The residue was co-evaporated with toluene (2 × 2 mL), dried in vacuo, dissolved in water (1 mL), and applied on a small column (ID 5 mm) packed with Amberlite MB-3 mixed-bed ion-exchange resin (1 mL), which was eluted with H2O (15 mL). The eluate was lyophilized and additionally purified by reversed phase chromatography on a Sep-Pak C18 cartridge (particle size: 55–105 μm, pore size: 125 Å, sorbent substrate: silica, sorbent weight: 360 mg), gradient: 0→100% MeCN in H2O) to give deprotected decasaccharide 5 (0.8 mg, 85%). Rf = 0.50 (MeCN–H2O 7.5:2.5); +15.1 (c 0.2 in MeOH); 1H NMR (600 MHz, CD3OD): δ 3.54–3.58 (m, 2H, CH2N3), 3.61–3.88 (m, 21H, H-5I-Xa, H-4VIII, X, H-5I-V, VII-Xb), 3.89–3.95 (m, 5H, H-5VIb, H-3II-V), 3.99–4.06 (m, 12H, H-2II-V, VIII, X, H-3I, VI-X), 4.06–4.11 (m, 4H, H-4II-V), 4.12 (dd, 2H, J 5.4 Hz, J 4.4 Hz, CH2O), 4.13–4.15 (m, 3H, H-2VII, H-2IX, H-4I), 4.16 (dd, 1H, J 2.7 Hz, J 1.5 Hz, H-2VI), 4.17–4.21 (m, 1H, H-4VI), 4.21 (dd, 1H, J 4.0 Hz, J 1.8 Hz, H-2I), 4.94–4.98 (m, 5H, H-1II-VI), 5.02–5.06 (m, 2H, H-1VIII, H-1X), 5.09 (d, 1H, J 2.2 Hz, H-1VII), 5.17 (d, 1H, J 2.4 Hz, H-1IX), 5.42 (d, 1H, J 2.0 Hz, H-1I), 6.86–6.91 (m, 2H, OC6H4O (H-3, H-5)), 6.97–7.03 (m, 2H, OC6H4O (H-2, H-6)); 13C NMR (151 MHz, CD3OD): δ 51.4 (CH2N3), 62.45 (C-5VII, C-5IX), 62.53 (C-5VII, C-5IX), 64.38 (C-5VIII, C-5X), 64.41 (C-5VIII, C-5X), 67.9 (2C, C-5I-VI), 68.0 (C-5I-VI), 68.3 (3C, C-5I-VI), 69.0 (CH2O), 75.8 (C-3VIII, C-3X), 75.9 (C-3VIII, C-3X), 76.3 (C-3VII, C-3IX), 76.5 (C-3VII, C-3IX), 78.8 (3C, C-2VIII, C-2X, C-3I-V), 78.9 (C-2VIII, C-2X, C-3I-V), 79.2 (3C, C-2VIII, C-2X, C-3I-V), 81.6 (C-2VI), 83.03 (C-4VI), 83.3 (C-2II-V), 83.8 (C-2I), 83.95 (C-4I-V, C-4VII-X), 84.03 (C-4I-V, C-4VII-X), 84.2 (4C, C-4I-V, C-4VII-X), 84.3 (C-4I-V, C-4VII-X), 84.4 (2C, C-4I-V, C-4VII-X), 84.6 (C-3VI), 89.3 (C-2VII), 89.6 (C-2IX), 102.5 (C-1VIII, C-1X), 102.6 (C-1VIII, C-1X), 107.1 (C-1IX), 107.4 (C-1VII), 108.7 (C-1I), 109.58 (C-1II-VI), 109.63 (C-1II-VI), 109.70 (2C, C-1II-VI), 109.74 (C-1II-VI), 116.6 (OC6H4O (C-3, C-5)), 119.3 (OC6H4O (C-2, C-6)), 152.7 (OC6H4O (C-1)), 155.2 (OC6H4O (C-4)); HRMS (ESI): m/z [M + NH4]+ Calcd for C58H93N4O42+ 1517.5259; found: 1517.5244.
p-Tolyl 2,3,5-tris-O-(triisopropylsilyl)-β-d-arabinofuranosyl-(1→2)-1-thio-3,5-bis-O-(triisopropylsilyl)-α-d-arabinofuranoside (6)
(1) To a solution of acetate 10 (41 mg, 0.037 mmol) in ClCH2CH2Cl (1 mL), p-TolSH (9 mg, 0.074 mmol) and BF3·Et2O (2 μL, 0.02 mmol) were added at 20 °C. The reaction mixture was stirred at 40 °C for 1 h. Then, the reaction mixture was diluted with CH2Cl2 (50 mL) and washed with H2O (50 mL). The organic phase was filtered through a cotton wool plug, concentrated, and dried in vacuo. The residue was dissolved in 2,4,6-collidine (1 mL), then i-Pr3SiOTf (450 mL, mmol) was added. The reaction mixture was stirred at 70 °C for 1 h and then diluted with CH2Cl2 (50 mL), washed with 1 M KHSO4 (50 mL), H2O (50 mL), and NaHCO3 (50 mL). Combined organic extracts were filtered through a cotton wool plug, concentrated, and dried in vacuo. The residue was purified by silica gel chromatography (gradient: 0%→15% CH2Cl2 in petroleum ether) to give known [28] silylated disaccharide 6 (27 mg, 63%).
(2) To a solution of CPP-glycoside 13 (91 mg, 0.075 mmol) in ClCH2CH2Cl (2 mL), p-TolSH (19 mg, 0.15 mmol) and BF3·Et2O (4 μL, 0.02 mmol) at −5 °C were added. Then, the temperature was raised to 0 °C and the reaction mixture was stirred at 0 °C for 2.5 h. Then, the reaction mixture was diluted with CH2Cl2 (50 mL) and washed with H2O (50 mL). The organic extract was filtered through a cotton wool plug, concentrated, and dried in vacuo. The residue was purified by silica gel chromatography (gradient: 0%→15% CH2Cl2 in petroleum ether) to give known [28] silylated disaccharide 6 (44 mg, 50%; 54% with respect to the reacted starting material).
2,3,5-Tris-O-(triisopropylsilyl)-β-d-arabinofuranosyl-(1→2)-1-O-acetyl-3,5-bis-O-(triisopropylsilyl)-α-d-arabinofuranose (10)
To a solution of known arabinofuranose 9 [28] (48 mg, 0.045 mmol) in anhydrous Py (1 mL), Ac2O (1 mL) was added at 0 °C (ice–water bath). The reaction mixture was stirred at 20 °C for 24 h. The reaction was quenched by the addition of MeOH (1 μL) at 0 °C (ice–water bath), then concentrated under reduced pressure, co-evaporated with toluene (5×5 mL), and dried in vacuo to give acetate 10 (42 mg, 84%); Rf = 0.50 (light petroleum–CH2Cl2 1:1); −1.7 (c 2.1 in CHCl3); 1H NMR (300 MHz, CDCl3): δ 0.99–1.20 (m, 105H, 5 × ((CH3)2CH)3Si), 2.05 (s, 3H, CH3CO), 3.68 (dd, 1H, J 8.4 Hz, J 3.6 Hz, H-5IIa), 3.71–3.82 (m, 2H, H-5Ia, H-5Ib), 3.82–3.99 (m, 2H, H-4II, H-5IIb), 4.04 (dd, 1H, J 2.7 Hz, J 1.0 Hz, H-2II), 4.21 (d, 1H, J 0.8 Hz, H-2I), 4.25 (td, 1H, J 6.4 Hz, J 2.4 Hz, H-4I), 4.32 (d, 1H, J 1.1 Hz, H-3II), 4.56 (d, 1H, J 2.5 Hz, H-3I), 5.25 (d, 1H, J 2.7 Hz, H-1II), 6.16 (s, 1H, H-1I); 13C NMR (76 MHz, CDCl3): δ 12.0 (2 × ((CH3)2CH)3Si), 12.2 (2 × ((CH3)2CH)3Si), 12.3 ((CH3)2CH)3Si), 17.9 (2 × ((CH3)2CH)3Si), 17.97 (2 × ((CH3)2CH)3Si), 18.01 ((CH3)2CH)3Si), 18.0 ((CH3)2CH)3Si), 18.07 ((CH3)2CH)3Si), 18.11 (3 × ((CH3)2CH)3Si), 21.2 (CH3CO), 63.6 (C-5II), 64.2 (C-5I), 77.1 (C-3I), 78.0 (C-2II), 78.1 (C-3II), 86.0 (C-4II), 90.4 (C-2I), 90.8 (C-4I), 101.0 (C-1I), 105.3 (C-1II), 170.1 (CO); 29Si INEPT NMR (60 MHz, CDCl3) δ 13.67 (5I-O-TIPS, 5II-O-TIPS), 13.80 (5I-O-TIPS, 5II-O-TIPS), 13.96 (3II-O-TIPS), 14.02 (3I-O-TIPS), 14.29 (2II-O-TIPS); HRMS (ESI): m/z [M + NH4]+ Calcd for C57H124NO10Si5+ 1122.8066; Found: 1122.8063; [M + K]+ Calcd for C57H120KO10Si5+: 1143.7359; found: 1143.7357.
4-(3-Chloropropoxy)phenyl 2,3,5-tris-O-(triisopropylsilyl)-β-d-arabinofuranosyl-(1→2)-3,5-bis-O-(triisopropylsilyl)-α-d-arabinofuranoside (13)
Known 4-(3-chloropropoxy)phenyl 3,5-O-(di-tert-butylsilylene)-2-O-[2-O-(triisopropylsilyl)-β-d-arabinofuranosyl]-α-d-arabinofuranoside 11 [28] (147 mg, 0.19 mmol) was dissolved in THF (2 mL), then 1 M TBAF in THF (0.77 mL, 0.77 mmol) was added to the reaction mixture at 0 °C. The reaction mixture was kept at 0 °C for 3 h. After that, the reaction mixture was concentrated under reduced pressure, co-evaporated with toluene (2 × 2 mL), and dried in vacuo. The residue was purified by silica gel chromatography (gradient: 5%→10% MeOH in CH2Cl2) to give 4-(3-chloropropoxy)phenyl 2-O-(β-d-arabinofuranosyl)-α-d-arabinofuranoside 12, containing 3 mol. % of 4-(3-fluoropropoxy)phenyl 2-O-(β-d-arabinofuranosyl)-α-d-arabinofuranoside (72 mg, 81%) and 15 mol. % of n-Bu4N+ salts. Selected signals for 12: 1H NMR (300 MHz, CD3OD): δ 2.18 (p, 2H, J 6.2 Hz, CH2), 3.62–3.86 (m, 7H), 3.94–4.09 (m, 5H), 4.13–4.20 (m, 1H, H-3I), 4.33–4.38 (m, 1H, H-2I), 5.03 (d, 1H, J 4.2 Hz, H-1II), 5.56 (d, 1H, J 2.5 Hz, H-1I), 6.79–6.92 (m, 2H, OC6H4O), 6.93–7.05 (m, 2H, OC6H4O); selected signals for minor 4-(3-fluoropropoxy)phenyl 2-O-(β-d-arabinofuranosyl)-α-d-arabinofuranoside: 1H NMR (300 MHz, CD3OD): 4.60 (dt, 2H, J 47.3 Hz, J 5.9 Hz, CH2F), 19F NMR (282 MHz, CD3OD): δ −223.86 (tt, 2JH–F 47.3 Hz, 3JH–F 25.4 Hz, CH2F). Then, i-Pr3SiOTf (0.42 mL, 1.57 mmol) was added to the solution of crude pentaol 12 (72 mg, 0.16 mmol) in 2,4,6-collidine (1.5 mL) at 20 °C. The reaction mixture was stirred at 80 °C for 20 h and then diluted with CH2Cl2 (30 mL), washed with 1 M KHSO4 (3 × 30 mL), and satd aq NaHCO3 (3 × 30 mL). Organic extracts were filtered, concentrated under reduced pressure, and purified by silica gel chromatography (gradient: petroleum ether–EtOAc, 0%→6%) to give silylated disaccharide 13, containing 3% 4-(3-fluoropropoxy)phenyl derivative (155 mg, 80%). Rf = 0.80 (light petroleum–EtOAc 10:1); +21.1 (c 0.25 in CHCl3); 1H NMR (600 MHz, CDCl3): δ 1.03–1.22 (m, 105H, ((CH3)2CH)3Si), 2.15 (dt, 1H, J 25.8 Hz, J 6.0 Hz, CH2CH2F), 2.22 (p, 2H, J 6.1 Hz, CH2), 3.71 (dd, 1H, J 9.3 Hz, J 4.5 Hz, H-5IIa), 3.75 (t, 2H, J 6.4 Hz, CH2Cl), 3.78 (dd, 1H, J 10.7 Hz, J 5.6 Hz, H-5Ia), 3.87 (dd, 1H, J 10.7 Hz, J 5.3 Hz, H-5Ib), 3.88 (dd, 1H, J 10.4 Hz, J 4.5 Hz, H-4II), 3.94 (dd, 1H, J 10.4 Hz, J 9.3 Hz, H-5IIb), 4.01 (d, 1H, J 2.9 Hz, H-2II), 4.07 (t, 2H, J 5.8 Hz, CH2O), 4.20 (td, 1H, J 5.5 Hz, J 4.4 Hz, H-4I), 4.33 (d, 1H, J 1.1 Hz, H-3II), 4.41 (dd, 1H, J 2.2 Hz, J 1.0 Hz, H-2I), 4.52 (dd, 1H, J 4.5 Hz, J 2.2 Hz, H-3I), 4.65 (dt, 1H, J 47.1 Hz, J 5.8 Hz, CH2F), 5.29 (d, 1H, J 2.8 Hz, H-1II), 5.53 (d, 1H, J 0.8 Hz, H-1I), 6.78–6.84 (m, 2H, OC6H4O (H-3, H-5)), 6.94–7.00 (m, 2H, OC6H4O (H-2, H-6)); 13C NMR (151 MHz, CDCl3): δ 11.98 (((CH3)2CH)3Si), 12.03 (((CH3)2CH)3Si), 12.30 (((CH3)2CH)3Si), 12.32 (((CH3)2CH)3Si), 12.4 (((CH3)2CH)3Si), 17.96 (((CH3)2CH)3Si), 17.98 (((CH3)2CH)3Si), 18.08 (((CH3)2CH)3Si), 18.11 (((CH3)2CH)3Si), 18.14 (((CH3)2CH)3Si), 18.15 (((CH3)2CH)3Si), 32.4 (CH2), 41.6 (CH2Cl), 63.7 (C-5II), 63.8 (C-5I), 64.9 (CH2O), 77.1 (C-3I), 78.1 (C-2II), 78.3 (C-3II), 85.9 (C-4II), 87.9 (C-4I), 91.2 (C-2I), 104.8 (C-1II), 105.6 (C-1I), 115.3 (OC6H4O (C-3, C-5)), 118.1 (OC6H4O (C-2, C-6)), 151.6 (OC6H4O (C-1)), 153.7 (OC6H4O (C-4)); 29Si INEPT NMR (60 MHz, CDCl3): δ 13.36 (3I-O-TIPS), 13.65 (5I-O-TIPS, 5II-O-TIPS), 13.70 (3II-O-TIPS), 14.05 (2II-O-TIPS); HRMS (ESI): m/z [M + NH4]+ Calcd for C64H131ClNO10Si5+ 1248.8302; found: 1248.8296.
4-(2-Chloroethoxy)phenyl 2,3,5-tris-O-(triisopropylsilyl)-β-d-arabinofuranosyl-(1→2)-3,5-bis-O-(triisopropylsilyl)-α-d-arabinofuranosyl-(1→5)-2-O-benzoyl-α-d-arabinofuranosyl-(1→5)-2,3-di-O-benzoyl-α-d-arabinofuranoside (14)
A mixture of disaccharide thioglycoside 6 (82 mg, 0.07 mmol) and disaccharide diol 8 [28] (19 mg, 0.25 mmol) was dried in vacuo for 2 h, then anhydrous CH2Cl2 (2 mL) was added under argon. Freshly activated powdered MS 4 Å (200 mg) (100 mg per 1 mL of solvent) was added under argon to the resulting solution. The suspension was stirred under argon at ~22 °C for 1 h, cooled to −60 °C, then NIS (16 mg, 0.7 mmol) and, after 10 min, AgOTf (1 mg) was added. Then, the temperature was allowed to rise slowly to −20 °C and was kept at −20 °C for 1 h. Then, the reaction was quenched by the addition of satd aq NaHCO3 (50 μL), diluted with CH2Cl2 (15 mL), and filtered through a Celite pad. The solids were washed with CH2Cl2 (5 × 10 mL), and the filtrate was washed with a mixture of satd aq Na2S2O3 (50 mL) and satd aq NaHCO3 (50 mL). The aqueous layer was extracted with CH2Cl2 (2 × 5 mL). The combined organic extracts were filtered through a cotton wool plug, concentrated and dried in vacuo, then dissolved in toluene (2 mL) and subjected to chromatography on Bio-Beads S-X1 in toluene. The fractions eluted just after the void volume were collected, concentrated under reduced pressure, and the residue was purified by silica gel chromatography (gradient: EtOAc in petroleum ether, 0%→30%) to give α-linked tetrasaccharide 14 (36 mg, 80%). Rf = 0.20 (light petroleum–EtOAc 10:1); 1H NMR (600 MHz, CDCl3): δ 0.89 –1.14 (m, 105H, 5 × ((CH3)2CH)3Si), 3.43 (d, 1H, J 4.1 Hz, HO-3II), 3.66 (dd, 1H, J 9.5 Hz, J 4.6 Hz, H-5IVa), 3.68 (dd, 1H, J 11.1 Hz, J 3.9 Hz, H-5IIa), 3.73 (dd, 1H, J 10.4 Hz, J 6.4 Hz, H-5IIIa), 3.77–3.80 (m, 1H, H-5IIIb), 3.79 (t, 2H, J 5.9 Hz, CH2Cl), 3.82 (dd, 1H, J 10.3 Hz, J 4.6 Hz, H-4IV), 3.90 (dd, 1H, J 10.2 Hz, J 9.4 Hz, H-5IVb), 3.92 (dd, 1H, J 11.1 Hz, J 3.7 Hz, H-5Ia), 3.98 (d, 1H, J 3.0 Hz, H-2IV), 4.00 (dd, 1H, J 11.2 Hz, J 3.5 Hz, H-5IIb), 4.07 (td, 1H, J 6.0 Hz, J 3.6 Hz, H-4III), 4.17 (d, 1H, J 1.2 Hz, H-2III), 4.19 (t, 2H, J 6.0 Hz, CH2O), 4.21 (dd, 1H, J 11.0 Hz, J 4.5 Hz, H-5Ib), 4.29 (d, 1H, J 1.1 Hz, H-3IV), 4.31 (dt, 1H, J 7.1 Hz, J 3.5 Hz, H-3II), 4.34–4.38 (m, 2H, H-4II, H-3III), 4.58–4.63 (m, 1H, H-4I), 5.06 (s, 1H, H-1III), 5.17 (dd, 1H, J 3.7 Hz, J 1.4 Hz, H-2II), 5.20 (d, 1H, J 2.6 Hz, H-1IV), 5.37 (d, 1H, J 1.4 Hz, H-1II), 5.68 (dd, 1H, J 5.0 Hz, J 1.8 Hz, H-3I), 5.75 (d, 1H, J 1.7 Hz, H-2I), 5.83 (s, 1H, H-1I), 6.84–6.91 (m, 2H, OC6H4O (H-3, H-5)), 7.04–7.11 (m, 2H, OC6H4O (H-2, H-6)), 7.41–7.51 (m, 6H, 3 × PhCO (H-3, H-5)), 7.55–7.64 (m, 3H, 3 × PhCO (H-4)), 7.99–8.04 (m, 2H, 2II-O-PhCO (H-2, H-6)), 8.07–8.11 (m, 2H, 2I-O-PhCO (H-2, H-6)), 8.09–8.14 (m, 2H, 3I-O-PhCO (H-2, H-6)); 13C NMR (151 MHz, CDCl3): δ 11.9 (((CH3)2CH)3Si), 12.0 (((CH3)2CH)3Si), 12.1 (((CH3)2CH)3Si), 12.2 (((CH3)2CH)3Si), 12.3 (((CH3)2CH)3Si), 17.9 (2 × ((CH3)2CH)3Si), 17.95 (3 × ((CH3)2CH)3Si), 17.97 (((CH3)2CH)3Si), 18.06 (((CH3)2CH)3Si), 18.09 (((CH3)2CH)3Si), 18.13 (((CH3)2CH)3Si), 18.14 (((CH3)2CH)3Si), 41.9 (CH2Cl), 63.7 (C-5IV), 64.4 (C-5II), 64.6 (C-5III), 66.0 (C-5I), 68.7 (CH2O), 76.1 (C-3II), 77.3 (C-3I), 77.9 (C-3III), 78.0 (C-2IV), 78.1 (C-3IV), 82.1 (C-2I, C-4II), 82.5 (C-4I), 85.8 (C-4IV), 86.9 (C-2II), 88.2 (C-4III), 90.8 (C-2III), 104.8 (C-1I), 105.0 (C-1IV), 105.4 (C-1II), 106.3 (C-1III), 115.8 (OC6H4O (C-3, C-5)), 118.3 (OC6H4O (C-2, C-6)), 128.4 (PhCO (C-3, C-5)), 128.5 (PhCO (C-3, C-5)), 128.6 (PhCO (C-3, C-5)), 128.9 (PhCO (C-1)), 129.1 (PhCO (C-1)), 129.3 (PhCO (C-1)), 129.87 (PhCO (C-2, C-6)), 129.92 (PhCO (C-2, C-6)), 129.94 (PhCO (C-2, C-6)), 133.4 (PhCO (C-4)), 133.5 (PhCO (C-4)), 133.6 (PhCO (C-4)), 150.7 (OC6H4O (C-1)), 153.7 (OC6H4O (C-4)), 165.5 (2I-O-PhCO), 165.7 (3I-O-PhCO), 166.8 (2II-O-PhCO); 29Si INEPT NMR (60 MHz, CDCl3): δ 13.54, 13.59, 13.70, 13.80, 13.89; HRMS (ESI): m/z [M + NH4]+ Calcd for C94H193ClNO21Si5+ 1810.9777; found: 1810.9764; [M + K]+ Calcd for C94H153ClKO21Si5+: 1831.9071; found: 1831.9063.
2,3,5-Tris-O-(triisopropylsilyl)-β-d-arabinofuranosyl-(1→2)-3,5-bis-O-(triisopropylsilyl)-α-d-arabinofuranosyl-(1→3)-[2,3,5-tris-O-(triisopropylsilyl)-β-d-arabinofuranosyl-(1→2)-3,5-bis-O-(triisopropylsilyl)-α-d-arabinofuranosyl-(1→5)]-2-O-benzoyl-α-d-arabinofuranosyl-(1→5)-2,3-di-O-benzoyl-d-arabinofuranose (15) and 2,3,5-tris-O-(triisopropylsilyl)-β-d-arabinofuranosyl-(1→2)-3,5-bis-O-(triisopropylsilyl)-α-d-arabinofuranosyl-(1→3)-[2,3,5-tris-O-(triisopropylsilyl)-β-d-arabinofuranosyl-(1→2)-3,5-bis-O-(triisopropylsilyl)-α-d-arabinofuranosyl-(1→5)]-2-O-benzoyl-α-d-arabinofuranosyl-(1→5)-1,3-di-O-benzoyl-β-d-arabinofuranose (16)
CEP glycoside 2 (44 mg, 0.016 mmol) was dissolved in CH2Cl2 (2 mL) and MeCN (1 mL), then H2O (0.1 mL) was added at 0 °C, followed by (NH4)2Ce(NO3)6 (52.6 mg, 0.096 mmol). The reaction mixture was stirred at 0 °C for 4 h. Then, Na2SO3 (110 mg) and H2O (1 mL) were added to the reaction mixture at 0 °C, and the reaction mixture was kept at 0 °C for 16 h. Then, the reaction mixture was diluted with CH2Cl2 (50 mL), washed with H2O (50 mL), concentrated under reduced pressure and purified by silica gel column chromatography (gradient: petroleum ether–EtOAc, 3%→8%) to give hemiacetal 15 (29 mg, 70%) as a mixture of anomers 15, and contaminated with the product 16 of migration of benzoyl group from O-2 to O-1. The ratio of 15α:15β:16 = 1:0.45:0.28 according to NMR; Rf = 0.50 (light petroleum–EtOAc, 8.5:1.5); selected signals: 1H NMR (300 MHz, CDCl3): δ 0.75–1.18 (m, 363H, ((CH3)2CH)3Si), 3.40 (s, 1H, HO-1Iα), 4.65 (ddd, 1H, J 6.3 Hz, J 5.2 Hz, J 3.3 Hz, H-4Iα), 5.05 (s, 1H, H-1III of 16), 5.10 (s, 1H, H-1IIIβ), 5.10 (s, 1H, H-1IIIα), 5.16 (s, 1H, H-1Vβ), 5.17–5.18 (m, 2H, H-1IIβ, H-1II or H-1V of 16), 5.31 (d, 1H, J 3.2 Hz, H-2IIβ), 5.35 (d, 1H, J 2.0 Hz, H-2IIα), 5.40–5.48 (m, 2H, H-3Iα, H-3I of 16), 5.51 (d, 1H, J 2.4 Hz, H-2Iα), 5.56 (dd, 1H, J 6.2 Hz, J 4.7 Hz, H-2Iβ), 5.63 (s, 1H, H-1Iα), 5.72 (dd, 1H, J 5.5 Hz, J 5.5 Hz, H-1Iβ), 5.86 (dd, 1H, J 6.2 Hz, J 5.0 Hz, H-3Iβ), 6.60 (d, 1H, J 4.5 Hz, H-1I of 16), 7.33–7.66 (m, 16H, PhCO (H-3, H-4, H-5)), 7.92–8.19 (m, 11H, PhCO (H-2, H-6)); 13C NMR (151 MHz, CDCl3): δ 11.97 (((CH3)2CH)3Si), 11.99 (((CH3)2CH)3Si), 12.02 (((CH3)2CH)3Si), 12.03 (((CH3)2CH)3Si), 12.2 (((CH3)2CH)3Si), 12.28 (((CH3)2CH)3Si), 12.31 (((CH3)2CH)3Si), 12.36 (((CH3)2CH)3Si), 12.39 (((CH3)2CH)3Si), 17.95 (((CH3)2CH)3Si), 17.97 (((CH3)2CH)3Si), 17.99 (((CH3)2CH)3Si), 18.01 (((CH3)2CH)3Si), 18.03 (((CH3)2CH)3Si), 18.05 (((CH3)2CH)3Si), 18.08 (((CH3)2CH)3Si), 18.09 (((CH3)2CH)3Si), 18.11 (((CH3)2CH)3Si), 18.13 (((CH3)2CH)3Si), 18.14 (((CH3)2CH)3Si), 18.17 (((CH3)2CH)3Si), 18.18 (((CH3)2CH)3Si), 18.20 (((CH3)2CH)3Si), 63.7 (C-5), 63.76 (2 × C-5), 63.82 (C-5), 64.16 (C-5), 64.18 (C-5), 64.5 (C-5), 64.7 (C-5), 65.3 (C-5II minor), 65.4 (C-5IIα), 66.0 (C-5IIβ), 66.6 (C-5Iα), 67.4 (C-5I of 16), 67.6 (C-5Iβ), 76.5 (C-3Iβ), 76.7 (C-2I of 16), 77.4, 77.5, 77.7, 78.08, 78.14, 78.19, 78.21, 78.23, 78.25, 78.28, 78.32, 78.37, 78.43, 78.47, 78.52, 79.7, 80.06 (C-3IIβ), 80.12, 80.6, 80.7 (C-3IIα), 81.0 (C-3I of 16), 81.1, 81.8 (C-4Iα), 82.1, 82.2, 82.6 (C-2Iα), 83.6 (C-2IIα), 83.7 (C-2II of 16), 84.1 (C-2IIβ), 85.7 (C-4IV, C-4VI), 85.7 (C-4IV, C-4VI), 85.6 (C-4IV, C-4VI), 85.7 (C-4IV, C-4VI), 85.8 (C-4IV, C-4VI), 87.5 (C-4III), 87.54 (C-4III), 87.59 (C-4III of 16), 87.8 (C-4V), 88.3 (C-4V), 88.4 (C-4V of 16), 90.5 (C-2III), 90.70 (C-2III), 90.74 (C-2III of 16), 91.2 (C-2V), 91.4 (C-2V of 16), 91.6 (C-2V), 95.4 (C-1Iβ), 97.0 (C-1I of 16), 100.9 (C-1Iα), 104.7 (C-1), 104.75 (C-1), 104.78 (C-1 minor), 105.2 (C-1), 105.5 (C-1), 105.48 (C-1 of 16), 105.93 (C-1), 105.94 (C-1 of 16), 105.98 (C-1), 106.04 (C-1), 106.2 (C-1), 106.4 (C-1 of 16), 106.69 (C-1III of 16), 106.73 (C-1IIIβ), 106.8 (C-1IIIα), 128.20 (PhCO (C-3, C-5) of 16), 128.23 (PhCO (C-3, C-5)), 128.27 (PhCO (C-3, C-5)), 128.30 (PhCO (C-3, C-5)), 128.37 (2 × PhCO (C-3, C-5)), 128.44 (PhCO (C-3, C-5) of 16), 128.5 (PhCO (C-3, C-5)), 129.3 (PhCO (C-1)), 129.4 (PhCO (C-1)), 129.48 (PhCO (C-1)), 129.49 (PhCO (C-1)), 129.54 (PhCO (C-1)), 129.8 (PhCO (C-1)), 129.85 (PhCO (C-2, C-6)), 129.86 (PhCO (C-2, C-6)), 129.92 (PhCO (C-2, C-6) of 16), 129.94 (PhCO (C-2, C-6)), 130.0 (2 × PhCO (C-2, C-6)), 130.1 (PhCO (C-2, C-6)), 132.9 (PhCO (C-4)), 133.07 (PhCO (C-4)), 133.12 (PhCO (C-4)), 133.2 (PhCO (C-4)), 133.27 (PhCO (C-4)), 133.29 (PhCO (C-4)), 133.5 (PhCO (C-4) of 16), 165.16 (PhCO of 16), 165.20 (PhCO), 165.58 (PhCO), 165.59 (PhCO), 165.7 (PhCO), 165.8 (2 × PhCO); HRMS (ESI): m/z [M + NH4]+ Calcd for C141H266NO28Si10+ 2701.7109; found: 2701.7093.
4-(2-Chloroethoxy)phenyl 2,3,5-tris-O-(triisopropylsilyl)-β-d-arabinofuranosyl-(1→2)-3,5-bis-O-(triisopropylsilyl)-α-d-arabinofuranosyl-(1→3)-[2,3,5-tris-O-(triisopropylsilyl)-β-d-arabinofuranosyl-(1→2)-3,5-bis-O-(triisopropylsilyl)-α-d-arabinofuranosyl-(1→5)]-2-O-benzoyl-α-d-arabinofuranosyl-(1→5)-2,3-di-O-benzoyl-α-d-arabinofuranosyl-(1→5)-2,3-di-O-benzoyl-α-d-arabinofuranosyl-(1→5)-2,3-di-O-benzoyl-α-d-arabinofuranosyl-(1→5)-2,3-di-O-benzoyl-α-d-arabinofuranosyl-(1→5)-2,3-di-O-benzoyl-α-d-arabinofuranoside (18)
A mixture of hexasaccharide imidate 5 (32 mg, 0.011 mmol) and known tetrasaccharide glycosyl acceptor 17 [33] (21 mg, 0.013 mmol) was dried in vacuo for 2 h, then anhydrous CH2Cl2 (2 mL) was added under argon. Freshly activated powdered MS 4 Å (200 g) (100 mg per 1 mL of solvent) was added under argon to the resulting solution. The suspension was stirred under argon at ~22 °C for 1 h, then cooled to −60 °C, followed by the addition of TfOH (1 μL). Then, the temperature was allowed to rise during 1 h to −40 °C and this temperature was kept for 1 h. After an additional 20 min, the reaction was quenched by the addition of Py (50 µL). The reaction mixture was diluted with CH2Cl2 (15 mL) and filtered through a Celite pad. The solids were washed with CH2Cl2 (5 × 10 mL), and the filtrate was washed with satd aq NaHCO3 (20 mL). The aqueous layer was extracted with CH2Cl2 (2 × 5 mL). The combined organic layer was filtered through a cotton wool plug, concentrated under reduced pressure, the residue was dried in vacuo, then dissolved in toluene (2 mL) and subjected to chromatography on Bio-Beads S-X1 in toluene. The fractions eluted just after the void volume were collected and concentrated under reduced pressure to give decasaccharide 18 (34 mg, 72%; 51% over 3 steps starting from 2). Rf = 0.28 (light petroleum–EtOAc 6:1); +31.9 (c 1.13 in CHCl3); 1H NMR (600 MHz, CDCl3): δ 0.91–1.16 (m, 210H, 10 × ((CH3)2CH)3Si), 3.63 (dd, 1H, J 9.3 Hz, J 4.4 Hz, H-5VIIIa or H-5Xa), 3.65 (dd, 1H, J 10.7 Hz, J 5.5 Hz, H-5IXa), 3.78 (t, 2H, J 5.9 Hz, CH2Cl), 3.69–3.98 (m, 16H, H-5Xa or H-5VIIIa, H-5I-VIIa, H-5VI-Xb, H-4VIII-X), 4.02–4.04 (m, 2H, H-2VIII, H-2X), 4.06 (d, 1H, J 1.2 Hz, H-2IX), 4.08–4.12 (m, 2H, H-4VII, H-5Vb), 4.13 (s, 1H, H-2VII), 4.16 (t, 2H, J 5.9 Hz, CH2O), 4.14–4.24 (m, 5H, H-5I-IV, H-3VI), 4.26–4.28 (m, 1H, H-4VI), 4.29 (s, 1H, H-3X), 4.32 (s, 1H, H-3VIII), 4.34 (d, 1H, J 3.9 Hz, H-3VII), 4.45 (d, 1H, J 3.4 Hz, H-3IX), 4.55–4.64 (m, 5H, H-4I-V), 5.10 (s, 1H, H-1VII), 5.15 (d, 1H, J 2.6 Hz, H-1X), 5.23 (s, 1H, H-1IX), 5.24 (d, 1H, J 2.8 Hz, H-1VIII), 5.29 (s, 1H, H-1VI), 5.37 (s, 2H, 2 × H-1), 5.38–5.41 (m, 3H, H-2VI, 2 × H-1), 5.50 (d, 1H, J 4.4 Hz, H-3V), 5.58 (d, 1H, J 1.1 Hz, H-2V), 5.62–5.67 (m, 6H, H-3II-IV, H-2II-IV), 5.75–5.79 (m, 2H, H-3I, H-2I), 5.82 (s, 1H, H-1I), 6.80–6.86 (m, 2H, OC6H4O (H-3, H-5)), 7.02–7.08 (m, 2H, OC6H4O (H-2, H-6)), 7.18–7.27 (m, 8H, 4 × PhCO (H-3, H-5)), 7.34–7.55 (m, 24H, 7 × PhCO (H-3, H-5), 10 × PhCO (H-4)), 7.55–7.61 (m, 1H, PhCO (H-4)), 7.84–7.92 (m, 8H, 4 × PhCO (H-2, H-6)), 7.97–8.06 (m, 10H, 5 × PhCO (H-2, H-6)), 8.06–8.11 (m, 2H, PhCO (H-2, H-6)), 8.09–8.14 (m, 2H, PhCO (H-2, H-6));13C NMR (151 MHz, CDCl3): δ 11.97 (((CH3)2CH)3Si), 11.99 (((CH3)2CH)3Si), 12.01 (((CH3)2CH)3Si), 12.1 (((CH3)2CH)3Si), 12.2 (((CH3)2CH)3Si), 12.27 (2 × ((CH3)2CH)3Si), 12.29 (2 × ((CH3)2CH)3Si), 12.4 (((CH3)2CH)3Si), 17.94 (((CH3)2CH)3Si), 17.96 (((CH3)2CH)3Si), 17.97 (((CH3)2CH)3Si), 18.02 (((CH3)2CH)3Si), 18.04 (((CH3)2CH)3Si), 18.06 (((CH3)2CH)3Si), 18.09 (((CH3)2CH)3Si), 18.13 (((CH3)2CH)3Si), 18.19 (((CH3)2CH)3Si), 18.20 (((CH3)2CH)3Si), 41.9 (CH2Cl), 63.8 (C-5VIII, C-5X), 63.9 (C-5VIII, C-5X), 64.3 (C-5IX), 64.9 (C-5VII), 65.2 (C-5VI), 65.3 (C-5V), 65.8 (2C, C-5I, C-5II, C-5III, C-5IV), 65.86 (C-5I, C-5II, C-5III, C-5IV), 65.91 (C-5I, C-5II, C-5III, C-5IV), 68.8 (CH2O), 77.2 (C-3I), 77.2 (2C, C-3II, C-3III, C-3IV, C-3V), 77.3 (C-3II, C-3III, C-3IV, C-3V), 77.6 (C-3II, C-3III, C-3IV, C-3V), 77.7 (C-3IX), 78.2 (C-2VIII or C-2X), 78.3 (C-3X), 78.4(C-2VIII or C-2X), 78.5 (2C, C-3VII, C-3VIII), 81.0 (C-3VI), 81.5 (2C, C-2II, C-2III, C-2IV, C-2V), 81.6 (2C, C-2II, C-2III, C-2IV, C-2V), 81.9 (C-2I), 82.2 (3C, C-4II, C-4III, C-4IV, C-4V, C-4VI), 82.26 (C-4II, C-4III, C-4IV, C-4V, C-4VI), 82.30 (C-4II, C-4III, C-4IV, C-4V, C-4VI), 82.8 (C-4I), 84.0 (C-2VI), 85.7 (C-4VIII, C-4X), 85.6 (C-4VIII, C-4X), 87.7 (C-4VII), 88.6 (C-4IX), 90.6 (C-2VII), 91.4 (C-2IX), 104.8 (C-1VIII), 104.9 (C-1I), 105.5 (C-1X), 105.85 (C-1VI), 105.89 (C-1), 106.0 (3C, C-1II, C-1III, C-1IV), 106.2 (C-1IX), 106.9 (C-1VII), 115.9 (OC6H4O (C-3, C-5)), 118.3 (OC6H4O (C-2, C-6)), 128.1 (PhCO (C-3, C-5)), 128.15 (PhCO (C-3, C-5)), 128.17 (PhCO (C-3, C-5)), 128.20 (PhCO (C-3, C-5)), 128.3 (PhCO (C-3, C-5)), 128.4 (PhCO (C-3, C-5)), 128.6 (PhCO (C-3, C-5)), 128.49 (3 × PhCO (C-3, C-5)), 128.54 (PhCO (C-3, C-5)), 129.1–129.29 (PhCO (C-1)), 129.29 (PhCO (C-1)), 129.36 (PhCO (C-1)), 129.41 (PhCO (C-1)), 129.5 (PhCO (C-1)), 129.78 (3 × PhCO (C-2, C-6)), 129.81 (2 × PhCO (C-2, C-6)), 129.83 (3 × PhCO (C-2, C-6)), 129.86 (PhCO (C-2, C-6)), 129.92 (PhCO (C-2, C-6)), 130.0 (PhCO (C-2, C-6)), 132.75 (PhCO (C-4)), 132.82 (PhCO (C-4)), 132.9 (PhCO (C-4)), 133.0 (PhCO (C-4)), 133.1 (2 × PhCO (C-4)), 133.2 (PhCO (C-4)), 133.3 (PhCO (C-4)), 133.35 (PhCO (C-4)), 133.44 (PhCO (C-4)), 133.5 (PhCO (C-4)), 150.8 (OC6H4O (C-1)), 153.7 (OC6H4O (C-4)), 165.0 (PhCO), 165.2 (PhCO), 165.2 (PhCO), 165.2 (PhCO), 165.2 (PhCO), 165.3 (PhCO), 165.37 (PhCO), 165.44 (PhCO), 165.5 (PhCO), 165.57 (PhCO), 165.64 (PhCO); 29Si INEPT NMR (60 MHz, CDCl3): δ 12.30 (3VII-O-TIPS), 12.84 (3IX-O-TIPS), 13.21 (5-O-TIPS), 13.44 (5-O-TIPS), 13.49 (3X-O-TIPS, 5-O-TIPS), 13.50 (3X-O-TIPS, 5-O-TIPS), 13.54 (5-O-TIPS), 13.76 (3VIII-O-TIPS), 13.82 (2VIII-O-TIPS, 2X-O-TIPS), 13.86 (2VIII-O-TIPS, 2X-O-TIPS); HRMS (ESI): m/z [M + 2NH4]2+ Calcd for C225H341ClN2O53Si102+ 2117.0710; found: 2117.0702.
4-(2-Azidoethoxy)phenyl 2,3,5-tris-O-(triisopropylsilyl)-β-d-arabinofuranosyl-(1→2)-3,5-bis-O-(triisopropylsilyl)-α-d-arabinofuranosyl-(1→3)-[2,3,5-tris-O-(triisopropylsilyl)-β-d-arabinofuranosyl-(1→2)-3,5-bis-O-(triisopropylsilyl)-α-d-arabinofuranosyl-(1→5)]-2-O-benzoyl-α-d-arabinofuranosyl-(1→5)-2,3-di-O-benzoyl-α-d-arabinofuranosyl-(1→5)-2,3-di-O-benzoyl-α-d-arabinofuranosyl-(1→5)-2,3-di-O-benzoyl-α-d-arabinofuranosyl-(1→5)-2,3-di-O-benzoyl-α-d-arabinofuranosyl-(1→5)-2,3-di-O-benzoyl-α-d-arabinofuranoside (19)
A mixture of decasaccharide CEP glycoside 18 (30 mg, 0.007 mmol), NaN3 (3 mg, 0.042 mmol), and 18-crown-6 (2 mg, 0.006 mmol) in DMF (1 mL) was stirred at 80 °C for 16 h. The reaction mixture was concentrated under reduced pressure, co-evaporated with toluene (2×2 mL), and dried in vacuo. The residue was dissolved in EtOAc (50 mL), washed with water (50 mL), and the aqueous layer was extracted with EtOAc (5 mL). The combined organic extracts were filtered through a cotton wool plug, concentrated under reduced pressure, and purified by silica gel column chromatography (gradient: EtOAc in petroleum ether, 2%→20%) to give azide 19 (25 mg, 84%); +29.7 (c 2.55 in CHCl3); 1H NMR (600 MHz, CDCl3): δ 0.91–1.13 (m, 210H, 10 × ((CH3)2CH)3Si), 3.56 (t, 2H, J 5.0 Hz, CH2N3), 3.63 (dd, 1H, J 9.3 Hz, J 4.4 Hz, H-5VIIIa or H-5Xa), 3.66 (dd, 1H, J 10.5 Hz, J 5.5 Hz, H-5IXa), 3.69–3.98 (m, 16H, H-5Xa or H-5VIIIa, H-5I-VIIa, H-5VI-Xb, H-4VIII-X), 4.03 (d, 1H, J 2.8 Hz, H-2VIII), 4.04 (d, 1H, J 2.7 Hz, H-2X), 4.07 (d, 1H, J 1.4 Hz, H-2IX), 4.08 (t, 2H, J 5.1 Hz, CH2O), 4.07–4.13 (m, 2H, H-4VII, H-5Vb), 4.13 (d, 1H, J 1.2 Hz, H-2VII), 4.14–4.24 (m, 5H, H-5I-IVb, H-3VI), 4.27 (ddd, 1H, J 7.2 Hz, J 5.0 Hz, J 1.9 Hz, H-4VI), 4.29 (d, 1H, J 1.0 Hz, H-3X), 4.32 (d, 1H, J 0.9 Hz, H-3VIII), 4.34 (dt, 1H, J 4.0 Hz, J 0.8 Hz, H-3VII), 4.45 (dd, 1H, J 3.3 Hz, J 1.6 Hz, H-3IX), 4.54–4.64 (m, 5H, H-4I-V), 5.09 (s, 1H, H-1VII), 5.16 (d, 1H, J 2.7 Hz, H-1X), 5.23 (s, 1H, H-1IX), 5.24 (d, 1H, J 2.8 Hz, H-1VIII), 5.29 (s, 1H, H-1VI), 5.37 (s, 1H, H-1), 5.37 (s, 1H, H-1), 5.37–5.41 (m, 3H, H-2VI, 2 × H-1), 5.50 (d, 1H, J 4.4 Hz, H-3V), 5.58 (d, 1H, J 1.2 Hz, H-2V), 5.61–5.67 (m, 6H, H-3II-IV, H-2II-IV), 5.74–5.79 (m, 1H, H-3I), 5.77 (s, 1H, H-2I), 5.82 (s, 1H, H-1I), 6.80–6.86 (m, 2H, OC6H4O (H-3, H-5)), 7.02–7.08 (m, 2H, OC6H4O (H-2, H-6)), 7.19–7.27 (m, 8H, 4 × PhCO (H-3, H-5)), 7.34–7.55 (m, 24H, 7 × PhCO (H-3, H-5), 10 × PhCO (H-4)), 7.55–7.61 (m, 1H, PhCO (H-4)), 7.84–7.92 (m, 8H, 4 × PhCO (H-2, H-6)), 7.97–8.06 (m, 10H, 5 × PhCO (H-2, H-6)), 8.06–8.11 (m, 2H, PhCO (H-2, H-6)), 8.09–8.14 (m, 2H, PhCO (H-2, H-6)); 13C NMR (151 MHz, CDCl3): δ 11.97 (((CH3)2CH)3Si), 11.99 (((CH3)2CH)3Si), 12.01 (((CH3)2CH)3Si), 12.04 (((CH3)2CH)3Si), 12.2 (((CH3)2CH)3Si), 12.26 (2 × ((CH3)2CH)3Si), 12.28 (((CH3)2CH)3Si), 12.29 (((CH3)2CH)3Si), 12.4 (((CH3)2CH)3Si), 17.9 (((CH3)2CH)3Si), 17.96 (((CH3)2CH)3Si), 17.97 (((CH3)2CH)3Si), 18.02 (((CH3)2CH)3Si), 18.04 (((CH3)2CH)3Si), 18.06 (((CH3)2CH)3Si), 18.09 (((CH3)2CH)3Si), 18.13 (((CH3)2CH)3Si), 18.19 (((CH3)2CH)3Si), 18.20 (((CH3)2CH)3Si), 50.2 (CH2N3), 63.8 (C-5VIII, C-5X), 63.9 (C-5VIII, C-5X), 64.3 (C-5IX), 64.8 (C-5VII), 65.2 (C-5V, C-5VI), 65.3 (C-5V, C-5VI), 65.76 (2C, C-5I, C-5II, C-5III, C-5IV), 65.83 (C-5I, C-5II, C-5III, C-5IV), 65.9 (C-5I, C-5II, C-5III, C-5IV), 67.6 (CH2O), 77.10 (C-3I), 77.2 (2C, C-3II, C-3III, C-3IV, C-3V), 77.3 (C-3II, C-3III, C-3IV, C-3V), 77.6 (C-3II, C-3III, C-3IV, C-3V), 77.7 (C-3IX), 78.15 (C-2VIII or C-2X), 78.24 (C-3X), 78.4 (C-2VIII or C-2X), 78.51 (C-3VII, C-3VIII), 78.53 (C-3VII, C-3VIII), 81.0 (C-3VI), 81.5 (2C, C-2II, C-2III, C-2IV, C-2V), 81.59 (C-2II, C-2III, C-2IV, C-2V), 81.61 (C-2II, C-2III, C-2IV, C-2V), 81.9 (C-2I), 82.15 (C-4II, C-4III, C-4IV, C-4V, C-4VI), 82.17 (C-4II, C-4III, C-4IV, C-4V, C-4VI), 82.19 (C-4II, C-4III, C-4IV, C-4V, C-4VI), 82.26 (C-4II, C-4III, C-4IV, C-4V, C-4VI), 82.30 (C-4II, C-4III, C-4IV, C-4V, C-4VI), 82.8 (C-4I), 84.0 (C-2VI), 85.7 (C-4VIII, C-4X), 85.8 (C-4VIII, C-4X), 87.6 (C-4VII), 88.3 (C-4IX), 90.6 (C-2VII), 91.4 (C-2IX), 104.8 (C-1VIII), 104.9 (C-1I), 105.6 (C-1X), 105.8 (C-1VI), 105.87 (C-1II, C-1III, C-1IV, C-1V), 105.94 (C-1II, C-1III, C-1IV, C-1V), 105.97 (2C, C-1II, C-1III, C-1IV, C-1V), 106.2 (C-1IX), 106.9 (C-1VII), 115.6 (OC6H4O (C-3, C-5)), 118.3 (OC6H4O (C-2, C-6)), 128.10 (PhCO (C-3, C-5)), 128.14 (PhCO (C-3, C-5)), 128.16 (PhCO (C-3, C-5)), 128.20 (PhCO (C-3, C-5)), 128.3 (PhCO (C-3, C-5)), 128.4 (PhCO (C-3, C-5)), 128.46 (PhCO (C-3, C-5)), 128.49 (2 × PhCO (C-3, C-5)), 128.50 (PhCO (C-3, C-5)), 128.54 (PhCO (C-3, C-5)), 129.0 (PhCO (C-1)), 129.1 (PhCO (C-1)), 129.15 (PhCO (C-1)), 129.17 (3 × PhCO (C-1)), 129.22 (PhCO (C-1)), 129.28 (PhCO (C-1)), 129.34 (PhCO (C-1)), 129.4 (PhCO (C-1)), 129.76 (PhCO (C-2, C-6)), 129.78 (2 × PhCO (C-2, C-6)), 129.80 (PhCO (C-2, C-6)), 129.81 (PhCO (C-2, C-6)), 129.83 (3 × PhCO (C-2, C-6)), 129.86 (PhCO (C-2, C-6)), 129.92 (PhCO (C-2, C-6)), 130.0 (PhCO (C-2, C-6)), 132.7 (PhCO (C-4)), 132.8 (PhCO (C-4)), 132.9 (PhCO (C-4)), 133.0 (PhCO (C-4)), 133.1 (PhCO (C-4)), 133.2 (PhCO (C-4)), 133.2 (PhCO (C-4)), 133.3 (PhCO (C-4)), 133.4 (PhCO (C-4)), 133.44 (PhCO (C-4)), 133.49 (PhCO (C-4)), 150.7 (OC6H4O (C-1)), 153.6 (OC6H4O (C-4)), 165.01 (PhCO), 165.11 (PhCO), 165.14 (PhCO), 165.18 (PhCO), 165.23 (PhCO), 165.3 (PhCO), 165.37 (PhCO), 165.44 (PhCO), 165.5 (PhCO), 165.57 (PhCO), 165.64 (PhCO); 29Si INEPT NMR (60 MHz, CDCl3): δ 12.27, 12.83, 13.20, 13.44, 13.48, 13.50, 13.53, 13.76, 13.81, 13.85; HRMS (ESI): m/z [M + 2NH4]2+ Calcd for C225H341N5O53Si102+ 2120.5912; Found: 2120.5897.
4-(2-Azidoethoxy)phenyl 2,3,5-tri-O-acetyl-β-d-arabinofuranosyl-(1→2)-3,5-di-O-acetyl-α-d-arabinofuranosyl-(1→3)-[2,3,5-tri-O-acetyl-β-d-arabinofuranosyl-(1→2)-3,5-di-O-acetyl-α-d-arabinofuranosyl-(1→5)]-2-O-acetyl-α-d-arabinofuranosyl-(1→5)-2,3-di-O-acetyl-α-d-arabinofuranosyl-(1→5)-2,3-di-O-benzoyl-α-d-arabinofuranosyl-(1→5)-2,3-di-O-acetyl-α-d-arabinofuranosyl-(1→5)-2,3-di-O-acetyl-α-d-arabinofuranosyl-(1→5)-2,3-di-O-acetyl-α-d-arabinofuranoside (21)
Protected AEP decasaccharide 19 (25 mg, 0.059 mmol) was dissolved in THF (1 mL), then AcOH (4 μL, 0.059 mmol) and 1 M TBAF in THF (178 μL, 0.18 mmol) was added to the reaction mixture at 0 °C. The reaction mixture was stirred at 40 °C for 3 h. Then, the reaction mixture was concentrated under reduced pressure, co-evaporated with toluene (2 × 2 mL), dried in vacuo, and purified by silica gel column chromatography (gradient: MeOH in CH2Cl2, 2%→20%) to give benzoylated polyol of decasaccharide 20 (12 mg) isolated as a mixture with n-Bu4N+ salts according to MS and NMR data. Rf = 0.42 (CH2Cl2–MeOH 4:1); HRMS (ESI): m/z [M + Na]+ Calcd for C135H133N3NaO53 2666.7696; found: 2666.7635. Then, the obtained reaction mixture was dissolved in MeOH (1 mL), followed by the addition of 1 M methanolic MeONa (50 µL, 0.05 mmol). The reaction mixture was kept at ~20 °C for 16 h. Then, the reaction mixture was neutralized with Dowex 50W×8 (H+) ion-exchange resin (the resin was washed with MeOH before addition) and then filtered. The resin was washed with MeOH (50 mL). The filtrate was concentrated under reduced pressure, and the residue was dried in vacuo. The obtained crude product was dissolved in anhydrous Py (1 mL), then Ac2O (1 mL) was added at 0 °C (ice–water bath). The reaction mixture was stirred at 20 °C for 24 h. The reaction was quenched by the addition of MeOH (1 μL) at 0 °C (ice–water bath), then concentrated under reduced pressure, co-evaporated with toluene (5 × 5 mL), and dried in vacuo. The residue was purified by silica gel chromatography (gradient: 5%→70% acetone in petroleum ether) to give acetylated decasaccharide 21 (2 mg, 14% over 3 steps). Rf = 0.20 (light petroleum–acetone 1:1); +53.9 (c 0.15 in CHCl3); 1H NMR (600 MHz, CDCl3): δ 2.08 (s, 9H, 3 × CH3CO), 2.08 (s, 9H, 3 × CH3CO), 2.09 (s, 6H, 2 × CH3CO), 2.09 (s, 3H, CH3CO), 2.10 (s, 3H, CH3CO), 2.11 (s, 3H, CH3CO), 2.11 (s, 15H, 5 × CH3CO), 2.11 (s, 3H, CH3CO), 2.12 (s, 6H, 2 × CH3CO), 2.14 (s, 3H, CH3CO), 2.14 (s, 3H, CH3CO), 3.58 (t, 2H, J 5.0 Hz, CH2N3), 3.70–3.78 (m, 6H, H-5I-VIa), 3.86–3.90 (m, 1H, H-5VIb), 3.92–3.98 (m, 5H, H-5I-Vb), 4.11–4.13 (m, 3H, CH2O), 4.10–4.26 (m, 15H, H-4II-X, H-3VI, H-5VII-Xa, H-5VIIb or H-5IXb), 4.25 (d, 1H, J 2.0 Hz, H-2VII), 4.29 (d, 1H, J 1.7 Hz, H-2IX), 4.32 (dd, 1H, J 11.5 Hz, J 3.9 Hz, H-5VIIb or H-5IXb), 4.34–4.39 (m, 3H, H-4I, H-5VIIIb, H-5Xb), 4.95–5.01 (m, 4H, H-2VIII, H-2X, H-3VII, H-3IX), 5.02 (s, 1H, H-1VII), 5.04–5.07 (m, 3H, H-3V, H-2VI, H-1IX), 5.12–5.18 (m, 12H, H-1II-VI, H-2II-V, H-3II-IV), 5.29 (dd, 1H, J 5.3 Hz, J 1.9 Hz, H-3I), 5.32–5.37 (m, 3H, H-3VIII, H-3X, H-2I), 5.39 (d, 1H, J 4.7 Hz, H-1X), 5.44 (d, 1H, J 4.7 Hz, H-1VIII), 5.61 (s, 1H, H-1I), 6.84–6.88 (m, 2H, OC6H4O (H-3, H-5)), 6.98–7.03 (m, 2H, OC6H4O (H-2, H-6)); 13C NMR (151 MHz, CDCl3): δ 20.3 (CH3CO), 20.4 (CH3CO), 20.6 (CH3CO), 20.67 (CH3CO), 20.69 (CH3CO), 20.73 (CH3CO), 20.75 (CH3CO), 20.80 (CH3CO), 50.2 (CH2N3), 63.4 (C-5VII, C-5IX), 63.7 (C-5VII, C-5IX), 64.2 (C-5VI), 65.0 (C-5I-V, C-5VIII, C-5X), 65.1 (C-5I-V, C-5VIII, C-5X), 65.18 (C-5I-V, C-5VIII, C-5X), 65.24 (3C, C-5I-V, C-5VIII, C-5X), 65.7 (C-5I-V, C-5VIII, C-5X), 67.6 (CH2O), 75.5 (C-3VIII, C-3X), 75.7 (C-3VIII, C-3X), 76.4 (C-3I), 76.6 (C-3II-V, C-3VII, C-3IX, C-2VIII, C-2X), 76.7 (C-3II-V, C-3VII, C-3IX, C-2VIII, C-2X), 76.8 (C-3II-V, C-3VII, C-3IX, C-2VIII, C-2X), 76.9 (C-3II-V, C-3VII, C-3IX, C-2VIII, C-2X), 77.1 (C-3II-V, C-3VII, C-3IX, C-2VIII, C-2X), 77.4 (C-3II-V, C-3VII, C-3IX, C-2VIII, C-2X), 77.7 (C-3II-V, C-3VII, C-3IX, C-2VIII, C-2X), 78.9 (C-4VIII, C-4X), 78.6 (C-4VIII, C-4X), 80.3 (C-4II-VII, C-4IX, C-3VI, C-2II-V), 80.7 (C-4II-VII, C-4IX, C-3VI, C-2II-V), 80.8 (C-4II-VII, C-4IX, C-3VI, C-2II-V), 81.1 (C-4II-VII, C-4IX, C-3VI, C-2II-V), 81.2 (C-4II-VII, C-4IX, C-3VI, C-2II-V), 81.47 (C-4II-VII, C-4IX, C-3VI, C-2II-V), 81.51 (C-4II-VII, C-4IX, C-3VI, C-2II-V), 81.53 (C-4II-VII, C-4IX, C-3VI, C-2II-V), 81.68 (2C, C-4II-VII, C-4IX, C-3VI, C-2II-V), 81.73 (C-4II-VII, C-4IX, C-3VI, C-2II-V), 81.9 (C-4I), 82.0 (C-2I), 82.2 (C-4II-VII, C-4IX, C-3VI, C-2II-V), 83.4 (C-2VI), 83.9 (C-2IX), 84.2 (C-2VII), 99.3 (C-1X), 99.5 (C-1VIII), 104.8 (C-1I), 105.2 (C-1II-VII, C-1IX), 105.35 (3C, C-1II-VII, C-1IX), 105.39 (C-1II-VII, C-1IX), 105.43 (C-1II-VII, C-1IX), 105.7 (C-1II-VII, C-1IX), 115.6 (OC6H4O (C-3, C-5)), 118.3 (OC6H4O (C-2, C-6)), 150.4 (OC6H4O (C-1)), 153.8 (OC6H4O (C-4)), 169.7 (CO), 169.7 (CO), 169.9 (CO), 170.00 (CO), 170.02 (CO), 170.1 (CO), 170.2 (CO), 170.18 (CO), 170.20 (CO), 170.3 (CO), 170.46 (CO), 170.51 (CO), 170.6 (CO); HRMS (ESI): m/z [M + 2NH4]2+ Calcd for C100H139N5O632+ 1208.8908; found: 1208.8948.
5. Conclusions
In summary, a synthesis of the branched α-(1→3)-, α-(1→5), β-(1→2)-linked hexaarabinofuranoside with CEP aglycone using silylated Ara-β-(1→2)-Ara disaccharide was accomplished. The CEP aglycone in hexaarabinofuranoside was cleaved, and the obtained hemiacetal was converted to the new glycosyl donor with N-phenyltrifluoroacetimidoyl leaving group. The coupling of N-phenyltrifluoroacetimidate of hexaarabinofuranoside and benzoylated tetraarabinofuranoside α-(1→5)-linked glycosyl acceptor promoted by TfOH successfully led to the branched α-(1→5)-, α-(1→3)-, β-(1→2)-linked decaarabinofuranoside with CEP aglycone. After the replacement of the chlorine atom with an azido group in the aglycone of the resulting decaarabinofuranoside and following removal of silyl and acyl groups, the deprotected decaarabinofuranoside bearing 4-(2-azidoethoxy)phenyl aglycone (AEP) was obtained.
The synthesized decaarabinofuranoside and neoglycoconjugates derived from them expand the library of antigens based on oligoarabinofuranosides related to LAM and AG fragments of mycobacteria developed by us. It is important to note that such libraries hold promise for the creation of new multi-antigen/multi-epitope assays for the serodiagnosis of tuberculosis and leprosy, as it has been established that tests based on a single antigen may not be equally sensitive in all regions endemic for tuberculosis and leprosy [10].
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules30153295/s1, Supplementary Materials include NMR data for all synthesised compounds.
Author Contributions
P.I.A.: writing—original draft, validation, supervision, resources, project administration, methodology, investigation, funding acquisition, data curation, conceptualization; N.N.M.: investigation; M.Y.K.: investigation; D.S.N.: investigation; A.I.Z.: data curation, validation; N.G.K.: investigation; L.O.K.: writing—review and editing, validation, supervision, methodology, conceptualization. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation (project No. 21-73-20164-P).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
This work was financially supported by the Russian Science Foundation (project No. 21-73-20164-P). The NMR and Mass experiments were performed using the equipment of the Center for Collective Use of N.D. Zelinsky Institute of Organic Chemistry of the Russian Academy of Sciences.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of this study; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Paolo, W.F.; Nosanchuk, J.D. Tuberculosis in New York city: Recent lessons and a look ahead. Lancet Infect. Dis. 2004, 4, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.D.O. The world-wide increase in tuberculosis: How demographic changes, HIV infection and increasing numbers in poverty are increasing tuberculosis. Ann. Med. 2009, 35, 235–243. [Google Scholar] [CrossRef]
- World Health Organization. Tuberculosis. Available online: https://www.who.int/en/news-room/fact-sheets/detail/tuberculosis (accessed on 30 June 2025).
- Hamasur, B.; Ka, G.; Svenson, S.B. Synthesis and immunologic characterisation of Mycobacterium tuberculosis lipoarabinomannan specific oligosaccharide-protein conjugates. Vaccine 1999, 17, 2853–2861. [Google Scholar] [CrossRef]
- Zheng, R.B.; Jegouzo, S.A.F.; Joe, M.; Bai, Y.; Tran, H.A.; Shen, K.; Saupe, J.; Xia, L.; Ahmed, M.F.; Liu, Y.H.; et al. Insights into Interactions of Mycobacteria with the Host Innate Immune System from a Novel Array of Synthetic Mycobacterial Glycans. ACS Chem. Biol. 2017, 12, 2990–3002. [Google Scholar] [CrossRef]
- Tong, M.; Jacobi, C.E.; van de Rijke, F.M.; Kujiper, S.; van de Werken, S.; Lowary, T.L.; Hokke, C.H.; Appelmelk, B.J.; Nagelkerke, N.J.D.; Tanke, H.J.; et al. A multiplexed and miniaturized serological tuberculosis assay identifies antigens that discriminate maximally between TB and non-TB sera. J. Immunol. Methods 2005, 301, 154–163. [Google Scholar] [CrossRef]
- Kim, H.-S.; Ng, E.S.M.; Zheng, R.B.; Whittal, R.M.; Schriemer, D.C.; Lowary, T.L. Studies toward the development of anti-tuberculosis vaccines based on mycobacterial lipoarabinomannan. In Carbohydrate-Based Vaccines; Roy, R., Ed.; ACS Symposium Series; American Chemical Society: Columbus, OH, USA, 2008; Volume 989, pp. 184–198. [Google Scholar] [CrossRef]
- Chen, T.T.; Blanc, C.; Liu, Y.Y.; Ishida, E.; Singer, S.; Xu, J.Y.; Joe, M.; Jenny-Avital, E.R.; Chan, J.; Lowary, T.L.; et al. Capsular glycan recognition provides antibody-mediated immunity against tuberculosis. J. Clin. Investig. 2020, 130, 1808–1822. [Google Scholar] [CrossRef]
- Li, Z.H.; Bavaro, T.; Tengattini, S.; Bernardini, R.; Mattei, M.; Annunziata, F.; Cole, R.B.; Zheng, C.P.; Sollogoub, M.; Tamborini, L.; et al. Chemoenzymatic synthesis of arabinomannan (AM) glycoconjugates as potential vaccines for tuberculosis. Eur. J. Med. Chem. 2020, 204, 112578. [Google Scholar] [CrossRef] [PubMed]
- Korolyova-Ushakova, A.G.; Baranova, E.V.; Ignatov, S.G.; Soloviev, P.V.; Kondakov, N.N.; Mel’nikova, T.M.; Abronina, P.I.; Podval’nyi, N.M.; Kononov, L.O.; Biketov, S.F. Comparative Characteristics of the Diagnostic Potential of Mycobacterial Synthetic Antigens for the Seroriagnosis of Lepra and Tuberculosis. Appl. Biochem. Microbiol. 2019, 55, 696–703. [Google Scholar] [CrossRef]
- Mereyala, H.B.; Hotha, S.; Gurjar, M.K. Synthesis of pentaarabinofuranosyl structure motif A of Mycobacterium tuberculosis. Chem. Commun. 1998, 685–686. [Google Scholar] [CrossRef]
- Ishiwata, A.; Akao, H.; Ito, Y. Stereoselective synthesis of a fragment of mycobacterial arabinan. Org. Lett. 2006, 8, 5525–5528. [Google Scholar] [CrossRef]
- Joe, M.; Bai, Y.; Nacario, R.C.; Lowary, T.L. Synthesis of the docosanasaccharide arabinan domain of mycobacterial arabinogalactan and a proposed octadecasaccharide biosynthetic precursor. J. Am. Chem. Soc. 2007, 129, 9885–9901. [Google Scholar] [CrossRef]
- Sahloul, K.; Lowary, T.L. Development of an Orthogonal Protection Strategy for the Synthesis of Mycobacterial Arabinomannan Fragments. J. Org. Chem. 2015, 80, 11417–11434. [Google Scholar] [CrossRef]
- Islam, M.; Gayatri, G.; Hotha, S. Influence of Steric Crowding on Diastereoselective Arabinofuranosylations. J. Org. Chem. 2015, 80, 7937–7945. [Google Scholar] [CrossRef]
- Lowary, T.L. Twenty years of mycobacterial glycans: Furanosides and beyond. Acc. Chem. Res. 2016, 49, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Manmode, S.; Panda, R.R.A.; Hotha, S. Expedient Synthesis of a Linear Nonadecaarabinofuranoside of the Mycobacterium tuberculosis Cellular Envelope. Eur. J. Org. Chem. 2017, 2017, 4794–4802. [Google Scholar] [CrossRef]
- Wu, Y.; Xiong, D.C.; Chen, S.C.; Wang, Y.S.; Ye, X.S. Total synthesis of mycobacterial arabinogalactan containing 92 monosaccharide units. Nat. Commun. 2017, 8, 14851. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Vargas, A.; Bharate, P.; Delbianco, M.; Seeberger, P.H. Automated glycan assembly of arabinomannan oligosaccharides from Mycobacterium tuberculosis. Beilstein J. Org. Chem. 2019, 15, 2936–2940. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Guo, Z. An extensive review of studies on mycobacterium cell wall polysaccharide-related oligosaccharides—Part I: Synthetic studies on arabinofuranosyl oligosaccharides. J. Carbohydr. Chem. 2019, 38, 269–334. [Google Scholar] [CrossRef]
- Han, L.; Wang, L.; Guo, Z. An extensive review of studies on mycobacterium cell wall polysaccharide-related oligosaccharides—Part II: Synthetic studies on complex arabinofuranosyl oligosaccharides carrying other functional motifs and related derivatives and analogs. J. Carbohydr. Chem. 2019, 38, 335–382. [Google Scholar] [CrossRef]
- Liu, K.; Wang, L.; Guo, Z. An extensive review of studies on mycobacterium cell wall polysaccharide-related oligosaccharides—Part III: Synthetic studies and biological applications of arabinofuranosyl oligosaccharides and their analogs, derivatives and conjugates. J. Carbohydr. Chem. 2019, 38, 414–469. [Google Scholar] [CrossRef]
- Islam, M.; Shinde, G.P.; Hotha, S. Expedient synthesis of the heneicosasaccharyl mannose capped arabinomannan of the Mycobacterium tuberculosis cellular envelope by glycosyl carbonate donors. Chem. Sci. 2017, 8, 2033–2038. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, R.P.; Lowary, T.L. 2.08—Glycosylation with Furanosides. In Comprehensive Glycoscience, 2nd ed.; Barchi, J.J., Vidal, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 2, pp. 267–285. [Google Scholar] [CrossRef]
- Mayfield, A.B.; Metternich, J.B.; Trotta, A.H.; Jacobsen, E.N. Stereospecific Furanosylations Catalyzed by Bis-thiourea Hydrogen-Bond Donors. J. Am. Chem. Soc. 2020, 142, 4061–4069. [Google Scholar] [CrossRef]
- Xu, H.; Schaugaard, R.N.; Li, J.; Schlegel, H.B.; Nguyen, H.M. Stereoselective 1,2-cis Furanosylations Catalyzed by Phenanthroline. J. Am. Chem. Soc. 2022, 144, 7441–7456. [Google Scholar] [CrossRef]
- Abronina, P.I.; Fedina, K.G.; Podvalnyy, N.M.; Zinin, A.I.; Chizhov, A.O.; Kondakov, N.N.; Torgov, V.I.; Kononov, L.O. The use of O-trifluoroacetyl protection and profound influence of the nature of glycosyl acceptor in benzyl-free arabinofuranosylation. Carbohydr. Res. 2014, 396, 25–36. [Google Scholar] [CrossRef]
- Abronina, P.I.; Malysheva, N.N.; Stepanova, E.V.; Shvyrkina, J.S.; Zinin, A.I.; Kononov, L.O. Five triisopropylsilyl substituents in Ara-β-(1→2)-Ara disaccharide glycosyl donor make unselective glycosylation reaction stereoselective. Eur. J. Org. Chem. 2022, 2022, e202201110. [Google Scholar] [CrossRef]
- Abronina, P.I.; Novikov, D.S.; Malysheva, N.N.; Zinin, A.I.; Chizhov, A.O.; Kononov, L.O. Stereocontrolled 1,2-trans-arabinofuranosylation in the absence of 2-O-acyl group in glycosyl donor. Carbohydr. Res. 2024, 544, 109252. [Google Scholar] [CrossRef]
- Abronina, P.I.; Malysheva, N.N.; Zinin, A.I.; Kolotyrkina, N.G.; Kononov, L.O. Synthesis of hexaarabinofuranoside bearing the 4-(3-azidopropoxy)phenyl aglycone related to the terminal fragment of polysaccharides of mycobacteria. Russ. Chem. Bull. 2024, 73, 1417–1425. [Google Scholar] [CrossRef]
- Walther, A.; Muller, A.H. Janus particles: Synthesis, self-assembly, physical properties, and applications. Chem. Rev. 2013, 113, 5194–5261. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Abronina, P.I.; Malysheva, N.N.; Zinin, A.I.; Novikov, D.S.; Panova, M.V.; Kononov, L.O. Unusual triflic acid-promoted oligomerization of arabinofuranosides during glycosylation. Carbohydr. Res. 2024, 540, 109141. [Google Scholar] [CrossRef] [PubMed]
- Armarego, W.L.F. Purification of Laboratory Chemicals, 8th ed.; Butterworth-Heinemann: Oxford, UK, 2017; p. 1198. [Google Scholar] [CrossRef]
- Huchel, U.; Tiwari, P.; Schmidt, R.R. N-Aryl-O-glycosyl Haloacetimidates as Glycosyl Donors. J. Carbohydr. Chem. 2010, 29, 61–75. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).