The Influence of Recipe Modification and the Technological Method on the Properties of Multigrain Snack Bars
Abstract
1. Introduction
2. Results and Discussion
2.1. Evaluation of the Effect of the Addition of a Fibre Preparation and NFC Juices on the Physical Properties of Baked and Dried Bars
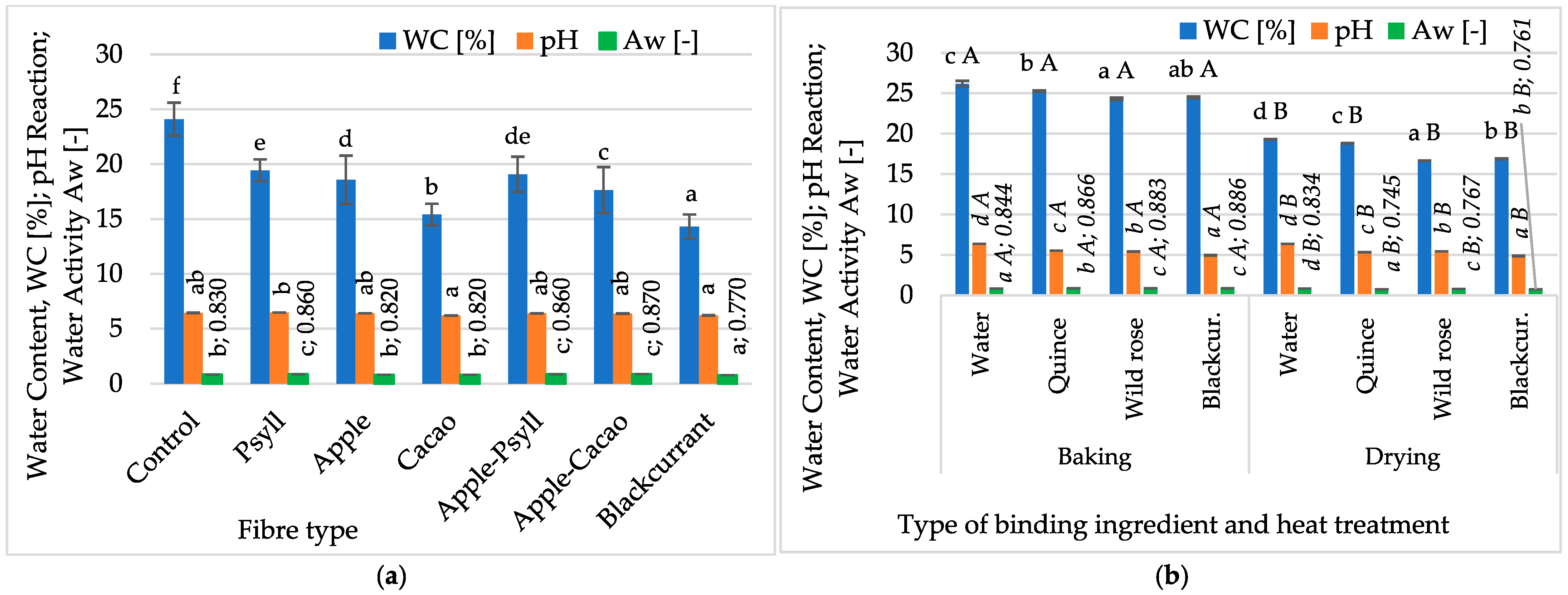
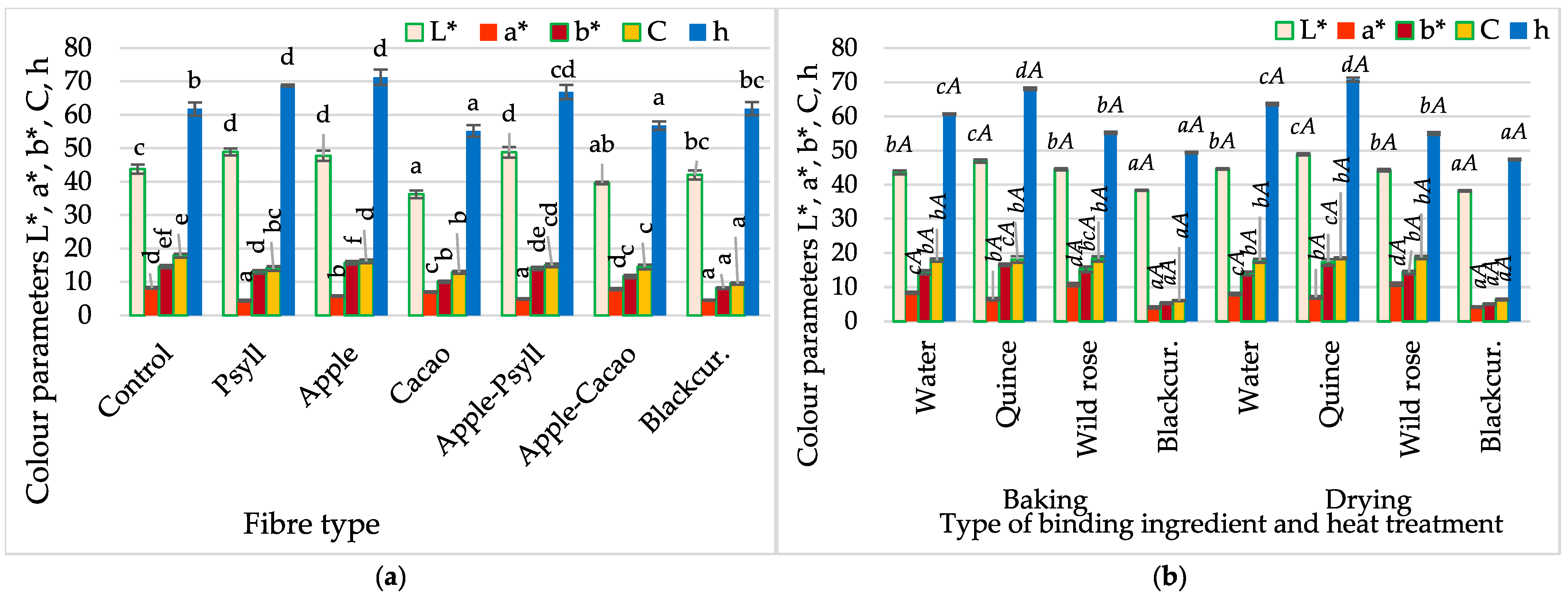
2.1.1. Water Content and Activity, pH, and Colour
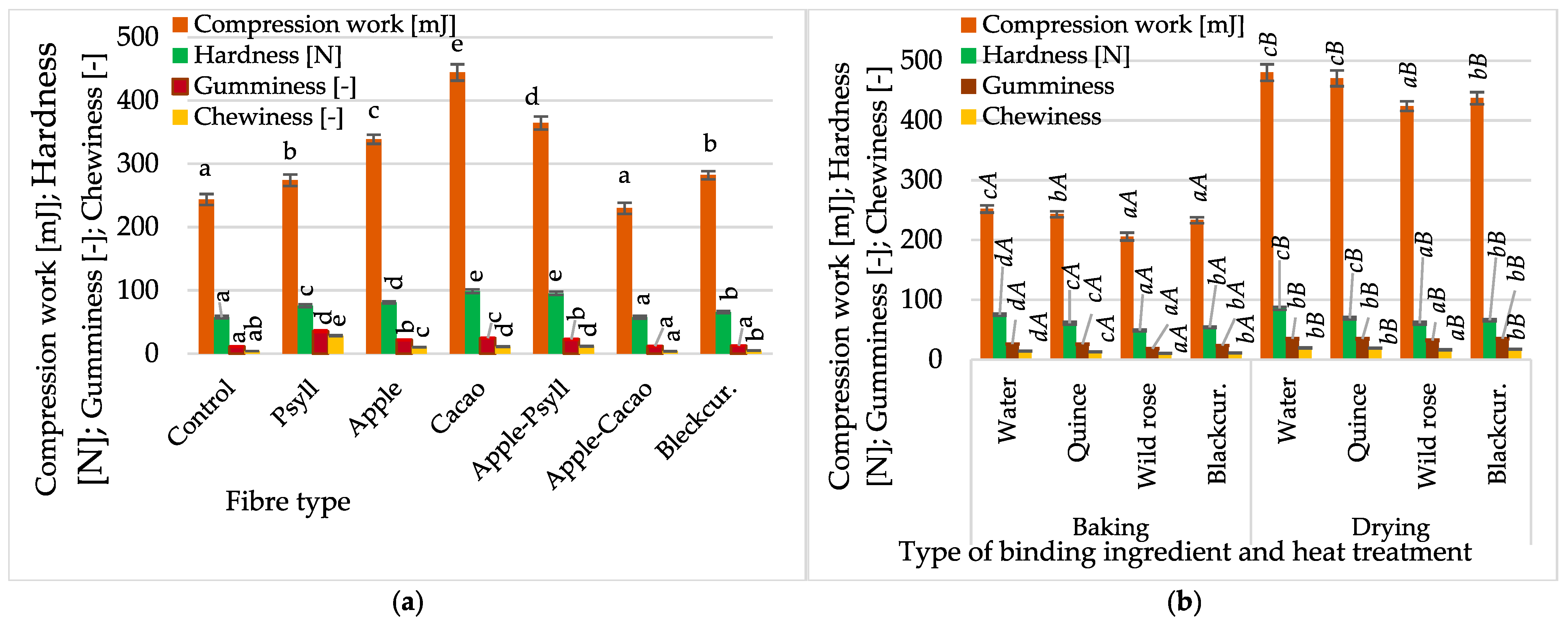
2.1.2. Texture of Bars
2.2. Evaluation of the Addition of Fibre Preparation and NFC Juices on the Chemical Properties of Baked and Dried Bars
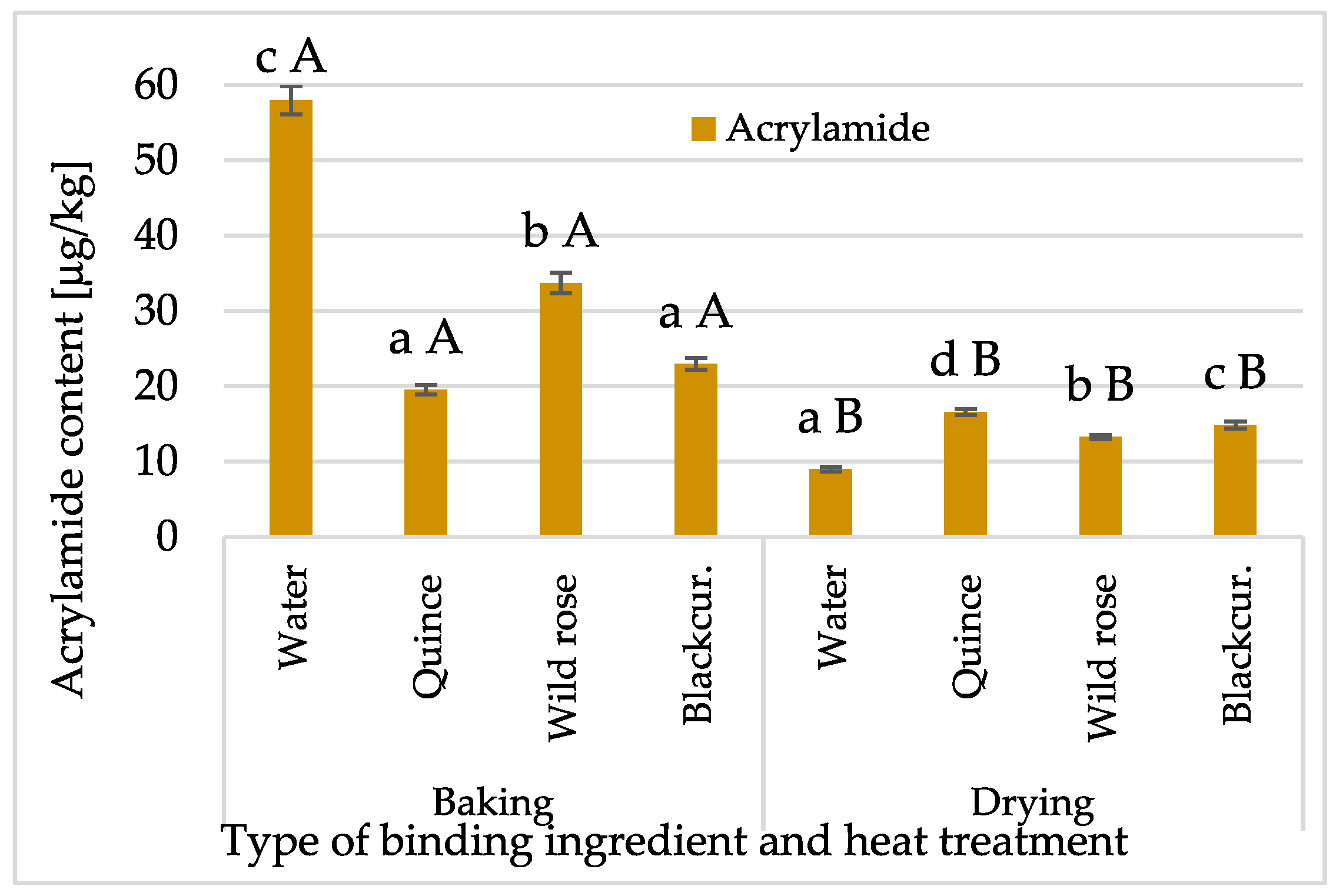
2.2.1. Acrylamide Content in Bars
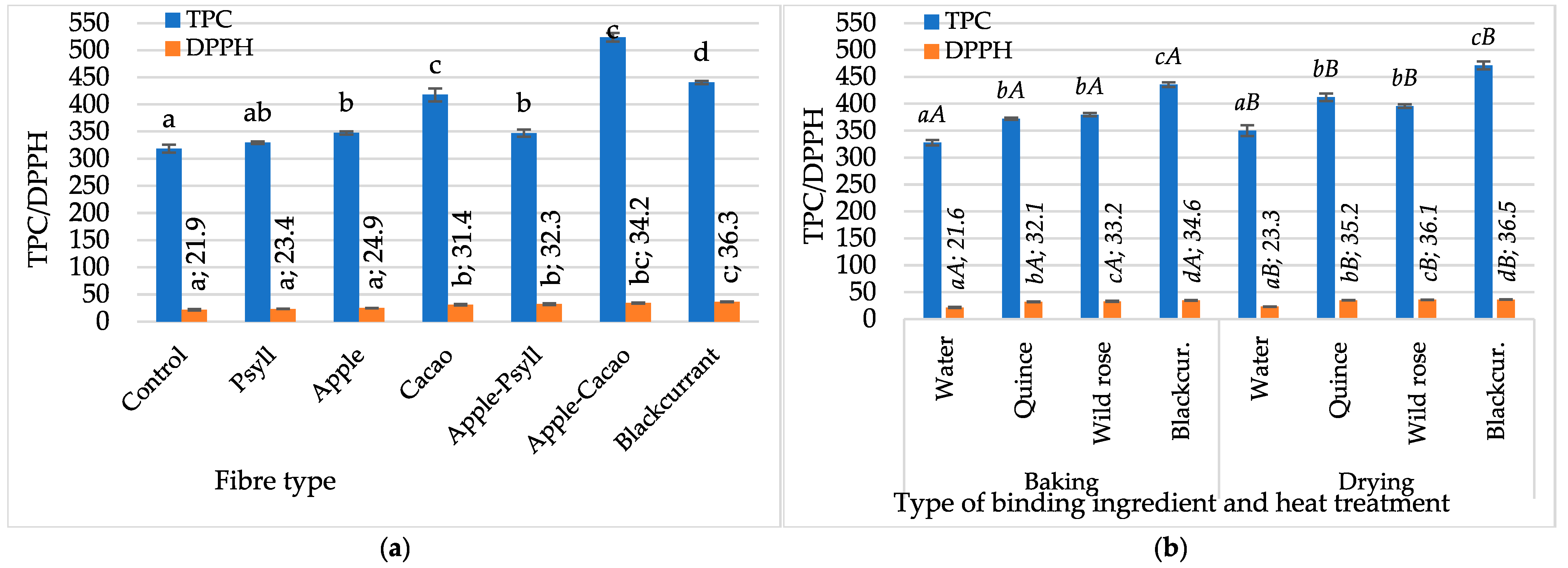
2.2.2. Polyphenol Content and Antioxidant Activity
2.2.3. Nutritional Content of Selected Bars
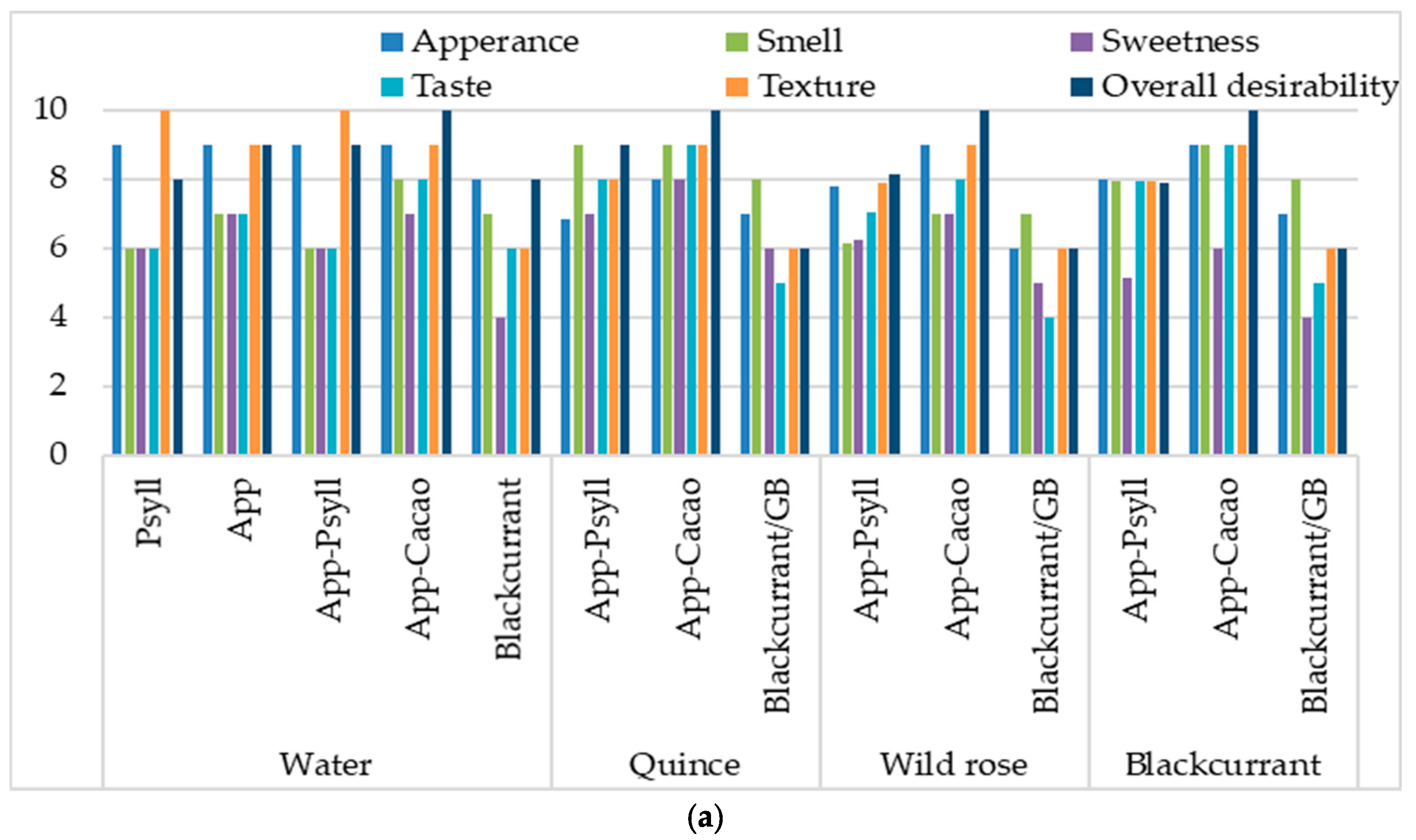
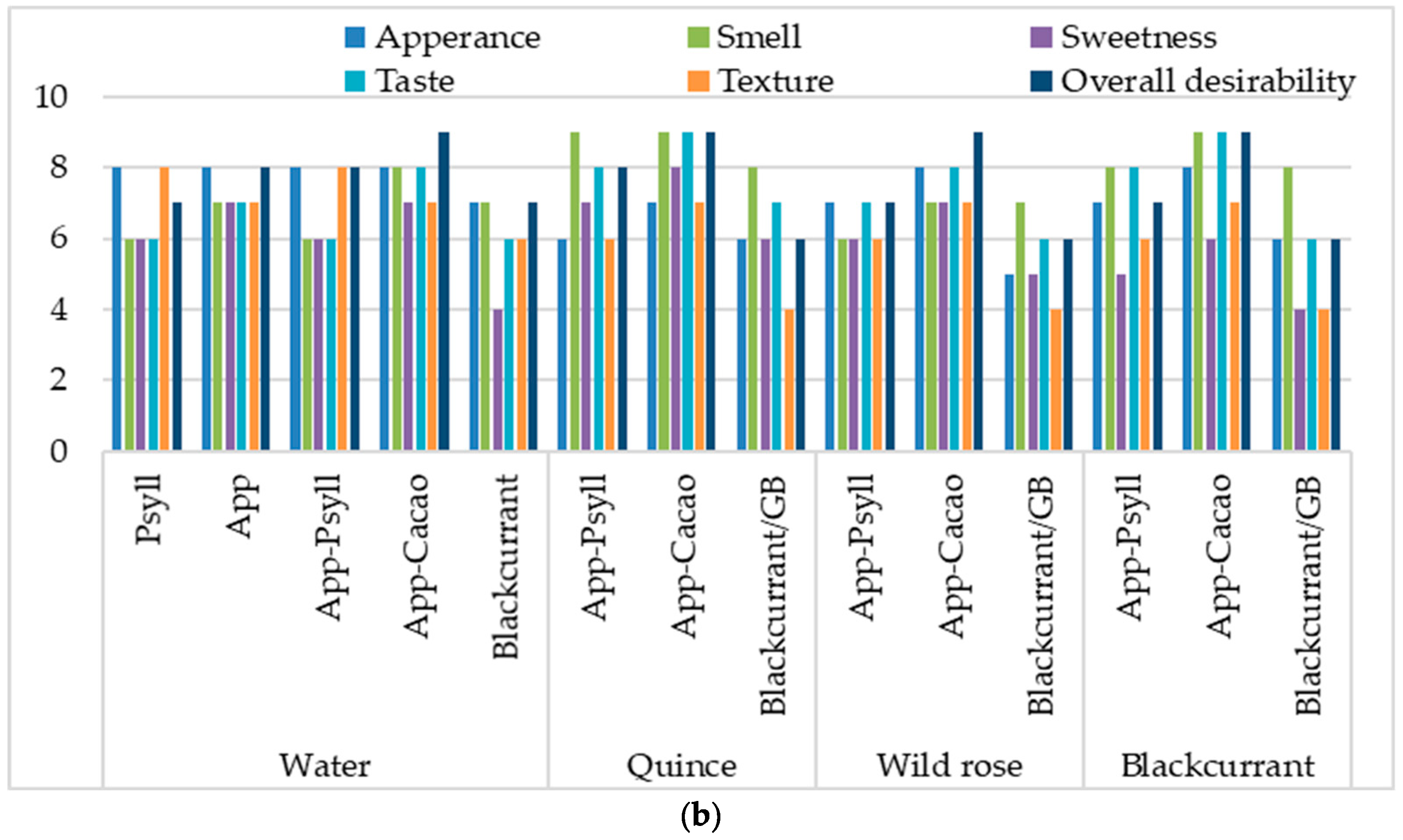
2.3. The Influence of Fibre and the Binder on the Sensory Quality of Baked and Dried Bars
2.4. Microbiological Quality of Selected Bars After Production and Storage
3. Materials and Methods
3.1. Materials
3.2. Technological Methods
3.3. Analytical Methods
3.4. Statistical Methods
4. Conclusions
- The type of fibre (Psyllium, apple, cocoa) and replacing water as a binding component with NFC juice (quince, wild rose, blackcurrant), as well as the method of thermal processing (baking, drying), significantly affected the properties of multigrain bars. Compared to the control sample, adding fibre and NFC juices caused an approximately 10% decrease in water content and activity, especially when using cocoa fibre, blackcurrant fibre, and blackcurrant juice. Thermal processing by microwave–convection drying (40 °C/microwave power 230 W/45 min) caused a significantly greater decrease in these indicators than in baked bars (180 °C/25 min). The pH values of the bars were less diverse (4.9–6.5) and did not depend on the type of fibre. Drying and replacing water with blackcurrant juice caused a beneficial decrease in the pH of the bars, allowing for the assumption of increased microbiological safety.
- Regardless of the processing method, the colour of the bars was shaped by both the addition of the fibre preparation and the NFC juice.
- Compared to other fibres, Psyllium and its mixture with another fibre resulted in a significantly lower hardness and higher gumminess and chewiness. However, regardless of the type of fibre and NFC juice, and the thermal production method, the general acceptability of the bars, including texture, was positively assessed by panellists.
- Despite the different heat treatment temperatures and chemical compositions of the bars that favour the formation of acrylamide, its content, reaching 59 µg/kg, was many times lower than the permissible level established in the Regulation of the European Commission 2017/2158/EC. The share of NFC juices significantly reduced its content in the bars, especially dried ones.
- The bars are a source of nutrients such as carbohydrates, fat, and a high proportion of essential unsaturated fatty acids (EFAs), protein, fibre, minerals, and polyphenolic compounds, especially in the case of bars with cacao fibre. Compared to commercial bars, all bars showed a moderate caloric value (290–310 kcal/100 g).
- The basic raw materials and those used to modify the recipe of multigrain bars were characterised by high microbiological quality.
- Wider use of fibre preparations produced from by-products may help in the development of sustainable technologies for the production of bar snacks.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Voss, G.B.; Campos, D.A.; Pintado, M.M. Cereals bars added with probiotics and prebiotics. In Probiotics and Prebiotics in Foods: Challenges, Innovations, and Advances; Cruz, A.G.D., Ranadheera, C.S., Nazzaro, F., Mortazavian, A., Eds.; Academic Press Inc.: Cambridge, MA, USA, 2021; pp. 201–217. [Google Scholar] [CrossRef]
- Klerks, M.; Román, S.; Verkerk, R.; Sanchez-Siles, L. Are cereal bars significantly healthier and more natural than chocolate bars? A preliminary assessment in the German market. J. Funct. Foods 2022, 89, 104940. [Google Scholar] [CrossRef]
- Souiy, Z.; Zakhama, N.; Cheraief, I.; Hammami, M. Nutritional, physical, microbial, and sensory characteristics of gluten-and sugar-free cereal bar enriched with spirulina and flavored with neroli essential oil. LWT 2022, 169, 113955. [Google Scholar] [CrossRef]
- Konopacka, D.; Rutkowski, K.P.; Płocharski, W.J. Fruits and vegetables as a component of a healthy human diet. In Bioactive Components of Plant Raw Materials and Products; Słupski, J., Tarko, T., Drożdż, I., Eds.; Malopolska Branch of the Polish Society of Food Technologists: Cracow, Poland, 2014; pp. 46–57. [Google Scholar]
- Kowalska, H.; Kowalska, J.; Ignaczak, A.; Masiarz, E.; Domian, E.; Galus, S.; Ciurzyńska, A.; Salamon, A.; Zając, A.; Marzec, A. Development of a high-fibre multigrain bar technology with the addition of curly kale. Molecules 2021, 26, 3939. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, H.; Masiarz, E.; Ignaczak, A.; Marzec, A.; Hać-Szymańczuk, E.; Salamon, A.; Cegiełka, A.; Żbikowska, A.; Kowalska, J.; Galus, S. Advances in multigrain snack bar technology and consumer expectations: A review. Food Rev. Int. 2022, 39, 93–118. [Google Scholar] [CrossRef]
- Ribeiro, J.C.; Santos, C.; Lima, R.C.; Pintado, M.E.; Cunha, L.M. Impact of defatting and drying methods on the overall liking and sensory profile of a cereal bar incorporating edible insect species. Future Foods 2022, 6, 100190. [Google Scholar] [CrossRef]
- Zafar, A.; Shaheen, M.; Tahir, A.B.; da Silva, A.P.G.; Manzoor, H.Y.; Zia, S. Unraveling the nutritional, biofunctional, and sustainable food application of edible crickets: A comprehensive review. Trends Food Sci. Technol. 2024, 143, 104254. [Google Scholar] [CrossRef]
- Xu, R.; Ye, H.; Zeng, D.; Zhang, H.; Xu, X.; Wu, F. Oat flour and β-glucan regulate the quality of cereal flour and cereal products: Unveiling novel physicochemical insights with future perspectives. Int. J. Biol. Macromol. 2025, 307, 142362. [Google Scholar] [CrossRef]
- Mathur, M.; Kumari, A.; Grewal, R.B.; Panghal, A.; Rani, M.; Kargwal, R. Development and optimization of ingredients for multigrain fibre and protein enriched composite bars using sensory evaluation. Pharma Innov. J. 2020, 9, 143–149. Available online: https://www.thepharmajournal.com/archives/2020/vol9issue11/PartC/9-10-31-398.pdf (accessed on 10 June 2025).
- Olagunju, A.I.; Arigbede, T.I.; Makanjuola, S.A.; Oyebode, E.T. Nutritional compositions, bioactive properties, and in-vivo glycemic indices of amaranth-based optimized multigrain snack bar products. Meas. Food 2022, 7, 100039. [Google Scholar] [CrossRef]
- Meethal, S.M.; Kaur, N.; Singh, J.; Gat, Y. Effect of addition of jackfruit seed flour on nutrimental, phytochemical and sensory properties of snack bar. Curr. Nutr. Food Sci. 2017, 5, 154–158. [Google Scholar] [CrossRef]
- Williamson, G.; Kay, C.D.; Crozier, A. The bioavailability, transport, and bioactivity of dietary flavonoids: A Review from a historical perspective. Comper. Rev. Food Sci. Food Saf. 2018, 17, 1054–1112. [Google Scholar] [CrossRef]
- Agbaje, R.; Hassan, C.Z.; Arifin, N.; Rahman, A.A. Sensory preference and mineral contents of cereal bars made from glutinous rice flakes and sunnah foods. IOSR-JESTFT 2014, 8, 26–31. [Google Scholar] [CrossRef]
- Showkat, S.; Dar, A.H.; Khan, S.; Gani, M. Effect of mung bean and rice on physico-chemical, sensory and microstructural properties of cereal bars. Carpathian J. Food Sci. Technol. 2018, 10, 70–78. [Google Scholar]
- Subiria-Cueto, R.; Coria-Oliveros, A.J.; Wall-Medrano, A.; Rodrigo-García, J.; González-Aguilar, G.A.; Martinez-Ruiz, N.D.R.; Alvarez-Parrilla, E. Antioxidant dietary fiber-based bakery products: A new alternative for using plant-by-products. Food Sci. Technol. 2021, 42, ctaAR57520. [Google Scholar] [CrossRef]
- Budin, A.C.; Wensing, C.S.; Cruz, C.L.V.; Ruffi, C.R.G.; Garcia, A.O.; de Moura, S.C.S.R. Stability study of bioactive compounds from yerba mate extract encapsulated by ionic gelation and application of microparticles in fruit and cereal bars. LWT 2025, 215, 117245. [Google Scholar] [CrossRef]
- Laricheva, K.; Mikhailova, O. Development of scientifically-based recipe and technology for the production of natural honey-based muesli bar. Environ. Earth Sci. 2020, 613, 012067. [Google Scholar] [CrossRef]
- Estévez, A.M.; Escobar, B.; Vasquez, M.; Castillo, E.; Araya, E.; Zacarías, I. Cereal and nut bars, nutritional quality and storage stability. Plant Foods Hum. Nutr. 1995, 47, 309–317. [Google Scholar] [CrossRef]
- Dutcosky, S.D.; Grossmann, M.V.E.; Silva, R.S.S.; Welsch, A.K. Combined sensory optimization of a prebiotic cereal product using multicomponent mixture experiments. Food Chem. 2006, 98, 630–638. [Google Scholar] [CrossRef]
- Farinazzi-Machado, F.M.V.; Barbalho, S.M.; Oshiiwa, M.; Goulart, R.; Pessan Junior, O. Use of cereal bars with quinoa (Chenopodium quinoa W.) to reduce risk factors related to cardiovascular diseases. Food Sci. Technol. 2012, 32, 239–244. [Google Scholar] [CrossRef]
- Damasceno, K.A.; Gonçalves, C.A.A.; Pereira, G.D.S.; Costa, L.L.; Campagnol, P.C.B.; Almeida, P.; Arantes-Pereira, L. Development of cereal bars containing pineapple peel flour (Ananas comosus L. Merril). J. Food Qual. 2016, 39, 417–424. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Teoh, A.; Massarotto, C.; Wibisono, R.; Wadhwa, S. Comparative analysis of fruit-based functional snack bars. Food Chem. 2010, 119, 1369–1379. [Google Scholar] [CrossRef]
- Garcia, M.C.; Lobato, L.P.; Benassi, M.T.; Soares Júnior, M.S. Application of roasted rice bran in cereal bars. Food Sci. Technol. 2012, 32, 718–724. [Google Scholar] [CrossRef]
- Sung, Y.-Y.; Kim, S.-H.; Kim, D.-S.; Park, S.H.; Yoo, B.W.; Kim, H.K. Nutritional composition and anti-obesity effects of cereal bar containing Allium fistulosum (welsh onion) extract. J. Funct. Foods 2014, 6, 428–437. [Google Scholar] [CrossRef]
- Silva, S.B.; Formigoni, M.A.; Zorzenon, M.R.; Milani, P.G.; Dacome, A.S.; Seixas, F.A.V.; Costa, S.C. Development of diet cereal bar sweetened with stevia leaves pre-treated with ethanol. Food Sci. Technol. 2020, 40, 894–901. [Google Scholar] [CrossRef]
- Melati, J.; Lucchetta, L.; Prado, N.V.; Oliveira, D.F.; Tonial, I.B. Physical and sensory characteristics of salty cereal bar with different binding agents. Food Sci. Technol. 2021, 41, 150–154. [Google Scholar] [CrossRef]
- Rögner, N.S.; Mall, V.; Steinhaus, M. Impact of malt extract addition on odorants in wheat bread crust and crumb. J. Agric. Food Chem. 2021, 69, 13586–13595. [Google Scholar] [CrossRef]
- Timm, T.G.; de Lima, G.G.; Matos, M.; Magalhães, W.L.E.; Tavares, L.B.B.; Helm, C.V. Nanosuspension of pinhão seed coat development for a new high-functional cereal bar. J. Food Process. Preserv. 2020, 44, E14464. [Google Scholar] [CrossRef]
- Mirpoor, S.F.; Giosafatto, C.V.L.; Porta, R. Biorefining of seed oil cakes as industrial co-streams for production of innovative bioplastics. A review. Trends Food Sci. Technol. 2021, 109, 259–270. [Google Scholar] [CrossRef]
- Godula, K.; Czerniejewska-Surma, B.; Dmytrów, I.; Plust, D.; Surma, O. Possible applications of dietary fibre in functional food production. Food Sci. Technol. Qual. 2019, 26, 5–17. (In Polish) [Google Scholar]
- Orrego, C.E.; Salgado, N.; Botero, C.A. Developments and trends in fruit bar production and characterization. Crit. Rev. Food Sci. Nutr. 2014, 54, 84–97. [Google Scholar] [CrossRef] [PubMed]
- González, L.; Morante-Zarcero, S.; Pérez-Quintanilla, D.; Sierra, I. Hydroxymethylfurfural determination in cereal and insect bars by high-performance liquid chromatography-mass spectrometry employing a functionalized mesostructured silica as sorbent in solid-phase extraction. J. Chromatogr. A 2020, 1622, 461124. [Google Scholar] [CrossRef]
- Niaz, W.; Iqbal, S.Z.; Ahmad, K.; Majid, A.; Haider, W.; Li, X. Mycotoxins: A comprehensive review of its global trends in major cereals, advancements in chromatographic detections and future prospectives. Food Chem. 2025, 27, 102350. [Google Scholar] [CrossRef] [PubMed]
- Borkowska, B.; Opolska, J. Assessment of sensory quality of selected fruit and cereal bars. Sci. J. Gdyn. Marit. Univ. 2017, 99, 149–155. (In Polish) [Google Scholar]
- Safvi, A.F.; Ahmad, A.; Younis, K.; Yousuf, O. Development of energy bar by adding underutilized Chironji (Buchanania lanzan) seeds. Food Humanit. 2023, 1, 44–50. [Google Scholar] [CrossRef]
- Samakradhamrongthai, R.S.; Jannu, T.; Renaldi, G. Physicochemical properties and sensory evaluation of high energy cereal bar and its consumer acceptability. Heliyon 2021, 7, e07776. [Google Scholar] [CrossRef]
- Nawirska, A.; Kwaśniewska, M. Dietary fibre fractions from fruit processing waste. Acta Sci. Pol. Technol. Aliment. 2004, 3, 13–20. (In Polish) [Google Scholar] [CrossRef]
- Iuliano, L.; González, G.; Casas, N.; Moncayo, D.; Cote, S. Development of an organic quinoa bar with amaranth and chia. Food Sci. Technol. 2019, 39, 218–224. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2017/2158 of 20 November 2017 Establishing Mitigation Measures and Benchmark Levels for the Reduction of the Presence of Acrylamide in Food. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32017R2158 (accessed on 10 June 2025).
- Žilić, S.; Sarić, B.; Mogol, B.A.; Kravić, N.; Hamzalıoğlu, A.; Simić, M.; Nikolić, V.; Gökmen, V. Assessment of acrylamide content in corn-based snack products marketed in Serbia. J. Food Compost. Anal. 2024, 135, 106652. [Google Scholar] [CrossRef]
- Rifai, L.; Saleh, F.A. A review on acrylamide in food: Occurrence, toxicity, and mitigation strategies. Int. J. Toxicol. 2020, 39, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Becalski, A.; Brady, B.; Feng, S.; Gauthier, B.R.; Zhao, T. Formation of acrylamide at temperatures lower than 100 °C: The case study of prunes and a model study. Food Addit. Contam. Part A 2011, 28, 726–730. [Google Scholar] [CrossRef]
- Gökmen, V.; Senyuva, H.Z. Improved method for the determination of hydroxymethylfurfural in baby foods using liquid chromatography-mass spectrometry. J. Agric. Food Chem. 2006, 54, 2845–2849. [Google Scholar] [CrossRef]
- Paradiman, A.Z.; Tahir, M.M.; Dirpan, A. Formation of acrylamide compounds in food products from Maillard reactions: A review article. In BIO Web of Conferences; EDP Sciences: Les Ulis, France, 2024; Volume 96, p. 01030. [Google Scholar] [CrossRef]
- Stadler, R.H.; Blank, I.; Varga, N.; Robert, F.; Hau, J.; Guy, P.A.; Robert, M.-C.; Riediker, S. Acrylamide from Maillard reaction products. Nature 2002, 419, 449–450. [Google Scholar] [CrossRef]
- Mottram, D.S.; Wedzicha, B.L.; Dodson, A.T. Acrylamide is formed in the Maillard reaction. Nature 2002, 419, 448–449. [Google Scholar] [CrossRef] [PubMed]
- Žilić, S.; Dodig, D.; Basić, Z.; Vančetović, J.; Titan, P.; Đurić, N.; Tolimir, N. Free asparagine and sugars profile of cereal species: The potential of cereals for acrylamide formation in foods. Food Addit. Contam. Part A 2017, 34, 705–713. [Google Scholar] [CrossRef]
- Czajkowska, K.; Kowalska, H. Methods of manufacturing fruit snacks enriched in natural ingredients. Technol. Prog. Food Process. 2017, 27, 110–115. (In Polish) [Google Scholar]
- Moczkowska, M.; Karp, S.; Niu, Y.; Kurek, M.A. Enzymatic, enzymatic-ultrasonic and alkaline extraction of soluble dietary fibre from flaxseed–A physicochemical approach. Food Hydrocoll. 2019, 90, 105–112. [Google Scholar] [CrossRef]
- Walkowiak, M.; Spasibionek, S.; Krótka, K. Variation and genetic analysis of fatty acid composition in flax (Linum usitatissimum L.). Euphytica 2022, 218, 2. [Google Scholar] [CrossRef]
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary fibre and fibre-rich by-products of food processing: Characterisation, technological functionality and commercial applications: A Review. Food Chem. 2011, 124, 411–421. [Google Scholar] [CrossRef]
- Urbańska, B.; Szafrański, T.; Kowalska, H.; Kowalska, J. Study of polyphenol content and antioxidant properties of various mix of chocolate milk masses with different protein content. Antioxidants 2020, 9, 299. [Google Scholar] [CrossRef]
- Zhou, M.; Sun, Y.; Luo, L.; Pan, H.; Zhang, Q.; Yu, C. Road to a bite of rosehip: A comprehensive review of bioactive compounds, biological activities, and industrial applications of fruits. Trends Food Sci. Technol. 2023, 136, 76–91. [Google Scholar] [CrossRef]
- Qin, Z.; Liu, H.-M.; Lv, T.-T.; Wang, X.-D. Structure, rheological, thermal and antioxidant properties of cell wall polysaccharides from Chinese quince fruits. Int. J. Biol. Macromol. 2020, 147, 1146–1155. [Google Scholar] [CrossRef]
- Berktas, S.; Cam, M. Effects of acid, alkaline and enzymatic extraction methods on functional, structural and antioxidant properties of dietary fibre fractions from quince (Cydonia oblonga Miller). Food Chem. 2025, 464, 141596. [Google Scholar] [CrossRef]
- Bu, X.; Xu, Y.; Zhao, M.; Li, D.; Zou, J.; Wang, L.; Bai, J.; Yang, Y. Simultaneous extraction of polysaccharides and polyphenols from blackcurrant fruits: Comparison between response surface methodology and artificial neural networks. Ind. Crops Prod. 2021, 170, 113682. [Google Scholar] [CrossRef]
- Trojanowicz, P. The dynamic development of NFC juice market in Poland. Ferment. Fruit-Veg. Process. Ind. 2015, 59, 3–4. (In Polish) [Google Scholar]
- Benjakul, S.; Pisuchpen, S.; O’Brien, N.; Karnjanapratum, S. Effect of antioxidants, and packing conditions on storage stability of cereal bar fortified with hydrolyzed collagen from seabass skin. Ital. J. Food Sci. 2019, 31, 347–366. [Google Scholar] [CrossRef]
- Langston, F.M.A.; Nash, G.R.; Bows, J.R. The retention and bioavailability of phytochemicals in the manufacturing of baked snacks. Crit. Rev. Food Sci. Nutr. 2023, 63, 2141–2177. [Google Scholar] [CrossRef] [PubMed]
- Stankiewicz, J.; Wieczorkiewicz, B. The content of vitamin C in short-shelf-life (24 hrs) juices available commercially and produced at home. Sci. J. Gdyn. Marit. Univ. 2017, 99, 62–70. (In Polish) [Google Scholar]
- Przygoda, B.; Wojtasik, A.; Pietraś-Krzyżewska, E.; Kunachowicz, H. Carbohydrates. In Nutrition Standards for the Polish Population; Rychlik, E., Stoś, K., Woźniak, A., Mojska, H., Eds.; National Institute of Public Health PZH—National Research Institute: Warsaw, Poland, 2024; pp. 111–144. (In Polish) [Google Scholar]
- Eswaran, S.; Muir, J.; Chey, W.D. Fiber and functional gastrointestinal disorders. Am. J. Gastroenterol. 2013, 108, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Naveed, H.; Sultan, W.; Awan, K.A.; Imtiaz, A.; Yaqoob, S.; Al-Asmari, F.; Faraz, A.; Qian, J.-Y.; Sharma, A.; Mugabi, R.; et al. Glycemic impact of cereal and legume-based bakery products: Implications for chronic disease management. Food Chem. X 2024, 24, 101959. [Google Scholar] [CrossRef]
- Boukid, F.; Mefleh, M.; Mameri, H.; Rosell, C.M. Plant protein innovations in snacks and bakery: Synergy of market trends and scientific advances. Food Biosci. 2024, 62, 105580. [Google Scholar] [CrossRef]
- Singh, K.K.; Mridula, D.; Rehal, J.; Barnwal, P. Flaxseed: A potential source of food, feed and fiber. Crit. Rev. Food Sci. Nutr. 2011, 51, 210–222. [Google Scholar] [CrossRef]
- Derewiaka, D.; Górska, J. Characteristics of fats occurring in the selected cereal bars. Bromatol. Toxicol. Chem. 2012, 45, 408–413. (In Polish) [Google Scholar]
- Aranceta, J.; Pérez-Rodrigo, C. Recommended dietary reference intakes, nutritional goals and dietary guidelines for fat and fatty acids: A systematic review. Br. J. Nutr. 2012, 107, S8–S22. [Google Scholar] [CrossRef]
- Mojska, H.; Jasińska-Melon, E.; Ołtarzewski, M.; Szponar, L. Fats. In Nutrition Standards for the Polish Population; Rychlik, E., Stoś, K., Woźniak, A., Mojska, H., Eds.; National Institute of Public Health PZH—National Research Institute: Warsaw, Poland, 2024; pp. 77–109. (In Polish) [Google Scholar]
- Woźniak, A.; Matczuk, E.; Kłys, W.; Pachocka, L. Proteins. In Nutrition Standards for the Polish Population; Rychlik, E., Stoś, K., Woźniak, A., Mojska, H., Eds.; National Institute of Public Health PZH—National Research Institute: Warsaw, Poland, 2024; pp. 41–75. (In Polish) [Google Scholar]
- Ajandouz, E.H.; Desseaux, V.; Tazi, S.; Puigserver, A. Effects of temperature and pH on the kinetics of caramelisation, protein cross-linking and Maillard reactions in aqueous model systems. Food Chem. 2008, 107, 1244–1252. [Google Scholar] [CrossRef]
- Dar, B.N. Cereal brans: Transforming upcycled ingredients for sustainable food solutions aligned with SDGs. Trends Food Sci. Technol. 2024, 153, 104738. [Google Scholar] [CrossRef]
- Senarathna, S.; Mel, R.; Malalgoda, M. Utilization of cereal-based protein ingredients in food applications. J. Cereal Sci. 2024, 116, 103867. [Google Scholar] [CrossRef]
- Amin, M.Z.; Islam, T.; Uddin, P.R.; Uddin, M.J.; Rahman, M.M.; Satter, M.A. Comparative study on nutrient contents in the different parts of indigenous and hybrid varieties of pumpkin (Cucurbita maxima Linn.). Heliyon 2019, 5, E02462. [Google Scholar] [CrossRef] [PubMed]
- Roszko, M.Ł.; Szczepańska, M.; Szymczyk, K.; Rzepkowska, M. Dietary risk evaluation of acrylamide intake with bread in Poland, determined by two comparable cleanup procedures. Food Addit. Contam. Part B Surveill. 2020, 13, 1–9. [Google Scholar] [CrossRef]
- Żbikowska, A.; Kowalska, M.; Żbikowska, K.; Onacik-Gür, S.; Łempicka, U.; Turek, P. Study on the incorporation of oat and yeast β-Glucan into shortbread biscuits as a basis for designing healthier and high quality food products. Molecules 2022, 27, 1393. [Google Scholar] [CrossRef] [PubMed]
- ISO 5508; Animal and Vegetable Fats and Oils—Analysis by Gas Chromatography of Methyl Esters of Fatty Acids. International Organization for Standardization: Geneva, Switzerland, 2000.
- ISO 5509; Animal and Vegetable Fats and Oils—Preparation of Methyl Esters of Fatty Acids. International Organization for Standardization: Geneva, Switzerland, 2000.
- AOAC 991.43; Total, Soluble, and Insoluble Dietary Fiber in Foods: Enzymatic-Gravimetric Method, MES-TRIS Buffer. AOAC: Rockville, MD, USA, 1995.
- AACC 32-07.01; Soluble, Insoluble, and Total Dietary Fiber in Foods and Food Products. AOAC: Rockville, MD, USA, 1999.
- PN-EN ISO 20483:2014:02; Determination of Nitrogen Content and Conversion to Protein Content—Kjeldahl Method. Polish Committee for Standardization (PKN): Warsaw, Poland, 2014.
- PN-EN ISO 11085:2015-10; Determination of Crude Fat and Total Fat Content by Randall Extraction Method. Polish Committee for Standardization (PKN): Warsaw, Poland, 2015.
- EN ISO 2171:2023-09; Determination of Ash Yield by Incineration. The International Organization for Standardization (ISO): Geneva, Switzerland, 2023.
- Regulation (EU) 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the Provision of Food Information to Consumers. Available online: https://data.europa.eu/eli/reg/2011/1169/oj (accessed on 10 May 2025).
- Baryłko-Pikielna, N.; Matuszewska, I. Sensory Food Testing: Fundamentals, Methods, Applications; Scientific Publishing House PTTŻ (Polish Society of Food Technologists): Cracow, Poland, 2009. (In Polish) [Google Scholar]
- Handa, C.; Goomer, S.; Siddhu, A. Pilot-scale technology development, nutritional and consumer assessment of whole-multigrain cookies as influenced by fructan inclusion. J. Food Sci 2011, 76, S198–S202. [Google Scholar] [CrossRef]
- Regulation, Protection. Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data. Off. J. Eur. Union 2016, L119, 177–184. Available online: http://data.europa.eu/eli/reg/2016/679/oj (accessed on 10 May 2025).
- PN-EN ISO 6887-4:2017-05; Cereal Grains, Legume Seeds and Their Products—Determination of Ash Content by Combustion Method. The International Organization for Standardization (ISO): Geneva, Switzerland, 2017.
- PN-EN ISO 4833-1:2013-12; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms. Polish Committee for Standardization (PKN): Warsaw, Poland, 2013.
- Beuchat, L.R.; Mann, D.A.; Gurtler, J.B. Comparison of dry sheet media and conventional agar media methods for enumerating yeasts and molds in food. J. Food Prot. 2007, 70, 2661–2664. [Google Scholar] [CrossRef] [PubMed]
- PN-EN ISO 6888-1:2022-03; Horizontal Method for Enumerating Coagulase-Positive Staphylococci. Polish Committee for Standardization (PKN): Warsaw, Poland, 2022.
- PN-EN ISO 21528-2:2017-08; Horizontal Method for the Detection and Enumeration of Enterobacteriaceae. Polish Committee for Standardization (PKN): Warsaw, Poland, 2017.
- PN-EN ISO 7932:2005; Horizontal Method for Enumerating Presumptive Bacillus Cereus. Polish Committee for Standardization (PKN): Warsaw, Poland, 2005.

| Apple Pomace Share [%] | Carbohydrates [%] | Fat [%] | Proteins [%] | Total Fibre [%] | Fibre Insoluble [%] | Fibre Soluble [%] | Ash [%] | TPC [mg GAE/100 g d.m.]/DPPH [µM Trolox/100 g d.m.] |
|---|---|---|---|---|---|---|---|---|
| 0 | 31.22 ± 0.98 | 26.62 c ± 0.67 | 16.57 ± 0.47 | 9.36 b ± 0.18 | 7.66 b ± 0.12 | 1.70 b ± 0.04 | 3.05 c ± 0.11 | 334.60 c ± 11.10/23.06 c ± 1.10 |
| 2 | 30.45 ± 0.86 | 24.77 a ± 0.61 | 16.15 ± 0.64 | 9.96 a ± 0.21 | 6.68 a ± 0.14 | 3.28 a ± 0.06 | 2.86 a ± 0.12 | 286.68 b ± 7.17/90.76 b ± 19.15 |
| 6 | 30.36 ± 1.23 | 23.59 a ± 0.55 | 16.22 ± 0.40 | 9.99 a ± 0.22 | 6.34 a ± 0.26 | 3.67 a ± 0.04 | 2.79 a ± 0.12 | 264.47 ab ± 12.14/87.52 ab ± 21.04 |
| 12 | 30.09 ± 1.33 | 21.92 b ± 0.47 | 15.18 ± 0.34 | 10.47 a ± 0.32 | 6.37 a ± 0.23 | 4.08 a ± 0.04 | 2.60 b ± 0.12 | 249.52 a ± 8.81/86.16 a ± 16.20 |
| Fibre/Binder/Thermal Method | Linoleic Acid (EFAs) | Oleic Acid (EFAs) | γ-Linolenic Acid (EFAs) | Palmitic Acid (Saturated) | Stearic Acid (Saturated) | Behenic Acid (Saturated) | |
|---|---|---|---|---|---|---|---|
| Ap-P | Wild rose/BAK | 35.73 acA ± 0.03 | 33.22 aaA ± 0.13 | 15.22 bcA ± 0.03 | 7.45 aA ± 0.02 | 4.52 bA ± 0.03 | 1.58 b ± 0.10 |
| Wild rose/MC | 37.28 acB ± 0.10 | 34.73 aaB ± 0.13 | 12.25 bcB ± 0.01 | 8.03 aB ± 0.07 | 4.685 bB ± 0.02 | 1.24 b ± 0.09 | |
| Ap-C | Quince/BAK | 38.14 bbA± 0.03 | 34.21 bbA ± 0.00 | 11.01 aaA ± 0.03 | 8.03 bA ± 0.01 | 4.67 aA ± 0.08 | 1.61 a ± 0.01 |
| Wild rose/BAK | 37.05 abA ± 0.01 | 33.03 abA ± 0.03 | 13.53 abA ± 0.03 | 8.18 bA ± 0.08 | 4.98 bA ± 0.02 | 1.25 b ± 0.01 | |
| Ap-P | Quince/BAK | 39.14 bcA ± 0.01 | 31.31 baA ± 0.01 | 13.68 acA ± 0.07 | 7.80 aA± 0.06 | 4.70 aA ± 0.03 | 1.46 a ± 0.06 |
| Ap-BC | 37.43 baA ± 0.09 | 33.62 bcA ± 0.07 | 12.35 abA ± 0.08 | 8.02 bA ± 0.04 | 4.82 aA ± 0.01 | 1.46 a ± 0.08 | |
| Type of Binding Ingredient | Type of Fibre | ||
|---|---|---|---|
| Apple–Psyllium | Apple–Cacao | Blackcurrant | |
| Water | 289 ± 13 | 295 ± 12 | 290 ± 15 |
| NFC juices: wild rose, quince, blackcurrant | 302 ± 11 | 309 ± 12 | 304 ± 16 |
| Fibre/NFC Juice | Yeasts and Moulds CFU/g | Aerobic Mesophilic Bacteria CFU/g | Spore-Forming Bacteria CFU/g | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Storage Period [Days] | |||||||||
| 0 | 7 | 14 | 0 | 7 | 14 | 0 | 7 | 14 | |
| Psyll/Quince | nd A | 2.3 × 104 g B ± 3.5 × 101 | 5.5 × 106 g C ± 1.4 × 104 | 3.5 × 102 e B ± 1.4 × 101 | 2.5 × 104 e B ± 0.0 | 1.1 × 105 e C ± 1.4 × 104 | 1.1 × 102 a A ± 3.1 × 100 | 1.1 × 102 a B ± 1.4 × 101 | 2.4 × 102 a C ± 1.4 × 101 |
| Psyll/Wild Rose | nd A | 2.6 × 104 b B ± 1.4 × 101 | 3.8 × 102 b C ± 2.1 × 101 | 2.6 × 102 d A ± 1.4 × 101 | 2.5 × 104 d B ± 1.1 × 102 | 8.8 × 104 d C ± 2.2 × 103 | 1.3 × 102 bc A ± 2.1 × 101 | 2.1 × 102 bc B ± 1.0 × 101 | 5.2 × 102 bc C ± 2.3 × 101 |
| Psyll/Blackcurrant | nd A | 2.1 × 102 f B ± 7.1 × 100 | 4.5 × 106 f C ± 7.1 × 103 | 5.9 × 102 c A ± 6.1 × 100 | 3.7 × 102 c B ± 5.1 × 100 | 8.7 × 104 c C ± 5.1 × 102 | 1.3 × 102 d A ± 2.4 × 101 | 1.4 × 102 d B ± 2.1 × 101 | 7.1 × 102 d C ± 1.1 × 100 |
| Apple–Cacao/Quince | nd A | 6.0 × 102 d B ± 0.0 | 2.4 × 105 d C ± 7.0 × 103 | 6.1 × 102 b A ± 1.1 × 101 | 1.5 × 103 b B ± 1.1 × 102 | 6.6 × 104 b C ± 2.4 × 103 | 1.7 × 102 cd A ± 2.4 × 101 | 2.2 × 102 cd B ± 1.1 × 101 | 5.4 × 102 cd C ± 1.1 × 101 |
| Apple–Cacao/Wild Rose | nd A | 1.2 × 102 cd B ± 2.8 × 101 | 2.3 × 105 cd C ± 1.3 × 104 | 2.5 × 102 a A ± 5.3 × 100 | 8.7 × 102 a B ± 2.2 × 101 | 1.2 × 104 a C ± 2.8 × 103 | 3.4 × 102 ef A ± 1.4 × 101 | 3.6 × 102 ef B ± 4.1 × 101 | 5.5 × 102 ef C ± 1.3 × 101 |
| Apple–Cacao/Blackcurrant | nd A | 8.2 × 102 c B ± 2.8 × 101 | 2.1 × 105 c C ± 1.4 × 104 | 4.4 × 102 a A ± 5.1 × 100 | 4.6 × 102 a B ± 1.4 × 101 | 2.4 × 104 a C ± 1.4 × 103 | 3.3 × 102 b A ± 2.8 × 101 | 1.8 × 102 b B ± 2.0 × 101 | 3.3 × 102 b C ± 7.0 × 100 |
| Blackcurrant/Quince | nd A | nd a B | 4.2 × 105 a C ± 2.1 × 104 | 3.2 × 102 b A ± 7.1 × 100 | 4.1 × 102 b B ± 5.3 × 100 | 4.9 × 104 b C ± 7.1 × 102 | 2.1 × 102 e A ± 3.1 × 100 | 2.8 × 102 e B ± 2.2 × 101 | 7.3 × 102 e C ± 2.2 × 101 |
| Blackcurrant/Wild Rose | nd A | nd ab B | 5.8 × 105 ab C ± 7.1 × 103 | 4.6 × 102 d A ± 1.1 × 101 | 3.8 × 103 d B ± 7.0 × 101 | 1.1 × 105 d C ± 1.4 × 104 | 1.4 × 102 b A ± 2.0 × 100 | 3.3 × 102 b B ± 1.4 × 101 | 3.4 × 102 b C ± 7.1 × 100 |
| Blackcurrant/Blackcurrant | nd A | 2.2 × 102 e B ± 2.1 × 101 | 5.2 × 105 e C ± 1.4 × 104 | 2.3 × 102 e A ± 1.4 × 101 | 1.4 × 103 e B ± 6.4 × 101 | 1.4 × 105 e C ± 6.1 × 103 | 2.1 × 102 f A ± 7.3 × 100 | 2.7 × 102 f B ± 1.1 × 101 | 8.3 × 102 f C ± 7.1 × 100 |
| Nutritional Content/Bar Ingredients | Fat, Including Saturated Fatty Acids | Carbohydrates, Including Sugars | Protein | Fibre | Energy Value |
|---|---|---|---|---|---|
| Whole-grain oat flakes | 7.6 (1.4) | 69.0 (1.3) | 14.0 | 9.0 | 1764/418 |
| Pumpkin seeds | 41.0 (7.3) | 5.6 (1.6) | 35.0 | 8.8 | 2273/548 |
| Linseed | 42.0 (3.7) | 1.0 (0.5) | 20.0 | 26.0 | 2119/514 |
| Sunflower seeds | 51.0 (5.4) | 14.0 (2.3) | 21.0 | 7.8 | 2557/618 |
| NFC quince juice | 0 | 11.0 (8.7) | 0 | - | 186/44 |
| NFC wild rose | 0 | 10.0 (5.5) | 0 | 3.3 | 209/50 |
| NFC blackcurrant | 0 | 8.6 (6.7) | 0 | 0.9 | 162/38 |
| Distinguishing Feature | Boundary Terms; Point Scores * | |
|---|---|---|
| Definition | ||
| Apperance | Colour of bars (colouration) | 1—exceptionally undesirable, discolouration; 10—exceptionally desirable |
| Smell | Intensity of the scent sensed | 1—exceptionally unnoticeable, foreign; 10—exceptionally characteristic |
| Sweetness | Intensity of sweetness | 1—exceptionally not sweet, 10—exceptionally desirable sweet |
| Taste | Felt after biting and chewing | 1—exceptionally undesirable, foreign; 10—exceptionally desirable, characteristic |
| Texture | Brittleness and porosity | 1—exceptionally undesirable, too hard, too compact; 10—exceptionally desirable, brittle |
| Desirability | The overall impression | 1—exceptionally undesirable; 10—exceptionally desirable |
 .
.Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalska, H.; Masiarz, E.; Hać-Szymańczuk, E.; Żbikowska, A.; Marzec, A.; Salamon, A.; Kozłowska, M.; Ignaczak, A.; Chobot, M.; Sobocińska, W.; et al. The Influence of Recipe Modification and the Technological Method on the Properties of Multigrain Snack Bars. Molecules 2025, 30, 3160. https://doi.org/10.3390/molecules30153160
Kowalska H, Masiarz E, Hać-Szymańczuk E, Żbikowska A, Marzec A, Salamon A, Kozłowska M, Ignaczak A, Chobot M, Sobocińska W, et al. The Influence of Recipe Modification and the Technological Method on the Properties of Multigrain Snack Bars. Molecules. 2025; 30(15):3160. https://doi.org/10.3390/molecules30153160
Chicago/Turabian StyleKowalska, Hanna, Ewelina Masiarz, Elżbieta Hać-Szymańczuk, Anna Żbikowska, Agata Marzec, Agnieszka Salamon, Mariola Kozłowska, Anna Ignaczak, Małgorzata Chobot, Wioletta Sobocińska, and et al. 2025. "The Influence of Recipe Modification and the Technological Method on the Properties of Multigrain Snack Bars" Molecules 30, no. 15: 3160. https://doi.org/10.3390/molecules30153160
APA StyleKowalska, H., Masiarz, E., Hać-Szymańczuk, E., Żbikowska, A., Marzec, A., Salamon, A., Kozłowska, M., Ignaczak, A., Chobot, M., Sobocińska, W., & Kowalska, J. (2025). The Influence of Recipe Modification and the Technological Method on the Properties of Multigrain Snack Bars. Molecules, 30(15), 3160. https://doi.org/10.3390/molecules30153160







