Lingonberry Leaves Modify Rumen Protozoa Population, Carbohydrate Digestion, and Morphology of Gastrointestinal Tract in Sheep: A Preliminary Study
Abstract
1. Introduction
2. Results
3. Discussion
4. Material and Methods
4.1. Animal Diets
4.2. Slaughter Procedure and Sampling
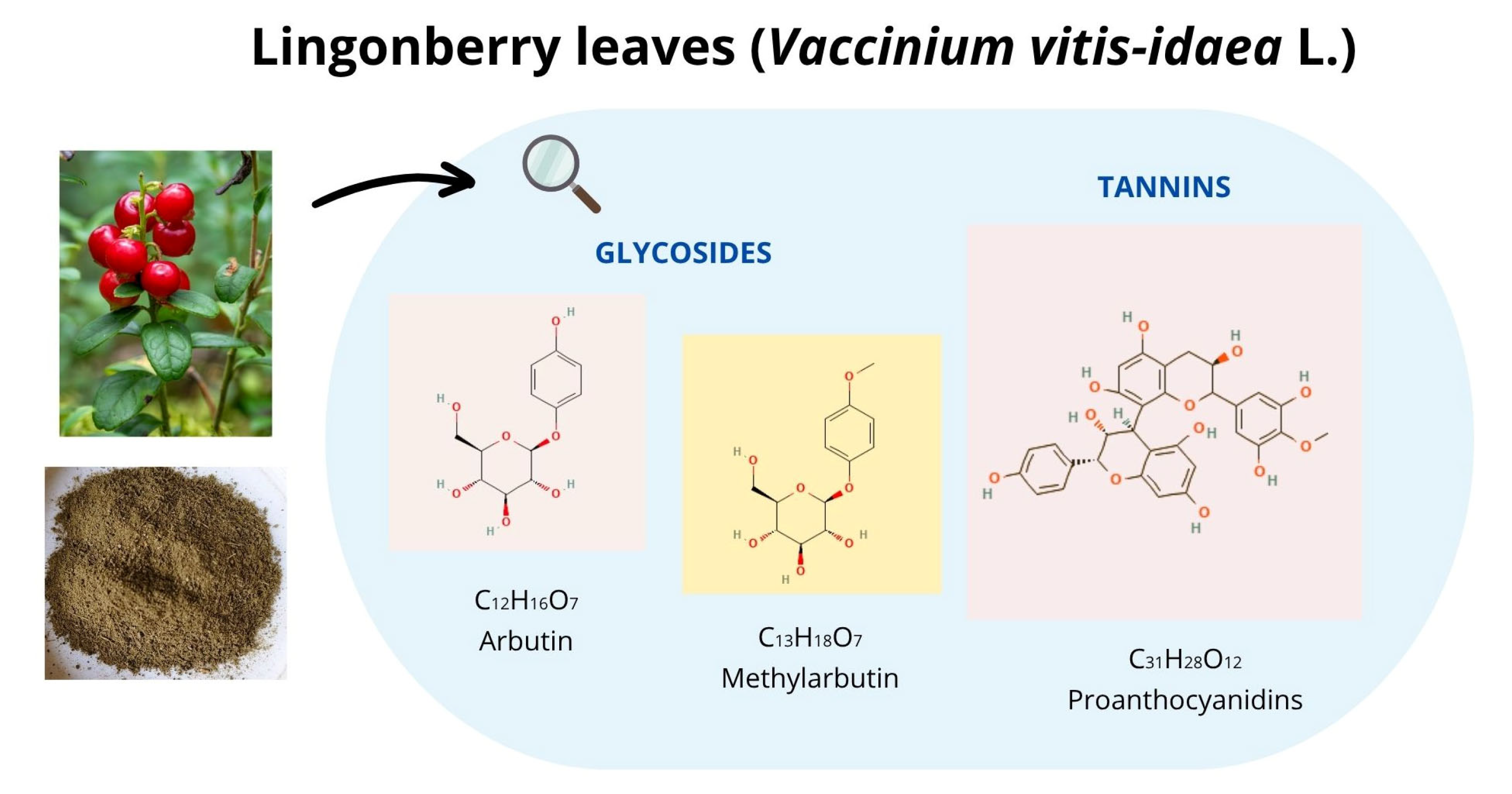
4.3. Analyses of Bioactive Compounds in LLs
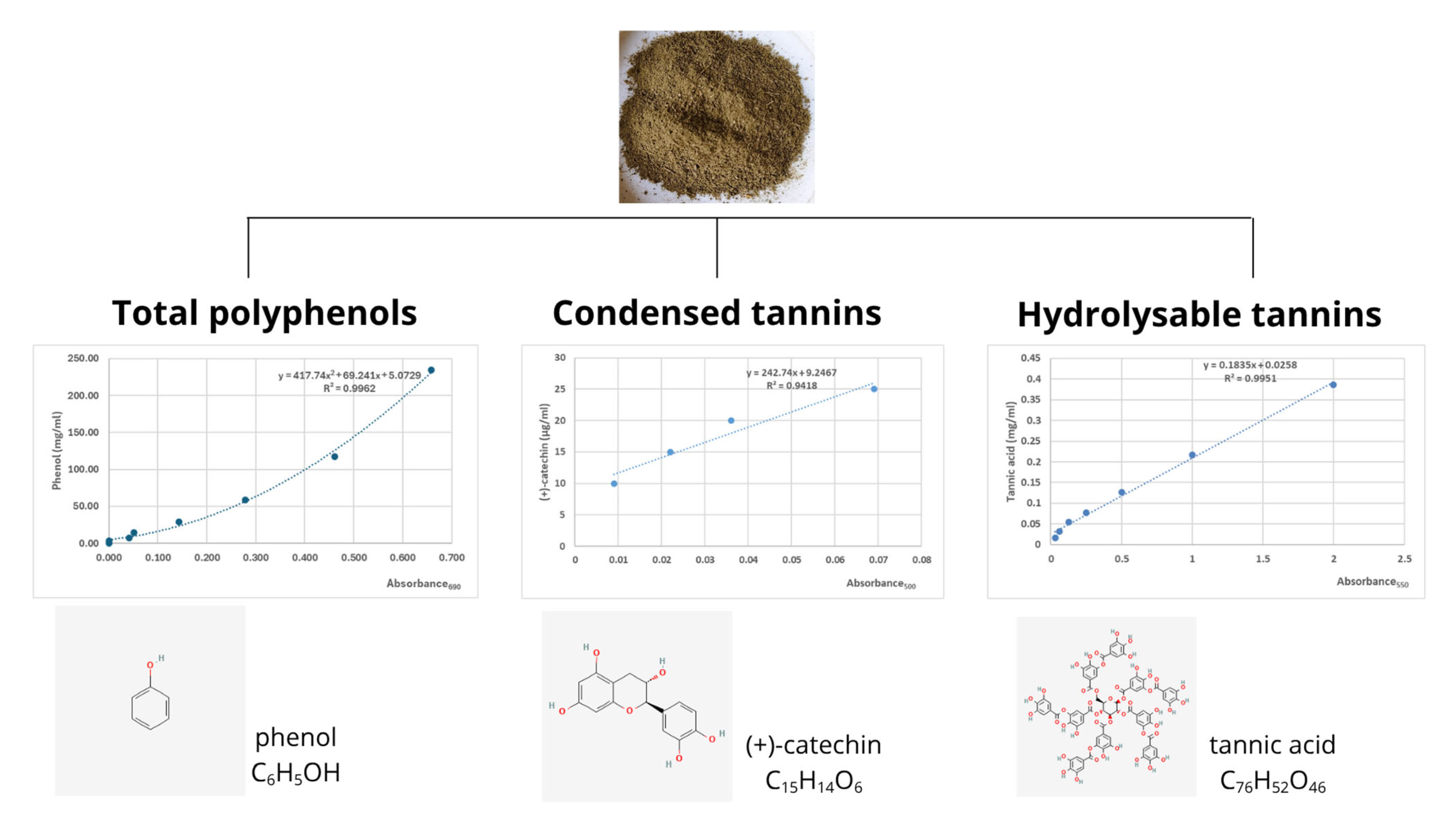
4.3.1. Analysis of Total Phenols Concentration
4.3.2. Analysis of Condensed Tannins Concentration
4.3.3. Analysis of Hydrolysable Tannins Concentration
4.4. Determination of Protozoa Number
4.5. Enzymatic Analysis
4.6. Determination of Short-Chain Fatty Acid Concentration
4.7. Methane Production
4.8. Histological Analyses
4.9. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADF | Acid detergent fibre |
| ADL | Acid detergent lignin |
| DM | Dry matter |
| DW | Dry weight |
| LLs | Lingonberry leaves |
| NDF | Neutral detergent fibre |
| RT | Retention time |
| SCFA | Short-chain fatty acid |
| SEM | Standard error of mean |
References
- Majewska, M.P.; Kędzierska, A.; Miltko, R.; Bełżecki, G.; Kowalik, B. Does humate supplementation affect ciliate population and fermentation parameters in the sheep rumen? J. Anim. Feed Sci. 2022, 31, 371–378. [Google Scholar] [CrossRef]
- Waqas, M.; Salman, M.; Sahrif, M.S. Application of polyphenolic compounds in animal nutrition and their promising effects. J. Anim. Feed Sci. 2023, 32, 233–256. [Google Scholar] [CrossRef]
- Prodanović, R.; Nedić, S.; Bošnjaković, D.; Nedić, S.; Jovanović, L.; Arsić, S.; Bojkovski, J.; Borozan, S.; Kirovski, D.; Vujanac, I. Chestnut tannin supplementation can improve rumen response and kidney function in prepartum dairy cows. J. Anim. Feed Sci. 2024, 33, 185–192. [Google Scholar] [CrossRef]
- Kowalska, K. Lingonberry (Vaccinium vitis-ideae L.) Fruit as a Source of Bioactive Compounds with Health-Promoting Effects-A Review. Int. J. Mol. Sci. 2021, 22, 5126. [Google Scholar] [CrossRef]
- Stefănescu, B.-E.; Călinoiu, L.F.; Ranga, F.; Fetea, F.; Mocan, A.; Vodnar, D.C.; Crișan, G. Chemical Composition and Biological Activities of the Nord-West Romanian Wild Berry (Vacinnium myrtillus L.) and Lingonberry (Vaccinium vitis-idaea L.) Leaves. Antioxidants 2020, 9, 495. [Google Scholar] [CrossRef]
- Kylli, P.; Nohynek, L.; Puupponen-Pimiä, R.; Westerlund-Wikström, B.; Leppänen, T.; Welling, J.; Moilanen, E.; Heinonen, M. Lingonberry (Vaccinium vitis-idaea) and European cranberry (Vaccinium microcarpon) proanthocyanidins: Isolation, identification, and bioactivities. J. Agric. Food Chem. 2011, 59, 3373–3384. [Google Scholar] [CrossRef]
- Ferlemi, A.-V.; Lamari, F.N. Berry Leaves: An Alternative Source of Bioactive Natural Products of Nutritional and Medicinal Value. Antioxidants 2016, 5, 17. [Google Scholar] [CrossRef]
- Stefănescu, B.E.; Szabo, K.; Mocan, A.; Crisan, G. Phenolic Compounds from Five Ericaceae Species Leaves and Their Related Bioavailability and Health Benefits. Molecules 2019, 24, 2046. [Google Scholar] [CrossRef]
- Schofield, P.; Mbugua, D.M.; Pell, A.N. Analysis of condensed tannins: A review. Anim. Feed Sci. Technol. 2001, 91, 21–40. [Google Scholar] [CrossRef]
- Wang, Y.; McAllister, T.A.; Acharya, S. Condensed tannins in sainfoin: Composition, concentration, and effects on nutritive and feeding value of sainfoin forage. Crop Sci. 2015, 55, 13–22. [Google Scholar] [CrossRef]
- Liu, P.; Lindstedt, A.; Markkinen, N.; Sinkkonen, J.; Suomela, J.-P.; Yang, B. Characterization of Metabolite Profiles of Leaves of Bilberry (Vaccinium myrtillus L.) and Lingonberry (Vaccinium vitis-idaea L.). J. Agric. Food Chem. 2014, 62, 12015–12026. [Google Scholar] [CrossRef]
- Russell, J.B.; Rychlik, J.L. Factors that alter rumen microbial ecology. Science 2001, 292, 1119–1122. [Google Scholar] [CrossRef] [PubMed]
- Miltko, R.; Bełżecki, G.; Kowalik, B.; Skomiał, J. Presence of carbohydrate-digesting enzymes throughout the digestive tract of sheep. Turk. J. Vet. Anim. Sci. 2016, 40, 271–277. [Google Scholar] [CrossRef]
- McSweeney, C.S.; Palmer, B.; McNeill, D.M.; Krause, D.O. Microbial interactions with tannins: Nutritional consequences for ruminants. Anim. Feed Sci. Technol. 2001, 91, 83–93. [Google Scholar] [CrossRef]
- Patra, A.K.; Saxena, J. Dietary phytochemicals as rumen modifiers: A review of the effects on microbial populations. Antonie Van Leeuwenhoek 2009, 96, 363–375. [Google Scholar] [CrossRef]
- Majewska, M.P.; Miltko, R.; Bełżecki, G.; Kędzierska, A.; Kowalik, B. Rumen protozoa population and carbohydrate-digesting enzymes in sheep fed a diet supplemented with hydrolysable tannins. Ann. Anim. Sci. 2023, 23, 561–570. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Jones, G.A.; McAllister, T.A.; Muir, A.D.; Cheng, K.J. Effects of sainfoin (Onobrychis viciifolia Scop.) condensed tannins on growth and proteolysis by four strains of ruminal bacteria. Appl. Environ. Microbiol. 1994, 60, 1374–1378. [Google Scholar] [CrossRef]
- Min, B.R.; Attwood, G.T.; Reilly, K.; Sun, W.; Peters, J.S.; Barry, T.N.; McNabb, W.C. Lotus corniculatus condensed tannins decrease in vivo populations of proteolytic bacteria and affect nitrogen metabolism in the rumen of sheep. Can. J. Microbial. 2002, 48, 911–921. [Google Scholar] [CrossRef]
- Jayanegara, A.; Togtokhbayar, N.; Makkar, H.P.S.; Becker, K. Tannins determined by various methods as predictors of methane production reduction potential of plants by an in vitro rumen fermentation system. Anim. Feed Sci. Technol. 2009, 150, 230–237. [Google Scholar] [CrossRef]
- Majewska, M.P.; Miltko, R.; Bełżecki, G.; Kędzierska, A.; Kowalik, B. Protozoa population and carbohydrate fermentation in sheep fed diet with different plant additives. Anim. Biosci. 2021, 34, 1146–1156. [Google Scholar] [CrossRef]
- Terrill, T.H.; Douglas, G.B.; Foote, A.G.; Purchas, R.W.; Wilson, G.F.; Barry, T.N. Effect of condensed tannins upon body growth and rumen metabolism in sheep grazing sulla (Hedysarum coronarium) and perennial pasture. J. Agric. Sci. Camb. 1992, 119, 265–273. [Google Scholar] [CrossRef]
- Carulla, J.E.; Kreuzer, M.; Machmüller, A.; Hess, H.D. Supplementation of Acacia mearnsii tannnins decreases methanogenesis and urinary nitrogen in forage-fed sheep. Aust. J. Agric. Res. 2005, 56, 961–970. [Google Scholar] [CrossRef]
- Sarnataro, C.; Spanghero, M. In vitro rumen fermentation of feed substrates added with chestnut tannins or an extract from Stevia rebaudiana Bertoni. Anim. Nutr. 2020, 6, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Barbehenn, R.V.; Constabel, C.P. Tannins in plant-herbivore interactions. Phytochemistry 2011, 72, 1551–1565. [Google Scholar] [CrossRef] [PubMed]
- Pérez, V.; Doce, R.R.; García-Pariente, C.; Hervás, G.; Ferreras, M.C.; Mantecón, Á.R.; Frutos, P. Oak leaf (Quercus pyrenaica) poisoning in cattle. Res. Vet. Sci. 2011, 91, 269. [Google Scholar] [CrossRef] [PubMed]
- Kelln, B.M.; Penner, G.B.; Acharya, S.N.; McAllister, T.A.; Lardner, H.A. Impact of condensed tannin-containing legumes on ruminal fermentation, nutrition, and performance in ruminants: A review. Can. J. Anim. Sci. 2021, 101, 210–223. [Google Scholar] [CrossRef]
- Moss, A.R.; Jouany, J.P.; Newbold, J. Methane production by ruminants: Its contribution to global warming. Ann. Zootech. 2000, 49, 231–253. [Google Scholar] [CrossRef]
- Vyas, P.; Curran, N.H.; Igamberdiev, A.U.; Debnath, S.C. Antioxidant properties of lingonberry (Vaccinium vitis-idaea L.) leaves within a set of wild clones and cultivars. Can. J. Plant Sci. 2015, 95, 663–669. [Google Scholar] [CrossRef]
- Akyurt, B.; Başyiğit, B.; Çam, M. Phenolic compounds content, antioxidant and antidiabetic potentials of seven edible leaves. GIDA 2018, 43, 876–885. [Google Scholar] [CrossRef]
- Vrancheva, R.; Ivanov, I.; Badjakov, I.; Dincheva, I.; Georgiev, V.; Pavlov, A. Optimization of polyphenols extraction process with antioxidant properties from wild Vaccinium myrtillus L. (bilberry) and Vaccinium vitis-idaea L. (lingonberry) leaves. Food Sci. Appl. Biotechnol. 2020, 3, 149–156. [Google Scholar] [CrossRef]
- Cieślak, A.; Zmora, P.; Pers-Kamczyc, E.; Stochmal, A.; Sadowinska, A.; Salem, A.Z.M.; Kowalczyk, D.; Zbonik, P.; Szumacher-Strabel, M. Effect of two sources of tannins (Quercus L. and Vaccinium vitis idaea L.) on rumen microbial fermentation: An in vitro study. Ital. J. Anim. Sci. 2014, 13, 3133. [Google Scholar] [CrossRef]
- Tan, H.Y.; Sieo, C.C.; Abdullah, N.; Liang, J.B.; Huang, X.D.; Ho, Y.W. Effects of condensed tannins from Leucaena on methane production, rumen fermentation and populations of methanogens and protozoa in vitro. Anim. Feed Sci. Technol. 2011, 169, 185–193. [Google Scholar] [CrossRef]
- Salami, S.A.; Valenti, B.; Bella, M.; O’Grady, M.; Luciano, G.; Kerry, J.P.; Jones, E.; Priolo, A.; Newbold, J.C. Characterisation of the ruminal fermentation and microbiome in lambs supplemented with hydrolysable and condensed tannins. FEMS Microbiol. Ecol. 2018, 94, fiy061. [Google Scholar] [CrossRef]
- Chiquette, J.; Cheng, K.J.; Rode, L.M.; Milligan, L.P. Effect of tannin content in two isosynthetic strains of birdsfoot trefoil (Lotus corniculatus L.) on feed digestibility and rumen fluid composition in sheep. Can. J. Anim. Sci. 1989, 69, 1031–1039. [Google Scholar] [CrossRef]
- Seigler, D.S. Chapter 12: Tannins. In Plant Secondary Metabolism; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1998; pp. 193–214. [Google Scholar]
- Björck, I.M.; Nyman, M.E. In vitro effects of phytic acid and polyphenols on starch digesting and fiber degradation. J. Food Sci. 1987, 52, 1588–1594. [Google Scholar] [CrossRef]
- Ozkose, E.; Kuloğlu, R.; Omlekcioglu, U.; Kar, B.; Akyol, I.; Ekinci, M.S. Effects of tannic acid on the fibrolytic enzyme activity and survival of some ruminal bacteria. Int. J. Agric. Biol. 2011, 13, 386–390. [Google Scholar]
- Cheng, K.J.; Forsberg, C.W.; Minato, H.; Costerton, J.W. Microbial ecology and physiology of feed degradation within the rumen. In Physiological Aspects of Digestion and Metabolism in Ruminants; Tsuda, T., Sasaki, Y., Kawashima, R., Eds.; Academic Press: Toronto, ON, Canada, 1991; pp. 595–624. [Google Scholar]
- Bae, H.D.; McAllister, T.A.; Yanke, L.J.; Cheng, K.J.; Muir, A.D. Effect of condensed tannins on endoglucanase activity and filter paper digestion by Fibrobacter succinogenes S85. Appl. Environ. Microbiol. 1993, 59, 2132–2138. [Google Scholar] [CrossRef]
- Patra, A.K.; Min, B.R.; Saxena, J. Dietary tannins on microbial ecology of the gastrointestinal tract in ruminants. In Dietary Phytochemicals and Microbes; Patra, A., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 237–262. [Google Scholar]
- Yang, C.M.J. Response of forage fiber degradation by ruminal microorganisms to branched-chain volatile fatty acids, amino acids, and dipeptides. J. Dairy Sci. 2002, 85, 1183–1190. [Google Scholar] [CrossRef]
- Cieślak, A.; Zmora, P.; Pers-Kamczyc, E.; Szumacher-Strabel, M. Effects of tannins source (Vaccinium vitis idaea L.) on rumen microbial fermentation in vivo. Anim. Feed Sci. Technol. 2012, 176, 102–106. [Google Scholar] [CrossRef]
- Hess, H.D.; Tiemann, T.T.; Noto, F.; Carulla, J.E.; Kreuzer, M. Strategic use of tannins as means to limit methane emission from ruminant livestock. Int. Congr. Ser. 2006, 1293, 164–167. [Google Scholar] [CrossRef]
- Tavendale, M.H.; Meagher, L.P.; Pacheco, D.; Walker, N.; Attwood, G.T.; Sivakumaran, S. Methane production from in vitro rumen incubations with Lotus pedunculatus and Medicago sativa, and effects of extractable condensed tannins fractions on methanogenesis. Anim. Feed Sci. Technol. 2005, 123, 403–419. [Google Scholar] [CrossRef]
- Tedeschi, L.O.; Ramírez-Restrepo, C.A.; Muir, J.P. Developing a conceptual model of possible benefits of condensed tannins for ruminant production. Animal 2014, 8, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Newbold, C.J.; de la Fuente, G.; Belanche, A.; Ramos-Morales, E.; McEwan, N.R. The role of ciliate protozoa in the rumen. Front. Microbiol. 2015, 6, 1313. [Google Scholar] [CrossRef] [PubMed]
- Chishti, G.; Salfer, I.J.; Nedelkov, K.V.; Felix, T.L. Impacts of time-fed concentrate-based diets on plasma metabolites, rumen histology, and mRNA expression of hepatic enzymes of wethers. Animals 2020, 10, 686. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Seyfert, H.-M.; Löhrke, B.; Schneider, F.; Zitnan, R.; Chudy, A.; Kuhla, S.; Hammon, H.M.; Blum, J.W.; Martens, H.; et al. An energy-rich diet causes rumen papillae proliferation associated with more IGF type 1 receptors and increased plasma IGF-1 concentrations in young goats. J. Nutr. 2004, 134, 11–17. [Google Scholar] [CrossRef]
- Redoy, M.R.A.; Shuvo, A.A.S.; Cheng, L.; Al-Mamun, M. Effect of herbal supplementation on growth, immunity, rumen histology, serum antioxidants and meat quality of sheep. Animal 2020, 14, 2433–2441. [Google Scholar] [CrossRef]
- Petrič, D.; Mravčáková, D.; Kucková, K.; Kišidayová, S.; Cieslak, A.; Szumacher-Strabel, M.; Huang, H.; Kolodziejski, P.; Lukomska, A.; Slusarczyk, S.; et al. Impact of Zinc and/or Herbal Mixture on Ruminal Fermentation, Microbiota, and Histopathology in Lambs. Front. Vet. Sci. 2021, 8, 630971. [Google Scholar] [CrossRef]
- Mueller-Harvey, I. Unravelling the conundrum of tannins in animal nutrition and health. J. Sci. Food Agric. 2006, 86, 2010–2037. [Google Scholar] [CrossRef]
- Mbatha, K.R.; Downs, C.T.; Nsahlai, I.V. The effects of graded levels of dietary tannin on the epithelial tissue of the gastrointestinal tract and liver and kidney masses of Boer goats. Anim. Sci. 2002, 74, 579–586. [Google Scholar] [CrossRef]
- Li, H.; Ran, T.; He, Z.; Yan, Q.; Tang, S.; Tan, Z. Postnatal developmental changes of the small intestinal villus height, crypt depth and hexose transporter mRNA expression in supplemental feeding and grazing goats. Small. Rumin. Res. 2016, 141, 106–112. [Google Scholar] [CrossRef]
- Zhao, M.D.; Di, L.F.; Tang, Y.Z.; Jiang, W.; Li, C.Y. Effect of tannins and cellulose on growth performance, nutrients digestibility, blood profiles, intestinal morphology and carcass characteristics in Hu sheep. Asian-Australas. J. Anim. Sci. 2019, 32, 1540–1547. [Google Scholar] [CrossRef] [PubMed]
- Frutos, P.; Raso, M.; Hervás, G.; Mantecón, Á.R.; Pérez, V.; Giráldez, F.J. Is there any detrimental effect when a chestnut hydrolysable tannin extract is included in the diet of finishing lambs? Anim. Res. 2004, 53, 127. [Google Scholar] [CrossRef]
- Yáñez-Ruiz, D.R.; Molina-Alcaide, E. A comparative study of the effect of two-stage olive cake added to alfalfa on digestion and nitrogen losses in sheep and goats. Animal 2007, 1, 227–232. [Google Scholar] [CrossRef]
- Strzetelski, J.A.; Brzóska, F.; Kowalski, Z.M.; Osięgłowski, S. Feeding Recommendations for Ruminants and Feed Tables; IZ PIB: Kraków, Poland, 2014. (In Polish) [Google Scholar]
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis, 18th ed.; AOAC: Arlington, VA, USA, 2011. [Google Scholar]
- Animal Protection Act of 21 August 1997. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU19971110724 (accessed on 17 July 2025). (In Polish)
- Regulation of the Minister of Agriculture and Rural Development of 15 February 2010 on Requirements and Procedures for the Keeping of Livestock Species. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20100560344 (accessed on 17 July 2025). (In Polish)
- Council Regulation (EC) No 1099/2009 of 24 September 2009 on the Protection of Animals at the Time of Killing. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32009R1099 (accessed on 17 July 2025).
- FAO (Food and Agricultural Organization). Animal Production and Health Paper. Guidelines for Slaughtering, Meat Cutting and Further Processing; Agriculture and Consumer Protection: Rome, Italy, 1991. [Google Scholar]
- Miltko, R.; Pietrzak, M.; Bełżecki, G.; Wereszka, K.; Michałowski, T.; Hackstein, J.H.P. Isolation and in vitro cultivation of the fibrolytic rumen ciliate Eremoplastron (Eudiplodinium) dilobum. Eur. J. Protistol. 2015, 51, 109–117. [Google Scholar] [CrossRef]
- Barszcz, M.; Taciak, M.; Tuśnio, A.; Święch, E.; Skomiał, J.; Čobanová, K.; Grešáková, L. The effect of organic and inorganic zinc source, used with lignocellulose or potato fiber, on microbiota composition, fermentation, and activity of enzymes involved in dietary fiber breakdown in the large intestine of pigs. Livest. Sci. 2021, 245, 104429. [Google Scholar] [CrossRef]
- Kuhla, S.; Ebmeier, C. Investigations of the tannin content in horse beans. I. Methods for determining condensed tannins in horse beans. Arch. Tierernähr. 1981, 31, 573–588. (In German) [Google Scholar] [CrossRef]
- Willis, R.B.; Allen, P.R. Improved method for measuring hydrolysable tannins using potassium iodate. Analyst 1998, 123, 435–439. [Google Scholar] [CrossRef]
- Dehority, B.A. Laboratory Manual for Classification and Morphology of Rumen Ciliate Protozoa; CRC Press Inc.: London, UK, 1993. [Google Scholar]
- Miltko, R.; Kowalik, B.; Majewska, M.P.; Kędzierska, A.; McEwan, N.R.; Bełżecki, G. The effect of protozoa on the bacterial composition and hydrolytic activity of the roe deer rumen. Animals 2020, 10, 467. [Google Scholar] [CrossRef]
- Miltko, R.; Rozbicka-Wieczorek, J.A.; Więsyk, E.; Czauderna, M. The influence of different chemical forms of selenium added to the diet including carnosic acid, fish oil and rapeseed oil on the formation of volatile fatty acids and methane in the rumen, and fatty acid profiles in the rumen content and muscles of lambs. Acta Vet. 2016, 66, 373–391. [Google Scholar] [CrossRef]
- Odongo, N.E.; AlZahal, O.; Lindinger, M.I.; Duffield, T.F.; Valdes, E.V.; Terrell, S.P.; McBride, B.W. Effects of mild heat stress and grain challenge on acid-base balance and rumen tissue histology in lambs. J. Anim. Sci. 2006, 84, 447–455. [Google Scholar] [CrossRef]

| Item | Control | Experimental Diet | SD | p-Value | Cohen’s d |
|---|---|---|---|---|---|
| Total protozoa | 163.7 | 140.0 | 24.86 | 0.389 | 0.953 |
| Entodinium | 143.6 | 127.9 | 24.74 | 0.554 | 0.613 |
| Diplodinium | 4.9 | 4.9 | 0.40 | 0.860 | −0.193 |
| Ophryoscolex | 1.9 | 1.3 | 0.40 | 0.167 | 1.630 |
| Isotricha | 2.4 A | 1.3 B | 0.16 | <0.001 | 7.038 |
| Dasytricha | 10.9 A | 4.6 B | 0.62 | <0.001 | 8.214 |
| Enzyme Activity | Control | Experimental Diet | SD | p-Value | Cohen’s d |
|---|---|---|---|---|---|
| Cellulolytic 1 | 41.4 | 37.4 | 4.08 | 0.210 | 0.994 |
| Xylanolytic 2 | 60.0 | 56.8 | 4.95 | 1.000 | 0.633 |
| Amylolytic 1 | 47.7 | 40.7 | 3.38 | 0.167 | 1.249 |
| Pectinolytic 3 | 6.5 A | 4.5 B | 1.14 | 0.043 | 1.814 |
| Inulinolytic 4 | 7.7 | 7.6 | 0.75 | 0.850 | 0.140 |
| Item | Control | Experimental Diet | SD | p-Value | Cohen’s d |
|---|---|---|---|---|---|
| Total SCFA | 6.3 | 7.8 | 1.91 | 0.369 | −0.825 |
| Acetate | 4.4 | 5.7 | 1.50 | 0.361 | −0.839 |
| Propionate | 0.8 | 0.9 | 0.17 | 0.437 | −0.879 |
| Butyrate | 0.6 | 0.9 | 0.27 | 0.176 | −1.307 |
| Valerate | 0.06 | 0.05 | 0.01 | 0.576 | 0.707 |
| Isoacids 1 | 0.3 | 0.2 | 0.03 | 0.069 | 1.820 |
| Methane 2 | 2.0 | 2.7 | 0.63 | 0.663 | −1.045 |
| Item | Control | Experimental Diet | SD | p-Value | Cohen’s d |
|---|---|---|---|---|---|
| Rumen | |||||
| Papilla height, mm | 1.5 | 1.6 | 0.60 | 0.108 | −0.045 |
| Papilla width, mm | 0.3 B | 0.4 A | 0.08 | <0.001 | −1.014 |
| Papilla surface area, mm2 * | 0.5 B | 0.6 A | 0.23 | <0.001 | −0.644 |
| Duodenum | |||||
| Villus height, μm | 1078.2 A | 879.5 B | 154.71 | <0.001 | 1.284 |
| Villus width, μm | 116.1 B | 124.5 A | 13.89 | <0.008 | −0.609 |
| Crypt depth, μm | 168.1 | 167.8 | 31.11 | 0.967 | 0.009 |
| Thickness of the muscular layer, μm | 259.4 A | 189.7 B | 50.57 | <0.001 | 1.379 |
| Item | Control | Experimental Diet |
|---|---|---|
| Components (g/kg DM) | ||
| Meadow hay | 548.0 | 548.0 |
| Barley meal | 260.6 | 260.6 |
| Soybean meal | 90.3 | 90.3 |
| Polfamix O-K 1 | 19.0 | 19.0 |
| Lingonberry leaves (LLs) | - | 9.3 |
| LL composition (mg/g DW) | ||
| Total phenols 2 | - | 167 |
| Condensed tannins 3 | - | 19.5 |
| Hydrolysable tannins 4 | - | 4.5 |
| Chemical composition (g/kg DM) | ||
| Dry matter | 899.9 | 900.2 |
| Organic matter | 846.8 | 847.3 |
| Crude protein 5 | 136.9 | 136.2 |
| Crude fat | 18.0 | 18.6 |
| Starch | 222.1 | 220.0 |
| Crude fibre | 182.7 | 182.6 |
| NDF | 460.2 | 459.3 |
| ADF | 233.5 | 234.1 |
| ADL | 35.8 | 37.6 |
| Crude ash | 34.6 | 34.6 |
| LL chemical composition (g/kg DM) | ||
| Dry matter | - | 9.0 |
| Organic matter | - | 8.8 |
| Crude protein 5 | - | 0.6 |
| Crude fat | - | 0.6 |
| Crude fibre | - | 1.7 |
| NDF | - | 3.6 |
| ADF | - | 2.8 |
| ADL | - | 1.6 |
| Crude ash | - | 0.3 |
| Nutrient intake (g/d) | ||
| Dry matter | 917.9 | 927.2 |
| Organic matter | 863.7 | 872.7 |
| Crude protein | 139.6 | 140.3 |
| Crude fat | 18.3 | 19.1 |
| Starch | 226.6 | 226.6 |
| Crude fibre | 186.3 | 188.0 |
| NDF | 469.4 | 473.1 |
| ADF | 238.2 | 241.1 |
| ADL | 36.5 | 38.7 |
| Crude ash | 35.3 | 35.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majewska, M.P.; Miltko, R.; Bełżecki, G.; Barszcz, M.; Kinsner, M.; Kowalik, B. Lingonberry Leaves Modify Rumen Protozoa Population, Carbohydrate Digestion, and Morphology of Gastrointestinal Tract in Sheep: A Preliminary Study. Molecules 2025, 30, 3161. https://doi.org/10.3390/molecules30153161
Majewska MP, Miltko R, Bełżecki G, Barszcz M, Kinsner M, Kowalik B. Lingonberry Leaves Modify Rumen Protozoa Population, Carbohydrate Digestion, and Morphology of Gastrointestinal Tract in Sheep: A Preliminary Study. Molecules. 2025; 30(15):3161. https://doi.org/10.3390/molecules30153161
Chicago/Turabian StyleMajewska, Małgorzata P., Renata Miltko, Grzegorz Bełżecki, Marcin Barszcz, Misza Kinsner, and Barbara Kowalik. 2025. "Lingonberry Leaves Modify Rumen Protozoa Population, Carbohydrate Digestion, and Morphology of Gastrointestinal Tract in Sheep: A Preliminary Study" Molecules 30, no. 15: 3161. https://doi.org/10.3390/molecules30153161
APA StyleMajewska, M. P., Miltko, R., Bełżecki, G., Barszcz, M., Kinsner, M., & Kowalik, B. (2025). Lingonberry Leaves Modify Rumen Protozoa Population, Carbohydrate Digestion, and Morphology of Gastrointestinal Tract in Sheep: A Preliminary Study. Molecules, 30(15), 3161. https://doi.org/10.3390/molecules30153161








