2.1. Electrochemical Characterization of Hybrid Silica-Modified Electrodes
The electrochemical performance of ITO/silica-PSS and ITO/silica-PDADMAC was evaluated by cyclic voltammetry in a 0.3 mM FcPF
6 Trizma aqueous solution. A schematic illustration of the electroassisted accumulation process of ferrocene within the sol–gel hybrid silica film is provided in (
Figure S1 in the Supporting Information), offering a visual representation of how ferrocene species are retained within the hybrid silica film structure.
Figure 1a shows the ferrocenium redox response using a silica-PSS-modified electrode.
The first scan (dashed line) displays two overlapping anodic peaks centered at 0.94 V and 1.35 V. These peaks are attributed to mass transport limitations of ferrocenium cations within the silica-modified films [
26]. The first oxidation peak is attributed to the oxidation of ferrocene species retained in the inner layers of the silica matrix. The second oxidation peak is assigned to the oxidation of ferrocene species retained in the outer layers of the silica matrix [
17]. The presence of two oxidation peaks is related to the compensation of positive charges within the silica film induced by ferrocenium cations [
25,
26]. During the backward scan, the counter process (reduction of ferrocenium cations to neutral ferrocene) was observed as a single cathodic peak centered at 0.73 V. The cathodic and anodic peak intensities increased stepwise with the repetitive cycling (see the 10th and 60th cycles in
Figure 1a), indicating a continuous accumulation of ferrocenium species within the silica-PSS film. After 20 cycles, the two anodic peaks merged, meaning that both processes overlapped in a single bell-shaped peak, shown by dotted line in
Figure 1a. The voltammogram reached a steady state after 60 cycles, as shown by the solid line in
Figure 1a.
The peak-to-peak separation of the stabilized voltammogram was 0.61 V, and the half-wave potential was 1.04 V. Here, the large peak-to-peak separation could be attributed to a sluggish heterogeneous electron transfer between the electrode and the redox probe. The anodic and cathodic peak intensities obtained after 60 scans were around 207 µA and −282 µA, respectively. This corresponds to an anodic/cathodic peak ratio of 0.73.
It is worth noting that this anodic/cathodic peak ratio is higher than that obtained with silica polyelectrolyte-free modified electrodes [
17], which indicates an enhanced accumulation of ferrocenium cations near the electrode surface. This enhancement was due to the electrostatic attraction between the ferrocenium species and the sulfonic groups present in the PSS polymer chain. The presence of negatively charged sulfonate groups (SO
3−) in the PSS film likely facilitates positive charge compensation during the oxidation of ferrocene. When ferrocene is oxidized to ferrocenium, the positive charge excess is locally balanced by the available sulfonate groups within the film, thereby decreasing the charge transfer limitation within the film and facilitating the oxidation process.
The pore size of silica-PSS was obtained by applying the DFT method to the adsorption isotherm (See
Figure S2 and Table S1 in the Supporting Information). The analysis of pore size distribution in the silica-PSS sample indicates the presence of both micropores, with a diameter of 1.46 nm, and mesopores, with a diameter of 6.5 nm. Notably, these values are smaller by 0.04 nm and 0.5 nm, respectively, compared to those reported for polyelectrolyte-free silica. [
17] This slight reduction in both pore types indicates that the incorporation of PSS alters the internal structure of the silica matrix. It is likely that PSS interacts with the silica network during the sol–gel process, leading to decreased pore dimensions and modified porosity.
Figure 1b shows the ferrocenium redox response using a silica-PDADMAC-modified electrode. The first voltammetric scan also showed two anodic peaks, centered at 0.96 V and 1.19 V, respectively. A single cathodic peak at 0.73 V was observed during the backward scan. The presence of the two oxidation peaks was attributed to mass transport limitations, as discussed
vide supra for silica-PSS-modified electrodes. A single bell-shaped peak was observed after the 85th repetitive voltammetric scan.
The cathodic and anodic peak intensities exhibited a stepwise increase with repetitive cycling, as observed in the CV cycles from 10th to 85th in
Figure 1b. The stabilized CV cycle showed a peak-to-peak separation of 0.63 V, and the half-wave potential was 1.03 V. The anodic and cathodic peak intensities after 85 scans were approximately 125 µA and −219 µA, respectively. The anodic/cathodic peak ratio was 0.57. The latter indicates unbalanced concentrations of ferrocene and ferrocenium at the interface of the silica-PDADMAC-modified electrode. Specifically, the concentration of ferrocenium cations was higher than that of neutral ferrocene, which was expected given that the electrode was immersed in a solution containing only ferrocenium cations. It is worth noting that the repulsion of ferrocenium ions from silica film containing the positively charged polyelectrolyte will produce a lower initial concentration of ferrocenium ions near the electrode surface, leading to a decrease in the concentration of ferrocene. This imbalance in the concentration of redox species can affect electron transfer kinetics, as reported in a previous work [
17].
The pore characteristics of the silica-PDADMAC film were also evaluated using the DFT model applied to the adsorption isotherm (see
Figure S2 and Table S1 in the Supporting Information). The resulting distribution showed the presence of micropores with a diameter of 1.56 nm and mesopores measuring 7.7 nm. Compared to pure silica [
17], the micropores were slightly wider by 0.06 nm, and the mesopores by 0.7 nm, indicating that PDADMAC incorporation leads to a more open porous structure. This structural expansion may be attributed to electrostatic interactions between the cationic PDADMAC and the negatively charged silica walls during the sol–gel process. Furthermore, we carried out experiments at varying scan rates, confirming the presence of two different kinetics of heterogeneous electron transfer attributed to mass transport limitations within the hybrid silica films (see
Figure S3 in the Supporting Information). For both silica-PSS and silica-PDADMAC-modified electrodes, the cyclic voltammograms recorded at low scan rates (10–20 mV s
−1) displayed a single anodic peak with a bell-shaped profile. This feature is indicative of ferrocene species either adsorbed at the electrode interface or slowly diffusing through the porous silica matrix. Under these conditions, the diffusion layer is sufficiently thick to mask mass transport limitations within the films. As the scan rate increased to 50 mV s
−1, a noticeable shoulder developed on the anodic wave for both hybrid silica-modified electrodes, signaling the emergence of a secondary oxidation process, most likely involving ferrocene species confined deeper in the silica films. At higher scan rates (500 and 1000 mV s
−1), pronounced peak broadening and distortion were evident in both hybrid silica-modified electrodes, reflecting growing mass transport limitation and slower heterogeneous electron transfer kinetics across the hybrid silica films.
The capacity to accumulate Ferrocene within silica-PSS-modified electrodes was higher than using silica-PDADMAC-films (almost two-fold). Furthermore, we observed that the addition of a cationic polyelectrolyte still permitted the accumulation of ferrocene species within the silica film, meaning that a partial neutralization of the negatively charged pores of the silica walls occurred by the presence of the positively charged PDADMAC polyelectrolyte.
It is worth mentioning that in the case of PDADMAC-modified electrodes, the first anodic peak showed a low current, while the subsequent oxidation peak showed a higher intensity. This contrasted with the voltammograms recorded with silica-PSS films, where the first oxidation peak displays the highest current intensity. This divergence can be attributed to the repulsion between positive charge species and the restricted charge compensation within the inner layer of the films during ferrocene oxidation.
In silica-PSS films, the negatively charged sulfonate groups interact with the ferrocenium ions, influencing their distribution and promoting their accumulation primarily in the inner layers of the film. This leads to a dominant first oxidation peak because charge compensation occurs efficiently from the start of the oxidation reaction. Over several voltammetric cycles, ferrocenium species keep accumulating, gradually fading the difference between the inner and outer layer oxidation peaks.
In silica-PDADMAC films, the situation is different due to electrostatic repulsion between ferrocenium ions and the positively charged PDADMAC. This repulsion hinders ferrocenium accumulation in the inner layers, resulting in a weaker first oxidation peak. However, as oxidation progresses and charge redistribution occurs, ferrocenium species accumulate more efficiently in the outer layers of the film, where there is less repulsion. This results in a more pronounced second oxidation peak, which remains dominant throughout the cycling process.
In summary, silica-PSS presents a larger accumulation capacity for ferrocene compared to the PDADMAC-modified electrode, as quantified by the current reduction peaks in the voltammogram (−282 µA for PSS vs. −219 µA for PDADMAC).
To evaluate how stable ferrocenium is retained within the hybrid silica films, electrodes were removed from the electrochemical cell and thoroughly rinsed with abundant ultrapure water (See
Figure S4 in the Supporting Information). The electrodes were characterized by cyclic voltammetry in a ferrocenium-free solution. A schematic representation of the electroassisted desorption process is provided in
Figure S5 in the Supporting Information, illustrating the gradual release of ferrocene species from the hybrid films during potential cycling.
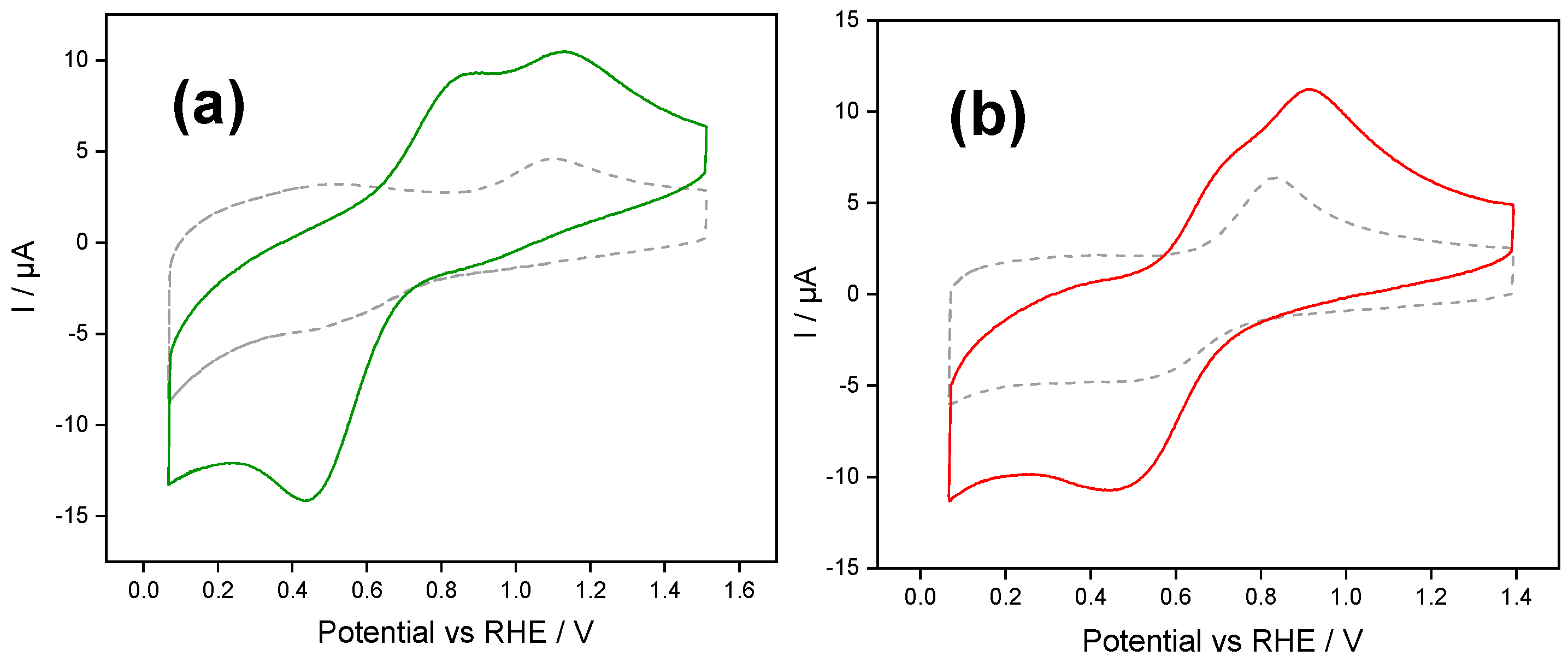
Figure 2 shows repetitive cyclic voltammograms obtained with (a) PSS and (b) PDADMAC Class I films after the ferrocene accumulation stage. The first notable aspect of these voltammograms, in comparison to the final voltammograms of
Figure 1, was the lowering of the intensity of the redox peaks (as expected by the rinsing stage) and the modification of the shape of the voltammogram.
During the first voltammetric scan, both electrodes presented a single ferrocene-ferrocenium diffusional-like oxidation peak, in contrast to the bell-shaped peak previously observed in
Figure 1 when ferrocenium was dissolved in the aqueous buffer solution. We consider this peak to correspond to the confined ferrocene species’ response within the silica films’ inner layers, while loosely retained ferrocene was removed during the rinsing.
Considering the first PSS-functionalized silica (
Figure 2a), we observed a continued decrease in the anodic and cathodic peak intensities during the voltammetric cycling due to the partial leaching of the remaining ferrocene. After 30 cycles, a stable signal was reached with a peak-to-peak separation equal to 215 mV and a reduction peak current intensity of −14 µA (solid line in
Figure 2a), that is, only the 5% of the current detected after the accumulation step (final scan of
Figure 1a). We determined the apparent ferrocene concentration within the silica film making use of the Randles–Sevcik equation [
29,
30]:
where
Ip (
A) is the peak current, υ is the scan rate (V s
−1),
n is the number of electrons transferred,
A the electrode surface area (cm
2), D the diffusion coefficient of the analyte (cm
2 s
−1), and C the concentration of the analyte (mol cm
−3) in the bulk solution.
We assume a value for the diffusion coefficient of 1.2 × 10
−6 cm
2 s
−1 as determined in the trizma buffer solution [
17]. It should be noted that this value represents an approximation and not the true diffusion coefficient within the film. Therefore, the calculated concentrations are not absolute but serve as relative estimates to compare the retention of ferrocene in the different hybrid silica films. In this context, the peak current value (
Ip) was obtained from the reduction peak of the stabilized cyclic voltammogram. By applying the Randles–Sevcik equation with the known parameters (scan rate, electrode area, and estimated diffusion coefficient), we calculated the apparent concentration of electroactive ferrocene. Based on this analysis, the apparent concentration of electroactive ferrocene in the silica-PSS film was approximately 0.03 mM.
Similar experiments performed with silica functionalized with PDADMAC are shown in
Figure 2b. In the first scan towards positive potentials, a single peak at 0.96 V corresponds to the oxidation of the confined ferrocene species within the inner layers of the silica-PDADMAC film. During the backwards scan, the counter process was observed as a cathodic peak centered at 0.74 V. The anodic and cathodic peak intensities decreased stepwise throughout the voltametric cycling due to the leaching of the ferrocene species into the solution. After 50 cycles a stable signal was reached. The stabilized cyclic voltammogram shows a reduction peak with a current intensity of −46 µA. The apparent ferrocene concentration determined by Randles–Sevcik analysis was estimated to be 0.08 mM; that is more than double the concentration determined for silica-PSS films.
The lower retention of ferrocene species in silica-PSS compared to silica-PDADMAC is notable and can be explained by differences in electrostatic interactions and film stability.
In silica-PSS films, the negatively charged sulfonate groups should facilitate the initial accumulation of ferrocenium ions; however, weaker retention forces and the high hydrophilicity of the film contribute to increased leaching during rinsing and cycling. Conversely, in silica-PDADMAC films, the positively charged matrix initially hinders ferrocenium incorporation due to electrostatic repulsion, but once retained, stronger affinity due to electrostatic and hydrophobic interactions minimize ferrocenium loss. As a result, the final concentration of ferrocene species within silica-PDADMAC films is higher than that observed in silica-PSS films.
The results indicate that the relevant interaction between the encapsulated ferrocenium/ferrocene couple and the hybrid silica is not the electrostatic one.
These findings can be further rationalized by distinguishing the dominant interaction at each stage of ferrocene processing within the films. During the initial accumulation step, electrostatic forces govern the incorporation of ferrocenium ions: the negatively charged sulfonate groups in silica-PSS films attract ferrocenium ions efficiently, while the electrostatic repulsion in PDADMAC-modified films limits their accumulation. This explains the initially lower voltammetric response in PDADMAC-modified films (
Figure 1b). However, once incorporated, neutral ferrocene exhibits higher affinity for the hydrophobic PDADMAC matrix, likely due to favorable hydrophobic interactions with the polymer backbone. This affinity improves ferrocene retention during rinsing and voltammetric cycling, as evidenced by the higher stabilized current intensities and calculated electroactive concentrations in
Figure 2b. In contrast, silica-PSS films, though efficient in accumulating ferrocenium, exhibit lower retention due to their hydrophilic environment, which facilitates ferrocene leaching. Therefore, while electrostatics dominate the initial loading, retention is predominantly governed by hydrophobic interactions within the film matrix.
The backbone of PDADMAC is relatively hydrophobic, and ferrocene in its neutral form is also moderately hydrophobic. Such interactions may favor the adsorption or retention of ferrocene within the hydrogel layer, potentially influencing its diffusion dynamics and redox behavior, especially under conditions where electrostatic effects are minimized or screened by counterions.
It is worth noting that the ITO electrode modification with silica-PSS and silica-PDADMAC significantly enhanced the reduction peak intensity by approximately 5-fold and 3-fold, respectively, compared to bare ITO [
17]. Additionally, the hybrid silica-modified electrodes effectively prevented electrode fouling [
17]. Importantly, no noticeable increase in resistance or loss of capacitive behavior was observed after surface modification, indicating that the silica layer does not hinder charge transport at the electrode interface (See
Figure S6 in the Supporting Information).
Figure 3 shows the in situ UV-vis spectra of Fc@hybrid silica modified-electrodes.
After ferrocenium stabilization (as described for experiments shown in
Figure 2), the stabilized Fc@silica-modified electrodes were extracted from the electrochemical cell at 1.42 V to ensure that ferrocenium stays in the oxidized state within the silica. Then, the electrodes were transferred to the UV-vis spectroelectrochemical cell containing a blank solution.
Figure 3a shows the response of a Fc@silica-PSS electrode for an applied potential of 1.42 V. The spectrum exhibits an absorption band centered at 618 nm attributed to the ligand-to-metal charge transfer band of ferrocenium incorporated within the silica pores [
17,
31]. From the absorbance, the apparent concentration of ferrocenium within the film was determined to be 1.40 mM (
Figure 3a). Details on the determination of this concentration were given elsewhere [
17].
We studied the electrochemical reduction process of encapsulated ferrocenium by applying a potential of +0.22 V vs. RHE. We kept this potential constant to ensure the complete reduction of electroactive ferrocenium within the film. The final stabilized spectrum obtained (dashed line in
Figure 3a) shows a decrease in the intensity of the ferrocenium absorption band at 618 nm, confirming that a fraction of ferrocenium species was reduced in the film. The intensity of the absorption band allows us to determine the portion of encapsulated ferrocenium that was effectively reduced to ferrocene. The final concentration of encapsulated Fc
+ species was still 1.17 mM. The difference between both concentrations is related to the concentration of ferrocenium that is electroactive within the film. In this case, the amount of electroactive ferrocenium was 0.23 mM, a value much higher than that determined from the voltammetric Randles–Sevcik analysis (0.03 mM).
Figure 3b shows the in situ UV-vis spectra of a Fc@silica-PDADMAC electrode. The spectrum obtained is very similar to the one obtained with Fc@silica-PSS. From the absorbance, the apparent concentration of encapsulated ferrocenium was 1.87 mM. After applying a potential of +0.22 V vs. RHE (dashed line spectrum in
Figure 3b) a decrease in the ferrocenium intensity band was observed reaching a concentration of 1.76 mM. The difference between oxidized and reduced ferrocenium species corresponds to the electroactive species within the film, that was 0.11 mM closer to the voltammetric estimation (0.08 mM).
Table 1 summarizes the electroactive ferrocene species incorporated in silica films measured by both techniques. For comparative purposes, we also included the data obtained with conventional silica (with no polyelectrolyte incorporated).
Cyclic voltammetry measured a lower concentration of electroactive ferrocenium than the concentration determined by in situ UV-vis. This discrepancy is particularly apparent in the silica modified with negatively charged sulfonated moieties (PSS).
Concentration derived from CV in silica functionalized with positively charged groups (PDADMAC) closely aligns with the spectroelectrochemical results. Since the ferrocenium concentration was determined by using the Randles–Sevcik equation (Equation (1)), we considered that the diffusion coefficient of ferrocenium was similar to that in solution. However, in previous works, we demonstrated that the silica pores can effectively modulate the diffusion of charged redox probes [
32]. Indeed, negatively charged redox species (like ferrocyanide species or aromatic sulfonates) show enhanced diffusivities across the SiO
2 pores functionalized with PSS, in contrast to positively charged redox species, which show lower diffusivities [
33].
Since silica is negatively charged under working conditions, adding PSS increases the number of negative charges on the silica walls. Consequently, the diffusion coefficient used to determine ferrocenium concentration is likely higher than the basal value within the silica films, resulting in an underestimation of the ferrocenium concentration in the films via voltammetry means.
Table 1 also shows the concentration of ferrocene species within polyelectrolyte-free silica films [
17]. Similarly, the estimation from CV measurements indicated a local concentration of 0.12 mM, which is lower than the UV-vis estimate of 0.19 mM. The difference is less pronounced when compared to Fc@silica-PSS, but it follows a similar trend as discussed for Class I films.
A different effect is inferred for Fc@silica-PDADMAC films. At neutral pH, the cationic polyelectrolyte (PDADMAC) partially neutralizes the negative charges on the silica walls, resulting in a neutral-like pore that has a less pronounced effect on the diffusion of redox species. Thus, the ferrocenium concentration measured by CV (0.08 mM) was much closer to the value obtained by UV-Vis (0.11 mM). This suggests that the discrepancy between electrochemical and spectroscopic methods could be attributed to the negative charge density within the silica pores.
The comparison between the concentration from in situ UV-vis experiments and cyclic voltammetry suggests that the diffusion coefficient of ferrocenium/ferrocene couple was underestimated in the voltammetric measurement. In previous publications, we determined that silica-PSS-modified electrodes modify the apparent diffusion coefficient of positively charged redox probes (such as Fe3+/2+ or dopamine). Under these circumstances, the electrostatic interactions between the positively charged species and the films result in lower diffusion coefficients, leading to retention of the probes.
However, the observed decrease in the apparent diffusion coefficient observed in the presence of PDADMAC suggests that hydrophobic interactions between ferrocene and the hybrid film are the most significant. Likely, the positively charged segments of PDADMAC interact electrostatically with the negatively charged silanol and siloxane groups (Si–OH, Si–O
−) on the silica surface, resulting in the orientation of the hydrophobic alkyl chains of PDADMAC toward the interior of the pores. This configuration creates a hydrophobic microenvironment that favors hydrophobic interactions with ferrocene, thereby retarding its diffusion through the film [
34]. These findings highlight the dominant role of hydrophobic interactions in governing the diffusion behavior of ferrocene within the hybrid films studied [
35,
36,
37].
To investigate how confined ferrocene species improve electron transfer, we studied a redox species with restricted direct electron interaction with the electrode, such as Cytochrome c (Cyt c). Therefore, the following section discusses the interaction between confined ferrocene species within Class I silica films and Cyt c in solution. It is worth noting that the results shown in
Figure 4 were obtained after determining the concentration of ferrocenium/ferrocene within the silica films after immersion in PBS, i.e., the electrochemical response of Cyt c in solution depended on the local concentration of ferrocene within the films.