In Vitro and In Vivo Efficacy of the Essential Oil from the Leaves of Annona amazonica R.E. Fries (Annonaceae) Against Liver Cancer
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Constituents of the EO from the Leaves of A. amazonica
2.2. In Vitro Cytotoxic Effects of EO from the Leaves of A. amazonica
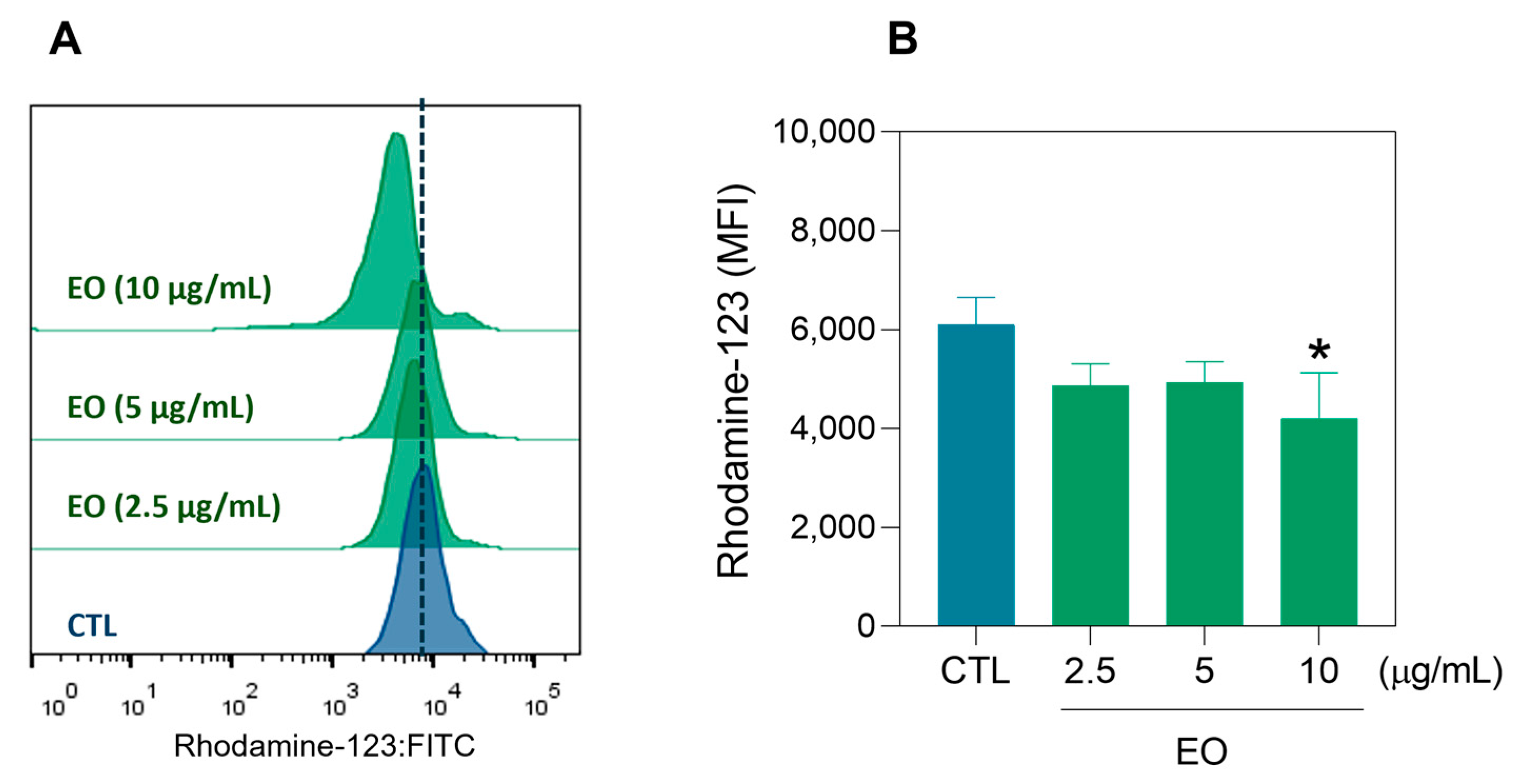
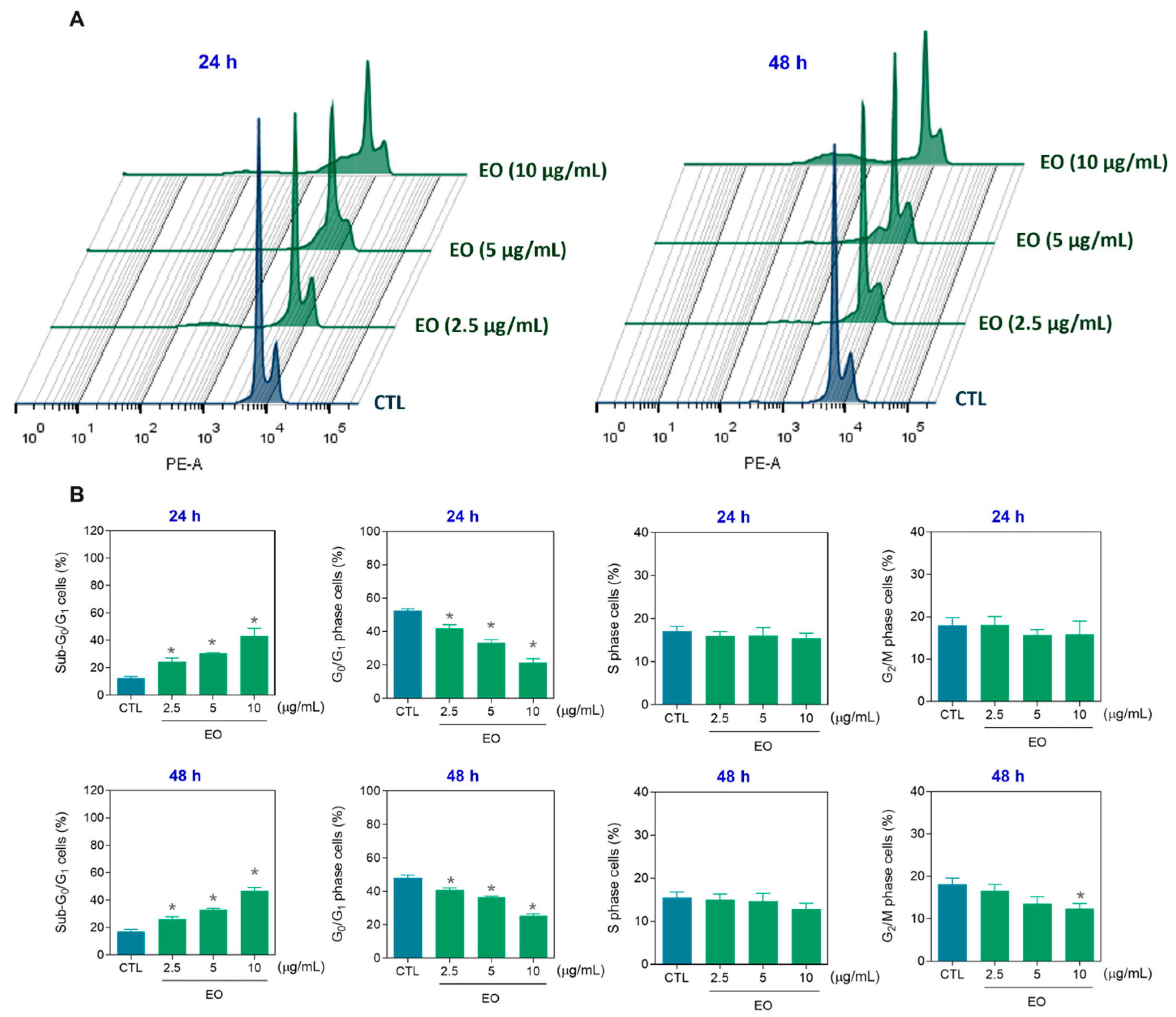
2.3. Apoptosis Induction by A. amazonica Leaf EO
2.4. In Vivo Effects of A. amazonica Leaf EO on Liver Cancer Development in a Xenograft Model
3. Conclusions
4. Materials and Methods
4.1. Botanical Material
4.2. EO Extraction
4.3. Chemical Analysis of the EO
4.4. Cells
4.5. Alamar Blue Assay
4.6. Flow Cytometry Assays
4.7. Animals
4.8. Human Liver Tumor Xenograft Model
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef]
- Brown, Z.J.; Tsilimigras, D.I.; Ruff, S.M.; Mohseni, A.; Kamel, I.R.; Cloyd, J.M.; Pawlik, T.M. Management of hepatocellular carcinoma: A review. JAMA Surg. 2023, 158, 410–420. [Google Scholar] [CrossRef]
- Shin, H.; Yu, S.J. A concise review of updated global guidelines for the management of hepatocellular carcinoma: 2017-2024. J. Liver Cancer 2025, 25, 19–30. [Google Scholar] [CrossRef]
- American Cancer Society. Cancer Facts & Figures 2025. Atlanta, Ga: American Cancer Society; 2025. Available online: https://www.cancer.org/cancer/types/liver-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 20 June 2025).
- Sharma, M.; Grewal, K.; Jandrotia, R.; Batish, D.R.; Singh, H.P.; Kohli, R.K. Essential oils as anticancer agents: Potential role in malignancies, drug delivery mechanisms, and immune system enhancement. Biomed. Pharmacother. 2022, 146, 112514. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.C.D.; Nogueira, M.L.; Oliveira, F.P.; Costa, E.V.; Bezerra, D.P. Essential oils of Duguetia species A. St. Hill (Annonaceae): Chemical diversity and pharmacological potential. Biomolecules 2022, 12, 615. [Google Scholar] [CrossRef] [PubMed]
- Abdoul-Latif, F.M.; Ainane, A.; Aboubaker, I.H.; Mohamed, J.; Ainane, T. Exploring the potent anticancer activity of essential oils and their bioactive compounds: Mechanisms and prospects for future cancer therapy. Pharmaceuticals 2023, 16, 1086. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, D.P.; Damasceno, R.O.S.; Amorati, R.; Elshabrawy, H.A.; de Castro, R.D.; Bezerra, D.P.; Nunes, V.R.V.; Gomes, R.C.; Lima, T.C. Essential oils: Chemistry and pharmacological activities. Biomolecules 2023, 13, 1144. [Google Scholar] [CrossRef]
- Alabrahim, O.A.A.; Lababidi, J.M.; Fritzsche, W.; Azzazy, H.M.E. Beyond aromatherapy: Can essential oil loaded nanocarriers revolutionize cancer treatment? Nanoscale Adv. 2024, 6, 5511–5562. [Google Scholar] [CrossRef]
- Maas, P.J.M.; Maas, H.; Miralha, J.M.S.; Junikka, L. Flora da reserva Ducke, Amazonas, Brasil: Annonaceae. Rodriguésia 2007, 58, 617. [Google Scholar] [CrossRef]
- The Herbarium Catalogue, Royal Botanic Gardens, Kew. Published on the Internet. Available online: http://www.kew.org/herbcat (accessed on 2 June 2025).
- Mendes-Silva, I.; Lopes, J.C.; Silva, L.V.; Bazante, M.L. Annona in flora e funga do Brasil. Jardim Botânico do Rio de Janeiro. Available online: https://floradobrasil.jbrj.gov.br/FB117059 (accessed on 9 June 2025).
- Thomsen, K.; Brimer, L. Cyanogenic constituents in woody plants in natural lowland rain forest in Costa Rica. Bot. J. Linnean Soc. 1997, 124, 273–294. [Google Scholar] [CrossRef][Green Version]
- Pinheiro, M.L.B.; Xavier, C.M.; De Souza, A.D.; Rabelo, D.D.M.; Batista, C.L.; Batista, R.L.; Costa, E.V.; Campos, F.R.; Barison, A.; Valdez, R.H.; et al. Acanthoic acid and other constituents from the stem of Annona amazonica (Annonaceae). J. Braz. Chem. Soc. 2009, 20, 1095–1102. [Google Scholar] [CrossRef]
- Alcântara, J.M.; Lucena, J.M.V.M.; Marques, M.O.M.; Lima, J.A.O.; Lima, M.P. Chemical composition and antibacterial activity of the essential oils of the Amazon Annona species. Orbital: Electron. J. Chem. 2024, 16, 263–266. [Google Scholar] [CrossRef]
- Van Den Dool, H.A.N.D.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Adams, R.P. 2007Identification of Essential oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Publishing, Corp: Carol Stream, IL, USA, 2007; 803p. [Google Scholar]
- Costa, E.V.; Pinheiro, M.L.B.; Silva, J.R.A.; Maia, B.H.L.N.S.; Duarte, M.C.T.; Amaral, A.C.F.; Machado, G.M.C.; Leon, L.L. Antimicrobial and antileishmanial activity of essential oil from the leaves of Annona foetida (Annonaceae). Quim. Nova 2009, 32, 78–81. [Google Scholar] [CrossRef]
- Costa, E.V.; Dutra, L.M.; Salvador, M.J.; Ribeiro, L.H.; Gadelha, F.R.; de Carvalho, J.E. Chemical composition of the essential oils of Annona pickelii and Annona salzmannii (Annonaceae), and their antitumour and trypanocidal activities. Nat. Prod. Res. 2013, 27, 997–1001. [Google Scholar] [CrossRef]
- Elhawary, S.S.; El Tantawy, M.E.; Rabeh, M.A.; Fawaz, N.E. DNA fingerprinting, chemical composition, antitumor and antimicrobial activities of the essential oils and extractives of four Annona species from Egypt. J. Nat. Sci. Res. 2013, 3, 59–68. [Google Scholar]
- Campos, F.G.; Baron, D.; Marques, M.O.M.; Ferreira, G.; Boaro, C.S.F. Caracterização do perfil químico do óleo essencial de Annona emarginata (Schltdl.) H. Rainer ‘terra-fria’ and Annona squamosa L. Rev. Bras. Frutic. 2014, 36, 202–208. [Google Scholar] [CrossRef][Green Version]
- Brito, M.T.; Ferreira, R.C.; Beltrao, D.M.; Moura, A.P.G.; Xavier, A.L.; Pita, J.C.L.R.; Batista, T.M.; Longato, G.B.; Ruiz, A.L.T.G.; Carvalho, J.E.D.; et al. Antitumor activity and toxicity of volatile oil from the leaves of Annona leptopetala. Rev. Bras. Farmacogn. 2018, 28, 602–609. [Google Scholar] [CrossRef]
- Souza, M.P.; de Castro, M.V.L.; Barbosa, G.A.d.C.; Carvalho, S.G.; Coelho, A.M.R.M.; Dias, R.B.; Soares, M.B.P.; Costa, E.V.; Bezerra, D.P. Essential oil from the leaves of Annona neoinsignis H. Rainer (Annonaceae) against liver cancer: In vitro and in vivo studies. Molecules 2025, 30, 2971. [Google Scholar] [CrossRef]
- Bomfim, L.M.; Menezes, L.R.; Rodrigues, A.C.; Dias, R.B.; Rocha, C.A.; Soares, M.B.; Neto, A.F.; Nascimento, M.P.; Campos, A.F.; Silva, L.C.; et al. Antitumour activity of the microencapsulation of Annona vepretorum essential oil. Basic Clin. Pharmacol. Toxicol. 2016, 118, 208–213. [Google Scholar] [CrossRef]
- Rabelo, S.V.; Oliveira, F.G.D.S.; Lira, M.M.C.; Dutra, L.M.; Sartoratto, A.; Duarte, M.C.T.; Luciano, M.C.D.S.; Silva, M.F.S.; Pessoa, C.D.O.; Moraes Filho, M.O.; et al. Non-polar chemical constituents of atemoya and evaluation of the cytotoxic and antimicrobial activity. Phyton 2021, 90, 921–931. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Elzefzafy, N.; El-Khadragy, M.F.; Alzahrani, A.; Yehia, H.M.; Kachlicki, P. Comprehensive tools of alkaloid/volatile compounds-metabolomics and DNA profiles: Bioassay-role-guided differentiation process of six Annona sp. Grown in Egypt as anticancer therapy. Pharmaceuticals 2024, 17, 103. [Google Scholar] [CrossRef]
- Soares, E.R.; Almeida, R.A.; Lima, B.R.; Pereira Junior, R.C.; Freitas, F.A.; Mafra, H.R.; Araujo, N.F.; Maciel, J.B.; Leão, L.Q.S.; Souza, A.D.L.; et al. Chemical composition of essential oils of three species of the genus Bocageopsis (Annonaceae) Amazon region. Rev. Vir. Quím 2022, 14, 947–953. [Google Scholar] [CrossRef]
- Ahmed, A.L.; Bassem, S.E.; Mohamed, Y.H.; Gamila, M.W. Cytotoxic essential oil from Annona sengalensis Pers. leaves. Pharmacogn. Res. 2010, 2, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Formagio, A.S.; Vieira, M.C.; Dos Santos, L.A.; Cardoso, C.A.; Foglio, M.A.; de Carvalho, J.E.; Andrade-Silva, M.; Kassuya, C.A. Composition and evaluation of the anti-inflammatory and anticancer activities of the essential oil from Annona sylvatica A. St.-Hil. J. Med. Food. 2013, 16, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Peng, C.X.; Hu, Y.; Bu, C.; Guo, S.C.; Li, X.; Chen, Y.; Chen, J.W. Studies on chemical constituents and anti-hepatoma effects of essential oil from Annona squamosa L. pericarps. Nat. Prod. Res. 2017, 31, 1305–1308. [Google Scholar] [CrossRef] [PubMed]
- Idziorek, T.; Estaquier, J.; De Bels, F.; Ameisen, J.C. YOPRO-1 permits cytofluorometric analysis of programmed cell death (apoptosis) without interfering with cell viability. J. Immunol. Methods 1995, 185, 249–258. [Google Scholar] [CrossRef]
- Ahmed, E.A. The Potential therapeutic role of beta-caryophyllene as a chemosensitizer and an inhibitor of angiogenesis in cancer. Molecules 2025, 30, 1751. [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.; Asif, M.; Ahmed, M.; Babu, D.; Hassan, L.E.; Ahamed, M.B.K.; Sandai, D.; Barakat, K.; Siraki, A.; et al. β-Caryophyllene induces apoptosis and inhibits angiogenesis in colorectal cancer models. Int. J. Mol. Sci. 2021, 22, 10550. [Google Scholar] [CrossRef]
- Arul, S.; Rajagopalan, H.; Ravi, J.; Dayalan, H. Beta-caryophyllene suppresses ovarian cancer proliferation by inducing cell cycle arrest and apoptosis. Anticancer Agents Med. Chem. 2020, 20, 1530–1537. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Liao, B.; Zhu, P.; Cheng, S.; Du, Z.; Jiang, G. β-Caryophyllene induces apoptosis and inhibits cell proliferation by deregulation of STAT-3/mTOR/AKT signaling in human bladder cancer cells: An in vitro study. J. Biochem. Mol. Toxicol. 2021, 35, e22863. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.S.; Hong, J.Y.; Lee, J.H.; Lee, H.J.; Park, J.Y.; Choi, J.H.; Park, H.J.; Hong, J.; Lee, K.T. β-Caryophyllene in the essential oil from Chrysanthemum Boreale induces G1 phase cell cycle arrest in human lung cancer cells. Molecules 2019, 24, 3754. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Wang, Q.; Li, G.; Li, Y.; Zhang, P.; Xu, G. β-Caryophyllene from chilli pepper inhibits the proliferation of non-small cell lung cancer cells by affecting miR-659-3p-targeted sphingosine kinase 1 (SphK1). Int. J. Gen. Med. 2021, 14, 9599–9613. [Google Scholar] [CrossRef]
- Ahmed, E.A.; Abu Zahra, H.; Ammar, R.B.; Mohamed, M.E.; Ibrahim, H.M. Beta-caryophyllene enhances the anti-tumor activity of cisplatin in lung cancer cell lines through regulating cell cycle and apoptosis signaling molecules. Molecules 2022, 27, 8354. [Google Scholar] [CrossRef]
- Jung, J.I.; Kim, E.J.; Kwon, G.T.; Jung, Y.J.; Park, T.; Kim, Y.; Yu, R.; Choi, M.S.; Chun, H.S.; Kwon, S.H.; et al. β-Caryophyllene potently inhibits solid tumor growth and lymph node metastasis of B16F10 melanoma cells in high-fat diet-induced obese C57BL/6N mice. Carcinogenesis 2015, 36, 1028–1039. [Google Scholar] [CrossRef]
- Silva, T.B.; Menezes, L.R.A.; Sampaio, M.F.C.; Meira, C.S.; Guimaraes, E.T.; Soares, M.B.P.; Prata, A.P.N.; Nogueira, P.C.L.; Costa, E.V. Chemical composition and anti-Trypanosoma cruzi activity of essential oils obtained from leaves of Xylopia frutescens and X. laevigata (Annonaceae). Nat. Prod. Commun. 2013, 8, 403–406. [Google Scholar] [CrossRef]
- ATCC Animal Cell Culture Guide: Get Time-Tested Tips for Culturing ATCC Animal Cells. Available online: https://www.atcc.org/resources/culture-guides/animal-cell-culture-guide (accessed on 9 June 2024).
- Ahmed, S.A.; Gogal, R.M.; Walsh, J.E. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes an alternative to [3H] thymidine incorporation assay. J. Immunol. Methods 1994, 170, 211–224. [Google Scholar] [CrossRef]
- Sureda, F.X.; Escubedo, E.; Gabriel, C.; Comas, J.; Camarasa, J.; Camins, A. Mitochondrial membrane potential measurement in rat cerebellar neurons by flow cytometry. Cytometry 1997, 28, 74–80. [Google Scholar] [CrossRef]
- Nicoletti, I.; Migliorati, G.; Pagliacci, M.C.; Grignani, F.; Riccardi, C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods 1991, 139, 271–279. [Google Scholar] [CrossRef] [PubMed]




| Compounds | AI a | AI b | Peak Area (%) | |
|---|---|---|---|---|
| 1 | α-pinene | 929 | 932 | 5.13 ± 1.72 |
| 2 | α-fenchene | 943 | 945 | 0.32 ± 0.20 |
| 3 | sabinene | 969 | 969 | 0.11 ± 0.02 |
| 4 | β-pinene | 971 | 974 | 1.72 ± 0.38 |
| 5 | myrcene | 990 | 988 | 0.62 ± 0.24 |
| 6 | α-terpinene | 1013 | 1014 | 0.12 ± 0.03 |
| 7 | O-cymene | 1021 | 1022 | 0.18 ± 0.02 |
| 8 | 1,8-cineole | 1027 | 1026 | 13.93 ± 0.84 |
| 9 | (Z)-β-ocimene | 1038 | 1032 | 0.11 ± 0.05 |
| 10 | (E)-β-ocimene | 1048 | 1044 | 0.21 ± 0.08 |
| 11 | γ-terpinene | 1056 | 1054 | 0.15 ± 0.02 |
| 12 | terpinolene | 1085 | 1086 | 1.04 ± 0.42 |
| 13 | linalool | 1099 | 1095 | 2.91 ± 0.79 |
| 14 | terpinen-4-ol | 1174 | 1174 | 0.23 ± 0.09 |
| 15 | α-terpineol | 1188 | 1186 | 2.16 ± 0.76 |
| 16 | geraniol | 1254 | 1249 | 0.73 ± 0.41 |
| 17 | δ-elemene | 1336 | 1335 | 0.19 ± 0.08 |
| 18 | α-cubebene | 1349 | 1348 | 0.32 ± 0.04 |
| 19 | cyclosativene | 1365 | 1369 | 0.31 ± 0.04 |
| 20 | α-copaene | 1375 | 1374 | 7.77 ± 0.36 |
| 21 | β-elemene | 1391 | 1389 | 0.60 ± 0.36 |
| 22 | (E)-caryophyllene | 1420 | 1417 | 32.01 ± 3.98 |
| 23 | β-copaene | 1427 | 1430 | 1.10 ± 0.18 |
| 24 | γ-elemene | 1433 | 1434 | 0.15 ± 0.01 |
| 25 | trans-α-bergamotene | 1435 | 1432 | 0.40 ± 0.19 |
| 26 | aromadendrene | 1437 | 1439 | 0.56 ± 0.03 |
| 27 | 6,9-guaiadiene | 1442 | 1442 | 0.24 ± 0.10 |
| 28 | α-humulene | 1452 | 1452 | 7.15 ± 1.10 |
| 29 | allo-aromadendrene | 1459 | 1458 | 0.12 ± 0.02 |
| 30 | γ-muurolene | 1475 | 1478 | 0.76 ± 0.07 |
| 31 | germacrene D | 1480 | 1480 | 1.86 ± 1.43 |
| 32 | β-selinene | 1484 | 1489 | 0.36 ± 0.03 |
| 33 | cis-β-guaiene | 1490 | 1492 | 0.12 ± 0.05 |
| 34 | bicyclogermacrene | 1495 | 1500 | 2.07 ± 0.35 |
| 35 | α-muurolene | 1499 | 1500 | 0.77 ± 0.05 |
| 36 | γ-cadinene | 1512 | 1513 | 0.29 ± 0.04 |
| 37 | δ-cadinene | 1522 | 1522 | 1.17 ± 0.05 |
| 38 | α-calacorene | 1541 | 1544 | 0.14 ± 0.03 |
| 39 | elemol | 1548 | 1548 | 0.88 ± 0.09 |
| 40 | germacrene B | 1555 | 1559 | 0.37 ± 0.08 |
| 41 | caryophyllene oxide | 1579 | 1582 | 0.52 ± 0.09 |
| 42 | guaiol | 1596 | 1600 | 1.40 ± 0.30 |
| 43 | eremoligenol | 1627 | 1629 | 0.43 ± 0.14 |
| 44 | γ-eudesmol | 1630 | 1630 | 1.54 ± 0.48 |
| 45 | hinesol | 1637 | 1640 | 0.12 ± 0.07 |
| 46 | cubenol | 1640 | 1645 | 0.17 ± 0.11 |
| 47 | β-eudesmol | 1648 | 1649 | 1.76 ± 0.62 |
| 48 | α-eudesmol | 1651 | 1652 | 2.59 ± 0.90 |
| 49 | bulnesol | 1665 | 1670 | 0.26 ± 0.09 |
| Monoterpenes | 29.67 | |||
| Sesquiterpenes | 68.50 | |||
| Total not identified | 1.83 | |||
| Total identified | 98.17 | |||
| Cells | Histological Type | IC50 and 95% CIs (in μg/mL) | |
|---|---|---|---|
| DOX | EO | ||
| Cancer cells | |||
| HCT116 | Human colon cancer | 0.20 0.11–0.35 | 21.42 17.60–26.07 |
| HepG2 | Human liver cancer | 0.04 0.03–0.05 | 14.72 7.54–28.75 |
| MDA-MB-231 | Human breast cancer | 0.62 0.37–1.02 | 16.20 6.59–29.85 |
| MCF-7 | Human breast cancer | 0.36 0.20–0.63 | 44.07 39.37–48.52 |
| 4T1 | Mouse breast cancer | 0.60 0.31–0.96 | 21.65 16.41–29.90 |
| B16-F10 | Mouse melanoma | 0.06 0.03–0.09 | 32.16 27.46–38.34 |
| Noncancerous cells | |||
| MRC-5 | Human lung fibroblast | 0.43 0.18–1.03 | 39.41 30.88–50.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Castro, M.V.L.; de Lima, M.C.F.; da C. Barbosa, G.A.; Carvalho, S.G.; Coelho, A.M.R.M.; de S. Santos, L.; Silva, V.R.; Dias, R.B.; Soares, M.B.P.; Costa, E.V.; et al. In Vitro and In Vivo Efficacy of the Essential Oil from the Leaves of Annona amazonica R.E. Fries (Annonaceae) Against Liver Cancer. Molecules 2025, 30, 3248. https://doi.org/10.3390/molecules30153248
de Castro MVL, de Lima MCF, da C. Barbosa GA, Carvalho SG, Coelho AMRM, de S. Santos L, Silva VR, Dias RB, Soares MBP, Costa EV, et al. In Vitro and In Vivo Efficacy of the Essential Oil from the Leaves of Annona amazonica R.E. Fries (Annonaceae) Against Liver Cancer. Molecules. 2025; 30(15):3248. https://doi.org/10.3390/molecules30153248
Chicago/Turabian Stylede Castro, Maria V. L., Milena C. F. de Lima, Gabriela A. da C. Barbosa, Sabrine G. Carvalho, Amanda M. R. M. Coelho, Luciano de S. Santos, Valdenizia R. Silva, Rosane B. Dias, Milena B. P. Soares, Emmanoel V. Costa, and et al. 2025. "In Vitro and In Vivo Efficacy of the Essential Oil from the Leaves of Annona amazonica R.E. Fries (Annonaceae) Against Liver Cancer" Molecules 30, no. 15: 3248. https://doi.org/10.3390/molecules30153248
APA Stylede Castro, M. V. L., de Lima, M. C. F., da C. Barbosa, G. A., Carvalho, S. G., Coelho, A. M. R. M., de S. Santos, L., Silva, V. R., Dias, R. B., Soares, M. B. P., Costa, E. V., & Bezerra, D. P. (2025). In Vitro and In Vivo Efficacy of the Essential Oil from the Leaves of Annona amazonica R.E. Fries (Annonaceae) Against Liver Cancer. Molecules, 30(15), 3248. https://doi.org/10.3390/molecules30153248








