Gastrointestinal Digestion Impact on Phenolics and Bioactivity of Tannat Grape Pomace Biscuits
Abstract
1. Introduction
2. Results and Discussion
2.1. Phenolic Compounds Profile in the Colonic Fraction (CF)
2.2. Total Phenolic Content (TPC)
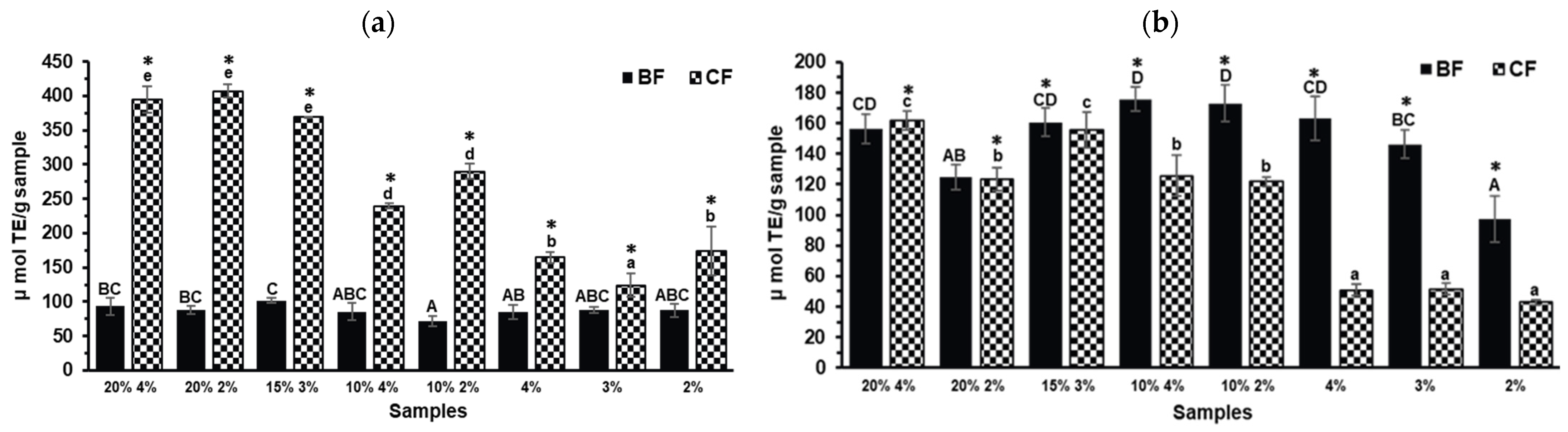
2.3. Antioxidant Capacity
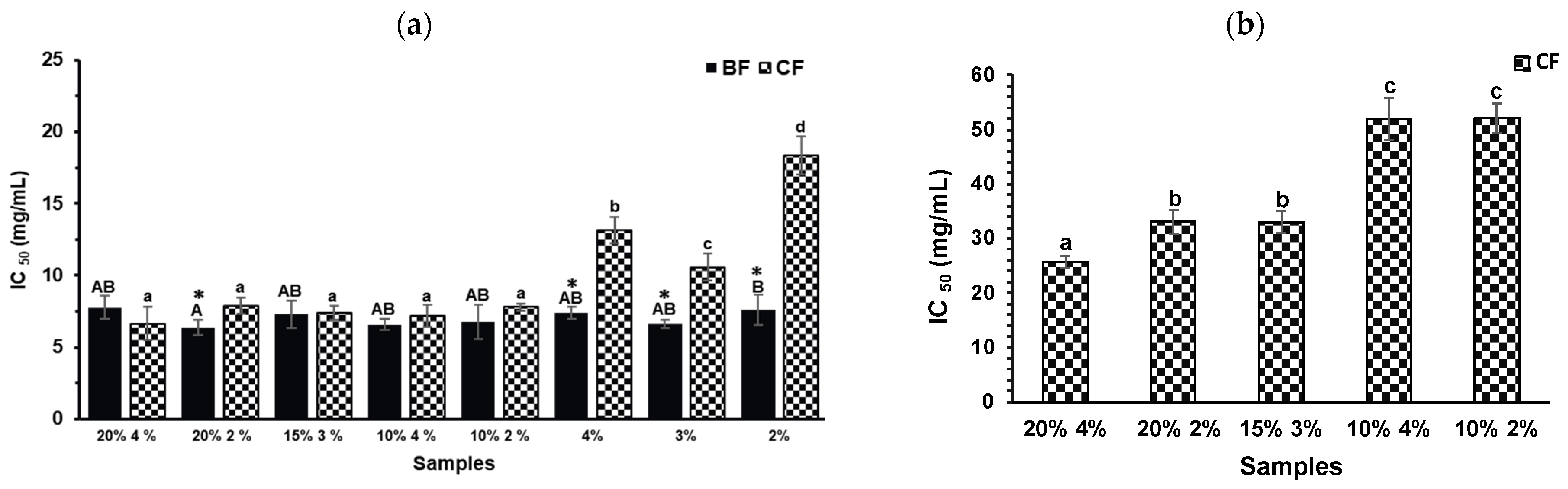
2.4. Anti-Diabetic and Anti-Obesity Capacities
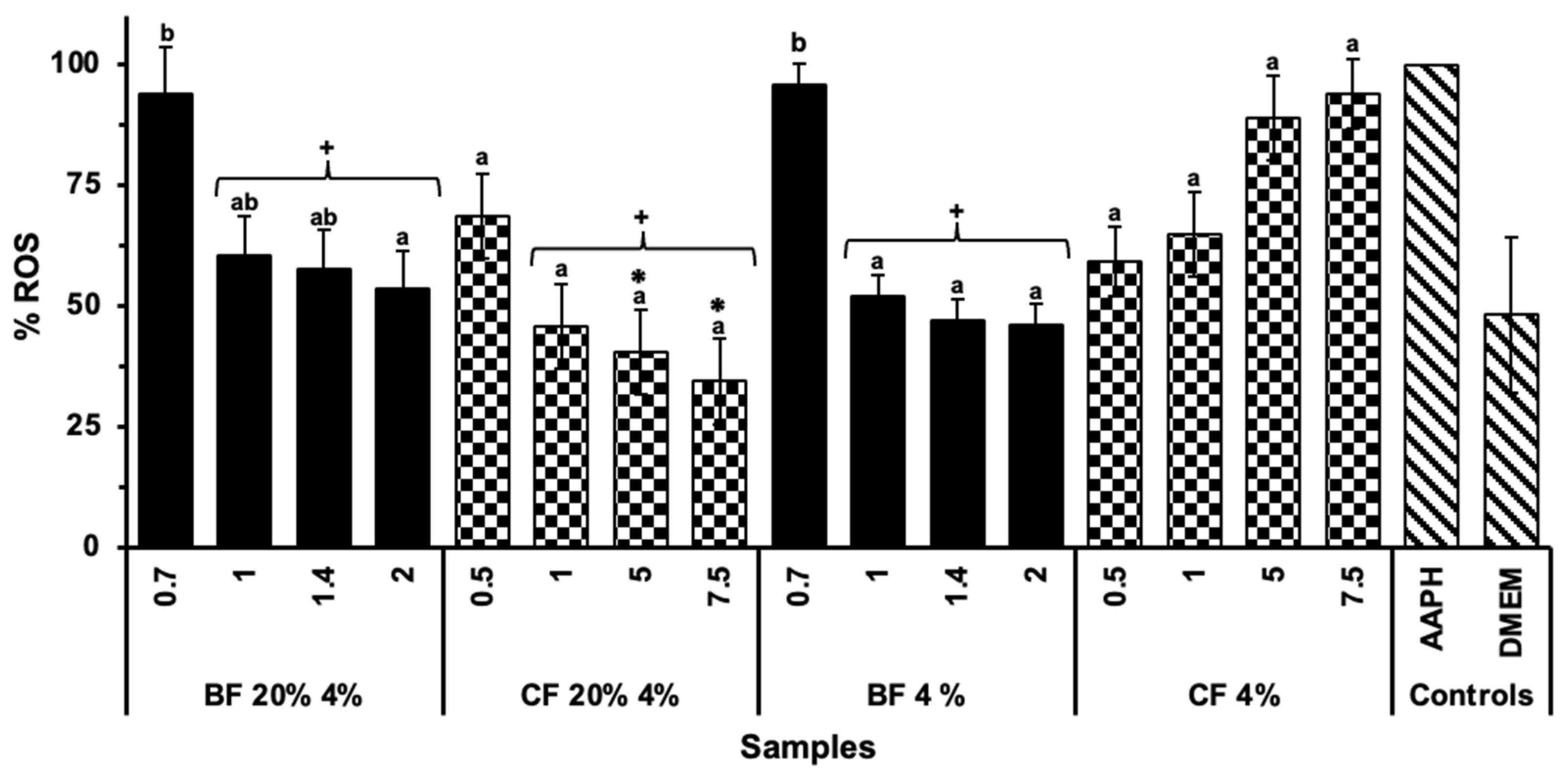
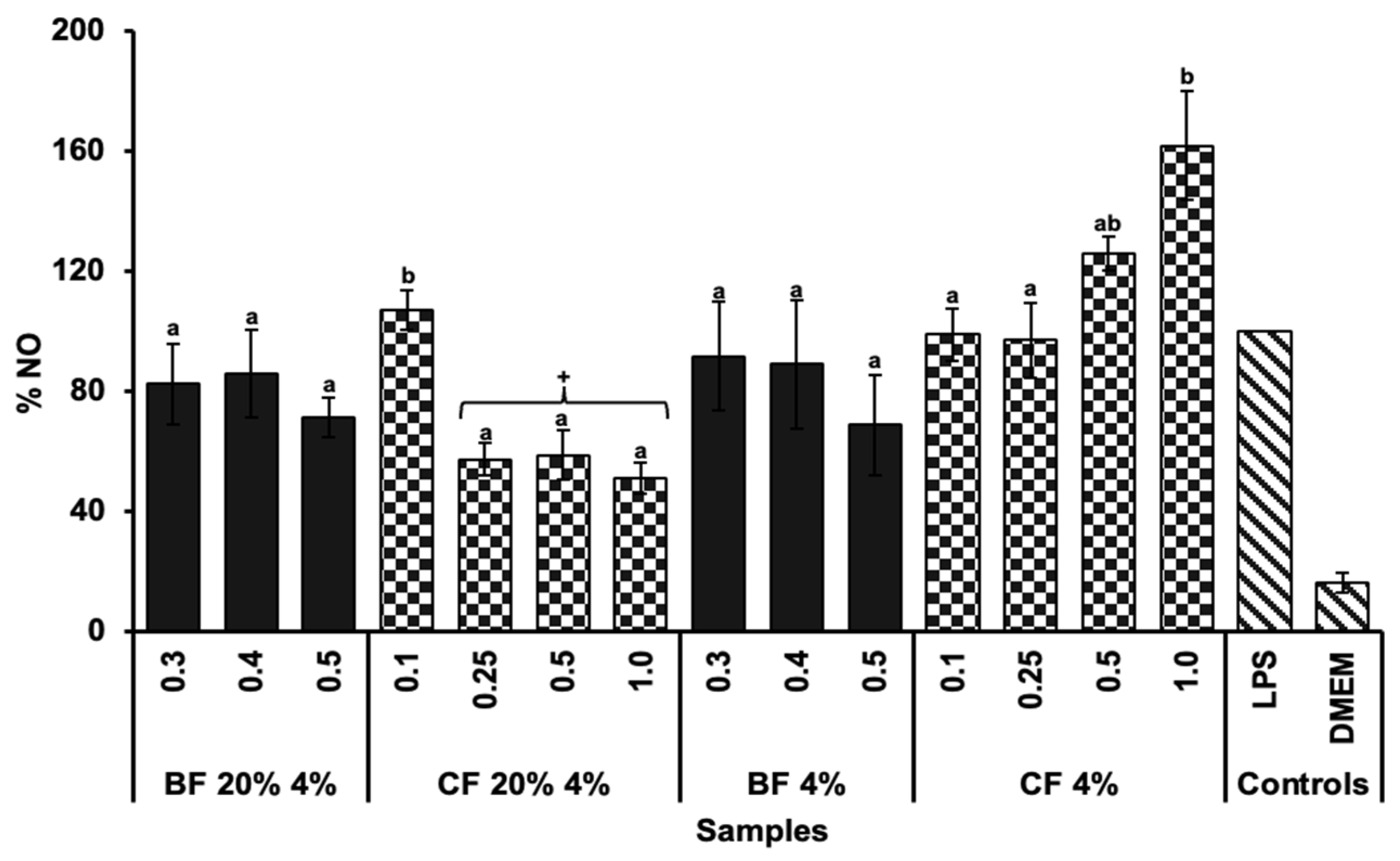
2.5. Anti-Inflammatory Capacity
3. Materials and Methods
3.1. Materials and Reagents
3.2. Samples
3.3. Simulated Digestion of the Biscuits
3.4. Identification of Phenolic Compounds in the Colonic Fraction
3.5. Total Phenolic Content
3.6. Antioxidant Capacity
3.7. Anti-Diabetic and Anti-Obesity Capacity
3.8. Anti-Inflammatory Capacity
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 4-MUG | 4-methylumbelliferyl-α-D-glucopyranoside |
| 4-MUO | 4-methylumbelliferyl oleate |
| AAPH | 2,2′-Azo- bis (2-methylpropionamidine) dihydrochloride |
| ABTS | 2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) |
| CCD 841 CoN | Cell line isolated from colon tissue of a healthy donor |
| FBS | Fetal bovine serum |
| GAE | Gallic acid equivalents |
| DCFH-DA | 2′,7′-dichlorodihydrofluorescein diacetate |
| DMEM | Dulbecco’s modified eagle medium |
| DMSO | Dimetilsulfoxide |
| HAT | Hydrogen atom transfer |
| HPLC-DAD-MS | High-performance liquid chromatography with diode array detection and mass spectrometry |
| IC50 | Inhibitory concentration 50% |
| LPS | Lipopolysaccharide |
| MTT | 3-(4,5-dimethylthiazole-y)-2,5-diphenyltetrazolium bromide |
| NO | Nitric oxide |
| ORAC | Oxygen radical absorbance capacity |
| RAW 264.7 | Mouse macrophage cell line |
| ROS | Reactive oxygen species |
| TPC | Total phenolic content |
| TGP | Tannat grape pomace |
| TROLOX | 6-hydroxy-2,5,7,8-tetramethylch-roman-2-acid |
| SET | Single electron transfer |
References
- Balli, D.; Cecchi, L.; Innocenti, M.; Bellumori, M.; Mulinacci, N. Food By-Products Valorisation: Grape Pomace and Olive Pomace (Pâté) as Sources of Phenolic Compounds and Fiber for Enrichment of Tagliatelle Pasta. Food Chem. 2021, 355, 129642. [Google Scholar] [CrossRef]
- Gerardi, G.; Cavia-Saiz, M.; Muñiz, P. From Winery By-Product to Healthy Product: Bioavailability, Redox Signaling and Oxidative Stress Modulation by Wine Pomace Product. Crit. Rev. Food Sci. Nutr. 2022, 62, 7427–7448. [Google Scholar] [CrossRef]
- Antonic, B.; Janciková, S.; Dani, D.; Tremolová, B. Grape Pomace Valorization: A Systematic Review and Meta-Analysis. Foods 2020, 9, 1627. [Google Scholar] [CrossRef]
- Caponio, G.R.; Minervini, F.; Tamma, G.; Gambacorta, G.; De Angelis, M. Promising Application of Grape Pomace and Its Agri-Food Valorization: Source of Bioactive Molecules with Beneficial Effects. Sustainability 2023, 15, 9075. [Google Scholar] [CrossRef]
- Da Silva, C.; Molin, A.D.; Ferrarini, A.; Boido, E.; Gaggero, C.; Delledonne, M.; Carrau, F. The Tannat Genome: Unravelling Its Unique Characteristics. BIO Web Conf. 2019, 12, 01016. [Google Scholar] [CrossRef]
- Olt, V.; Báez, J.; Curbelo, R.; Boido, E.; Amarillo, M.; Gámbaro, A.; Alborés, S.; García, N.G.; Cesio, M.V.; Heinzen, H.; et al. Tannat Grape Pomace as an Ingredient for Potential Functional Biscuits: Bioactive Compound Identification, In Vitro Bioactivity, Food Safety, and Sensory Evaluation. Front. Nutr. 2023, 10, 1241105. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Gervasi, T.; Calderaro, A.; Barreca, D.; Tellone, E.; Trombetta, D.; Ficarra, S.; Smeriglio, A.; Mandalari, G.; Gattuso, G. Biotechnological Applications and Health-Promoting Properties of Flavonols: An Updated View. Int. J. Mol. Sci. 2022, 23, 1710. [Google Scholar] [CrossRef]
- El Rayess, Y.; Nehme, N.; Azzi-Achkouty, S.; Julien, S.G. Wine Phenolic Compounds: Chemistry, Functionality and Health Benefits. Antioxidants 2024, 13, 1312. [Google Scholar] [CrossRef]
- Kurćubić, V.S.; Stanišić, N.; Stajić, S.B.; Dmitrić, M.; Živković, S.; Kurćubić, L.V.; Živković, V.; Jakovljević, V.; Mašković, P.Z.; Mašković, J. Valorizing Grape Pomace: A Review of Applications, Nutritional Benefits, and Potential in Functional Food Development. Foods 2024, 13, 4169. [Google Scholar] [CrossRef] [PubMed]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of In Vitro Digestion on Composition, Bioaccessibility and Antioxidant Activity of Food Polyphenols—A Non-Systematic Review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef] [PubMed]
- Ed Nignpense, B.; Francis, N.; Blanchard, C.; Santhakumar, A.B. Bioaccessibility and Bioactivity of Cereal Polyphenols: A Review. Foods 2021, 10, 1595. [Google Scholar] [CrossRef]
- Yang, C.; Han, Y.; Tian, X.; Sajid, M.; Mehmood, S.; Wang, H.; Li, H. Phenolic Composition of Grape Pomace and Its Metabolism. Crit. Rev. Food Sci. Nutr. 2022, 64, 4865–4881. [Google Scholar] [CrossRef] [PubMed]
- Tamargo, A.; Cueva, C.; Taladrid, D.; Khoo, C.; Moreno-Arribas, M.V.; Bartolomé, B.; González de Llano, D. Simulated Gastrointestinal Digestion of Cranberry Polyphenols under Dynamic Conditions. Impact on Antiadhesive Activity against Uropathogenic Bacteria. Food Chem. 2022, 368, 130871. [Google Scholar] [CrossRef] [PubMed]
- Duda-Chodak, A.; Tarko, T. Possible Side Effects of Polyphenols and Their Interactions with Medicines. Molecules 2023, 28, 2536. [Google Scholar] [CrossRef]
- Abia, R.; Fry, S.C. Degradation and Metabolism of 14C-Labelled Proanthocyanidins from Carob (Ceratonia Siliqua) Pods in the Gastrointestinal Tract of the Rat. J. Sci. Food Agric. 2001, 81, 1156–1165. [Google Scholar] [CrossRef]
- Nieto, J.A.; Fern, I.; Siles-s, N.; Jaime, L.; Santoyo, S. Implication of the Polymeric Phenolic Fraction and Matrix Effect on the Antioxidant Activity, Bioaccessibility, and Bioavailability of Grape Stem Extracts. Molecules 2023, 28, 2461. [Google Scholar] [CrossRef]
- Chedea, V.S.; Palade, L.M.; Marin, D.E.; Pelmus, R.S.; Habeanu, M.; Rotar, M.C.; Gras, M.A.; Pistol, G.C.; Taranu, I. Intestinal Absorption and Antioxidant Activity of Grape Pomace Polyphenols. Nutrients 2018, 10, 588. [Google Scholar] [CrossRef]
- Unusan, N. Proanthocyanidins in Grape Seeds: An Updated Review of Their Health Benefits and Potential Uses in the Food Industry. J. Funct. Foods 2020, 67, 103861. [Google Scholar] [CrossRef]
- Ferreyra, S.; Torres-Palazzolo, C.; Bottini, R.; Camargo, A.; Fontana, A. Assessment of In-Vitro Bioaccessibility and Antioxidant Capacity of Phenolic Compounds Extracts Recovered from Grapevine Bunch Stem and Cane by-Products. Food Chem. 2021, 348, 129063. [Google Scholar] [CrossRef]
- Pešić, M.B.; Milinčić, D.D.; Kostić, A.; Stanisavljević, N.S.; Vukotić, G.N.; Kojić, M.O.; Gašić, U.M.; Barać, M.B.; Stanojević, S.P.; Popović, D.A.; et al. In Vitro Digestion of Meat- and Cereal-Based Food Matrix Enriched with Grape Extracts: How Are Polyphenol Composition, Bioaccessibility and Antioxidant Activity Affected? Food Chem. 2019, 284, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Milinčić, D.D.; Stanisavljević, N.S.; Kostić, A.Z.; Gašić, U.M.; Stanojević, S.P.; Tešić, Ž.L.; Pešić, M.B. Bioaccessibility of Phenolic Compounds and Antioxidant Properties of Goat-Milk Powder Fortified with Grape-Pomace-Seed Extract after In Vitro Gastrointestinal Digestion. Antioxidants 2022, 11, 2164. [Google Scholar] [CrossRef]
- Núñez-Gómez, V.; González-Barrio, R.; Periago, M.J. Interaction between Dietary Fibre and Bioactive Compounds in Plant By-Products: Impact on Bioaccessibility and Bioavailability. Antioxidants 2023, 12, 976. [Google Scholar] [CrossRef]
- González-Aguilar, G.A.; Blancas-Benítez, F.J.; Sáyago-Ayerdi, S.G. Polyphenols Associated with Dietary Fibers in Plant Foods: Molecular Interactions and Bioaccessibility. Curr. Opin. Food Sci. 2017, 13, 84–88. [Google Scholar] [CrossRef]
- Martini, S.; Conte, A.; Cattivelli, A.; Tagliazucchi, D. Domestic Cooking Methods Affect the Stability and Bioaccessibility of Dark Purple Eggplant (Solanum Melongena) Phenolic Compounds. Food Chem. 2021, 341, 128298. [Google Scholar] [CrossRef]
- Ou, J.; Wang, M.; Zheng, J.; Ou, S. Positive and Negative Effects of Polyphenol Incorporation in Baked Foods. Food Chem. 2019, 284, 90–99. [Google Scholar] [CrossRef]
- Makarewicz, M.; Drożdż, I.; Tarko, T.; Duda-Chodak, A. The Interactions between Polyphenols and Microorganisms, Especially Gut Microbiota. Antioxidants 2021, 10, 188. [Google Scholar] [CrossRef]
- Taladrid, D.; de Llano, D.G.; Zorraquín-Peña, I.; Tamargo, A.; Silva, M.; Molinero, N.; Moreno-Arribas, M.V.; Bartolomé, B. Gastrointestinal Digestion of a Grape Pomace Extract: Impact on Intestinal Barrier Permeability and Interaction with Gut Microbiome. Nutrients 2021, 13, 2467. [Google Scholar] [CrossRef] [PubMed]
- Salazar-López, N.J.; Salmerón-Ruiz, M.L.; Domínguez-Avila, J.A.; Villegas-Ochoa, M.A.; Ayala-Zavala, J.F.; González-Aguila, G.A. Phenolic Compounds from “Hass” Avocado Peel Are Retained in the Indigestible Fraction after an In Vitro Gastrointestinal Digestion. J. Food Meas. Charact. 2021, 15, 1982–1990. [Google Scholar] [CrossRef]
- Milinčić, D.D.; Stanisavljević, N.S.; Pešić, M.M.; Kostić, A.; Stanojević, S.P.; Pešić, M.B. The Bioaccessibility of Grape-Derived Phenolic Compounds: An Overview. Foods 2025, 14, 607. [Google Scholar] [CrossRef]
- Luo, X.; Tian, M.; Cheng, Y.; Ji, C.; Hu, S.; Liu, H.; Lu, J.; Ren, J. Effects of Simulated In Vitro Gastrointestinal Digestion on Antioxidant Activities and Potential Bioaccessibility of Phenolic Compounds from K. Coccinea Fruits. Front Nutr. 2022, 9, 1024651. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, V.; Tagliamonte, S.; Ferracane, R.; Vitaglione, P. Potential Antidiabetic Effect of Muffins Formulated with Different Protein Hydrolysates: Role of Bioactive Peptides Formed during Digestion. J. Funct. Foods 2023, 109, 105810. [Google Scholar] [CrossRef]
- Moreno-Fernández, S.; Garcés-Rimón, M.; Miguel, M. Egg-Derived Peptides and Hydrolysates: A New Bioactive Treasure for Cardiometabolic Diseases. Trends Food Sci. Technol. 2020, 104, 208–218. [Google Scholar] [CrossRef]
- Fernández-Fernández, A.M.; Dellacassa, E.; Nardin, T.; Larcher, R.; Ibañez, C.; Terán, D.; Gámbaro, A.; Medrano-Fernandez, A.; Del Castillo, M.D. Tannat Grape Skin: A Feasible Ingredient for the Formulation of Snacks with Potential for Reducing the Risk of Diabetes. Nutrients 2022, 14, 419. [Google Scholar] [CrossRef]
- Odriozola-Serrano, I.; Nogueira, D.P.; Esparza, I.; Vaz, A.A.; Jiménez-Moreno, N.; Martín-Belloso, O.; Ancín-Azpilicueta, C. Stability and Bioaccessibility of Phenolic Compounds in Rosehip Extracts during In Vitro Digestion. Antioxidants 2023, 12, 1035. [Google Scholar] [CrossRef] [PubMed]
- Furger, C. Live Cell Assays for the Assessment of Antioxidant Activities of Plant Extracts. Antioxidants 2021, 10, 944. [Google Scholar] [CrossRef]
- Tonolo, F.; Coletta, S.; Fiorese, F.; Grinzato, A.; Albanesi, M.; Folda, A.; Ferro, S.; De Mario, A.; Piazza, I.; Mammucari, C.; et al. Sunflower Seed-Derived Bioactive Peptides Show Antioxidant and Anti-Inflammatory Activity: From in Silico Simulation to the Animal Model. Food Chem. 2023, 439, 138124. [Google Scholar] [CrossRef]
- Martins, I.M.; Macedo, G.A.; Macedo, J.A.; Roberto, B.S.; Chen, Q.; Blumberg, J.B.; Chen, C.-Y.O. Tannase Enhances the Anti-Inflammatory Effect of Grape Pomace in Caco-2 2 Cells Treated with IL-1β. J. Funct. Foods. 2017, 29, 69–76. [Google Scholar] [CrossRef]
- Liu, Y.F.; Oey, I.; Bremer, P.; Carne, A.; Silcock, P. Bioactive Peptides Derived from Egg Proteins: A Review. Crit. Rev. Food Sci. Nutr. 2018, 58, 2508–2530. [Google Scholar] [CrossRef]
- Aleixandre, A.; Gil, J.V.; Sineiro, J.; Rosell, C.M. Understanding Phenolic Acids Inhibition of α-Amylase and α-Glucosidase and Influence of Reaction Conditions. Food Chem. 2022, 372, 131231. [Google Scholar] [CrossRef]
- Cisneros-Yupanqui, M.; Lante, A.; Mihaylova, D.; Krastanov, A.I.; Rizzi, C. The α-Amylase and α-Glucosidase Inhibition Capacity of Grape Pomace: A Review. Food Bioprocess Technol. 2023, 16, 691–703. [Google Scholar] [CrossRef]
- Dwibedi, V.; Jain, S.; Singhal, D.; Mittal, A.; Rath, S.K.; Saxena, S. Inhibitory Activities of Grape Bioactive Compounds against Enzymes Linked with Human Diseases. Appl. Microbiol. Biotechnol. 2022, 106, 1399–1417. [Google Scholar] [CrossRef] [PubMed]
- Sales, A.L.; Iriondo-DeHond, A.; DePaula, J.; Ribeiro, M.; Ferreira, I.M.P.L.V.O.; Miguel, M.A.L.; del Castillo, M.D.; Farah, A. Intracellular Antioxidant and Anti-Inflammatory Effects and Bioactive Profiles of Coffee Cascara and Black Tea Kombucha Beverages. Foods 2023, 12, 1905. [Google Scholar] [CrossRef]
- Bocsan, I.C.; Măgureanu, D.C.; Pop, R.M.; Levai, A.M.; Macovei Ștefan, O.; Pătrașca, I.M.; Chedea, V.S.; Buzoianu, A.D. Antioxidant and Anti-Inflammatory Actions of Polyphenols from Red and White Grape Pomace in Ischemic Heart Diseases. Biomedicines 2022, 10, 2337. [Google Scholar] [CrossRef]
- Fernández-Fernández, A.M.; Iriondo-DeHond, A.; Dellacassa, E.; Medrano-Fernandez, A.; del Castillo, M.D. Assessment of Antioxidant, Antidiabetic, Antiobesity, and Anti-Inflammatory Properties of a Tannat Winemaking by-Product. Eur. Food Res. Technol. 2019, 245, 1539–1551. [Google Scholar] [CrossRef]
- Fernández-Fernández, A.M.; Iriondo-DeHond, A.; Nardin, T.; Larcher, R.; Dellacassa, E.; Medrano-Fernandez, A.; Castillo, M.D. del In Vitro Bioaccessibility of Extractable Compounds from Tannat Grape Skin Possessing Health Promoting Properties with Potential to Reduce the Risk of Diabetes. Foods 2020, 9, 1575. [Google Scholar] [CrossRef]
- Serreli, G.; Deiana, M. Role of Dietary Polyphenols in the Activity and Expression of Nitric Oxide Synthases: A Review. Antioxidants 2023, 12, 147. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Liu, X.; Feng, H.; Zhao, S.; Gao, H. Grape Seed Proanthocyanidin Extracts Enhance Endothelial Nitric Oxide Synthase Expression through 5′-AMP Activated Protein Kinase/Surtuin 1-Krüpple like Factor 2 Pathway and Modulate Blood Pressure in Ouabain Induced Hypertensive Rats. Biol. Pharm. Bull. 2012, 35, 2192–2197. [Google Scholar] [CrossRef]
- Stevens, J.; Miranda, C.; Wolthers, K.; Schimerlik, M.; Deinzer, M.; Buhler, D. Identification and In Vitro Biological Activities of Hop Proanthocyanidins: Inhibition of NNOS Activity and Scavenging of Reactive Nitrogen Species. J. Agric. Food Chem. 2002, 50, 3435–3443. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Du, B.; Li, J.; Yang, Y.; Zhu, F. An Insight into Anti-Inflammatory Activities and Inflammation Related Diseases of Anthocyanins: A Review of Both in Vivo and In Vitro Investigations. Int. J. Mol. Sci. 2021, 22, 11076. [Google Scholar] [CrossRef]
- Singh, A.; Yau, Y.F.; Leung, K.S.; El-Nezami, H.; Lee, J.C.Y. Interaction of Polyphenols as Antioxidant and Anti-Inflammatory Compounds in Brain–Liver–Gut Axis. Antioxidants 2020, 9, 669. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static In Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Peña-Vázquez, G.I.; Dominguez-Fernández, M.T.; Camacho-Zamora, B.D.; Hernandez-Salazar, M.; Urías-Orona, V.; De Peña, M.P.; de la Garza, A.L. In Vitro Simulated Gastrointestinal Digestion Impacts Bioaccessibility and Bioactivity of Sweet Orange (Citrus Sinensis) Phenolic Compounds. J. Funct. Foods 2022, 88, 104891. [Google Scholar] [CrossRef]
- Fernández-Fernández, A.M.; Dumay, E.; Lazennec, F.; Migues, I.; Heinzen, H.; Lema, P.; López-Pedemonte, T.; Medrano-Fernandez, A. Antioxidant, Antidiabetic, and Antiobesity Properties, Tc7-Cell Cytotoxicity and Uptake of Achyrocline Satureioides (Marcela) Conventional and High Pressure-Assisted Extracts. Foods 2021, 10, 893. [Google Scholar] [CrossRef] [PubMed]

| Compound | CF TGP | CF Biscuit 20% 4% | ||||
|---|---|---|---|---|---|---|
| Rt (min) | Area | Rt (min) | Area | |||
| Chromatogram 280 nm | Phenolic acids | Trans-caftaric acid | 6.911 | 16,981 | ||
| Flavan-3-ols | Procyanidin trimer C2 | 4.62 | 4624 | 4.867 | 1495 | |
| Procyanidin dimer B1 | 7.334 | 9819 | 7.224 | 2166 | ||
| Procyanidin dimer B3 | 7.795 | 63,891 | 7.768 | 21,267 | ||
| (+)-Catechin | 8.335 | 130,356 | 8.274 | 41,044 | ||
| Procyanidin trimer | 9.008 | 185,897 | 9.109 | 271,845 | ||
| Procyanidin trimer | 10.554 | 99,312 | 10.509 | 30,335 | ||
| Procyanidin dimer B4 | 11.397 | 35,471 | 11.299 | 9612 | ||
| Procyanidin dimer B6 | 11.952 | 22,016 | 11.826 | 3210 | ||
| (−)-Epicatechin | 12.442 | 84,897 | 12.379 | 28,992 | ||
| Galloylated procyanidin dimer | 15.364 | 19,076 | 15.346 | 14,467 | ||
| (−)-Epicatechin gallate | 18.389 | 6723 | ||||
| Flavonols | Myricetin-3-O-glucoside | 21.816 | 9007 | |||
| Quercetin-3-O-glucoside | 23.388 | 60,556 | 23.385 | 4589 | ||
| Quercetin-7-O-neohesperidoside | 28.161 | 26,998 | 28.204 | 8073 | ||
| Quercetin (aglycone) | 35.086 | 44,860 | 35.165 | 15,043 | ||
| Chromatogram 520 nm | Anthocyanins | Delphinidin-3-O-glucoside | 14.221 | 6325 | 14.236 | 1490 |
| Cyanidin-3-O-glucoside | 16.208 | 2038 | ||||
| Petunidin-3-O-glucoside | 17.65 | 54,033 | 17.709 | 6960 | ||
| Peonidin-3-O-glucoside | 19.669 | 36,138 | 19.771 | 1733 | ||
| Malvidin-3-O-glucoside | 20.771 | 398,827 | 20.925 | 42,507 | ||
| Petunidin-3-O-(6′-acetyl)glucoside | 26.555 | 10,056 | 26.61 | 1621 | ||
| Peonidin-3-O-(6′-acetyl)glucoside | 28.907 | 6793 | ||||
| Malvidin-3-O-(6′-acetyl)glucoside | 29.508 | 39,730 | 29.555 | 4603 | ||
| Delfinidin-3-O-(6′-p-coumaroyl)glucoside | 30.471 | 26,936 | 30.527 | 9299 | ||
| Malvidin-3-O-(6′-caffeoyl)glucoside | 32.356 | 30,092 | 32.39 | 3240 | ||
| Cyanidin-3-O-(6′-p-coumaroyl)glucoside | 32.766 | 8846 | 32.812 | 2113 | ||
| Petunidin-3-O-(6′-p-coumaroyl)glucoside | 33.448 | 55,245 | 33.495 | 17,043 | ||
| Peonidin-3-O-(6′-p-coumaroyl)glucoside | 35.865 | 37,621 | 35.913 | 10,862 | ||
| Malvidin-3-O-(6′-p-coumaroyl)glucoside | 36.19 | 362,321 | 36.236 | 121,228 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olt, V.; Báez, J.; Curbelo, R.; Boido, E.; Dellacassa, E.; Medrano, A.; Fernández-Fernández, A.M. Gastrointestinal Digestion Impact on Phenolics and Bioactivity of Tannat Grape Pomace Biscuits. Molecules 2025, 30, 3247. https://doi.org/10.3390/molecules30153247
Olt V, Báez J, Curbelo R, Boido E, Dellacassa E, Medrano A, Fernández-Fernández AM. Gastrointestinal Digestion Impact on Phenolics and Bioactivity of Tannat Grape Pomace Biscuits. Molecules. 2025; 30(15):3247. https://doi.org/10.3390/molecules30153247
Chicago/Turabian StyleOlt, Victoria, Jessica Báez, Romina Curbelo, Eduardo Boido, Eduardo Dellacassa, Alejandra Medrano, and Adriana Maite Fernández-Fernández. 2025. "Gastrointestinal Digestion Impact on Phenolics and Bioactivity of Tannat Grape Pomace Biscuits" Molecules 30, no. 15: 3247. https://doi.org/10.3390/molecules30153247
APA StyleOlt, V., Báez, J., Curbelo, R., Boido, E., Dellacassa, E., Medrano, A., & Fernández-Fernández, A. M. (2025). Gastrointestinal Digestion Impact on Phenolics and Bioactivity of Tannat Grape Pomace Biscuits. Molecules, 30(15), 3247. https://doi.org/10.3390/molecules30153247











